Abstract
Context:
Alopecia areata is a chronic non-scarring alopecia that involves scalp and/or body. Corticosteroids are the most popular drugs for its treatment.
Aim:
The aim of the study was to evaluate the therapeutic efficacy of intralesional injection of triamcinolone acetonide and platelet-rich plasma (PRP) in alopecia areata and to compare the efficacy of these modalities in alopecia areata.
Settings and Design:
This was a randomized controlled comparative study.
Subjects and Methods:
Forty patients were enrolled from the outpatient department and divided into two groups of 20 patients each. Group A and B randomly received intradermal triamcinolone acetonide suspension (10 mg/mL) and PRP, respectively, into the lesion using an insulin syringe in multiple 0.1 mL injections 1cm apart. The injections were repeated every 3 weeks till 12 weeks. The patients were evaluated by Severity of Alopecia Tool (SALT) score and photographically every 3 weeks till the end of 12 weeks and then at the end of 6 months. Statistical analysis used descriptive analysis along with Pearson chi-square test or Fisher exact test, paired samples, and independent samples t test or their nonparametric analogs for continuous variables.
Results:
The reduction in SALT score at each visit with respect to baseline was greater in the triamcinolone group as compared to PRP group. This signifies greater effect of triamcinolone in alopecia areata. Around 50% patients in triamcinolone group and 5% patients in PRP group showed grade V improvement. Pain during intralesional injection was higher in the PRP group.
Conclusion:
Both intralesional triamcinolone and PRP were found to be efficacious in alopecia areata but the latter produced lesser improvement.
Keywords: Alopecia areata, intralesional triamcinolone, intralesional platelet-rich plasma
Key message: • Intralesional steroids still remain the first-line drugs for the treatment of patchy alopecia areata.; • PRP does not have direct immunosuppressive action, despite being rich in growth factors.
INTRODUCTION
Alopecia areata (AA) is a chronic, inflammatory disorder that causes non-cicatricial hair loss of scalp and/or body. The severity varies from small patches of hair loss, which may undergo spontaneous recovery, to complete alopecia where chances of regrowth are poor. It is a T cell–mediated autoimmune disease that occurs in genetically predisposed individuals, which is triggered by environmental factors.
Most of the Asians have onset before 40 years of age, and there is no sex predilection.[1,2] It is associated with autoimmune diseases such as thyroid disease, diabetes, lupus erythematosus, vitiligo, and psoriasis. Poor prognostic factors include younger age of onset, presence of atopy, positive family history of AA, severe disease such as alopecia totalis/universalis, ophiasis pattern, disease duration >1 year, and associated autoimmune or nail disease.[3,4]
No definitive cure or preventive treatment has been established. Treatment is challenging as none of the available therapies is curative or preventive. Most therapies are immunosuppressive as AA is autoimmune and occurs because of loss of the immune privilege of the hair follicle. Intralesional steroids are the treatment of choice in adults with patchy AA.[5] Finding new options for this condition is of utmost importance as it severely impacts the quality of life, especially in young individuals. Autologous platelet-rich plasma (PRP) has emerged as a new modality of treatment, and it might have a beneficial role in hair growth as suggested by the preliminary evidence. PRP means abundant platelets concentrated into a small volume of plasma. It has growth-promoting, immunomodulatory, and anti-inflammatory effects and is claimed to be a simple yet effective modality for the treatment of AA.[6,7,8] In view of the paucity of data on the efficacy of PRP in patients with AA, we carried out this study. This study was conducted to compare the therapeutic efficacy of intralesional injection of triamcinolone acetonide versus intralesional autologous PRP in AA.
SUBJECTS AND METHODS
Forty patients clinically diagnosed as AA were enrolled from the outpatient department.
Inclusion criteria consisted of subjects aged 18–50 years with AA, AA on the scalp (<25% involvement), patients who had not taken any form of treatment in the last 6 months, and subjects who were willing to sign the written consent form before participating in the study.
Exclusion criteria consisted of subjects aged <18 or >50 years, patients with a history of diabetes, hypertension, thromboembolism, bleeding disorders, and abnormal coagulogram; patients who were already on steroids; and patients with active infection at the local site and with keloidal tendency.
A total of 40 patients were enrolled in the study. They were randomly divided into two groups of 20 patients each. Disease activity was assessed by the history of disease progression and by hair pull test performed at the margins of patches of AA. A written informed consent was obtained from each patient before starting the study. A detailed history and general examination were conducted in each case. Each patient was subjected to routine blood investigations at the time of reporting along withbleeding time (BT), clotting time (CT) and international normalised ratio (INR).
Treatment protocol
Group A: Under aseptic conditions, triamcinolone acetonide suspension (10 mg/mL) was administered intradermally into the active lesion using an insulin syringe in multiple 0.1 mL injections approximately 1cm apart [Figure 1].
Figure 1.
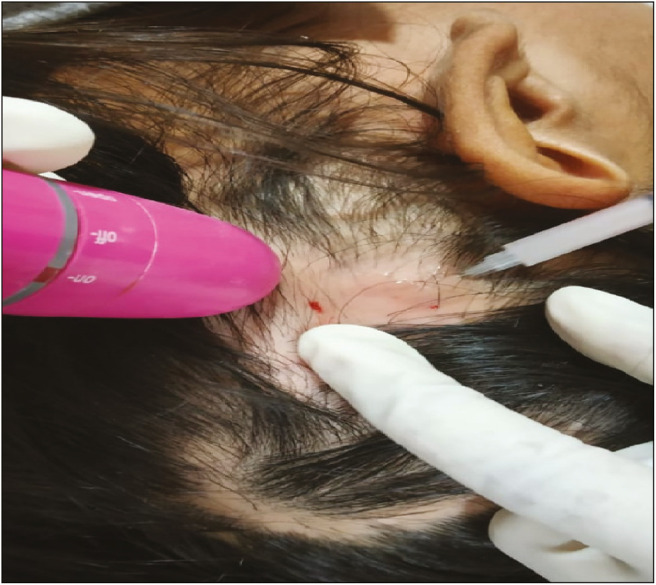
Intradermal injection being administered. A handheld vibrator placed close to the site of injection
Group B: Under aseptic conditions, autologous PRP was administered intradermally into the active lesion using an insulin syringe in multiple 0.1 mL injections approximately 1cm apart.
A handheld vibratory device placed adjacent to the area of hair loss was used as a distraction in both the groups to minimize discomfort from the injection.
Follow-up
There were a total of five sittings at an interval of 3 weeks each till 12 weeks with a follow-up at the end of 6 months.
Method of preparation of PRP
PRP was prepared from 20 mL of blood by centrifugation method. Blood was collected in citrate vials and centrifuged for 3 min at 2000rpm. After centrifugation, the supernatant was used as PRP. Platelet count was on an average 3.5 times higher than the whole blood count.
Severity of Alopecia Tool score
National Alopecia Areata Foundation working committee devised the “Severity of Alopecia Tool score” (SALT score).[9] Scalp is divided into four areas, namely vertex, 40% (0.4) of scalp surface area; right profile of scalp, 18% (0.18) of scalp surface area; left profile of scalp, 18% (0.18) of scalp surface area; and posterior aspect of scalp, 24% (0.24) of scalp surface area. Percentage of hair loss in each area is determined independently and multiplied by the percentage of scalp covered in that area of the scalp. SALT score is obtained by summing the products of each area. All the patients were evaluated both objectively by SALT score and photographically at baseline, then at every 3-week intervals till the end of 12 weeks, and then at the end of 6 months. Percentage change from baseline at each visit was calculated as follows:
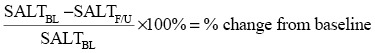
where BL = baseline and F/U = follow-up [Table 1].
Table 1.
Grades of improvement by the reduction in SALT score
| S. no. | Grades of improvement | Reduction in SALT score |
|---|---|---|
| I | No response | No improvement |
| II | Mild response | <25% reduction in SALT score |
| III | Moderate response | 25%–49% reduction in SALT score |
| IV | Good response | 50%–74% reduction in SALT score |
| V | Very good response | >75% reduction in SALT score |
Side effects known to be associated with these therapeutic modalities as well as other side effects that seem relevant to the treatment were evaluated at every visit.
The pain experienced during intralesional injection was rated using the visual analog scale (VAS) for pain. The pain VAS is a continuous scale consisting of a horizontal (HVAS) or vertical (VVAS) line, which is 10cm (100 mm) in length and anchored by two verbal descriptors for symptom extremes.[10,11] The symptom extremes were taken as “no pain” (score = 0) and “worst imaginable pain” (score = 100 [100‐mm scale]).
No pain______________________Worst imaginable pain
The patients were asked to place a line perpendicular to the VAS line at the point corresponding to their pain intensity.[10,12,13] Using a ruler, the score was determined by measuring the distance in millimeters between the “no pain” anchor and the patient’s mark, providing a range of scores from 0 to 100.[11]
RESULTS
The pretreatment characteristics of all patients were noted [Table 2]. The two groups were comparable in terms of age and gender distribution, disease duration, distribution as per Ikeda’s classification, and number of patches [Tables 3–7]. The most common site of involvement was occiput (45%), followed by vertex (27.5%), parietal/temporal (15%), vertex with occiput (7.5%), parietal with occiput (2.5%), and parietal with vertex (2.5%). Personal history of atopy was noted in 25% patients. The difference between the two groups with regard to the history of atopy was not statistically significant. The two groups were comparable as far as associated nail disease was concerned. Nail disease was noted in 57.5% of patients. The common findings were pitting, leukonychia, longitudinal ridging, and trachyonychia.
Table 2.
Pretreatment characteristics of all patients
| Characteristics | No. of patients | |
|---|---|---|
| Gender | Male | 18 |
| Female | 22 | |
| M:F | 0.81:1 | |
| Age | Range (years) | 18–49 |
| Mean (years) | 27.1 ± 7.08 | |
| Duration of disease | <6 months | 36 |
| >6 months | 12 | |
| SALT score | Range | 0.54–21.4 |
| Mean | 6.72 ± 5.19 | |
| History of atopy | Present | 10 |
| Family history of AA | Present | 4 |
| Associated autoimmune disease | Present | 2 |
| Nail disease | Present | 23 |
Table 3.
Age-wise distribution of subjects in Group A and Group B
| Age (years) | Group A (triamcinolone) | Group B (PRP) | ||
|---|---|---|---|---|
| N = 20 | N = 20 | |||
| No. | % | No. | % | |
| 18–30 | 12 | 60 | 18 | 90 |
| 31–40 | 6 | 30 | 2 | 10 |
| 41–50 | 2 | 10 | 0 | 0 |
| Mean ± SD | 28.80 ± 8.56 | 25.40 ± 4.85 | ||
SD = standard deviation, PRP = platelet-rich plasma
P value = 0.079 (Fisher exact test)
In both the groups, the majority of patients were in the age-group of 18–30 years. The mean age of total 40 patients was 27.1 years
Table 7.
Distribution of subjects according to number of patches
| Number of patches | Group A (triamcinolone) | Group B (PRP) | ||
|---|---|---|---|---|
| N = 20 | N = 20 | |||
| No. | % | No. | % | |
| Single | 8 | 40.00 | 12 | 60.00 |
| Multiple | 12 | 60.00 | 8 | 40.00 |
P value = 0.206 (Pearson chi-square test), χ2 = 1.600, df = 1
Table 4.
Gender-wise distribution of subjects
| Sex | Group A (triamcinolone) | Group B (PRP) | ||
|---|---|---|---|---|
| N = 20 | N = 20 | |||
| No. | % | No. | % | |
| Female | 13 | 65.00 | 9 | 45.00 |
| Male | 7 | 35.00 | 11 | 55.00 |
P value = 0.204 (Pearson chi-square test), χ2 = 1.616, df = 1
Table 5.
Distribution of subjects according to duration of disease
| Duration of disease | Group A (triamcinolone) | Group B (PRP) | ||
|---|---|---|---|---|
| N = 20 | N = 20 | |||
| No. | % | No. | % | |
| <6 months | 13 | 65.00 | 15 | 75.00 |
| >6 months | 7 | 35.00 | 5 | 25.00 |
P value = 0.490 (Pearson chi-square test), χ2 = 0.476, df = 1
Table 6.
Distribution of subjects according to Ikeda’s classification
| Ikeda’s classification | Group A (triamcinolone) | Group B (PRP) | ||
|---|---|---|---|---|
| N = 20 | N = 20 | |||
| No. | % | No. | % | |
| Atopic type | 3 | 15.00 | 5 | 25.00 |
| Prehypertensive type | 6 | 30.00 | 4 | 20.00 |
| Autoimmune type | 2 | 10.00 | 0 | 0 |
| Common type | 9 | 45.00 | 11 | 55.00 |
P value = 0.523, Fisher exact test
Trends in SALT score and mean SALT score difference with respect to baseline were analyzed in the two groups [Tables 8–10, Figures 2 and 3]. The percentage reduction in baseline SALT score was used to assess the grade of improvement [Table 11].
Table 8.
Trends in SALT score with treatment in Group A
| Time | Group A | P value* | Statistical significance | ||
|---|---|---|---|---|---|
| N = 20 | |||||
| Mean | SD | Median | |||
| Baseline | 9.01 | 1.37 | 7.20 | - | - |
| 3 weeks | 8.16 | 1.23 | 6.60 | 0.001 | Significant |
| 6 weeks | 6.57 | 1.00 | 5.82 | 0.00009 | Significant |
| 9 weeks | 4.51 | 0.85 | 4.00 | 0.00009 | Significant |
| 12 weeks | 3.10 | 0.82 | 1.90 | 0.00009 | Significant |
| 24 weeks | 2.27 | 0.78 | 1.07 | 0.0001 | Significant |
SD = standard deviation, SALT = Severity of Alopecia Tool
*Related samples Wilcoxon signed-rank test
In Group A, the mean SALT score reduced from 9.01 at baseline to 2.27 at 24 weeks. A statistically significant difference was observed in the distribution of SALT scores at each visit with respect to baseline
Table 10.
Trends in mean SALT score difference in Group A and B
| Difference in SALT score at each visit with respect to baseline | Group A | Group B | P value* | ||
|---|---|---|---|---|---|
| Mean ± SD | Median | Mean ± SD | Median | ||
| Baseline–3 weeks | 0.848 ± 1.112 | 0.440 | 0.268 ± 0.342 | 0.19 | 0.114 |
| Baseline–6 weeks | 2.444 ± 2.384 | 1.920 | 0.580 ± 0.442 | 0.480 | 0.000 |
| Baseline–9 weeks | 4.502 ± 4.356 | 2.880 | 0.911 ± 0.616 | 0.900 | 0.000 |
| Baseline–12 weeks | 5.906 ± 5.520 | 3.840 | 1.233 ± 0.821 | 0.930 | 0.000 |
| Baseline–24 weeks | 6.735 ± 5.787 | 5.040 | 1.339 ± 0.981 | 1.140 | 0.000 |
SD = standard deviation, SALT = Severity of Alopecia Tool
*Independent samples Mann–Whitney U test
A statistically significant difference in the distribution of SALT score difference across the two groups at all visits with respect to baseline except at 3 weeks
Figure 2.
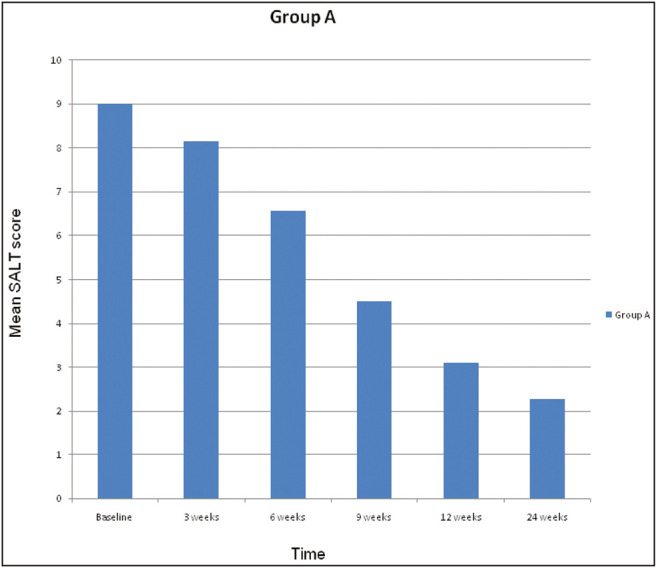
Trends in mean SALT score with treatment in Group A
Figure 3.

Trends in mean SALT score with treatment in Group B
Table 11.
Distribution of subjects according to grades of improvement
| S. no | Grades of improvement | Group A (triamcinolone) | Group B (PRP) | ||
|---|---|---|---|---|---|
| N = 20 | N = 20 | ||||
| No. | % | No. | % | ||
| I. | No response | 1 | 5.00 | 1 | 5.00 |
| II. | Mild | 2 | 10.00 | 5 | 25.00 |
| III. | Moderate | 2 | 10.00 | 12 | 60.00 |
| IV. | Good | 5 | 25.00 | 1 | 5.00 |
| V. | Very good | 10 | 50.00 | 1 | 5.00 |
P value = 0.0002 (Fisher exact test)
A statistically significant difference in Group A and B with regard to the response to treatment
Table 9.
Trends in SALT score with treatment in Group B
| Time | Group B | P value* | Statistical significance | ||
|---|---|---|---|---|---|
| N = 20 | |||||
| Mean | SD | Median | |||
| Baseline | 4.42 | 2.48 | 4.00 | - | - |
| 3 weeks | 4.15 | 2.49 | 3.60 | 0.002 | Significant |
| 6 weeks | 3.83 | 2.41 | 3.30 | 0.0001 | Significant |
| 9 weeks | 3.50 | 2.22 | 3.18 | 0.00009 | Significant |
| 12 weeks | 3.18 | 2.04 | 3.00 | 0.00009 | Significant |
| 24 weeks | 3.07 | 1.86 | 2.64 | 0.00013 | Significant |
SD = standard deviation, SALT = Severity of Alopecia Tool
*Related samples Wilcoxon signed-rank test
In Group B, the mean SALT score reduced from 4.42 at baseline to 3.07 at 24 weeks. A statistically significant difference was observed in the distribution of SALT scores at each visit with respect to baseline
Table 12 shows the distribution of subjects according to side effects.
Table 12.
Distribution of subjects according to side effects
| Side effects | Group A (triamcinolone) | Group B (PRP) | |
|---|---|---|---|
| N = 20 | N = 20 | ||
| Atrophy | 5 | 0 | |
| VAS score | Mean ± SD | 2.25 ± 3.27 | 25.6 ± 10.65 |
| Median | 1.00 | 26.00 | |
SD = standard deviation, VAS = visual analog scale, PRP = platelet-rich plasma
The difference in the distribution of VAS score was statistically significant across the two groups, (independent samples Mann–Whitney U test, P value = 0.000)
Atrophy was seen in only five patients of Group A and this difference was statistically significant (Fisher exact test, P value = 0.047)
DISCUSSION
Intralesional steroids are considered as the first-line treatment for limited patch stage AA with varying side effects. PRP has been explored in the treatment of hair disorders and is a potential therapeutic tool for AA. It is an effective concentration of various growth factors by virtue of platelets and plasma proteins such as fibrin, fibronectin, and vitronectin.[14] These growth factors stimulate proliferation and differentiation of stem cells in hair follicle.[6] Vascular endothelial growth factor in PRP has an important role in anagen-associated angiogenesis.[15] Li et al. observed that PRP-treated dermal papilla cells had increased levels of protein kinase B and phosphorylated extracellular signal regulated kinase (ERK). ERK pathway promotes cell growth, whereas protein kinase B pathway increases cell survival and decreases apoptosis.[6] Fibroblast growth factor 7 levels were found to be higher in cells treated with PRP. This may cause prolongation of the anagen phase of cell cycle.[6,16] PRP also augments the expression of antiapoptotic B cell lymphoma protein and β-catenin, which is a mediator for hair follicle genesis.[6] Another study found significantly higher levels of Ki 67, a marker of cellular proliferation in hair treated with PRP.[17] Anti-inflammatory effects of PRP suppress the release of cytokines and reduce tissue inflammation. It is probable that the anti-inflammatory effects may be beneficial in AA.[8]
The SALT scores in the two groups were compared. The mean SALT score decreased from 9.01 at baseline in Group A to 2.27 at the end of 24 weeks. Similarly, the mean SALT score in Group B decreased from 4.42 at baseline to 3.07 at the end of 24 weeks. A statistically significant difference was observed in the distribution of SALT scores at each visit with respect to the baseline, in both the groups.
The trends in the difference of SALT score with treatment were analyzed across the two groups. A statistically significant difference was observed in the treatment effect across the two groups at all visits with respect to baseline except at 3 weeks. The reduction in SALT score at each visit with respect to baseline was greater in the triamcinolone group as compared to PRP group, therefore signifying greater effect of triamcinolone in AA.
Kaur et al.[18] in their study on patients of AA observed more than 50% regrowth in 67.5% of patients treated with intralesional steroid at the end of 12 weeks. This finding is comparable to our study as we noted that 11 (55%) of 20 patients in Group A attained more than 50% reduction in SALT score at the end of 12 weeks.
Amirnia et al.[19] administered intralesional steroid in 120 patients of AA. Patients were treated with intralesional triamcinolone acetonide for four sessions with an interval of 3 weeks. It was noted that there was 60%–90% regrowth in 26.7% of patients at the end of 12 weeks.[19] In our study, grade V improvement, that is, >75% reduction in SALT score was observed in 35% of patients treated with intralesional triamcinolone at the end of 12 weeks. These findings are comparable.
Singh[20] in their prospective study on 20 patients of biopsy-proven AA also reported successful treatment with PRP. All the patients received six sessions of PRP at 4-week intervals.[20]
Kumar et al.[21] in their study on the role of PRP in AA observed a significant difference in mean SALT score after intralesional PRP treatment. The mean SALT score decreased from 36.41 before treatment to 25.59 after treatment.[21] We noted a statistically significant difference in the distribution of SALT scores at each visit with respect to baseline in patients treated with intralesional injection of PRP.
Both the groups were compared with regard to the grade of improvement at the end of 24 weeks. A total of 50% patients in Group A showed grade V improvement followed by 25% with grade IV, 10% with grade III and II each, and 5% with grade I improvement. In Group B, 5% patients had grade V and IV improvement each, followed by 60%, 25%, and 5% patients with grade III, II, and I improvement, respectively. Majority of patients in Group A had very good improvement at the end of 24 weeks, whereas the majority of patients in Group B achieved moderate-grade improvement.
Trink et al.[17] evaluated the use of PRP for AA by a double-blind, split-scalp study. They noted that PRP increased hair regrowth significantly and decreased hair dystrophy and burning or itching sensation compared with triamcinolone acetonide or placebo. In our study, the results were more in favor of intralesional triamcinolone acetonide as compared to PRP.[17]
Shumez et al.[22] allocated 74 patients of AA into two groups, 48 patients were treated with triamcinolone injections and 26 patients were treated with PRP injections at 3-week intervals each with a follow-up at 3 months. At the end of 3 weeks, the comparison of overall improvement between the two groups was not significant.[22] This finding is comparable to our study. We also observed that the distribution of the difference in SALT score with respect to the baseline and at 3 weeks across the two groups was statistically insignificant. In a study by Shumez et al.,[22] a higher percentage of complete resolution was observed in the PRP group at the 6th week but the difference was not statistically significant. Also, all their patients achieved complete regrowth of hair at the end of 9th week and 3 months in both the groups.
Ovidio and Roberto[23] noted the limited effectiveness of PRP in chronic severe AA. None of the patients achieved noticeable cosmetic results.[23]
Mubki[24] used intralesional PRP combined with triamcinolone acetonide in a patient of AA. The right half of the scalp was treated with both TrA and intradermal PRP injections. The left half was treated only with intralesional injections of TrA. There was a minimal difference (4%) in the number of regrowing terminal hairs between the two treatment protocols. Thus, PRP was not found to increase the number of hair in the AA patches.[24]
The adverse effects observed during treatment were noted. Mild erythema and bleeding at the injection site occurred in most patients treated with intralesional PRP or triamcinolone, but this was transient. Pain during intralesional injection was significantly higher in the PRP group. The difference in the distribution of VAS score was statistically significant across the two groups [Table 12].
Skin atrophy was noted in five patients treated with intralesional triamcinolone at the end of 12 weeks. The atrophy did not persist at 24-week follow-up. None of the patients in the PRP group developed this side effect. This finding is similar to the study conducted by Kuldeep et al.[25] who noted atrophy in 6 of 25 cases of AA treated with intralesional triamcinolone acetonide.
In our study, one patient in Group A and two patients in Group B relapsed 3 months after treatment discontinuation.
No standard guidelines are available for the preparation of PRP, number of platelets in PRP, number of PRP applications, the amount to be injected in each session, or the interval between injections. It is produced by different methods of platelet concentration through centrifugation and cell separation. Several commercial kits are also available to prepare the same but no devices have been approved yet for PRP preparation. It is a relatively new modality in the treatment of alopecia. Despite the growing interest of this therapy, only a limited number of studies that have investigated the safety and efficacy of PRP in AA are available. Also, there is heterogeneity of interventions and outcome assessment. The duration and follow-up of studies is also varied. Lack of standardized therapeutic protocols and methods to assess response makes it challenging to assess the potential benefit of PRP. We noted that both intralesional triamcinolone and PRP are safe and efficacious in AA but response with PRP was relatively less [Figures 4 and 5]. This can possibly be explained by the fact that steroids have immunosuppressive and strong inhibitory action on T lymphocyte activation, which is not seen with PRP. Also, centrifugation of blood does not remove autoantibodies to hair follicle tissue, thought to be present at increased frequency in patients of AA.
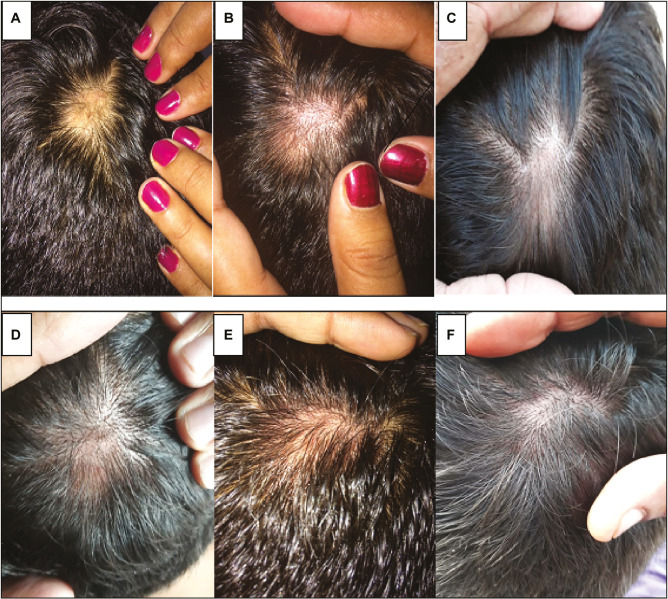
Figure 4.
Patient treated with intralesional triamcinolone acetonide injection. (A) Baseline. (B) 3 weeks. (C) 6 weeks. (D) 9 weeks. (E) 12 weeks. (F) 24 weeks
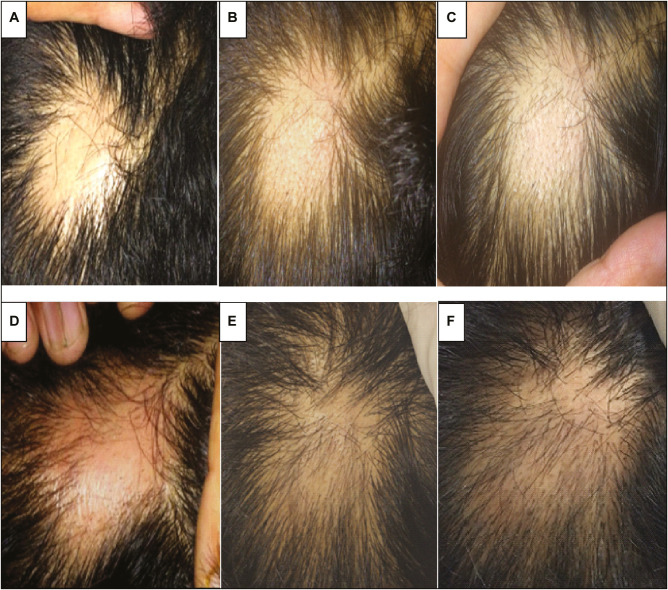
Figure 5.
Patient treated with intralesional autologous PRP injection. (A) Baseline. (B) 3 weeks. (C) 6 weeks. (D) 9 weeks. (E) 12 weeks. (F) 24 weeks
Larger studies with approved centrifugation devices and evidence-based data regarding concentration, dosing, and depth of injection can add to the knowledge regarding the clinical efficacy of a newer modality, that is, PRP, compared to the conventional therapeutic options.
Limitations of our study
It was an unblinded study.
A small number of patients were included in the study.
The duration of follow-up was short.
Interobserver variability was possible during SALT score calculation.
No control arm was present in the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tan E, Tay YK, Goh CL, Chin Giam Y. The pattern and profile of alopecia areata in Singapore—a study of 219 Asians. Int J Dermatol. 2002;41:748–53. doi: 10.1046/j.1365-4362.2002.01357.x. [DOI] [PubMed] [Google Scholar]
- 2.Wasserman D, Guzman-Sanchez DA, Scott K, McMichael A. Alopecia areata. Int J Dermatol. 2007;46:121–31. doi: 10.1111/j.1365-4632.2007.03193.x. [DOI] [PubMed] [Google Scholar]
- 3.Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J. Alopecia areata update: part I. Clinical picture, histopathology, and pathogenesis. J Am Acad Dermatol. 2010;62:177–88. doi: 10.1016/j.jaad.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 4.Madani S, Shapiro J. Alopecia areata update. J Am Acad Dermatol. 2000;42:549–66. [PubMed] [Google Scholar]
- 5.Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J. Alopecia areata update: part II. Treatment. J Am Acad Dermatol. 2010;62:191–202. doi: 10.1016/j.jaad.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 6.Li ZJ, Choi HI, Choi DK, Sohn KC, Im M, Seo YJ, et al. Autologous platelet-rich plasma: a potential therapeutic tool for promoting hair growth. Dermatol Surg. 2012;38:1040–6. doi: 10.1111/j.1524-4725.2012.02394.x. [DOI] [PubMed] [Google Scholar]
- 7.Donovan J. Successful treatment of corticosteroid-resistant ophiasis-type alopecia areata (AA) with platelet-rich plasma (PRP) JAAD Case Rep. 2015;1:305–7. doi: 10.1016/j.jdcr.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Sharkawy H, Kantarci A, Deady J, Hasturk H, Liu H, Alshahat M, et al. Platelet-rich plasma: growth factors and pro- and anti-inflammatory properties. J Periodontol. 2007;78:661–9. doi: 10.1902/jop.2007.060302. [DOI] [PubMed] [Google Scholar]
- 9.Olsen EA, Hordinsky MK, Price VH, Roberts JL, Shapiro J, Canfield D, et al. Alopecia areata investigational assessment guidelines–part II. National Alopecia Areata Foundation. J Am Acad Dermatol. 2004;51:440–7. doi: 10.1016/j.jaad.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 10.Huskisson EC. Measurement of pain. Lancet. 1974;2:1127–31. doi: 10.1016/s0140-6736(74)90884-8. [DOI] [PubMed] [Google Scholar]
- 11.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27:117–26. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 12.Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976;2:175–84. [PubMed] [Google Scholar]
- 13.Joyce CR, Zutshi DW, Hrubes V, Mason RM. Comparison of fixed interval and visual analogue scales for rating chronic pain. Eur J Clin Pharmacol. 1975;8:415–20. doi: 10.1007/BF00562315. [DOI] [PubMed] [Google Scholar]
- 14.Arshdeep Kumaran MS. Platelet-rich plasma in dermatology: boon or a bane? Indian J Dermatol Venereol Leprol. 2014;80:5–14. doi: 10.4103/0378-6323.125467. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Man X, Li C, Chen JQ, Zhou J, Cai SQ, et al. VEGF induces proliferation of human hair follicle dermal papilla cells through VEGFR2 mediated activation of ERK. Exp Cell Res. 2012;318:1633–4. doi: 10.1016/j.yexcr.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Danilenko DM, Ring BD, Yanagihara D, Benson W, Wiemann B, Starnes CO, et al. Keratinocyte growth factor is an important endogenous mediator of hair follicle growth, development, and differentiation. Normalization of the nu/nu follicular differentiation defect and amelioration of chemotherapy-induced alopecia. Am J Pathol. 1995;147:145–54. [PMC free article] [PubMed] [Google Scholar]
- 17.Trink A, Sorbellini E, Bezzola P, Rodella L, Rezzani R, Ramot Y, et al. A randomized, double-blind, placebo- and active-controlled, half-head study to evaluate the effects of platelet-rich plasma on alopecia areata. Br J Dermatol. 2013;169:690–4. doi: 10.1111/bjd.12397. [DOI] [PubMed] [Google Scholar]
- 18.Kaur S, Mahajan BB, Mahajan R. Comparative evaluation of intralesional triamcinolone acetonide injection, narrow band ultraviolet B, and their combination in alopecia areata. Int J Trichology. 2015;7:148–55. doi: 10.4103/0974-7753.171568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amirnia M, Mahmoudi SS, Karkon-Shayan F, Alikhah H, Piri R, Naghavi-Behzad M, et al. Comparative study of intralesional steroid injection and cryotherapy in alopecia areata. Niger Med J. 2015;56:249–52. doi: 10.4103/0300-1652.165034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh S. Role of platelet-rich plasma in chronic alopecia areata: our centre experience. Indian J Plast Surg. 2015;48:57–9. doi: 10.4103/0970-0358.155271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar A, Sharma RP, Bali S, Arya P. Role of platelet plasma therapy in alopecia areata—a prospective study. Int J Contemp Med Res. 2016;3:2499–502. [Google Scholar]
- 22.Shumez H, Prasad PVS, Kaviarasan PK, Deepika R. Intralesional platelet rich plasma vs. intralesional triamcinolone in the treatment of alopecia areata: a comparative study. Int J Med Res Health Sci. 2015;4:118–22. [Google Scholar]
- 23.Ovidio R, Roberto M. Limited effectiveness of platelet-rich-plasma treatment on chronic severe alopecia areata. Hair Ther Transplant. 2014;4:116. [Google Scholar]
- 24.Mubki T. Platelet-rich plasma combined with intralesional triamcinolone acetonide for the treatment of alopecia areata: a case report. J Dermatol Dermatol Surg. 2016;20:87–90. [Google Scholar]
- 25.Kuldeep C, Singhal H, Khare AK, Mittal A, Gupta LK, Garg A. Randomized comparison of topical betamethasone valerate foam, intralesional triamcinolone acetonide and tacrolimus ointment in management of localized alopecia areata. Int J Trichology. 2011;3:20–4. doi: 10.4103/0974-7753.82123. [DOI] [PMC free article] [PubMed] [Google Scholar]