Abstract
Importance
After menopause, estradiol (E2) is predominately an intracrine hormone circulating in very low serum concentrations.
Objective
The objective of this work is to examine determinants of E2 concentrations in women beyond age 70 years.
Design and Setting
A cross-sectional, community-based study was conducted.
Participants
A total of 5325 women participated, with a mean age of 75.1 years (± 4.2 years) and not using any sex steroid, antiandrogen/estrogen, glucocorticoid, or antiglycemic therapy.
Main Outcome Measures
Sex steroids were measured by liquid chromatography–tandem mass spectrometry. Values below the limit of detection (LOD; E2 11 pmol/L [3 pg/mL] were assigned a value of LOD/√2 to estimate total E2.
Results
E2 and estrone (E1) were below the LOD in 66.1% and 0.9% of women, respectively. The median (interdecile ranges) for E1 and detectable E2 were 181.2 pmol/L (range, 88.7-347.6 pmol/L) and 22.0 pmol/L (range, 11.0-58.7 pmol/L). Women with undetectable E2 vs detectable E2 were older (median age 74.1 years vs 73.8, P = .02), leaner (median body mass index [BMI] 26.8 kg/m2 vs 28.5, P < .001), and had lower E1, testosterone and DHEA concentrations (P < .001). A linear regression model, including age, BMI, E1, and testosterone, explained 20.9% of the variation in total E2, but explained only an additional 1.2% of variation over E1 alone. E1 and testosterone made significant contributions (r2 = 0.162, P < .001) in a model for the subset of women with detectable E2.
Conclusions
Our findings support E1 as a principal circulating estrogen and demonstrate a robust association between E1 and E2 concentrations in postmenopausal women. Taken together with prior evidence for associations between E1 and health outcomes, E1 should be included in studies examining associations between estrogen levels and health outcomes in postmenopausal women.
Keywords: estradiol, estrone, postmenopause
In premenopausal women, estradiol (E2), primarily produced by the ovaries, functions as a circulating hormone acting on distal target tissues. In contrast, following menopause, E2 is produced in extragonadal sites predominantly from adrenal precursors, where it acts locally, in a paracrine or intracrine manner (1, 2). Consequently, in postmenopausal women circulating E2 is from spillover from E2 made in peripheral tissues that has escaped local metabolism (1, 2). We recently reported that serum E2 was below 11 pmol/L (3 pg/mL) in two-thirds of community-dwelling women age 70 years and older (3). This is in line with other studies, of predominantly younger postmenopausal women, that have reported median E2 levels between 3 and 10.4 pg/mL (4-9). In contrast, serum estrone (E1) concentrations were similar to those found in healthy premenopausal women, although the significance of this is uncertain (5, 10, 11). The final precursors for E2 biosynthesis are testosterone (T) and E1, which in postmenopausal women are produced peripherally from the adrenal steroid dehydroepiandrosterone (DHEA). To elucidate what best predicts serum E2 concentrations in older women, we have systematically examined the contributions of several factors that have previously been associated with E2 concentrations, namely age (3), body mass index (BMI) (12, 13), E114, DHEA (2, 15), and T (2, 16).
Methods
Study participants
The Sex Hormones in Older Women (SHOW) study comprised 6392 of the 9180 Australian women participants in the ASPREE (ASPirin in Reducing Events in the Elderly) study who provided biobank specimens at enrollment and consent to measurement of an array of biomarkers, including sex steroids. To be eligible for the ASPREE study, women were required to be age at least 70 years, not current regular aspirin users, and free of documented cardiovascular or cerebrovascular disease, dementia or cognitive impairment (17), severe difficulty or inability to perform any of the 6 Katz activities of daily living (18), a high risk of bleeding or anemia, uncontrolled hypertension (systolic ≥ 180 mm Hg and/or diastolic ≥ 105 mm Hg), and any chronic illness that would limit expected survival to less than 5 years (19).
The SHOW study was approved by the Monash Human Research Ethics Committee (CF16/10-2016000001) and the Alfred Hospital Human Research Ethics Committee (616/15). All participants provided written informed consent to contribute biospecimens to the ASPREE Healthy Ageing Biobank.
Sex steroid measurement
Sex steroids were measured in plasma samples drawn at recruitment of women included in SHOW and stored under nitrogen vapor. Measurement was by liquid chromatography and tandem mass spectrometry (LC-MS/MS) at the ANZAC Research Institute, Sydney, New South Wales, within a single run without derivatization as previously described in full (3). Assay standards were certified reference materials for T and DHEA (National Measurement Institute, NMI) and for E2 and E1 (Cerillant) and steroid internal standards were d3-T and d2-DHEA (NMI), d4-E2 (Cambridge Isotope Laboratories), and (d4-E1) (Steraloids). The assay limits of detection (LODs), limits of quantification, within-run and between-run coefficient of variations were T (35 pmol/L, 0.09 nmol/L, 2.0%, 3.9-6.5%), E2 (11 pmol/L, 18 pmol/L, 6.6%, 4.8-8.6%), E1 (3.7 pmol/L, 11 pmol/L, 4.7%, 4.6-7.5%), and DHEA (0.07 nmol/L, 0.17 nmol/L, < 10%, < 10%) (20).
Statistical analysis
We defined a “normative group” within the 6392 women who had sex steroids measured to investigate sex steroid physiology. Participants were excluded from this group if they were using systemic or topical sex steroid therapy (estrogen, progestogen, tibolone, DHEA, or T), a selective estrogen receptor modulator, aromatase inhibitor, antiandrogen therapy (spironolactone or cyproterone acetate), glucocorticosteroid therapy, or any antiglycemic agent.
For any samples with an E2 or E1 concentration below the LOD, the concentration was estimated using the formula of LOD/√2, because this has been shown to be a minimally biased method to overcome left censoring (21). The imputed value for any sample with an E2 concentration below the LOD was 7.7 pmol/L. Analyses were performed for the detectable E2 (dE2) values and the dE2 plus imputed values (total E2). A similar approach was adopted for E1; however, less than 1% of the sample had an E1 value below the LOD, hence only total E1 values are reported. The Kruskal-Wallis test was used for comparison of the distribution of continuous variables between the undetectable E2 and dE2 groups. Associations between E2 and age, BMI, and other sex steroids were explored using Spearman correlations (rho, r). Multivariable linear regression was performed using log-transformed (ln) data for the sex steroids. A backward manual stepwise elimination approach was used, and a P value of less than .05 was required for retention of each variable in the model. For models for ln E2, β coefficients and CIs, with r2 values to indicate the proportion of variation explained by each model, are both reported. Analyses were performed using statistical software package Stata (version 15.0; StataCorp LP).
Results
The normative study group included 5325 of the 6392 women age 70 to 94.7 years. Their mean age was 75.1 (SD 4.2) years, 98.9% were Caucasian/white, 40.1% were overweight, and 30.1% were obese (Table 1). Serum E2 and E1 concentrations were below the LOD in 66.1% and 0.9% of the women, respectively (Table 2). The median and interdecile ranges (IDRs) for E1 and dE2 groups were 181.2 pmol/L (range, 88.7-347.6 pmol/L) and 22.0 pmol/L (range, 11.0-58.7 pmol/L), respectively.
Table 1.
Characteristics of women included in the study sample
| n = 5325 | |
|---|---|
| Age, y | |
| Mean (SD), range | 75.1 (4.2), 70-94.8 |
| 70-74, n (%) | 3121 (58.6%) |
| 75-79, n (%) | 1442 (27.1%) |
| 80-84, n (%) | 592 (11.1%) |
| ≥ 85, n (%) | 170 (3.2%) |
| Ethnicity, n (%) | |
| Caucasian/white | 5266 (98.9%) |
| Asian | 30 (0.6%) |
| Other | 29 (0.5%) |
| Body mass index, kg/m2, mean (SD) | 28.0 (5.0) |
| < 18.5, n (%) | 46 (0.87%) |
| Normal 18.5 to < 25, n (%) | 1533 (28.91%) |
| 25 to < 30a, n (%) | 2128 (40.14%) |
| ≥ 30b, n (%) | 1595 (30.08%) |
| Waist circumference, cm, mean (SD) | 92.6 (12.5) |
| Current smoker, n (%) | 156 (2.93%) |
aOverweight.
bObese.
Table 2.
Serum estrone and estradiol concentrations for the study sample
| Age, y | 70-74 | 75-79 | 80-85 | ≥ 85 | Total ≥ 70 y |
|---|---|---|---|---|---|
| Estrone, pmol/L | |||||
| n | 3093 | 1425 | 589 | 170 | 5277 |
| Below LOD (3.7 pmol/L) | 28 (0.9%) | 17 (1.2%) | 3 (0.5%) | 0 (0.0%) | 48 (0.9%) |
| Median | 181.2 | 181.20 | 196.0 | 205.2 | 181.2 |
| 10th percentile | 88.8 | 85.1 | 99.8 | 83.2 | 88.7 |
| 90th percentile | 340.2 | 351.3 | 362.4 | 369.8 | 347.6 |
| Estradiol, pmol/L | |||||
| n | 1097 | 480 | 179 | 47 | 1803 |
| Below LOD (11 pmol/L) | 2024 (64.8%) | 962 (66.7%) | 413 (69.8%) | 123 (72.3%) | 3522 (66.1%) |
| Median | 22.0 | 22.0 | 22.0 | 22.0 | 22.0 |
| 10th percentile | 11.0 | 11.0 | 11.0 | 11.0 | 11.0 |
| 90th percentile | 58.7 | 58.7 | 62.4 | 58.7 | 58.7 |
To convert from pmol/L to pg/mL, divide by 3.671.
Abbreviation: LOD, limit of detection.
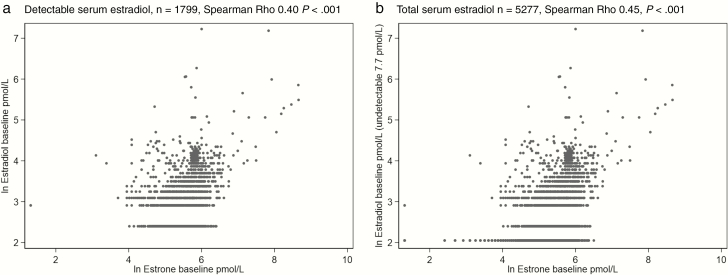
We first explored the associations between serum E2 and each of age, BMI, serum E1, DHEA, and T for both the dE2 and total E2 groups. There was a weak but significant correlation between total E2 and age (Spearman r = 0.03, P = .03) but not between dE2 and age (r = 0.00, P = .89). Serum concentrations both for total E2 and dE2 were significantly correlated with BMI (r = 0.16, P < .001 and r = 0.09, P < .001, respectively), DHEA (r = 0.20, P < .001 and 0.22, P < .001, respectively), and T (r = 0.26, P < .001 and r = 0.27, P < .001, respectively). The strongest correlations were seen between total E2 and dE2, and E1 (r = 0.45, P < .001 and r = 0.40, P < .001, respectively) (Fig. 1).
Figure 1.
Associations between serum estrone and A, detectable estradiol concentrations and B, total estradiol concentrations with undetectable values imputed.
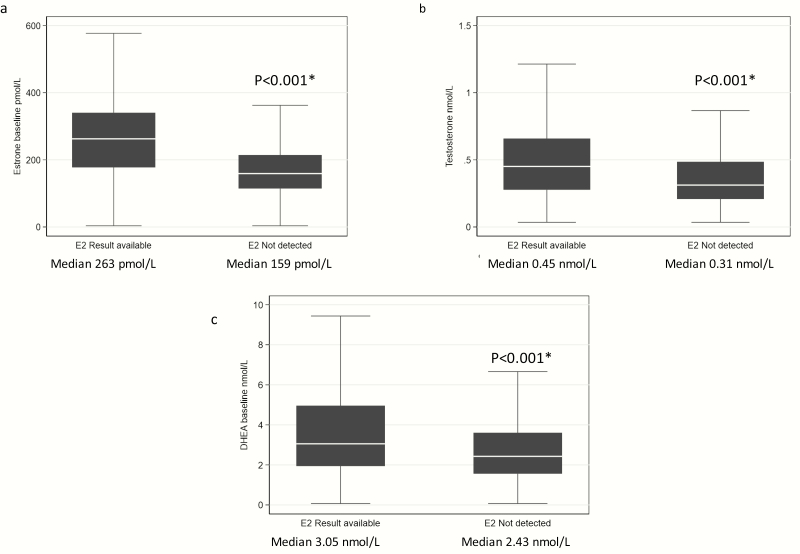
We next examined whether women with dE2 concentrations differed from women with undetectable E2 concentrations in terms of age, BMI, and other sex steroids. The women with dE2 concentrations were younger (median 73.8 years vs 74.1, respectively, Kruskal-Wallis P = .02), and had a higher BMI (median BMI 28.5 kg/m2 vs 26.8 kg/m2 respectively, Kruskal-Wallis P < .001). Serum E1, testosterone and DHEA concentrations were all lower in women with undetectable vs dE2 concentrations (Fig. 2).
Figure 2.
A, Estrone, B, testosterone, and C, dehydroepiandrosterone (DHEA) concentrations in women with and without detectable estradiol (E2) levels. In the box and whisker plots (outliers not included), the box represents the interquartile range (IQR); the line in the box is the median. The whiskers extend to the upper and lower adjacent values. The upper adjacent value is defined as the largest data point less than the 75th percentile + 1.5 × IQR. The lower adjacent value is defined as the smallest data point greater than the 25th percentile –1.5 × IQR. Outliers are any values beyond the whiskers. *Kruskal-Wallis test.
In the univariate analyses, E1 was positively associated with total E2 (P < .001) and explained 19.7% of the variation in total E2, whereas T and DHEA, also positively associated (P < .001), each explained 8.7% and 4.1% of the variation of total E2, respectively (Table 3). BMI explained approximately 3% of the variation in total E2 (P < .001), and age, which was negatively associated, explained less than 1% of the variation in total E2 (P = .036).
Table 3.
Univariable associations between serum estradiol and other variables
| ln total E2 | |||||
|---|---|---|---|---|---|
| n | β | 95% CI for β | P for β | R2 | |
| ln E1 | 5277 | .495 | 0.468 to 0.521 | < .001 | 0.197 |
| ln T | 5247 | .279 | 0.254 to 0.303 | < .001 | 0.087 |
| ln DHEA | 5290 | 0.187 | 0.163 to 0.211 | < .001 | 0.041 |
| Age, y | 5325 | –.004 | –0.009 to –0.0003 | .036 | 0.001 |
| BMI, kg/m2 | 5302 | .021 | 0.018 to 0.024 | < .001 | 0.026 |
| ln dE2 | |||||
| n | β | 95% CI for β | P for β | R2 | |
| ln E1 | 1799 | .453 | 0.402 to 0.505 | < .001 | 0.142 |
| ln T | 1792 | .293 | 0.249 to 0.336 | < .001 | 0.088 |
| ln DHEA | 1798 | .172 | 0.129 to 0.216 | < .001 | 0.033 |
| Age, y | 1803 | .001 | –0.006 to 0.009 | .754 | 0.000 |
| BMI, kg/m2 | 1789 | .009 | 0.004 to 0.015 | .001 | 0.006 |
Abbreviations: BMI, body mass index; dE2, detectable estradiol; DHEA, dehydroepiandrosterone; E1, estrone; E2, estradiol; ln, log-transformed; T, testosterone.
The contribution of the variables of interest to serum E2 was examined by multivariable regression with a backward elimination approach (Table 4). In the multivariable model for total E2 that included age, BMI, E1, T, and DHEA, DHEA did not make a significant contribution (P = .06). After removing DHEA from this model, all the remaining variables were significant (r2 = 0.209, P ≤ .001). This model explained an additional 1.2% of the variation in total E2 over ln E1 alone. Only E1 and T made significant contributions (r2 = 0.162, P < .001) in the multivariable model for dE2 (n = 1788).
Table 4.
Multivariable analysis of associations between total and detectable serum estradiol and other variables
| β (95% CI) | P for β | R2 | |
|---|---|---|---|
| Models for ln total E2, n = 5190 (backward elimination) | |||
| ln E1 | 0.432 (0.398 to 0.466) | < .001 | 0.211 |
| ln T | 0.115 (0.087 to 0.142) | < .001 | |
| ln DHEA | –0.026 (–0.053 to 0.001) | .057 | |
| Age, y | –0.007 (–0.011 to –0.003) | < .001 | |
| BMI, kg/m2 | 0.010 (0.007 to 0.013) | < .001 | |
| After removal of ln DHEA | |||
| ln E1 | 0.416 (0.383 to 0.448) | < .001 | 0.210 |
| ln T | 0.107 (0.081 to 0.134) | < .001 | |
| Age, y | –0.006 (–0.010 to –0.003) | < .001 | |
| BMI, kg/m2 | 0.010 (0.007 to 0.013) | < .001 | |
| Models for ln dE2, n = 1774 (backward elimination) | |||
| ln E1 | 0.368 (0.307 to 0.428) | < .001 | 0.164 |
| ln T | 0.196 (0.145 to 0.246) | < .001 | |
| ln DHEA | –0.035 (–0.085 to 0.015) | .167 | |
| Age, y | –0.004 (–0.011 to 0.003) | .301 | |
| BMI, kg/m2 | 0.003 (–0.003 to 0.008) | .334 | |
| After removal of BMI | |||
| ln E1 | 0.373 (0.314 to 0.432) | < .001 | 0.164 |
| ln T | 0.198 (0.147 to 0.248) | < .001 | |
| ln DHEA | –0.038 (–0.087 to 0.012) | .134 | |
| Age, y | –0.004 (–0.011 to 0.003) | .241 | |
| After removal of age | |||
| ln E1 | 0.371 (0.312 to 0.430) | < .001 | 0.164 |
| ln T | 0.194 (0.145 to 0.244) | < .001 | |
| ln DHEA | –0.034 (–0.083 to 0.015) | .175 | |
| After removal of ln DHEA | |||
| ln E1 | 0.360 (0.303 to 0.417) | < .001 | 0.163 |
| ln T | 0.180 (0.135 to 0.226) | < .001 |
Abbreviations: BMI, body mass index; dE2, detectable estradiol; E1, estrone; E2, estradiol; DHEA, dehydroepiandrosterone; ln, log-transformed; T, testosterone.
Discussion
This study demonstrates serum E1 is potentially an important determinant of serum E2 in older women. Although age, BMI, and DHEA were each significantly associated with serum E2, none made strong independent contributions to E2 concentrations after accounting for E1.
In contrast to very low circulating E2 being a hallmark of the postmenopausal period, serum E1 concentrations are maintained into older age and are of the same order of magnitude as those seen in premenopausal women. The IDR for E1 in our study was lower than that reported by other studies that have measured E1 by liquid chromatography and mass spectrometry in postmenopausal women. Falk et al (5) reported an IDR for E1 in 215 postmenopausal women, mean age 59.8 years of 96.5 to 842.7 pmol/L, and Trabert and colleagues (10) in 67 women, mean age 63, reported an IDR for E1 of 156 to 1208 pmol/L. Hence 10% of the participants’ serum E1 concentrations in the studies by Falk et al (5) and Trabert et al (10) were above 842 pmol/L and 1208 pmol/L, respectively. As in our study, Frederiksen and colleagues (9) reported unexplained elevated E1 levels in their sample of postmenopausal women up to age 61 years. The very high E1 levels in some postmenopausal women are potentially due to unreported hormone use, despite women having been asked to report all prescription medication. Although dietary factors such as high phytoestrogen intake, or the use of supplements, might explain some of the high E1 levels observed, this is unlikely with the specificity of LC-MS/MS. Furthermore, the consistency of the finding of high E1 levels in postmenopausal women across other studies (5, 9, 10, 22), and the present study, suggests that some women continue to produce large amounts of E1 throughout their adult life. Furthermore, the wide ranges of E1 in otherwise healthy women is a reminder that although reference ranges offer clinical guidance, for values outside a reference range to be considered abnormal, they should first be associated with pathophysiology.
After menopause, circulating E2 arises from extragonadal biosynthesis from the adrenal precursor DHEA. Through this pathway E2 is produced either by aromatization of T or following aromatization of androstenedione to E1, and then from E1 by 17β-hydroxysteroid dehydrogenase (HSD) enzymes in multiple extragonadal tissues (23). Aromatase is regulated by various tissue-specific promotors, which in turn are activated or inhibited by other hormones or local tissue factors, so the control of E2 production is complex (24). Aromatase gene expression in fat increases with age (25, 26). Therefore, it might be expected that age, BMI, and T would each be a strong predictor of circulating E2 concentrations in older women. However, in the univariate analyses of these factors, only T explained what might be considered a meaningful contribution to the variation in E2. The extragonadal reduction of E1 to E2 is less well understood, although 17β–HSD type 12 has been implicated as having a key role in E2 biosynthesis in human adipose (27). Our finding of E1 alone accounting for 19.7% of the variation in total E2 in older women, with only a 1.2% additional benefit of adding in age, BMI, or T to the model, highlights the physiological importance of E1 in E2 biosynthesis in older women.
Nonetheless, E1 has often been dismissed as a weak estrogen of little physiological significance. Although a weaker estrogen than E2 in conventional ligand-binding assays and in vitro estrogen bioassays, the estimation of in vivo estrogen potency is complex. Net E2 biosynthesis and metabolism need to be taken into account along with the variability in tissue-specific coactivator recruitment that modulates estrogen receptor gene expression (28). With circulating E1 concentrations in postmenopausal women on the order of 100-fold greater than E2, E1 is likely to exert biologically significant effects, even with estimates of relative potency of less than 10% of that of E2 (28). Consistent with this, serum E1 concentrations have been positively correlated with bone mineral density (29) and breast cancer risk (30) and inversely with colon cancer risk (31) in postmenopausal women. Hence, taken together, serum E1 concentrations should be included in studies investigating estrogen associations with postmenopausal health outcomes.
The present study has several strengths, including being a large, community-based sample of older women, providing statistical power to report hormonal ranges and measurement of sex steroids by LC-MS/MS, and providing high sensitivity and specificity (31), 32). A study limitation is its cross-sectional design that rendered us unable to report changes in hormone concentrations with age. With our study sample comprising predominantly causcasian/white women, our findings cannot be generalized to other populations. Regarding past oophorectomy, these data were not collected; therefore, we were unable to assess their impact on hormone levels in these older postmenopausal women.
In conclusion, our findings support E1 as a principal circulating estrogen and demonstrate a robust association between E1 and E2 concentrations in postmenopausal women. E1 should be measured in future studies seeking an association between endogenous estrogen concentrations and health outcomes in postmenopausal women.
Acknowledgments
Financial Support: The ASPREE trial was supported by the National Institute on Aging and the National Cancer Institute at the National Institutes of Health (Grant U01AG029824), the National Health and Medical Research Council (NHMRC) of Australia (Grant 34047, 1127060), Monash University (Australia), and the Victorian Cancer Agency (Australia). The ASPREE Healthy Ageing Biobank was funded by the CSIRO (Flagship Grant), the National Cancer Institute (Grant U01 AG029824), and Monash University. This analysis of sex hormones was funded by an NHMRC of Australia Project Grant (No. 1105305). S.R.D. is an Australian NHMRC Senior Principal Research Fellow (Grant 1135843).
Trial Registration: International Standard Randomized Controlled Trial registration No. ISRCTN83772183 (July 14, 2005) and clinicaltrials.gov No. NCT01038583 (December 24, 2009).
Glossary
Abbreviations
- ASPREE
ASPirin in Reducing Events in the Elderly study
- BMI
body mass index
- dE2
detectable estradiol
- DHEA
dehydroepiandrosterone
- E1
estrone
- E2
estradiol
- HSD
17β-hydroxysteroid dehydrogenase
- IDR
interdecile range
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- ln
log-transformed
- LOD
limit of detection
- SHOW
Sex Hormones in Older Women study
- T
testosterone
Additional Information
Disclosure Summary: Dr Davis reports having received honoraria from Besins Healthcare and Pfizer Australia and has been a consultant to Mayne Pharmaceuticals, Lawley Pharmaceuticals and Que Oncology. Dr Handelsman has received institutional grant funding (but no personal income) for investigator-initiated clinical testosterone pharmacology studies (Lawley, Besins Healthcare) and has provided expert testimony to antidoping and professional standards tribunals and testosterone litigation. No other potential conflict of interest relevant to this article are reported.
Data Availability: Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Simpson E, Rubin G, Clyne C, et al. . The role of local estrogen biosynthesis in males and females. Trends Endocrinol Metab. 2000;11(5):184-188. [DOI] [PubMed] [Google Scholar]
- 2. Mauvais-Jarvis F. Estrogen sulfotransferase: intracrinology meets metabolic diseases. Diabetes. 2012;61(6):1353-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davis SR, Bell RJ, Robinson PJ, et al. . Testosterone and estrone increase from the age of 70 years; findings from the Sex Hormones in Older Women study. J Clin Endocrinol Metab. 2019;104(12):6291-6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pauwels S, Antonio L, Jans I, et al. . Sensitive routine liquid chromatography–tandem mass spectrometry method for serum estradiol and estrone without derivatization. Anal Bioanal Chem. 2013;405(26):8569-8577. [DOI] [PubMed] [Google Scholar]
- 5. Falk RT, Brinton LA, Dorgan JF, et al. . Relationship of serum estrogens and estrogen metabolites to postmenopausal breast cancer risk: a nested case-control study. Breast Cancer Res. 2013;15(2):R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dallal CM, Lacey JV Jr, Pfeiffer RM, et al. ; B~FIT Research Group Estrogen metabolism and risk of postmenopausal endometrial and ovarian cancer: the B~FIT Cohort. Horm Cancer. 2016;7(1):49-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simon JA, Archer DF, Constantine GD, et al. . A vaginal estradiol softgel capsule, TX-004HR, has negligible to very low systemic absorption of estradiol: efficacy and pharmacokinetic data review. Maturitas. 2017;99:51-58. [DOI] [PubMed] [Google Scholar]
- 8. Kushnir MM, Rockwood AL, Bergquist J, et al. . High-sensitivity tandem mass spectrometry assay for serum estrone and estradiol. Am J Clin Pathol. 2008;129(4):530-539. [DOI] [PubMed] [Google Scholar]
- 9. Frederiksen H, Johannsen TH, Andersen SE, et al. . Sex-specific estrogen levels and reference intervals from infancy to late adulthood determined by LC-MS/MS. J Clin Endocrinol Metab. 2020;105(3):754-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trabert B, Brinton LA, Anderson GL, et al. . Circulating estrogens and postmenopausal ovarian cancer risk in the Women’s Health Initiative Observational study. Cancer Epidemiol Biomarkers Prev. 2016;25(4):648-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Skiba MA, Bell RJ, Islam RM, Handelsman DJ, Desai R, Davis SR. Androgens during the reproductive years: what is normal for women? J Clin Endocrinol Metab. 2019;104(11):5382-5392. [DOI] [PubMed] [Google Scholar]
- 12. Key TJ, Appleby PN, Reeves GK, et al. ; Endogenous Hormones and Breast Cancer Collaborative Group Steroid hormone measurements from different types of assays in relation to body mass index and breast cancer risk in postmenopausal women: reanalysis of eighteen prospective studies. Steroids. 2015;99(Pt A):49-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Weelden WJ, Fasmer KE, Tangen IL, et al. . Impact of body mass index and fat distribution on sex steroid levels in endometrial carcinoma: a retrospective study. BMC Cancer. 2019;19(1):547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hetemäki N, Savolainen-Peltonen H, Tikkanen MJ, et al. . Estrogen metabolism in abdominal subcutaneous and visceral adipose tissue in postmenopausal women. J Clin Endocrinol Metab. 2017;102(12):4588-4595. [DOI] [PubMed] [Google Scholar]
- 15. Labrie F. All sex steroids are made intracellularly in peripheral tissues by the mechanisms of intracrinology after menopause. J Steroid Biochem Mol Biol. 2015;145:133-138. [DOI] [PubMed] [Google Scholar]
- 16. Braunstein GD, Johnson BD, Stanczyk FZ, et al. . Relations between endogenous androgens and estrogens in postmenopausal women with suspected ischemic heart disease. J Clin Endocrinol Metab. 2008;93(11):4268-4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314-318. [PubMed] [Google Scholar]
- 18. Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv. 1976;6(3):493-508. [DOI] [PubMed] [Google Scholar]
- 19. McNeil JJ, Woods RL, Nelson MR, et al. ; ASPREE Investigator Group Baseline characteristics of participants in the ASPREE (ASPirin in Reducing Events in the Elderly) study. J Gerontol A Biol Sci Med Sci. 2017;72(11):1586-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hsu B, Cumming RG, Hirani V, et al. . Temporal trend in androgen status and androgen-sensitive outcomes in older men. J Clin Endocrinol Metab. 2016;101(4):1836-1846. [DOI] [PubMed] [Google Scholar]
- 21. Handelsman DJ, Ly LP. An accurate substitution method to minimize left censoring bias in serum steroid measurements. Endocrinology. 2019;160(10):2395-2400. [DOI] [PubMed] [Google Scholar]
- 22. Sampson JN, Falk RT, Schairer C, et al. . Association of estrogen metabolism with breast cancer risk in different cohorts of postmenopausal women. Cancer Res. 2017;77(4):918-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Simpson ER, Davis SR. Minireview: aromatase and the regulation of estrogen biosynthesis—some new perspectives. Endocrinology. 2001;142(11):4589-4594. [DOI] [PubMed] [Google Scholar]
- 24. Zhao H, Zhou L, Shangguan AJ, Bulun SE. Aromatase expression and regulation in breast and endometrial cancer. J Mol Endocrinol. 2016;57(1):R19-R33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Misso ML, Jang C, Adams J, et al. . Adipose aromatase gene expression is greater in older women and is unaffected by postmenopausal estrogen therapy. Menopause. 2005;12(2):210-215. [DOI] [PubMed] [Google Scholar]
- 26. Bulun SE, Simpson ER. Competitive reverse transcription-polymerase chain reaction analysis indicates that levels of aromatase cytochrome P450 transcripts in adipose tissue of buttocks, thighs, and abdomen of women increase with advancing age. J Clin Endocrinol Metab. 1994;78(2):428-432. [DOI] [PubMed] [Google Scholar]
- 27. Bellemare V, Laberge P, Noël S, Tchernof A, Luu-The V. Differential estrogenic 17beta-hydroxysteroid dehydrogenase activity and type 12 17beta-hydroxysteroid dehydrogenase expression levels in preadipocytes and differentiated adipocytes. J Steroid Biochem Mol Biol. 2009;114(3-5):129-134. [DOI] [PubMed] [Google Scholar]
- 28. Jeyakumar M, Carlson KE, Gunther JR, Katzenellenbogen JA. Exploration of dimensions of estrogen potency: parsing ligand binding and coactivator binding affinities. J Biol Chem. 2011;286(15):12971-12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Suzuki N, Yano T, Nakazawa N, Yoshikawa H, Taketani Y. A possible role of estrone produced in adipose tissues in modulating postmenopausal bone density. Maturitas. 1995;22(1):9-12. [DOI] [PubMed] [Google Scholar]
- 30. Miyoshi Y, Tanji Y, Taguchi T, Tamaki Y, Noguchi S. Association of serum estrone levels with estrogen receptor-positive breast cancer risk in postmenopausal Japanese women. Clin Cancer Res. 2003;9(6):2229-2233. [PubMed] [Google Scholar]
- 31. Murphy N, Strickler HD, Stanczyk FZ, et al. . A prospective evaluation of endogenous sex hormone levels and colorectal cancer risk in postmenopausal women. J Natl Cancer Inst. 2015;107(10):djv210. [DOI] [PMC free article] [PubMed] [Google Scholar]