Summary
Over the past decade, wingless-activated (WNT) medulloblastoma has been identified as a candidate for therapy de-escalation based on excellent survival; however, a paucity of relapses has precluded additional analyses of markers of relapse. To address this gap in knowledge, an international cohort of 93 molecularly confirmed WNT MB was assembled, where 5-year progression-free survival is 0.84 (95%, 0.763–0.925) with 15 relapsed individuals identified. Maintenance chemotherapy is identified as a strong predictor of relapse, with individuals receiving high doses of cyclophosphamide or ifosphamide having only one very late molecularly confirmed relapse (p = 0.032). The anatomical location of recurrence is metastatic in 12 of 15 relapses, with 8 of 12 metastatic relapses in the lateral ventricles. Maintenance chemotherapy, specifically cumulative cyclophosphamide doses, is a significant predictor of relapse across WNT MB. Future efforts to de-escalate therapy need to carefully consider not only the radiation dose but also the chemotherapy regimen and the propensity for metastatic relapses.
Keywords: medulloblastoma, WNT, subgroup, chemotherapy, radiation, wingless, recurrence, methylation, expression, survival
Graphical Abstract

Highlights
Maintenance chemotherapy regimen appears to affect survival in WNT medulloblastoma
WNT medulloblastoma recurs most frequently with metastasis in the lateral ventricles
Outcome of relapsed WNT medulloblastoma is poor, with limited salvage potential
Relapse of WNT medulloblastoma is not predicted by clinical risk stratification
Nobre et al. analyze a clinically annotated cohort of 93 WNT-activated medulloblastoma using an integrated genomic approach. A maintenance chemotherapy regimen is the strongest predictor of relapse, and cumulative cyclophosphamide doses confer a reduced risk of relapse. Unlike other medulloblastoma subgroups, WNT-activated medulloblastoma recurs most frequently in the lateral ventricles.
Introduction
The 2016 World Health Organization (WHO) classification has stratified medulloblastoma into four molecular groups (wingless-activated [WNT], sonic hedgehog-activated [SHH], group 3, and group 4) based on clear biological and clinical differences, and the current challenge is to define an optimal therapy regimen for each.1,2 Currently, all children with medulloblastoma are treated uniformly with surgery followed by craniospinal irradiation and combination chemotherapy, and although these treatments improve outcome, they also result in major life-long cognitive, neurological, and neuroendocrine side effects. Across most prospective and retrospective studies, WNT medullablastoma (MB) has been recognized as having excellent outcomes with current therapy, particularly in patients below the age of 16.3 Hence, WNT MB is the subject of trials of de-escalation of therapy,2,4 with three studies open worldwide for de-escalation of radiation therapy and one study investigating the role of chemotherapy-only regimens (15 Gy, NCT:NCT01878617; 18 Gy, NCT:NCT02724579 and NCT:NCT02066220; chemotherapy only, NCT: NCT02212574). Unfortunately, the study investigating post-surgical chemotherapy only, with maintenance chemotherapy according to the ACNS0331 protocol, was stopped prematurely because of an unacceptable number of early failures, suggesting that a deeper understanding of relapsed WNT MB is urgently required.5
Although most studies have shown that WNT MB has an excellent outcome, the relationship between treatment regimen and outcome is still an area of investigation. Adult WNT MB was first shown to portend to a poorer outcome, and it has been suggested that patients with WNT MB over the age of 16 should not be included in trials of de-escalation of therapy.6,7 A review of treatment failures in the European Society for Pediatric Oncology (SIOPe)-PNET4 study revealed a surprisingly high number of WNT MB relapses (8 of 58, 14%) using nuclear β-catenin as a marker of WNT activation.8,9 This dedicated study of relapsed individuals in the SIOPe-PNET4 study reveals that 5 of 8 WNT relapses involved individuals under the age of 16. However, the reliance on nuclear β-catenin to diagnose WNT MB and lack of genome-wide methylation-based subgrouping cast doubt on whether these were simply misdiagnosed.10 It has been postulated that WNT MB has improved survival because of an impaired blood-brain barrier, suggesting that chemotherapy is an important mainstay of therapy; however, there is a paucity of literature suggesting that survival may be different based on treatment.11 Although it is well recognized that WNT failures do occur, these instances have been poorly characterized, and specifically the pattern of relapse and markers of prognosis are unclear. To start addressing these questions, we assembled a cohort of 93 molecularly confirmed individuals with WNT MB having received heterogeneous treatment, examined whether particular treatment regimens might predict prognosis, and observed that use of high-dose cyclophosphamide-based adjuvant chemotherapy is a strong predictor of outcome.
Results
Demographics and Survival of the Entire Cohort
Ninety-three cases of WNT MB were collected with accompanying clinical information between 1990 and 2017 (Table S1). The diagnosis of WNT MB was established using genome-wide methylation profiling, with WNT MB assigned applying the Heidelberg Brain Tumor Classifier in 84 cases (https://www.molecularneuropathology.org/mnp). A calibrated score of 0.9 or above is regarded as WNT MB and considered the gold standard for diagnosis of WNT MB.12 In addition, t-distributed stochastic neighbor embedding (t-SNE) analysis of the top differentially methylated probes was performed with a cohort of 401 MBs, and all 84 cases cluster tightly with the WNT group. In 63 cases, nanoString limited gene expression profiling of 22 signature genes was also performed, showing a very strong WNT signature, with confidence scores exceeding 0.99 in all cases. In 8 cases, methylation profiling was unavailable, and at least two methods were employed. In two of these cases, WNT MB was ascribed using nanoString limited gene expression profiling, monosomy 6, and β-catenin nuclear immunopositivity, as determined by an expert pediatric neuropathologist (C.E.H.). In 6 cases, WNT MB was diagnosed by direct sequencing of exon 3 of CTNNB1 and fluorescence in situ hybridization (FISH) for monosomy 6. Full details of subgrouping methods are provided in Table S1.
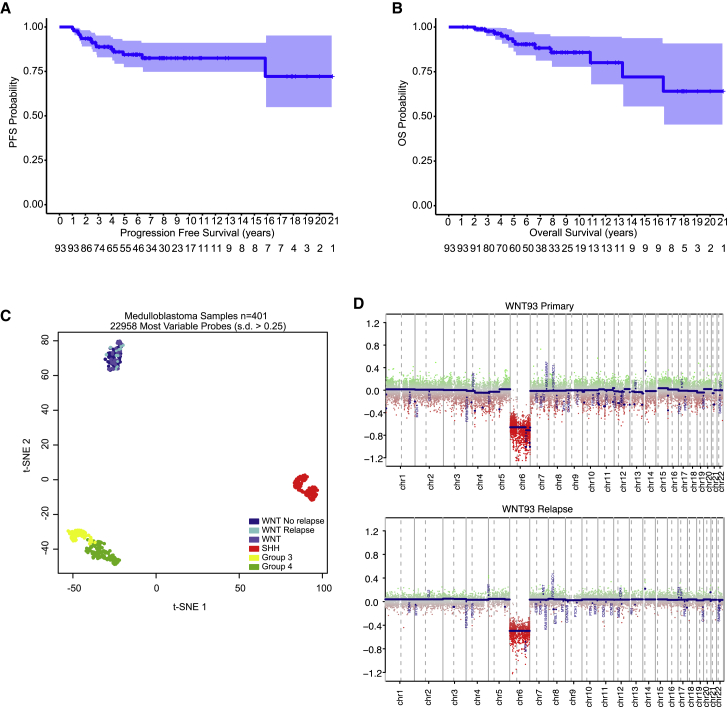
Across the entire cohort, 15 relapsed individuals were identified, with a 5-year progression-free survival of 0.84 (95% confidence interval [CI], 0.763–0.925) and 5-year overall survival across the cohort of 0.929 (95% CI, 0.871–0.992) (Figures 1A and 1B). Considering that this rate of relapse was higher than in the literature, we performed t-SNE analysis of WNT MB and confirmed that the 15 relapsed cases clustered tightly with all WNT MBs as a single entity (Figure 1C). This confirms that relapsed WNT MB does not form a separate entity and suggests that other factors are leading to relapse. One late relapse was identified 15.8 years after diagnosis, and methylation profiling of primary and relapse confirmed that it was still a WNT MB and not a radiation-induced glioblastoma. Only one relapsed case did not have methylation profiling available but was ascribed a WNT MB using three methods: β-catenin nuclear immunopositivity, monosomy 6, and nanoString. Gene expression arrays were available for 53 cases, including 7 relapses; however, only 18 differentially expressed genes could be identified between those with progression and those without, which were not associated with any single pathway. t-SNE analysis and unsupervised hierarchical clustering of the top 200 differentially expressed genes did not show WNT MB relapses segregating as a distinct group (Figure S1).
Figure 1.
Predictors of Relapse in WNT MB
(A and B) Progression-free (A) and overall (B) survival of WNT MB. Light blue shading represents 95% CIs.
(C) t-SNE visualization of 401 primary MB samples profiled by genome-wide methylation profiling. WNT cases are divided into those with no relapse, relapse, and no clinical information.
(D) Copy number plot of a late relapse after 16 years, confirming monosomy 6 (WNT subgroup) at diagnosis and relapse. One late death was not confirmed to be a relapse and was attributed to chronic respiratory failure secondary to long-standing neurological dysfunction and possible tracheostomy failure.
Genomic Profiling of a Very Late Relapse
Tissue from diagnosis and relapse was available from a subject with a relapse in the lateral ventricles 15.8 years post-diagnosis, treated under the closed protocol CCG9961B. Genome-wide methylation profiling of this case using the Molecular Neuropathology 2.0 classifier revealed that the primary tumor and the recurrence still classified as a WNT with a calibrated score of 0.99, with an identical copy number plot across primary and relapse (Figure 1D). Pathological review of the relapse sample was consistent with an MB with β-catenin nuclear immunopositivity.
Factors Predictive of Relapse
To determine whether any clinical risk factors of relapse could be identified, we then proceeded to compare relapsed and non-relapsed WNT MBs. Known risk factors, such as metastatic disease and extent of resection, showed no difference in survival (Table 1; Figure S2). Copy number profiles were available in 86 of 93 samples and did not differ between relapsed and non-relapsed individuals. Monosomy 6 was observed in 92 of 93 samples (98.92%), and 17p loss (a surrogate for TP53 mutational status) was observed in 9 of 86 samples (10.4%), with no differences in survival discerned (Table 1; Figure S2).13 Specifically, 14 of 15 relapsed individuals harbored monosomy 6 (p = 0.63, Fisher’s exact test), and 3 of 15 relapsed individuals had 17p loss (p = 0.61, Fisher’s exact test). Male gender was a significant predictor of relapse (p = 0.033). Full clinical details of all 15 relapsed WNT MBs are presented in Table 2. When separating centers by geographical location and/or affiliation with the SIOPe or Children’s Oncology Group (COG), 6 were COG centers, 6 were SIOPe centers, 1 was in Asia, and 2 were in Eastern Europe (Table S1). Eight of 14 relapsed individuals received chemotherapy according to CCG9961 regimen A, with one late relapse at 15.8 years treated with CCG9961 regimen B (Table 2).
Table 1.
Comparison of Demographics and Treatment of WNT MB, Stratified by Relapse
| No Relapse | Relapse | p Value | |
|---|---|---|---|
| Age years (median + IQR) | 10 (8–14.2) | 11 (9.75–14.35) | 0.45 |
| Gender | 0.09 | ||
| Male | 27 | 9 | |
| Female | 50 | 6 | |
| M-status | 0.65 | ||
| M0 | 65 | 13 | |
| M+ | 7 | 2 | |
| Extent of Resection | 0.34 | ||
| GTR | 64 | 15 | |
| STR | 8 | 0 | |
| Craniospinal Irradiation Dose | 1 | ||
| ≤23.4 Gy | 37 | 8 | |
| ≥23.4 Gya | 35 | 7 | |
| Chemotherapy | 0.032 | ||
| No chemotherapy or older protocols | 18 | 6 | |
| CCNU-based | 26 | 8 | |
| Cyclophosphamide/ifosfamide-based (>12 g/m2) | 30 | 1 | |
| Chromosome 6 | 0.68 | ||
| Balanced chromosome 6 | 10 | 1 | |
| Monosomy 6 | 66 | 14 | |
| Chromosome 17p | 0.19 | ||
| 17p balanced | 64 | 12 | |
| 17p loss | 6 | 3 |
GTR, gross total resection; STR, subtotal resection with residual >1.5 cm2. Cyclophosphamide/ifosfamide includes ICE regimens. CCNU-based includes treatment according to ACNS0331. Chromosomes 6 and 17 are arm-level losses. The p values are from a Fisher’s exact test, except age at diagnosis was determined using a Mann-Whitney U test. Bold p-values represent values below 0.05.
≥23.4-Gy craniospinal irradiation (CSI) includes 3 patients with 25-Gy CSI and 2 patients with 30.6-Gy CSI.
Table 2.
Subject Characteristics and Survival of 15 Relapsed WNT MBs
| ID | Age (years)/Gender | Stage | OS (Years) | PFS (Years) | Relapse Pattern | CSI Dose (Gy) | Initial Chemotherapy | Status |
|---|---|---|---|---|---|---|---|---|
| 1 | 15.0/M | M0 | 2 | 1.4 | diffuse mets | 23.4 | 8 in 1a | DOD |
| 2 | 18.5/M | M0 | 4.9b | 4.8 | frontal horn lateral ventricles | 40.0 | none | died |
| 3 | 10.0/F | M0 | 4.0 | 2.7 | tumor bed | 36.0 | carbo/CTX/VPc | DOD |
| 4 | 9.0/M | M1 | 13.2 | 6.3 | diffuse mets | 36.0 | carbo/CTX/VPc | DOD |
| 5 | 7.7/M | M0 | 1.0 | 1.0 | extraneural | 35.2 | none | AWD |
| 6 | 56.3/M | M2 | 8.6 | 4 | tumor bed | 34.2 | none | AWD |
| 7 | 10.5/F | M0 | 7.8 | 2.8 | frontal horn lateral ventricles | 23.4 | 9961Ad | DOD |
| 8 | 9.9/M | M0 | 3.5 | 1 | frontal horn lateral ventricles | 23.4 | ACNS0331e | DOD |
| 9 | 11/M | M0 | 2.5 | 2.5 | frontal horn lateral ventricles | 23.4 | 9961Ad | AWD |
| 10 | 6.4/M | M0 | 17 | 1.4 | frontal horn lateral ventricles | 23.4 | 9961Ad | alive |
| 11 | 16.0/F | M0 | 6.6 | 2.4 | frontal horn lateral ventricles | 23.4 | 9961Ad | DOD |
| 12 | 9.5/F | M0 | 5.0 | 4.1 | diffuse mets | 36.0 | cisplatin, vincristine | ANED |
| 13 | 11.2/F | M0 | 4.5 | 1.6 | tumor bed | 36.0 | 9961Ad | AWD |
| 14 | 13.7/M | M0 | 1.6 | 2.8 | lateral ventricles and spinal | 23.4 | 9961Ad | DOD |
| 15 | 12/F | M0 | 15.8 | 16.4 | lateral ventricles | 23.4 | 9961Bf | DOD |
AWD, alive with disease; ANED, alive with no evidence of disease; DOD, dead of disease; CSI, craniospinal irradiation; CTX, cyclophosphamide; VP, VP16.
Chemotherapy scheme with vincristine, hydroxyurea, procarbazine, CCNU, cisplatin, cytosine arabinoside (Ara-C), methylprednisolone, and either cyclophosphamide in 1 day.
Died of massive hemorrhage.
Neo-adjuvant chemotherapy (PNET III).
9961A chemotherapy consists of 8 cycles of cisplatin, CCNU, and vincristine.
ACNS0331 chemotherapy consists of 9 cycles of AABAABAAB, with A cycles having cisplatin, CCNU, and vincristine and B cycles having cyclophosphamide with vincristine.
9961B chemotherapy consists of 8 cycles of cisplatin, cyclophosphamide, and vincristine.
Of the 15 relapsed individuals, 10 were confirmed to have died, 4 were alive with disease, and 1 was a confirmed long-term survivor. The single individual where salvage was possible was initially treated with a gross total resection and 23.4 Gy of craniospinal irradiation with a posterior fossa boost to 55.8 Gy, followed by 8 cycles of cisplatin, CCNU, and vincristine. A relapse was observed along the ependymal surface of the frontal horn of the lateral ventricle 18 months post-completion of therapy and treated successfully with oral etoposide induction, followed by a single cycle of busulfan and thiotepa with autologous stem cell transplant and 30 Gy of focal radiation to the frontal lobes. This individual is currently 16 years post-relapse with no evidence of disease.
Survival Stratified by Treatment Protocol
Detailed chemotherapy protocols were available in 90 of 93 subjects, and detailed radiotherapy dosage with fields available in 87 of 93 subjects. Twenty-three individuals received craniospinal radiotherapy only, and although a trend of improved survival in those receiving maintenance chemotherapy was observed, it was not significant (hazard ratio [HR], 0.518; 95% CI, 0.1738–1.545; p = 0.17). Comparison between reduced-dose (23.4 Gy) and standard-dose (36 Gy or more) craniospinal irradiation did not result in different survival (Figure S1). Because 9 of 15 relapsed individuals received maintenance chemotherapy, we sought to determine if there were discrepancies in their treatment regimen. Strikingly, when we divided the cohort based on cyclophosphamide dosing, those with no or intermediate doses of cyclophosphamide and/or CCNU-based maintenance (CCNU ± cyclophosphamide 6 g/m2) and primarily high doses of nitrogen mustard-based chemotherapy (cyclophosphamide ≥ 12 g/m2 or an equivalent dose of ifosphamide), we observed a single very late failure in those receiving high-dose cyclophosphamide or ifosphamide therapy (Figure 2). Five-year progression-free survival with CCNU-based regimens was 0.723 (95% CI, 0.574–0.911), and 5-year overall survival was 0.878 (95% CI, 0.757–1), with 7 of 8 relapses occurring within 4 years of radiotherapy. A multivariable Cox regression analysis confirmed that this survival difference across maintenance chemotherapy regimens was independent of age at diagnosis, male gender, metastatic status, and radiation dose (Table S2). Restricting the analysis to children receiving modern chemotherapy protocols showed this same pattern, with children on cisplatin and CCNU-based protocols having significantly inferior survival than those treated with high-dose cyclophosphamide or ifosfamide (Table S3). Male gender remained a significant predictor of relapse in the multivariable analysis. An individual aged 2.7 years was treated using a radiation-sparing approach using CCG99703 comprising 3 induction cycles and 3 high-dose chemotherapy cycles with autologous stem cell support and is alive and progression-free 5 years post-completion of therapy. Taken together, this suggests that high-dose cyclophosphamide/ifosfamide-based chemotherapy regimens result in a significantly improved outcome.
Figure 2.
Effect of Maintenance Chemotherapy on Survival in WNT MB Treated with Modern Protocols
(A and B) Progression-free survival (A) and overall survival (B) of WNT MB stratified by individuals receiving no cyclophosphamide or high-dose cyclophosphamide/ifosfamide-based maintenance chemotherapy regimens. The p values were determined using a log rank test. Lighter shading around the survival curve represents 95% CIs
Anatomical Location of Relapse in WNT MB
Finally, we queried the clinical data for any trends suggestive of a pattern of relapse. The location of relapse was available for all 15 patients; 12 relapsed in the metastatic compartment, 3 relapsed in the surgical cavity, and one outside of the neuroaxis. Interestingly, of the 11 metastatic relapses, 8 were in the lateral ventricles, with 6 of the 8 restricted to the frontal horn (Table 2). The remaining 4 were diffuse metastatic relapses, with one being extraneural.
Discussion
Despite WNT MB considered as having an excellent prognosis, we observed a relatively high relapse rate of 15% in our cohort. Although the relapse rate we observed is likely an overestimate based on the retrospective design of the cohort, the finding that individuals receiving higher doses of cyclophosphamide therapy do not relapse suggests that the chemotherapy regimen administered will be crucial as we reduce radiation doses.
It has been shown previously that WNT-MB has a different vasculature suggestive of a unique microenvironment, leading to improved responses to chemotherapy, specifically vincristine.11 Most cohorts have suggested excellent outcomes for WNT patients with few relapses; however, analysis of relapses in the PNET4 study suggested that individuals with WNT MB relapse in almost 15% of cases.8 In PNET3, the 5-year event-free survival of individuals with WNT MB was 88.9% (3 of 27 relapses), where the cumulative dose of cyclophosphamide was 3 g/m2 in those treated with chemotherapy, and the dose of craniospinal irradiation was 36 Gy.14, 15, 16 In contrast, St. Jude's Medulloblastoma ′96 [SJMB96] showed 100% survival with high-dose cyclophosphamide therapy for individuals with WNT MB, but this was only a cohort of 10 subjects.17 Our observation of a confirmed very late relapse of WNT MB has not been described previously and warrants long-term follow-up, particularly with those enrolled in recently completed trials with available molecular subgrouping. A limitation of these studies could be the reliance on nuclear β-catenin rather than robust subgrouping using DNA methylation or multiple methods, but these results are indeed consistent with our findings. Unfortunately, previous trial cohorts of average-risk medulloblastoma specifically CCG9961 which randomized CCNU to cyclophosphamide, have not been subgrouped as tissue was not prospectively collected. As such, a definitive validation of the results of our study are not possible.18
Indeed, the eventual publication of recently closed trial cohorts may help validate our finding that high-alkylator therapy results in improved survival in WNT MB compared with CCNU-based treatments as prescribed in PNET4 and ACNS0331. Evaluating the geographic location of treatment in our cohort, WNT MB relapses were treated globally, with the majority of cases being treated at COG or SIOPe centers. In addition, half of the individuals receiving cyclophosphamide in this cohort were outside of SIOPe or COG centers, suggesting that location of therapy is not an unobserved variable accounting for our findings.19
In our study, clinical risk factors, such as extent of resection and metastatic disease, were not significant predictors of relapse in WNT MB, which is consistent with several previous reports16,19,20. A recent retrospective study across 78 WNT MBs treated at the Burdenko Neurosurgical Institute was also consistent with these clinical risk factors not being significant predictors of outcome.12 Interestingly, this study also had heterogeneous maintenance regimens, including treatment according to St. Jude's Medulloblastoma ′03 [SJMB03] and German Society of Pediatric Oncology and Hematology (HIT) protocols, but did not reveal a similar propensity for CCNU-based regimens having worse outcomes. However, one potential difference is that the majority of individuals in this series treated with CCG9961 regimen A maintenance chemotherapy (cisplatin, CCNU, vincristine) also received 36 Gy of craniospinal irradiation, and high-risk individuals received intrathecal methotrexate. Therefore, it is plausible that the intensification of therapy in the majority of subjects in this study accounts for the excellent survival observed. In our cohort, individuals treated with CCG9961A- or ACNS0331-based regimens received reduced-dose craniospinal irradiation of 23.4 Gy. It has been suggested that individuals with WNT MB over the age of 16 are a higher-risk group; however, in our cohort, age was not a significant risk factor for relapse, and 13 of 14 relapses were in individuals 16 years of age or younger.3,9 Moreover, previous observations that adults with WNT MB do poorly may pertain to the frequent omission of chemotherapy in this group rather than any biological risk factors.6
Our findings have profound implications for the design of de-escalation studies for WNT MB and suggest that serious consideration should be given to the chemotherapy regimen. Recently, a study of surgery and chemotherapy only was suspended to accrual after an unacceptable number of relapses (NCT: NCT02212574).5 In this study, only chemotherapy was administered, according to ACNS0331, with 6 g/m2 of cyclophosphamide and 450 mg/m2 of CCNU. Several studies of radiation reduction are ongoing through the Children's Oncology Group (ACNS1422 and NCT: NCT02724579), SIOPe (PNET5 and NCT: NCT02066220), and St. Jude’s (SJMB12 and NCT: NCT01878617). Completion of these studies with appropriate follow-up will take at least another decade; however, our findings should serve as a warning that future efforts to de-escalate the radiation dose in WNT MB will likely require careful consideration of the chemotherapy protocol and at least 12 g/m2 of cyclophosphamide. An intriguing possibility is use of radiation-sparing protocols, such as Headstart or CCG99703; however, WNT MB is rare in infancy, resulting in a paucity of preliminary data with this approach. Although cyclophosphamide-based protocols have a less favorable toxicity profile (specifically, ovarian failure in females, alopecia, and more profound myelosuppression), our results suggest that, when designing studies of de-escalation of therapy, the chemotherapy regimen needs to be accounted for.
Our study reveals that WNT MB has a unique pattern of relapse, with a significant proportion of relapses in the lateral ventricles, with the remainder of failures in the surgical tumor bed or the leptomeninges. We reported previously that the pattern of relapse in MB is highly subgroup specific, with SHH recurring in the surgical cavity in 50%–60% of instances and groups 3 and 4 recurring almost exclusively in the leptomeninges in previously irradiated individuals.21 In this previous study, the pattern of relapse in WNT MB could not be evaluated because of a paucity of cases; our observations add significantly to our understanding of the pattern of relapse and suggest that the ependymal lining is a unique microenvironment conducive to WNT MB. Generation of additional WNT MB models will be required to fully discern the biological implications of this finding, but potential options to de-escalate therapy may include direct intraommaya delivery of chemotherapy to the lateral ventricles.
A limitation of our findings is its retrospective design and lack of tissue from all relapsed samples. Indeed, it has become more apparent that a significant subset of MB relapses, particularly late relapses, are, in fact, radiation-induced glioblastoma, but our one relapse is still a WNT MB 15 years after diagnosis. Consideration for biopsy at relapse with genome-wide methylation profiling should be undertaken in future studies to exclude this possibility, although radiation-induced glioblastoma typically arises at least 5 years post-diagnosis.22,23 Furthermore, our study is limited by a lack of granularity regarding the quality of radiotherapy and adherence to protocol, which may account for unobserved variables portending relapse. Our retrospective design may also result in overestimation of relapses, suggesting that the difference between treatment regimens in trial cohorts may, in fact, be considerably less pronounced. However, the retrospective heterogeneous treatment across our cohort allowed us to identify that the chemotherapy protocol is potentially an important predictor of outcome. In the future, a pooled analysis of trial cohorts will be necessary to address this issue. Our study highlights the importance of incorporating specific treatment protocols when evaluating retrospective biologically stratified data, which can provide robust hypothesis generation to be tested and validated in prospective studies.
Overall, our findings significantly advance our understanding of the clinical behavior of WNT MB and provide several key insights to help inform future treatment protocols aimed at de-escalating therapy. Future studies of WNT MB medulloblastoma, particularly those aimed at therapy de-escalation, need to give careful consideration to the maintenance chemotherapy protocol and the unique pattern of relapse observed in this subgroup.
STAR★Methods
Key Resource Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological Samples | ||
| 93 primary medulloblastoma samples | 24; This study | N/A |
| Critical Commercial Assays | ||
| Infinium® HumanMethylation450 BeadChip Kit | Illumina, San Diego, USA | N/A |
| Infinium MethylationEPIC BeadChip Kit | Illumina, San Diego, USA | N/A |
| Deposited Data | ||
| Methylation array data (53 previously published samples) | 24 | GEO: GSE85218 (Table S1) |
| Expression array (53 previously published samples) | 24 | GEO: GSE85217 (Table S1) |
| Methylation array data (30 unpublished samples) | This study | Mendeley, https://doi.org/10.17632/cw37zdmgm3.2 (Table S1) |
| Software and Algorithms | ||
| minfi R Bioconductor package (v1.22.0) including Illumina normalization method | 25 | http://bioconductor.org/packages/release/bioc/html/minfi.html |
| Conumee (v.1.8.0) | 26 | https://bioconductor.org/packages/release/bioc/html/conumee.html |
| Molecular Neuropathology 2.0 | 27 | https://www.molecularneuropathology.org/mnp |
| Rtsne (v 0.11.17) | 28 | https://cran.r-project.org/web/packages/Rtsne/index.html |
| Multiple Experiment Viewer (MeV) | 29 | http://mev.tm4.org |
| R2: Genomics Analysis and Visualization Platform | R2 Support Team, Amsterdam Medical Center | https://hgserver1.amc.nl:443/ |
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Vijay Ramaswamy (vijay.ramaswamy@sickkids.ca), Assistant Professor, Departments of Medical Biophysics and Paediatrics, University of Toronto; Division of Haematology/Oncology, Hospital for Sick Children, Toronto, ON, Canada.
Materials Availability
This study did not generate new, unique reagents.
Data and Code Availability
The DNA methylation datasets unique to this study are available at Mendeley Data, https://doi.org/10.17632/cw37zdmgm3.2
Experimental Model and Subject Details
Human Subjects
We assembled a cohort of 93 cases of molecularly confirmed WNT medulloblastoma identified through the Medulloblastoma Advanced Genomics International Consortium, the Hospital for Sick Children, the Royal Children’s Hospital Melbourne and the University Hospital Motol in Prague. Treatment details including radiation dose and field, chemotherapy protocol, extent of surgical resection, gender and survival were available and discerned via retrospective chart review (Table 1; Complete Demographics available in Table S1). Updated clinical data with treatment annotations were obtained for WNT-MB cases previously published in Cavalli et al.24 WNT subgroup was ascribed using genome wide methylation arrays in 84 patients, specifically a calibrated score above 0.9 using the Molecular Neuropathology 2.0 algorithm (https://www.molecularneuropathology.org/mnp) and t-SNE analysis with a published cohort of medulloblastoma (GEO: GSE85218). Fifty-three samples were profiled using Affimetrix Human Gene 1.1 ST Arrays (GEO: GSE85217). Array number and .idat file identifiers are included in Table S1. An additional 2 samples were included based on the presence of clear nuclear beta-catenin accumulation, monosomy 6 by SNP array and a strong signature of WNT-MB by nanoString limited gene expression profiling, and an additional 6 samples were included based on concurrent mutation in exon 3 of beta-catenin and monosomy 6. All samples and clinical annotations were collected in accordance to research institutional review boards at participating institutions and at the Hospital for Sick Children.
Method Details
DNA Methylation Profiling
Samples were analyzed on the Illumina Infinium HumanMethylation450 or Illumina MethylationEPIC arrays at the PM-OICR Translational Genomics Laboratory or the Center for Applied Genomics (Toronto, ON) according to manufacturer’s instructions as previously described.24,30 All analysis was conducted in the R Statistical Environment (v3.4.1). Raw data files (.idat) generated by the Illumina iScan array scanner from both frozen and FFPE derived tissue were loaded and pre-processed using the minfi package (v1.22.1).25 Illumina pre-processing was selected to mimic the normalization performed in Illumina Genome Studio. Methylation beta values were calculated as described in Illumina’s protocols. Subsequently, the following filtering criteria were applied: Removal of probes targeting the X and Y chromosomes (n = 11,551), removal of probes containing a single nucleotide polymorphism (dbSNP132 Common) within five base pairs of and including the targeted CpG-site (n = 24,536), and probes not mapping uniquely to the human reference genome (hg19) allowing for one mismatch (n = 9,993). In total, 438,370 probes were kept for analysis. Medulloblastoma subgroup was ascribed using the Heidelberg brain tumor classifier (https://www.molecularneuropathology.org/mnp).
In order to consider distinct molecular signatures, we performed unsupervised clustering of DNA methylation data generated from previously published cases clearly identified as one of the four subgroups by the Heidelberg Brain Tumor Classifier.24,27 In addition, we repeated unsupervised clustering only for samples which were classified as WNT MB. For unsupervised clustering, we selected the most variable probes with a median absolute deviation > 0.25. Distance between samples was calculated using Pearson correlation coefficient as the distance measure and the same distance matrix was used to perform the t-distributed stochastic neighbor embedding (tSNE) analysis using the Rtsne package version 0.11.17. The following nondefault parameters were used: theta = 0, is_distance = T, pca = F, max_iter = 10000.28 Copy number profiles were generated using the ‘conumee’ package (v1.8.0) as previously described.26
Gene Expression Analysis
Fifty-three samples were previously published using Affymetrix Human Gene 1.1 ST Arrays (GEO: GSE85217), and analysis conducted in the R2: Genomics Analysis and Visualization Platform (https://hgserver1.amc.nl:443/) and within the Multi Experiment Viewer. Samples were normalized using RMA in R2, and t-SNE analysis performed within R2 comparing recurrent versus non-recurrent WNT-MB. Differential gene expression was performed comparing relapsed and non-relapsed WNT-MB using ANOVA and an FDR of 0.01, and pathway analysis performed using KEGG and GO terms. Unsupervised hierarchical clustering was performed in the Multi Experiment Viewer (MeV_4_8) using the top 200 differentially expressed genes ranked by median absolute deviation.29
Quantification and Statistical Analysis
Complete subject data can be found in Table S1, with details of statistical tests found in the figure legends and table footers. Progression-free survival and overall survival were analyzed by the Kaplan-Meier method and p values reported using the log-rank test. Associations between covariates and risk groups were tested by the Fishers exact test. Multivariable cox proportional hazard regression was performed using Firth’s penalized maximum likelihood bias reduction method to estimate hazard ratios including 95% confidence intervals. The proportional-hazards assumption was tested using the cox.zph function in the survival package and graphical inspection of Schoenfeld residual plots, and was not statistically significant for any of the co-variates. All statistical analyses were performed in the R statistical environment (v3.6.0), using R packages survival (v2.41-3), coxphf (v1.13) and ggplot2 (v2.2.1).
Acknowledgments
L.N. was supported by a Meagan’s Walk fellowship in pediatric neuro-oncology and the Hospital for Sick Children Clinician Scientist Training Program. V.R. is supported by operating funds from the Canadian Institutes of Health Research, the Brain Tumour Foundation of Canada, the American Brain Tumor Association, the C.R. Younger Foundation, Nelina’s Hope, Meagan’s Walk, the Garron Family Cancer Center, and b.r.a.i.n.child. V.R. and M.D.T. are supported by a Stand Up To Cancer (SU2C) St. Baldrick’s Pediatric Dream Team translational research grant (SU2C-AACR-DT1113). M.D.T. is supported by operating funds from the National Institutes of Health (5R01CA159859-08 and R01NS106155-01) and the Pediatric Brain Tumor Foundation. E.B. is supported by the Garron Family Chair in Childhood Cancer Research of the Hospital for Sick Children and University of Toronto. M. Zapotocky is supported by the Charles University Grant Agency (PRIMUS/19/MED/06) and MH CZ – DRO, University Hospital Motol, Prague, Czech Republic 00064203. M. Zollo is supported by the Italian Association for Cancer Research (AIRC 2019–2024) grant IG #2219. E.G.V.M. is supported by NIH grants R01NS096236 and R01CA235162 and the CURE Childhood Cancer Foundation. M.M. is supported by Associazione Con Lorenzo per Mano, Como, Italy. Tumor samples and coded data were supplied by the Children’s Cancer Centre Tissue Bank at the Murdoch Children’s Research Institute and The Royal Children’s Hospital. Establishment and running of the Children’s Cancer Centre Tissue Bank is made possible through generous support from Cancer In Kids @ RCH (http://cika.org.au/), The Royal Children's Hospital Foundation, and the Murdoch Children's Research Institute. This study was partially funded with support provided by the Government of Ontario Ministry of Research, Innovation and Science and the Princess Margaret Cancer Foundation.
Author Contributions
Conceptualization, L.N., E.B., and V.R.; Methodology, L.N., M.Z. (Michal Zapotocky), and V.R.; Investigation, L.N. and V.R.; Data Curation, M.Z. (Michal Zapotocky), S.K., D.S., A.V., W.A.G., J.T., K.K.W.L., H.-K.N., L.M., J.Y.L., S.-K.K., S.Z., A.V., C.F.-C., P.H., B.L., M.-L.v.V.-V., P.J.F., E.G.V.M., W.A.W., N.G., I.F.P., R.L.H., A.A.N.R., C.G., J.B.R., A.S.M., L.B.C., R.V., Y.S.R., M.M., R.E.M., H.W., M.Z. (Massimo Zollo), V.F., T.K., C.C.F., J.S., S.J., E.L.-A., J.M., C.G.C., J.M.O., S.L., J.C., L.K., J.Z., C.E.H., U.T., A.H., U.B., M.D.T., J.R.H., and V.R.; Formal Analysis, L.N., M.Z.(Michal Zapotocky), K.F., A.F., and V.R.; Writing – Review & Editing, L.N., E.B., and V.R.; Resources, M.Z. (Michal Zapotocky), S.K., D.S., A.V., T.M., W.A.G., J.T., K.K.W.L., H.-K.N., L.M., J.Y.L., S.-K.K., S.Z., A.V., C.F.-C., P.H., B.L., M.L.v.V.-V., P.J.F., E.G.V.M., W.A.W., N.G., I.F.P., R.L.H., A.A.N.R., C.G., J.B.R., A.S.M., L.B.C., R.V., Y.S.R., M.M., R.E.M., H.W., M.Z. (Massimo Zollo), V.F., T.K., C.C.F., J.S., S.J., E.L.-A., J.M., C.G.C., J.M.O., S.L., J.C., L.K., J.Z., C.E.H., U.T., A.H., U.B., M.D.T., S.Y., J.R.H., and V.R., Project Administration, E.B. and V.R.; Supervision, V.R.; Funding Acquisition, M.Z. (Michal Zapotocky), J.C., M.D.T., and VR.
Declaration of Interests
The authors declare no competing interests.
Published: June 23, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.xcrm.2020.100038.
Supplemental Information
References
- 1.Nör C., Ramaswamy V. Clinical and pre-clinical utility of genomics in medulloblastoma. Expert Rev. Neurother. 2018;18:633–647. doi: 10.1080/14737175.2018.1503536. [DOI] [PubMed] [Google Scholar]
- 2.Ramaswamy V., Taylor M.D. Medulloblastoma: From Myth to Molecular. J. Clin. Oncol. 2017;35:2355–2363. doi: 10.1200/JCO.2017.72.7842. [DOI] [PubMed] [Google Scholar]
- 3.Ramaswamy V., Remke M., Bouffet E., Bailey S., Clifford S.C., Doz F., Kool M., Dufour C., Vassal G., Milde T. Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Acta Neuropathol. 2016;131:821–831. doi: 10.1007/s00401-016-1569-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramaswamy V., Remke M., Adamski J., Bartels U., Tabori U., Wang X., Huang A., Hawkins C., Mabbott D., Laperriere N. Medulloblastoma subgroup-specific outcomes in irradiated children: who are the true high-risk patients? Neuro-oncol. 2016;18:291–297. doi: 10.1093/neuonc/nou357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen K., Bandopadhayay P., Chi S., London W., Rodriguez F., Hawkins C., Yang E., Aguilera D., Castellino R., Macdonald T. MEDU-34. PILOT STUDY OF A SURGERY AND CHEMOTHERAPY-ONLY APPROACH IN THE UPFRONT THERAPY OF CHILDREN WITH WNT-POSITIVE STANDARD RISK MEDULLOBLASTOMA. Neuro-oncol. 2019;21:ii110. ii110. [Google Scholar]
- 6.Remke M., Hielscher T., Northcott P.A., Witt H., Ryzhova M., Wittmann A., Benner A., von Deimling A., Scheurlen W., Perry A. Adult medulloblastoma comprises three major molecular variants. J. Clin. Oncol. 2011;29:2717–2723. doi: 10.1200/JCO.2011.34.9373. [DOI] [PubMed] [Google Scholar]
- 7.Zhao F., Ohgaki H., Xu L., Giangaspero F., Li C., Li P., Yang Z., Wang B., Wang X., Wang Z. Molecular subgroups of adult medulloblastoma: a long-term single-institution study. Neuro-oncol. 2016;18:982–990. doi: 10.1093/neuonc/now050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabel M., Fleischhack G., Tippelt S., Gustafsson G., Doz F., Kortmann R., Massimino M., Navajas A., von Hoff K., Rutkowski S., SIOP-E Brain Tumour Group Relapse patterns and outcome after relapse in standard risk medulloblastoma: a report from the HIT-SIOP-PNET4 study. J. Neurooncol. 2016;129:515–524. doi: 10.1007/s11060-016-2202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clifford S.C., Lannering B., Schwalbe E.C., Hicks D., O’Toole K., Nicholson S.L., Goschzik T., Zur Mühlen A., Figarella-Branger D., Doz F., SIOP-Europe PNET Group Biomarker-driven stratification of disease-risk in non-metastatic medulloblastoma: Results from the multi-center HIT-SIOP-PNET4 clinical trial. Oncotarget. 2015;6:38827–38839. doi: 10.18632/oncotarget.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pietsch T., Schmidt R., Remke M., Korshunov A., Hovestadt V., Jones D.T., Felsberg J., Kaulich K., Goschzik T., Kool M. Prognostic significance of clinical, histopathological, and molecular characteristics of medulloblastomas in the prospective HIT2000 multicenter clinical trial cohort. Acta Neuropathol. 2014;128:137–149. doi: 10.1007/s00401-014-1276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phoenix T.N., Patmore D.M., Boop S., Boulos N., Jacus M.O., Patel Y.T., Roussel M.F., Finkelstein D., Goumnerova L., Perreault S. Medulloblastoma Genotype Dictates Blood Brain Barrier Phenotype. Cancer Cell. 2016;29:508–522. doi: 10.1016/j.ccell.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korshunov A., Sahm F., Zheludkova O., Golanov A., Stichel D., Schrimpf D., Ryzhova M., Potapov A., Habel A., Meyer J. DNA methylation profiling is a method of choice for molecular verification of pediatric WNT-activated medulloblastomas. Neuro-oncol. 2019;21:214–221. doi: 10.1093/neuonc/noy155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhukova N., Ramaswamy V., Remke M., Pfaff E., Shih D.J., Martin D.C., Castelo-Branco P., Baskin B., Ray P.N., Bouffet E. Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J. Clin. Oncol. 2013;31:2927–2935. doi: 10.1200/JCO.2012.48.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellison D.W., Onilude O.E., Lindsey J.C., Lusher M.E., Weston C.L., Taylor R.E., Pearson A.D., Clifford S.C., United Kingdom Children’s Cancer Study Group Brain Tumour Committee beta-Catenin status predicts a favorable outcome in childhood medulloblastoma: the United Kingdom Children’s Cancer Study Group Brain Tumour Committee. J. Clin. Oncol. 2005;23:7951–7957. doi: 10.1200/JCO.2005.01.5479. [DOI] [PubMed] [Google Scholar]
- 15.Taylor R.E., Bailey C.C., Robinson K., Weston C.L., Ellison D., Ironside J., Lucraft H., Gilbertson R., Tait D.M., Walker D.A., International Society of Paediatric Oncology. United Kingdom Children’s Cancer Study Group Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: The International Society of Paediatric Oncology/United Kingdom Children’s Cancer Study Group PNET-3 Study. J. Clin. Oncol. 2003;21:1581–1591. doi: 10.1200/JCO.2003.05.116. [DOI] [PubMed] [Google Scholar]
- 16.Ellison D.W., Kocak M., Dalton J., Megahed H., Lusher M.E., Ryan S.L., Zhao W., Nicholson S.L., Taylor R.E., Bailey S., Clifford S.C. Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J. Clin. Oncol. 2011;29:1400–1407. doi: 10.1200/JCO.2010.30.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gajjar A., Chintagumpala M., Ashley D., Kellie S., Kun L.E., Merchant T.E., Woo S., Wheeler G., Ahern V., Krasin M.J. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 18.Packer R.J., Gajjar A., Vezina G., Rorke-Adams L., Burger P.C., Robertson P.L., Bayer L., LaFond D., Donahue B.R., Marymont M.H. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J. Clin. Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 19.Thompson E.M., Hielscher T., Bouffet E., Remke M., Luu B., Gururangan S., McLendon R.E., Bigner D.D., Lipp E.S., Perreault S. Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: a retrospective integrated clinical and molecular analysis. Lancet Oncol. 2016;17:484–495. doi: 10.1016/S1470-2045(15)00581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Bueren A.O., Kortmann R.D., von Hoff K., Friedrich C., Mynarek M., Müller K., Goschzik T., Zur Mühlen A., Gerber N., Warmuth-Metz M. Treatment of Children and Adolescents With Metastatic Medulloblastoma and Prognostic Relevance of Clinical and Biologic Parameters. J. Clin. Oncol. 2016;34:4151–4160. doi: 10.1200/JCO.2016.67.2428. [DOI] [PubMed] [Google Scholar]
- 21.Ramaswamy V., Remke M., Bouffet E., Faria C.C., Perreault S., Cho Y.J., Shih D.J., Luu B., Dubuc A.M., Northcott P.A. Recurrence patterns across medulloblastoma subgroups: an integrated clinical and molecular analysis. Lancet Oncol. 2013;14:1200–1207. doi: 10.1016/S1470-2045(13)70449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phi J.H., Park A.K., Lee S., Choi S.A., Baek I.P., Kim P., Kim E.H., Park H.C., Kim B.C., Bhak J. Genomic analysis reveals secondary glioblastoma after radiotherapy in a subset of recurrent medulloblastomas. Acta Neuropathol. 2018;135:939–953. doi: 10.1007/s00401-018-1845-8. [DOI] [PubMed] [Google Scholar]
- 23.Packer R.J., Zhou T., Holmes E., Vezina G., Gajjar A. Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: results of Children’s Oncology Group trial A9961. Neuro-oncol. 2013;15:97–103. doi: 10.1093/neuonc/nos267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavalli F.M.G., Remke M., Rampasek L., Peacock J., Shih D.J.H., Luu B., Garzia L., Torchia J., Nor C., Morrissy A.S. Intertumoral Heterogeneity within Medulloblastoma Subgroups. Cancer Cell. 2017;31:737–754.e6. doi: 10.1016/j.ccell.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aryee M.J., Jaffe A.E., Corrada-Bravo H., Ladd-Acosta C., Feinberg A.P., Hansen K.D., Irizarry R.A. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics. 2014;30:1363–1369. doi: 10.1093/bioinformatics/btu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hovestadt V., Remke M., Kool M., Pietsch T., Northcott P.A., Fischer R., Cavalli F.M., Ramaswamy V., Zapatka M., Reifenberger G. Robust molecular subgrouping and copy-number profiling of medulloblastoma from small amounts of archival tumour material using high-density DNA methylation arrays. Acta Neuropathol. 2013;125:913–916. doi: 10.1007/s00401-013-1126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Capper D., Jones D.T.W., Sill M., Hovestadt V., Schrimpf D., Sturm D., Koelsche C., Sahm F., Chavez L., Reuss D.E. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555:469–474. doi: 10.1038/nature26000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Maaten L. Accelerating t-SNE using Tree-Based Algorithms. J. Mach. Learn. Res. 2014;14:3221–3245. [Google Scholar]
- 29.Saeed A.I., Sharov V., White J., Li J., Liang W., Bhagabati N., Braisted J., Klapa M., Currier T., Thiagarajan M. TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003;34:374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- 30.Cavalli F.M.G., Hübner J.M., Sharma T., Luu B., Sill M., Zapotocky M., Mack S.C., Witt H., Lin T., Shih D.J.H. Heterogeneity within the PF-EPN-B ependymoma subgroup. Acta Neuropathol. 2018;136:227–237. doi: 10.1007/s00401-018-1888-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The DNA methylation datasets unique to this study are available at Mendeley Data, https://doi.org/10.17632/cw37zdmgm3.2