Abstract
Background:
This network meta-analysis assessed the comparative risk of grade 3–5 and grade 5 treatment-related adverse events (TRAEs) for immune checkpoint inhibitors (ICIs), either alone or in combination with other modalities, for cancer treatment.
Methods:
PubMed, Embase, Cochrane Library, Web of Science, and recent predominant oncology congresses were searched for relevant phase II and phase III randomized controlled trials (RCTs). As outcomes, grade 3–5, and grade 5 TRAE outcomes were reported as odds ratios and 95% confidence intervals.
Results:
In 67 RCTs involving 36,422 patients and 19 ICIs, the incidence of grade 3–5 and grade 5 TRAEs was 17.9% and 0.8% with ICI monotherapy and 46.3% and 1.4%, respectively, with combinatorial therapy. Pneumonitis was the most common cause of grade 5 TRAEs following either monotherapy (16.3%) or combinatorial therapy (11.4%). Regarding grade 3–5 TRAEs, atezolizumab + chemotherapy (CT) and antiangiogenic therapy (AT) (atezolizumab + CAT), pembrolizumab + CT, ipilimumab + CT, and atezolizumab + CT were more toxic than any ICI monotherapy, pembrolizumab or nivolumab + radiotherapy (RT), and ICIs dual therapy (durvalumab + tremelimumab and nivolumab + ipilimumab). Tremelimumab, ipilimumab, durvalumab, and pembrolizumab were, however, associated with higher grade 5 TRAEs than combinatorial treatments. Atezolizumab + CAT was the most toxic and nivolumab + RT was the least toxic of combinatorial treatments; among monotherapies, tremelimumab and avelumab were the most and least toxic, respectively. The toxicity ranking changed with type of grade 3–5 TRAEs.
Conclusions:
Compared with combinatorial therapy, ICI monotherapy caused lower grade 3–5 TRAEs, but some monotherapies resulted in a higher incidence of fatal TRAEs. Atezolizumab + CAT and nivolumab + RT were the most and least toxic of combinatorial treatments, respectively, and tremelimumab and avelumab were the most and least toxic of the monotherapies, respectively.
Keywords: antiangiogenic therapy, chemotherapy, immune checkpoint inhibitor, network meta-analysis, radiotherapy, treatment-related adverse events
Introduction
Immune checkpoint inhibitors (ICIs), including programmed cell death-1 (PD-1), programmed cell death ligand-1 (PD-L1), and cytotoxic T lymphocyte antigen-4 (CTLA-4) inhibitors, have revolutionized the treatment of many cancers. These agents can upregulate T cell activity, leading to an immune response against cancer cells. However, the increased activity of T cells can also induce autoimmune toxicities by unbalancing the immune system.1 ICIs have been reported to cause a wide spectrum of immune-related adverse events (irAEs), such as skin, gastrointestinal, endocrine, hepatic, pulmonary, and cardiovascular toxicities.2 In general, most irAEs are mild and can be well controlled with supportive treatment and glucocorticoids. However, the incidence of irAEs appears to have increased with the rapidly growing number of patients receiving ICIs, and some irAEs are serious with fatal outcomes.
Currently, the United States Food and Drug Administration (FDA) has approved several ICIs for the treatment of various cancers, including two PD-1 inhibitors (nivolumab and pembrolizumab), three PD-L1 inhibitors (atezolizumab, avelumab, and durvalumab), and two CTLA-4 inhibitors (ipilimumab and tremelimumab). As individual ICIs influence different immunologic mechanisms, the frequencies, severities, and profiles of the irAEs may vary.2–6 Moreover, ICIs in combination with conventional therapy [chemotherapy (CT), antiangiogenic therapy (AT), or their combinations] or with a second ICI, are being increasingly used, and these combinations have demonstrated survival advantage over monotherapy in several tumors.7–10 However, combinatorial therapy may also result in an increased risk of treatment-related adverse events (TRAEs). To date, evidence regarding head-to-head comparisons among ICIs is lacking, and therefore, determining which monotherapy or combinatorial therapy has the most or the least toxicity remains undefined.
Safety is the critical factor for drug evaluation. A better understanding of the comparative safety profiles between the ICIs would be helpful in clinical decision making. In this study, we performed a systematic review and network meta-analysis to assess the comparative risk of grade 3–5 and grade 5 TRAEs among 19 ICIs used in cancer treatment.
Methods
Literature search strategy
This network meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria (Supplemental Table S1).11 We systematically searched PubMed, Embase, the Cochrane Library, Web of Science, and the most recent oncology congresses (American Society of Clinical Oncology, American Society of Radiation Oncology, European Society for Medical Oncology, and World Conference on Lung Cancer) for available studies published before 1 November 2019. The search strategy is detailed in Supplemental Table S2. The reference lists of all relevant publications were also assessed for additional eligible studies.
Inclusion and exclusion criteria
Studies were included if they met all of the following criteria: (a) phase II or phase III randomized controlled trials (RCTs) of patients with cancer; (b) at least one treatment arm received an FDA-approved ICI, alone or in combination with another ICI or conventional therapy; (c) reported data of grade 3–5 and/or grade 5 TRAEs in each arm; and (d) published in English. When multiple publications covered the same study population, the one with the most recent and comprehensive data was used. Studies that failed to meet the above criteria were excluded from the network meta-analysis.
Data extraction
To better assess the toxicity of ICIs, especially in combinatorial treatments, we evaluated TRAEs instead of irAEs as the outcomes of interest. Two investigators independently extracted the following data from each study: the first author or the name of the RCT, year of publication, region, cancer type, study design, follow-up time, number of patients, interventions, and the number of grade 3–5 and grade 5 TRAEs.
Quality assessment
Two investigators independently assessed the risk of bias of the included RCTs using the Cochrane Collaboration’s tool,12 which includes the following five domains: sequence generation, allocation concealment, blinding, incomplete data, and selective reporting. Blinding cannot be applied in studies with specific designs (such as open-label or cross-over) owing to unavoidable reasons. If such reasons were clearly stated in the included studies, these studies were rated as “+.” An RCT was judged to have a “low risk of bias,” a “high risk of bias,” or an “unclear risk of bias” if all domains indicated low risk, one or more domains indicated high risk, or more than three domains indicated as unclear risk, respectively.
Statistical analysis
The outcomes of interest were grade 3–5 and grade 5 TRAEs. Odds ratios (ORs) and their 95% confidence intervals (CIs) were used as summary statistics to estimate treatment effects. If a study reported zero grade 5 TRAEs in any arm, a half integer continuity correction (adding a 0.5 to each cell) was applied to calculate ORs. For direct comparisons, a standard pairwise meta-analysis was performed using R (version 3.5.0). The heterogeneity among studies was assessed using the chi-squared (χ2) and I-squared (I2) tests. A p value greater than 0.10 or an I2 value greater than 50% indicated substantial heterogeneity, and a random-effects model was used; otherwise, a fixed-effects model was used.
The Bayesian network meta-analysis was conducted using a random-effects model (generalized linear model) using the Markov chain Monte Carlo method in OpenBUGS (version 3.2.3).13 For each outcome measure, four independent Markov chains were simultaneously run for 20,000 burn-ins and 100,000 inference iterations per chain to obtain the posterior distribution. The traces plot and Gelman–Rubin method were used to assess the convergence of the model.14 Relative toxicity rankings were assessed according to the surface under the cumulative ranking curve (SUCRA) method.15 SUCRA equals one if the treatment is certain to be the worst, and zero if it is certain to be the best. The transitivity assumption was assessed by comparing the distribution of potential effect modifiers (sample size, median age, and median follow-up time) across treatment comparisons.16 Global inconsistency was evaluated by comparing the fit of the consistency and inconsistency models using a design-by-treatment test15; local inconsistency was assessed by calculating the difference between the direct and indirect estimates in the treatment loops using the loop-specific approach,15 and by testing between direct and indirect results within the treatment loops using node-split models17; p < 0.05 indicated significant inconsistency. We assumed a common heterogeneity parameter for all comparisons and used the between-study heterogeneity variance, τ², to assess the extent of heterogeneity for each outcome.18,19 We conducted subgroup meta-regressions (sample size, treatment line, tumor type, drug dose, control arm, and study risk of bias) to search for the sources of heterogeneity.
Sensitivity analyses were conducted to assess the stability of the results by omitting studies with a high overall risk of bias, a sample size <100, or a placebo-controlled trial as well as by dividing the trials of nivolumab + ipilimumab into two dose groups [nivolumab (3 mg) + ipilimumab (1 mg) and nivolumab (1 mg) + ipilimumab (3 mg)] and the trials of pembrolizumab into three dose groups (200 mg, 2 mg/kg, 10 mg/kg). In addition, we performed subgroup analyses based on the nature and severity of TRAEs. Publication bias was examined using funnel plots.20
Results
Literature search results and characteristics of included RCTs
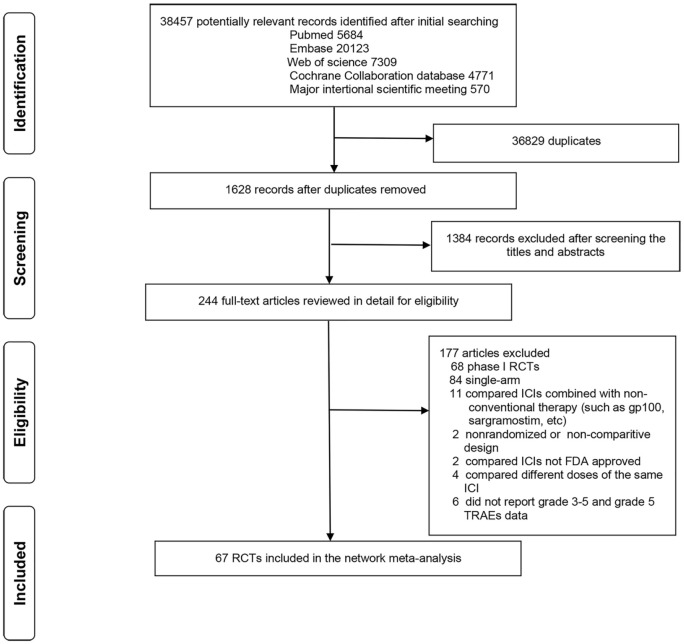
The details of our literature search and study selection process are shown in Figure 1. The initial literature search identified 38,457 studies, of which 244 were deemed potentially eligible and were thus retrieved for detailed assessment. The relevant references were also reviewed for any missed studies. Finally, 67 RCTs involving 36,422 patients and evaluating 22 treatments (including CT, AT, placebo, and 19 ICIs) were included in the network meta-analysis.8–10,21–95 Among the 19 ICI-based treatments, 7 were monotherapies (nivolumab,21–31 pembrolizumab,32–43 atezolizumab,44–47 avelumab,48,49 durvalumab,91,93,95 ipilimumab,50,51 and tremelimumab52,53) and 12 were combinatorial therapies (nivolumab + RT,54 pembrolizumab + AT,55 pembrolizumab + CT,56–61 pembrolizumab + RT,62,63 atezolizumab + AT,64,65 atezolizumab + CT,66–70 atezolizumab + CT + AT (atezolizumab + CAT),71 avelumab + AT,72 durvalumab + CT,73 ipilimumab + CT,74–80 nivolumab + ipilimumab,8–10,81–90 and durvalumab + tremelimumab91–95). The baseline characteristics of the included trials are shown in Table 1. A total of 46 studies (68.7%) were phase III trials, and 64 (95.5%) were multinational trials. Cancer types assessed in the trials included lung (n = 30), melanoma (n = 10), gastric and esophageal (n = 8), head and neck (n = 6), renal cell (n = 5), urothelial (n = 3), prostate (n = 2), breast (n = 1), endometrial (n = 1), and malignant mesothelioma (n = 1). The mean sample size for toxicity analysis was 515 participants (range, 36–1274). The mean age was 62.3 years (range, 55.5–69.5 years). The median follow-up time was 13.9 months (range, 5.1–57.7 months).
Figure 1.
Literature search and selection.
FDA, United States Food and Drug Administration; ICIs, immune checkpoint inhibitors; RCTs, randomized control trials; TRAEs, treatment-related adverse events.
Table 1.
Characteristics of included trials.
| Trial/year | Region | Design | Cancer type | Treatment line |
Treatment | Total No. |
Toxicity analysis No. |
Median follow-up (months) |
G 3–5 TRAEs No. |
G 5 TRAEs No. |
Median age |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CheckMate 067/20178,9 | Multicenter | Ⅱ | Melanoma | First | Niv+Ipi | 945 | 313 | NR | 187 | 2 | 59.3 |
| Niv | 313 | 71 | 1 | 58.7 | |||||||
| Ipi | 311 | 87 | 1 | 59.6 | |||||||
| CheckMate 032-2/201910 | Multicenter | Ⅱ | UC | Second | Niv+Ipi | 274 | 196 | NR | 69 | 1 | 63.5 |
| Niv | 78 | 22 | 1 | 65.5 | |||||||
| CheckMate 026/201721 | Multicenter | Ⅲ | NSCLC | First | Niv | 423 | 267 | 13.5 | 49 | 2 | 63 |
| PC/PP/GP | 263 | 13.5 | 136 | 3 | 65 | ||||||
| CheckMate 057/201522 | Multicenter | Ⅲ | NSCLC | Second | Niv | 592 | 287 | NR | 31 | 1 | 61 |
| Doc | 268 | 145 | 1 | 64 | |||||||
| CheckMate 017/201523 | Multicenter | Ⅲ | NSCLC | Second | Niv | 272 | 131 | NR | 9 | 0 | 62 |
| Doc | 129 | 74 | 3 | 64 | |||||||
| CheckMate 078/201924 | Multicenter | Ⅲ | NSCLC | Second | Niv | 504 | 337 | 10.4 | 44 | 4 | 60 |
| Doc | 156 | 8.8 | 77 | 3 | 60 | ||||||
| CheckMate 066/201425,26 | Multicenter | Ⅲ | Melanoma | First | Niv | 418 | 206 | 38.4 | 31 | 0 | 64 |
| DTIC | 205 | 38.5 | 36 | 0 | 66 | ||||||
| CheckMate 037/201527,28 | Multicenter | Ⅲ | Melanoma | Second | Niv | 405 | 268 | 48 | 37 | 0 | 59 |
| DTIC/PC | 102 | 48 | 35 | 0 | 62 | ||||||
| CheckMate 141/201629 | Multicenter | Ⅲ | HNC | Second | Niv | 361 | 236 | 5.1 | 33 | 2 | 59 |
| Met/Doc/Cet | 111 | 5.1 | 40 | 1 | 61 | ||||||
| ATTRACTION-3/201930 | Multicenter | Ⅲ | GEC | Second | Niv | 419 | 209 | 10.5 | 38 | 2 | 64 |
| Pac/Doc | 208 | 8 | 131 | 3 | 67 | ||||||
| ATTRACTION-2/201731 | Multicenter | Ⅲ | GEC | Second | Niv | 493 | 330 | 8.9 | 39 | 5 | 62 |
| Placebo | 161 | 8.6 | 9 | 2 | 61 | ||||||
| KEYNOTE-024/201632,33 | Multicenter | Ⅲ | NSCLC | First | Pem | 205 | 154 | 11.2 | 48 | 2 | 64.5 |
| PC/PP/GP | 150 | 11.2 | 80 | 3 | 66 | ||||||
| KEYNOTE-042/201934 | Multicenter | Ⅲ | NSCLC | First | Pem | 1274 | 636 | 12.8 | 113 | 13 | 63 |
| PC/PP | 615 | 12.8 | 252 | 14 | 63 | ||||||
| KEYNOTE-010/201635 | Multicenter | Ⅱ/Ⅲ | NSCLC | Second | Pem | 991 | 682 | 13.1 | 98 | 6 | 63 |
| Doc | 309 | 13.1 | 109 | 5 | 62 | ||||||
| KEYNOTE-002/201536,37 | Multicenter | Ⅱ | Melanoma | Second | Pem | 540 | 357 | 28 | 54 | 1 | 61 |
| PC/DTIC/Tem | 171 | 28 | 45 | 0 | 63 | ||||||
| KEYNOTE-045/201738 | Multicenter | Ⅲ | UC | Second | Pem | 542 | 266 | 14.1 | 40 | 4 | 67 |
| Pac/Doc/Vin | 255 | 14.1 | 126 | 4 | 65 | ||||||
| KEYNOTE-181/201939 | Multicenter | Ⅲ | GEC | Second | Pem | 628 | 314 | 7.1 | 57 | 5 | NR |
| Pac/Doc/Iri | 296 | 6.9 | 129 | 5 | NR | ||||||
| KEYNOTE-061/201840 | Multicenter | Ⅲ | GEC | Second | Pem | 592 | 294 | 7.5 | 42 | 3 | 62.5 |
| Pac | 276 | 7.1 | 96 | 1 | 60 | ||||||
| KEYNOTE-040/201941 | Multicenter | Ⅲ | HNC | Mix | Pem | 595 | 246 | 7.5 | 33 | 4 | 60 |
| Met/Doc/Cet | 234 | 7.1 | 85 | 2 | 60 | ||||||
| KEYNOTE-006/201542,43 | Multicenter | Ⅲ | Melanoma | Mix | Pem | 834 | 555 | 57.7 | 97 | 1 | 62 |
| Ipi | 256 | 57.7 | 50 | 0 | 62 | ||||||
| OAK/201744 | Multicenter | Ⅲ | NSCLC | Second | Ate | 1225 | 609 | 28 | 90 | 0 | 63 |
| Doc | 578 | 28 | 248 | 1 | 64 | ||||||
| POPLAR/201645 | Multicenter | Ⅱ | NSCLC | Second | Ate | 144 | 142 | 14.8 | 17 | 1 | 62 |
| Doc | 135 | 15.7 | 55 | 3 | 62 | ||||||
| IMpower110/201946 | Multicenter | Ⅲ | NSCLC | First | Ate | 572 | 286 | 15.7 | 37 | 0 | NR |
| PP/GP | 263 | 15.7 | 117 | 1 | NR | ||||||
| IMvigor211/201847 | Multicenter | Ⅲ | UC | Second | Ate | 931 | 459 | 17.3 | 95 | 4 | 67 |
| Pac/Doc/Vin | 443 | 17.3 | 198 | 9 | 67 | ||||||
| JAVELIN Lung 200/201848 | Multicenter | Ⅲ | NSCLC | Second | Ave | 792 | 393 | 18.3 | 39 | 4 | 64 |
| Doc | 365 | 18.3 | 180 | 14 | 63 | ||||||
| JAVELIN Gastric 300/201849 | Multicenter | Ⅲ | GEC | Second | Ave | 371 | 184 | 10.6 | 17 | 0 | 59 |
| Pac/Iri | 177 | 10.6 | 56 | 1 | 61 | ||||||
| Beer/201750 | Multicenter | Ⅲ | PC* | First | Ipi | 602 | 399 | 24 | 167 | 9 | 70 |
| Placebo | 199 | 24 | 11 | 0 | 69 | ||||||
| CA184-043/201451 | Multicenter | Ⅲ | PC* | Second | Ipi | 799 | 393 | 9.9 | 105 | 4 | 69 |
| Placebo | 396 | 9.3 | 11 | 0 | 67.5 | ||||||
| DETERMINE/201752 | Multicenter | Ⅱ | MM | Second | Tre | 571 | 380 | NR | 110 | 5 | 66 |
| Placebo | 189 | 12 | 0 | 67 | |||||||
| Ribas/201353 | Multicenter | Ⅲ | Melanoma | First | Tre | 655 | 325 | NR | NR | 7 | 57 |
| Tem/DTIC | 319 | NR | 1 | 56 | |||||||
| ASCO6009/201854 | Single center | Ⅱ | HNC | Mix | Niv+RT | 53 | 27 | 12.8 | 3 | NR | NR |
| Niv | 26 | 12.8 | 4 | NR | |||||||
| KEYNOTE-426/201955 | Multicenter | Ⅲ | RCC | First | Pem+Axi | 861 | 429 | 12.8 | 270 | 4 | 62 |
| Sun | 425 | 12.8 | 247 | 7 | 61 | ||||||
| KEYNOTE-189/201856 | Multicenter | Ⅲ | NSCLC | First | Pem+PP | 616 | 405 | 10.5 | 36() | 3 | 65 |
| PP | 202 | 10.5 | 9() | 0 | 63.5 | ||||||
| KEYNOTE-407/201957 | Multicenter | Ⅲ | NSCLC | First | Pem+PC/CnP | 559 | 278 | 7.8 | 30 | 1 | 65 |
| PC/CnP | 280 | 7.8 | 9 | 1 | 65 | ||||||
| KEYNOTE-021/201658,59 | Multicenter | Ⅱ | NSCLC | First | Pem+PP | 123 | 59 | 23.9 | 24 | 1 | 62.5 |
| PP | 62 | 23.9 | 17 | 2 | 63.2 | ||||||
| KEYNOTE-048/201860 | Multicenter | Ⅲ | HNC | First | Pem+FP | 582 | 281 | 17 | 200 | NR | NR |
| Pem | 301 | 17 | 51 | NR | |||||||
| KEYNOTE-062/201961 | Multicenter | Ⅲ | GEC | First | Pem+FP/Cap | 763 | 257 | 11.3 | 188 | NR | NR |
| Pem | 256 | 11.3 | 44 | NR | |||||||
| FP/Cap | 250 | 11.3 | 173 | NR | |||||||
| ASCO9104/201962 | Single center | Ⅱ | NSCLC | First | Pem+RT | 124 | 36 | 15.4 | 11 | 0 | NR |
| Pem | 36 | 15.4 | 5 | 0 | NR | ||||||
| PEMBRO-RT/201963 | Multicenter | Ⅱ | NSCLC | Second | Pem+RT | 92 | 35 | 23.6 | 5 | 0 | 62 |
| Pem | 37 | 23.6 | 11 | 1 | 62 | ||||||
| IMmotion151/201964 | Multicenter | Ⅲ | RCC | First | Ate+Bev | 915 | 451 | 15 | 187 | 5 | 62 |
| Sun | 446 | 15 | 241 | 1 | 60 | ||||||
| IMmotion150/201865 | Multicenter | Ⅱ | RCC | First | Ate+Bev | 305 | 100 | 20.7 | 59 | 2 | 62 |
| Ate | 103 | 20.7 | 17 | 0 | 61 | ||||||
| Sun | 101 | 20.7 | 41 | 1 | 61 | ||||||
| IMpower130/201966 | Multicenter | Ⅲ | NSCLC | First | Ate+CnP | 724 | 473 | 18.5 | 354 | 8 | 64 |
| CnP | 232 | 19.2 | 141 | 1 | 65 | ||||||
| IMpower131/201867 | Multicenter | Ⅲ | NSCLC | First | Ate+CnP | 683 | 334 | 17.1 | 231 | 4 | 65 |
| CnP | 334 | 17.1 | 196 | 3 | 65 | ||||||
| IMpower132/201868 | Multicenter | Ⅲ | NSCLC | First | Ate+PP | 578 | 291 | 14.8 | 167 | 11 | 64 |
| PP | 274 | 14.8 | 114 | 7 | 63 | ||||||
| IMpower133/201869 | Multicenter | Ⅲ | SCLC | First | Ate+EP | 403 | 198 | 13.9 | 115 | 3 | 64 |
| EP | 196 | 13.9 | 113 | 3 | 64 | ||||||
| IMpassion130/201870 | Multicenter | Ⅲ | BC | First | Ate+nap-Pac | 902 | 452 | 13 | 182 | 3 | 55 |
| nap-Pac | 438 | 12.5 | 133 | 1 | 56 | ||||||
| IMpower150/201871 | Multicenter | Ⅲ | NSCLC | First | Ate+Bev+PC | 793 | 393 | 20 | 234 | 11 | 63 |
| Ate+PC | 400 | 20 | 176 | 4 | 63 | ||||||
| JAVELIN Renal 101/201972 | Multicenter | Ⅲ | RCC | First | Ave+Axi | 886 | 434 | 11.6 | 246 | 3 | 62 |
| Sun | 439 | 10.7 | 243 | 1 | 61 | ||||||
| CASPIAN/201973 | Multicenter | Ⅲ | SCLC | First | Dur+EP | 575 | 265 | 14.2 | 126 | 5 | 62 |
| EP | 266 | 14.2 | 140 | 2 | 63 | ||||||
| CA184-024/201174,75 | Multicenter | Ⅲ | Melanoma | First | Ipi+DTIC | 502 | 247 | NR | 103 | 0 | 57.5 |
| DTIC | 251 | 16 | 1 | 56.4 | |||||||
| Lynch/201276 | Multicenter | Ⅱ | NSCLC | First | Ipi+PC | 204 | 138 | NR | 56 | 1 | 60 |
| PC | 65 | 24 | 1 | 62 | |||||||
| Govindan/201777 | Multicenter | Ⅲ | NSCLC | First | Ipi+PC | 749 | 388 | 12.5 | 205 | 7 | 64 |
| PC | 361 | 12.5 | 129 | 1 | 64 | ||||||
| Reck/201378 | Multicenter | Ⅱ | SCLC | First | Ipi+PC | 130 | 84 | NR | 40 | 1 | NR |
| PC | 44 | 13 | 0 | NR | |||||||
| Reck/201679 | Multicenter | Ⅲ | SCLC | First | Ipi+EP | 954 | 478 | 10.5 | 231 | 5 | 62 |
| EP | 476 | 10.2 | 214 | 2 | 63 | ||||||
| Hersh/201180 | Multicenter | Ⅱ | Melanoma | First | Ipi+DTIC | 72 | 35 | 20.9 | 9 | 1 | 60 |
| Ipi | 39 | 16.4 | 6 | 1 | 66 | ||||||
| CheckMate 227/201881,82 | Multicenter | Ⅲ | NSCLC | First | Niv+Ipi | 1537 | 576 | 28.3 | 221 | 8 | NR |
| Niv | 391 | 28.3 | 95 | 2 | NR | ||||||
| PP/GP | 570 | 28.3 | 248 | 6 | NR | ||||||
| Lung-MAP Sub-Study/201983 | Multicenter | Ⅲ | NSCLC | Second | Niv+Ipi | 275 | 124 | 17.4 | 48 | 5 | NR |
| Niv | 123 | 17.4 | 38 | 1 | NR | ||||||
| CheckMate 032-1/201984 | Multicenter | Ⅱ | SCLC | Second | Niv+Ipi | 243 | 96 | 11.2 | 40 | 4 | 65 |
| Niv | 147 | 11.9 | 20 | 1 | 63 | ||||||
| CheckMate 032/201885 | Multicenter | Ⅱ | GEC | Second | Niv+Ipi | 160 | 101 | 22–28 | 37 | 1 | 55.5 |
| Niv | 59 | 28 | 10 | 0 | 60 | ||||||
| Long/201886 | Multicenter | Ⅱ | Melanoma | First | Niv+Ipi | 63 | 35 | 17 | 19 | 0 | 59 |
| Niv | 25 | 17 | 4 | 0 | 63 | ||||||
| CheckMate 069/201587,88 | Multicenter | Ⅱ | Melanoma | First | Niv+Ipi | 142 | 94 | 24 | 54 | 3 | 64 |
| Ipi | 46 | 24 | 9 | 0 | 67 | ||||||
| CheckMate 214/201889,90 | Multicenter | Ⅲ | RCC | First | Niv+Ipi | 1096 | 547 | 25.2 | 263 | 8 | 62 |
| Sun | 535 | 25.2 | 346 | 4 | 62 | ||||||
| MYSTIC/201891 | Multicenter | Ⅲ | NSCLC | First | Dur+Tre | 1118 | 373 | NR | 82 | 0 | NR |
| Dur | 373 | 54 | 0 | NR | |||||||
| PP | 373 | 126 | 0 | NR | |||||||
| CONDOR/201892 | Multicenter | Ⅱ | HNC | Second | Dur+Tre | 267 | 133 | 6.5 | 22 | 1 | 62 |
| Dur | 65 | 6 | 8 | 0 | 62 | ||||||
| Tre | 65 | 5.2 | 11 | 0 | 61 | ||||||
| EAGLE/201993 | Multicenter | Ⅲ | HNC | Second | Dur+Tre | 736 | 247 | NR | 40 | NR | NR |
| Dur | 240 | 24 | NR | ||||||||
| FP/Cet/Tax/Met | 249 | 60 | NR | ||||||||
| ASCO5582/201994 | Single center | Ⅱ | EC | NR | Dur+Tre | 56 | 27 | NR | 12 | 0 | NR |
| Dur | 27 | 3 | 0 | NR | |||||||
| Kelly/201995 | Multicenter | Ⅱ | GEC | Second | Tre | 88 | 12 | 9.2 | 5 | 0 | 64 |
| Dur+Tre | 52 | 9.2 | 9 | 0 | 60 | ||||||
| Dur | 24 | 3.5 | 1 | 0 | 54 |
Ate, atezolizumab; Ave, avelumab; Axi, axitinib; BC, breast cancer; Bev, bevacizumab; Cap, capecitabine; Cet, cetuximab; CnP, paclitaxel-nanoparticle albumin-bound-carboplatin; DOC, docetaxel; DTIC, dacarbazine; Dur, durvalumab; EC, endometrial carcinoma; EP, etoposide-cisplatin/carboplatin; FP, fluorouracil-cisplatin/carboplatin; GEC, gastric or esophageal cancer; GP, gemcitabine-cisplatin/carboplatin; HNC, head and neck cancer; Ipi, ipilimumab; Iri, irinotecan; Met, methotrexate; MM, malignant mesothelioma; Nap, nedaplatin; Niv, nivolumab; No., number; NR, not reported ; NSCLC, non-small cell lung cancer; Pac, paclitaxel; PC, paclitaxel-cisplatin /carboplatin; PC*, prostate cancer; Pem, pembrolizumab; RCC, renal cell carcinoma; RT, radiotherapy; SCLC, small cell lung cancer; Sun, sunitinib; Tax, taxane; Tem, temozolomide; TRAEs, treatment-related adverse events; Tre, tremelimumab; UC, urothelial carcinoma; Vin, vinflunine.
Assessment of included trials
The risks of bias for the included RCTs are summarized in Supplemental Figure S1. Overall, the risk of bias across studies was relatively low; 12 RCTs were rated with a high risk of bias.22–24,35,42–45,48,55,72,86,92 The funnel plot analysis did not indicate any evident risk of publication bias for grade 5 TRAEs, but it did suggest a probability of publication bias for grade 3–5 TRAEs (Supplemental Figure S2).
Incidence of grade 3–5 and grade 5 TRAEs
The overall incidence of grade 3–5 and grade 5 TRAEs were 34.4% (12,297 of 35,778 patients from 66 studies) and 1.0% (352 of 34,288 patients from 63 studies), respectively, and, for patients receiving ICIs, the incidence rates were 30.5% (6,793 of 22,256 patients) and 1.1% (221 of 20,946 patients) for grade 3–5 and grade 5 TRAEs, respectively. Further analysis revealed that, with monotherapy, the incidence of grade 3–5 and grade 5 TRAEs were 17.9% (2220 of 12,373 patients from 47 studies) and 0.8% (98 of 11,875 patients from 44 studies), respectively, and combinatorial therapy resulted in 46.3% (4573 of 9883 from 39 studies) and 1.4% (123 of 9071 from 36 studies), respectively.
The causes of the grade 5 TRAEs are presented in Supplemental Table S3. Of the 98 cases of grade 5 TRAEs that occurred in the monotherapy cohort, the leading causes were respiratory (n = 36; 36.7%), gastroenteropancreatic (n = 10; 10.2%), and cardiovascular (n = 9; 9.2%) diseases. Of the 123 cases in the combinatorial treatment cohort, the leading causes were respiratory (n = 26; 21.1%), cardiovascular (n = 10; 8.1%), and infectious (n = 10; 8.1%) diseases. Pneumonitis was the most common cause of grade 5 TRAEs in patients receiving either monotherapy (16 out of 98; 16.3%) or combinatorial therapy (14 out of 123; 11.4%).
Conventional pairwise meta-analysis
The results of the pairwise meta-analysis are shown in Table 2. In terms of grade 3–5 TRAEs, monotherapies, including atezolizumab (OR = 0.25, 95% CI: 0.21–0.29), avelumab (OR = 0.14, 95% CI: 0.10–0.19), durvalumab (OR = 0.34, 95% CI: 0.25–0.45), nivolumab (OR = 0.21, 95% CI: 0.13–0.34), and pembrolizumab (OR = 0.27, 95% CI: 0.20–0.36), and the combination of durvalumab + tremelimumab (OR = 0.57, 95% CI: 0.47–0.74) were safer than CT. In addition, ICIs in combination with CT, including atezolizumab + CT (OR = 1.59, 95% CI: 1.37–1.84), ipilimumab + CT (OR = 2.24, 95% CI: 1.13–4.47), and pembrolizumab + CT (OR = 1.89, 95% CI: 1.15–3.09) were more toxic than CT alone. The durvalumab + tremelimumab combination was more toxic than durvalumab monotherapy (OR = 1.76, 95% CI: 1.33–2.34), the nivolumab + ipilimumab combination was more toxic than ipilimumab monotherapy (OR = 4.04, 95% CI: 2.96–5.51) and nivolumab monotherapy (OR = 2.69, 95% CI: 1.69–4.28), and the pembrolizumab + CT combination was more toxic than pembrolizumab monotherapy (OR = 12.57, 95% CI: 9.40–16.80). Atezolizumab and avelumab caused less grade 5 TRAEs than CT alone (OR = 0.38, 95% CI: 0.15–0.98 and OR = 0.26, 95% CI: 0.09–0.76, respectively); the nivolumab + ipilimumab combination caused more grade 5 TRAEs than nivolumab monotherapy (OR = 2.64, 95% CI: 1.13–6.14). Obvious heterogeneity was observed for grade 3–5 TRAEs in avelumab versus CT, nivolumab versus CT, pembrolizumab versus CT, durvalumab versus tremelimumab, ipilimumab + CT versus CT, pembrolizumab + CT versus CT, nivolumab + ipilimumab versus nivolumab monotherapy, and durvalumab + tremelimumab versus tremelimumab monotherapy (I2 = 56–90%). No heterogeneity was observed for grade 5 TRAEs in all comparisons, except atezolizumab + AT versus AT (I2 = 51%).
Table 2.
Results of direct comparison meta-analysis.
| Treatment | No. of study | No. of patients (E/C) | OR(95%CI) | Heterogeneity I2 (%) |
|---|---|---|---|---|
| Grade 3–5 TRAEs | ||||
| Ate versus CT | 4 | 1496/1419 | 0.25(0.21–0.29) | 48 |
| Ave versus CT | 2 | 577/542 | 0.14(0.10–0.19) | 70 |
| Dur versus CT | 2 | 613/622 | 0.34(0.25–0.45) | 0 |
| Niv versus CT | 9 | 2332/2012 | 0.21(0.13–0.34) | 90 |
| Pem versus CT | 9 | 3205/2556 | 0.27(0.20–0.36) | 80 |
| Dur versus Tre | 2 | 89/77 | 0.26(0.02–2.69) | 72 |
| Ate+CT versus CT | 5 | 1748/1474 | 1.59(1.37–1.84) | 43 |
| Ipi+CT versus CT | 5 | 1335/1197 | 2.24(1.13–4.47) | 92 |
| Pem+CT versus CT | 4 | 999/794 | 1.89(1.15–3.09) | 57 |
| Dur+Tre versus CT | 2 | 620/622 | 0.57(0.44–0.74) | 0 |
| Dur+Tre versus Dur | 4 | 780/705 | 1.76(1.33–2.34) | 14 |
| Niv+Ipi versus Ipi | 2 | 407/357 | 4.04(2.96–5.51) | 0 |
| Niv+Ipi versus Niv | 7 | 1441/1136 | 2.69(1.69–4.28) | 82 |
| Pem+CT versus Pem | 2 | 538/557 | 12.57(9.40–16.80) | 0 |
| Pem+RT versus Pem | 2 | 71/73 | 1.04(0.16–6.91) | 81 |
| Dur+Tre versus Tre | 2 | 185/77 | 0.73(0.37–1.44) | 56 |
| Ate+AT versus AT | 2 | 552/546 | 0.58(0.45–0.73) | 0 |
| Ipi versus placebo | 2 | 792/595 | 12.5(8.0–19.7) | 0 |
| Grade 5 TRAEs | ||||
| Ate versus CT | 4 | 1498/1421 | 0.38(0.15–0.98) | 0 |
| Ave versus CT | 2 | 577/542 | 0.26(0.09–0.76) | 0 |
| Niv versus CT | 9 | 2335/2015 | 0.56(0.28–1.11) | 0 |
| Pem versus CT | 8 | 2950/2307 | 0.94(0.59–1.50) | 0 |
| Dur versus Tre | 2 | 91/79 | 0.72(0.04–11.86) | 0 |
| Ate+CT versus CT | 5 | 1748/1474 | 1.68(0.88–3.18) | 0 |
| Ipi+CT versus CT | 5 | 1337/1199 | 2.14(0.83–5.51) | 0 |
| Pem+CT versus CT | 3 | 743/545 | 1.21(0.30–4.95) | 0 |
| Dur+Tre versus Dur | 4 | 589/493 | 0.98(0.16–6.09) | 0 |
| Niv+Ipi versus Ipi | 2 | 408/358 | 2.61(0.41–16.65) | 0 |
| Niv+Ipi versus Niv | 7 | 1443/1138 | 2.64(1.13–6.14) | 0 |
| Pem+RT versus Pem | 2 | 73/75 | 0.51(0.05–5.77) | 0 |
| Dur+Tre versus Tre | 2 | 187/79 | 0.80(0.07–8.64) | 0 |
| Ate+AT versus AT | 2 | 553/547 | 1.85(0.50–6.83) | 51 |
| Ipi versus placebo | 2 | 794/597 | 9.5(1.2–73.9) | 0 |
Significant results are in bold.
AT, antiangiogenic therapy; Ate, atezolizumab; Ave, avelumab; CI, confidence interval; CT, chemotherapy; Dur, durvalumab; E/C, experimental/control; Ipi, ipilimumab; Niv, nivolumab; No., number; OR, odds ratio; Pem, pembrolizumab; TRAEs, treatment-related adverse events; Tre, tremelimumab; RT, radiotherapy.
Network meta-analysis
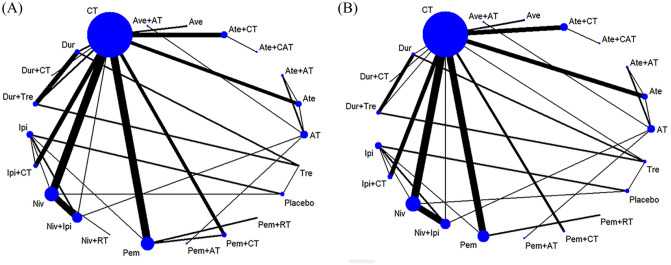
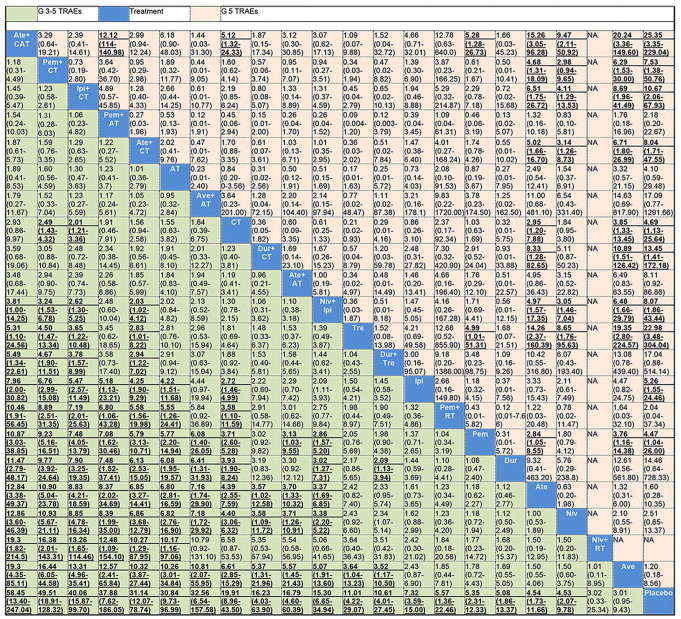
Figure 2 shows the network of eligible comparisons for grade 3–5 and grade 5 TRAEs. Results of the network meta-analysis are presented in Figure 3. In terms of grade 3–5 TRAEs, ICIs in combination with CT (atezolizumab + CAT, pembrolizumab + CT, ipilimumab + CT, and atezolizumab + CT) were more toxic than all monotherapies (pembrolizumab, nivolumab, durvalumab, atezolizumab, avelumab, ipilimumab, and tremelimumab); pembrolizumab + AT, avelumab + AT, and nivolumab + ipilimumab were more toxic than all ICI monotherapy regimens except tremelimumab and ipilimumab; durvalumab + CT and atezolizumab + AT were more toxic than atezolizumab, nivolumab, and avelumab; and durvalumab + tremelimumab was more toxic than durvalumab and avelumab. CT was more toxic than all ICIs when used as monotherapy (except tremelimumab), and pembrolizumab + RT. Pembrolizumab + CT and ipilimumab + CT were more toxic than CT. Among the combinatorial treatments, ICIs in combination with CT (except durvalumab + CT) were more toxic than dual ICI therapy (nivolumab + ipilimumab and durvalumab + tremelimumab) as well as ICI + RT (pembrolizumab + RT and nivolumab + RT). Moreover, pembrolizumab + AT and avelumab + AT were more toxic than pembrolizumab + RT and nivolumab + RT, respectively. Among ICIs used as monotherapies, tremelimumab was more toxic than avelumab. With regard to grade 5 TRAEs, atezolizumab + CAT, ipilimumab + CT, atezolizumab + CT, and nivolumab + ipilimumab showed higher risk of grade 5 TRAEs than nivolumab, atezolizumab, and avelumab; atezolizumab + CAT also had a higher risk of grade 5 TRAEs than pembrolizumab, CT, and pembrolizumab + AT; durvalumab + CT, pembrolizumab + CT, and CT alone were associated with a higher risk of grade 5 TRAEs than atezolizumab and avelumab. Tremelimumab was more toxic than the other ICIs when used as monotherapy, except durvalumab and ipilimumab; pembrolizumab was more toxic than atezolizumab and avelumab. All results mentioned above were statistically significant with the ORs and lower limits of 95% CIs greater than 1.
Figure 2.
Network of eligible comparisons for the network meta-analysis. (A) Grade 3–5 TRAEs. (B) Grade 5 TRAEs.
AT, antiangiogenic therapy; Ate, atezolizumab; Ave, avelumab; CAT, CT+AT; CT, chemotherapy; Dur, durvalumab; Ipi, ipilimumab; Niv, nivolumab; Pem, pembrolizumab; RT, radiotherapy; TRAEs, treatment-related adverse events; Tre, tremelimumab.
Figure 3.
Treatments are reported in order of risk of grade 3–5 TRAEs ranking from high to low according to SUCRAs. Comparisons should be read from left to right. Data are ORs (95% CI) in the column-defining treatment compared with the row-defining treatment. An OR over 1 favors the row-defining treatment. Significant results are in bold and underlined.
AT, antiangiogenic therapy; Ate, atezolizumab; Ave, avelumab; CAT, CT+AT; CI, confidecned interval; CT, chemotherapy; Dur, durvalumab; Ipi, ipilimumab; Niv, nivolumab; OR, odds ratio; Pem, pembrolizumab; RT, radiotherapy; SUCRA, surface under the cumulative ranking; TRAEs, treatment-related adverse events; Tre, tremelimumab.
Results of the toxicity ranking based on SUCRA are presented in Table 3, and ranking curves are shown in Supplemental Figure S3. Atezolizumab + CAT (91.2%) was ranked the most toxic treatment in terms of grade 3–5 TRAEs, followed by pembrolizumab + CT (90.9%), ipilimumab + CT (85.7%), pembrolizumab + AT(81.9%), and atezolizumab + CT (78.2%); avelumab (11.6%) was the least toxic treatment except placebo; and the nivolumab + RT combination was the least toxic combinatorial treatment. In terms of grade 5 TRAEs, atezolizumab + CAT (86.6%) was the most toxic treatment, followed by tremelimumab (84.5%), avelumab + AT (74.2%), durvalumab + CT (72.9%), and durvalumab + tremelimumab (72.2%); avelumab (10.6%) was also the least toxic treatment except placebo.
Table 3.
SUCRA values of grade 3–5 and grade 5 TRAEs for overall and sensitivity analysis.
| Treatment | Overall | Sensitivity analysis |
||||
|---|---|---|---|---|---|---|
| Excluding trials of high risk of bias | Excluding trials of sample size <100 | Excluding trials of placebo-controlled | Dividing Niv+Ipi into two dose groups | Dividing Pem into three dose groups | ||
| Grade3–5 | ||||||
| Ate+CAT | 91.2 | 91.0 | 90.8 | 91.0 | 93.0 | 92.0 |
| Pem+CT | 90.9 | 90.3 | 89.9 | 90.4 | 92.9 | 90.7 |
| Ipi+CT | 85.7 | 87.9 | 87.3 | 84.2 | 88.4 | 87.2 |
| Pem+AT | 81.9 | 81.6 | 79.5 | 79.8 | 77.8 | 83.8 |
| Ate+CT | 78.2 | 78.0 | 76.4 | 76.7 | 80.6 | 79.9 |
| AT | 78.1 | 77.6 | 74.9 | 75.4 | 73.1 | 80.4 |
| Ave+AT | 77.9 | 77.7 | 75.3 | 75.4 | 73.5 | 80.1 |
| CT | 64.7 | 65.3 | 62.3 | 61.4 | 66.1 | 67.3 |
| Dur+CT | 58.6 | 59.5 | 56.3 | 55.4 | 59.6 | 60.7 |
| Ate+AT | 58.5 | 56.7 | 54.8 | 54.3 | 53.6 | 61.6 |
| Niv+Ipi | 56.2 | 55.8 | 50.9 | 50.2 | a (44.8), b (70.9) | 60.1 |
| Tre | 47.8 | 47.4 | 43.0 | 60.5 | 48.8 | 50.6 |
| Dur+Tre | 46.5 | 47.4 | 41.5 | 47.4 | 47.0 | 49.0 |
| Ipi | 35.9 | 32.2 | 27.7 | 23.0 | 38.9 | 40.6 |
| Pem+RT | 27.9 | 28.5 | - | 23.3 | 27.2 | 25.2 |
| Pem | 25.9 | 26.9 | 23.0 | 21.9 | 24.8 | c (21.0), d (32.2) e (40.4) |
| Dur | 23.7 | 24.5 | 23.5 | 25.6 | 23.2 | 24.5 |
| Ate | 20.3 | 21.1 | 17.4 | 16.6 | 17.6 | 20.6 |
| Niv | 19.6 | 19.7 | 16.1 | 16.2 | 19.2 | 20.5 |
| Niv+RT | 17.9 | 17.9 | - | 14.1 | 17.5 | 18.4 |
| Ave | 11.6 | 12.3 | 9.3 | 7.4 | 10.6 | 11.3 |
| Placebo | 1.0 | 0.8 | 0.1 | - | 1.0 | 1.0 |
| Grade 5 | ||||||
| Ate+CAT | 86.6 | 86.9 | 87.2 | 85.1 | 87.1 | 88.0 |
| Tre | 84.5 | 86.2 | 83.9 | 86.6 | 84.7 | 86.7 |
| Ave+AT | 74.2 | 75.3 | 71.0 | 73.9 | 73.6 | 71.2 |
| Dur+CT | 72.9 | 73.9 | 72.2 | 71.5 | 72.0 | 74.7 |
| Dur+Tre | 72.2 | 73.0 | 68.7 | 73.3 | 70.9 | 72.7 |
| Ipi+CT | 69.7 | 70.4 | 70.4 | 65.9 | 67.4 | 70.7 |
| Dur | 69.1 | 68.9 | 64.6 | 71.1 | 68.0 | 70.0 |
| Niv+Ipi | 62.2 | 64.6 | 62.5 | 58.9 | a (63.0), b (57.5) | 64.4 |
| Ate+CT | 62.1 | 62.7 | 62.3 | 60.5 | 61.6 | 64.9 |
| Ate+AT | 60.1 | 34.0 | 60.0 | 58.8 | 60.3 | 61.3 |
| Pem+CT | 59.4 | 59.7 | 58.8 | 57.8 | 59.5 | 61.5 |
| Ipi | 48.1 | 47.9 | 43.0 | 30.9 | 46.9 | 49.7 |
| CT | 42.1 | 42.8 | 41.7 | 40.0 | 41.4 | 44.5 |
| Pem | 41.4 | 42.3 | 41.0 | 39.2 | 40.5 | c (46.3), d (30.3) e (40.4) |
| AT | 38.3 | 45.2 | 37.4 | 36.7 | 38.2 | 39.6 |
| Pem+RT | 26.9 | 27.4 | - | 25.4 | 28.5 | 29.7 |
| Niv | 23.6 | 24.7 | 22.6 | 21.8 | 23.1 | 25.0 |
| Pem+AT | 23.2 | 29.2 | 22.5 | 21.9 | 23.0 | 23.8 |
| Ate | 14.3 | 14.5 | 13.9 | 12.5 | 14.2 | 15.4 |
| Ave | 10.6 | 10.8 | 8.8 | 7.9 | 10.0 | 10.0 |
| Placebo | 8.7 | 9.7 | 7.7 | - | 8.5 | 9.0 |
AT, antiangiogenic therapy; Ate, atezolizumab; Ave, avelumab; CAT, CT+AT; CT, chemotherapy; Dur, durvalumab; Ipi, ipilimumab; Niv, nivolumab; Pem, pembrolizumab; RT, radiotherapy; SUCRA, surface under the cumulative ranking; TRAEs, treatment-related adverse events; Tre, tremelimumab; a, Niv(3 mg)+Ipi(1 mg); b, Niv(1 mg)+Ipi(3 mg); c, Pem(200 mg); d, Pem(2 mg/kg); e, Pem(10 mg/kg).
Transitivity, inconsistency, heterogeneity, and sensitivity analysis
Assessment of transitivity for grade 3–5 TRAEs indicated that the sample size, median age, and median follow-up times across treatment comparisons were relatively similar (Supplemental Figure S4). There were 13 independent closed loops with 32 comparisons in the network for grade 3–5 TRAEs, and 15 independent closed loops with 31 comparisons for grade 5 TRAEs. The design-by-treatment test for grade 3–5 TRAEs showed that there was no significantly global inconsistency (p = 0.102). However, tests of local inconsistency (loop-specific method and node-split model) showed that two of the loops (ipilimumab-nivolumab-placebo, p = 0.003; and pembrolizumab + CT-pembrolizumab-CT, p = 0.009) (Supplemental Table S4) and three of the comparisons (nivolumab + ipilimumab versus nivolumab, p = 0.017; nivolumab + ipilimumab versus ipilimumab monotherapy, p = 0.005; and ipilimumab versus placebo, p = 0.018) (Supplemental Table S5) were inconsistent. No significantly global (p = 0.976) or local inconsistencies (Supplemental Tables S4 and S5) were observed for grade 5 TRAEs.
The median heterogeneity, τ², were estimated at 0.29 (95% CI: 0.17–0.49) for grade 3–5 TRAEs, suggesting moderate heterogeneity; and 0.02 (95% CI: 0.01–0.23) for grade 5 TRAEs, suggesting low heterogeneity. The common heterogeneity standard deviation (SD) was 0.54 (95% CI: 0.41–0.70) for grade 3–5 TRAEs, and 0.14 (95% CI: 0.01–0.48) for grade 5 TRAEs. Subgroup meta-regression analyses for grade 3–5 TRAEs (Supplemental Table S6) revealed that the treatment choice and tumor type were the main sources of heterogeneity. Exclusion of patients receiving first-line therapy or including only patients with lung cancer resulted in 24.1% or 20.4%, respectively, relative reduction in heterogeneity SD. Sample size, control arm, and drug dose were also potential sources of heterogeneity. Excluding trials with a sample size <100 participants, or trials with a placebo-controlled design, or dividing treatments of nivolumab + ipilimumab into two dose groups resulted in 3.7%, 3.7%, or 5.6% relative reduction in heterogeneity SD, respectively.
Sensitivity analysis (Table 3) conducted by omitting trials with high risk of bias (n = 12), with sample size <100 (n = 6), or with placebo-controlled arms (n = 4) did not affect the main results of toxicity ranking substantially for both grade 3–5 and grade 5 TRAEs. Sensitivity analysis dividing treatments of nivolumab + ipilimumab into two dose groups or pembrolizumab into three dose groups resulted in slight changes in the ranking order of nivolumab + ipilimumab or pembrolizumab for either grade 3–5 or grade 5 TRAEs, without obvious changes in the ranking order of other treatments.
Subgroup analysis according to the type and severity grade 3–5 TRAEs
Results of the subgroup analyses are shown in Supplemental Tables S7–14. In term of grade 3–5 respiratory TRAEs, pembrolizumab was more toxic than CT. In terms of grade 3–5 gastroenteropancreatic TRAEs, atezolizumab + CAT, pembrolizumab + CT, ipilimumab + CT, atezolizumab + CT, pembrolizumab + AT, atezolizumab + AT, avelumab + AT, nivolumab + ipilimumab, ipilimumab monotherapy, pembrolizumab monotherapy, CT, and AT were more toxic than monotherapy with nivolumab, atezolizumab, or avelumab; atezolizumab + CAT, pembrolizumab + CT, ipilimumab + CT, atezolizumab + CT, nivolumab + ipilimumab, and CT were also more toxic than pembrolizumab monotherapy; atezolizumab + CAT, pembrolizumab + CT, and ipilimumab + CT were also more toxic than durvalumab + CT; the combination of ipilimumab + CT was also more toxic than atezolizumab + CT, nivolumab + ipilimumab, CT, ipilimumab monotherapy, atezolizumab + AT, and pembrolizumab + RT; atezolizumab + AT was more toxic than avelumab monotherapy; tremelimumab was also more toxic than monotherapy with avelumab or atezolizumab. As for grade 3–5 hepatic TRAEs, ipilimumab + CT and nivolumab + ipilimumab were more toxic than monotherapy with ipilimumab, pembrolizumab, nivolumab, avelumab, or CT; durvalumab + CT and atezolizumab + CT were more toxic than CT. Regarding grade 3–5 neurological TRAEs, atezolizumab + CT was more toxic than monotherapy with pembrolizumab, nivolumab, atezolizumab, or avelumab; CT was more toxic than monotherapy with pembrolizumab or atezolizumab. As for grade 3–5 endocrine TRAEs, durvalumab + CT, nivolumab + ipilimumab, pembrolizumab + CT, atezolizumab + CT, ipilimumab, and pembrolizumab monotherapy were more toxic than CT; nivolumab + ipilimumab and ipilimumab monotherapy were also more toxic than nivolumab monotherapy; pembrolizumab + AT was more toxic than AT. For grade 3–5 skin TRAEs, nivolumab + ipilimumab and ipilimumab + CT were more toxic than monotherapy with pembrolizumab, nivolumab, CT, and AT; ipilimumab monotherapy was also more toxic than CT. With regard to grade 3–5 hematological TRAEs, durvalumab + CT, atezolizumab + CT, pembrolizumab + CT, ipilimumab + CT, CT, and AT were more toxic than avelumab monotherapy; durvalumab + CT, atezolizumab + CT, ipilimumab + CT, and CT were also more toxic than monotherapy with nivolumab or pembrolizumab. All results mentioned above were statistically significant with the ORs and lower limits of 95% CIs greater than 1. No significant differences were observed in grade 3–5 renal TRAEs among all treatments.
The safety ranking based on SUCRA (Table 4) showed that pembrolizumab monotherapy, atezolizumab + CAT, durvalumab + CT, avelumab, nivolumab + ipilimumab, pembrolizumab + AT, atezolizumab + CT, and AT were ranked the most toxic regimens for respiratory, gastroenteropancreatic, hepatic, renal, skin, endocrine, neurological, and hematological grade 3–5 TRAEs, respectively.
Table 4.
SUCRA values according to type of grade3–5 TRAEs.
| Respiratory | Gastroentero-pancreatic |
Hepatic |
Renal |
Skin |
Endocrine |
Neurological |
Hematological |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | SUCRA | Treatment | SUCRA | Treatment | SUCRA | Treatment | SUCRA | Treatment | SUCRA | Treatment | SUCRA | Treatment | SUCRA | Treatment | SUCRA |
| Pem | 81.2 | Ate+CAT | 90.2 | Dur+CT | 85.8 | Ave | 73.6 | Niv+Ipi | 87.8 | Pem+AT | 87.2 | Ate+CT | 91.9 | Dur+CT | 81.9 |
| Pem+CT | 68.1 | Ipi+CT | 88.7 | Ipi+CT | 84.7 | Ipi+CT | 72.6 | Ipi+CT | 84.2 | Dur+CT | 73.4 | Ipi+CT | 77 | AT | 79.2 |
| Niv+Ipi | 67.3 | Pem+CT | 79.9 | Niv+Ipi | 84.3 | Niv+Ipi | 68.4 | Pem+RT | 83.7 | Ipi | 68.6 | CT | 71.8 | Ate+CT | 76.5 |
| Dur+CT | 61.4 | Pem+AT | 76.3 | Ate+CAT | 67.2 | Ate+CT | 62.3 | Ipi | 75.4 | Niv+Ipi | 67.3 | Niv+Ipi | 42.8 | CT | 72.5 |
| Pem+RT | 61.1 | Tre | 73.4 | Pem+CT | 58.9 | Pem+CT | 55.2 | Ate+CT | 63.7 | Pem+CT | 65.7 | Niv | 41.7 | Pem+CT | 70.7 |
| Ate+CAT | 56.5 | Ave+AT | 70.3 | Ate+CT | 55.1 | Pem | 54.7 | Ate+CAT | 51.7 | Ate+CAT | 64.3 | Pem | 32.3 | Ipi+CT | 70.6 |
| Niv | 48.2 | Ate+CT | 66.1 | Pem+RT | 43.5 | Pem+RT | 51.1 | Pem+CT | 48.4 | Ave | 51 | Ate | 21.5 | Ate+AT | 48.6 |
| Ate+CT | 46.9 | AT | 64.6 | Niv | 41 | Ipi | 39.4 | Pem | 47.3 | Ate+CT | 48.8 | Ave | 21.1 | Ate | 42.5 |
| Ipi | 35.5 | Dur+Tre | 63.3 | Pem | 38.3 | Dur+CT | 39.1 | Niv | 46.0 | Ave+AT | 47.2 | Ave+AT | 36.6 | ||
| CT | 32.4 | Niv+Ipi | 62.9 | Ipi | 35.2 | Niv | 32.2 | Dur+CT | 44.3 | Pem+RT | 46.9 | Pem+AT | 34.2 | ||
| Ave | 25.8 | CT | 52.2 | Ave | 30.2 | Placebo | 25.9 | Pem+AT | 34.7 | AT | 45.5 | Niv+Ipi | 33.9 | ||
| Placebo | 15.6 | Ipi | 51.6 | CT | 15.3 | CT | 25.4 | Placebo | 34.1 | Ate+AT | 44.5 | Ipi | 32.5 | ||
| Ate+AT | 41.2 | Placebo | 10.5 | CT | 31.9 | Pem | 44.3 | Niv | 31.9 | ||||||
| Pem | 35.3 | Ate+AT | 27.7 | Ate | 39.7 | Pem | 30.3 | ||||||||
| Dur+CT | 31.1 | Ave+AT | 21.5 | Placebo | 33.4 | Ave | 8.1 | ||||||||
| Pem+RT | 27.1 | AT | 17.7 | Niv | 31.8 | ||||||||||
| Dur | 27 | Ipi+CT | 24.7 | ||||||||||||
| Niv | 23.1 | CT | 15.6 | ||||||||||||
| Ave | 11.1 | ||||||||||||||
| Ate | 9.9 | ||||||||||||||
| Placebo | 4.8 | ||||||||||||||
AT, antiangiogenic therapy; Ate, atezolizumab; Ave, avelumab; CT, chemotherapy; Dur, durvalumab; Ipi, ipilimumab; Niv, nivolumab; Pem, pembrolizumab; RT, radiotherapy; SUCRA, surface under the cumulative ranking; TRAEs, treatment-related adverse events; Tre, tremelimumab.
Discussion
To our knowledge, this is the largest and most comprehensive network meta-analysis conducted to assess the comparative safety of ICIs. Compared with the previous meta-analysis on this subject, our network meta-analysis included more recent studies, as well as the information reported in the predominant oncology congresses of 2019, more patients, and compared nearly all ICI-based treatments used in cancers. Moreover, this network meta-analysis focused on individual ICIs rather than ICI classes, selecting TRAEs instead of irAEs as the outcome of interest, and assessing the risk of grade 3–5 and grade 5 TRAEs separately. This network meta-analysis included 67 RCTs involving 36,422 patients and compared 19 ICIs. The incidence of grade 3–5 and grade 5 TRAEs were 17.9% and 0.8%, respectively, for monotherapy with an ICI, and were 46.3% and 1.4%, respectively, for combinatorial therapy. Pneumonitis was the most common cause of grade 5 TRAEs for patients receiving either monotherapy (16 out of 98; 16.3%) or combinatorial therapy (14 out of 123; 11.4%). Most of combinatorial treatments (ICI + CT, or AT, or another ICI) showed a significantly higher risk for grade 3–5 TRAEs than most of ICI-based monotherapy regimens. However, no significant differences were observed between several monotherapy regimens (tremelimumab, ipilimumab, durvalumab, and pembrolizumab) and combinatorial treatments in risk of grade 5 TRAEs, and tremelimumab was ranked the second-most toxic treatment among all treatments. Compared with grade 3–4 TRAEs, grade 5 TRAEs are uncommon. Individual clinical trials cannot characterize these rare toxic effects comprehensively, and the comparative risk of fatal TRAEs in ICI-based therapies is still not fully understood. Our findings suggested that although monotherapy was generally safer than a combinatorial treatment, a number of them seemed to be associated with an even higher risk of grade 5 TRAEs, which suggests that monitoring for adverse events is important.
Although CTLA-4 inhibitors are generally considered to be more toxic, and PD-L1 inhibitors are generally considered to be better tolerated because of their programmed cell death ligand-2-sparing ability that preserves the normal immunological homeostasis among ICIs used as monotherapy,3,5,96,97 the lack of head-to-head comparisons prevents us from making a firm conclusion. In a systematic analysis of the toxicity profile of PD-1 versus PD-L1 Inhibitors in non-small cell lung cancer,98 patients treated with PD-1 inhibitors had an increased rate of irAEs (16% versus 11%, p = 0.07) and pneumonitis (4% versus 2%, p = 0.01) compared with patients who received PD-L1 inhibitors. However, in our network meta-analysis, no significant differences in the risk of grade 3–5 TRAEs were observed between PD-1 and PD-L1 inhibitors. Tremelimumab showed a significantly higher risk of grade 3–5 TRAEs than avelumab. The toxicity of ICIs as monotherapy, in terms of grade 3–5 TRAEs ranked from high to low was: tremelimumab, ipilimumab, pembrolizumab, durvalumab, atezolizumab, nivolumab, and avelumab. In terms of grade 5 TRAEs, tremelimumab was more toxic than other ICIs except durvalumab and ipilimumab; and pembrolizumab was more toxic than atezolizumab and avelumab. The toxicity ranking of ICIs as monotherapy based on the risk of grade 5 TRAEs from high to low was: tremelimumab, durvalumab, ipilimumab, pembrolizumab, nivolumab, atezolizumab, and avelumab. These results suggested that tremelimumab and avelumab seemed to be the most and least toxic ICIs monotherapy, respectively, and that different ICIs in the same class might be related to different risks of serious TRAEs.
To date, few trials have directly compared the safety between ICI-based combinatorial treatments. In their network meta-analysis, Xu et al. concluded no significant difference was observed in the risk of all-grade and grade 3–5 TRAEs between the combination of two ICIs and one ICI with conventional therapy.6 In our network meta-analysis, 12 combinatorial treatments were compared. There were no significant differences in either the risk of grade 3–5 or grade 5 TRAEs among combinatorial treatments with CT or AT, while two ICIs (durvalumab + tremelimumab or nivolumab + ipilimumab) showed lower risk of grade 3–5 TRAEs than ICIs in combination with CT (except durvalumab + CT). Based on toxicity rankings, atezolizumab + CAT, pembrolizumab + CT, and ipilimumab + CT were ranked the most, second-most, and third-most toxic regimens in term of grade 3–5 TRAEs, respectively. Moreover, we found that the comparative risk of grade 3–5 TRAEs for ICIs based treatments varied depending on the nature and degree of severity TRAEs. Pembrolizumab, atezolizumab + CAT, durvalumab + CT, avelumab, nivolumab + ipilimumab, pembrolizumab + AT, atezolizumab + CT, and durvalumab + CT were ranked the most toxic treatments in risk of respiratory, gastroenteropancreatic, hepatic, renal, skin, endocrine, neurological, and hematological grade 3–5 TRAEs, respectively. These findings will be helpful for physicians to tailor an ICI-based therapy strategy for patients with different clinical backgrounds. For example, although the overall risk of grade 3–5 TRAEs for pembrolizumab and avelumab monotherapy were lower than combinatorial treatments, they seemed to have the highest risk of respiratory and renal grade 3–5 TRAEs in our study respectively, and should be used with caution in patients with chronic lung or kidney diseases.
Several recent clinical trials have evaluated combinations of ICIs with RT in cancers.54,62,63,99 The available data suggests that the combination has significantly improved survival compared with ICIs or RT alone. However, it is still not clear if combining ICIs with RT will increase the risk of TRAEs. In the present network meta-analysis, the risk of grade 3–5 TRAEs for pembrolizumab + RT or nivolumab + RT was similar to ICI monotherapy and was lower than other combinatorial treatments. Of note, current trials only represent a small fraction of the potential therapeutic combinations of ICIs with RT. Some factors such as treatment schedules of ICIs plus RT (concurrent or sequential), RT technique (SBRT or conventional RT), anatomic location irradiated (internal organs, bone, or brain), interval between treatments, and type of ICI used might affect the outcomes. Further clinical studies are needed to address these issues.
Some limitations of our network meta-analysis should be stated. First, heterogeneity was observed in the results of grade 3–5 TRAEs. Subgroup meta-regression analyses revealed that trials with a sample size <100 patients, cancer type, treatment line, and drug dose were potential sources of heterogeneity. However, sensitivity analysis showed that the main results for both grade 3–5 and grade 5 TRAEs were not markedly altered when removing trials of high risk of bias, sample size <100, or placebo-controlled, or dividing treatments of nivolumab + ipilimumab and pembrolizumab into different dose groups. Second, some trials reported TRAEs without the necessary details, and excluded reporting on TRAEs which occurred underneath a certain threshold (for example 1% or 5%). The missing information might result in bias. Moreover, different CT regimens and schedules used in individual trials might also lead to heterogeneity. Third, some of the newer data were extracted from recent conference abstracts. This could lead to a selection bias because the comprehensive toxicity data might be reported in the full publication. Fourth, TRAES refer to those adverse events which occur during the treatment, while irAEs mean those which have a putative immunological basis, and irAEs/TRAEs incidence might differ from each other. We selected TRAEs instead of irAEs as the outcome of interest in this study because TRAEs are more suitable for identifying and describing the safety profiles of chemo-immunotherapy combinations. However, not using the irAEs profiles might result in missing/overlooking the true nature of the monotherapy safety profile (at least for clinical practice). Finally, the network meta-analysis was conducted based on results reported from trials rather than individual patient data, and they were based on indirect comparisons but not direct comparisons. Thus, interpretation of the network meta-analysis results and drawing conclusions should be done with caution.
Conclusion
Compared with ICI-based combinatorial therapy, monotherapy with an ICI had a lower risk of grade 3–5 TRAEs, but some of them resulted in an even higher risk of fatal TRAEs. Some ICIs combined with CT seemed to be more toxic than the combination with RT or combination of two ICIs. Atezolizumab + CAT seemed to be the most toxic and nivolumab + RT seemed to be the least toxic among the combinatorial treatments, and among the monotherapy regimens, tremelimumab and avelumab seemed to be the most and least toxic, respectively. The toxicity ranking of some treatments changed depending on the nature and degree of severity of grade 3–5 TRAEs.
Supplemental Material
Supplemental material, Supplementary_File for Comparative risk of serious and fatal treatment-related adverse events caused by 19 immune checkpoint inhibitors used in cancer treatment: a network meta-analysis by Tingting Liu, Bo Jin, Jun Chen, Hui Wang, Shuiyu Lin, Jun Dang and Guang Li in Therapeutic Advances in Medical Oncology
Footnotes
Author contributions: DJ contributed to the conception and design, data interpretation, and statistical analysis. LT and JB contributed to data acquisition, data interpretation, and statistical analysis and drafting of the manuscript. CJ, WH, LS, and LG contributed to data acquisition, data interpretation, and drafting of the manuscript. All authors read and approved the final manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jun Dang  https://orcid.org/0000-0002-1408-4749
https://orcid.org/0000-0002-1408-4749
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Tingting Liu, Department of Radiation Oncology, The First Hospital of China Medical University, Shenyang, China Department of Radiation Oncology, Anshan Cancer Hospital, Anshan, China.
Bo Jin, Department of Medical Oncology, The First Hospital of China Medical University, Shenyang, China.
Jun Chen, Department of Radiation Oncology, Shenyang Chest Hospital, Shenyang, China.
Hui Wang, Department of Radiation Oncology, General Hospital of Benxi Iron & Steel Industry Group of Liaoning Health Industry Group, Shenyang, China.
Shuiyu Lin, Department of Radiation Oncology, The First Hospital of China Medical University, Shenyang, China.
Jun Dang, Department of Radiation Oncology, The First Hospital of China Medical University, 155 Nanjing Road, Heping District, Shenyang, 110001, China.
Guang Li, Department of Radiation Oncology, The First Hospital of China Medical University, Shenyang, China.
References
- 1. Marrone KA, Ying W, Naidoo J. Immune-related adverse events from immune checkpoint inhibitors. Clin Pharmacol Ther 2016; 100: 242–251. [DOI] [PubMed] [Google Scholar]
- 2. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018; 378: 158–168. [DOI] [PubMed] [Google Scholar]
- 3. Wang Y, Zhou S, Yang F, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol 2019; 5: 1008–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers 2020; 6: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khoja L, Day D, Wei-Wu Chen T, et al. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol 2017; 28: 2377–2385. [DOI] [PubMed] [Google Scholar]
- 6. Xu C, Chen YP, Du XJ, et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ 2018; 363: k4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dafni U, Tsourti Z, Vervita K, et al. Immune checkpoint inhibitors, alone or in combination with chemotherapy, as first-line treatment for advanced non-small cell lung cancer. A systematic review and network meta-analysis. Lung Cancer 2019; 134: 127–140. [DOI] [PubMed] [Google Scholar]
- 8. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 2018; 19: 1480–1492. [DOI] [PubMed] [Google Scholar]
- 10. Sharma P, Siefker-Radtke A, de Braud F, et al. Nivolumab alone and with ipilimumab in previously treated metastatic urothelial carcinoma: checkmate 032 nivolumab 1 mg/kg plus ipilimumab 3 mg/kg expansion cohort results. J Clin Oncol 2019; 37: 1608–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010; 8: 336–341. [DOI] [PubMed] [Google Scholar]
- 12. Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gelman A, Rubin D. Markov chain Monte Carlo methods in biostatistics. Stat Methods Med Res 1996; 5: 339–355. [DOI] [PubMed] [Google Scholar]
- 14. Brooks S, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat 1998; 7: 434–455. [Google Scholar]
- 15. Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS One 2013; 8: e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods 2012; 3: 80–97. [DOI] [PubMed] [Google Scholar]
- 17. Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010; 29: 932–944. [DOI] [PubMed] [Google Scholar]
- 18. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS One 2014; 9: e99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Turner RM, Davey J, Clarke MJ, et al. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol 2012; 41: 818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carbone DP, Reck M, Paz-Ares L, et al. ; CheckMate 026 Investigators. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med 2017; 376: 2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu YL, Lu S, Cheng Y, et al. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced NSCLC: CheckMate 078 randomized phase III clinical trial. J Thorac Oncol 2019; 14: 867–875. [DOI] [PubMed] [Google Scholar]
- 25. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372: 320–330. [DOI] [PubMed] [Google Scholar]
- 26. Ascierto PA, Long GV, Robert C, et al. Survival outcomes in patients with previously untreated BRAF wild-type advanced melanoma treated with nivolumab therapy: three-year follow-up of a randomized phase 3 trial. JAMA Oncol 2019; 5: 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015; 16: 375–384. [DOI] [PubMed] [Google Scholar]
- 28. Larkin J, Minor D, D’Angelo S, et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in CheckMate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol 2018; 36: 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016; 375: 1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019; 20: 1506–1517. [DOI] [PubMed] [Google Scholar]
- 31. Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 390: 2461–2471. [DOI] [PubMed] [Google Scholar]
- 32. Reck M, Rodríguez-Abreu D, Robinson AG, et al. ; KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375: 1823–1833. [DOI] [PubMed] [Google Scholar]
- 33. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol 2019; 37: 537–546. [DOI] [PubMed] [Google Scholar]
- 34. Lopes G, Wu Y-L, Kudaba I, et al. Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ⩾ 1%: open-label, phase 3 KEYNOTE-042 study. J Clin Oncol 2018; 36(Suppl. 18): abstract LBA4. [Google Scholar]
- 35. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 36. Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015; 16: 908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hamid O, Puzanov I, Dummer R, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer 2017; 86: 37–45. [DOI] [PubMed] [Google Scholar]
- 38. Bellmunt J, de Wit R, Vaughn DJ, et al. ; KEYNOTE-045 Investigators. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017; 376: 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shah MA, Adenis A, Enzinger PC, et al. Pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer: phase 3 KEYNOTE-181 study. J Clin Oncol 2019; 37(Suppl. 4): abstract 4010. [Google Scholar]
- 40. Shitara K, Özgüroğlu M, Bang YJ, et al. ; KEYNOTE-061 investigators. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 2018; 392: 123–133. [DOI] [PubMed] [Google Scholar]
- 41. Cohen EEW, Soulières D, Le Tourneau C, et al. ; KEYNOTE-040 investigators. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 2019; 393: 156–167. [DOI] [PubMed] [Google Scholar]
- 42. Robert C, Schachter J, Long GV, et al. ; KEYNOTE-006 investigators. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015; 372: 2521–2532. [DOI] [PubMed] [Google Scholar]
- 43. Robert C, Ribas A, Schachter J, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol 2019; 20: 1239–1251. [DOI] [PubMed] [Google Scholar]
- 44. Rittmeyer A, Barlesi F, Waterkamp D, et al. ; OAK Study Group. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fehrenbacher L, Spira A, Ballinger M, et al. ; POPLAR Study Group. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016; 387: 1837–1846. [DOI] [PubMed] [Google Scholar]
- 46. Spigel DR, Marinis FD, Giaccone G, et al. Interim OS analysis of a phase III study of atezolizumab (atezo) vs platinum-based chemotherapy (chemo) as 1L treatment (tx) in PD-L1–selected NSCLC. Ann Oncol 2019; 30 (Suppl. 5): v915. [Google Scholar]
- 47. Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018; 391: 748–757. [DOI] [PubMed] [Google Scholar]
- 48. Barlesi F, Vansteenkiste J, Spigel D, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol 2018; 19: 1468–1479. [DOI] [PubMed] [Google Scholar]
- 49. Bang YJ, Ruiz EY, Van Cutsem E, et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol 2018; 29: 2052–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Beer TM, Kwon ED, Drake CG, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol 2017; 35: 40–47. [DOI] [PubMed] [Google Scholar]
- 51. Kwon ED, Drake CG, Scher HI, et al. ; CA184-043 Investigators. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2014; 15: 700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Maio M, Scherpereel A, Calabrò L, et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol 2017; 18:1261–1273. [DOI] [PubMed] [Google Scholar]
- 53. Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol 2013; 31: 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McBride SM, Sherman EJ, Tsai CJ, et al. A phase II randomized trial of nivolumab with stereotactic body radiotherapy (SBRT) versus nivolumab alone in metastatic (M1) head and neck squamous cell carcinoma (HNSCC). J Clin Oncol 2018; 36(Suppl. 15): abstract 6009. [Google Scholar]
- 55. Rini BI, Plimack ER, Stus V, et al. ; KEYNOTE-426 Investigators. Pembrolizumab plus Axitinib versus Sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019; 380: 1116–1127. [DOI] [PubMed] [Google Scholar]
- 56. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. ; KEYNOTE-189 Investigators. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018; 378: 2078–2092. [DOI] [PubMed] [Google Scholar]
- 57. Paz-Ares L, Luft A, Vicente D, et al. ; KEYNOTE-407 Investigators. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 2018; 379: 2040–2051. [DOI] [PubMed] [Google Scholar]
- 58. Langer CJ, Gadgeel SM, Borghaei H, et al. ; KEYNOTE-021 Investigators. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016; 17: 1497–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Borghaei H, Langer CJ, Gadgeel S, et al. 24-month overall survival from KEYNOTE-021 Cohort G: pemetrexed and carboplatin with or without pembrolizumab as first-line therapy for advanced nonsquamous non-small cell lung cancer. J Thorac Oncol 2019; 14: 124–129. [DOI] [PubMed] [Google Scholar]
- 60. Burtness B, Harrington KJ, Greil R, et al. KEYNOTE-048: phase III study of first-line pembrolizumab (P) for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). Ann Oncol 2019; 30 (Suppl. 9): ix97–ix106. [Google Scholar]
- 61. Josep TJ, Cutsem EV, Bang YJ, et al. Pembrolizumab with or without chemotherapy versus chemotherapy for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: the phase III KEYNOTE-062 study. J Clin Oncol 2019; 37(Suppl. 18): abstract LBA4007. [Google Scholar]
- 62. Welsh JW, Menon H, Tang C, Verma V, Altan M, Hess KR., et al. ; Randomized phase I/II trial of pembrolizumab with and without radiotherapy for metastatic non-small cell lung cancer. J Clin Oncol 2019; 37(Suppl. 15): abstract 9104. [Google Scholar]
- 63. Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. Epub ahead of print 11 July 2019. DOI: 10.1001/jamaoncol.2019.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rini BI, Powles T, Atkins MB, et al. ; IMmotion151 Study Group. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet 2019; 393: 2404–2415. [DOI] [PubMed] [Google Scholar]
- 65. McDermott DF, Huseni MA, Atkins MB, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med 2018; 24: 749–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019; 20: 924–937. [DOI] [PubMed] [Google Scholar]
- 67. Jotte RM, Cappuzzo F, Vynnychenko I, et al. IMpower131: primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol 2018; 36(Suppl. 18): abstract LBA9000. [Google Scholar]
- 68. Papadimitrakopoulou VA, Cobo M, Bordoni R, et al. IMpower132: PFS and safety results with 1L atezolizumab + carboplatin/cisplatin + pemetrexed in stage IV non-squamous NSCLC. J Thorac Oncol 2018; 13(Suppl. 10): S332–S333. [Google Scholar]
- 69. Horn L, Mansfield AS, Szczęsna A, et al. ; IMpower133 Study Group. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018; 379: 2220–2229. [DOI] [PubMed] [Google Scholar]
- 70. Schmid P, Adams S, Rugo HS, et al. ; IMpassion130 Trial Investigators. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018; 379: 2108–2121. [DOI] [PubMed] [Google Scholar]
- 71. Socinski MA, Jotte RM, Cappuzzo F, et al. ; IMpower150 Study Group. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018; 378: 2288–2301. [DOI] [PubMed] [Google Scholar]
- 72. Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019; 380: 1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Paz-Ares L, Dvorkin M, Chen Y, et al. ; CASPIAN investigators. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019; 394: 1929–1939. [DOI] [PubMed] [Google Scholar]
- 74. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364: 2517–2526. [DOI] [PubMed] [Google Scholar]
- 75. Maio M, Grob JJ, Aamdal S, et al. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J Clin Oncol 2015; 33: 1191–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol 2012; 30: 2046–2054. [DOI] [PubMed] [Google Scholar]
- 77. Govindan R, Szczesna A, Ahn MJ, et al. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J Clin Oncol 2017; 35: 3449–3457. [DOI] [PubMed] [Google Scholar]
- 78. Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol 2013; 24: 75–83. [DOI] [PubMed] [Google Scholar]
- 79. Reck M, Luft A, Szczesna A, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol 2016; 34: 3740–348. [DOI] [PubMed] [Google Scholar]
- 80. Hersh EM, O’Day SJ, Powderly J, et al. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naïve patients with advanced melanoma. Invest New Drugs 2011; 29: 489–498. [DOI] [PubMed] [Google Scholar]
- 81. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018; 378: 2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Borghaei H, Hellmann MD, Paz-Ares LG, et al. Nivolumab (Nivo) + platinum-doublet chemotherapy (chemo) vs chemo as first-line (1L) treatment (Tx) for advanced non-small cell lung cancer (NSCLC) with <1% tumor PD-L1 expression: results from CheckMate 227. J Clin Oncol 2018; 36(Suppl. 15): abstract 9001. [Google Scholar]
- 83. Bazhenova L, Redman MW, Gettinger SN, et al. A phase III randomized study of nivolumab plus ipilimumab versus nivolumab for previously treated patients with stage IV squamous cell lung cancer and no matching biomarker (Lung-MAP Sub-Study S1400I, NCT02785952). J Clin Oncol 2019; 37(Suppl. 15): abstract 9014. [Google Scholar]
- 84. Ready NE, Ott PA, Hellmann MD, et al. Nivolumab monotherapy and nivolumab plus ipilimumab in recurrent small cell lung cancer: results from the CheckMate 032 randomized cohort. J Thorac Oncol. Epub ahead of print 17 October 2019. DOI: 10.1016/j.jtho.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 85. Janjigian YY, Bendell J, Calvo E, et al. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol 2018; 36: 2836–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol 2018; 19: 672–681. [DOI] [PubMed] [Google Scholar]
- 87. Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015; 372: 2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hodi FS, Chesney J, Pavlick AC, et al. Two-year overall survival rates from a randomised phase 2 trial evaluating the combination of nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma. Lancet Oncol 2016; 17: 1558–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Motzer RJ, Tannir NM, McDermott DF, et al. ; CheckMate 214 Investigators. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018; 378: 1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Motzer RJ, Rini BI, McDermott DF, et al. ; CheckMate 214 Investigators. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol 2019; 20: 1370–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rizvi NA, Cho BC, Reinmuth N, et al. Durvalumab with or without tremelimumab vs platinum-based chemotherapy as first-line treatment for metastatic non-small cell lung cancer: MYSTIC. Ann Oncol 2018; 29(Suppl_10): x39–x43. [Google Scholar]
- 92. Siu LL, Even C, Mesía R, et al. Safety and efficacy of durvalumab with or without tremelimumab in patients with PD-L1-low/negative recurrent or metastatic HNSCC: the phase 2 CONDOR randomized clinical trial. JAMA Oncol 2019; 5: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Licitra LF, Robert I, Haddad RI, et al. A phase 3, randomized, open-label study of durvalumab (D) with or without tremelimumab (T) in patients (pts) with recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC). J Clin Oncol 2019; 37(Suppl. 15): abstract 6012. [Google Scholar]
- 94. Rubinstein MM, Caird I, Zhou Q, et al. A phase II trial of durvalumab with or without tremelimumab in patients with persistent or recurrent endometrial carcinoma and endometrial carcinosarcoma. J Clin Oncol 2019; 37(Suppl. 15): abstract 5582. [Google Scholar]
- 95. Kelly RJ, Lee J, Bang YJ, et al. Safety and efficacy of durvalumab and tremelimumab alone or in combination in patients with advanced gastric and gastroesophageal junction adenocarcinoma. Clin Cancer Res. Epub ahead of print 1 November 2019. DOI: 10.1158/1078-0432.CCR-19-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 2018; 4: 1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Stewart R, Morrow M, Hammond SA, et al. Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol Res 2015; 3: 1052–1062. [DOI] [PubMed] [Google Scholar]
- 98. Pillai RN, Behera M, Owonikoko TK, et al. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: a systematic analysis of the literature. Cancer 2018; 124: 271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Antonia SJ, Villegas A, Daniel D, et al. ; PACIFIC Investigators. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017; 377: 1919–1929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_File for Comparative risk of serious and fatal treatment-related adverse events caused by 19 immune checkpoint inhibitors used in cancer treatment: a network meta-analysis by Tingting Liu, Bo Jin, Jun Chen, Hui Wang, Shuiyu Lin, Jun Dang and Guang Li in Therapeutic Advances in Medical Oncology