Abstract
Introduction
There are very few studies investigating the dermoscopic aspect of nail involvement in lichen planus and these studies described dermoscopic features of only clinically visible nail involvement in lichen planus.
Aim
To reveal subtle dermoscopic nail findings in patients with lichen planus.
Material and methods
The study included 40 patients with lichen planus and 40 healthy volunteers. All fingernails of the patients and healthy volunteers were examined by a handheld dermoscope and the findings detected were recorded. The patients were grouped by age, gender, disease duration and extent of the disease. The statistical analysis was performed using Chi square test.
Results
82.5% of the patients and 17.5% of the healthy volunteers showed at least one dermoscopic nail finding. The frequency of multiple splinter haemorrhage (p < 0.05), multiple leukonychia (p < 0.05), longitudinal erythronychia (p < 0.05), prominent hyponychial vascular structures (p < 0.05) and onycholysis (p < 0.05) observed in the patients group was statistically significant. The other findings included distal short longitudinal lines, onychorrhexis, solitary splinter haemorrhage and solitary punctate leukonychia and showed no statistically significant differences between the two groups.
Conclusions
To the best of our knowledge, this is the first study focusing on uncovering subtle nail involvement in lichen planus. The presence of dilated hyponychial vascular structures in lichen planus was first described in the present study. It can be concluded that the frequency of nail involvement in lichen planus is more than known. Dermoscopic imaging of the nails of all patients with lichen planus may help establish early diagnosis and treatment to avoid permanent nail damage.
Keywords: dermoscopy, lichen planus, nail
Introduction
Lichen planus (LP) is a pruritic eruption involving the skin, nails, hair and mucosa [1]. The exact etiopathogenesis of the disease is unknown but it is thought to be a cell-mediated immune response associated with environmental triggers [2]. Violaceous, polygonal, flat-topped itchy papules and plaques located on flexural surfaces of the extremities are typical presentations of the cutaneous LP. Mucous membrane involvement is also common and may be found in patients who do not show cutaneous involvement [1].
The prevalence of LP is reported to be 0.5–1% of the population [3]. No significant geographical variation and racial predispositions have been noted for the disease. Some recent studies have shown that adult females are affected more than adult males [4]. LP can occur at any age including in children but most of the patients are aged 30–60 years [5].
Nail involvement is usually a manifestation of disseminated LP and can be seen up to 10% of patients with LP [6]. It may also be the only finding of the disease [7]. Nail LP affects the fingernails more than the toenails. Longitudinal ridging, pitting, distal splitting, onychorrhexis, brown discoloration, onycholysis and trachyonychia are known manifestations of nail LP [1].
Dermoscopy may be helpful in evaluation of disease progress and prognosis as the manifestations of nail matrix, nail bed and perionychium involvement can be observed clearly with this technique [6]. Nail LP may have a very progressive course, so early diagnosis and proper treatment are important [1].
There are very few studies about dermoscopic findings of nail LP [6, 8].
Aim
Here we aimed to reveal dermoscopic nail findings in patients with cutaneous and mucosal lichen planus regarding the importance of early diagnosis. To the best of our knowledge, the present study is the first study focused on demonstrating subtle nail involvement without clinically visible changes.
Material and methods
The study included 40 patients with histopathologically confirmed mucocutaneous lichen planus and 40 age- and gender-matched healthy volunteers. The patients with any dermatological and systemic disease which may affect nails were not included. The patients who had a history of systemic treatment during the last 3 years were also not included. All fingernails of the patients and healthy volunteers were examined by a polarized-light handheld dermoscope with 10-fold magnification (DermLite DL4; 3Gen, San Juan Capistrano, CA). Dermoscopic photographing was performed with a dermoscope-adopted camera phone with high resolution (iPhone 7 plus, Apple, California, USA). The images were obtained after 2× optical zoom. All of the dermoscopic images were reviewed and the findings identified were recorded. The patients grouped by age, gender, disease duration and extent of the disease (localized/generalized).
Statistical analysis
The statistical analysis was performed with χ2 test using SPSS pocket program (SPSS Inc., Chicago, IL). P < 0.05 was considered as statistically significant. All the procedures followed were in accordance with the Helsinki Declaration and the study was approved by the local clinical research ethics committee.
Results
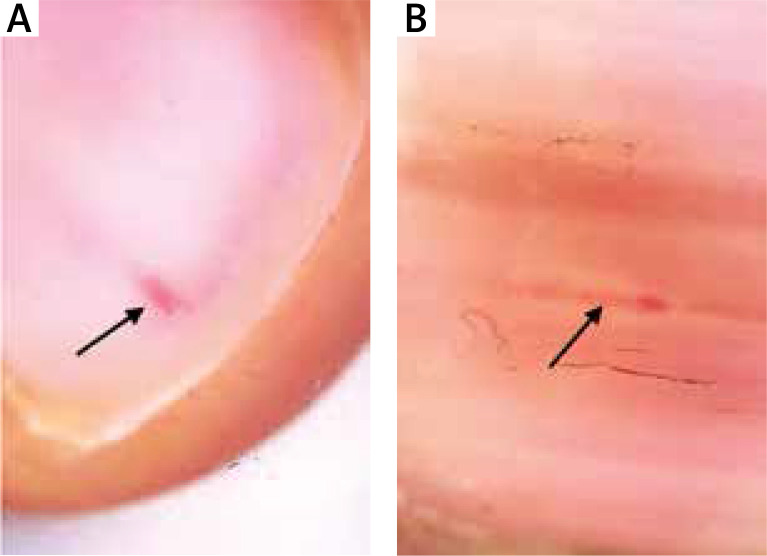
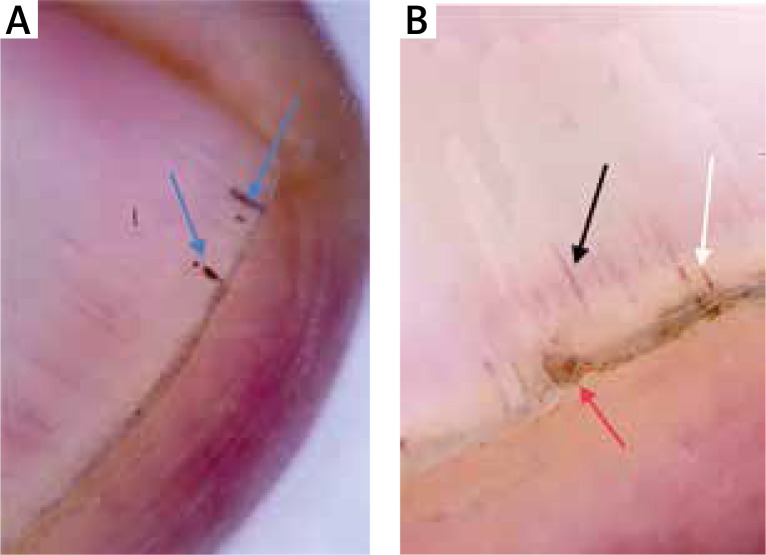
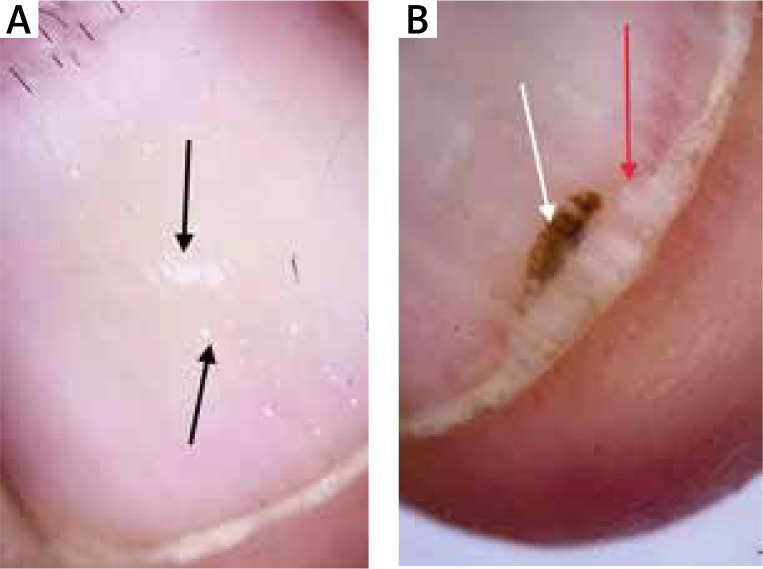
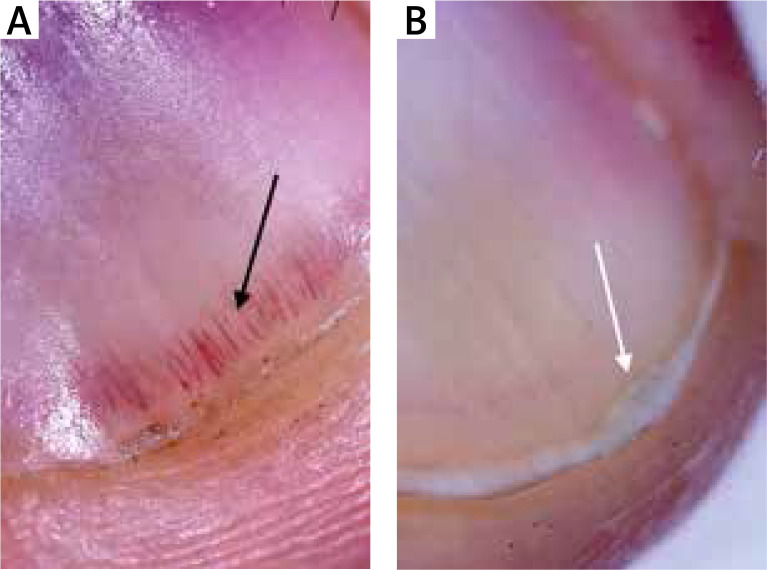
The mean age of the patients and healthy volunteers were 46 ±13 and 44 ±14 years, respectively. Eighteen (45%) patients were male and 22 (55%) were female. Twenty (50%) healthy volunteers were male and 20 (50%) were female. There was no statistically significant difference between patients and control groups regarding the distribution of age and gender. The mean disease duration was 27 months. Thirty-three (82.5%) of the patients and 12 (17.5%) of the healthy volunteers showed at least one dermoscopic nail finding. Thirty-three (82.5%) patients had localized and 7 (17.5%) patients had generalized mucocutaneous involvement. The dermoscopic findings detected are detailed in Table 1. The presence of longitudinal erythronychia (p < 0.05) (Figures 1 A, B), multiple splinter haemorrhage (p < 0.05) (Figures 2 A, B), prominent hyponychial vascular structures (p < 0.05) (Figures 2 B, 3 A), multiple leukonychia (p < 0.05) (Figure 4 A) and onycholysis (p < 0.05) (Figures 3 B, 4 B) in the patients group was statistically significant. The other findings included distal short longitudinal lines, onychorrhexis, solitary splinter haemorrhage and solitary punctate leukonychia and showed no statistically significant difference between the two groups. There was no statistically significant difference between male and female patients regarding the number of the nail findings detected.
Table 1.
Dermoscopic findings detected in the patients and control groups
| Dermoscopic finding | The patients group (n = 40) | The control group (n = 40) | P-values |
|---|---|---|---|
| Multiple splinter haemorrhage | 12 (30%) | 3 (7.5%) | < 0.05 |
| Multiple leukonychia | 11 (27.5%) | 4 (10%) | < 0.05 |
| Longitudinal erythronychia | 6 (15%) | None | < 0.05 |
| Onycholysis | 6 (15%) | 1 (2.5%) | < 0.05 |
| Prominent hyponychial vascular structures | 7 (17.5%) | 1 (2.5%) | < 0.05 |
| Melanonychia | 3 (7.5 %) | None | > 0.05 |
| Onychomadesis | 1 (2.5%) | None | > 0.05 |
| Distal splitting | 1 (2.5%) | None | > 0.05 |
| Solitary splinter haemorrhage | 6 (15%) | 6 (15%) | > 0.05 |
| Solitary punctate leukonychia | 7 (17.5%) | 8 (20%) | > 0.05 |
| Onychorrhexis | 11 (27.5%) | 8 (20%) | > 0.05 |
| Distal short longitudinal white lines | 6 (15 %) | 4 (10%) | > 0.05 |
Figure 1.
Longitudinal erythronychia (black arrow)
Figure 2.
Splinter haemorrhage (blue arrows), prominent hyponychial vascular structures (black arrow), splinter haemorrhage (white arrow) and distal splitting (red arrow)
Figure 4.
Punctate leukonychia (black arrows), melanonychia (white arrow), onycholysis (red arrow)
Figure 3.
Prominent hyponychial vascular structures (black arrow) and onycholysis (white arrow)
When the patients grouped by age into four groups as 16–24 years, 25–35 years, 36–44 years and 45–68 years; the number of the nail findings in the 45–68 years group were higher and statistically significant (p < 0.05) when compared with the other age groups. When the disease duration was grouped as 1–3 months, 4–12 months, 13–24 months and 24–120 months; the number of the nail findings detected in the 24–120 months group was statistically significant (p < 0.05). When the patients were grouped as localized (n = 32) and generalized (n = 8) mucocutaneous involvement, the number of the nail findings detected showed no statistically significant difference. In 5 (12.5%) cases, only oral mucosa was involved. Mucocutaneous and only cutaneous involvement were seen in 22 (55%) and 13 cases (32.5%), respectively. Eighty percent of the cases with only oral mucosal involvement, 86% of the cases with mucocutaneous involvement and 77 cases with only cutaneous involvement showed dermoscopic nail involvement. There was no statistically significant difference between these three groups regarding the presence of dermoscopic findings.
Discussion
Nail LP shows variable progression including nail dystrophy, severe damage and even anonychia. So, early diagnosis is very important for proper management of the disease. Recently, dermoscopy of the nail, also known as onychoscopy, has become an important tool providing early diagnosis in many non-neoplastic and neoplastic onychopathies including subungual hematoma, onychomycosis, nail psoriasis, melanonychia, subungual melanoma, onychopapilloma, onychomatricoma etc. [8].
To the best of our knowledge, there is just one study focusing on dermoscopic features of nail LP, in the literature. In this descriptive study, Nakamura et al. reviewed 79 affected nails of 11 patients with clinically visible nail LP [6]. Differently, here we focused on uncovering subtle and clinically invisible nail involvement in mucocutaneous LP to detect nail involvement as soon as possible.
When the literature is reviewed, it seems that the frequency of nail involvement has been reported in up to 10% of patients with LP [6, 9, 10]. In the present study 25 (60%) of the patients showed at least one statistically significant dermoscopic finding (at least one of the multiple splinter haemorrhage, multiple leukonychia, longitudinal erythronychia, prominence of hyponychial vascular structures and onycholysis). Sixteen (40%) of these 25 patients had no clinically visible nail finding. So, it can be concluded that 40% of the patients showed a clinically invisible nail involvement.
Splinter haemorrhage can be described as tiny, linear, non-blanchable, reddish-brown to black, longitudinal streaks under the nail plate. SH of the nails can be seen in a wide variety of dermatological and internal diseases including psoriasis, lichen planus, vasculitis, connective tissue disorders, infectious diseases and renal failure [11]. In the study of Nakamura et al., the frequency of SH was found to be 35.44% of 11 cases with nail lichen planus [6]. In the present study, SH was considered in two groups as solitary (monodactylous) and multiple (polydactylous). The presence of solitary SH was not statistically significant between the patients and control group. However, 30% of the patients and 7.5% of the control group showed multiple SH and this difference was statistically significant (p < 0.05).
Leukonychia are small white areas in the nail plate caused by formation of deeper pits. It can be seen in different dermatological entities. Punctate leukonychia (PL) is usually found in nail plates of normal individuals [12]. Here we also considered PL in two groups as solitary and multiple. The presence of solitary PL was not statistically significant between the patients and control group. However, 27.55 of the patients and 10% of the control group showed multiple PL and this difference was statistically significant (p < 0.05).
Longitudinal erythronychia (LE) can be described as the presence of red bands in the nail unit and it can be monodactylous or polydactylous. The monodactylous form is usually caused by neoplastic conditions, however, the polydactylous form can occur in many disorders including LP, Darier’s disease, amyloidosis, graft versus host disease, and epidermolysis bullosa [13]. In the present study, 6 (15%) of the patients showed monodactylous LE. There was no dermoscopic finding which may be associated with a neoplastic process in these cases. LE was very subtle clinically in all of these 6 cases. In the study of Nakamura et al., none of the cases showed LE.
Onycholysis can be defined as separation of the nail plate from the nail bed. Onychomycosis, trauma and psoriasis are the main causes of onycholysis [14]. In our study, we detected onycholysis in 6 (15%) patients and 1 (2.5%) healthy volunteer and this difference was statistically significant (p < 0.05). In the study of Nakamura et al., 27.8% of the patients showed onycholysis.
Dilated hyponychial vascular structures can be seen in nail psoriasis and it was not described previously for LP [15]. We detected this finding in 7 (17.5%) of the patients and one healthy volunteer. This difference was also statistically significant.
Onychorrhexis, also known as brittle nails, may be caused by physical factors like frequent soap and water exposure, nail polish remover and hypothyroidism [16]. Up to 20% of the population may be affected. In the present study, 27.5% of the patients and 20% of the healthy volunteers showed onychorrhexis. This difference was not significant statistically.
Onychomadesis is usually a late manifestation of the hand, foot and mouth disease. This finding was previously described for LP in a case report [17]. We also detected clinically evident onychomadesis in 1 patient.
Melanonychia is one of the previously reported clinical findings of nail lichen planus [18]. In our study, 7.5% of the patients showed subtle melanonychia but this finding was not statistically significant.
The main limitation of our study was the lack of long-term follow-up of the patients with subtle nail involvement.
Conclusions
The novel aspects of the present study can be summarized as follows:
To the best of our knowledge, this is the first study focusing on uncovering subtle nail involvement in LP.
Statistically significant dermoscopic findings were detected in 40% of the patients. It can be concluded that the frequency of nail involvement in LP is more than known.
The presence of dilated hyponychial vascular structures in LP, to the best of our knowledge, was first described in the present study.
We described the second case of onychomadesis associated with LP.
Dermoscopic imaging of the nails of all patients with lichen planus may help establish early diagnosis and treatment to avoid permanent nail damage.
We suggest that short-term monitoring of the patients with subtle nail involvement may lead to more effective management of the disease.
Conflict of interest
The author declares no conflict of interest.
References
- 1.Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140–9. doi: 10.1016/j.ijwd.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehman JS, Tollefson MM, Gibson LE. Lichen planus. Int J Dermatol. 2009;48:682–94. doi: 10.1111/j.1365-4632.2009.04062.x. [DOI] [PubMed] [Google Scholar]
- 3.Pereira ALC, Azulay RD. Líquen plano. In: Azulay RD, Azulay DR, Azulay-Abulafia L, Guanabara RJ, Kogan SA, editors. Dermatologia. 5th edn. Guanabara, RJ: Kogan SA; 2008. pp. 124–7. Azulay. [Google Scholar]
- 4.Kyriakis KP, Terzoudi S, Palamaras I, et al. Sex and age distribution of patients with lichen planus. J Eur Acad Dermatol Venereol. 2006;20:625–6. doi: 10.1111/j.1468-3083.2006.01513.x. [DOI] [PubMed] [Google Scholar]
- 5.Balasubramaniam P, Ogboli M, Moss C. Lichen planus in children: review of 26 cases. Clin Exp Dermatol. 2008;33:457–9. doi: 10.1111/j.1365-2230.2008.02694.x. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura R, Broce AA, Palencia DP, et al. Dermatoscopy of nail lichen planus. Int J Dermatol. 2013;52:684–7. doi: 10.1111/j.1365-4632.2011.05283.x. [DOI] [PubMed] [Google Scholar]
- 7.Tosti A, Peluso AM, Fanti PA, Piraccini BM. Nail lichen planus: clinical and pathologic study of twenty-four patients. J Am Acad Dermatol. 1993;28:724–30. doi: 10.1016/0190-9622(93)70100-8. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura RC, Costa MC. Dermatoscopic findings in the most frequent onychopathies: descriptive analysis of 500 cases. Int J Dermatol. 2012;51:483–5. doi: 10.1111/j.1365-4632.2010.04720.x. [DOI] [PubMed] [Google Scholar]
- 9.Daoud MS, Pittelkow MR. Lichen planus. In: Gilchrest BA, Paller AS, Wolff K, et al., editors. Fitzpatrick’s Dermatology in General Medicine. 7 th. New York, NY: McGraw-Hill; 2008. pp. 244–55. [Google Scholar]
- 10.Baran R, Dawber R, Haneke E, Tosti A. In: Treatment in common nail disorders. In: A Text Atlas of Nail Disorders: Techniques in Investigation and Diagnosis. 3rd. Tosti A, Baran R, Dawber R, et al., editors. London: Martin Dunitz; 2003. pp. 320–1. [Google Scholar]
- 11.Haber R, Khoury R, Kechichian E, Tomb R. Splinter hemorrhages of the nails: a systematic review of clinical features and associated conditions. Int J Dermatol. 2016;55:1304–10. doi: 10.1111/ijd.13347. [DOI] [PubMed] [Google Scholar]
- 12.Tüzün Y, Karakuş Ö. Leukonychia. J Turk Acad Dermatol. 2009;3.1:93101r. [Google Scholar]
- 13.Scher RK. Evaluation of nail lines: color and shape hold clues. Cleve Clin J Med. 2016;83:385–91. doi: 10.3949/ccjm.83a.14187. [DOI] [PubMed] [Google Scholar]
- 14.Alessandrini A, Starace M, Piraccini BM. Dermoscopy in the evaluation of nail disorders. Skin Append Disord. 2017;3:70–82. doi: 10.1159/000458728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yorulmaz A, Artuz F. A study of dermoscopic features of nail psoriasis. Adv Dermatol Allergol. 2017;34:28–35. doi: 10.5114/ada.2017.65618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uyttendaele H, Geyer A, Scher RK. Brittle nails: pathogenesis and treatment. J Drugs Dermatol. 2003;2:48–9. [PubMed] [Google Scholar]
- 17.Grover C, Vohra S. Onychomadesis with lichen planus: an under-recognized manifestation. Indian J Dermatol. 2015;60:420. doi: 10.4103/0019-5154.160512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juhlin L, Baran R. Longitudinal melanonychia after healing of lichen planus. Acta Derm Venereol. 1989;69:338–9. [PubMed] [Google Scholar]