Abstract
Immunotoxins are antibody–toxin fusion proteins under development as cancer therapeutics. In early clinical trials, immunotoxins constructed with domains II and III of Pseudomonas exotoxin (termed PE38), have produced a high rate of complete remissions in Hairy Cell Leukemia and objective responses in other malignancies. Cholera exotoxin (also known as cholix toxin) has a very similar three-dimensional structure to Pseudomonas exotoxin (PE) and when domains II and III of each are compared at the primary sequence level, they are 36% identical and 50% similar. Here we report on the construction and activity of an immunotoxin made with domains II and III of cholera exotoxin (here termed CET40). In cell viability assays, the CET40 immunotoxin was equipotent to tenfold less active compared to a PE-based immunotoxin made with the same single-chain Fv. A major limitation of toxin-based immunotoxins is the development of neutralizing antibodies to the toxin portion of the immunotoxin. Because of structure and sequence similarities, we evaluated a CET40 immunotoxin for the presence of PE-related epitopes. In western blots, three-of-three anti-PE antibody preparations failed to react with the CET40 immunotoxin. More importantly, in neutralization studies neither these antibodies nor those from patients with neutralizing titers to PE38, neutralized the CET40-immunotoxin. We propose that the use of modular components such as antibody Fvs and toxin domains will allow a greater flexibility in how these agents are designed and deployed including the sequential administration of a second immunotoxin after patients have developed neutralizing antibodies to the first.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-009-0794-4) contains supplementary material, which is available to authorized users.
Keywords: Exotoxin, Cholix, Immunotoxin, Neutralization, Antibody
Introduction
Antibody-based therapies of human cancer have become first-line treatments in certain settings. By way of example: Her2-positive breast cancer patients are treated with Herceptin [1] while individuals with certain B-cell malignancies receive Rituxan [2]. These antibodies are given either alone or in combination with chemotherapy. The potential benefit of using antibody-based therapy is an effective treatment with low side effects. However, when the administration of an unmodified antibody is not effective, several options are available to make the antibody a ‘cytotoxic’ agent [3]. Radionuclides, small molecular weight drugs (including prodrugs), enzymes, homing partners (such as bispecific antibodies), and protein toxins have each been “attached” to tumor-binding antibodies as adjuncts to increase their effectiveness [4–12]. Each type of modified antibody has benefits and limitations [3, 13].
Recombinant immunotoxins are antibody-based therapeutics composed of Fv fragments fused with protein toxins [11, 14–16]. The protein toxins are usually derived from bacterial or plant cytotoxic proteins and act enzymatically within the cytosol of mammalian cells. Advantages of toxin-based agents relate to their potency, lack of mutagenic activity, and the fact that cancer cells rarely exhibit toxin resistance. The main disadvantage is immunogenicity [17–24]. Typically, patients make neutralizing antibodies within weeks of receiving their first course of treatment.
Pseudomonas exotoxin has been investigated for a number of years as a cytotoxic partner with antibody fragments in the development of anticancer immunotoxins [11, 16]. Typically, a 38-kDa fragment of PE, encompassing domains II and III of the parental toxin, is fused genetically to either a single-chain Fv (scFv) or disulfide stabilized Fv (dsFv). With the exception of treating individuals with B-cell malignancies [25], most immunotoxin trials have been limited to one or sometimes two cycles of therapy because patients make neutralizing antibodies, usually within 3 weeks of initiating treatment [23, 26]. Potential strategies to make such toxin-based agents less immunogenic include the co-administration of immunosuppressive agents [27–29] and the re-engineering of the parent molecule to remove major epitopes [21, 24]. While the co-administration of immunosuppressive agents is simple in concept, it is difficult to accomplish in Phase I/II trials due to the confounding problem of mixing two agents where the properties of one, in this case the immunotoxin, are not well understood. The prospect of engineering a bacterial toxin to render it non-immunogenic is also challenging. However, by removing the most potent antigenic epitopes, it may be possible to administer several cycles of therapy before a neutralizing response develops [21]. The replacement of the toxin portion of the immunotoxin with a closely related but immunologically distinct ‘molecular cousin’ may allow for a third approach. This strategy should work best in situations where structural similarities are close enough to allow for domain swapping and the use of a ‘modular replacement strategy’.
Here, we report on the development of a novel recombinant immunotoxin constructed from domains II and III of an exotoxin from Vibrio cholerae (also termed cholix toxin [30]) that shares ~36% identity and 50% similarity with the comparable domains of Pseudomonas exotoxin.
To construct this immunotoxin, a synthetic gene encoding amino acids 270 to 634 of cholera exotoxin (CET) was combined with the single-chain Fv antibody (HB21scFv) directed to the human transferrin receptor. HB21-CET40 was potently toxic for a number of human cancer cell lines. And despite a high level of structural and sequence similarity between PE40 and CET40, anti-PE antibodies did not recognize or neutralize the CET40 immunotoxin. Thus, it is now possible to develop a distinct anti-cancer therapeutic platform centered on CET-based immunotoxins that potentially can be administered as a first-line therapeutic agent or to individuals with prior exposure to PE-based immunotoxins.
Methods
Construction of HB21-CET40
A synthetic gene fragment (sequence provided in Fig. 1a) encoding putative domains II and III of Cholera exotoxin was produced (at Blue Heron Biotechnology) with HindIII and EcoRI restriction sites flanking the gene, and provided as a pUC19 plasmid. An expression vector (pRB2506) encoding the single-chain Fv fragment of the HB21 antibody fused with PE40 (HB21-PE40) was provided by Richard Beers and Ira Pastan. The vector DNA was digested with the appropriate restriction endonucleases, separated via 0.9% agarose gel electrophoresis and the fragments gel purified using a Qiaquick gel extraction kit. Ligations of DNA fragments were performed using an approximate (3:1) insert to vector molar ratio with T4 DNA ligase (New England Biolabs) in 1× ligation buffer, at 37°C for 1.5 or 16 h at 16°C. Clones were screened by diagnostic restriction digests and positive clones confirmed by DNA sequencing (Johns Hopkins Sequencing Facility, Baltimore, MD).
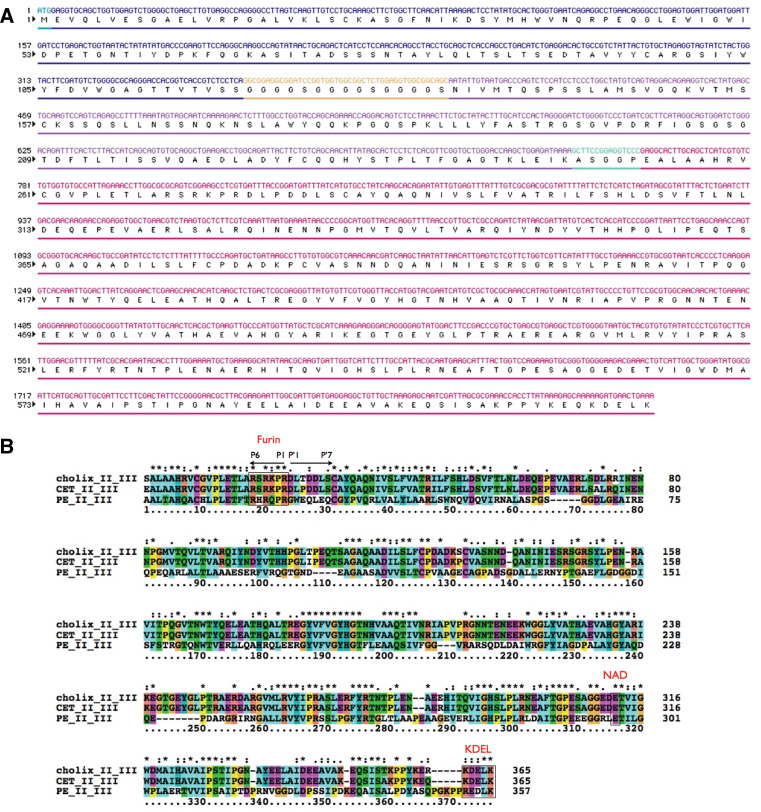
Fig. 1.
CET40 immunotoxin. a Shown is the DNA and protein sequence of the immunotoxin HB21-CET40. The initiating methionine is followed by the variable portion of the heavy chain, a glycine-serine linker, the variable portion of the light chain, a short connector sequence (including the HindIII site), and amino acids 270–634 of cholera exotoxin. b Sequence alignment via ‘ClustalX’ (2.09) analysis. Shown in descending order are amino acids 270–634 of cholix toxin [30], amino acids 270–634 of CET (corresponding to domains II and III from the exotoxin gene of Vibrio cholerae strain 1587), and finally domains II and III of exotoxin A, PE40. Key common features include the location of a furin cleavage site, an NAD binding glutamic acid, and a KDEL-like motif at the C-terminus. Coloring was per the default settings for ClustalX as defined by ‘colprot.xml’ (colprot.par). For alignment: “*” = fully conserved; “:” = conserved within a ‘strong’ group; and “.” = conserved within a ‘weak’ group
Expression of HB21-CET40
The backbone of the expression vector has an inducible T7 promoter and carries a gene encoding chloramphenicol resistance. Expression of the single-chain immunotoxin was carried out in BL21-Star (DE3) E. coli cells (Invitrogen) grown at 37°C in baffled Fernbach flasks at 275 rpm. Cells were grown in Superbroth (KD Medical) supplemented with chloramphenicol at 25 µg/ml (Sigma) and ‘Overnight Express’ additives (Novagen). This medium was inoculated with freshly transformed cells and grown overnight (~17 h). Final culture OD600’s were ~5–6. Cells were harvested by centrifugation at 4,000×g for 10 min in a Sorvall 3B centrifuge. Cell pellets were stored frozen at −80°C.
Preparation of inclusion bodies
Frozen cells (up to 10,000 OD units) were resuspended in 25 ml of Tris–EDTA (TE 50/20-mM/mM, pH 8.0) and dispersed using a tissuemizer. Cells were then lysed with the addition of chicken egg white lysozyme (Sigma) to a final concentration of 200 µg/ml for 1 h at RT. Lysed cells were incubated further for 30 min with the addition of 3.3 ml of 5.0 M NaCl and 3.3 ml of 25% Triton X-100. Inclusion bodies (IB) were then recovered in the pellet following centrifugation for 45 min at 15,000×g (Sorvall SS-34 rotor). The pellet was resuspended in 25 ml TE 50/20, 1% vol/vol Triton X-100, dispersed using a tissuemizer and centrifuged as above three more times. To remove the detergent, the inclusion bodies were washed four times in TE 50/20. The IB pellet preparation was stored frozen at −80°C.
Solubilization of IB’s
IBs were solubilized initially in 6 M Guanidine–HCl, 0.1 M Tris–HCl, 2 mM EDTA at pH 8.0. After 1–4 h, dithioerythretol (DTE) was added to a final concentration of 65 mM (10 mg/ml) and solubilization allowed to proceed on a rocking platform overnight at RT.
Renaturation refolding
Solubilized immunotoxin was centrifuged to remove non-soluble material and the supernatant diluted (~1:100 vol/vol) into a refolding buffer: 0.1 M Tris, 0.5 M l-Arginine–HCl, 2 mM EDTA, 0.9 mM GSSG, pH 8.0 at 10°C. After 24 h, additional GSSG, (9 mM final), was added for another 24 h. The refolded protein was then dialyzed against 20 mM Tris–HCl,100 mM Urea pH 8.0.
Anion exchange chromatography
The post dialysis immunotoxin preparation was adjusted to 2 l with deionized water and batch-adsorbed onto 50 ml of Q-sepharose. The resin was recovered on a 2-l-Buchner funnel and washed with four volumes of 20 mM Tris–HCl,1 mM EDTA, pH 8.0 and then eluted using the same buffer supplemented with 0.1, 0.35, and 0.5 M NaCl. The immunotoxin eluting with 0.35 M NaCl was retained for additional chromatography. The retained material was diluted in low salt buffer (buffer A, 20 mM Tris, pH 8.0) and pumped onto Mono-Q 5/5 column at 1 ml/min. Protein was eluted from the resin with a linear gradient 0–100% of buffer B (20 mM Tris pH 8.0, 1.0 M NaCl), over 30 ml, collecting 1 ml fractions. Peak fractions were concentrated using an Amicon Ultra (10,000 MWCO) concentrator (Amicon) to a volume ≤1 ml for gel filtration.
Gel filtration chromatography
The concentrated sample was loaded onto a TSKgel G3000-SWxl column (Tosoh Bioscience) at 0.5 ml/min, using PBS, pH 7.4, as the mobile phase. Fractions of 0.5 ml were collected and analyzed by SDS-PAGE (Supp Fig. 1).
Cell lines
KB3-1, A549, DLD-1, Raji, 293TT (from C. Buck NCI), HUT102, and L929 cells were grown in RPMI-1640 or DMEM and 10% fetal bovine serum supplemented with penicillin, streptomycin, glutamine, and pyruvate.
Cytotoxicity assay
The WST-1 (Roche) was used to assess cytotoxicity. Cells were seeded in 96-well plates at 5 × 103 per well. After 24 h, immunotoxins or immunotoxin-antibody mixtures were added to cells for a further 48 h. Dye-containing media was removed and replaced with a 10% vol/vol of WST-1 reagent in dye-free RPMI-1640 growth media. Absorbance measurements were made at 450 nm at 30 and 60 min. Replicates of five were used for each data point and all experiments were conducted independently at least twice. Cycloheximide (Sigma) was added at 10 µg/ml as a positive control in all experiments. For competition experiments with excess antibody, DLD-1 cells were pretreated with 10 ug/ml of HB21 for 30 min and then HB21-CET40 was added to a final concentration of either 10 or 1 ng/ml.
Anti-PE antibodies
Several anti-PE antibody preparations were evaluated. A mouse monoclonal antibody, termed M40-1, was originally described as a neutralizing antibody that also recognized PE via western blots [31]. Two rabbit polyclonal antibody preparations that reacted with PE via western blot and neutralized the toxin were also employed. One of these was a lab reagent originally generated to formaldehyde-treated native PE. The other was purchased from Sigma (P2318). We also used human sera from patients treated with the immunotoxin SS1P-PE38 (see below).
Antibodies to CET40
To make antibodies reactive for CET40, a rabbit was hyperimmunized with an enzymatically inactive form of CET40 (E581A) fused to HB21scFv. Immunizations and antisera production were carried out at Convance. Because these sera contained antibodies to both the scFv and CET40, western blots were conducted on full-length PE and CET proteins. Both PE and CET were expressed in E. coli and purified using the same protocol used to prepared HB21-CET40.
Western blots
Immunotoxin proteins, either HB21-PE40 or HB21-CET40 (30 ng) were separated via SDS-PAGE (8–16% gradient), transferred to PVDF membranes and probed with anti-PE antibodies. CET and PE was similarly separated and transferred to PVDF membranes. Either donkey anti-mouse IgG-HRP or donkey anti-rabbit IgG-HRP (Jackson, Immunoresearch) was used to detect the primary antibodies. Reactive bands were detected by ECL and visualized on Amersham Hyperfilm.
Neutralization assay
Rabbit or mouse antibodies were diluted 1:100 and mixed with either 5 or 1 ng/ml of either HB21-PE40 or HB21-CET40 for 1 h at room temperature. At the end of the incubation the immunotoxin–antibody mixture was diluted 1:1 with media over cells. Cells were incubated with immunotoxin and antibodies for 48 h and then evaluated for viability using the WST-1 assay.
Human sera (from four individuals) were obtained with informed consent before and after treatment with the PE38 immunotoxin, SS1P, which is directed to the surface antigen mesothelin [26]. Immunotoxin treatment was at the dose level of 45 μg/kg for each the four individuals [26]. The post treatment sample was documented as having neutralizing titers to PE38. The immunotoxin at 5 or 1 ng/ml was mixed with a 1:100 dilution of patient sera and incubated for 1 h at room temperature. After this incubation, 50 μl was added to each well giving a final immunotoxin concentration of 2.5 and 0.5 ng/ml, respectively, and a final serum dilution of 1:200.
Results
Construction and initial characterization of a CET40 immunotoxin
A single-chain immunotoxin was constructed from a cDNA encoding the Fv portion of the HB21 antibody recognizing the human transferrin receptor [32] and a synthetic gene encoding domains II and III of cholera exotoxin1 (here called CET) which is very similar to the toxin named ‘cholix toxin’ (accession number; AY876053) by Jorgensen et al. [30]. The synthetic gene, corresponding to amino acids 270–634 of CET (the annotated DNA and protein sequences are provided in Fig. 1a) was derived from the sequenced genome of V. cholerae strain1587 (accession number for CET is ZP_01950668) and differs from cholix toxin in domains II and III by ten amino acids. Figure 1b shows a clustalX sequence alignment of domains II and III of cholix toxin (V. cholerae strain TP [33]), CET (V. cholerae strain1587) and PE40. Key features of each toxin include a consensus furin cleavage site (with strong conservation on the N-terminal site of the scissile bond and weak conservation on the C-terminal side), a conserved glutamic acid marking the NAD-binding pocket and a C-terminal a KDEL-like sequence followed by a terminal lysine. Also the four half cysteines are completely conserved as are several stretches of residues within domain III (from residues 187–336 in Fig. 1b).
Expression of HB21-CET40 was driven by a T7 promoter and accomplished via growth in ‘autoinduction media’ under Cm selection (see “Methods”). After an overnight culture, the insoluble protein was recovered in inclusion bodies and purified according to our standard laboratory protocol (see “Methods” and [34, 35]). Briefly, inclusion bodies were solubilized with 6 M guanidine and a reducing agent, refolded into a redox shuffling buffer, and purified using anion exchange and gel filtration chromatography. An SDS-PAGE analysis of gel filtration fractions revealed that ~20% of HB21-CET40 eluted as a monomer (Supp Fig. 1). Fractions 28 and 29 were used for experiments presented below.
Cytotoxic activity of the HB21-CET40
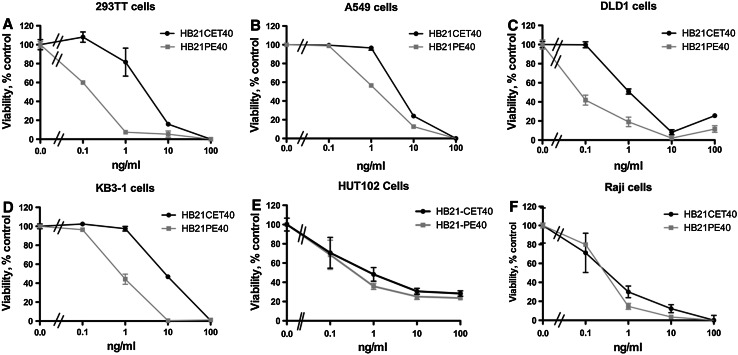
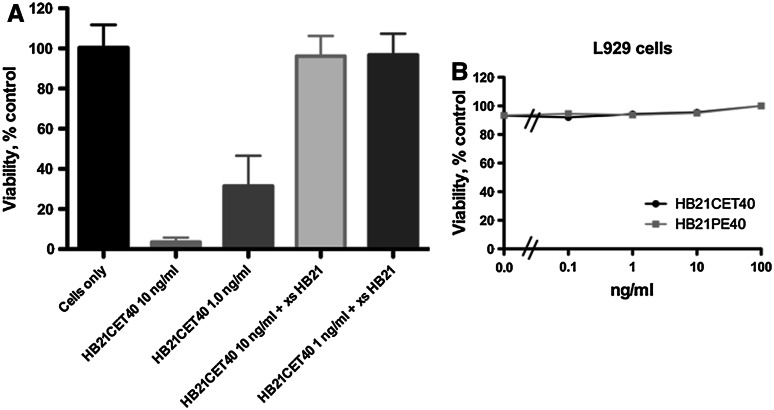
HB21-CET40 was assayed for cell-killing activity against several cell lines and compared directly with HB21-PE40. The following lines of various tissue origins were tested: DLD-1, colon; A549, lung; KB3-1, epidermoid; 293TT, kidney; Raji, B-cell; and HUT102, T-cell. In all cells tested, HB21-CET40 was equipotent to ten times less active when compared to HB21-PE40 (Fig. 2a–f). Generally, the adherent epithelial cancer cell lines (a–d) were approximately fivefold more sensitive to the PE40 immunotoxin, while lymphoid cancers (e and f) exhibited equal sensitivity the PE40/CET40 immunotoxins. To confirm specificity of activity, excess HB21 antibody was used in competition experiments to block the human transferrin receptor (huTFR) and reduce immunotoxin activity (Fig. 3a). The pretreatment of cells with 10 µg/ml of HB21 completely abrogated the toxicity seen with either 1 or 10 ng/ml of HB21-CET40. Because there is no cross-reactivity of the HB21 antibody with the murine TFR, HB21-CET40 was added to the mouse L929 cell line to assess any non-specific toxicity that might be contributed by CET40 (Fig. 3b). No reduction in viability was noted in concentrations up to 100 ng/ml. From the data above, we conclude that CET40 can be used to construct potent and antigen-specific recombinant immunotoxins.
Fig. 2.
Cytotoxicity of HB21-CET40 compared with HB21-PE40. Immunotoxin concentrations from 0.1 to 100 ng/ml were added to each of six cell lines for 48 h. DLD-1 (colon), A549 (lung), KB 3-1 (epidermoid), 293TT (kidney), Raji (B-cell), or HUT102 (T-cell) cells were used as representative cell lines of various common cancers. Cell viability was then determined using the WST-1 reagent. Error bars represent one standard deviation (SD) of five replicate wells per data point
Fig. 3.
Immunotoxin specificity. a Excess HB21 antibody competes for killing activity on DLD1 cells. Cells were pretreated or not with the HB21 antibody (10 µg/ml) for 1 h at 37°C and then incubated with HB21-CET40 at 10 and 1 ng/ml. b Immunotoxin activity on a mouse cell line. HB21-CET40 or HB21-PE40 was added to L929 cells at concentrations from 0.1 to 100 ng/ml. Cell viability was assessed after 48 h using the WST-1 reagent. Error bars represent one SD of five replicate wells per data point
Cross-reactivity of anti-PE antibodies
Because of the close structural and sequence similarity of PE40 to CET40, we probed preparations of CET40 with anti-PE antibodies looking for evidence of cross-reactivity. However, two distinct rabbit anti-PE polyclonal antibody preparations and one monoclonal antibody, each strongly reactive for HB21-PE40, were unreactive for HB21-CET40 (Fig. 4a). In an attempt to conduct the reciprocal experiment, we produced rabbit antibodies to CET40 by hyperimmunizing with a preparation of HB21-CET40E581A, an enzymatically inactive form of CET40. Because this antibody preparation contained antibodies to both CET40 and the HB21scFv (data not shown) we could not use western blot analysis of PE40 immunotoxins to assess cross-reactivity. Instead, we assessed reactivity using full length CET and PE. As shown in Fig. 4b, the rabbit anti-CET40 preparation reacted with CET, but not PE, providing further evidence that the two toxins are immunologically distinct.
Fig. 4.
Toxin reactivity via western blot analysis. a Western blot analysis of HB21-PE40 and HB21-CET40. Immunotoxins ~30 ng per lane were separated on a reducing 8–16% Gel and transferred to a PVDF membrane. Immunotoxins and a lane with molecular weight (MW) markers were each probed with one of three anti-PE antibodies (from left to right: M40-1, rabbit anti-PE from Sigma–Aldrich and rabbit anti-PE raised at NCI. b Western blot analysis of CET and PE probed with anti-CET40 antibodies. CET or PE at 30 and 3 ng per lane was probed with a rabbit anti-HB21-CET40 antibody preparation
Because conformational epitopes may not be recognized in western blots, a neutralization assay was performed where antibodies and immunotoxins were mixed in solution and then added to cells. To assess neutralization activity, HB21-PE40 and HB21-CET40 at either 5 or 1 ng/ml were each mixed with either 20 µg/ml of Rabbit anti-PE IgG (a lab reagent raised many years ago to formaldehyde-treated full length PE) or 20 µg/ml of the monoclonal antibody M40-1 [31] or with a 1:100 dilution of commercial antisera to PE available from Sigma–Aldrich. Mixtures of antibody and immunotoxin were incubated for 1 h at room temp after which aliquots were added to cells. Addition to cells resulted in a twofold dilution of all reagents, so that immunotoxins at 2.5 and 0.5 ng/ml were incubated with target cells in the continued presence of the test antibodies. The rabbit anti-PE antibodies neutralized completely the PE40 immunotoxin but not the CET40 version (see thin black lines on Fig. 5 comparing immunotoxin activity in the absence and presence of anti-PE antibodies). The mouse monoclonal antibody showed modest neutralizing activity against the PE40 immunotoxin and none against the CET40 immunotoxin (see black lines on Supp Fig. 2). Together, these results suggested that the two toxins do not share a common epitope that is recognized by neutralizing antibodies.
Fig. 5.
a, b Neutralization activity of anti-PE antibody preparations. Antibody preparations were mixed with twice the desired final immunotoxin concentration for 1 h at room temp. At the end of the incubation the mixture was added to each well of DLD-1 cells in a 96-well format. After a 48-h incubation, cell viability was assessed using the WST-1 reagent. Each bar represents a replicate of five with the error bar indicating one SD. Comparisons of immunotoxin activity with and without anti-PE antibodies are indicated with thin black lines. Each experiment was conducted independently at least twice per antibody preparation
Activity of human anti-PE38 Sera
The administration of PE38 immunotoxins to non-immunosuppressed cancer patients usually (>90% of the time) results in the formation of neutralizing antibodies [26]. Human anti-immunotoxin response is most often directed to the toxin portion of the immunotoxin [23, 36]. As mentioned earlier, when compared at the level of primary sequence, Domains II and III of PE and CET are 36% identical. It was therefore of some interest to learn whether conserved residues included those that generated human neutralizing antibodies. To examine this, sera from four patients that had developed neutralizing anti-PE38 antibodies were each evaluated for neutralizing activity to HB21-CET40. These patients had each been treated with SS1P, a PE38 immunotoxin directed to the surface differentiation antigen mesothelin, and had developed a neutralizing antibody response to PE38. Therefore, we tested a pre- and post-treatment sample from each patient and assessed neutralization activity against 5 and 1 ng/ml of HB21-PE40 and HB21-CET40 (see thin black lines on Fig. 6a–d indicating comparisons between pre- and post-treatment samples). Each serum sample gave essentially the same result: full neutralization of HB21-PE40 at 5 and 1 ng/ml by the post-treatment sample but no neutralization with the pretreatment sample. In contrast, there was no neutralization of HB21-CET40 by either the pre- or post- treatment sera, confirming that, in (four-of-four) humans, PE38 was immunologically distinct from CET40 with respect to the production of neutralizing antibodies.
Fig. 6.
Neutralizing activity of pre and post-treatment sera from four individual patients treated with a PE38-immunotoxin. Patient sera (at 1:100) were mixed with either 5 or 1 ng/ml of immunotoxin for 1 h at room temp. a Reports activity with HB21-PE40 and b with HB21-CET40. At the end of the incubation the mixture was added to DLD-1 cells in a 96-well format. After a 48-h incubation, cell viability was assessed using the WST-1 reagent. Each bar represents a replicate of five with the error bar indicating one SD. Direct comparisons of pre- and post-treatment serum samples are indicated with thin black lines. Each experiment was conducted independently twice per patient sample
Discussion
Vibrio cholerae is best known for the expression of classic cholera toxin with its A-chain that ADPR-ribosylates the Gs alpha subunit of the heterotrimeric G protein leading to increased cAMP and a pentameric B-chain for cell binding. Recently, evidence that certain strains of V. cholerae encode an unrelated exotoxin similar to PE from Pseudomonas has been supported by tissue culture experiments, bioinformatic comparisons of sequenced genomes, and direct structural comparisons of the two toxins [30, 33, 37]. Here, we confirm functional similarity in another way by showing that domains II and III of CET can be used to generate immunotoxins with cell killing activities roughly equivalent to that of PE-based proteins. While a role for cholera exotoxin in contributing to human disease has not been firmly established, at least one report documents an outbreak of diarrhea caused by V. cholerae isolate (strain 1587) that was negative for the structural genes encoding classical cholera toxin and positive for CET [37]. With several V. cholerae strains to choose from, we focused on strain 1587 [37] because it had been isolated from a disease outbreak rather than cholix toxin that had been derived from an environmental isolate. CET and cholix differ by 13 amino acids and consequence of this is not yet known.
To make a recombinant immunotoxin, the gene fragment of domains II and III of this exotoxin (CET40) was manufactured synthetically and then inserted into an expression vector routinely used to produce PE-based immunotoxins. HB21-CET40 was made primarily as proof of concept that CET could be directed to kill cells expressing the human transferrin receptor, a cell surface receptor known to be efficiently internalized. Original Pseudomonas exotoxin-based immunotoxins were constructed with domains II and III together with the subdomain termed domain Ib and were called PE40 [38]. However, recent iterations have been made with a deletion of a portion of Ib (amino acids 365–380) and are termed PE38 [39]. Because we were concerned that making an exactly comparable Ib deletion in CET40 might not be accomplished reliably, we chose to compare CET40 with PE40 immunotoxins. In assays against various cell lines, HB21-CET40 was equipotent or less potent (usually by 2–10 fold—see below) when compared to the same immunotoxin constructed with PE40. We conclude that CET40 is a potent cytotoxic molecule that can be targeted using an antibody Fv to antigens on the surface of cancer cells. In the current study, two specificity controls were included. We show that mouse cells, not recognized by the antibody to the human transferrin receptor, were not killed by HB21-CET40. We also added excess of the parent antibody, HB21, and showed that this blocked cytotoxic activity of the immunotoxin (Fig. 3). These latter two experiments confirm that cell binding is via the targeting antibody Fv and not mediated by CET40 residues.
The reason or reasons for reduced cell killing by CET40 compared with PE40 have not yet been elucidated. However, we have considered differences in three key sequences that are known to be important in PE-based immunotoxins. Both toxins have a consensus furin cleavage site, a glutamic acid for binding NAD, and a KDEL-like sequence at the end of the molecule (see Fig. 1b). The furin recognition site has a P1 and P4 arginine in cholix, CET, and PE. Also present is a P6 arginine that represents an extended furin site (Fig. 1b). The P2 residue is a proline, which is not usual for substrates of furin but apparently is functional at this location [40]. While residues P1–6 appear well conserved, P’1–7 residues are not (Fig. 1b). A key tryptophan [41] at the P’2 position of PE (residue 281 in native PE) is replaced with a leucine while the P’1 glycine of PE is replaced with an aspartic acid in CET. These residues and others in the vicinity may contribute to an altered efficiency of translocation to the cytosol. NAD binding relies on a glutamic acid in all three toxins. However, in cholix and CET there are two negatively charged residues immediately preceding this residue. In PE there is an arginine and leucine instead. Finally, PE is known to require a KDEL-like sequence for cytoxic activity presumably because retrograde transport to the ER is essential for toxicity. Both cholix and CET end in the sequence, ‘KDELK’, while PE finishes with ‘REDLK’. At this time it is not known if these variants behave equally well for retrieval to the ER and whether they influence translocation efficiency to the cytosol. In sum there are several differences between the two toxins and these may contribute to altered efficiencies in different cell types. One approach to study this in the future is to make hybrid toxin molecules where domains or domain segments are swapped and activities compared.
A secondary goal was to investigate the immunological similarities of the two toxins. This was especially important because of the potential of using immunotoxins sequentially. Despite exhibiting segments of high sequence homology and being very similar structurally, there appears to be very little immunological cross-reactivity between PE40 and CET40. None of the anti-PE antibodies reacted with the CET40 immunotoxin and, using a recently generated antibody to a CET40 immuntoxin, we showed no cross-reactivity with PE. Perhaps the most relevant test was the lack of a cross-neutralizing response by patients who had received PE38 immunotoxins. In four-of-four samples, post treatment but not pretreatment sera neutralized the cytotoxic activity of HB21-PE40 but not the CET40 immunotoxin (Fig. 6). This suggests that the major neutralizing epitopes of PE38 are not shared with CET40. If this initial trend extends to the majority of patients treated with PE38 immunotoxins, then we can devise a strategy whereby PE38 immunotoxins are administered first and then treatment is switched to a CET40 immunotoxin using the same targeting antibody. In our experience with PE38 immunotoxins, patients rarely make antibodies to the Fv fragment of the immunotoxin, so this strategy should allow one or two additional cycles of treatment [23].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig 1. Fractions of HB21-CET40 eluted from TSK G3000 column. Fractions 19-30 are shown after migration through a 4-20% Tris–glycine precast gel under reducing and non-reducing conditions. Fractions 28 and 29, marked with an asterisk, were used for experiments described in this paper (EPS 14682 kb)
Supplemental Fig 2. The monoclonal antibody M40-1 was mixed with twice the desired final immunotoxin concentration for 1 hr at room temp and then added to each well of DLD-1 cells in a 96-well format. After a 48-hr incubation, cell viability was assessed using the WST-1 reagent. Each bar represents a replicate of 5 with the error bar indicating one SD. Comparisons of immunotoxin activity with and without antibody incubations are indicated with thin black lines (JPG 84 kb)
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Conflict of interest statement
No conflicts are noted.
Footnotes
(for results sections) Amino acids 270–634 of CET encompass domains II, III and a small sub-domain termed, Ib. For simplicity, domain Ib is not routinely mentioned (also see “Discussion”).
References
- 1.Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 2.Cheson BD, Leonard JP. Monoclonal antibody therapy for B-cell non-Hodgkin’s lymphoma. N Engl J Med. 2008;359:613–626. doi: 10.1056/NEJMra0708875. [DOI] [PubMed] [Google Scholar]
- 3.Heimann DM, Weiner LM. Monoclonal antibodies in therapy of solid tumors. Surg Oncol Clin N Am. 2007;16:775–792. doi: 10.1016/j.soc.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Green DJ, Pagel JM, Pantelias A, et al. Pretargeted radioimmunotherapy for B-cell lymphomas. Clin Cancer Res. 2007;13:5598s–5603s. doi: 10.1158/1078-0432.CCR-07-1223. [DOI] [PubMed] [Google Scholar]
- 5.Rybak SM. Antibody–onconase conjugates: cytotoxicity and intracellular routing. Curr Pharm Biotechnol. 2008;9:226–230. doi: 10.2174/138920108784567272. [DOI] [PubMed] [Google Scholar]
- 6.Liu XY, Pop LM, Vitetta ES. Engineering therapeutic monoclonal antibodies. Immunol Rev. 2008;222:9–27. doi: 10.1111/j.1600-065X.2008.00601.x. [DOI] [PubMed] [Google Scholar]
- 7.Singh Y, Palombo M, Sinko PJ. Recent trends in targeted anticancer prodrug and conjugate design. Curr Med Chem. 2008;15:1802–1826. doi: 10.2174/092986708785132997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brumlik MJ, Daniel BJ, Waehler R, et al. Trends in immunoconjugate and ligand-receptor based targeting development for cancer therapy. Expert Opin Drug Deliv. 2008;5:87–103. doi: 10.1517/17425247.5.1.87. [DOI] [PubMed] [Google Scholar]
- 9.Carter PJ, Senter PD. Antibody–drug conjugates for cancer therapy. Cancer J. 2008;14:154–169. doi: 10.1097/PPO.0b013e318172d704. [DOI] [PubMed] [Google Scholar]
- 10.Goldenberg DM, Sharkey RM. Novel radiolabeled antibody conjugates. Oncogene. 2007;26:3734–3744. doi: 10.1038/sj.onc.1210373. [DOI] [PubMed] [Google Scholar]
- 11.Pastan I, Hassan R, FitzGerald DJ, et al. Immunotoxin treatment of cancer. Annu Rev Med. 2007;58:221–237. doi: 10.1146/annurev.med.58.070605.115320. [DOI] [PubMed] [Google Scholar]
- 12.Kreitman RJ, Pastan I. Immunotoxins in the treatment of refractory hairy cell leukemia. Hematol Oncol Clin North Am. 2006;20:1137–1151. doi: 10.1016/j.hoc.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Ricart AD, Tolcher AW. Technology insight: cytotoxic drug immunoconjugates for cancer therapy. Nat Clin Pract Oncol. 2007;4:245–255. doi: 10.1038/ncponc0774. [DOI] [PubMed] [Google Scholar]
- 14.Frankel AE, Kreitman RJ, Sausville EA. Targeted toxins. Clin Cancer Res. 2000;6:326–334. [PubMed] [Google Scholar]
- 15.Frankel AE, Neville DM, Bugge TA, et al. Immunotoxin therapy of hematologic malignancies. Semin Oncol. 2003;30:545–557. doi: 10.1016/S0093-7754(03)00241-0. [DOI] [PubMed] [Google Scholar]
- 16.Pastan I, Hassan R, Fitzgerald DJ, et al. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–565. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 17.Schnell R, Borchmann P, Staak JO, et al. Clinical evaluation of ricin A-chain immunotoxins in patients with Hodgkin’s lymphoma. Ann Oncol. 2003;14:729–736. doi: 10.1093/annonc/mdg209. [DOI] [PubMed] [Google Scholar]
- 18.Schnell R, Staak O, Borchmann P. A Phase I study with an anti-CD30 ricin A-chain immunotoxin (Ki-4.dgA) in patients with refractory CD30+ Hodgkin’s and non-Hodgkin’s lymphoma. Clin Cancer Res. 2002;8:1779–1786. [PubMed] [Google Scholar]
- 19.Frankel AE. Reducing the immune response to immunotoxin. Clin Cancer Res. 2004;10:13–15. doi: 10.1158/1078-0432.CCR-1216-3. [DOI] [PubMed] [Google Scholar]
- 20.Messmer D, Kipps TJ. Treatment of solid tumors with immunotoxins. Breast Cancer Res. 2005;7:184–186. doi: 10.1186/bcr1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onda M, Beers R, Xiang L, et al. An immunotoxin with greatly reduced immunogenicity by identification and removal of B cell epitopes. Proc Natl Acad Sci USA. 2008;105:11311–11316. doi: 10.1073/pnas.0804851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onda M, Nagata S, FitzGerald DJ, et al. Characterization of the B cell epitopes associated with a truncated form of Pseudomonas exotoxin (PE38) used to make immunotoxins for the treatment of cancer patients. J Immunol. 2006;177:8822–8834. doi: 10.4049/jimmunol.177.12.8822. [DOI] [PubMed] [Google Scholar]
- 23.Posey JA, Khazaeli MB, Bookman MA, et al. A phase I trial of the single-chain immunotoxin SGN-10 (BR96 sFv-PE40) in patients with advanced solid tumors. Clin Cancer Res. 2002;8:3092–3099. [PubMed] [Google Scholar]
- 24.Weldon JE, Xiang L, Chertov O, et al. A protease-resistant immunotoxin against CD22 with greatly increased activity against CLL and diminished animal toxicity. Blood. 2009;113(16):3792–3800. doi: 10.1182/blood-2008-08-173195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kreitman RJ, Wilson WH, Bergeron K, et al. Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N Engl J Med. 2001;345:241–247. doi: 10.1056/NEJM200107263450402. [DOI] [PubMed] [Google Scholar]
- 26.Hassan R, Bullock S, Premkumar A, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 27.Hassan R, Williams-Gould J, Watson T, et al. Pretreatment with rituximab does not inhibit the human immune response against the immunogenic protein LMB-1. Clin Cancer Res. 2004;10:16–18. doi: 10.1158/1078-0432.CCR-1160-3. [DOI] [PubMed] [Google Scholar]
- 28.Knechtle SJ. Treatment with immunotoxin. Philos Trans R Soc Lond B Biol Sci. 2001;356:681–689. doi: 10.1098/rstb.2001.0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pai LH, FitzGerald DJ, Tepper M, et al. Inhibition of antibody response to Pseudomonas exotoxin and an immunotoxin containing Pseudomonas exotoxin by 15-deoxyspergualin in mice. Cancer Res. 1990;50:7750–7753. [PubMed] [Google Scholar]
- 30.Jorgensen R, Purdy AE, Fieldhouse RJ, et al. Cholix toxin, a novel ADP-ribosylating factor from Vibrio cholerae . J Biol Chem. 2008;283:10671–10678. doi: 10.1074/jbc.M710008200. [DOI] [PubMed] [Google Scholar]
- 31.Ogata M, Pastan I, FitzGerald D. Analysis of Pseudomonas exotoxin activation and conformational changes by using monoclonal antibodies as probes. Infect Immun. 1991;59:407–414. doi: 10.1128/iai.59.1.407-414.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batra JK, Fitzgerald DJ, Chaudhary VK, et al. Single-chain immunotoxins directed at the human transferrin receptor containing Pseudomonas exotoxin A or diphtheria toxin: anti-TFR(Fv)-PE40 and DT388-anti-TFR(Fv) Mol Cell Biol. 1991;11:2200–2205. doi: 10.1128/mcb.11.4.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purdy A, Rohwer F, Edwards R, et al. A glimpse into the expanded genome content of Vibrio cholerae through identification of genes present in environmental strains. J Bacteriol. 2005;187:2992–3001. doi: 10.1128/JB.187.9.2992-3001.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchner J, Brinkmann U, Pastan I. Renaturation of a single-chain immunotoxin facilitated by chaperones and protein disulfide isomerase. Biotechnology (N Y) 1992;10:682–685. doi: 10.1038/nbt0692-682. [DOI] [PubMed] [Google Scholar]
- 35.Buchner J, Pastan I, Brinkmann U. A method for increasing the yield of properly folded recombinant fusion proteins: single-chain immunotoxins from renaturation of bacterial inclusion bodies. Anal Biochem. 1992;205:263–270. doi: 10.1016/0003-2697(92)90433-8. [DOI] [PubMed] [Google Scholar]
- 36.Kreitman RJ, Wilson WH, White JD, et al. Phase I trial of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) in patients with hematologic malignancies. J Clin Oncol. 2000;18:1622–1636. doi: 10.1200/JCO.2000.18.8.1622. [DOI] [PubMed] [Google Scholar]
- 37.Dalsgaard A, Albert MJ, Taylor DN, et al. Characterization of Vibrio cgolerae non-O1 serogroups obtained from an outbreak of diarrhea in Lima, Peru. J Clin Microbiol. 1995;33:2715–2722. doi: 10.1128/jcm.33.10.2715-2722.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaudhary VK, Queen C, Junghans RP, et al. A recombinant immunotoxin consisting of two antibody variable domains fused to Pseudomonas exotoxin. Nature. 1989;339:394–397. doi: 10.1038/339394a0. [DOI] [PubMed] [Google Scholar]
- 39.Brinkmann U, Pai LH, FitzGerald DJ, et al. B3(Fv)-PE38KDEL, a single-chain immunotoxin that causes complete regression of a human carcinoma in mice. Proc Natl Acad Sci USA. 1991;88:8616–8620. doi: 10.1073/pnas.88.19.8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matthews DJ, Goodman LJ, Gorman CM, et al. A survey of furin substrate specificity using substrate phage display. Protein Sci. 1994;3:1197–1205. doi: 10.1002/pro.5560030805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zdanovsky AG, Chiron M, Pastan I, et al. Mechanism of action of Pseudomonas exotoxin. Identification of a rate-limiting step. J Biol Chem. 1993;268:21791–21799. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig 1. Fractions of HB21-CET40 eluted from TSK G3000 column. Fractions 19-30 are shown after migration through a 4-20% Tris–glycine precast gel under reducing and non-reducing conditions. Fractions 28 and 29, marked with an asterisk, were used for experiments described in this paper (EPS 14682 kb)
Supplemental Fig 2. The monoclonal antibody M40-1 was mixed with twice the desired final immunotoxin concentration for 1 hr at room temp and then added to each well of DLD-1 cells in a 96-well format. After a 48-hr incubation, cell viability was assessed using the WST-1 reagent. Each bar represents a replicate of 5 with the error bar indicating one SD. Comparisons of immunotoxin activity with and without antibody incubations are indicated with thin black lines (JPG 84 kb)