Abstract
Background:
Experimental and clinical evidence slupport the role of macrophage Toll-like receptor (TLR) signaling in maternal anti-SSA/Ro-mediated congenital heart block (CHB).
Objectives:
Hydroxychloroquine (HCQ), an orally administered TLR antagonist widely used in lupus including during pregnancy, was evaluated for efficacy in reducing the historical 18% recurrence rate of CHB.
Methods:
This multicenter, open-label, single-arm, two-stage clinical trial was designed using Simon’s optimal approach. Anti-SSA/Ro-positive mothers with a previous pregnancy complicated by CHB were recruited (N=19 first stage; N=35 second stage). Patients received 400mg daily of HCQ prior to completion of gestational week 10 which was maintained through pregnancy. The primary outcome was 2° or 3° CHB any time during pregnancy and secondary outcomes included isolated endocardial fibroelastosis and/or 1°CHB at birth and/or skin rash.
Results:
By intention-to-treat (ITT) analysis, 4/54 evaluable pregnancies resulted in a primary outcome (7.4%; 90%CI:3.4%−15.9%). Since 9 mothers took potentially confounding medications (fluorinated glucocorticoids and/or intravenous gamma globulin) after enrollment but prior to a primary outcome, to evaluate HCQ alone, 9 additional mothers were recruited and followed the identical protocol. In the per-protocol analysis restricted to pregnancies exposed to HCQ alone, 4/54 (7.4%) fetuses developed a primary outcome as in the ITT. Secondary outcomes included mild endocardial fibroelastosis (N=1) and cutaneous neonatal lupus (N=4).
Conclusions:
These prospective data support that HCQ significantly reduces the recurrence of CHB below the historical rate by more than 50%, suggesting that this drug be prescribed for secondary prevention of fetal cardiac disease in anti-SSA/Ro-exposed pregnancies.
Keywords: Anti-SSA/Ro antibodies, Congenital heart block, Neonatal lupus, Hydroxychloroquine, Prevention
CONDENSED ABSTRACT
Given the irreversibility of anti-SSA/Ro-mediated congenital heart block (CHB), prevention with hydroxychloroquine (a Toll-like receptor antagonist reducing inflammation) was evaluated in high risk pregnancies defined as mothers with a previously affected child. In an open label prospective study, mothers received 400mg daily of HCQ prior to completion of gestational week 10 and were followed for the primary outcome of 2° or 3° CHB. 4/54 evaluable pregnancies resulted in a primary outcome (7.4%; 90%CI:3.4%−15.9%), a reduction of more than 50% compared to the historical rate of 18%. These results suggest that hydroxychloroquine be prescribed to prevent the recurrence of CHB.
Introduction
Congenital heart block (CHB), detected in the second trimester in an otherwise normally developing fetus without cardiac structural abnormalities, is almost universally associated with exposure to maternal anti-SSA/Ro antibodies transplacentally passaged via FcγRn (1,2). The risk of CHB is estimated at 2% if the mother is primigravid or has no previous pregnancies with affected children (3–6). Recurrence rates of CHB are six to tenfold higher (2,7–14). Maternal health status does not influence the risk of congenital heart block recurrence (14). Third degree (3°) CHB carries a substantial case fatality rate (17.5%, primarily fetal/neonatal) and morbidity (>70% require pacing before adulthood) (15). To date there is no cure for established disease or proven preventative treatment.
Histopathologic studies constitute a major basis for formulating hypotheses regarding the pathogenesis of CHB, which in turn guide the rationale for therapeutic approaches. Autopsy studies reveal that fibrosis is extensive and can replace the AV node (16,17), affirming the clinical observation that 3° block is irreversible. The identification of a macrophage infiltration in fetuses dying most proximate to the time of diagnosis provides a critical clue to preventative treatments and suggests that inflammation may be integral to the development of fibrosis (16,17). In vitro evidence suggests that immune complexes containing SSA/Ro-associated ssRNA phagocytosed by macrophages ligate Toll-like receptors (TLR7/8) which leads to the secretion of cytokines capable of transdifferentiating fibroblasts to myofibroblasts culminating in fibrosis (18,19). This inflammatory anti-SSA/Ro-induced pathway (recently implicated to involve type I interferon activation (20,21)) is suppressed by hydroxychloroquine (HCQ), the mechanism of which is inhibition of endosomal acidification or direct binding to nucleic acids (22,23).
Previous retrospective studies provide translational support for HCQ in the management of CHB. A case-control study of anti-SSA/Ro pregnancies in SLE mothers demonstrated a reduced risk of fetal cardiac disease in mothers prescribed HCQ (24). In a subsequent multinational historical cohort study, HCQ was associated with a reduction in the recurrence rate of CHB by 60% (14). Additional smaller studies are supportive (25–27) with benefit extended to cutaneous NL (28). Thus, given biologic plausibility and observational data, a Phase II study was initiated to address whether HCQ reduces the recurrence of CHB below the historical rate of 18% (14).
Methods
Trial Design and Oversight
The Preventive Approach To Congenital Heart Block with Hydroxychloroquine (PATCH) study is an open_label single-arm Phase II trial to assess whether HCQ is effective for the prevention of CHB recurrence. Treatment with HCQ 400mg was required by completion of 10 weeks of gestation based on last menstrual period (LMP) and maintained throughout pregnancy. If the mother was already on HCQ the dose remained at 400mg, and if the mother was taking 200mg the dose had to be escalated to 400mg by 10 weeks. Given the inherent inaccuracies of dating the LMP, patients were considered screen failures if 400mg HCQ had not been started before completion of 11 weeks. The protocol was approved by the NYU School of Medicine (NYUSOM) Institutional Review Board (IRB). The trial was overseen by a data and safety monitoring board (DSMB) of independent experts which convened every six months by teleconference.
The trial was designed using Simon’s 2_stage optimal approach which allows for early termination due to the absence of treatment efficacy (29). The first stage required 19 study subjects treated with HCQ and if fewer than 3 cases developed CHB, the study would enroll 35 additional mothers in the second stage for a total of 54 subjects. The treatment would be considered efficacious if fewer than 6 of 54 mothers had a fetus or child with advanced CHB. Six or more fetuses developing advanced block would terminate enrollment. With this design, if the true recurrence rate is ≥ 18%, the historical control rate, the probability of terminating the trial early is 69% and the probability of concluding that the treatment is effective is at most 5% (Type 1 error rate). In contrast, there is a 90% probability of concluding that the treatment is effective (i.e., power) if the true recurrence rate is ≤ 5%.
Trial Population
Patients were prospectively recruited into the PATCH trial between 2011 and 2018 from across the US including one from the United Kingdom. All patients signed informed consents approved by the NYUSOM IRB. For patients not seen in person at NYUSOM by either JPB or PMI, the consent was specifically to allow for the release of medical records and blood specimens to NYUSOM. For these patients HCQ was prescribed by their treating physicians who were comfortable doing so based on the existing literature.
Patients were eligible if they had anti-SSA/Ro with or without anti-SSB/La antibodies irrespective of maternal diagnosis and a previous pregnancy with any of the following complications: 2° or 3° CHB, serious cardiac injury defined as autopsy evidence of a mononuclear infiltrate in the endocardium, myocardium and pericardium and/or the presence of severe endocardial fibroelastosis (EFE) associated with cardiac dysfunction seen on fetal echocardiography. Patients were excluded if a structural fetal cardiac defect was present or if the mother was treated with >20mg prednisone/day or any dose of fluorinated steroids at the time of enrollment.
Fetal echocardiograms were recommended starting at 16–18 weeks and weekly thereafter until 26 weeks and then biweekly until 34 weeks as per the treating physicians (6,30). However, over the course of the trial, the recommendation for echocardiograms beyond 26 weeks diminished given the rarity of advanced block observed de novo in monitored patients after that time (2). Echocardiograms were performed according to standard methods (6,11,30) and recorded on DVD/Optical Disc (and reviewed by DF). Infants had an EKG and/or echocardiogram performed at birth and/or up to one year after birth.
Maternal blood was obtained at baseline, second trimester, third trimester and birth to determine antibody levels by ELISA using native Ro60, recombinant Ro52 and recombinant La48 in the lab of JPB as previously described (11). HCQ levels, to assess for patient adherence, were measured in Paris, France as previously described (31,32).
End Points
The primary endpoint was recurrence of 2° or 3° block in utero or at birth. The secondary endpoints consisted of: a) prolonged fetal Doppler mechanical PR interval (>150msec) documented on the fetal echocardiogram (AV interval) or PR interval on EKG done after birth; b) isolated myocardial injury, without change in cardiac rate or rhythm defined as: shortening fraction <28%=2 SD below normal mean or qualitatively reduced systolic function; cardio-thoracic area ratio >0.33; or moderate/severe tricuspid regurgitation; c) echocardiographic densities consistent with EFE confirmed postnatally; d) prematurity (<37 weeks at birth); e) birth weight <10th percentile for gestational age; f) neonatal lupus rash.
All primary outcomes or serious adverse events were communicated to the DSMB within 24 hours of being reported to the study PI.
Statistical Analysis
The null and alternative hypotheses of interest were specified as H0:π ≥18% (historical rate) and HA:π <18%, respectively, where π is the true CHB recurrence rate with HCQ. The historical rate of 18% was chosen based on the largest study of pregnancies following a child with CHB, which included 257 pregnancies from the U.S, France, and U.K (14). There were 49 recurrent cases of cardiac disease resulting in a recurrence rate of 19.1%. However, given that our primary outcome was advanced conduction disease, this would eliminate 4 cases of isolated mild endocardial fibroelastosis which did not require medications or confer functional consequence, reducing the rate to 17.5%.
The CHB recurrence rate in this study was estimated two ways: the simple proportion of pregnancies that resulted in CHB (), and the biased corrected estimator () proposed by Jung and Kim(33) which accounts for the two-stage trial design. The p-value for testing H0, and 2-sided 90% CI (consistent with a 1-sided α=0.05 level) were computed using the approach of Koyama and Chen (34). Primary analyses were conducted according to intention-to-treat (ITT). To account for the effects of any potentially confounding conditions, a per-protocol (PP) analysis was also performed to evaluate the efficacy of HCQ alone. Estimation of CHB recurrence rates and statistical inference in PP analyses were based on standard approaches for single proportions using the exact binomial distribution. Secondary endpoints (e.g., occurrence of 1° block) were analyzed by similar approaches. Titers of anti-Ro60 and 52 were summarized as median (IQR) and compared between the CHB and non-CHB groups using the Wilcoxon rank sum test, and between visits in the combined sample using the Wilcoxon signed rank test.
Results
Patients
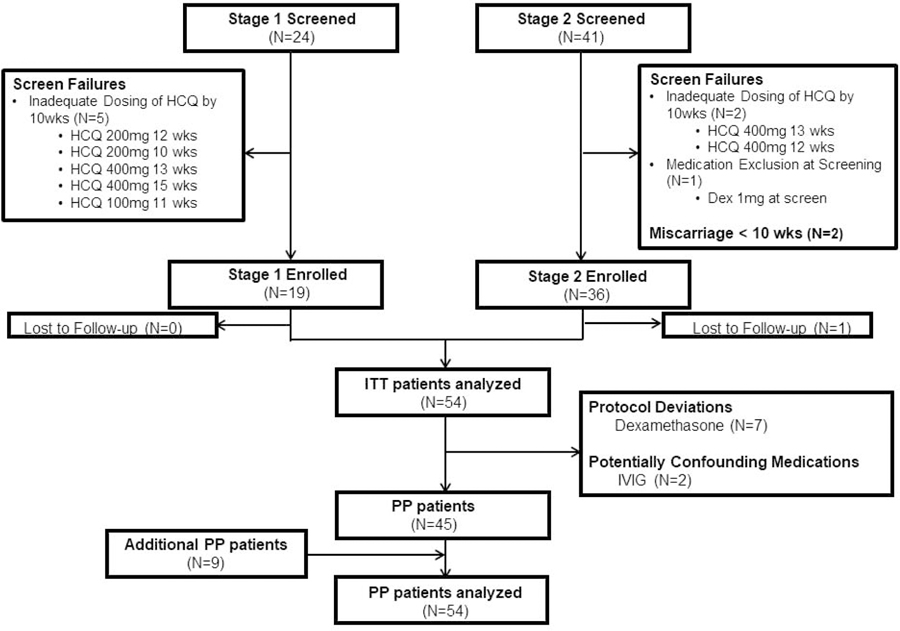
Initially, a total of 55 mothers from both stages of the trial were enrolled. One patient was lost to follow up, resulting in 54 patients who were included in the ITT analysis (Figure 1; demographics provided in Table 1). The DSMB requested and the IRB approved enrollment of an additional nine mothers to evaluate efficacy of HCQ alone, since nine original mothers had been treated with other medications in addition to HCQ (Figure 1, Table 1). Mothers were predominately white. One-half of the enrollees were completely asymptomatic or had an undifferentiated autoimmune disease. In 7, HCQ had been taken during the previously affected pregnancy. Three mothers had >1 child with CHB. The fetal mortality rate in the prior CHB pregnancies was 35%, twice that reported (15).
Figure 1.
CONSORT diagram of PATCH study enrollment. In 13 enrolled mothers, despite being on 400mg HCQ by 10 weeks, consents were signed after 10 weeks, 11 before the first echocardiogram was performed. Two of these 13 agreed to be in the study prior to the first echo and taking 400mg HCQ, but consents were signed after the first echocardiogram.
Dex=dexamethasone; HCQ=hydroxychloroquine; ITT=intention-to-treat; IVIG=intravenous immunoglobulin; PP=per-protocol
Table 1.
Demographics of PATCH participants.
| Intention-to-treat pregnancies N=54 |
Per protocol pregnancies N=54 |
Overall pregnancies evaluated N=63 |
|
|---|---|---|---|
| Ethnicity | |||
| Hispanic/Latino | 3 (5.6%) | 3 (5.6%) | 3 (4.8%) |
| Race | |||
| White | 37 (68.5%) | 38 (70.4%) | 45 (71.4%) |
| Black | 6 (11.1%) | 4 (7.4%) | 6 (9.5%) |
| Asian | 7 (13.0%) | 8 (14.8%) | 8 (12.7%) |
| Not available | 4 (7.4%) | 4 (7.4%) | 4 (6.3%) |
| Maternal diagnosis* | |||
| Asymptomatic | 16 (29.6%) | 17 (31.5%) | 20 (31.8%) |
| Undifferentiated autoimmune syndrome | 12 (22.2%) | 12 (22.2%) | 13 (20.6%) |
| Systemic lupus erythematosus | 3 (5.6%) | 4 (7.4%) | 5 (7.9%) |
| Sjögren’s syndrome† | 11 (20.4%†) | 11 (20.4%†) | 12 (19.1%†) |
| Sjögren’s syndrome/systemic lupus erythematosus | 12 (22.2%) | 10 (18.5%) | 13 (20.6%) |
| Maternal Autoantibodies | |||
| Ro60 (% +) | 54 (100%) | 54 (100%) | 63 (100%) |
| Ro52 (% +) | 51 (94.4%) | 51 (94.4%) | 60 (95.2%) |
| La48 (% +) | 29 (53.7%) | 27 (50.0%) | 31 (49.2%) |
| Death of previous CHB child | 19 (35.2%) | 18 (33.3%) | 23 (36.5%) |
| More than 1 previous CHB child | 3 (5.6%) | 2 (3.7%) | 3 (4.8%) |
| Use of HCQ in previous CHB pregnancy‡ | 7 (13.0%) | 5 (9.3%) | 8 (12.7%) |
The 54 intention-to-treat cases included nine participants who, despite strong discouragement from study personnel, were prescribed the potentially confounding medications dexamethasone or intravenous immunoglobulin. Accordingly, the DSMB recommended and the IRB approved additional per protocol enrollment until 54 pregnancies exposed only to HCQ could be evaluated; none of the nine subsequently enrolled patients took confounding medications and thus the study concluded. In total, 63 pregnancies occurred in the PATCH study, which are evaluated cumulatively in the rightmost column.
A diagnosis of systemic lupus erythematosus (SLE) was assigned based on fulfillment of either American College of Rheumatology (45,46) or Systemic Lupus International Collaborating Clinics classification criteria (47); a diagnosis of Sjögren’s Syndrome (SS) was assigned based on fulfillment of American-European Consensus Group’s criteria for probable SS (48) or American College of Rheumatology/European League Against Rheumatism criteria for definite SS (49); mothers that did not fulfill SS or SLE classification criteria were diagnosed as undifferentiated autoimmune syndrome (UAS) or asymptomatic.
Three mothers had Sjögren’s syndrome in addition to rheumatoid arthritis.
Of the three mothers with two previous CHB pregnancies, two did not take HCQ during either pregnancy; the third mother was on HCQ for the second affected pregnancy but not the first.
CHB=congenital heart block; HCQ=hydroxychloroquine.
End Points
In Stage I, of 24 mothers screened, five were screen failures, resulting in 19 enrolled patients (Figure 1). A primary endpoint of 2° block occurred in two pregnancies at 19 weeks and 18 weeks (detailed in Table 2), the latter initially detected by maternal home Doppler monitoring (35). Since fewer than three cases developed CHB in the first stage, the study proceeded to Stage II.
Table 2.
Descriptions of Primary Endpoints.
| Timing | Echo | Treatment | In Utero Course and Birth Outcome | Follow up |
|---|---|---|---|---|
| Stage I | ||||
| 19w5d | 2° block | Dex 4mg | In 3 days progressed to 3° block Born=39 weeks | At 4.5 months, pacemaker placed |
| 18w3d | 2° block initially identified by home Doppler monitoring | Dex 4 then increased to 8mg within 6 hrs | Within 48 hrs reverted to NSR. EKG at birth NSR Born=37 weeks |
At 2 yrs: EKG - PR interval 184ms Holter-predominant sinus with 1° and 2° Type I block. |
| Stage II | ||||
| 18w6d | 2° block early EFE | Dex 8mg IVIG 1gm/kg | Within 48 hrs progressed to 3° block, cardiomyopathy, terminated (50) | - |
| 19w0d | AV interval 160ms | Dex 8 mg (confounding med since given before primary endpoint) | Within 1 wk, progressed to 2° block Birth echo NSR Born=37 weeks | At 4 weeks, EKG HR 126 and Mobitz Type I, 2° block At 1 and 2.5yrs – EKG – Mobitz Type II, 2° block |
| Additional Per Protocol | ||||
| 20w4d | 3° block, EFE (resolved EFE in 1wk) | Dex 4mg | EKG at birth 3° block Born=37 weeks | At birth pacemaker placed |
Stage I = first stage of Simon’s two step design
Stage II = second stage of Simon’s two step design
Additional Per Protocol – the addition of pregnancies to evaluate exposure only to HCQ without and potentially confounding medications
AV=atrioventricular; Dex=dexamethasone; EFE=endocardial fibroelastosis; EKG=electrocardiogram; IVIG=intravenous immunoglobulin; NSR=normal sinus rhythm;
In Stage II (Figure 1), of 41 mothers screened, three were considered screen failures and two mothers miscarried in the first trimester. Thirty six mothers were enrolled and one patient moved from the initial enrollment site after stopping HCQ at 13 weeks and no further medical records were obtained. Of the 35 evaluable pregnancies in Stage II, two fetuses developed advanced block (detailed in Table 2), at 18 weeks and 20 weeks. The latter fetus was initially identified at 19 weeks with an AV interval of 160ms and treated with dexamethasone which then became a confounding medication since it was initiated prior to advanced block.
Combining Stages I and II, a total of 4/54 of pregnancies in the ITT analysis resulted in CHB (;; 90%CI:3.4%−15.9%; p=0.02 for H0). Since this satisfied the Simon’s two-stage criterion for concluding that the treatment is effective at the end of the trial (<6/54 CHB), and the upper limit of the CI was <18%, HCQ was considered effective in decreasing the CHB recurrence rate.
Primary and Secondary Outcomes of HCQ with Potentially Confounding Medications
Although strongly discouraged, initiation of medications after enrollment, which could potentially confound interpretation of data, occurred in nine cases, one in Stage I and eight in Stage II consisting of dexamethasone in seven and IVIG in two. Five mothers were treated with dexamethasone for fetal cardiac concerns raised during the echocardiographic surveillance (detailed in Table 3). One fetus treated for a prolonged AV interval described above progressed to 2° block one week later thus constituting a primary outcome. In one, mild EFE persisted at birth, constituting a secondary outcome. The remaining three had normal cardiac evaluations in the neonatal period. Two mothers were given dexamethasone for lupus flares; two were given intravenous immunoglobulin (IVIG) for perceived added prevention of CHB despite the absence of any observed echocardiographic abnormalities.
Table 3.
Reasons for Confounding Medications and Outcomes.
| Drug/Dose | Week Initiated | Week Discontinued | Reason for Initiation | Outcome at Birth |
|---|---|---|---|---|
| Confounding Medications for Potential Fetal Cardiac Concerns | ||||
| Dex 4mg | 23 | 37 | AV interval 143 | EKG normal |
| Dex 4mg | 27 | 27 (3 days) | Isolated Atrial Premature Contractions and runs of un-sustained SVT at 220–230 bpm | EKG normal |
| Dex 4mg | 20 | 29 | Mild mitral regurgitation; EFE (“echobrightness” of crux heart) trivial pericardial effusion All resolved in utero. | EKG normal |
| Dex 4mg | 19 | 23 | AV interval 160msec which progressed to 2° | EKG Mobitz Type I, 2° block (Primary Outcome - Stage II 2nd case) |
| Dex 4mg | 22 | 24 | Echo densities in right ventricle along the intraventricular septum and crux of the heart | 37 weeks – mother developed pre-eclampsia necessitating urgent C-section. Neonate cardiac arrest and severe hypoxia, after resuscitation EKG RBBB and echo minimal EFE* (Secondary Outcome) |
| Confounding Medications for Non-cardiac Considerations | ||||
| Dex 4mg | 12 | 34 | Leukocytoclastic vasculitis legs | EKG normal |
| Dex 6mg | 14 | 32 | Lupus flare | EKG normal |
| IVIG 1g/kg q 3 weeks | 13 | 25 | Added prophylaxis | EKG normal |
| IVIG 2g/kg q week | 15 | 36 | Added prophylaxis | EKG normal |
Evaluation by pediatric cardiology and a specialist in electrophysiology concluded that attribution to anti-SSA/Ro antibodies versus severe hypoxia could not be determined.
AV=atrioventricular; CHB=congenital heart block; Dex=dexamethasone; EFE=endocardial fibroelastosis; EKG=electrocardiogram; IVIG=intravenous immunoglobulin; NSR=normal sinus rhythm; RBBB=right bundle branch block; SVT=supraventricular tachycardia
Evaluation of HCQ without any Potentially Confounding Medications
To evaluate the efficacy of HCQ alone, the DSMB requested, and the IRB approved enrollment of nine additional patients as described in the overall enrollment above (Figure 1). Of the 45 patients on HCQ alone in Stages I and II, three met the primary endpoint of 2° CHB. Of the nine additionally enrolled patients on HCQ alone, one fetus developed 3° block at 20 weeks (Table 2). The recurrence rate of CHB in the 54 pregnancies exposed only to HCQ in the per-protocol (PP) population was 7.4%, which was the same as the result obtained in the ITT analyses (90%CI:2.6%−16.2%; p=0.02 for H0).
In an analysis of all evaluable pregnancies inclusive of those with potentially confounding medications, a total of 5/63 pregnancies (7.9%; 90%CI:3.2%−16.0%; p=0.02 for H0) resulted in 2° or 3°CHB (primary outcome), and one in persistent mild EFE (secondary outcome). All remaining ECGs of the unaffected children, including those with rash, had normal sinus rhythm and normal PR intervals.
Non-Cardiac Secondary Outcomes
Table 4 provides details of the non-cardiac outcomes. Cutaneous neonatal lupus occurred in 4/63 cases (6.4%; 90%CI:2.2%−13.4%). Nine of 63 cases (14.3%; 90%CI:7.7%−23.6%) were born at <37 weeks gestation. All but one neonate, independent of gestational age at birth, had appropriate weights defined as ±2 standard deviations of the expected weight for gestational age.
Table 4.
Secondary Outcomes
| Outcome | N (%) | 90% Confidence Interval | Details |
|---|---|---|---|
| Cutaneous neonatal lupus | 4/63 (6.4%) | 2.2% - 13.9% | Occurred at 3, 5, 6 and 12 weeks after birth |
| Weights below normal gestational age (<±2 standard deviations) | 1/63 (1.6%) | 0.08% - 7.3% | Born to a mother with SLE/SS weight=1.995 kg at 37 weeks |
| Premature birth | 9/63 (14.3%) | 7.7% - 23.6% | |
| Premature rupture of membranes | 2/9 | 35 weeks, SLE/SS; 35 weeks, UAS | |
| Low amniotic fluid & intrauterine growth restriction | 2/9 | 35 weeks, SLE/SS; 35 weeks, UAS | |
| Pre-eclampsia | 1/9 | 36 weeks, SLE | |
| Placental insufficiency | 1/9 | 32 weeks, SLE | |
| Placental abruption | 1/9 | 35 weeks, SS | |
| Partial placenta previa with recurrent bleeding | 1/9 | 35 weeks, SLE/SS | |
| Vaginal bleeding of unknown cause | 1/9 | 36 weeks, UAS |
SLE=systemic lupus erythematosus; SS=Sjogren’s syndrome; UAS=undifferentiated autoimmune syndrome
Results of Laboratory Testing
HCQ levels were obtained on all patients at least once (Online Figure 1). During the 2nd trimester, 60 of 61 HCQ levels were >200ng/ml confirming adherence. The one patient with low values at the 2nd trimester did have adherent levels at enrollment and delivery. The two patients without blood draws during the 2nd trimester had levels >200ng at baseline and delivery.
Median baseline titers of anti-Ro60 were similar in the CHB and non-CHB groups (26,325 (IQR 12,740) ELISA units (EU), N=5 versus 27,480 (IQR 26,274) EU, N=55 as 3 were only done later in pregnancy, respectively; p=0.99). Anti-Ro52 titers were lower in the CHB group but not significantly (16,455 (IQR 18,080) EU versus 26,600 (IQR 50,858) EU; p=0.40). Overall, although titers of anti-Ro60 decreased between baseline and the second trimester (27,480 (IQR 21,627) EU versus 25,046 (IQR 22,928) EU respectively; N=57, p=0.11), they remained very high titer as did anti-Ro52 (24,470 (IQR 42,088) EU versus 23,418 (IQR 38,660) EU, respectively; N=56; P=0.004).
Discussion
PATCH is the first prospective study to evaluate the use of HCQ in mothers who have previously had a pregnancy complicated by CHB. Adherence to medication was confirmed by 2nd trimester HCQ levels in 98%. Both the ITT and PP analyses demonstrated that the recurrence rate of CHB was reduced by more than half: from 18% to 7.4% (Central Illustration). Thus, HCQ is the first medication shown to favorably alter the fetal cardiac consequences associated with maternal anti-SSA/Ro antibodies.
Central Illustration.
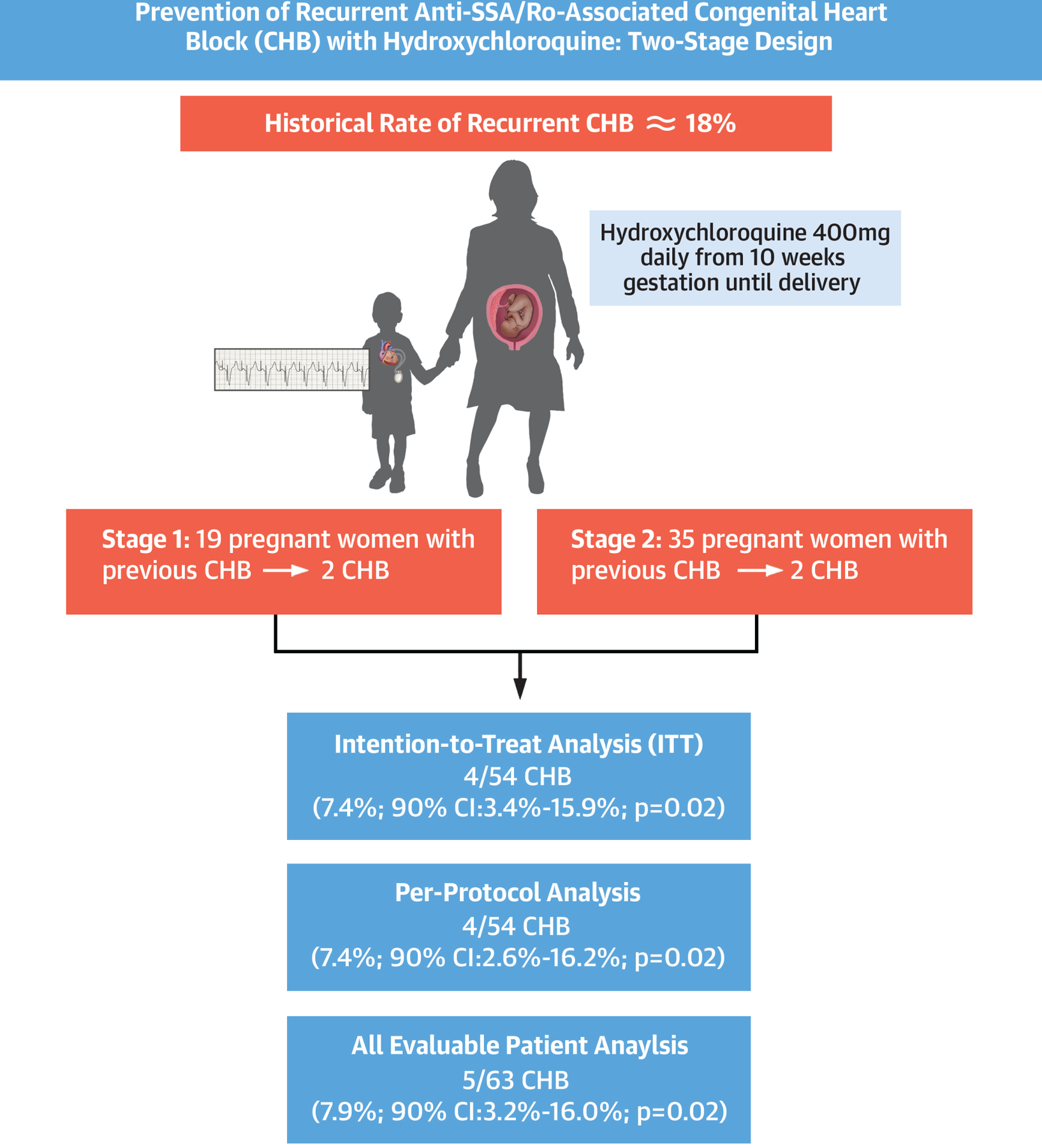
Prevention of recurrent anti-SSA/Ro-associated congenital heart block with hydroxychloroquine: two-stage design with results for intention-to-treat and per-protocol analyses.
As described in the methods, the historical recurrence rate chosen in this study was 18% based on our own previous study which comprised the largest to date at the time PATCH was iniatiated (14). However, we initially reviewed the existing literature but were concerned given the variable recurrence rates, degree of block in the initial CHB case, limited sample sizes, inclusion of replicate cases in multiple studies as registries expanded, and the varied spectrum of the cardiac manifestations considered associated with autoimmunity (2,7–13). Herein, we sought clinically relevant recurrences defined as 2° or 3° block, not 1° or isolated mild EFE (not compromising cardiac function or requiring medication), which might be of less concern. In two prospective studies evaluating the efficacy of 400mg/kg IVIG given at 12,15,18 and 21 weeks, the combined recurrence rate of 2° or 3° CHB was 6/33 (18.1%) in mothers receiving IVIG and 7/42 (16.7%) when including 9 pregnancies not exposed to IVIG (11,12). In our initial review of recurrences restricted to the US Research Registry for Neonatal Lupus, a recurrence rate of a spectrum of cardiac manifestations of neonatal lupus in 129 pregnancies immediately following the birth of an affected child was 18% and 17.4% if all 161 total pregnancies following an affected child were included (10). Restricting outcomes to only advanced block reduced that rate to 14.7%; however 1° block was included in the initial CHB calculation which may have diluted the recurrence rate. In a major review of several studies, the combined recurrence rate was 15.8% in 317 pregancies (2) but methodologies varied and included prospective (6,12) and retrospective studies (8,9,13) and differing inclusion criteria. Even if persistent mild EFE was included as a primary outcome, thus increasing our recurrence rate to 9.3%, our study would still be considered successful given our threshold of <6/54 cases. Most relevant to our findings, with the exception of the study we used for the historical recurrence rate (14), no published study factored the influence of HCQ on these rates. Focusing only on cases which would meet our primary outcome, the recurrence rate was 2/40 (5.0%) in mothers taking HCQ and 43/217 (19.8%) in mothers not taking HCQ, the latter considered most relevant to our study hypothesis (14). Thus, historical estimates might be diluted if HCQ exposure was not considered. It should also be pointed out that the recurrence rate can approach 50% if a mother has had more than one previously affected child (10). None of the 3 mothers with two prior CHB children had a primary outcome in this study.
Efficacy of HCQ needs to be balanced against potential safety concerns. The literature suggests that HCQ is safe to use during pregnancy (36–38) with no increased frequency of congenital malformation (36,37,39) or hearing or visual abnormalities in the offspring (37). However, prolonged use of HCQ carries the risk of maculopathy (40). Although longer-term safety of HCQ was not assessed in PATCH, an anatomical survey of the retina performed via optical coherence tomography (OCT) in the children who have reached age five is ongoing. In this study prematurity occurred in 14.3% of all 63 evaluable pregnancies which is consistent with rates observed in a prospective study evaluating IVIG in anti-SSA/Ro positive mothers with a previous CHB child (11) and was not considered attributable to HCQ per se. All mothers of the premature infants had an underlying rheumatologic condition, with 5 having SLE which may confer an increased risk of prematurity (41).
Limitations
Limitations of this study include absence of randomization and potentially confounding medications given to some of the mothers. Based on our previous experience with treatment studies (11,30), it was deemed unlikely that mothers having a previous child with CHB would agree to randomization with observation alone knowing a potentially effective therapy was under evaluation. In addition, randomization in a placebo-controlled study would require at least 100 subjects per arm, which would be prohibitive given CHB is a rare disease. Finally, given the recommendation to prescribe HCQ to all pregnant women with SLE to prevent disease flares, it would be potentially unethical to discontinue HCQ in any mother carrying a diagnosis of SLE (42,43). Since recruitment took place across the country with referral both by treating physicians and patients themselves, discussion of the study occurred over the phone and by email. In two cases, although the patient was on HCQ per protocol and agreed to participate before any cardiac evaluation, consents were signed after obtaining a first surveillance echocardiogram. Since all women agreeing to the study did return a consent form and knowledge of the echocardiogram results were not known to the investigators, introduction of bias in favor of HCQ efficacy was not likely.
The use of potentially confounding medication after enrollment was another limitation of the study. This was challenging to prevent given the high-risk nature of these pregnancies with over a third having previously affected offspring dying with CHB, causing heightened anxieties in both the mothers and their treating physicians. Despite perceived benefits, data regarding the efficacy of dexamethasone and IVIG as prophylactic therapy are limited. One retrospective study reported on prophylactic fluorinated steroids in 6 mothers with previously affected CHB children with 2 pregnancies ending in a spontaneous abortion, 2 stillbirths and 2 live births with IUGR and mild adrenal insufficiency (44). In the initial study that analyzed HCQ’s effects on the CHB recurrence rate, prophylactic fluorinated steroids were not associated with a reduction in recurrent CHB (14). With regard to IVIG, two prospective studies evaluated the use of prophylactic IVIG at 400mg/kg to prevent recurrent CHB using similar methodology to the PATCH study and neither showed reduction in the recurrence rate (11,12). Nevertheless, concerns regarding the influence of confounding medications were addressed by enrolling additional cases treated only with HCQ. That this strategy was successful was seen in the equivalent results of the ITT and the PP studies.
Conclusion
In conclusion, data from this prospective single-arm clinical trial support that HCQ reduces the recurrence of CHB in anti-SSA/Ro exposed pregnancies by more than half and should be considered for secondary prevention.
Supplementary Material
Clinical Perspectives.
Competency in Medical Knowledge:
The risk of congenital heart block in a fetus exposed to maternal anti-SSA/Ro antibodies is approximately 2% if the mother has never had an affected child and 18% if she has. In an open-label prospective trial, hydroxychloroquine, 400 mg daily, initiated at or before 10 weeks gestation, was associated with a recurrence rate less than half that of historical controls.
Translational Outlook:
Further studies are needed to confirm the safety and efficacy of hydroxychloroquine during pregnancies involving maternal anti-SSA/Ro antibodies for prevention of fetal congenital heart block.
Acknowledgements:
The authors would like to thank all the patients and doctors who participated in the study. In addition we would like to thank the Data and Safety Monitoring Board. Finally, we want to acknowledge prior coordinators who worked on the study, Zoey Smith and Tishaun Middleton.
Funding: This reserch was supported by the Lupus Foundation of Minnesota (Bloomington, MN), the Lupus Foundation of America (LIFELINE Grant, Washington, DC), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R03HD069986, J.P.B.; R01HD079951, J.P.B., Bethesda, MD).
ABBREVIATIONS
- Anti-SSA/Ro
Anti-Sjogren’s syndrome type A/Ro autoantibodies
- CHB
congenital heart block
- Dex
dexamethasone
- EFE
endocardial fibroelastosis
- EKG
electrocardiogram
- HCQ
hydroxychloroquine
- IVIG
intravenous immunoglobulin
- LMP
Last menstrual period
- SLE
systemic lupus erythematosus
- TLR 7/8
Toll-like receptor 7/8
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None.
Tweet: Anti-Ro+ women who have had babies with congenital heart block have ~18% risk in subsequent pregnancies. The PATCH study shows HCQ significantly reduces risk to 7.4%.
CLINICAL TRIAL: NCT01379573
References
- 1.Jaeggi ET, Hornberger LK, Smallhorn JF, Fouron JC. Prenatal diagnosis of complete atrioventricular block associated with structural heart disease: combined experience of two tertiary care centers and review of the literature. Ultrasound Obstet Gynecol 2005;26:16–21. [DOI] [PubMed] [Google Scholar]
- 2.Brito-Zeron P, Izmirly PM, Ramos-Casals M, Buyon JP, Khamashta MA. The clinical spectrum of autoimmune congenital heart block. Nat Rev Rheumatol 2015;11:301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brucato A, Frassi M, Franceschini F et al. Risk of congenital complete heart block in newborns of mothers with anti-Ro/SSA antibodies detected by counterimmunoelectrophoresis: a prospective study of 100 women. Arthritis Rheum 2001;44:1832–5. [DOI] [PubMed] [Google Scholar]
- 4.Cimaz R, Spence DL, Hornberger L, Silverman ED. Incidence and spectrum of neonatal lupus erythematosus: a prospective study of infants born to mothers with anti-Ro autoantibodies. The Journal of pediatrics 2003;142:678–83. [DOI] [PubMed] [Google Scholar]
- 5.Costedoat-Chalumeau N, Amoura Z, Lupoglazoff JM et al. Outcome of pregnancies in patients with anti-SSA/Ro antibodies: a study of 165 pregnancies, with special focus on electrocardiographic variations in the children and comparison with a control group. Arthritis Rheum 2004;50:3187–94. [DOI] [PubMed] [Google Scholar]
- 6.Friedman DM, Kim MY, Copel JA et al. Utility of cardiac monitoring in fetuses at risk for congenital heart block: the PR Interval and Dexamethasone Evaluation (PRIDE) prospective study. Circulation 2008;117:485–93. [DOI] [PubMed] [Google Scholar]
- 7.Buyon JP, Hiebert R, Copel J et al. Autoimmune-associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol 1998;31:1658–66. [DOI] [PubMed] [Google Scholar]
- 8.Julkunen H, Eronen M. The rate of recurrence of isolated congenital heart block: a population-based study. Arthritis Rheum 2001;44:487–8. [DOI] [PubMed] [Google Scholar]
- 9.Gladman G, Silverman ED, Yuk L et al. Fetal echocardiographic screening of pregnancies of mothers with anti-Ro and/or anti-La antibodies. American journal of perinatology 2002;19:73–80. [DOI] [PubMed] [Google Scholar]
- 10.Llanos C, Izmirly PM, Katholi M et al. Recurrence rates of cardiac manifestations associated with neonatal lupus and maternal/fetal risk factors. Arthritis Rheum 2009;60:3091–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman DM, Llanos C, Izmirly PM et al. Evaluation of fetuses in a study of intravenous immunoglobulin as preventive therapy for congenital heart block: Results of a multicenter, prospective, open-label clinical trial. Arthritis Rheum 2010;62:1138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pisoni CN, Brucato A, Ruffatti A et al. Failure of intravenous immunoglobulin to prevent congenital heart block: Findings of a multicenter, prospective, observational study. Arthritis and rheumatism 2010;62:1147–52. [DOI] [PubMed] [Google Scholar]
- 13.Ambrosi A, Salomonsson S, Eliasson H et al. Development of heart block in children of SSA/SSB-autoantibody-positive women is associated with maternal age and displays a season-of-birth pattern. Ann Rheum Dis 2012;71:334–40. [DOI] [PubMed] [Google Scholar]
- 14.Izmirly PM, Costedoat-Chalumeau N, Pisoni CN et al. Maternal use of hydroxychloroquine is associated with a reduced risk of recurrent anti-SSA/Ro-antibody-associated cardiac manifestations of neonatal lupus. Circulation 2012;126:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izmirly PM, Saxena A, Kim MY et al. Maternal and fetal factors associated with mortality and morbidity in a multi-racial/ethnic registry of anti-SSA/Ro-associated cardiac neonatal lupus. Circulation 2011;124:1927–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Llanos C, Friedman DM, Saxena A et al. Anatomical and pathological findings in hearts from fetuses and infants with cardiac manifestations of neonatal lupus. Rheumatology (Oxford) 2012;51:1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clancy RM, Kapur RP, Molad Y, Askanase AD, Buyon JP. Immunohistologic evidence supports apoptosis, IgG deposition, and novel macrophage/fibroblast crosstalk in the pathologic cascade leading to congenital heart block. Arthritis Rheum 2004;50:173–82. [DOI] [PubMed] [Google Scholar]
- 18.Clancy RM, Markham AJ, Reed JH, Blumenberg M, Halushka MK, Buyon JP. Targeting downstream transcription factors and epigenetic modifications following Toll-like receptor 7/8 ligation to forestall tissue injury in anti-Ro60 associated heart block. J Autoimmun 2016;67:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed JH, Sim S, Wolin SL, Clancy RM, Buyon JP. Ro60 Requires Y3 RNA for Cell Surface Exposure and Inflammation Associated with Cardiac Manifestations of Neonatal Lupus. J Immunol 2013. [DOI] [PMC free article] [PubMed]
- 20.Clancy RM, Halushka M, Rasmussen SE, Lhakhang T, Chang M, Buyon JP. Siglec-1 Macrophages and the Contribution of IFN to the Development of Autoimmune Congenital Heart Block. J Immunol 2019;202:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hedlund M, Thorlacius GE, Ivanchenko M et al. Type I IFN system activation in newborns exposed to Ro/SSA and La/SSB autoantibodies in utero. RMD Open 2020;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuznik A, Bencina M, Svajger U, Jeras M, Rozman B, Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol 2011;186:4794–804. [DOI] [PubMed] [Google Scholar]
- 23.Lamphier M, Zheng W, Latz E et al. Novel small molecule inhibitors of TLR7 and TLR9: mechanism of action and efficacy in vivo. Mol Pharmacol 2014;85:429–40. [DOI] [PubMed] [Google Scholar]
- 24.Izmirly PM, Kim MY, Llanos C et al. Evaluation of the risk of anti-SSA/Ro-SSB/La antibody-associated cardiac manifestations of neonatal lupus in fetuses of mothers with systemic lupus erythematosus exposed to hydroxychloroquine. Ann Rheum Dis 2010;69:1827–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tunks RD, Clowse ME, Miller SG, Brancazio LR, Barker PC. Maternal autoantibody levels in congenital heart block and potential prophylaxis with antiinflammatory agents. Am J Obstet Gynecol 2013;208:64 e1–7. [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Sanchez N, Perez-Pinto S, Robles-Marhuenda A et al. Obstetric and perinatal outcome in anti-Ro/SSA-positive pregnant women: a prospective cohort study. Immunol Res 2017;65:487–494. [DOI] [PubMed] [Google Scholar]
- 27.Barsalou J, Jaeggi E, Laskin CA et al. Prenatal exposure to antimalarials decreases the risk of cardiac but not non-cardiac neonatal lupus: a single-centre cohort study. Rheumatology (Oxford) 2017;56:1552–1559. [DOI] [PubMed] [Google Scholar]
- 28.Barsalou J, Costedoat-Chalumeau N, Berhanu A et al. Effect of in utero hydroxychloroquine exposure on the development of cutaneous neonatal lupus erythematosus. Ann Rheum Dis 2018. [DOI] [PMC free article] [PubMed]
- 29.Simon R Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989;10:1–10. [DOI] [PubMed] [Google Scholar]
- 30.Friedman DM, Kim MY, Copel JA, Llanos C, Davis C, Buyon JP. Prospective evaluation of fetuses with autoimmune-associated congenital heart block followed in the PR Interval and Dexamethasone Evaluation (PRIDE) Study. Am J Cardiol 2009;103:1102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costedoat-Chalumeau N, Galicier L, Aumaitre O et al. Hydroxychloroquine in systemic lupus erythematosus: results of a French multicentre controlled trial (PLUS Study). Ann Rheum Dis 2013;72:1786–92. [DOI] [PubMed] [Google Scholar]
- 32.Noe G, Amoura Z, Combarel D et al. Development and Validation of a Fast Ultra-High Performance Liquid Chromatography-Fluorescent Method for the Quantification of Hydroxychloroquine and Its Metabolites in Patients With Lupus. Ther Drug Monit 2019;41:476–482. [DOI] [PubMed] [Google Scholar]
- 33.Jung SH, Kim KM. On the estimation of the binomial probability in multistage clinical trials. Stat Med 2004;23:881–96. [DOI] [PubMed] [Google Scholar]
- 34.Koyama T, Chen H. Proper inference from Simon’s two-stage designs. Stat Med 2008;27:3145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cuneo BF, Sonesson SE, Levasseur S et al. Home Monitoring for Fetal Heart Rhythm During Anti-Ro Pregnancies. J Am Coll Cardiol 2018;72:1940–1951. [DOI] [PubMed] [Google Scholar]
- 36.Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis 2010;69:20–8. [DOI] [PubMed] [Google Scholar]
- 37.Costedoat-Chalumeau N, Amoura Z, Huong DL, Lechat P, Piette JC. Safety of hydroxychloroquine in pregnant patients with connective tissue diseases. Review of the literature. Autoimmun Rev 2005;4:111–5. [DOI] [PubMed] [Google Scholar]
- 38.Gaffar R, Pineau CA, Bernatsky S, Scott S, Vinet E. Risk of Ocular Anomalies in Children Exposed In Utero to Antimalarials: A Systematic Literature Review. Arthritis Care Res (Hoboken) 2019;71:1606–1610. [DOI] [PubMed] [Google Scholar]
- 39.Vroom F, de Walle HE, van de Laar MA, Brouwers JR, de Jong-van den Berg LT. Disease-modifying antirheumatic drugs in pregnancy: current status and implications for the future. Drug Saf 2006;29:845–63. [DOI] [PubMed] [Google Scholar]
- 40.Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol 2014;132:1453–60. [DOI] [PubMed] [Google Scholar]
- 41.Skorpen CG, Lydersen S, Gilboe IM et al. Influence of disease activity and medications on offspring birth weight, pre-eclampsia and preterm birth in systemic lupus erythematosus: a population-based study. Ann Rheum Dis 2018;77:264–269. [DOI] [PubMed] [Google Scholar]
- 42.Clowse ME, Magder L, Witter F, Petri M. Hydroxychloroquine in lupus pregnancy. Arthritis Rheum 2006;54:3640–7. [DOI] [PubMed] [Google Scholar]
- 43.Gotestam Skorpen C, Hoeltzenbein M, Tincani A et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis 2016;75:795–810. [DOI] [PubMed] [Google Scholar]
- 44.Costedoat-Chalumeau N, Amoura Z, Le Thi Hong D et al. Questions about dexamethasone use for the prevention of anti-SSA related congenital heart block. Ann Rheum Dis 2003;62:1010–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 46.Tan EM, Cohen AS, Fries JF et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. [DOI] [PubMed] [Google Scholar]
- 47.Petri M, Orbai AM, Alarcon GS et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vitali C, Bombardieri S, Jonsson R et al. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002;61:554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shiboski CH, Shiboski SC, Seror R et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjogren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis 2017;76:9–16. [DOI] [PubMed] [Google Scholar]
- 50.Friedman D, Lovig L, Halushka M, Clancy RM, Izmirly PM, Buyon JP. No histologic evidence of foetal cardiotoxicity following exposure to maternal hydroxychloroquine. Clin Exp Rheumatol 2017;35:857–859. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.