Abstract
Objective
Benign epilepsy with centrotemporal spikes (BECTS) is the most common childhood idiopathic localization-related epilepsy syndrome. BECTS present normal routine magnetic resonance imaging (MRI), however, quantitative analytic techniques have captured subtle cortical and subcortical MR anomalies. Network science, including graph theory (GT) analyses, facilitates understanding of brain covariance patterns, potentially informing in important ways how this common self-limiting epilepsy syndrome may impact normal patterns of brain and cognitive development.
Methods
GT analyses examined the developmental covariance among cortical and subcortical regions in children with new/recent-onset BECTS (n=19) and typically developing healthy controls (n=22) who underwent high resolution MRI and cognitive assessment at baseline and 2 years later. Global (transitivity, global efficiency, and modularity index, Q) and regional measures (local efficiency and hubs) were investigated to characterize network development in each group. Associations between baseline-based GT measures and cognition at both time points explored the implications of GT analyses for cognition and prospective cognitive development. Furthermore, an individual contribution (IC) measure was investigated to observe how important is for BECTS to resemble controls regarding their correlation matrices.
Results
Groups exhibited similar Q and overall network configuration, with BECTS presenting significantly higher transitivity and both global and local efficiency. Furthermore, both groups presented a similar number of hubs with BECTS showing a higher number in temporal lobe regions compared to controls. The investigated measures were negatively associated to 2-year cognitive outcomes in BECTS.
Significance
Children with BECTS present a higher-than-normal global developmental configuration compared to controls, along with divergence from normality in terms of its regional configuration. Baseline GT measures demonstrate potential as a cognitive biomarker to predict cognitive outcome in BECTS two years after diagnosis. Similarities and differences in developmental network configurations and their implications for cognition and behavior across common epilepsy syndromes are of theoretical interest and clinical relevance.
Keywords: Benign Epilepsy with Centrotemporal Spikes (BECTS), Rolandic epilepsy, graph theory, cognition, brain volumes development
INTRODUCTION
Benign epilepsy with centrotemporal spikes (BECTS) is the most common idiopathic localization-related epilepsy (ILRE) syndrome in children1–4. Although classically referred to as a “benign” syndrome given its uncomplicated prognosis with remission of seizures typically prior to puberty, there is ample evidence that children affected by BECTS exhibit an increased rate of cognitive and academic problems compared to healthy peers5–8. Despite a wealth of investigations examining important dimensions of BECTS in cross-sectional research, less examined is the prospective course of brain development and its association with cognitive maturation.
Although BECTS is a focal epilepsy syndrome, structural and functional alterations have been observed in areas that extend beyond the primary centrotemporal epileptogenic regions. Cross-sectional studies examining children with chronic BECTS have reported anomalies that include both decreased cortical thickness in left perisylvian regions9 and increased cortical thickness in bilateral middle and inferior frontal gyri, bilateral supramarginal gyrus, and left insula10; along with hypertrophy of bilateral putamen11 and lower volume of the right caudate12. In addition, both white matter (WM) and functional discrepancies have been reported comprising lower fractional anisotropy in left inferior frontal and supramarginal regions, and lower functional connectivity between left sensorimotor and right inferior frontal gyrus, respectively13–14. Similarly, while interest has been longstanding in regard to the complications of language based abilities in children with BECTS15–17, cognitive complications of BECTS have been reported in diverse areas of mentation compared to age-matched control peers8,18–20, including not only language related skills but intelligence, memory, attention, and executive function (for reviews see [21–22]). Importantly, cognitive and MR abnormalities have been reported in new-onset cases as well, with the nature and range of anomalies varying across studies23–26, attesting to the fact that these abnormalities are not necessarily secondary to the effects of epilepsy chronicity and medication treatment20.
Prospective cognitive and imaging research in BECTS is much less common. The limited available evidence has suggested that anomalies in prospective brain development are characterized by slowed cortical thinning compared to typically developing children in bilateral frontal, parietal, occipital, and left insular regions, with persisting hypertrophy of the putamen bilaterally24. The cognitive anomalies that have been detected at baseline27 appear to persist over time without a definable pattern of deterioration or improvement24.
One issue that remains unexplored is how the initial cortical and subcortical structural irregularities may affect maturation of other brain regions and influence typical cognitive development. Using graph theory (GT), we address this central question of brain and cognitive development in order to better understand this common epilepsy syndrome. GT analyses have been used to investigate the covariance of cortical volumes cross-sectionally in healthy children28 and children with epilepsy29–30. This method of brain analysis measures associations of cortical volumes among diverse brain regions and clarifies which areas play a relatively more important role during development. Although informative, cross-sectional analyses tell only part of the story. Therefore, the purpose of this study is twofold. First, we prospectively investigate the covariance network development among cortical and subcortical structures in children with BECTS during the first two years following epilepsy diagnosis compared to healthy controls. Second, we examine the association between network metrics near epilepsy onset (baseline) and cognitive performance at baseline and two years later. This approach potentially informs the degree to which GT measures may serve as potential biomarkers of cognitive status and cognitive development in this common childhood epilepsy syndrome. As we demonstrate, GT measures calculated on baseline morphological networks appear to be promising as cognitive biomarker in BECTS, particularly for 2-year neuropsychological outcomes.
METHODS
Participants
Participants included 19 youth with recent-onset BECTS and 22 normally developing controls that were matched to BECTS in terms of age and sex. All participants completed neuropsychological assessment and neuroimaging at two different time points. The baseline assessment was performed within 12 months of epilepsy onset. In BECTS, the time between time points was 1.81–2.25 years (average 2.0 years), and in controls it was 1.86–2.15 years (average 1.9 years). Details and rationale for selection criteria for participants can be found in a previous publication31. In short, inclusion criteria comprised epilepsy diagnosis within 12 months, no other developmental disabilities, no other neurological disorder, and normal clinical MRI. A pediatric neurologist certified by the American Board of Psychiatry and Neurology diagnosed individuals with BECTS according to the International League Against Epilepsy (ILAE) international classification of epilepsy32. Criteria for inclusion of control participants included no neurological disorders, no history of seizures, no history of any classic precipitating injury (e.g., febrile seizures), no previous loss of consciousness for >5 min, and no other family history of a first-degree relative with epilepsy or febrile convulsions. All children with epilepsy were attending regular schools at the time of baseline evaluations, and intelligence quotients (IQ) were within normal levels. Details regarding demographic and clinical characteristics of the study participants are provided in Table 1. A flow-chart regarding retention of participants can be found in the supplementary document (Figure 1S). All procedures were conducted by research staff.
Table 1:
Demographic and Clinical Characteristics
| Control (n=22) | BECTS (n=19) | |
|---|---|---|
| Age (range: mean ± SD) | 8.4–14.9: 11.3 ± 2.0 | 8.1–14.9: 10.5 ± 1.9 |
| Sex (F/M) | 12/10 | 6/13 |
| SES (mean ± SD) | 4.4 ± 1.6 | 4.6 ± 1.3 |
| Grade (mean ± SD) | 5.2 ± 2.1 | 4.6 ± 1.9 |
| IQ (mean ± SD) | 108.9 ± 11.0 | 106.3 ± 15.9 |
| Epilepsy duration (months, mean ± SD) | - | 7.1 ± 3.2 |
| AED (Y/N) | - | 11/8 |
| Epilepsy comorbidities at baseline: | - | |
| ADHD (Y/N) | 3/16 | |
| Anxiety (Y/N) | 4/15 | |
SES=socioeconomic status; based on the mother’s education. AED: Antiepileptic drug.
The project protocol was reviewed and approved by the institutional review board of the University of Wisconsin School of Medicine and Public Health. Families and children gave written informed consent or assent, respectively, on the day of the study.
Neuropsychological assessment
In this investigation we included cognitive measures assessing the domains of immediate33 and delayed verbal memory33, psychomotor speed34, and speeded motor dexterity35 based on a previous investigation on the same group of BECTS27. In addition, we added tests of intelligence36 and executive function37, based on the usually-affected domains in BECTS21. These test scores were standardized according to age-based norms. Details of the tests and targeted cognitive domains are presented in Table 2. In summary, 10 cognitive tests were included for investigation of their relationship with GT results of morphological networks at baseline.
Table 2.
Cognitive tests for correlation analysis
| Test Name | Cognitive ability | Abbreviation | |
|---|---|---|---|
| 1 | WASI Vocabulary36 | Word knowledge (word definition) | IQVOCS |
| 2 | WASI Block Design36 | Visuoconstruction (block design reproduction) | IQBDS |
| 3 | WASI Similarities36 | Verbal reasoning (similarities between nouns) | IQSIMS |
| 4 | WASI Matrix Reasoning36 | Nonverbal reasoning (visual reasoning) | IQMRS |
| 5 | Children’s Memory Scale-III33 | Total list learning (cumulative number of words learned across trials) | WLLSS |
| 6 | Children’s Memory Scale-III33 | Delayed verbal memory (total words spontaneously remembered in delayed recall) | WLDSS |
| 7 | D-KEFS Card Sorting Test37 | Problem solving (number of correct sorts) | CORSORS |
| 8 | D-KEFS Inhibition37 | Response inhibition (color-word interference) | INHSS |
| 9 | WISC-IV Digit Symbol34 | Psychomotor speed (speeded digit-symbol substitution) | IQDSYMS |
| 10 | Grooved Pegboard35 | Speeded motor dexterity (dominant hand) | PGDOM |
MRI acquisition and processing
MR images were obtained on a 1.5-T GE Signa MRI scanner (GE Healthcare, Waukesha, WI, USA). T1-weighted images were acquired using a three-dimensional (3D) spoiled gradient recall (SPGR) with imaging parameters: repetition time (TR)=24ms, echo time (TE)=5ms, flip angle=40°, thickness=1.5mm, slices=124, plane=coronal, field-of-view (FOV)=20cm, matrix=256×256. Processing of T1-weighted images was performed with the software Freesurfer (http://freesurfer.net) (version 5.3) using the recon-all pipeline38–39. Details regarding image processing can be found in a previous publication24. In short, we performed automatic cortical surface parcellation and subcortical structures segmentation, with cortical volume changes calculated using Freesurfer’s processing stream for longitudinal images40. Once both baseline and follow-up scans were longitudinally processed, the change in volume between time points normalized to the baseline evaluation ((TP2-TP1)/TP1) was calculated. Aside from calculations of prospective development, baseline volumes also underwent GT investigations in order to study possible associations to neurocognitive measures. Baseline volumes were obtained from the longitudinally processed data.
Matrix and graph theory measures: calculations and statistical analyses
Methods for matrices creation and GT measures can be found in a previous investigation41. In short, we calculated a weighted undirected matrix of 85 nodes based on the correlation coefficients of the covariance between nodal volume changes for each group. Each matrix was resampled by replacement 500 times; and the null distribution was calculated based on 500 random matrices with the same number of nodes and degree distribution as the pertinent graphs. P-values were corrected for multiple comparisons (Bonferroni correction) and matrices were corrected for intracranial volume (ICV) and age. A combination of proportional thresholding with the Minimum Spanning Tree (MST) as its backbone was employed as graph thresholding. For example, a hybrid threshold of 25% means the MST (a subset of the whole graph that connects every node just once without making loops and not allowing disconnected nodes) plus a 25% of the rest of the strongest correlations from the matrix (see [42] for details). GT measures were obtained from each resampled matrix and averages were used for analyses. Table 1S of the supplementary file contains the list of the 85 nodes used and their abbreviations.
Global measures such as transitivity, global efficiency (GE), and Q were calculated in order to investigate the properties of network segregation, integration, and configuration, respectively. Transitivity characterizes the level of segregation of the network, i.e., the degree of clustering of nodes in the network. Global and local efficiency were calculated to examine network integration43. Finally, the community structure indexes the configuration of a network into segregated communities while Q speaks to how easily those communities are identified by the algorithm. Given that the modularity algorithm provides a statistical estimate for each output44, we calculated modularity 1000 times for each group and the highest proportion was chosen as the number of modules in that group. Next, we used the open source software Gephi 9.2 (https://gephi.org) for the 2D visualization of community structure using the Force Atlas 2 algorithm (scaling=2000). These global metrics were calculated over a range of hybrid thresholds (15%−35%) to ensure that the results were not driven by graph density.
Betweenness centrality (BC) was investigated here in order to identify the hubs of the networks; calculated for each group at a hybrid threshold of 25%. Hubs are nodes that are critical for the configuration of the network45. BC measures the relevance of a node for the communication between other nodes in the network46. Hubs for each group were identified as those nodes with BC higher than one standard deviation above the mean. Group differences were investigated by performing two-sample student’s t-test for the global and regional measures (both correcting for multiple comparisons using Bonferroni correction). No statistical comparisons were undertaken in the visualization of community structure since only qualitative observations are performed.
Correlation analyses of baseline GT with cognition
To determine whether MR-based GT measures might serve as potential biomarkers of cognition, GT metrics were also calculated at the baseline evaluation, and correlated to cognitive performance at both baseline and the 2-year follow-up assessments. To obtain a better sense of the associations, GT metrics were derived for adjacency matrices corresponding to each participant. This was done by applying the AOP (add-one-patient) approach formulated by Saggar et al.47. With this method one of the BECTS participants is added to the control group before the correlation matrix is calculated. Then the matrix of the control group is subtracted from the one containing the controls plus one BECTS and a matrix reflecting the effect of that single BECTS participant is obtained. This was done for each BECTS participant (n=19) and GT metrics were obtained for each. In this way each participant’s cognitive results at baseline and at 2-year follow-up would have a corresponding GT value at baseline.
Transitivity, GE, Q, and average BC were calculated from the matrix representing each of the BECTS participants at a hybrid threshold of 25%. In addition, the individual contribution (IC) measure was calculated47. This measure reflects the similarity between the matrix containing only controls and the matrix containing controls plus each BECTS participant. A value close to 1 means a high contribution of the BECTS participant to the control group matrix while a value close to 0 means low contribution. This measure was also calculated to see how beneficial it is for BECTS to be similar to controls in terms of cognition and, therefore, was also included in the correlation analysis. These calculations of subject-specific GT measures and IC were also obtained for each of the control participants; however, using the “Leave-one-out” approach47. Significant correlations were attested using False discovery rate (FDR) correction.
RESULTS
Groups were similar in age (F(1,40)=1.389, p=0.25), sex (χ2=2.183; p=0.122), socioeconomic status (SES) (F(1,40)=0.145, p=0.71), grade (F(1,40)=0.915, p=0.35), and IQ (F(1,40)=0.363, p=0.55). MR analyses were corrected for intracranial volume (ICV). Given that the average age between groups was one year different, albeit non significant, age was also included as a nuisance covariate. Differences in age, SES, grade, and IQ were tested with the independent samples t-test; differences in sex were tested with the chi-squared test of independence.
Community structure of morphological development
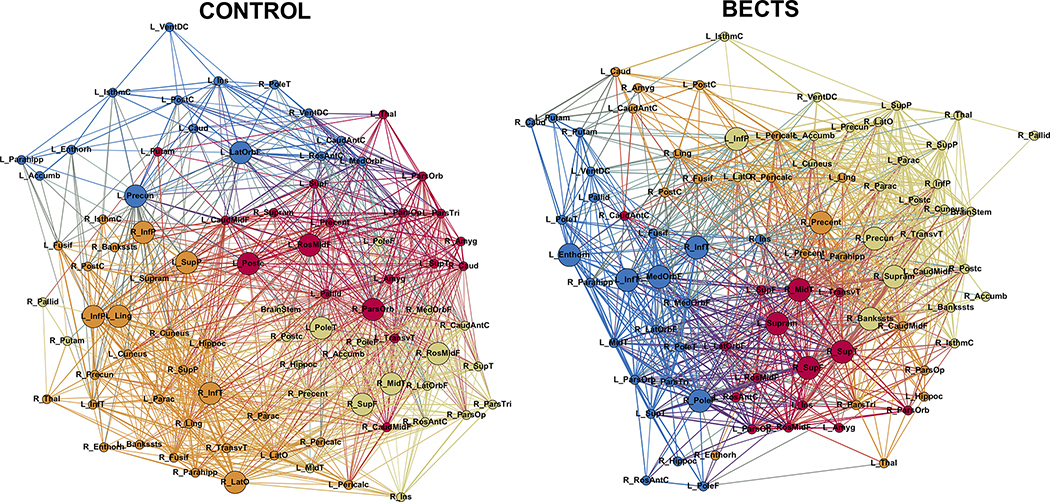
Figure 1 shows the modularity of the developmental networks for BECTS (right) and controls (left). Although both groups showed 4 modules the blue module in controls is less dense than the rest of the modules in controls, while BECTS are presenting similar density among modules. This could represent a higher modular development in BECTS than in controls. Figure 2S (supplementary document) shows the community structure consistency across different thresholds.
Figure 1:
Modularity in controls (left) and BECTS (right). Node abbreviations are the same as in Table 1S. Same color nodes belong to the same module. The spatial distribution of nodes was calculated using the force-atlas graph algorithm, where nodes that demonstrated stronger connections are located closer in space, while nodes with fewer connections tend to be farther in space. Bigger nodes represent the hubs of the network. Calculated at a hybrid threshold of 25%.
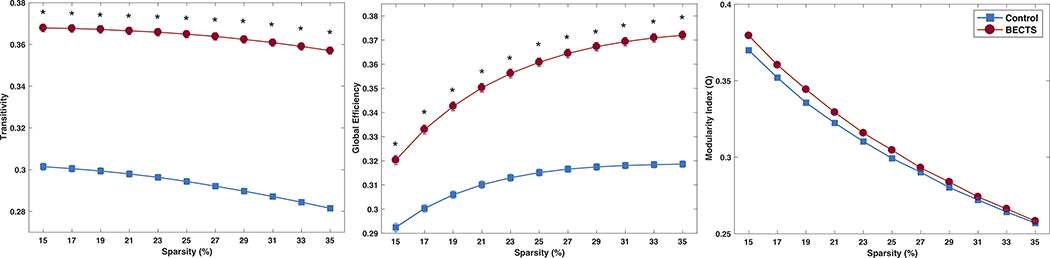
Global measures
Figure 2 shows the global measures for the morphological development of both groups. As can be seen, BECTS are presenting significantly higher transitivity and GE compared to controls, implying that different brain regions in BECTS seem to be developing in higher synchrony than controls. Although Q was higher in BECTS than in controls, such differences were not significant at any of the investigated thresholds.
Figure 2:
Transitivity (left), global efficiency (middle), and modularity index (right) in in controls (blue) and BECTS (red). Error bars represent the standard error of the mean. *Statistically significant between groups after Student’s t-test calculations. Each group was statistically significant against random at each density level; corrected for multiple comparisons (Bonferroni correction).
Regional measures and network hubs
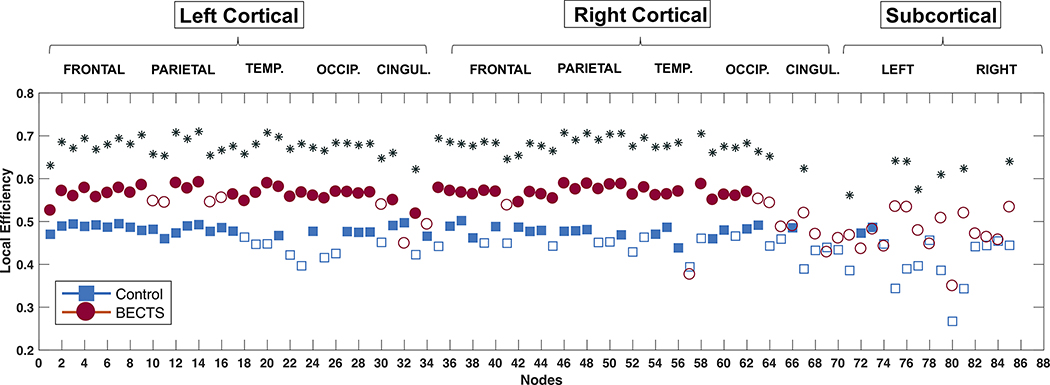
Local efficiency presented nodes with significantly higher values in BECTS (Figure 3) comprising most bilateral cortical regions and some subcortical areas (left putamen, left accumbens, left ventral DC, right thalamus, right caudate, right hippocampus, and the brainstem). Therefore, the higher developmental integration between brain regions in BECTS compared to controls seems to be driven by groups of local interactions involving temporal, parietal, and occipital areas.
Figure 3:
Local efficiency in controls (blue) and BECTS (red). Filled symbols represent statistical significance against random. *Statistically significantly after Student’s t-test analysis; corrected for multiple comparisons (Bonferroni correction). Calculated at a hybrid density level of 25%.
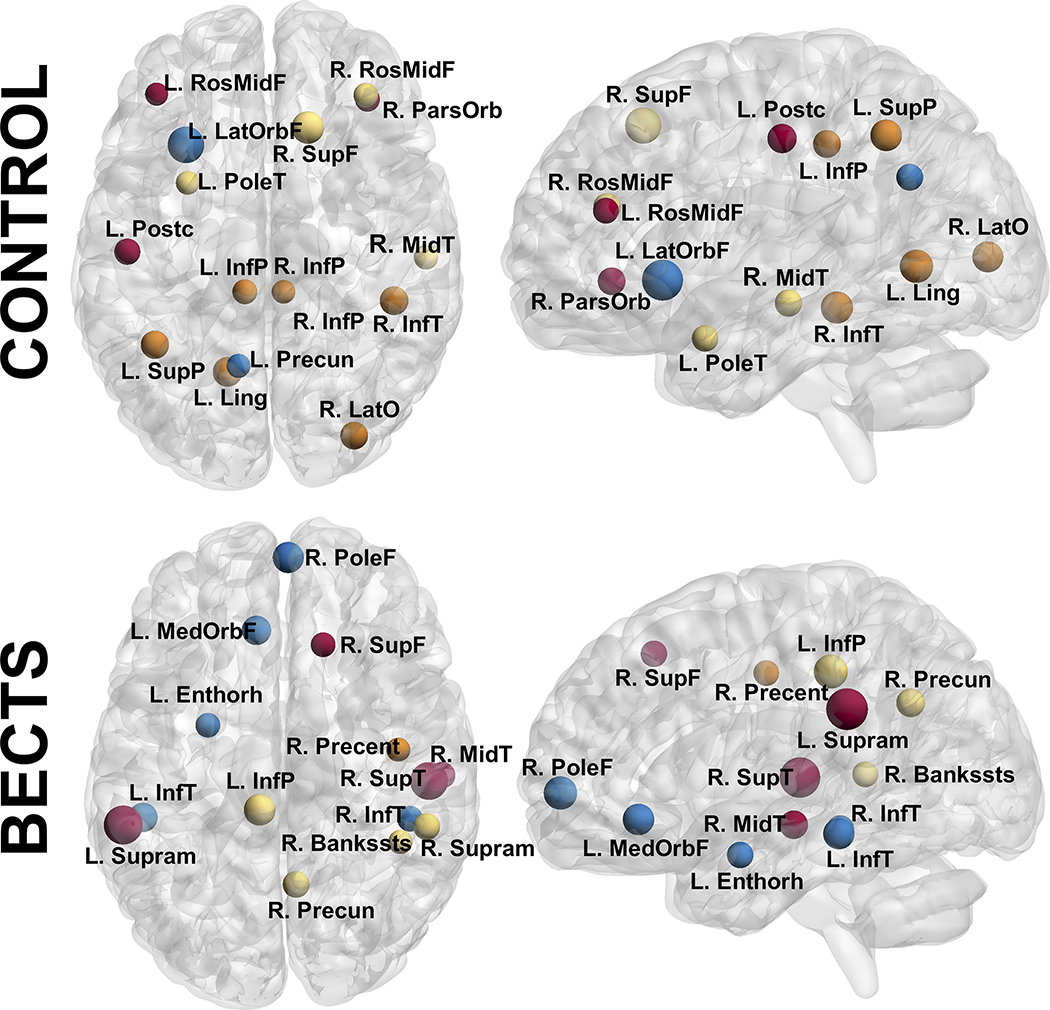
Regarding the hubs of the network, both groups presented a similar number, with a total of 15 in controls and 14 in BECTS. Specifically, controls showed 5 hubs in the frontal lobe versus 4 for BECTS, 5 hubs in the parietal lobe versus 2 in BECTS, 5 hubs in the temporal lobe versus 8 in BECTS, and 2 hubs in the occipital lobe versus none in BECTS. The configuration of hubs is evidently discrepant between groups, with different regions/lobes of the brain as mediators on its development.
Correlation analyses
GT measures were calculated on correlation matrices based on baseline brain volumes to determine associations with baseline and prospective cognitive performances. Since the test of motor function (Grooved Pegboard-dominant hand [PGDOM]) has reverse interpretation, scores were reversed prior to the analysis.
In controls, transitivity at baseline could not be included due to high skewness, along with the tests of motor speed/dexterity (PGDOM) and response inhibition (INHSS) at baseline, and the tests of word knowledge (IQVOCS), visuoconstruction (IQBDS), verbal reasoning (IQSIMS), and nonverbal reasoning (IQMRS) at follow-up. At baseline, controls showed word knowledge (IQVOCS) significantly associated to Q (R=−0.46, p=0.03), IQSIMS significantly associated to average BC (R=−0.53, p=0.012), and problem solving (CORSORS) significantly associated to local efficiency (R=−0.43, p=0.048); while at follow-up, none of the tests showed a significant correlation. In all instances, the associations were negative; however, none of them remained significant after FDR correction. In the group of BECTS, Q could not be included due to high skewness, as well as the tests of rote verbal learning (WLLSS) and INHSS at baseline, and the tests of delayed verbal memory (WLDSS) and INHSS at follow-up. At baseline, IQBDS showed a significant association to transitivity (R=−0.49, p=0.035), psychomotor speed (IQDSYMS) showed a significant association to global efficiency (R=−0.48, p=0.039), local efficiency (R=−0.49, p=0.032), and the IC measure (R=−0.46, p=0.047); and PGDOM to transitivity (R=−0.5, p=0.029), local efficiency (R=−0.49, p=0.034), and the IC measure (R=−0.51, p=0.026). However, none of the associations remained significant after FDR correction. At 2-year follow-up, IQVOCS showed a significant association to transitivity (R=−0.61, p=0.021), average BC (R=−0.68, p=0.008), and local efficiency (R=−0.55, p=0.044); IQMRS showed a significant association with transitivity (R=−0.61, p=0.022), average BC (R=−0.70, p=0.005), local efficiency (R=−0.63, p=0.016) and IC (R=−0.6, p=0.023); and PGDOM presented significant correlation to local efficiency (R=−0.54, p=0.047). In all instances, the associations were negative and remained significant after FDR correction. Scatter plots showing such significant associations can be found in Figure 3S of the supplementary document.
As can be seen, PGDOM was associated to GT measures in BECTS at both time points. Furthermore, regarding the IC measure, BECTS presented a negative association regarding the IC measure, meaning that a higher resemblance to controls renders a better cognitive score.
DISCUSSION
This study investigated the prospective covariance of brain volumetric development in cortical and subcortical structures, considered as a network based on GT analyses, in children with new/recent-onset BECTS compared to healthy controls. Furthermore, we examined the volumetric relationship of the same cortical and subcortical regions as a network at the baseline evaluation with concurrent and prospective cognitive outcomes. The results showed that the group of BECTS seems to be presenting higher-than-normal development –higher values than controls– at the global level, but with local efficiency and hubs configuration highly divergent from controls. Furthermore, specific baseline GT metrics showed associations between baseline and particularly prospective cognition in BECTS, relationships that were considerably attenuated in the control group. Thus, the disruptions in baseline and prospective network development as characterized by network science metrics has concurrent and potential significance as a biomarker for cognition and cognitive development.
Network configuration and global metrics
Groups presented dissimilarities in terms of transitivity and GE, which were significantly higher in BECTS across thresholds with no significant differences in Q. The community structure in BECTS was similar to controls in terms of the number of modules; however, in controls, one of the modules was sparser than the rest which could explain the higher Q observed in the BECTS group–although not significantly so. Previous studies that investigated differences in brain development regarding cortical thickness have demonstrated that certain regions tend to mature together, while other areas mature at different developmental epochs48–49. Specifically, Raznahan et al.48 showed that cortical thickness development is very heterogeneous in healthy subjects (from 9 years until adulthood) with frontal and temporal cortical regions changing in synchrony with many other cortical areas (mainly thinning with time). Such development could be interpreted as modular or comprising a combination of different sets of regions developing together. BECTS participants presented a distribution of modules with similar density across their 4 modules while controls practically showed only 3 modules with such integration; therefore, BECTS appear to be too modular compared to controls in terms of their cortical-subcortical developmental associations. In a previous publication by our group we examined changes in cortical thickness in children with BECTS and controls, and showed that controls tended to exhibit thinning across cortical regions comprising frontal and occipital areas, whereas BECTS only presented a small area of cortical thinning in the left isthmus cingulate, and a small region showing cortical thickening in the right precentral gyrus24. It should be noted that the studies of Raznahan et al.48 and our previous publication24 were based on cortical thickness instead of the cortical volumes investigated here; therefore, the developmental dynamics, although theoretically proportional, may not be the same. The apparent superior developmental modularity observed in BECTS could represent abnormal developmental associations (i.e., they might not be showing the expected regional development as the control group or their same direction) or might be suggesting the potential achievement of normality. This will be explored in the following subsection.
Regional measures and network hubs
Regarding local efficiency, BECTS participants showed higher local developmental synchronization than controls across many of the cortical nodes. This indicates a higher degree of synchronization in the maturation of these neighboring cortical regions in BECTS compared to controls.
When investigating network hubs, the differences between groups became more evident. Both groups presented a similar number; however, in controls, brain development seems highly influenced by frontal and parietal brain regions (5 on each) followed by temporal (3) and occipital areas (2). In BECTS, hubs were mainly from temporal regions (8), followed by frontal (4) and parietal (2) areas, with no hubs from the occipital lobe (contrary to controls). This is an evident disparity in terms of those areas that are developmentally bridging different brain regions indicating that even though overall brain development seems similar between groups, group differences are amplified at the regional level.
Comparison of epilepsy syndromes
We previously performed similar analyses in youth with Juvenile Myoclonic Epilepsy (JME)41 and the results revealed a prospective network characterized by highly correlated cortical regions that were unassociated with subcortical structures. Regarding network hubs, JME participants showed half the total number of hubs than controls. However, the most striking contrast between groups was that controls had ample representation of hubs from the different modules and lobes of the brain, while in JME hubs were mainly from one module, comprised mostly of subcortical structures, and excluded the parietal lobe and prefrontal regions entirely. Hence, the prospective network development observed in children with BECTS is strikingly different over the same time period compared to children with JME, and it seems that typically developing children are between BECTS and JME. These divergent patterns speak to the epilepsy syndrome specificity of GT findings. It is clear that children with BECTS present a developmental network that more closely approaches that of the normally developing controls than patients with JME. This could be viewed as consistent with population-based research that has reported that the long-term social outcomes of children with BECTS appear largely uncomplicated and more similar to controls compared to other epilepsy syndromes50.
Associations of network features and cognition
For the first time in the child epilepsy literature we examined the association of baseline GT measures with cognitive outcomes two years later. The fundamental issue addressed was whether these metrics might serve as biomarkers for cognitive development over the subsequent two years. For this study, we only focused on tests related to intelligence, verbal memory, executive function and speed. Controls showed negative correlations between baseline GT measures with baseline cognitive score that did not remain significant after FDR correction, with no significant associations between baseline GT measures and 2-year follow-up cognitive measures. However, regarding BECTS, GT measures from baseline morphological networks were associated with both baseline and 2-year follow-up cognitive testing, associations that remained significant after FDR correction. These preliminary analyses suggest that further validation of GT measures as biomarkers for cognitive function and its prospective development may be worth pursuing.
Study limitations
This study has its limitations. The first is the relatively modest number of subjects in each group. Larger groups may result in greater power and ability to detect additional developmental differences between groups. Second, more than half of the patients were treated with antiepileptic drugs (or AEDs) which might influence the results. We therefore performed additional analyses controlling for AED and the results were similar to that presented here (Figures 4S and 5S, supplementary document). Because this is a prospective observational study it becomes impossible to isolate AED effects. The important point is that this cohort of children with new-onset/recently diagnosed BECTS was treated at baseline as they were 7 months post-diagnosis (on average), so there is no confounding of time with treatment. Lastly, it is important to notice that the approach to obtain GT measures from subject-specific morphological matrices was validated by Saggar el at.47 under similar circumstances but with some differences such as (1) they examined cortical thickness rather than cortical volume; (2) they used path length and graph diameter as measures of network integration, and clustering coefficient as a measure of network segregation; and (3) their sample was older (mean age: 17 years).
CONCLUSIONS
Children with BECTS showed a developmental configuration of their brain volume network similar to that of controls, however, presenting higher global clustering and efficiency that might represent a higher-than-normal development. This pattern, however, is not system-wide as there were also notable discrepancies in the hub locations and local efficiency between groups. Investigations of associations between GT measures at baseline from extracted individual contributions of BECTS participants and cognitive testing two years later showed a number of negative associations in BECTS that were significant prospectively. This could make possible the use of GT measures as possible biomarkers of cognitive abilities in this epilepsy syndrome.
Supplementary Material
Figure 4:
Nodes with high BC in controls (top) and BECTS (bottom) at their approximate anatomical location. Nodes with the same color represent the same module (as in Figure 2). Labels are the node abbreviations from Table 1S. Calculated at a hybrid density level of 25%.
Highlights.
BECTS participants present a higher-than-normal global developmental configuration.
BECTS showed significantly higher local efficiency in most cortical regions compared to controls.
BECTS showed their majority of hubs from the temporal lobe and none from the occipital lobe compared to controls.
Baseline GT measures demonstrate potential as cognitive biomarkers in participants with BECTS.
Acknowledgements
All phases of this study were supported by the National Institute of Neurological Disorders and Stroke (NINDS) 3RO1-44351 and by the Clinical and Translational Science Award (CTSA) program, through the National Institutes of Health (NIH) National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. We thank Raj Sheth MD, Monica Koehn MD, and Jason Dozier MD for study participation and recruitment of participants. Also greatly appreciated are Dace Almane, Melissa Hanson, Kate Young, and Bjorn Hanson for overall study coordination, participant recruitment, cognitive assessment, and data management.
Footnotes
Disclosure
“Neither of the authors has any conflict of interest to disclose.”
‘We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.’
References
- 1.Panayiotopoulos CP. A Clinical Guide to Epileptic Syndromes and their Treatment: Based on the New ILAE Diagnostic Scheme. Bladon Medical Pub., Oxfordshire, UK; 2002. [Google Scholar]
- 2.Larsson K, Eeg-Olofsson O. A population based study of epilepsy in children from a Swedish county. Eur J Paediatr Neurol. 2006;10:107–13. [DOI] [PubMed] [Google Scholar]
- 3.Astradsson A, Olafsson E, Ludvigsson P, et al. Rolandic epilepsy: An incidence study in Iceland. Epilepsia. 1998;39:884–86. [DOI] [PubMed] [Google Scholar]
- 4.Sidenvall R, Forsgren L, Heijbel J. Prevalence and characteristics of epilepsy in children in northern Sweden. Seizure. 1996;5:139–46. [DOI] [PubMed] [Google Scholar]
- 5.Wickens S, Bowden SC, D’Souza W. Cognitive functioning in children with self-limited epilepsy with centrotemporal spikes: A systematic review and meta-analysis. Epilepsia. 2017;58:1673–1685. [DOI] [PubMed] [Google Scholar]
- 6.Besag F, Gobbi G, Aldenkamp A, et al. Psychiatric and Behavioural Disorders in Children with Epilepsy (ILAE Task Force Report): Behavioural and psychiatric disorders associated with epilepsy syndromes. Epileptic Disord. 2016. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Vannest J, Szaflarski JP, Eaton KP, et al. Functional magnetic resonance imaging reveals changes in language localization in children with benign childhood epilepsy with centrotemporal spikes. J Child Neurol 2013;28:435–45. [DOI] [PubMed] [Google Scholar]
- 8.Datta AN, Oser N, Bauder F, et al. Cognitive impairment and cortical reorganization in children with benign epilepsy with centrotemporal spikes. Epilepsia 2013;54:487–94. [DOI] [PubMed] [Google Scholar]
- 9.Overvliet GM, Besseling RM, Jansen JF, et al. Early onset of cortical thinning in children with rolandic epilepsy. Neuroimage Clin 2013;2:434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pardoe HR, Berg AT, Archer JS, et al. A neurodevelopmental basis for BECTS: evidence from structural MRI. Epilepsy Res 2013;105:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo C, Zhang Y, Cao W, et al. Altered structural and functional feature of striato-cortical circuit in benign epilepsy with centrotemporal spikes. Int J Neural Syst. 2015;25:1550027. [DOI] [PubMed] [Google Scholar]
- 12.Shakeri M, Datta AN, Malfait D, et al. Sub-cortical brain morphometry and its relationship with cognition in rolandic epilepsy. Epilepsy Res. 2017;138:39–45. [DOI] [PubMed] [Google Scholar]
- 13.Besseling RM, Jansen JF, Overvliet GM, et al. Reduced structural connectivity between sensorimotor and language areas in rolandic epilepsy. PLoS One. 2013;8:e83568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Besseling RM, Overvliet GM, Jansen JF, et al. Aberrant functional connectivity between motor and language networks in rolandic epilepsy. Epilepsy Res. 2013;107:253–62. [DOI] [PubMed] [Google Scholar]
- 15.Smith AB, Bajomo O, Pal DK. A meta-analysis of literacy and language in children with rolandic epilepsy. Dev Med Child Neurol. 2015;57:1019–26. [DOI] [PubMed] [Google Scholar]
- 16.Teixeira J, Santos ME. Language skills in children with benign childhood epilepsy with centrotemporal spikes: A systematic review. Epilepsy Behav. 2018;84:15–21. [DOI] [PubMed] [Google Scholar]
- 17.Currie NK, Lew AR, Palmer TM, et al. Reading comprehension difficulties in children with rolandic epilepsy. Dev Med Child Neurol. 2018;60:275–282. [DOI] [PubMed] [Google Scholar]
- 18.Smith AB, Bajomo O, Pal DK. A meta-analysis of literacy and language in children with rolandic epilepsy. Dev Med Child Neurol. 2015;57:1019–26. [DOI] [PubMed] [Google Scholar]
- 19.Overvliet GM, Besseling RM, van der Kruijs SJ, et al. Clinical evaluation of language fundamentals in Rolandic epilepsy, an assessment with CELF-4. Eur J Paediatr Neurol 2013;17:390–396. [DOI] [PubMed] [Google Scholar]
- 20.Overvliet GM, Aldenkamp AP, Klinkenberg S, et al. Impaired language performance as a precursor or consequence of Rolandic epilepsy? J Neurol Sci 2011;304:71–74. [DOI] [PubMed] [Google Scholar]
- 21.Wickens S, Bowden SC, D’Souza W. Cognitive functioning in children with self-limited epilepsy with centrotemporal spikes: A systematic review and meta-analysis. Epilepsia. 2017;58:1673–1685. [DOI] [PubMed] [Google Scholar]
- 22.Pal DK, Ferrie C, Addis L, et al. Idiopathic focal epilepsies: the “lost tribe”. Epileptic Disord. 2016;18:252–88. [DOI] [PubMed] [Google Scholar]
- 23.Vannest J, Maloney TC, Tenney JR, et al. Changes in functional organization and functional connectivity during story listening in children with benign childhood epilepsy with centro-temporal spikes. Brain Lang. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Ramos C, Jackson DC, Lin JJ, et al. Cognition and brain development in children with benign epilepsy with centrotemporal spikes. Epilepsia. 2015;56:1615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim EH, Yum MS, Shim WH, et al. Structural abnormalities in benign childhood epilepsy with centrotemporal spikes (BCECTS). Seizure 2015;27:40–46. [DOI] [PubMed] [Google Scholar]
- 26.Lin JJ, Riley JD, Hsu DA, et al. Striatal hypertrophy and its cognitive effects in new-onset benign epilepsy with centrotemporal spikes. Epilepsia 2012;53:677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson DC, Dabbs K, Walker NM, et al. The neuropsychological and academic substrate of new/recent-onset epilepsies. J Pediatr 2013;162:1047–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lerch JP, Worsley K, Shaw WP, et al. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage. 2006;31:993–1003. [DOI] [PubMed] [Google Scholar]
- 29.Bonilha L, Tabesh A, Dabbs K, et al. Neurodevelopmental alterations of large-scale structural networks in children with new-onset epilepsy. Hum Brain Mapp. 2014;35:3661–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernhardt BC, Chen Z, He Y, et al. Graph-theoretical analysis reveals disrupted small-world organization of cortical thickness correlation networks in temporal lobe epilepsy. Cereb Cortex. 2011;21:2147–57. [DOI] [PubMed] [Google Scholar]
- 31.Hermann B, Jones J, Sheth Ret al. Children with new-onset epilepsy: neuropsychological status and brain structure. Brain 2006;129:2609–2619. [DOI] [PubMed] [Google Scholar]
- 32.ILAE. Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy 1989. Epilepsia 1989;30:389–399. [DOI] [PubMed] [Google Scholar]
- 33.Cohen MJ. Children’s Memory Scale. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 34.Wechsler D Wechsler Intelligence Scale for Children. San Antonio, TX: The Psycholocal Corporation; 1991. [Google Scholar]
- 35.Trites RL. Neuropsychological Test Manual. Ottawa, Ontario, Canada: Royal Ottawa Hospital; 1977. [Google Scholar]
- 36.Wechsler D Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 37.Delis DC, Kaplan E, Kramer JH. The Delis-Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 38.Fischl B, Dale A. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences USA, 2000; 97:11044–11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cerebral Cortex, 2004;14:11–22. [DOI] [PubMed] [Google Scholar]
- 40.Reuter M, Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage 2011;57:19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia-Ramos C, Dabbs K, Lin JJ, et al. Progressive dissociation of cortical and subcortical network development in children with new-onset juvenile myoclonic epilepsy. Epilepsia. 2018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Ramos C, Lin JJ, Prabhakaran V, et al. Developmental reorganization of the cognitive network in pediatric epilepsy. PLoS ONE. 2015;10:e0141186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Zuo X, He Y. Graph-based network analysis of resting-state functional MRI. Front Syst Neurosci. 2010;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blondel VD, Guillaume J-L, Lambiotte R, et al. Fast unfolding of communities in large networks. J. Stat. Mech 2008, P10008. [Google Scholar]
- 45.Sporns O, Honey CJ, Kötter R. Identification and classification of hubs in brain networks. PLoS One. 2007;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boccaletti S, Latora V, Moreno Y, et al. Complex networks: Structure and dynamics. Phys Rep. 2006;424:175–308. [Google Scholar]
- 47.Saggar M, Hosseini SM, Bruno JL, et al. Estimating individual contribution from group-based structural correlation networks. Neuroimage. 2015;120:274–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raznahan A, Lerch JP, Lee N, et al. Patterns of coordinated anatomical change in human cortical development: a longitudinal neuroimaging study of maturational coupling. Neuron. 2011;72:873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaw P, Kabani NJ, Lerch JP, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Camfield C, Camfield P. Cognitive Disabilities and Long-term Outcomes in Children with Epilepsy: A Tangled Tail. Semin Pediatr Neurol. 2017. November;24:243–250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.