There is little doubt that biomechanics, considered here as the mechanics of body movement, including neuromuscular control, plays a role in the development of low back pain (LBP) and perhaps in the persistent and/or recurrent nature of this condition.66,100 There is consensus that LBP is a multifactorial problem, and many biopsychosocial factors affect the clinical presentation of LBP and treatment outcomes.19 Although biomechanics is acknowledged to be one aspect of the “bio” component, much of the biomechanics research in LBP has not considered other biopsychosocial factors (that have been discussed since the 1980s121) and their interactions. Notwithstanding all past accomplishments of biomechanics, it is timely to evaluate the role of biomechanics as a stand-alone discipline to address the LBP problem.
This commentary focuses on 2 questions. First, does biomechanics in isolation have the potential to advance treatment of LBP? Second, how likely is it that such an approach would improve treatment strategies for LBP? This commentary is presented as a point-counterpoint discussion. First, we present the view that biomechanics alone cannot lead to improved outcomes for LBP. Second, from among the various models that consider biomechanics, we select 3 that offer very different perspectives on how biomechanics may improve outcomes for LBP by guiding selection of clinical interventions. Third, we present the counterpoint reaction of the authors of the biomechanical models to the presented debate. The overall objective is to foster discussion to encourage fruitful development in this field.
POINT
Consideration of biomechanics alone is unlikely to lead to more effective treatment strategies for LBP. Many elegant biomechanical models of LBP have been developed based on clinical observation, basic research, and discovery of common biomechanical features in samples of individuals with LBP. Various treatment strategies were then designed to address these proposed mechanisms, many of them evaluated in clinical trials. For example, a deficit in the recruitment of the transversus abdominis was proposed in 1996 as an important mechanism associated with LBP.44,123 Additional observations of delayed trunk muscle reflex responses and mathematical formulation of static and dynamic models assessing spinal stability15,18,59 informed the development of various forms of motor control rehabilitation and trunk stabilization exercises.92,97 Other examples of models involving biomechanical factors in LBP include the movement system impairment107,113 and directional preference (eg, McKenzie method for treating LBP) approaches.70 These models are based on sound anatomical, biological, and mechanical principles, which provide a foundation for internal validity. Furthermore, the relationship between pain and the proposed biomechanical measures is supported by a body of research demonstrating, for example, differences between patients with LBP and healthy controls. The development and refinement of models of LBP such as these have increased our knowledge regarding biomechanics and LBP. The critical question is whether these biomechanical representations of LBP can lead to intervention strategies that are superior to other nonsurgical therapies.
Recent systematic reviews of randomized clinical trials (RCTs) have revealed that consideration of biomechanics alone does not produce a uniquely distinct and effective treatment20 (with the exception of exercise therapy, which can affect multiple systems beyond biomechanics114). Although treatment strategies based on these models are generally better than no treatment, clinical trials have generally not shown them to be superior to other forms of exercise or other types of treatment when applied to patients with chronic nonspecific LBP. On this basis, it has been broadly argued that the efficacy of interventions (including those based on biomechanics) would be greater if applied to specific patient subgroups that would be expected to respond best to specific interventions.32,57,91,104 Although identification of subgroups has been a goal of research for some time,31 a successful subgrouping method that guides treatment based on biomechanical factors remains elusive.26,32,57,91
There are several possible reasons why consideration of biomechanics has not led to improved outcomes. First, it seems likely that consideration of factors other than biomechanics will be required for effective patient selection and treatment allocation.42,104 Second, biomechanics-based interventions may not have reached adequate refinement to achieve their highest possible impact. Third, they may only be effective in a very narrow subset of patient presentations, and methods to select those patients may not be realized. Fourth, the identified biomechanical factors may not be the cause of nociceptive input contributing to the pain response, or pain may be continuing for reasons other than ongoing nociceptive input. For example, even when a “provocation” test reproduces a patient’s pain, or local injection of an anesthetic agent reduces pain, it cannot be concluded that the identified motions or structures are responsible for the maintenance of pain34 or, more uncertainly, whether targeting them with some intervention will lead to clinical improvement.13,43
Whether any of these alternatives explains the lack of strong evidence for efficacy of an approach based on biomechanics is not yet clear, but we suggest that the most likely explanation is that LBP is a multifactorial problem, in which any individual factor or mechanism plays a small role in the overall condition, and the outcomes of interventions based on any such mechanisms might easily be obscured. If we consider LBP a truly multifactorial problem17 (FIGURE 1), what potential is there for identification of individual biomechanical factors to play a role in the treatment of LBP? Numerical and analytical simulations of multifactorial presentation of LBP demonstrated that if a large number of factors contribute to an individual’s LBP, then any treatment strategy that seeks and treats the most dominant factor is less effective than treating any 2 or more factors chosen arbitrarily.16 Furthermore, the probability of identifying subgroups that might respond favorably to a specific intervention in such a population approaches zero as the number of factors contributing to the LBP presentation increases (FIGURE 2). If biomechanical factors are intertwined with many other factors across the biopsychosocial domains in individuals with LBP, then the most likely results from RCTs will be the inability to subgroup and effectively treat LBP based purely on biomechanical factors.
FIGURE 1.
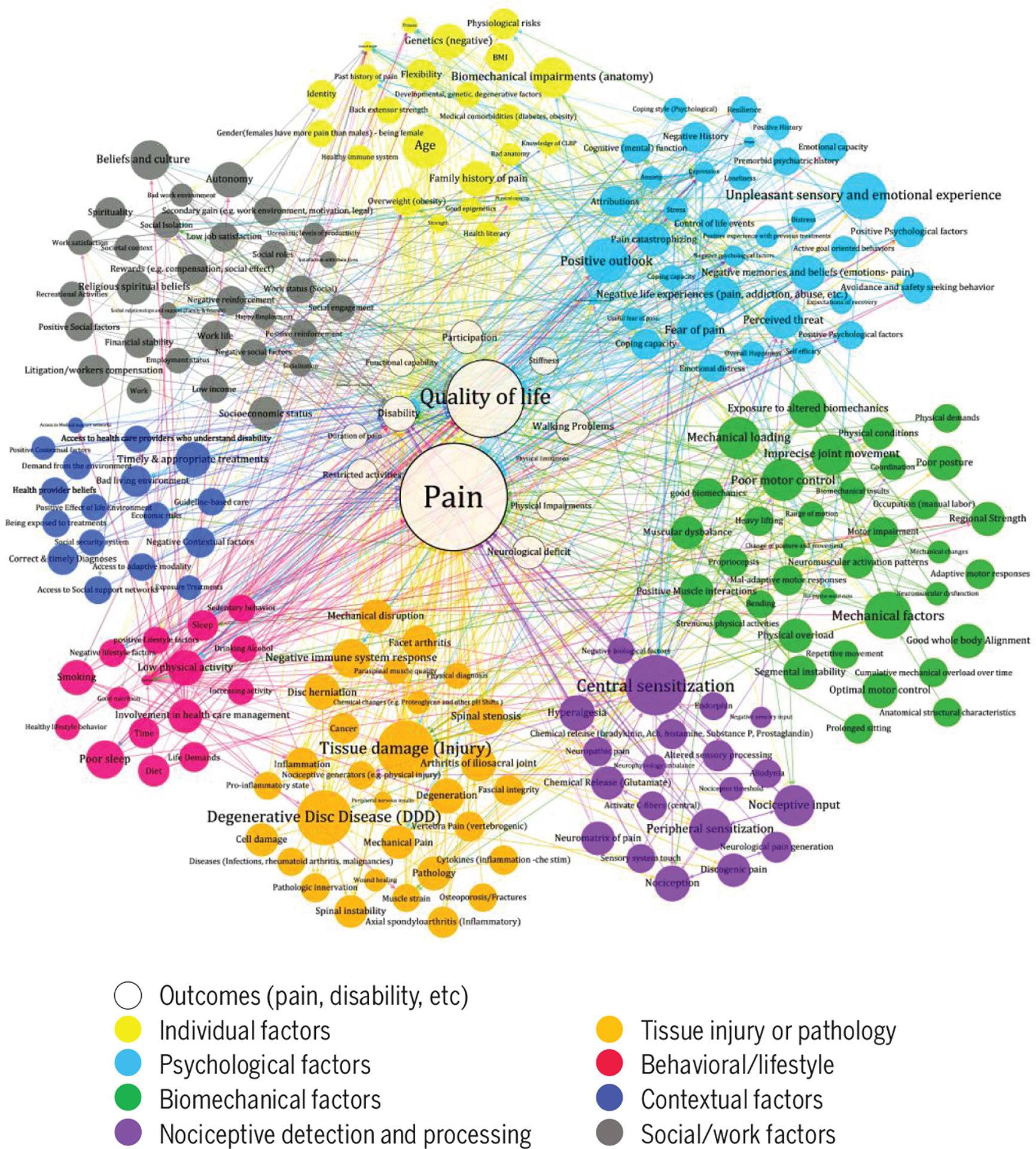
A metamodel illustrating factors (colored circles) contributing to low back pain, disability, quality of life, and other outcomes (white circles) and their interactions (colored lines). This metamodel was constructed with input from the multidisciplinary panel of 27 experts in preparation for the symposium at the 26th Annual Meeting of the North American Spine Society (2017). Diameters of the circles are proportional to the number of experts identifying these factors and the number and strength of connections with other factors.
FIGURE 2.
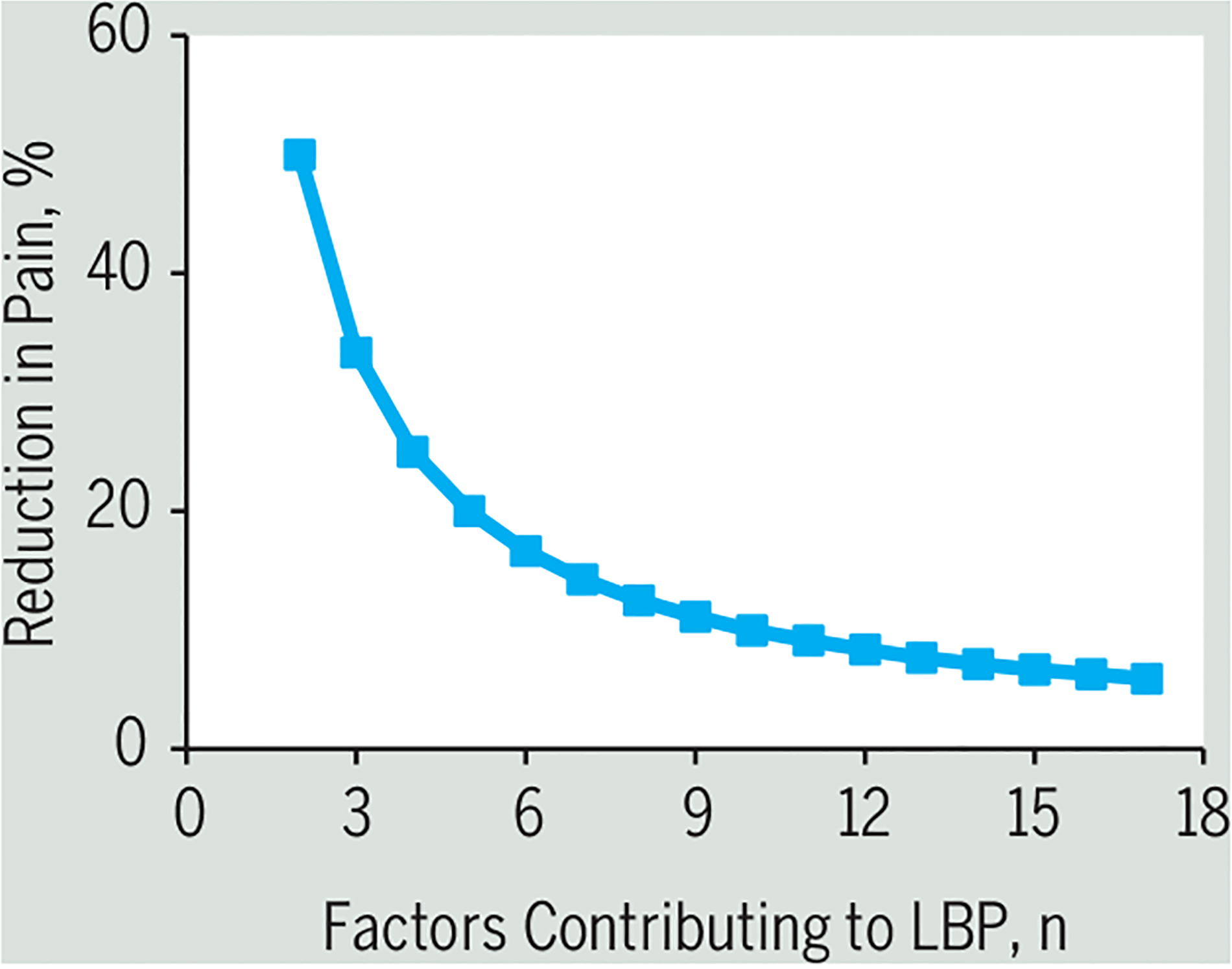
Mathematical simulation of the predicted reduction in pain when the number of factors contributing to LBP that must be addressed with treatment is considered. On the vertical axis is the predicted success when a single factor that contributes the most to LBP is addressed with treatment. As the number of factors contributing to LBP increases, the effectiveness of such an intervention decreases. Abbreviation: LBP, low back pain.
An additional concern about the evaluation of any intervention, including one based on biomechanics, is that the interpretation and synthesis of clinical trials designed to isolate an individual factor or a mechanism of LBP are complicated by nonspecific effects associated with various therapeutic modalities (eg, clinician-patient alliance,30 placebo effects,6 etc). Unfortunately, nonspecific effects of treatment present some unique challenges, because RCTs are ill equipped to estimate or control them.6 For most nonpharmacological interventions, it is not possible to design a double-blinded sham treatment where both the therapist and the patient are blinded as to the treatment administered and received. This problem makes it difficult to estimate the specific effect size attributable to any intervention, including one based on biomechanics.
For all the reasons described above, we argue that it is unlikely that biomechanical factors alone will be able to guide treatment with an effect size greater than that achieved by other therapies. Nevertheless, we believe that biomechanics has its place among the constellation of factors contributing to LBP. The challenge is to identify how best to systematically integrate knowledge from various fields of science and stakeholders, which appears necessary to achieve the goal of more effective management of LBP treatment and reduction in disability.
COUNTERPOINT
Biomechanics research can lead to more effective treatment strategies for LBP.
The following 3 biomechanical models present examples of how biomechanics has been used as a primary feature to guide management of LBP. These examples have been studied extensively and were selected to show the diversity of biomechanical concepts that have been considered.
Biomechanical Model 1: Intervertebral Mechanical Dysfunction in Nonspecific LBP
There is consensus that chronic nonspecific LBP is a biopsychosocial problem. Consideration of psychosocial factors alone explains little of the variance in outcome, and effects are small when they are used to guide treatment.41,86 In the biological domain, there is diversity across chemical, mechanical, and neuroplastic mechanisms, and it is questionable whether a mechanical phenomenon, such as lumbar movements, could alone provide a marker of sufficient influence to identify phenotypes and pain generators in LBP populations.33
As most LBP presentations are affected by movement or position, it is likely that mechanical factors play a role. Attempts have been made to measure mechanical factors to assess whether they are more prevalent in people with LBP than in those without LBP, and whether they cause pain episodes and/or moderate or mediate outcome. This counterpoint presents a view of LBP that includes lumbar segmental mechanics and how mechanical assessments may be used to identify factors important in back pain generation and perpetuation—and therefore in physical treatment.
Measurement of Intervertebral Motion
Lumbar segmental motion can be measured by digital registration of the positions of individual vertebral segments on fluoroscopic sequences, and the movement between them. This technique has been validated.7,10–12,28,78–80,102,125 Kinematic variables include intervertebral range of motion, anterior/posterior translation, disc height, finite center of rotation, intervertebral laxity (as initial rotational attainment rate), phase shift, and proportional motion pattern variability. There is little evidence that intervertebral range of motion, translation, disc height change, and laxity are different between people with and without LBP, but there is some evidence that translation and laxity are relevant for those with LBP related to injury.54,55,58,85
Proportional motion, representing the sharing of bending across segments, is more variable in people with LBP. Using flexion/extension radiographs, Abbott et al1 demonstrated that patients with LBP had proportional intervertebral range of motion and translation ranges outside of reference intervals of pain-free controls. Continuous fluoroscopic sequences that combine left and right and flexion and extension motion into a single summary measure of the variability of motion sharing show differences between patients with LBP and matched controls.80 Results have been replicated and expanded in recent studies (FIGURE 3).8,9
FIGURE 3.
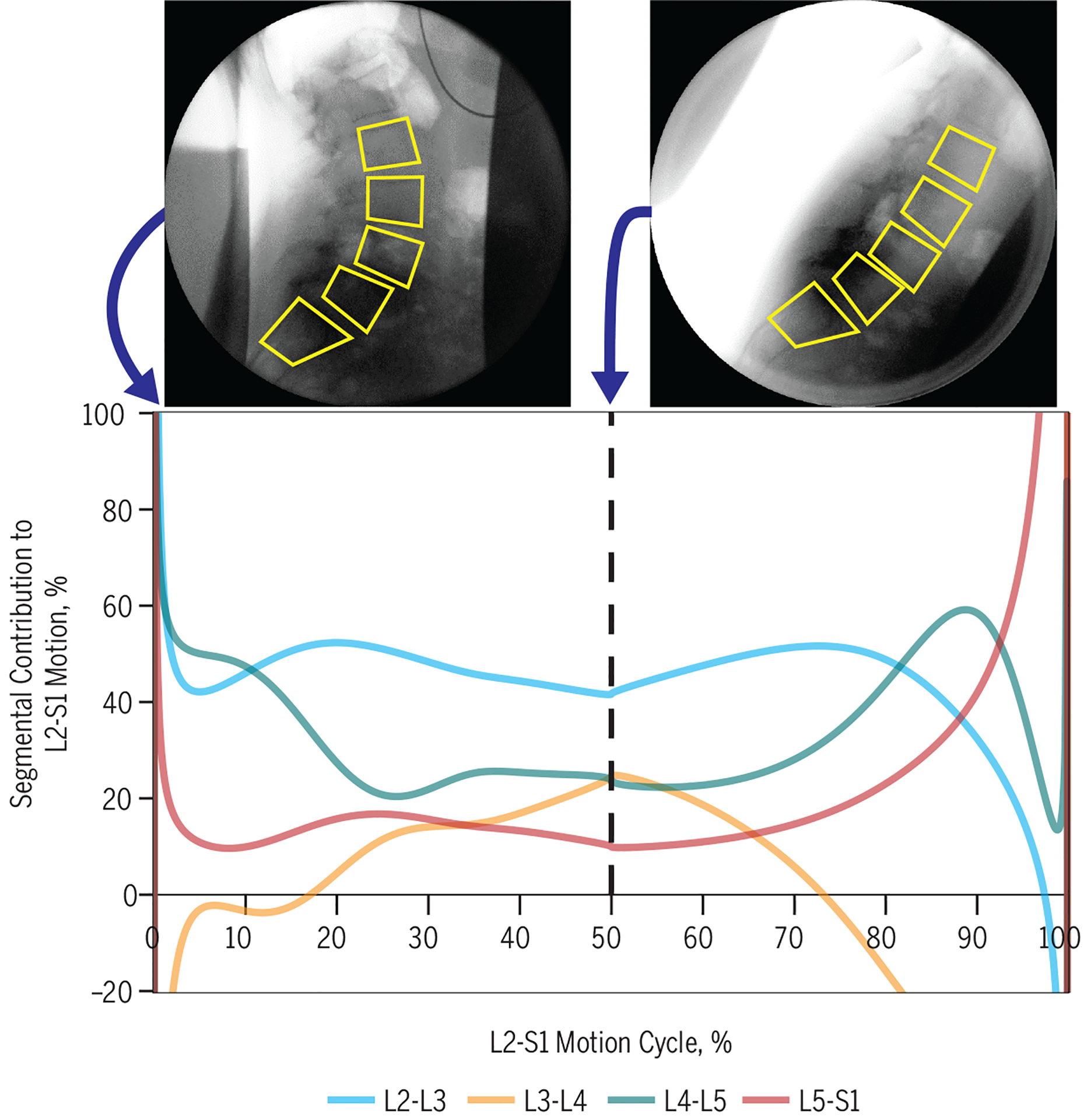
Continuous proportional weight-bearing flexion intervertebral motion in a 63-year-old female patient with spondylolisthesis. Note that the segmental contributions to the total L2-S1 motion change continuously. On average, L2-L3 makes a higher contribution than the upper reference range of a control population, and the L4-L5 average share is in the normal range.
Implications
The observation that motion sharing variability is significantly greater in people with LBP than in controls suggests that it is worthy of exploration as a biomechanical marker, and perhaps a moderator, of LBP outcomes. Good intrasubject reliability over 6 weeks makes it a suitable measure to identify subgroups. Poor agreement parameters suggest that it may be unsuitable as a mediator or outcome measure.24 Because data can be recorded during passive recumbent lumbar motion and during standardized weight-bearing active motion, results can be extrapolated to make inferences about both intervertebral restraint (passive mode) and performance (upright mode).
Uneven motion sharing at segmental levels is a consequence of heterogeneity in passive system restraint. This may be the result of structural factors, such as disc degeneration or ligament tighten ing.63 If these are related to abnormal motion patterns, then treatment options that alter the patterns could be tested as interventions. Likewise, relationships between aberrant motion in the upright position and the presence of LBP could be interpreted to suggest that pain is generated by the effects of untoward muscular effort and/or inconsistency of loading, where metabolic deficit in the former, or rapid displacements in the latter, could be responsible. Either way, they relate to the extent of control that is achieved at segmental levels.
The case for consideration of the importance of this biomechanical factor in guiding LBP management is supported by evidence of differences between patients and controls without any stratification based on factors in other biopsychosocial domains. The biomechanical model may benefit from expansion to include more diverse variables such as muscle metabolic factors,27 inflammatory markers,65 mathematical modeling to infer loads with input from combined quantitative fluoroscopy and 3-D magnetic resonance imaging,126 muscle activation patterns, lumbar configuration (eg, lordotic curve), and response to perturbation and changes in balance. Consideration of relationships between segmental motion patterns and patient-reported data (directional preference, pain impact, kinesiophobia, distress, and somatization) may also be important to outcomes.
Summary
Mechanical dysfunction has been rightly called into doubt as a single explanatory variable for LBP, and the view that a biological approach alone is inadequate is indisputable.124 However, through the mist of complexity, biomechanical features cannot be discounted, especially if they are measurable, frequently present, and able to discriminate individuals with LBP from pain-free individuals.
Biomechanical Model 2: The Kinesiopathologic Model
The kinesiopathologic model was designed specifically to describe the mechanically related processes proposed to contribute to the development and course of LBP (FIGURE 4). The basic premise is that LBP results from the repeated use of direction-specific (flexion, extension, rotation, lateral bending, or a combination of these) stereotypic movement and alignment patterns in the lumbar spine. The model proposes that the patterns begin as the result of adaptations of the musculoskeletal and neural systems due to repeated use of specific movements and alignments during daily activities. The nature and rate of the adaptations can be modified by intrinsic and extrinsic characteristics of the individual, for example, sex, anthropometrics, or typical activities of the person. The typical pattern is one in which, during performance of a movement (eg, forward bending) or assumption of a posture (eg, sitting), the lumbar spine moves into its available range in a specific direction more readily than other joints, such as the knees, hips, or thoracic spine, do.
FIGURE 4.
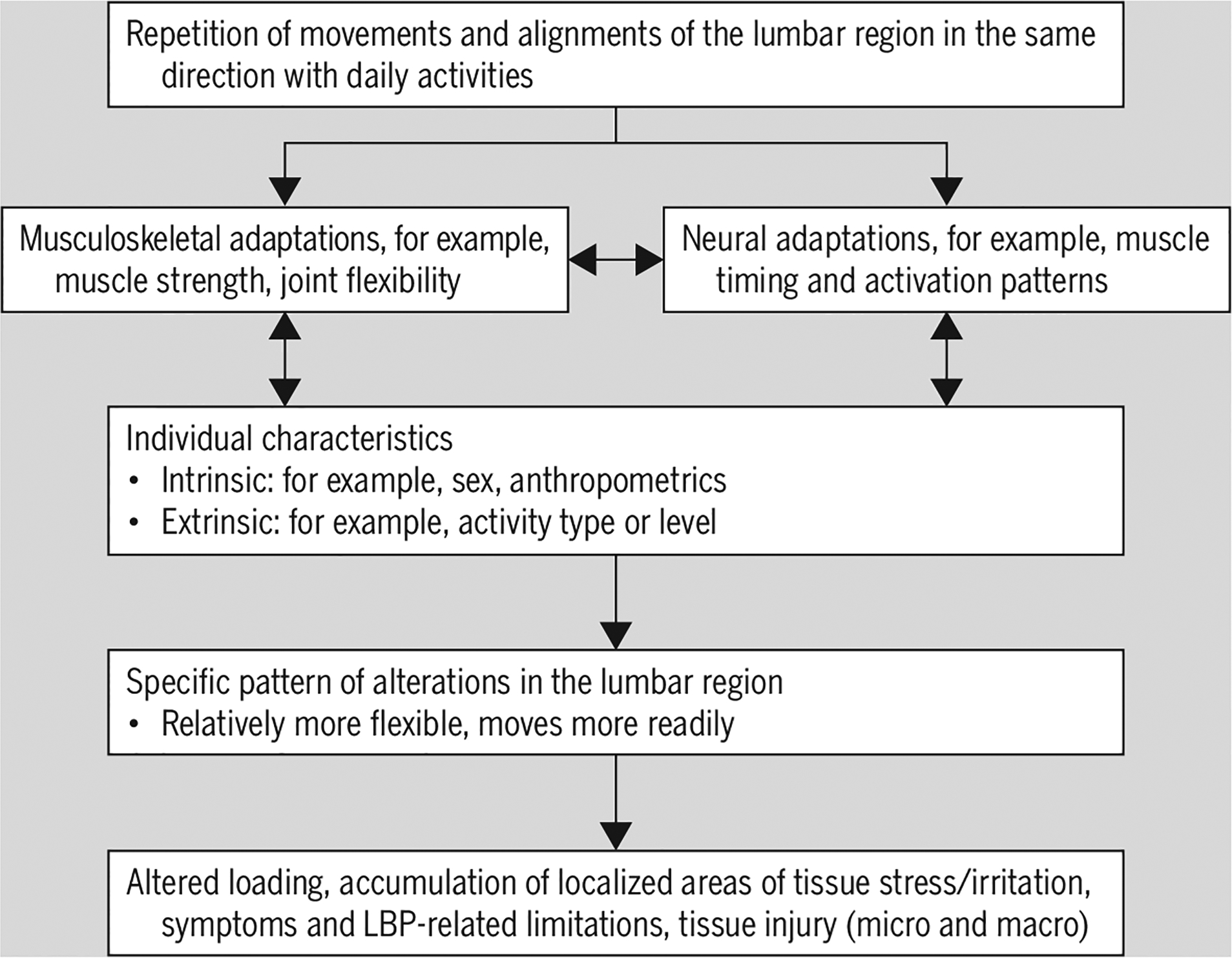
Illustration of the mechanically related processes proposed to contribute to the development and course of LBP based on the kinesiopathologic model. Abbreviation: LBP, low back pain.
In this context, movement occurs sooner or proceeds farther than is ideal. With the repetitive use of the same pattern across multiple activities, some/all of the lumbar joints become relatively more flexible than other joints. Repetitive use of the same relative flexibility pattern(s) decreases variability in the types of lumbar movements and alignments used throughout the day, contributing to repeated subfailure magnitude loading and accumulation of localized areas of lumbar spinal tissue stress. Over time, the rate of accumulation of tissue stress is proposed to be greater than the adaptive tissue remodeling needed to prevent tissue failure. The result is tissue irritation, LBP symptoms, microinjury, and potentially macroinjury. Finally, the model maintains that until the relative flexibility patterns are modified, LBP may persist or recur. The following sections present evidence that supports the model.
Existence of Direction-Specific Relative Flexibility Patterns
A major assumption of the kinesiopathologic model is that people with LBP display direction-specific relative flexibility patterns across clinical tests (eg, forward bend test) and daily activities, and that there are subgroups of people with LBP who differ based on their specific relative flexibility pattern.90
In a study of people with LBP, findings from standardized clinical tests designed to identify relative flexibility patterns111 revealed 3 intercorrelated groupings of tests related to 3 LBP subgroups: lumbar (1) extension, (2) rotation, and (3) rotation with extension.113 Subsequent evidence of examiner reliability to identify relative flexibility patterns and LBP subgroups with clinical tests supports the existence of the patterns and proposed subgroups.39,40,61,69,83,88,103 Additional studies using clinical tests reported that people with LBP display a greater number of relative flexibility findings than people without LBP,68,122 and in people with LBP the prevalence of the patterns differs between sexes.95 Laboratory-based studies quantifying patterns during individual clinical tests have documented more relative flexibility patterns in people with LBP than in people without LBP,29,60,61,76,83,93 subgroup-specific differences in patterns,37,46,60,75,83,105 and a different prevalence of patterns between sexes in those with LBP.36,47,48,83
Laboratory-based studies of patterns during daily activity tests have documented that people with LBP, compared to those without LBP, display a relative flexibility pattern during the activities of picking up an object and stand-to-sit.37,72,96 Further, the relative flexibility pattern with the picking-up-an-object test is used across other daily activity tests71 and is related to the pattern used during the clinical test of forward bending.72 Finally, subgroup-specific differences in relative flexibility patterns have been identified among LBP subgroups with the picking-up-an-object test, aspects of gait, and lumbar alignment in sitting.35,37,46,49
Relevance of Relative Flexibility Patterns to the LBP Condition
A second kinesiopathologic model assumption is that relative flexibility patterns are relevant to the person’s LBP. Two studies reported that systematically modifying relative flexibility patterns displayed during symptom-provoking clinical tests improved reported LBP during the tests.106,112 Within-session treatment of the relative flexibility pattern during the picking-up-an-object test resulted in both an improvement in the pattern and an improvement in reported LBP during the test.73 Moderate to large21 relationships also have been documented between the severity of the relative flexibility pattern across daily activity tests and LBP-related functional limitations.71,72 Finally, relative flexibility patterns during clinical tests are related to the LBP condition severity,25,68,110 a person’s ability to correct the pattern,94 and the risk for LBP development.88,98,99
Repetitive Activities and Relative Flexibility Patterns
A third assumption is that relative flexibility patterns are, in part, a consequence of repetition of movements and sustained alignments in the same direction with activities people perform regularly. Two studies of people with LBP showed that the type89,109 and number109 of relative flexibility patterns were related to an individual’s regular leisure activity. Another study compared findings during clinical tests among people without LBP who did not participate in a rotational sport and 2 groups who participated in a rotational sport—people with and people without LBP. People who participated in the sport (LBP and no LBP) displayed a greater number and asymmetry of relative flexibility patterns associated with lumbar rotation than people without LBP who did not participate in the sport.122 Of those who played the sport, those with LBP displayed more relative flexibility patterns with tests of limb movements122 and spent a greater proportion of their leisure time playing the sport than those without LBP.14
Summary
The kinesiopathologic model describes the mechanically related processes proposed to contribute to the development and course of LBP. A primary process proposed to contribute to LBP is the tendency for 1 or more of the lumbar joints to move more readily than other joints in a specific direction (ie, a relative flexibility pattern) when performing daily activities. Numerous studies support that relative flexibility patterns are prevalent in LBP, relevant to the person’s LBP condition, associated with increased risk of LBP, and associated with the activities people participate in regularly. Collectively, these findings suggest that targeting symptom-provoking, relative flexibility patterns used during repetitive daily activities may be an effective, efficient, and feasible method to improve outcomes and, potentially, maintain the improvement over time.
Biomechanical Model 3: Anatomy, Biomechanics, and Pathology of the Sacroiliac Joints
One specific subgroup of patients with lumbopelvic pain with a clearly defined anatomical/biomechanical model involves those with sacroiliac joint (SIJ) involvement. The SIJs are highly specialized joints that permit stable (yet flexible) upper-body support. In bipeds, the pelvis serves as a platform with 3 large levers acting on it (spine and legs). The tightness of the well-developed fibrous apparatus and the specific SIJ architecture limit mobility. Sacral movement involves the SIJ and directly influences the discs and higher lumbar joints; for example, forward and backward tilting of the sacrum between the iliac bones affects the joints between L5 and S1, as well as higher spinal levels.119,120
The SIJ is unique, with elements of a combined synarthrosis and diarthrosis, that is, amphiarthrosis (FIGURE 5). The joint’s main portion is surrounded by a complex capsule and lined with cartilage (diarthrosis). Its shape is auricular, and “opens” posteriorly. The sacrum and ilia have an extracapsular, dorsally located articulation (synarthrosis), which is augmented by the vast interosseous ligament that provides considerable internal stability. The SIJ is encased in a capsule, with a smooth anterior wall and irregular bands/ligaments comprising the posterior wall.118
FIGURE 5.
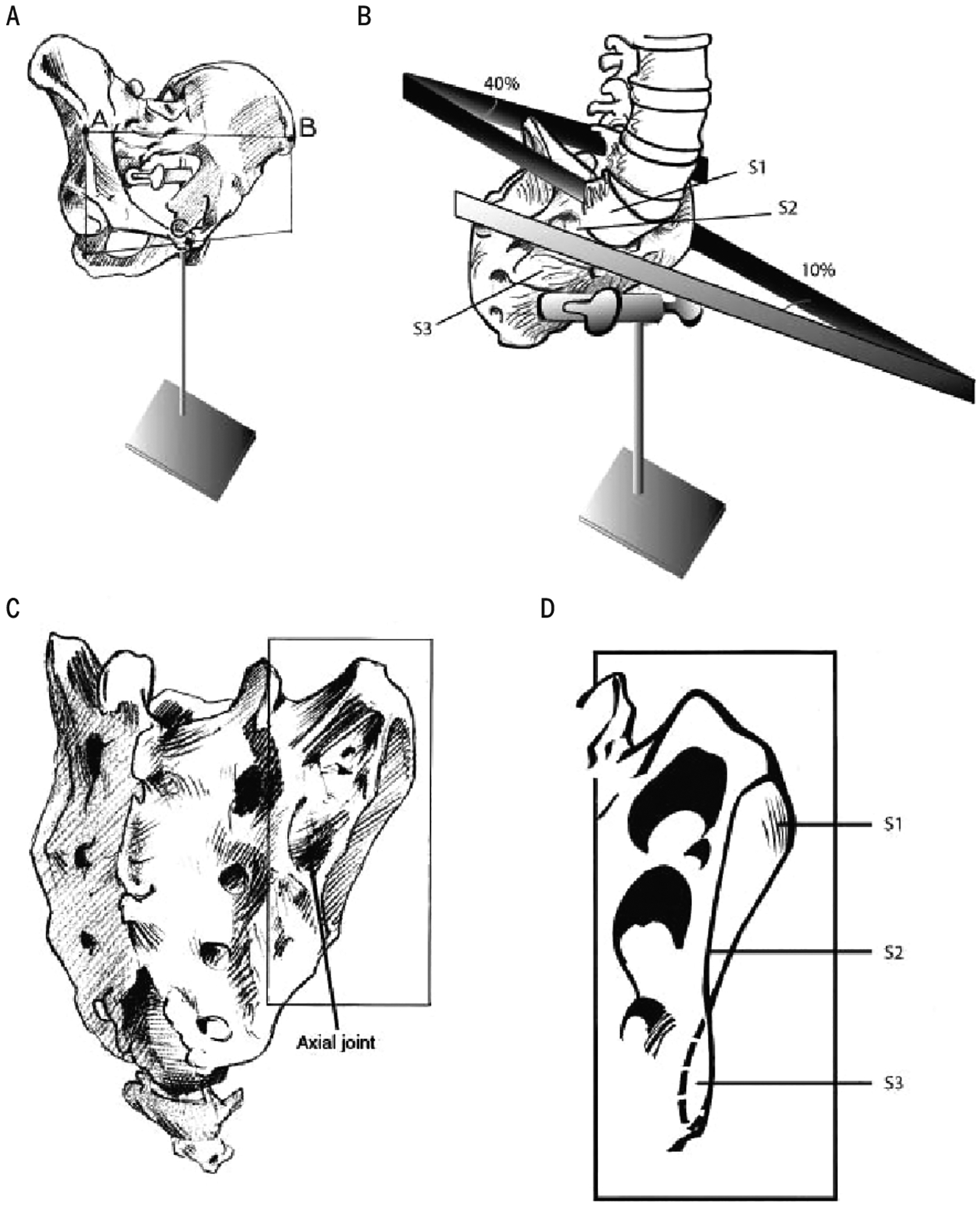
(A) The pelvis in erect posture. (B) View of the sacrum from the ventrolateral side, showing the different angles between left and right sacral articular surfaces. (C) Dorsolateral view of the sacrum. The pointer indicates a cavity in the sacrum, in which an iliac tubercle fits, called the “axial” sacroiliac joint. (D) Sacral articular surface at the right side. The different angles reflect the propeller-like shape of an adult sacroiliac joint.
Besides small internal pelvic motions of the SIJ and symphysis, substantial motion of the external pelvic platform takes place. Movements of the pelvic platform upon the hip joints, such as flexion and extension (pelvic anteversion and retroversion), rotation, and abduction/adduction, strongly influence lumbar and SIJ movement.118 Coupled hip flexion and extension play a key role in establishing lower spine lordosis and kyphosis.64 The SIJs are postulated to act as important stress relievers in the “force-motion” relationships between the trunk and legs. These joints ensure that the pelvic girdle is not a solid ring of bone that could easily fracture under the great forces to which it might be subjected, either from trauma or its many bipedal functions.67 Analysis of gait mechanics demonstrates that the SIJs provide sufficient flexibility for the intrapelvic forces to be transferred effectively to and from the lumbar spine and legs.64
The ventrally directed angle between L5 and the sacrum tends to become more acute when loaded as the sacrum nutates. Accordingly, the thick anterior longitudinal ligament spans the ventral aspect of L5 and S1, buttressing against excessive extension.118
Biomechanical calculations show the influence that a higher friction coefficient and greater wedge angle of the sacrum have on SIJ stability.119,120 It was suggested that during juvenile growth, lever arms like the spine and legs generate an increasing force until full body weight is reached. Consequently, the SIJ is dynamically modified by changing form closure in the direction and strength of imposed forces.120 Disturbed or excessive force transfer through the SIJ can exaggerate compressional or torsional stresses on these joints. Such altered transmission to the spine and legs can cause tissue effects with deleterious consequences.23,74
In contrast to excessive SIJ force closure, a counter-opposing condition of diminished stability occurs in pregnancy-related pelvic girdle pain (PGP).117 Insufficient and/or asymmetric compression of the SIJs can result in PGP.22,81,119,120 Nonoptimal load transfers and clinical effects would be expected to occur from either the suspected excessive pelvic and SIJ stiffness64,74 or the documented insufficient pelvic girdle stability with PGP.81,117 Sufficient SIJ force closure can be defined as the amount needed to provide the necessary stiffness for the particular demands of static or dynamic load transfer, at optimal utilization of energy.115,117,120 Thus, stability is an instantaneous phenomenon and is antagonistic to instability.77 Neither too little nor too much SIJ stability, from either mechanical stiffness properties or force closure/compression, is optimal.118
Biomechanics and PGP
European guidelines117 for PGP define sacroiliac and symphyseal pain: “PGP generally arises in relation to pregnancy, trauma, arthritis and osteoarthritis. Pain is experienced between the posterior iliac crest and the gluteal fold, particularly in the vicinity of the SIJ. The pain may radiate in the posterior thigh and can also occur in conjunction with/or separately in the symphysis. The endurance capacity for patients standing, walking and sitting is diminished. The diagnosis of PGP can be reached after exclusion of lumbar causes. The pain of functional disturbances in relation to PGP must be reproducible by evidence based specific clinical tests.”
The following clinical tests are recommended for PGP (European guideline117): the posterior pelvic pain provocation test (P4/thigh thrust test), the Patrick (flexion, abduction, and external rotation) test, the Gaenslen test, pain with palpation of the long dorsal SIJ ligament, the symphysis palpation test, and the modified Trendelenburg test. The recommended functional test is the active straight leg raise.
The biomechanical model of PGP is based on the concept that altered motor function of the deep abdominal muscles in PGP leads to insufficient bracing of the SIJs/pelvis. This is combined with weakened erector muscles and fascia over the lower lumbar spine and SIJ.118 Studies demonstrate that, among other muscles, contraction of the internal oblique and transversus abdominis results in force closing of the pelvic ring.4,5,52,53 Conversely, in patients with PGP, a maladaptive compensatory pattern occurs, characterized by diminished activity of these muscles and a subsequent failure to brace the pelvis.52
As evidence in support of the biomechanical model, Sturesson et al101 showed that painful movement in patients with the most severe PGP can be instantly reduced by an external Hoffmann-Slätis surgical frame. This finding agrees with studies using pelvic belts to normalize SIJ movement.81,82,119 Application of the external fixator in patients with chronic severe PGP generates an anterior compression on the ilia, leading to effective force closure.101 Using Roentgen stereophotogram-metric analysis, precise measurement shows how the surgical frame changes the position of the SIJ from counternutation to nutation, which reduces pain in the long dorsal ligament and, possibly, deeper dorsal SIJ ligaments.101,118 When successful external frame application is verified with diminished pain after several weeks, the frame is removed, followed by surgical SIJ arthrodesis. This methodology is only indicated in patients with severe PGP who failed intense rehabilitation, and with confirmation of the appropriateness of SIJ arthrodesis by a successful fixator frame trial. It is important to note that activation of transversely oriented abdominal muscles also reduces SIJ laxity,87 providing the foundation for an active approach to control these joints as an alternative to surgery for some.
In conclusion, as in other joints, the SIJ is highly innervated from L3 to S2, and when PGP occurs,118 the dorsal SIJ ligaments especially are targeted. This represents a clearly defined biomechanical subgroup of pain.
COUNTERPOINT RESPONSE
Authors of the point and counterpoint agree that chronic nonspecific LBP has biological, psychological, and social components to various extents in different people, and that biomechanics plays a role in the development of LBP. None of the biomechanical models presented above suggests that biomechanics research alone should be the desired approach. Whichever treatment approach is decided upon, it seems obvious that it should be based on a biopsychosocial assessment that includes biomechanics. The question, therefore, is not whether biomechanics alone should be the desired approach, but whether biomechanics is a sufficiently dominant factor in a high-enough proportion of cases to make it worth including as an explanation for pain generation, moderation, or mediation in LBP.
There are 2 primary sources of evidence to suggest that biomechanics is a sufficiently dominant factor in LBP. The first is that most LBP is aggravated or relieved by movements and postures. For example, there are reports that systematically correcting biomechanical impairments during symptom-provoking movements and postures results in an immediate improvement in LBP symptoms.73,106,112 Treatment directed at correcting the impairments, particularly training a person to make corrections during performance of daily activities, results in short- and long-term improvements in both functional and biomechanical outcomes.45,50,51,62,107,108 Importantly, people with LBP also are more likely to adhere to correcting performance of movements and postures during daily activities than traditional therapeutic exercise.107 Thus, if a biomechanical impairment contributes to a patient’s LBP presentation, then training the patient to correct the impairment during daily activities should facilitate both short- and long-term improvement because of the repeated opportunity to practice across the day.
The second source is the discovery of measurable biomechanical markers for LBP (eg, differences in intervertebral motion8,9,80). As for neuropathological and vascular disorders, the pursuit of quantitative biomarkers for LBP should continue and should include biomechanics. These should join research into inflammatory, neuropathic, and muscle metabolic markers, to name a few, to give a more complete picture of LBP by correlating them with each other and with symptomatology, clinical examination findings, and outcomes.
Subgroups of patients with LBP for use in RCTs based on mechanical biomarkers will require more and better research into their identification, validation, and interactions, as well as their roles as prognostic factors, moderators, and mediators. Subgrouping will also require greater sophistication in methods and a move beyond surface markers placed on the skin overlying the spine in the laboratory and cadaveric studies. There have been calls for biomechanics research to address dynamic, multiseg-mental issues in vivo as well as in cadaveric models.3,56,84 Although it seems to be agreed that biomechanics research has not yet reached adequate refinement to achieve its highest impact, we argue that it is on its way and will likely be relevant to more than a narrow subset of patient presentations.
We offer the following to address the 2 questions posed initially. In response to the question of whether current biomechanics research has the potential to advance treatment of LBP, it appears that the answer is yes, but mainly recently and not to the exclusion of other factors. In response to whether this will lead to better treatment strategies for LBP, the answer is quite likely, so long as individualized and intrinsic biomechanics is investigated with sufficient depth and rigor.
CONCLUSION
THREE MODELS PRESENTED IN THE counterpoint to illustrate how biomechanics is being used to understand the problem of LBP and guide treatment are based on sound anatomical, biological, and mechanical principles, which ensures internal validity. Furthermore, the described relationship between pain and biomechanical measures is supported by a body of research demonstrating, for example, differences between patients with LBP and controls. Each author has contributed to new knowledge by developing and refining these models of LBP. However, the question still remains whether these predominantly biomechanical representations of LBP can lead to intervention strategies that are superior to other interventions known to have only small to moderate effects on pain for a broad spectrum of patients.5
Whether any of these approaches, or others, based on biomechanics can advance outcomes is not yet clear. All do include some consideration of factors beyond biomechanics. This concurs with the view that a reductionist approach focusing only on biomechanics will not provide the solution for the LBP problem, and further underscores the need for a multidisciplinary approach. If LBP is indeed a very complex, multifactorial problem, then a much broader view, such as a systems approach,2,38 that accommodates other biopsychosocial factors and their interactions, even when biomechanical issues may be dominant presenting factors, is necessary. Work is under way to truly integrate understanding from across the diverse biopsychosocial domains,17 and approaches are being proposed to achieve this integration in guiding understanding and management of LBP.13,42,43,116 This new approach must consider the massively multifactorial character of LBP, including nonspecific treatment effects of various therapies for LBP.
SYNOPSIS:
Although biomechanics plays a role in the development and perhaps the persistent or recurrent nature of low back pain (LBP), whether biomechanics alone can provide the basis for intervention is debated. Biomechanics, which refers to the mechanics of the body, including its neuromuscular control, has been studied extensively in LBP. But, can gains be made in understanding LBP by research focused on this component of biology in the multifactorial biopsychosocial problem of LBP? This commentary considers whether biomechanics research has the potential to advance treatment of LBP, and how likely it is that this research will lead to better treatment strategies. A point-counterpoint format is taken to present both sides of the argument. First, the challenges faced by an approach that considers biomechanics in isolation are presented. Next, we describe 3 models that place substantial emphasis on biomechanical factors. Finally, reactions to each point are presented as a foundation for further research and clinical practice to progress understanding of the place for biomechanics in guiding treatment of LBP.
ACKNOWLEDGMENTS:
The forum on which this body of research was based, “State-of-the-Art in Motor Control and Low Back Pain: International Clinical and Research Expert Forum,” was supported by the National Health and Medical Research Council of Australia, in collaboration with the North American Spine Society. The forum was chaired by Dr Paul Hodges.
Drs Cholewicki, Reeves, and Popovich, Jr. were partially supported by National Center for Complementary and Integrative Health grant U19AT006057-01A1 from the US National Institutes of Health. Dr Reeves is the founder and president of Sumaq Life LLC. Dr Breen’s work is supported by the European Academy of Chiropractic, the UK Chiropractic Research Council, and the UK National Institute for Health Research. Dr Sahrmann receives book royalties from Elsevier. She receives honoraria, and her travel costs are reimbursed for teaching continuing education programs. Dr van Dillen was supported, in part, by funding from the National Center for Medical Rehabilitation Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, US National Institutes of Health (grant R01 HD047709). Dr Hodges receives book royalties from Elsevier. Professional and scientific bodies have reimbursed him for travel costs related to presentation of research on pain, motor control, and exercise therapy at scientific conferences/symposia. He has received fees for teaching practical courses on motor control training. He is also supported by a Senior Principal Research Fellowship from the National Health and Medical Research Council of Australia (APP1102905).
Footnotes
The authors certify that they have no affiliations with or financial involvement in any organization or entity with a direct financial interest in the subject matter or materials discussed in the article.
REFERENCES
- 1.Abbott JH, Fritz JM, McCane B, et al. Lumbar segmental mobility disorders: comparison of two methods of defining abnormal displacement kinematics in a cohort of patients with non-specific mechanical low back pain. BMC Musculoskelet Disord. 2006;7:45 10.1186/1471-2474-7-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn AC, Tewari M, Poon CS, Phillips RS. The limits of reductionism in medicine: could systems biology offer an alternative? PLoS Med 2006;3:e208 10.1371/journal.pmed.0030208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barz T, Melloh M, Lord SJ, Kasch R, Merk HR, Staub LP. A conceptual model of compensation/ decompensation in lumbar segmental instability. Med Hypotheses. 2014;83:312–316. 10.1016/j.mehy.2014.06.003 [DOI] [PubMed] [Google Scholar]
- 4.Beales DJ, O’Sullivan PB, Briffa NK. The effects of manual pelvic compression on trunk motor control during an active straight leg raise in chronic pelvic girdle pain subjects. Man Ther. 2010;15:190–199. 10.1016/j.math.2009.10.008 [DOI] [PubMed] [Google Scholar]
- 5.Beales DJ, O’Sullivan PB, Briffa NK. Motor control patterns during an active straight leg raise in chronic pelvic girdle pain subjects. Spine (Phila Pa 1976). 2009;34:861–870. 10.1097/BRS.0b013e318198d212 [DOI] [PubMed] [Google Scholar]
- 6.Bialosky JE, Bishop MD, Penza CW. Placebo mechanisms of manual therapy: a sheep in wolf’s clothing? J Orthop Sports Phys Ther. 2017;47:301–304. 10.2519/jospt.2017.0604 [DOI] [PubMed] [Google Scholar]
- 7.Breen A, Breen A. Accuracy and repeatability of quantitative fluoroscopy for the measurement of sagittal plane translation and finite centre of rotation in the lumbar spine. Med Eng Phys. 2016;38:607–614. [DOI] [PubMed] [Google Scholar]
- 8.Breen A, Breen A. Uneven intervertebral motion sharing is related to disc degeneration and is greater in patients with chronic, non-specific low back pain: an in vivo, cross-sectional cohort comparison of intervertebral dynamics using quantitative fluoroscopy. Eur Spine J. 2018;27:145–153. 10.1007/s00586-017-5155-y [DOI] [PubMed] [Google Scholar]
- 9.Breen A, Mellor F, Breen A. Aberrant intervertebral motion in patients with treatment-resistant nonspecific low back pain: a retrospective cohort study and control comparison. Eur Spine J. 2018;27:2831–2839. 10.1007/s00586-018-5666-1 [DOI] [PubMed] [Google Scholar]
- 10.Breen AC, Dupac M, Osborne N. Attainment rate as a surrogate indicator of the intervertebral neutral zone length in lateral bending: an in vitro proof of concept study. Chiropr Man Therap. 2015;23:28 10.1186/s12998-015-0073-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breen AC, Muggleton JM, Mellor FE. An objective spinal motion imaging assessment (OSMIA): reliability, accuracy and exposure data. BMC Musculoskelet Disord. 2006;7:1 10.1186/1471-2474-7-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breen AC, Teyhen DS, Mellor FE, Breen AC, Wong KW, Deitz A. Measurement of intervertebral motion using quantitative fluoroscopy: report of an international forum and proposal for use in the assessment of degenerative disc disease in the lumbar spine. Adv Orthop 2012;2012:802350 10.1155/2012/802350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brumagne S, Diers M, Danneels L, Moseley GL, Hodges PW. Neuroplasticity of sensorimotor control in low back pain. J Orthop Sports Phys Ther. 2019;49:402–414. 10.2519/jospt.2019.8489 [DOI] [PubMed] [Google Scholar]
- 14.Chimenti RL, Scholtes SA, Van Dillen LR. Activity characteristics and movement patterns in people with and people without low back pain who participate in rotation-related sports. J Sport Rehabil. 2013;22:161–169. 10.1123/jsr.22.3.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cholewicki J, McGill SM. Mechanical stability of the in vivo lumbar spine: implications for injury and chronic low back pain. Clin Biomech (Bristol, Avon) 1996;11:1–15. 10.1016/0268-0033(95)00035-6 [DOI] [PubMed] [Google Scholar]
- 16.Cholewicki J, Pathak PK, Reeves NP, Popovich JM, Jr. Model simulations challenge reductionist research approaches to studying chronic low back pain. J Orthop Sports Phys Ther. 2019;49:477–481. 10.2519/jospt.2019.8791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cholewicki J, Popovich JM Jr., Aminpour P, Gray SA, Lee AS, Hodges PW. Development of a collaborative model of low back pain: report from the 2017 NASS consensus meeting. Spine J In press. 10.1016/j.spinee.2018.11.014 [DOI] [PubMed] [Google Scholar]
- 18.Cholewicki J, Silfies SP, Shah RA, et al. Delayed trunk muscle reflex responses increase the risk of low back injuries. Spine (Phila Pa 1976). 2005;30:2614–2620. 10.1097/01.brs.0000188273.27463.bc [DOI] [PubMed] [Google Scholar]
- 19.Cholewicki J, van Dieën JH, Arsenault AB. Muscle function and dysfunction in the spine. J Electromyogr Kinesiol. 2003;13:303–304. 10.1016/S1050-6411(03)00038-5 [DOI] [PubMed] [Google Scholar]
- 20.Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492–504. 10.7326/0003-4819-147-7-200710020-00007 [DOI] [PubMed] [Google Scholar]
- 21.Cohen J Statistical Power Analysis for the Behavioral Sciences. 2nd ed Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 22.Damen L, Buyruk HM, Güler-Uysal F, Lotgering FK, Snijders CJ, Stam HJ. Pelvic pain during pregnancy is associated with asymmetric laxity of the sacroiliac joints. Acta Obstet Gynecol Scand. 2001;80:1019–1024. 10.1034/j.1600-0412.2001.801109.x [DOI] [PubMed] [Google Scholar]
- 23.DeRosa C, Porterfield JA. Anatomical linkages and muscle slings of the lumbopelvic region In: Vleeming A, Mooney V, Stoeckart R, eds. Movement, Stability and Lumbopelvic Pain: Integration of Research and Therapy. 2nd ed Edinburgh, UK: Elsevier/Churchill Livingstone; 2007:47–62. [Google Scholar]
- 24.de Vet HC, Terwee CB, Knol DL, Bouter LM. When to use agreement versus reliability measures. J Clin Epidemiol 2006;59:1033–1039. 10.1016/j.jclinepi.2005.10.015 [DOI] [PubMed] [Google Scholar]
- 25.De Vito G, Meroni R, Lanzarini C, Barindelli G, Della Morte GC, Valagussa G. Pelvic rotation and low back pain [abstract]. 6th Interdisciplinary World Congress on Low Back and Pelvic Pain; November 7–10, 2007; Barcelona, Spain. [Google Scholar]
- 26.Deyo RA, Dworkin SF, Amtmann D, et al. Report of the NIH Task Force on Research Standards for Chronic Low Back Pain. Spine (Phila Pa 1976). 2014;39:1128–1143. 10.1097/BRS.0000000000000434 [DOI] [PubMed] [Google Scholar]
- 27.Dickx N, D’Hooge R, Cagnie B, Deschepper E, Verstraete K, Danneels L. Magnetic resonance imaging and electromyography to measure lumbar back muscle activity. Spine (Phila Pa 1976). 2010;35:E836–E842. 10.1097/BRS.0b013e3181d79f02 [DOI] [PubMed] [Google Scholar]
- 28.du Rose A, Breen A. Relationships between lumbar inter-vertebral motion and lordosis in healthy adult males: a cross sectional cohort study. BMC Musculoskelet Disord. 2016;17:121 10.1186/s12891-016-0975-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esola MA, McClure PW, Fitzgerald GK, Siegler S. Analysis of lumbar spine and hip motion during forward bending in subjects with and without a history of low back pain. Spine (Phila Pa 1976) 1996;21:71–78. [DOI] [PubMed] [Google Scholar]
- 30.Ferreira PH, Ferreira ML, Maher CG, Refshauge KM, Latimer J, Adams RD. The therapeutic alliance between clinicians and patients predicts outcome in chronic low back pain. Phys Ther. 2013;93:470–478. 10.2522/ptj.20120137 [DOI] [PubMed] [Google Scholar]
- 31.Foster NE, Dziedzic KS, van der Windt DA,Fritz JM, Hay EM. Research priorities for non-pharmacological therapies for common musculoskeletal problems: nationally and internationally agreed recommendations. BMC Musculoskelet Disord. 2009;10:3 10.1186/1471-2474-10-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foster NE, Hill JC, Hay EM. Subgrouping patients with low back pain in primary care: are we getting any better at it? Man Ther 2011;16:3–8. 10.1016/j.math.2010.05.013 [DOI] [PubMed] [Google Scholar]
- 33.Foster NE, Pincus T, Underwood MR, Vogel S, Breen A, Harding G. Understanding the process of care for musculoskeletal conditions—why a biomedical approach is inadequate. Rheumatology (Oxford). 2003;42:401–404. 10.1093/rheumatology/keg165 [DOI] [PubMed] [Google Scholar]
- 34.Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69:119–130. 10.1037/a0035514 [DOI] [PubMed] [Google Scholar]
- 35.Gombatto SP, Brock T, DeLork A, Jones G, Madden E, Rinere C. Lumbar spine kinematics during walking in people with and people without low back pain. Gait Posture. 2015;42:539–544. 10.1016/j.gaitpost.2015.08.010 [DOI] [PubMed] [Google Scholar]
- 36.Gombatto SP, Collins DR, Sahrmann SA, Engsberg JR, Van Dillen LR. Gender differences in pattern of hip and lumbopelvic rotation in people with low back pain. Clin Biomech (Bristol, Avon). 2006;21:263–271. 10.1016/j.clinbiomech.2005.11.002 [DOI] [PubMed] [Google Scholar]
- 37.Gombatto SP, D’Arpa N, Landerholm S, et al. Differences in kinematics of the lumbar spine and lower extremities between people with and without low back pain during the down phase of a pick up task, an observational study. Musculoskelet Sci Pract. 2017;28:25–31. 10.1016/j.msksp.2016.12.017 [DOI] [PubMed] [Google Scholar]
- 38.Greene JA, Loscalzo J. Putting the patient back together—social medicine, network medicine, and the limits of reductionism. N Engl J Med. 2017;377:2493–2499. 10.1056/NEJMms1706744 [DOI] [PubMed] [Google Scholar]
- 39.Harris-Hayes M, Van Dillen LR. The inter-tester reliability of physical therapists classifying low back pain problems based on the Movement System Impairment classification system. PM R. 2009;1:117–126. 10.1016/j.pmrj.2008.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henry SM, Van Dillen LR, Trombley AR, Dee JM, Bunn JY. Reliability of novice raters in using the Movement System Impairment approach to classify people with low back pain. Man Ther. 2013;18:35–40. 10.1016/j.math.2012.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hill JC, Whitehurst DG, Lewis M, et al. Comparison of stratified primary care management for low back pain with current best practice (STarT Back): a randomised controlled trial. Lancet. 2011;378:1560–1571. 10.1016/S0140-6736(11)60937-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hodges PW. Hybrid approach to treatment tailoring for low back pain: a proposed model of care. J Orthop Sports Phys Ther. 2019;49:453–463. 10.2519/jospt.2019.8774 [DOI] [PubMed] [Google Scholar]
- 43.Hodges PW, Barbe MF, Loggia ML, Nijs J, Stone LS. Diverse role of biological plasticity in low back pain and its impact on sensorimotor control of the spine. J Orthop Sports Phys Ther. 2019;49:389–401. 10.2519/jospt.2019.8716 [DOI] [PubMed] [Google Scholar]
- 44.Hodges PW, Richardson CA. Inefficient muscular stabilization of the lumbar spine associated with low back pain. A motor control evaluation of transversus abdominis. Spine (Phila Pa 1976). 1996;21:2640–2650. [DOI] [PubMed] [Google Scholar]
- 45.Hoffman SL, Johnson MB, Zou D, Harris-Hayes M, Van Dillen LR. Effect of classification-specific treatment on lumbopelvic motion during hip rotation in people with low back pain. Man Ther. 2011;16:344–350. 10.1016/j.math.2010.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoffman SL, Johnson MB, Zou D, Van Dillen LR. Differences in end-range lumbar flexion during slumped sitting and forward bending between low back pain subgroups and genders. Man Ther. 2012;17:157–163. 10.1016/j.math.2011.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoffman SL, Johnson MB, Zou D, Van Dillen LR. Gender differences in modifying lumbopelvic motion during hip medial rotation in people with low back pain. Rehabil Res Pract. 2012;2012:635312 10.1155/2012/635312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoffman SL, Johnson MB, Zou D, Van Dillen LR. Sex differences in lumbopelvic movement patterns during hip medial rotation in people with chronic low back pain. Arch Phys Med Rehabil. 2011;92:1053–1059. 10.1016/j.apmr.2011.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hooker QL, Roles K, Lanier VM, Francois SJ, Van Dillen LR. Consistent differences in lumbar alignment during clinical tests and a functional task between low back pain subgroups [abstract]. J Orthop Sports Phys Ther. 2019;49:CSM104 10.2519/jospt.2019.49.1.CSM63 [DOI] [Google Scholar]
- 50.Hooker QL, Roles K, Lanier VM, Francois SJ, Van Dillen LR. Short- and long-term effects of skill training versus strength and flexibility exercise on knee, hip, and lumbar spine kinematics in people with chronic low back pain [abstract]. J Orthop Sports Phys Ther. 2019;49:CSM15 10.2519/jospt.2019.49.1.CSM1 [DOI] [Google Scholar]
- 51.Hooker QL, Roles K, Van Dillen LR. Skill training versus strength and flexibility exercise in people with chronic low back pain: effects on kinematics [abstract]. 42nd Annual Meeting of the American Society of Biomechanics; August 8–11, 2018; Rochester, MN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu H, Meijer OG, van Dieën JH, et al. Muscle activity during the active straight leg raise (ASLR), and the effects of a pelvic belt on the ASLR and on treadmill walking. J Biomech. 2010;43:532–539. 10.1016/j.jbiomech.2009.09.035 [DOI] [PubMed] [Google Scholar]
- 53.Hungerford B, Gilleard W. The pattern of intrapelvic motion and lumbopelvic muscle recruitment alters in the presence of pelvic girdle pain In: Vleeming A, Mooney V, Stoeckart R, eds. Movement, Stability and Lumbopelvic Pain: Integration of Research and Therapy 2nd ed. Edinburgh, UK: Elsevier/Churchill Livingstone; 2007:361–376. [Google Scholar]
- 54.Iguchi T, Kanemura A, Kasahara K, et al. Lumbar instability and clinical symptoms: which is the more critical factor for symptoms: sagittal translation or segment angulation? J Spinal Disord Tech. 2004;17:284–290. 10.1097/01.bsd.0000102473.95064.9d [DOI] [PubMed] [Google Scholar]
- 55.Iguchi T, Ozaki T, Chin T, et al. Intimate relationship between instability and degenerative signs at L4/5 segment examined by flexion–extension radiography. Eur Spine J. 2011;20:1349–1354. 10.1007/s00586-011-1793-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones AC, Wilcox RK. Finite element analysis of the spine: towards a framework of verification, validation and sensitivity analysis. Med Eng Phys 2008;30:1287–1304. [DOI] [PubMed] [Google Scholar]
- 57.Kamper SJ, Maher CG, Hancock MJ, Koes BW, Croft PR, Hay E. Treatment-based subgroups of low back pain: a guide to appraisal of research studies and a summary of current evidence. Best Pract Res Clin Rheumatol. 2010;24:181–191. 10.1016/j.berh.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 58.Kanemura A, Doita M, Kasahara K, Sumi M, Kurosaka M, Iguchi T. The influence of sagittal instability factors on clinical lumbar spinal symptoms. J Spinal Disord Tech. 2009;22:479–485. 10.1097/BSD.0b013e31818d1b18 [DOI] [PubMed] [Google Scholar]
- 59.Kiers H, van Dieën JH, Brumagne S, Vanhees L. Postural sway and integration of proprioceptive signals in subjects with LBP. Hum Mov Sci 2015;39:109–120. 10.1016/j.humov.2014.05.011 [DOI] [PubMed] [Google Scholar]
- 60.Kim MH, Yi CH, Kwon OY, et al. Comparison of lumbopelvic rhythm and flexion-relaxation response between 2 different low back pain sub-types. Spine (Phila Pa 1976). 2013;38:1260–1267. 10.1097/BRS.0b013e318291b502 [DOI] [PubMed] [Google Scholar]
- 61.Kim MH, Yoo WG, Choi BR. Differences between two subgroups of low back pain patients in lumbopelvic rotation and symmetry in the erector spinae and hamstring muscles during trunk flexion when standing. J Electromyogr Kinesiol. 2013;23:387–393. 10.1016/j.jelekin.2012.11.010 [DOI] [PubMed] [Google Scholar]
- 62.Lanier VM, Lang CE, Van Dillen LR. Motor skill training in musculoskeletal pain: a case report in chronic low back pain. Disabil Rehabil. In press. 10.1080/09638288.2018.1460627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lao L, Daubs MD, Scott TP, et al. Effect of disc degeneration on lumbar segmental mobility analyzed by kinetic magnetic resonance imaging. Spine (Phila Pa 1976). 2015;40:316–322. 10.1097/BRS.0000000000000738 [DOI] [PubMed] [Google Scholar]
- 64.Lee D, Vleeming A. An integrated therapeutic approach to the treatment of pelvic girdle pain In: Vleeming A, Mooney V, Stoeckart R, eds. Movement, Stability and Lumbopelvic Pain: Integration of Research and Therapy. 2nd ed Edinburgh, UK: Elsevier/Churchill Livingstone; 2007:621–638. [Google Scholar]
- 65.Li Y, Liu J, Liu ZZ, Duan DP. Inflammation in low back pain may be detected from the peripheral blood: suggestions for biomarker. Biosci Rep. 2016;36:e00361 10.1042/BSR20160187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lotz JC. The biomechanics of prevention and treatment for low back pain: 2nd international workshop. Clin Biomech (Bristol, Avon). 1999;14:220–223. 10.1016/S0268-0033(98)00087-4 [DOI] [PubMed] [Google Scholar]
- 67.Lovejoy CO. Evolution of human walking. Sci Am. 1988;259:118–125. [DOI] [PubMed] [Google Scholar]
- 68.Luomajoki H, Kool J, de Bruin ED, Airaksinen O. Movement control tests of the low back; evaluation of the difference between patients with low back pain and healthy controls. BMC Musculoskelet Disord. 2008;9:170 10.1186/1471-2474-9-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luomajoki H, Kool J, de Bruin ED, Airaksinen O. Reliability of movement control tests in the lumbar spine. BMC Musculoskelet Disord. 2007;8:90 10.1186/1471-2474-8-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Machado LA, de Souza M, Ferreira PH, Ferreira ML. The McKenzie method for low back pain:a systematic review of the literature with a meta-analysis approach. Spine (Phila Pa 1976) 2006;31:E254–E262. 10.1097/01.brs.0000214884.18502.93 [DOI] [PubMed] [Google Scholar]
- 71.Marich AV, Hwang CT, Salsich GB, Lang CE, Van Dillen LR. Consistency of a lumbar movement pattern across functional activities in people with low back pain. Clin Biomech (Bristol, Avon) 2017;44:45–51. https://doi.org/10.1016z/j.clinbiomech.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marich AV, Hwang CT, Sorensen CJ, Van Dillen LR. Examination of the lumbar movement pattern during a clinical test and a functional activity test in people with and people without low back pain. Phys Med Rehabil. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marich AV, Lanier VM, Salsich GB, Lang CE, Van Dillen LR. Immediate effects of a single session of motor skill training on the lumbar movement pattern during a functional activity in people with low back pain: a repeated-measures study. Phys Ther. 2018;98:605–615. 10.1093/ptj/pzy044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Masi AT, Benjamin M, Vleeming A. Anatomical, biomechanical, and clinical perspectives on sacroiliac joints: an integrative synthesis of biodynamic mechanisms related to ankylosing spondylitis In: Vleeming A, Mooney V, Stoeckart R, eds. Movement, Stability and Lumbopelvic Pain: Integration of Research and Therapy. 2nd ed Edinburgh, UK: Elsevier/Churchill Livingstone; 2007:205–227. [Google Scholar]
- 75.Mazzone B, Wood R, Gombatto S. Spine kinematics during prone extension in people with and without low back pain and among classification-specific low back pain subgroups. J Orthop Sports Phys Ther. 2016;46:571–579. 10.2519/jospt.2016.6159 [DOI] [PubMed] [Google Scholar]
- 76.McClure PW, Esola M, Schreier R, Siegler S. Kinematic analysis of lumbar and hip motion while rising from a forward, flexed position in patients with and without a history of low back pain. Spine (Phila Pa 1976). 1997;22:552–558. [DOI] [PubMed] [Google Scholar]
- 77.McGill SM, Grenier S, Kavcic N, Cholewicki J. Coordination of muscle activity to assure stability of the lumbar spine. J Electromyogr Kinesiol. 2003;13:353–359. 10.1016/S1050-6411(03)00043-9 [DOI] [PubMed] [Google Scholar]
- 78.Mellor FE, Muggleton JM, Bagust J, Mason W, Thomas PW, Breen AC. Midlumbar lateral flexion stability measured in healthy volunteers by in vivo fluoroscopy. Spine (Phila Pa 1976) 2009;34:E811–E817. 10.1097/BRS.0b013e3181b1feba [DOI] [PubMed] [Google Scholar]
- 79.Mellor FE, Thomas P, Breen A. Moving back: the radiation dose received from lumbar spine quantitative fluoroscopy compared to lumbar spine radiographs with suggestions for dose reduction. Radiography (Lond). 2014;20:251–257. 10.1016/j.radi.2014.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mellor FE, Thomas PW, Thompson P, Breen AC. Proportional lumbar spine inter-vertebral motion patterns: a comparison of patients with chronic, non-specific low back pain and healthy controls. Eur Spine J. 2014;23:2059–2067. 10.1007/s00586-014-3273-3 [DOI] [PubMed] [Google Scholar]
- 81.Mens JM, Damen L, Snijders CJ, Stam HJ. The mechanical effect of a pelvic belt in patients with pregnancy-related pelvic pain. Clin Biomech (Bristol, Avon) 2006;21:122–127. 10.1016/j.clinbiomech.2005.08.016 [DOI] [PubMed] [Google Scholar]
- 82.Mens JM, Vleeming A, Snijders CJ, Stam HJ, Ginai AZ. The active straight leg raising test and mobility of the pelvic joints. Eur Spine J. 1999;8:468–473. 10.1007/s005860050206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Norton BJ, Sahrmann SA, Van Dillen LR. Differences in measurements of lumbar curvature related to gender and low back pain. J Orthop Sports Phys Ther. 2004;34:524–534. 10.2519/jospt.2004.34.9.524 [DOI] [PubMed] [Google Scholar]
- 84.Oxland TR. Fundamental biomechanics of the spine—what we have learned in the past 25 years and future directions. J Biomech. 2016;49:817–832. 10.1016/j.jbiomech.2015.10.035 [DOI] [PubMed] [Google Scholar]
- 85.Panjabi MM. A hypothesis of chronic back pain: ligament subfailure injuries lead to muscle control dysfunction. Eur Spine J. 2006;15:668–676. 10.1007/s00586-005-0925-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pincus T, Burton AK, Vogel S, Field AP. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine (Phila Pa 1976) 2002;27:E109–E120. [DOI] [PubMed] [Google Scholar]
- 87.Richardson CA, Snijders CJ, Hides JA, Damen L, Pas MS, Storm J. The relation between the transversus abdominis muscles, sacroiliac joint mechanics, and low back pain. Spine (Phila Pa 1976) 2002;27:399–405. [DOI] [PubMed] [Google Scholar]
- 88.Roussel NA, Nijs J, Mottram S, Van Moorsel A, Truijen S, Stassijns G. Altered lumbopelvic movement control but not generalized joint hypermobility is associated with increased injury in dancers. A prospective study. Man Ther. 2009;14:630–635. 10.1016/j.math.2008.12.004 [DOI] [PubMed] [Google Scholar]
- 89.Sadeghisani M, Namnik N, Karimi MT, et al. Evaluation of differences between two groups of low back pain patients with and without rotational demand activities based on hip and lumbopelvic movement patterns. Ortop Traumatol Rehabil. 2015;17:51–57. 10.5604/15093492.1143536 [DOI] [PubMed] [Google Scholar]
- 90.Sahrmann SA. Diagnosis and Treatment of Movement Impairment Syndromes. St Louis, MO: Mosby; 2001. [Google Scholar]
- 91.Saragiotto BT, Maher CG, Hancock MJ, Koes BW. Subgrouping patients with nonspecific low back pain: hope or hype? J Orthop Sports Phys Ther. 2017;47:44–48. 10.2519/jospt.2017.0602 [DOI] [PubMed] [Google Scholar]
- 92.Saragiotto BT, Maher CG, Yamato TP, et al. Motor control exercise for chronic nonspecific low-back pain. Cochrane Database Syst Rev. 2016:CD012004 10.1002/14651858.CD012004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Scholtes SA, Gombatto SP, Van Dillen LR. Differences in lumbopelvic motion between people with and people without low back pain during two lower limb movement tests. Clin Biomech (Bristol, Avon). 2009;24:7–12. 10.1016/j.clinbiomech.2008.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scholtes SA, Norton BJ, Gombatto SP, Van Dillen LR. Variables associated with performance of an active limb movement following within-session instruction in people with and people without low back pain. Biomed Res Int. 2013;2013:867983 10.1155/2013/867983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Scholtes SA, Van Dillen LR. Gender-related differences in prevalence of lumbopelvic region movement impairments in people with low back pain. J Orthop Sports Phys Ther. 2007;37:744–753. 10.2519/jospt.2007.2610 [DOI] [PubMed] [Google Scholar]
- 96.Shum GL, Crosbie J, Lee RY. Effect of low back pain on the kinematics and joint coordination of the lumbar spine and hip during sit-to-stand and stand-to-sit. Spine (Phila Pa 1976) 2005;30:1998–2004. 10.1097/01.brs.0000176195.16128.27 [DOI] [PubMed] [Google Scholar]
- 97.Smith BE, Littlewood C, May S. An update of stabilisation exercises for low back pain: a systematic review with meta-analysis. BMC Musculoskelet Disord. 2014;15:416 10.1186/1471-2474-15-416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sorensen CJ, Johnson MB, Norton BJ, Callaghan JP, Van Dillen LR. Asymmetry of lumbopelvic movement patterns during active hip abduction is a risk factor for low back pain development during standing. Hum Mov Sci. 2016;50:38–46. 10.1016/j.humov.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sorensen CJ, Norton BJ, Callaghan JP, Hwang CT, Van Dillen LR. Is lumbar lordosis related to low back pain development during prolonged standing? Man Ther 2015;20:553–557. 10.1016/j.math.2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stokes IA. The contribution of biomechanics to the prevention and treatment of low back pain: 1st international workshop. Clin Biomech (Bristol, Avon). 1997;12:195–197. 10.1016/S0268-0033(97)00064-8 [DOI] [PubMed] [Google Scholar]
- 101.Sturesson B, Uden A, Onsten I. Can an external frame fixation reduce the movements in the sacroiliac joint? A radiostereometric analysis of 10 patients. Acta Orthop Scand. 1999;70:42–46. 10.3109/17453679909000956 [DOI] [PubMed] [Google Scholar]
- 102.Teyhen DS, Flynn TW, Childs JD, et al. Fluoroscopic video to identify aberrant lumbar motion. Spine (Phila Pa 1976) 2007;32:E220–E229. 10.1097/01.brs.0000259206.38946.cb [DOI] [PubMed] [Google Scholar]
- 103.Trudelle-Jackson E, Sarvaiya-Shah SA, Wang SS. Interrater reliability of a movement impairment-based classification system for lumbar spine syndromes in patients with chronic low back pain. J Orthop Sports Phys Ther. 2008;38:371–376. 10.2519/jospt.2008.2760 [DOI] [PubMed] [Google Scholar]
- 104.van Dieën JH, Reeves NP, Kawchuk G, van Dillen LR, Hodges PW. Analysis of motor control in patients with low back pain: a key to personalized care? J Orthop Sports Phys Ther. 2019;49:380–388. 10.2519/jospt.2019.7916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Van Dillen LR, Gombatto SP, Collins DR, Engsberg JR, Sahrmann SA. Symmetry of timing of hip and lumbopelvic rotation motion in2 different subgroups of people with low back pain. Arch Phys Med Rehabil. 2007;88:351–360. 10.1016/j.apmr.2006.12.021 [DOI] [PubMed] [Google Scholar]
- 106.Van Dillen LR, Maluf KS, Sahrmann SA. Further examination of modifying patient-preferred movement and alignment strategies in patients with low back pain during symptomatic tests. Man Ther. 2009;14:52–60. 10.1016/j.math.2007.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Van Dillen LR, Norton BJ, Sahrmann SA, et al. Efficacy of classification-specific treatment and adherence on outcomes in people with chronic low back pain. A one-year follow-up, prospective, randomized, controlled clinical trial. Man Ther 2016;24:52–64. 10.1016/j.math.2016.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Van Dillen LR, Norton BJ, Steger-May K, et al. Short- and long-term effects of skill training versus strength and flexibility exercise on functional limitations and pain in people with chronic low back pain [abstract]. J Orthop Sports Phys Ther. 2019;49:CSM147 10.2519/jospt.2019.49.1.CSM63 [DOI] [Google Scholar]
- 109.Van Dillen LR, Sahrmann SA, Caldwell CA, McDonnell MK, Bloom N, Norton BJ. Trunk rotation-related impairments in people with low back pain who participated in 2 different types of leisure activities: a secondary analysis. J Orthop Sports Phys Ther. 2006;36:58–71. 10.2519/jospt.2006.36.2.58 [DOI] [PubMed] [Google Scholar]
- 110.Van Dillen LR, Sahrmann SA, Norton BJ, et al. Effect of active limb movements on symptoms in patients with low back pain. J Orthop Sports Phys Ther. 2001;31:402–413; discussion 414–418. 10.2519/jospt.2001.31.8.402 [DOI] [PubMed] [Google Scholar]
- 111.Van Dillen LR, Sahrmann SA, Norton BJ, et al. Reliability of physical examination items used for classification of patients with low back pain. Phys Ther. 1998;78:979–988. 10.1093/ptj/78.9.979 [DOI] [PubMed] [Google Scholar]
- 112.Van Dillen LR, Sahrmann SA, Norton BJ, Caldwell CA, McDonnell MK, Bloom N. The effect of modifying patient-preferred spinal movement and alignment during symptom testing in patients with low back pain: a preliminary report. Arch Phys Med Rehabil. 2003;84:313–322. 10.1053/apmr.2003.50010 [DOI] [PubMed] [Google Scholar]
- 113.Van Dillen LR, Sahrmann SA, Norton BJ, Caldwell CA, McDonnell MK, Bloom NJ. Movement system impairment-based categories for low back pain: stage 1 validation. J Orthop Sports Phys Ther. 2003;33:126–142. 10.2519/jospt.2003.33.3.126 [DOI] [PubMed] [Google Scholar]
- 114.van Middelkoop M, Rubinstein SM, Verhagen AP, Ostelo RW, Koes BW, van Tulder MW. Exercise therapy for chronic nonspecific low-back pain. Best Pract Res Clin Rheumatol. 2010;24:193–204. 10.1016/j.berh.2010.01.002 [DOI] [PubMed] [Google Scholar]
- 115.van Wingerden JP, Vleeming A, Buyruk HM, Raissadat K. Stabilization of the sacroiliac joint in vivo: verification of muscular contribution to force closure of the pelvis. Eur SpineJ. 2004;13:199–205. 10.1007/s00586-003-0575-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vibe Fersum K, O’Sullivan P, Skouen JS, Smith A, Kvåle A. Efficacy of classification-based cognitive functional therapy in patients with non-specific chronic low back pain: a randomized controlled trial. Eur J Pain. 2013;17:916–928. 10.1002/j.1532-2149.2012.00252.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vleeming A, Albert HB, Östgaard HC, Sturesson B, Stuge B. European guidelines for the diagnosis and treatment of pelvic girdle pain. Eur Spine J. 2008;17:794–819. 10.1007/s00586-008-0602-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vleeming A, Schuenke MD, Masi AT, Carreiro JE, Danneels L, Willard FH. The sacroiliac joint: an overview of its anatomy, function and potential clinical implications. J Anat. 2012;221:537–567. 10.1111/j.1469-7580.2012.01564.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vleeming A, Stoeckart R, Volkers AC, Snijders CJ. Relation between form and function in the sacroiliac joint. Part I: clinical anatomical aspects. Spine (Phila Pa 1976). 1990;15:130–132. [DOI] [PubMed] [Google Scholar]
- 120.Vleeming A, Volkers AC, Snijders CJ, Stoeckart R. Relation between form and function in the sacroiliac joint. Part II: biomechanical aspects. Spine (Phila Pa 1976). 1990;15:133–136. [DOI] [PubMed] [Google Scholar]
- 121.Waddell G 1987 Volvo Award in clinical sciences. A new clinical model for the treatment of low-back pain. Spine (Phila Pa 1976) 1987;12:632–644. [DOI] [PubMed] [Google Scholar]
- 122.Weyrauch SA, Bohall SC, Sorensen CJ, Van Dillen LR. Association between rotation-related impairments and activity type in people with and without low back pain. Arch Phys Med Rehabil. 2015;96:1506–1517. 10.1016/j.apmr.2015.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wong AY, Parent EC, Funabashi M, Kawchuk GN. Do changes in transversus abdominis and lumbar multifidus during conservative treatment explain changes in clinical outcomes related to nonspecific low back pain? A systematic review. J Pain 2014;15:377.e1–377.e35. 10.1016/j.jpain.2013.10.008 [DOI] [PubMed] [Google Scholar]
- 124.Wood PHN. Understanding back pain In: Jayson MIV, ed. The Lumbar Spine and Back Pain.2nd ed Tunbridge Wells, UK: Pitman Medical; 1980:1–27. [Google Scholar]
- 125.Yeager MS, Cook DJ, Cheng BC. Reliability of computer-assisted lumbar intervertebral measurements using a novel vertebral motion analysis system. Spine J 2014;14:274–281. 10.1016/j.spinee.2013.10.048 [DOI] [PubMed] [Google Scholar]
- 126.Zanjani-Pour S, Meakin JR, Breen A, Breen A. Estimation of in vivo inter-vertebral loading during motion using fluoroscopic and magnetic resonance image informed finite element models. J Biomech. 2018;70:134–139. 10.1016/j.jbiomech.2017.09.025 [DOI] [PubMed] [Google Scholar]


