Abstract
Background:
Endovascular therapy and intravenous thrombolysis with recombinant tissue plasminogen activator are the 2 most recommended treatments for acute ischemic stroke (AIS). Glycoprotein (GP) IIb-IIIa inhibitors are short-acting selective reversible antiplatelet agents that emerged as promising therapeutic agents for AIS about 10 years ago. Given the unclear safety profile and application coverage of GP inhibitors, we conducted this meta-analysis to explore the same.
Methods:
We used GP IIb-IIIa inhibitors, intracranial hemorrhage, and mortality as the key words on Medline, Web of Science, and the Embase databases. Randomized controlled trials, prospective literatures, and retrospective studies in English published between 1990 and 2020 were screened. The outcomes were relative risk (RR) of death and 90-day intracerebral hemorrhage (ICH). We pooled the results in 2 categories and conducted a subgroup analysis stratified by different drugs. The choice of the effects model depended on the value of I 2.
Results:
In all, 3700 patients from 20 studies were included. No GP IIb-IIIa inhibitors were found to have a remarkable influence on the ICH rate. The RR values of symptomatic ICH for abciximab and eptifibatide were 4.26 (1.89, 9.59) and 0.17 (0.04, 0.69), respectively. Both tirofiban and abciximab could decrease the mortality rate within 90 days. Age > 70 years, National Institutes of Health Stroke Scale > 15, and overall dose > 10 mg are risk factors for ICH events with tirofiban usage. Thrombectomy combined with tirofiban was safe for arterial reocclusion prevention.
Conclusions:
In stroke-related treatment, administration of GP IIb-IIIa inhibitors could be safe, but care should be taken regarding drug species and doses. Abciximab can increase the risk of symptomatic intracranial hemorrhage. Tirofiban and eptifibatide can be considered safe in low doses. Suitable patients should be selected using strict criteria.
Keywords: glycoprotein IIb-IIIa inhibitors, ischemic stroke, intracranial hemorrhage, dose
Introduction
Stroke may occur in one of 6 people in their lifetime around the world, and around 5.8 million people die annually from stroke (http://world-stroke.org). About 70% of all strokes are ischemic in nature. Acute ischemic stroke (AIS) is defined as the sudden loss of blood flow to a brain area with resulting loss of neurologic function.1 Thus, early vessel recanalization has been the major target in AIS treatment.2 Over the past 2 decades, intravenous thrombolysis has been the main method to achieve vessel recanalization for ischemia–reperfusion.1 Therapy with intravenous recombinant tissue plasminogen activator (rt-PA) is the first-line option when contraindications are absent.3,4 Endovascular treatment proved to be effective in improving functional outcomes for selected patients with AIS5 and is recommended by current clinical practice guideline.6 However, the application of intravenous rt-PA is limited by a narrow therapeutic time window and relatively poor revascularization rates,7 and endovascular therapy can cause injury to endothelial cells leading to activation of local platelet aggregation and subsequent early reocclusion.8 Demands for more effective and safe thrombolytic agents are required.
Glycoprotein (GP) IIb-IIIa inhibitors are short-acting, reversible, selective platelet antagonists that act by antagonizing the GP IIb-IIIa receptors on the platelet surface and block the final common pathway to platelet aggregation by preventing fibrinogen molecules between adjacent platelets from binding. Thus, GP IIb-IIIa inhibitors could favor endogenous thrombolysis by suppressing thrombus growth and preventing thrombus reformation through competitive inhibition with fibrinogen. Since being approved by the Food and Drug Administration (FDA) for treatment of acute coronary syndrome, GP IIb-IIIa inhibitors have been widely used in percutaneous coronary intervention including angioplasty, thrombectomy, and stent implantation.9 Additional data showed that GP antagonists have neuroprotective properties and could improve cerebral microcirculatory blood flow.10 Hence, researchers have attempted to explore the applicability of GP IIb-IIIa inhibitors in AIS treatment on the basis of existing research in ischemic heart disease.
Tirofiban, eptifibatide, and abciximab are 3 GP IIa-IIIb receptor antagonists approved by the US FDA.11 Yet, their safety in treating stroke and preventing reocclusion after intervention remains debatable. A trial on abciximab therapy for stroke—Abciximab Emergent Stroke Treatment Trial (AbESTT-II)—which enrolled patients with stroke within 6 hours from onset, was stopped ahead of schedule owing to a 6-fold increase in the symptomatic intracranial hemorrhage (SICH) rate.12 Kellert et al reported that tirofiban raised the risk for fatal intracranial hemorrhage (ICH) in patients with stroke after mechanical thrombectomy in a prospective nonrandomized study,8 while another recent study found that half-dose abciximab is safe and effective as intravascular therapy for acute stroke.13 Ciccone et al14 last updated the Cochrane systematic review on GP antagonists in 2014 and concluded that GP antagonists not only did not reduce mortality but also significantly increased the risk of SICH. However, their study was based on only 4 randomized controlled trials (RCTs; 3 on abciximab and 1 on tirofiban). By contrast, our analysis addresses what was lacking in the Cochrane review, by discussing the efficacy of GP antagonists combined with mechanical thrombectomy in AIS treatment. Considering the fact that safety profile of GP antagonists is unclear in both clinical trials and retrospective studies, a more comprehensive systematic review is essential
The present meta-analysis aimed to present the respective ICH risk for 3 GP IIa-IIIb receptor antagonists. The initial analysis was conducted only on RCTs, followed by another one on all study types. For tirofiban, we reported its relationship with age, National Institute of Health stroke scale (NIHSS) at admission, and total dose through regression analysis. Overall, we intended to provide an overview on tirofiban, eptifibatide, and abciximab in AIS and discuss factors that might amplify the incidence of adverse events.
Methods
Search Strategy and Selection Criteria
Published literature from January 1, 1990, through January 30, 2020, were independently retrieved by 2 authors on Medline, Web of Science, and the Embase databases. We screened literature relevant to GP IIb-IIIa inhibitors (Tirofiban, Eptifibatide, Abciximab, etc), stroke, and ICH. Two reviewers independently screened relevant articles through titles and abstracts after duplicate records were eliminated on endnote. A review of the full text of eligible articles was conducted. A third reviewer was consulted if disagreement occurred. A trial or study was included if: (1) it focused on patients with AIS; (2) patients in the experiment group were given abciximab, eptifibatide, or tirofiban; (3) patients in the control group were given placebo (saline) or aspirin, or it was an open-control trial; (4) it was an RCT or a prospective nonrandomized study or a retrospective control study; (5) additional therapies such as thrombolytic therapy (eg, rt-PA), antiplatelet agents, nonsteroidal anti-inflammation drugs, and mechanical thrombectomy were applied in all study arms; (6) ICH, SICH, or death were reported within 90 days; and (7) drug administration was described with adequate details such as dose and infusion speed.
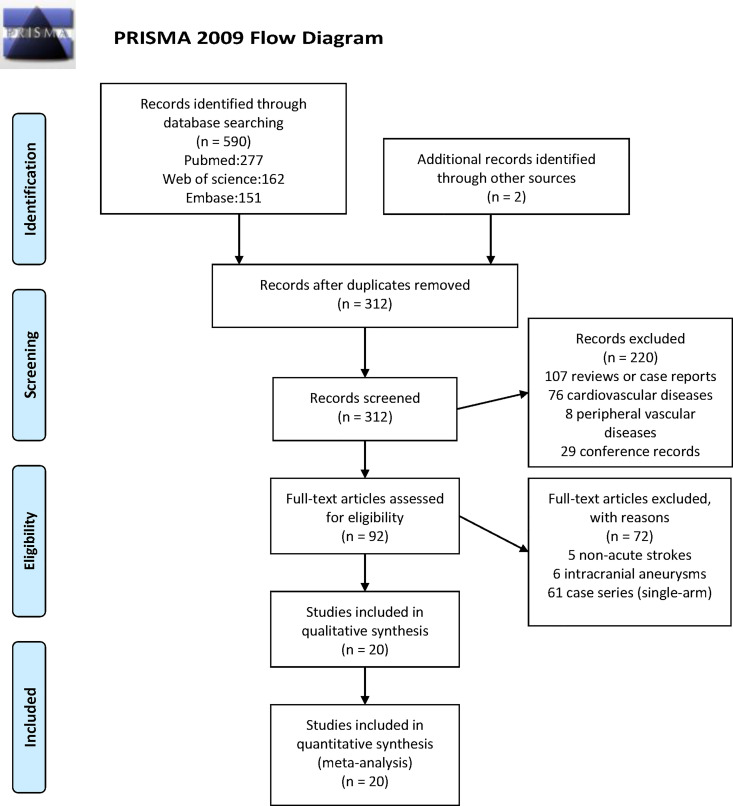
We use the Cochrane risk of bias assessment tool to assess the methodological quality of the selected RCTs and used the Newcastle-Ottawa score to assess the methodological quality of cohort studies. This systematic review reports Preferred Reporting Items for Systematic reviews and Meta-Analyses statement (Figure 1).15
Figure 1.
PRISMA 2009 flow diagram. PRISMA indicates Preferred Reporting Items for Systematic reviews and Meta-Analyses.
Data Extraction and Risk Outcomes
The following aspects were extracted from the included studies: (1) Basic demographic characteristics including sex, mean and median age, NIHSS at admission, number of cases in each group, and onset to treatment time; (2) Disease history: hypertension, hyperlipidemia, diabetes, atrial fibrillation; (3) Medication regimen: type of GP receptor inhibitor, total dose, infusion speed, combined thrombolysis, or interventional therapy schedule; and (4) Risk outcomes: caseload of any ICH, SICH, and death within 90 days in each group. Risk ratio (RR) of any ICH and SICH was calculated and analyzed separately. If cases of ICH or SICH in the experimental group was zero, the study was not considered for analysis of ICH or SICH.
Subgroup Analysis and Metaregression Analysis Strategy
We evaluated the GP receptor inhibitor risk in 2 sets. One merely incorporated RCTs, and another included all type of studies including RCTs, cohort, and case–control studies. Regardless of heterogeneity, we first conducted a subgroup analysis with different GP receptor inhibitor types. More comprehensive analysis was performed if heterogeneity in the subgroup was large (I 2 ≥ 75%). The total dose of tirofiban was divided into “>10 mg” and “≤10 mg”; patient age was divided as “<65 years,” “65 to 70 years,” and “>70 years”; NIHSS at admission was divided as “≤15” and “>15.” Further, based on whether thrombectomy was performed, there are groups of “thrombectomy” and “no thrombectomy”. Next, we conducted a metaregression analysis to further explore the effect of continuous variables such as age and NIHSS on outcomes.
Statistical Analysis
If the lower bound of the 95% CI was >1, we considered the GP receptor inhibitors to increase the risk for ICH and mortality. If the lower bound of the 95% CI was <1, we considered the GP receptor inhibitors as be a protective factor for ICH. Cochran Q test and I 2 test were used in our analysis to quantify the heterogeneity among trials. Cochran Q test was used to verify subgroup differences. If P ≤ .05, the between-subgroup differences were considered to be statistically significant. The random effect model was used for data analysis when I 2 > 75%; otherwise, the fixed effect model was used. We carried out the Z test to evaluate statistical significance in metaregression analysis and checked publication bias through funnel plots and Egger regression asymmetry test. All statistical analysis was performed using the R package (version 3.6.1).
Results
Characteristics of Analyzed Studies
Ultimately, 7 RCTs,16,17,12,18–21 11 prospective studies,22–24,8,25–28,2,29,30 and 2 retrospective studies31,32 with 3700 patients in total were included (Table 1). In all, 5 studies used abciximab, 2 used eptifibatide, and 13 used tirofiban. Patients in 9 studies received interventional therapy incorporating carotid stenting, angioplasty, or thrombectomy. The whole process of literature selection is presented in a flowchart in Figure 1. Results of assessing the methodological quality RCTs (by Cochrane risk of bias assessment tool) and cohort studies (by Newcastle-Ottawa score) are presented in Tables 2 and 3, respectively.
Table 1.
The Basic Characteristics of Included Studies.a
| Study (reference) | Agent | Study type | Intervention | Age (GPI vs control) | Women: Men (GPI vs control) |
Dose |
|---|---|---|---|---|---|---|
| Adams and Bogousslavsky16 arm 1 | Abciximab | RCT | No | 66 ± 13 vs 64 ± 17 | (10:5) vs (8:12) | 0.15 mg/kg bolus |
| Adams et al arm 2 | Abciximab | RCT | No | 63 ± 13 | (6:6) | 0.2 mg/kg bolus |
| Adams et al arm 3 | Abciximab | RCT | No | 64 ± 15 | (5:9) | 0.2 mg/kg bolus + 0.125 μg/kg/min (12 hours) |
| Adams et al arm 4 | Abciximab | RCT | No | 72 ± 14 | (5:8) | 0.25 mg/kg bolus + 0.125 μg/kg/min (12 hours) |
| Eckert et al23 | Abciximab | Prospective | Angioplasty/stenting | 69 (31-84) vs 61 (28-79) | (17:30) vs (7:34) | 0.25 mg/kg bolus + 0.125 μg/kg/min (12 hours) |
| AbESTT17 | Abciximab | RCT | No | 67 ± 13 vs 68 ± 12 | (80:120) vs (95:105) | 0.25 mg/kg bolus + 0.125 μg/kg/min (12 hours) |
| AbESTT-II12 | Abciximab | RCT | No | 68 ± 14 vs 70 ± 13 | (103:118) vs (97:121) | 0.25 mg/kg bolus + 0.125 μg/kg/min (12 hours) |
| Nagel et al24 | Abciximab | Prospective | No | 65 (28-83) vs 65 (19-86) | (17:26) vs (12:20) | 0.25 mg/kg bolus + 0.125 μg/kg/min (12 hours) |
| Pancioli et al (CLEAR)18 | Eptifibatide | RCT | No | 71 (62-77) vs 61 (55-74) | NA | 75 μg/kg bolus + 0.75 μg/kg/min (2 hours) |
| Pancioli et al (CLEAR-ER)21 | Eptifibatide | RCT | No | 71 (58-81) vs 75 (60-81) | (48:53) vs (12:13) | 135 μg/kg bolus + 0.75 μg/kg/min (2 hours) |
| Torgano et al (SETIS)19 | Tirofiban | RCT | No | 71 ± 13 vs 73 ± 8 | (39:36) vs (38:37) | 0.6 μg/kg/min (30 minutes) +0.15 μg/kg/min (72 hours) |
| Siebler et al (SaTIS)20 | Tirofiban | RCT | No | 67 (34-81) vs 65 (30-82) | (57:74) vs (48:81) | 0.4 μg/kg/min (30 minutes) +0.1 μg/kg/min (48 hours) |
| Junghans et al22 | Tirofiban | Prospective | No | 62 (36-80) vs 62 (27-77) | (4:14) vs (6:11) | 0.4 μg/kg/min (30 minutes) +0.1 μg/kg/min (24 hours) |
| Li et al25 | Tirofiban | Prospective | No | 66 (29-86) vs 68 (37-86) | (16:25) vs (17:24) | 0.4 μg/kg/min (30 minutes) +0.1 μg/kg/min (24 hours) |
| Lin et al26 | Tirofiban | Prospective | No | 63 (47-82) vs 70 (44-89) | (5:17) vs (5:17) | 0.4 μg/kg/min (30 minutes) +0.1 μg/kg/min (24 hours) |
| Zhao et al27 | Tirofiban | Prospective | Thrombectomy | 61 ± 13 vs 60 ± 12 | (21:69) vs (30:60) | 5 mg bolus + 0.2-0.25 mg/h (12-24 hours) |
| Wu et al28 | Tirofiban | Prospective | Thrombectomy | 70 (63-76) vs 71 (63-80) | (36:58) vs (56:68) | 3.3-10 μg/kg, 50 μg/min |
| Neuberger et al2 | Tirofiban | Prospective | Thrombectomy | NA | NA | 0.4 μg/kg/min (30 minutes) +0.1 μg/kg/min (48 hours) |
| Zhang et al32 | Tirofiban | Retrospective | Thrombectomy | 64 ± 13 vs 64 ± 12 | (62:92) vs (202:276) | 0.25-1.0 mg, 50 μg/min |
| Kellert et al8 | Tirofiban | Prospective | Thrombectomy | 64 ± 13 vs 67 ± 14 | NA | 0.4 μg/kg/min (30 minutes) + 0.1 μg/kg/min (48 hours) |
| Quan et al29 | Tirofiban | Prospective | Thrombectomy | NA | NA | 5 mg bolus + 0.2-0.25 mg/h (12-24 hours) |
| Sun et al30 | Tirofiban | Prospective | Thrombectomy | 66 ± 15 vs 66 ± 13 | (20:51) vs (38:86) | 0.25-0.5 mg, 1 mL/min |
| Yu et al31 | Tirofiban | Retrospective | Thrombectomy | 70 ± 10 vs 67 ± 10 | (14:12) vs (13:15) | 0.25-0.5 mg, 1 mL/min |
Abbreviations: AbESTT, Abciximab Emergent Stroke Treatment Trial; CLEAR, Combined Approach to Lysis Utilizing Eptifibatide and recombinant tissue plasminogen activator; CLEAR-ER, Combined Approach to Lysis Utilizing Eptifibatide and recombinant tissue plasminogen activator in acute ischemic stroke-enhanced regimen; GPI, glycoprotein inhibitors; NA, not applicable; RCT, randomized control trials; SaTIS, Safety of Tirofiban in acute Ischemic Stroke; SETIS, Study of Efficacy of Tirofiban in acute Ischemic Stroke.
a In total, 5 studies discussed abciximab, 3 discussed eptifibatide, 13 discussed tirofiban, and 1 discussed eptifibatide and abciximab.
Table 2.
Results of the Methodological Quality Assessment of Randomized Control Trials Included in Our Analyses.a
| Study (Reference) | Random sequence generation | Allocation concealment | Blinding of participant/personnel | Blinding of outcome assessment | Incomplete outcome data | Selective outcome reporting |
|---|---|---|---|---|---|---|
| Adams et al16 | Low | Low | Low | Low | Low | Low |
| AbESTT17 | Low | Low | Low | Low | Unclear | Low |
| AbESTT-II12 | Low | Low | Low | Unclear | Unclear | Low |
| CLEAR18 | Low | Low | Low | Low | Low | Low |
| CLEAR-ER21 | Low | Low | Low | Low | Low | Low |
| SETIS19 | Low | Unclear | Low | Low | Low | Low |
| SaTIS20 | Low | Low | Low | Low | Low | Low |
Abbreviations: AbESTT, Abciximab Emergent Stroke Treatment Trial; CLEAR, Combined Approach to Lysis Utilizing Eptifibatide and recombinant tissue plasminogen activator; CLEAR-ER, Combined Approach to Lysis Utilizing Eptifibatide and recombinant tissue plasminogen activator in acute ischemic stroke-enhanced regimen; SaTIS, Safety of Tirofiban in acute Ischemic Stroke; SETIS, Study of Efficacy of Tirofiban in acute Ischemic Stroke.
a Cochrane risk of bias assessment tool was used.
Table 3.
Results of the Methodological Quality Assessment of Prospective and Retrospective Cohort Studies Included in Our Analyses.a
| Study (year) | Selection (maximum 4 stars) | Comparability (maximum 2 stars) | Outcome (maximum 3 stars) | Total Newcastle-Ottawa score |
|---|---|---|---|---|
| Eckert et al23 | 4 | 1 | 2 | 7 |
| Nagel et al24 | 4 | 1 | 2 | 7 |
| Junghans et al22 | 3 | 2 | 2 | 7 |
| Li et al25 | 3 | 2 | 2 | 7 |
| Lin et al26 | 3 | 1 | 2 | 6 |
| Wu et al28 | 3 | 1 | 2 | 6 |
| Zhao et al27 | 3 | 2 | 3 | 7 |
| Neuberger et al2 | 4 | 1 | 2 | 7 |
| Zhang et al32 | 3 | 1 | 2 | 6 |
| Kellert et al8 | 3 | 1 | 2 | 6 |
| Quan et al29 | 3 | 1 | 2 | 6 |
| Sun et al30 | 3 | 2 | 2 | 7 |
| Yu et al31 | 3 | 2 | 2 | 7 |
a The Newcastle-Ottawa score was used. Most included studies carried out a 3-month follow-up. Zhao et al carried out a 1-year follow-up.
Risk Ratio for Any ICH
The pooled relative risk value for ICH was 1.05 (95% CI: 0.82-1.34) in RCTs and 1.21 [95% CI: 0.90-1.62] in all studies (Table 4). It indicates that GP receptor antagonists will not increase the risk of ICH. Furthermore, our results of the subgroup analysis on drug species showed that abciximab, eptifibatide, or tirofiban had any significantly influence on ICH.
Table 4.
The Risk Ratio of 3 Glycoprotein IIb-IIIa Inhibitors.a
| No. | ICH (95% CI) | No. | SICH (95% CI) | No. | Mortality (95% CI) | |
|---|---|---|---|---|---|---|
| Analysis for only randomized control trials | ||||||
| Total | 6 | 1.05 (0.82-1.34) | 6 | 1.78 (1.04-3.04) | 7 | 0.92 (0.66-1.28) |
| Abciximab | 2 | 1.87 (0.64-5.48) | 2 | 4.26 (1.89-9.59) | 3 | 0.93 (0.60-1.45) |
| Eptifibatide | 2 | 0.59 (0.33-1.08) | 2 | 0.17 (0.04-0.69) | 2 | 1.47 (0.69-3.09) |
| Tirofiban | 2 | 1.13 (0.77-1.63) | 2 | 0.50 (0.092.71) | 2 | 0.58 (0.28-1.20) |
| Analysis for all type studies | ||||||
| Total | 15 | 1.21 (0.90-1.62) | 16 | 1.16 (0.92-1.46) | 14 | 0.76 (0.65-0.89) |
| Abciximab | 3 | 1.27 (0.89-1.81) | 4 | 1.88 (1.17-3.00) | 5 | 0.70 (0.54-0.90) |
| Eptifibatide | 2 | 0.59 (0.32-1.08) | 2 | 0.17 (0.04-0.69) | 2 | 1.47 (0.69-3.09) |
| Tirofiban | 10 | 1.27 (0.84-1.93) | 10 | 1.04 (0.78-1.38) | 7 | 0.75 (0.61-0.93) |
Abbreviations: ICH, intracerebral hemorrhage; SICH, symptomatic intracranial hemorrhage.
a No. means number of studies analyzed.
The boldface values means the risk ratio is significant.
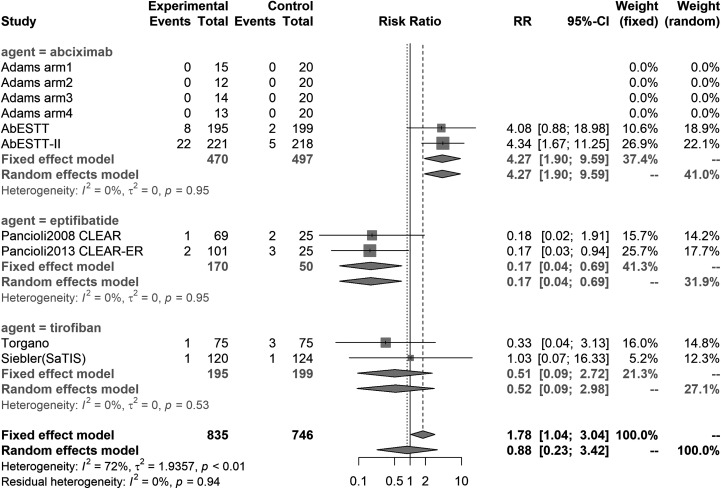
Risk Ratio for Symptomatic ICH
The pooled relative risk value for SICH was 1.78 (95% CI: 1.04-3.04) in RCTs (Figure 2) and 1.16 (95% CI: 0.92-1.46) in all studies (Table 4). This suggests that GP receptor antagonists can possibly induce SICH. In subsequent subgroup analysis, the consolidated results of both RCTs only and all studies showed that abciximab could significantly increase SICH risk (RR: 4.26 [1.89-9.59] in RCT and 1.88 [1.17-3.00] in all studies). Surprisingly, however, eptifibatide showed an effect of reducing SICH risk (RR: 0.17 [0.04-0.69] in RCT). Owing to the high heterogeneity of tirofiban, we subsequently performed a subgroup analysis in greater detail (I 2: 84.9% for ICH and 54.3% for SICH).
Figure 2.
Forest plot of meta-analysis for SICH in RCTs. No heterogeneity was observed for any agent. RCTs indicates randomized controlled trials; SICH, symptomatic intracranial hemorrhage.
Risk Ratio for Mortality Within 90 days
The results incorporating only RCTs suggested that GP receptor antagonists have no effect on mortality in general, whereas the results incorporating all included studies showed that abciximab and tirofiban showed lowered mortality (RR: 0.70 [0.54-0.90] for abciximab; 0.75 [0.61-0.93] for tirofiban). Moreover, eptifibatide did not seem to have any influence on mortality of patients with ischemic stroke (RR: 1.47 [0.69-3.09]).
Subgroup Analysis and Metaregression Analysis for Tirofiban
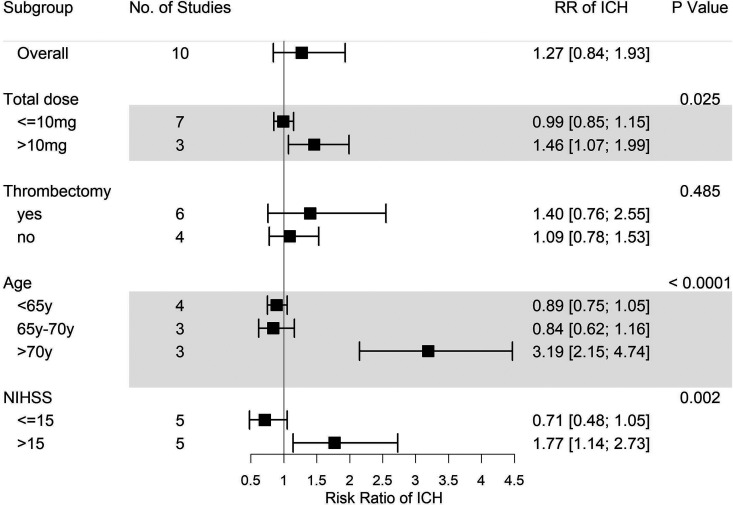
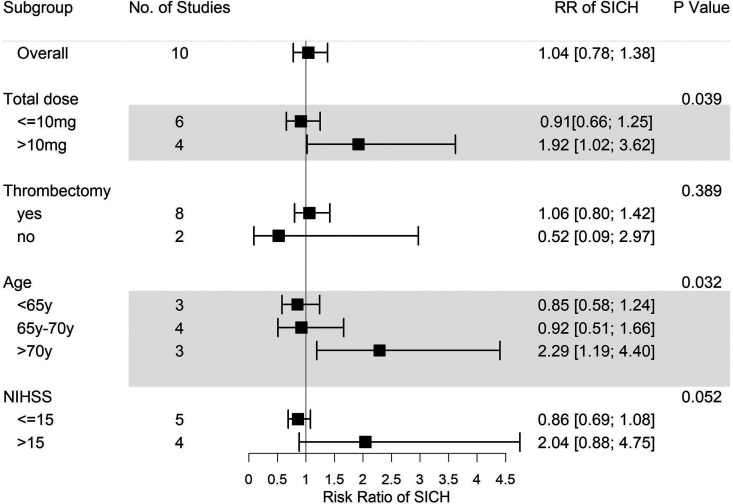
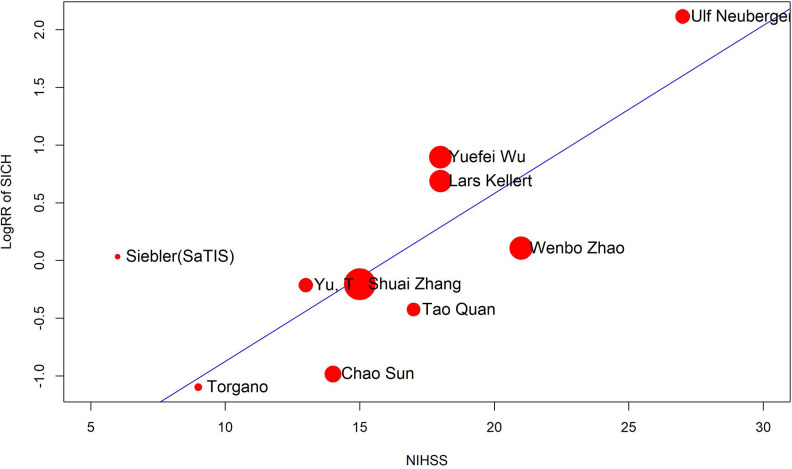
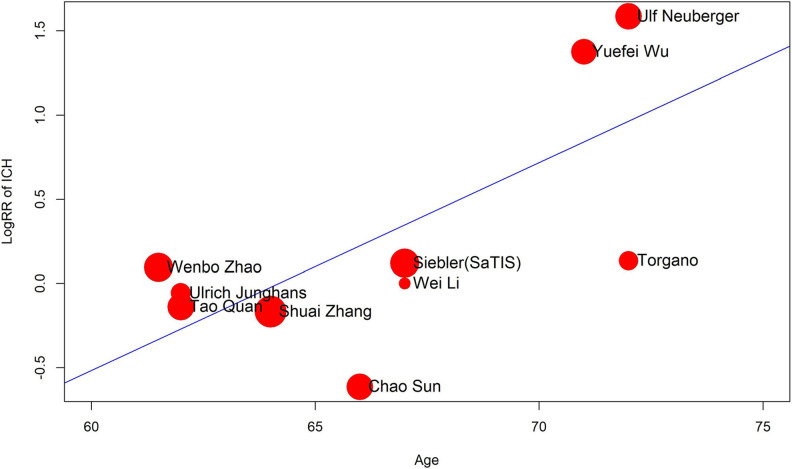
As mentioned above, a more comprehensive subgroup analysis was conducted to identify potential risk factors for tirofiban use. Eventually, we found that while thrombectomy is not one of the factors, overall tirofiban dose >10 mg, age > 70 years, and NIHSS score > 15 all play important roles in the incidence of ICH events (Figures 3 and 4). Thrombectomy combined with tirofiban showed no risk for both ICH and SICH. When the overall tirofiban dose is >10 mg, the RR value of ICH was 1.46 (1.07-1.99), while that of SICH is 1.92 (1.02-3.62). When the initial NIHSS score was >15, the RR for ICH was 1.77 (1.14-2.73), implying an evident hazard, and the RR for SICH was 2.04 (0.88-4.75). In the metaregression model, we found a positive correlation between NIHSS and RR for SICH (P = .001, Figure 5), as well as between age and RR for ICH (P = .0024, Figure 6). Thus, we believe that tirofiban is safe in low doses for selected patients. However, older patients or those with high NIHSS score should be treated with caution.
Figure 3.
Subgroup analysis of ICH for tirofiban. Total dose > 10 mg, age > 70 years, and baseline NIHSS score > 15 are risk factors for ICH. ICH indicates intracerebral hemorrhage; NIHSS, National Institute of Health stroke scale.
Figure 4.
Subgroup analysis of SICH for tirofiban. Total dose > 10 mg and age > 70 years are illustrated as risk factors for SICH. SICH indicates symptomatic intracranial hemorrhage.
Figure 5.
Bubble plot of metaregression analysis for baseline NIHSS score. The risk of SICH is positively related to baseline NIHSS. NIHSS indicates National Institute of Health stroke scale; SICH, symptomatic intracranial hemorrhage.
Figure 6.
Bubble plot of metaregression analysis for age. The risk of ICH is positively related to age. ICH indicates intracerebral hemorrhage.
Publication Bias
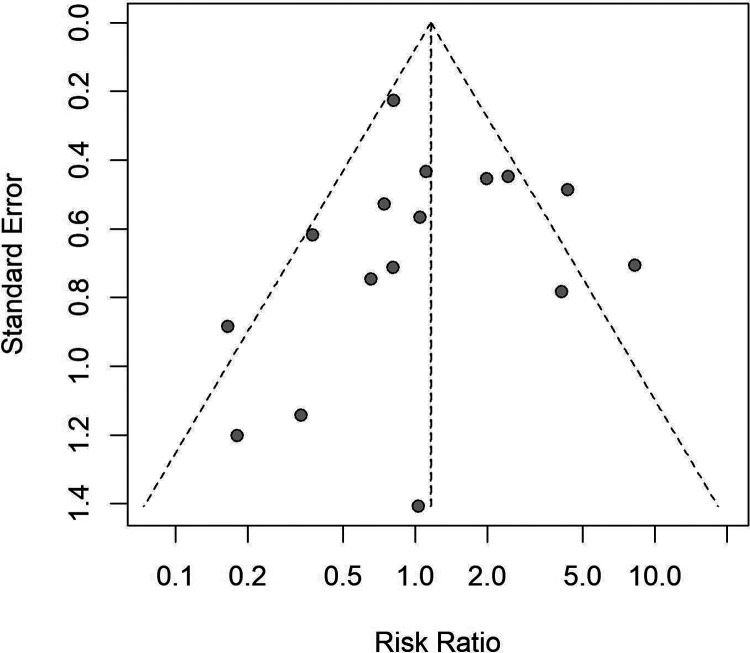
No significant publication bias was noted in the analysis by funnel plots (Figure 7) and Egger regression asymmetry test (P = .253 for ICH, P = .862 for SICH).
Figure 7.
Funnel plot of SICH, including all study types. This figure showed no publication bias existing among included studies. SICH indicates symptomatic intracranial hemorrhage.
Discussion
To assess the safety of combined treatment with GP IIb-IIIa inhibitors in stroke-related treatment, the ICH and mortality at 90 days were considered as the evaluation criteria. As SICH is a relatively fatal bleeding condition in ICH, short-term SICH incidence was also accepted as a separate evaluation standard. We chose 3 agents that are commonly used in clinical practice, namely tirofiban, eptifibatide, and abciximab; searched the relevant literature; pooled and compared results in category; and conducted further subgroup and regression analyses.
Unlike us, Ciccone et al excluded 2 eptifibatide RCTs because they speculated that a lower dose of rt-PA in the experimental group than in the control group might result in bias.14 No RCT yet has proven that low dose rt-PA is safer than the standard dose. Two systematic reviews (Liu et al33 and Liu et al34) also reported no significant difference in SICH incidence between low-dose rt-PA and standard-dose rt-PA. A recent prospective study showed that bleeding risk in the 0.6 mg/kg rt-PA group and 0.9 mg/kg rt-PA group was not significantly different.35 Based on their results, we overlooked the dose difference of rt-PA between the CLEAR and CLEAR-ER trials and included both these studies in our analysis. The Safety of Tirofiban in acute Ischemic Stroke (SaTIS) trial was also excluded in the Cochrane systematic review, because the maximum time from onset to inclusion in SaTIS was 22 hours.20 We speculated that with the same distribution in the experimental and control groups, the onset-to-inclusion time would not bias the final outcomes. Thus, we also included the SaTIS trial.
The American Heart Association published a new international guideline in 2019,15 in which the level of evidence of tirofiban and eptifibatide in AIS treatment is B-R and the class of recommendation is IIb, indicating that benefits for patients are generally greater than the inherent risks. According to our results, for younger patients with lower NIHSS scores, the ICH risk presented a tendency to decrease. Therefore, based on multiple studies, we thought our conclusion can be used as a reference for the development of guidelines and future clinical practice. Furthermore, the administration of abciximab was proposed to be potentially harmful in the guideline according to the results of the AbESTT trial. We will discuss later in the text when we discuss AbESTT trial.
Mechanical thrombectomy has been used for AIS treatment since 2005.36 Accordingly, concerns about postoperative arterial reocclusion were raised. Glycoprotein antagonists, with strong antiplatelet ability, were found superior to prevent arterial reocclusion. Owing to the lack of strong evidence provided by RCT, the Cochrane SR did not investigate its application in this area. Different from them, we included nonrandomized studies in this regard and carried out a preliminary discussion. The 2019 guideline suggested that the efficacy and safety of GP IIa-IIIb inhibitors combined with endovascular therapy are unclear. However, this conclusion was based on 3 retrospective single-arm studies (no control group) with an evidence level of C-LD (low evidence grade).37,38,13 Of 3 studies, 2 were about abciximab and 1 was about tirofiban and abciximab. Therefore, whether the level or the quantity of evidence was inadequate. Instead, we concluded that tirofiban is safe to use when combined with thrombectomy, especially at low doses.
Subgroup results showed that abciximab can remarkably increase a 4-fold risk for SICH but may reduce the 90-day mortality. Adam et al conducted the first clinical trial for abciximab with no occurrence of SICH. This likely ascribes to the low drug dose and small sample size (≤15 in each experimental group).16 In a large international trial (AbESTT II) conducted in 2008, Adams et al reported that the experiment group with abciximab presented a high proportion of hemorrhage, because of which the trial had to be discontinued. Although the superiority of abciximab in ischemic stroke was not demonstrated, the authors, based on their research in phase II, proposed a view that such hemorrhagic ratio is acceptable provided the ultimate outcome can be improved.12 In AbESTT, the experimental group had 8 cases of SICH out of 195 patients; however, the ratio increased to 22 of 221 in the AbESTT-II trial.16,12 In addition, 4 studies focused on carotid stent/angioplasty.39–42 In these studies, all control groups reported no ICH, while all abciximab groups reported ICH and 3 reported SICH. Our conclusions were consistent with their theory. However, the efficacy of abciximab needs more studies and analyses to be confirmed. In this article, we exclusively focused on the safety of drugs. Interestingly, the results of the AbESTT trial indicated that the therapeutic window of abciximab can extend to 24 hours,16 while AbESTT II in 2008 showed a reasonable and safe window within 6 hous from onset.12 Therefore, the time window of administration may also act as a factor for safety. It is also worth noting that AbESTT, AbESTT-II, and the aforementioned 4 case–control studies administrated full dose in cardiac trials, so the application of low-dose abciximab still deserves further research.
Although eptifibatide exhibited no statistically significant impact on any ICH and death, the incidence of SICH was reduced after administration of eptifibatide. The CLEAR trial, the first randomized blind trial on safety of low-dose rt-PA combined with GP IIb-IIIa, showed encouraging results of this combination regimen, perhaps owing to the extremely small dose in CLEAR. However, a limitation of the CLEAR-trial is that the eptifibatide group had a much lower baseline of NIHSS (median: 12.0) than the control group.18 In addition, the CLEAR-ER trial highlighted concerns regarding systemic bleeding, given that cases of fatal, moderate, and mild bleeding were 1, 4, and 12, respectively, in the eptifibatide group but 0 in the standard therapy group.21 Again, the time window of eptifibatide in stroke merits further discussion.
Tirofiban is a highly selective, fast-acting, nonpeptide GP IIa-IIIb inhibitor with short halftime and debatable safety in ischemic stroke. Our subgroup results indicated that tirofiban does not influence ICH or SICH and can lower the 90-day mortality. However, a significant interstudy heterogeneity was detected while merging data. Consequently, further analysis on tirofiban was conducted. Eventually, we found that total dose >10 mg, age >70 years, and NIHSS >15 are risks for ICH events, and thrombectomy was not one of them. Based on these findings, we believe that tirofiban is safe to use but in low doses and is not suggested in either patients with high NIHSS score or older individuals. Kellert et al reported that the occurrence of fatal hemorrhage significantly increased for patients who received tirofiban after mechanical thrombectomy. To this point, we believe it could be attributed to the relatively high drug dose and severe baseline NIHSS score. In their study, the authors noted that the risk was mainly demonstrated in patients with anterior circulation stroke.8 Considering those patients who had lower NIHSS score and shorter time window at admission than those with posterior circulation stroke, which could minimize the events of fatal ICH, the results contradicted our assumption. This correlation between tirofiban-induced hemorrhage and lesion location suggested by Kellert et al still needs validation. Jeong and Jin in 2013 reported that they administrated tirofiban through the arterial route and in a lower dose than recommended to avoid surgical complications such as ICH. The results eventually proved that small dose administration is safe and rewarding.43 A larger clinical trial in 2011 by Siebler et al also reported the safety of tirofiban in ischemic stroke. They believed tirofiban would not carry an extra risk of ICH compared to standard care. Furthermore, patients treated with tirofiban were associated with lower mortality in the 5-month follow-up and higher survival rate at 3 months posttreatment.20 Pharmacologically speaking, with only a 2-hour halftime period, tirofiban has a lower risk of inducing continuous bleeding. Considering the benefits for cardiovascular disease, it is very much likely that tirofiban may be more advantageous to a certain group of people. Other than these, we also found that the risk of SICH was positively associated with the NIHSS score. A similar relationship exists between age and ICH. Thus, age and NIHSS score at admission influence postoperative ICH. This is consistent with the conclusions of Kellert et al,8 in that age and NIHSS score of patients with stroke are both independent predictors of poor prognosis.
A common side effect of all types of GP IIb-IIIa inhibitors is thrombocytopenia. Currently, there has not been clear evidence to show that this could result in poor outcomes. In the AbESTT II trial, the incidence of thrombocytopenia was relatively low and no adverse consequences were reported.16 Moreover, as reported in Qureshi et al, the platelet function of patients could distinctly recover within 4 hours after eptifibatide was discontinued.44
Torgano et al concluded in a study of 150 patients with AIS that tirofiban administered within 6 hours of symptom onset did not further escalate the risk for these patients.19 Another simultaneous trial, the SaTIS, investigated the safety of low-dose tirofiban within 22 hours of symptom onset. Authors of the SaTIS trial concluded that compared to standard care, tirofiban does not increase ICH risk.20 A recent study suggested that because it is difficult to avoid the use of abciximab during stenting in AIS, more balance is required between the antiplatelet effects and safety of a given drug.13 Qureshi et al concluded that eptifibatide could be safely used intravenously after low-dose intra-arterial thrombolysis.44 The CLEAR trial, which assessed the safety of combination therapy within 3 hours from symptom onset, proposed that a reduced dose of rt-PA with eptifibatide was reasonably safe to allow further exploration of the dose range.18
Conclusion
Our results provided convincing evidence that abciximab enhances the risk of SICH when used for AIS treatment and prevention of artery reocclusion. Further, eptifibatide and tirofiban are promising therapeutic agents at a reduced dose. However, patient age and NIHSS score as independent risk factors should be taken into consideration by clinicians. Further investigations on the safety and efficacy of low-dose abciximab should be performed as the evidence available thus far is inadequate.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Genmao Cao  https://orcid.org/0000-0003-2199-6903
https://orcid.org/0000-0003-2199-6903
References
- 1. Phipps MS, Cronin CA. Management of acute ischemic stroke. BMJ. 2020;368:l6983 doi:10.1136/bmj.l6983 [DOI] [PubMed] [Google Scholar]
- 2. Neuberger U, Seker F, Schonenberger S, et al. Prediction of intracranial hemorrhages after mechanical thrombectomy of basilar artery occlusion. J Neurointerv Surg. 2019;11(12):1181–1186. doi:10.1136/neurintsurg-2019-014939 [DOI] [PubMed] [Google Scholar]
- 3. Lindsberg PJ, Mattle HP. Therapy of basilar artery occlusion: a systematic analysis comparing intra-arterial and intravenous thrombolysis. Stroke. 2006;37(3):922–928. [DOI] [PubMed] [Google Scholar]
- 4. Prabhakaran S, Ruff I, Bernstein RA. Acute stroke intervention: a systematic review. JAMA. 2015;313(14):1451–1462. doi:10.1001/jama.2015.3058 [DOI] [PubMed] [Google Scholar]
- 5. Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–1030. [DOI] [PubMed] [Google Scholar]
- 6. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46–e99. [DOI] [PubMed] [Google Scholar]
- 7. Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014384(9958):1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kellert L, Hametner C, Rohde S, et al. Endovascular stroke therapy: tirofiban is associated with risk of fatal intracerebral hemorrhage and poor outcome. Stroke. 2013;44(5):1453–1455. doi:10.1161/strokeaha.111.000502 [DOI] [PubMed] [Google Scholar]
- 9. Stangl PA, Lewis S. Review of currently available GP IIb/IIIa inhibitors and their role in peripheral vascular interventions. Semin Intervent Radiol. 2010;27(4):412–421. doi:10.1055/s-0030-1267856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choudhri TF, Hoh BL, Zerwes HG, et al. Reduced microvascular thrombosis and improved outcome in acute murine stroke by inhibiting GP IIb/IIIa receptor-mediated platelet aggregation. J Clin Invest. 1998;102(7):1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. King S, Short M, Harmon C. Glycoprotein IIb/IIIa inhibitors: the resurgence of tirofiban. Vasc Pharmacol. 2016;78:10–16. [DOI] [PubMed] [Google Scholar]
- 12. Adams HP, Jr, Effron MB, Torner J, et al. Emergency administration of abciximab for treatment of patients with acute ischemic stroke: results of an international phase III trial: abciximab in emergency treatment of stroke trial (AbESTT-II). Stroke. 2008;39(1):87–99. doi:10.1161/STROKEAHA.106.476648 [DOI] [PubMed] [Google Scholar]
- 13. Delgado F, Oteros R, Jimenez-Gomez E, Bravo Rey I, Bautista MD, Valverde Moyano R. Half bolus dose of intravenous abciximab is safe and effective in the setting of acute stroke endovascular treatment. J Neurointerv Surg. 2019;11(2):147–152. doi:10.1136/neurintsurg-2018-014163 [DOI] [PubMed] [Google Scholar]
- 14. Ciccone A, Motto C, Abraha I, Cozzolino F, Santilli I. Glycoprotein IIb-IIIa inhibitors for acute ischaemic stroke. Cochrane Database Syst Rev. 2014;(3):CD005208 doi:10.1002/14651858.CD005208.pub3 [DOI] [PubMed] [Google Scholar]
- 15. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2019;50(12):e344–e418. doi:10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 16. Adams HP, Jr, Bogousslavsky J. Abciximab in acute ischemic stroke: a randomized, double-blind, placebo-controlled, dose-escalation study. Stroke. 2000;31(3):601–609. [DOI] [PubMed] [Google Scholar]
- 17. Abciximab Emergent Stroke Treatment Trial (AbESTT) Investigators. Emergency administration of abciximab for treatment of patients with acute ischemic stroke: results of a randomized phase 2 trial. Stroke. 2005;36(4):880–890. doi:10.1161/01.Str.0000157668.39374.56 [DOI] [PubMed] [Google Scholar]
- 18. Pancioli AM, Broderick J, Brott T, et al. The combined approach to lysis utilizing eptifibatide and rt-PA in acute ischemic stroke: the CLEAR stroke trial. Stroke. 2008;39(12):3268–3276. doi:10.1161/strokeaha.108.517656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Torgano G, Zecca B, Monzani V, et al. Effect of intravenous tirofiban and aspirin in reducing short-term and long-term neurologic deficit in patients with ischemic stroke: a double-blind randomized trial. Cerebrovasc Dis. 2010;29(3):275–281. doi:10.1159/000275503 [DOI] [PubMed] [Google Scholar]
- 20. Siebler M, Hennerici MG, Schneider D, et al. Safety of tirofiban in acute ischemic stroke: the SaTIS trial. Stroke. 2011;42(9):2388–2392. doi:10.1161/strokeaha.110.599662 [DOI] [PubMed] [Google Scholar]
- 21. Pancioli AM, Adeoye O, Schmit PA, et al. Combined approach to lysis utilizing eptifibatide and recombinant tissue plasminogen activator in acute ischemic stroke-enhanced regimen stroke trial. Stroke. 2013;44(9):2381–2387. doi:10.1161/strokeaha.113.001059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Junghans U, Seitz RJ, Aulich A, Freund HJ, Siebler M. Bleeding risk of tirofiban, a nonpeptide GPIIb/IIIa platelet receptor antagonist in progressive stroke: an open pilot study. Cerebrovasc Dis. 2001;12(4):308–312. doi:10.1159/000047726 [DOI] [PubMed] [Google Scholar]
- 23. Eckert B, Koch C, Thomalla G, et al. Aggressive therapy with intravenous abciximab and intra-arterial rtPA and additional PTA/stenting improves clinical outcome in acute vertebrobasilar occlusion: combined local fibrinolysis and intravenous abciximab in acute vertebrobasilar stroke treatment (FAST): results of a multicenter study. Stroke. 2005;36(6):1160–1165. doi:10.1161/01.STR.0000165918.80812.1e [DOI] [PubMed] [Google Scholar]
- 24. Nagel S, Schellinger PD, Hartmann M, et al. Therapy of acute basilar artery occlusion intraarterial thrombolysis alone vs bridging therapy. Stroke. 2009;40(1):140–146. doi:10.1161/strokeaha.108.526566 [DOI] [PubMed] [Google Scholar]
- 25. Li W, Lin L, Zhang M, et al. Safety and preliminary efficacy of early tirofiban treatment after alteplase in acute ischemic stroke patients. Stroke. 2016;47(10):2649–2651. doi:10.1161/strokeaha.116.014413 [DOI] [PubMed] [Google Scholar]
- 26. Lin L, Li W, Liu CC, et al. Safety and preliminary efficacy of intravenous tirofiban in acute ischemic stroke patient without arterial occlusion on neurovascular imaging studies. J Neurol Sci. 2017;383:175–179. doi:10.1016/j.jns.2017.10.041 [DOI] [PubMed] [Google Scholar]
- 27. Zhao W, Che R, Shang S, et al. Low-dose tirofiban improves functional outcome in acute ischemic stroke patients treated with endovascular thrombectomy. Stroke. 2017;48(12):3289–3294. doi:10.1161/strokeaha.117.019193 [DOI] [PubMed] [Google Scholar]
- 28. Wu Y, Yin C, Yang J, Jiang L, Parsons MW, Lin L. Endovascular thrombectomy Tirofiban increases bleeding risk in acute stroke patients. Stroke. 2018;49(11):2783–2785. doi:10.1161/STROKEAHA.118.022919 [DOI] [PubMed] [Google Scholar]
- 29. Quan T, Hou H, Xue W, et al. Endovascular treatment of acute intracranial vertebrobasilar artery occlusion: a multicenter retrospective observational study. Neuroradiology. 2019;61(12):1477–1484. doi:10.1007/s00234-019-02282 -1 [DOI] [PubMed] [Google Scholar]
- 30. Sun C, Li X, Zhao Z, et al. Safety and efficacy of tirofiban combined with mechanical thrombectomy depend on ischemic stroke etiology. Front Neurol. 2019;10:1100 doi:10:1100. doi:10.3389/fneur.2019.01100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yu T, Lin Y, Jin A, et al. Safety and efficiency of low dose intra-arterial tirofiban in mechanical thrombectomy during acute ischemic stroke. Curr Neurovasc Res. 2018;15(2):145–150. doi:10.2174/1567202615666180605104931 [DOI] [PubMed] [Google Scholar]
- 32. Zhang S, Hao Y, Tian X, et al. Safety of intra-arterial tirofiban administration in ischemic stroke patients after unsuccessful mechanical thrombectomy. J Vasc Interv Radiol. 2019;30(2):141–147. e141. doi:10.1016/j.jvir.2018.08.021 [DOI] [PubMed] [Google Scholar]
- 33. Liu H, Zheng H, Cao Y, et al. Low- versus standard-dose intravenous tissue-type plasminogen activator for acute ischemic stroke: an updated meta-analysis. J Stroke Cerebrovasc Dis. 2018;27(4):988–997. doi:10.1016/j.jstrokecerebrovasdis.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 34. Liu M-D, Ning W-D, Wang R-C, et al. Low-dose versus standard-dose tissue plasminogen activator in acute ischemic stroke in Asian populations. Medicine. 2015;94(52):e2412 doi:10.1097/md.0000000000002412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wong YS, Sung SF, Wu CS, et al. The impact of loading dose on outcome in stroke patients receiving low-dose tissue plasminogen activator thrombolytic therapy. Drug Des Devel Ther. 2020;14:257–263. doi:10.2147/DDDT.S235388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith WS, Sung G, Starkman S, Saver JL, Marks MP. Safety and efficacy of mechanical embolectomy in acute ischemic stroke results of the MERCI Trial. Stroke. 2005;36(7):1432–1438. [DOI] [PubMed] [Google Scholar]
- 37. Heck DV, Brown MD. Carotid stenting and intracranial thrombectomy for treatment of acute stroke due to tandem occlusions with aggressive antiplatelet therapy may be associated with a high incidence of intracranial hemorrhage. J Neurointerv Surg. 2015;7(3):170–175. doi:10.1136/neurintsurg-2014-011224 [DOI] [PubMed] [Google Scholar]
- 38. Ernst M, Butscheid F, Fiehler J, et al. Glycoprotein IIb/IIIa Inhibitor Bridging and subsequent endovascular therapy in vertebrobasilar occlusion in 120 patients. Clin Neuroradiol. 2016;26(2):169–175. doi:10.1007/s00062-014-0341-3 [DOI] [PubMed] [Google Scholar]
- 39. Kapadia SR, Bajzer CT, Ziada KM, et al. Initial experience of platelet glycoprotein IIb/IIIa inhibition with abciximab during carotid stenting: a safe and effective adjunctive therapy. Stroke. 2001;32(10):2328–2332. doi:10.1161/hs1001.096003 [DOI] [PubMed] [Google Scholar]
- 40. Qureshi AI, Suri MFK, Ali Z, et al. Carotid angioplasty and stent placement: A prospective analysis of perioperative complications and impact of intravenously administered abciximab. Neurosurgery. 2002;50(3):466–475. doi:10.1097/00006123-200203000-00006 11841713 [Google Scholar]
- 41. Wholey MH, Wholey MH, Eles G, et al. Evaluation of glycoprotein IIb/IIIa inhibitors in carotid angioplasty and stenting. J Endovasc Ther. 2003;10(1):33–41. doi:10.1177/152660280301000108 [DOI] [PubMed] [Google Scholar]
- 42. Chan AW, Yadav JS, Bhatt DL, et al. Comparison of the safety and efficacy of emboli prevention devices versus platelet glycoprotein IIb/IIIa inhibition during carotid stenting. Am J Cardiol. 2005;95(6):791–795. doi:10.1016/j.amjcard.2004.11.041 [DOI] [PubMed] [Google Scholar]
- 43. Jeong HW, Jin SC. Intra-arterial infusion of a glycoprotein IIb/IIIa antagonist for the treatment of thromboembolism during coil embolization of intracranial aneurysm: a comparison of abciximab and tirofiban. AJNR Am J Neuroradiol. 2013;34(8):1621–1625. doi:10.3174/ajnr.A3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Qureshi AI, Hussein HM, Janjua N, Harris-Lane P, Ezzeddine MA. Postprocedure intravenous eptifibatide following intra-arterial reteplase in patients with acute ischemic stroke. J Neuroimaging. 2008;18(1):50–55. doi:10.1111/j.1552-6569.2007.00185.x [DOI] [PubMed] [Google Scholar]