Abstract
Zinc fingers and homeoboxes (ZHX) proteins are heterodimeric transcriptional factors largely expressed at the cell membrane in podocytes in vivo. We found ZHX2-based heterodimers in podocytes, with ZHX2-ZHX1 predominantly at the cell membrane of the podocyte cell body, and ZHX2-ZHX3 at the slit diaphragm. In addition to changes in overall ZHX2 expression, there was increased podocyte nuclear ZHX3 and ZHX2 in patients with focal segmental glomerulosclerosis, and increased podocyte nuclear ZHX1 in patients with minimal change disease. Zhx2 deficient mice had increased podocyte ZHX1 and ZHX3 expression. Zhx2 deficient mice and podocyte specific Zhx2 overexpressing transgenic rats develop worse experimental focal segmental glomerulosclerosis than controls, with increased nuclear ZHX3 and ZHX2, respectively. By contrast, podocyte specific Zhx2 overexpressing transgenic rats develop lesser proteinuria during experimental minimal change disease due to peripheral sequestration of ZHX1 by ZHX2. Using co-immunoprecipitation, the interaction of ZHX2 with aminopeptidase A in the podocyte body cell membrane, and EPHRIN B1 in the slit diaphragm were noted to be central to upstream events in animal models of minimal change disease and focal segmental glomerulosclerosis, respectively. Mice deficient in Enpep, the gene for aminopeptidase A, and Efnb1, the gene for ephrin B1 developed worse albuminuria in glomerular disease models. Targeting aminopeptidase A in Zhx2 deficient mice with monoclonal antibodies induced albuminuria and upregulation of the minimal change disease mediator angiopoietin-like 4 through nuclear entry of ZHX1. Thus, podocyte ZHX2 imbalance is a critical factor in human glomerular disease, with minimal change disease disparities mediated mostly through ZHX1, and focal segmental glomerulosclerosis deviations through ZHX3 and ZHX2.
Keywords: glomerulus, proteinuria, kidney injury, transcriptional regulation, albuminuria
Graphical Abstract
INTRODUCTION
Patients with primary glomerular diseases, such as minimal change disease (MCD) and focal and segmental glomerulosclerosis (FSGS), often present with nephrotic syndrome, a constellation of large amounts of proteinuria, hypoalbuminemia, edema, and hyperlipidemia.1 Some downstream pathways in these diseases have been clarified over the past two decades. For example, secretion of sialic acid deficient Angiopoietin-like 4 (ANGPTL4) from podocytes due to ANGPTL4 gene upregulation mediates early proteinuria and many structural and functional changes in MCD,2 whereas activation of the Wnt - β catenin pathway and downregulation of WT1 play a clear role in some forms of FSGS.3,4 However, upstream events leading to these downstream changes are not known. In addition, APOL1 variants are under investigation for their role in the pathogenesis of FSGS.5
The ZHX family transcriptional factors (ZHX1, ZHX2 and ZHX3) regulate a majority of the structurally and functionally important genes expressed in the podocyte, including, but not limited to, foot process expressed protein genes NPHS1, NEPH1, ZO1, CD2AP and ACTN4; glomerular basement membrane expressed protein genes COL4A3, COL4A4 and NID1; podocyte surface expressed proteins genes ENPEP, PODXL, DAG1, and ITGB1; secreted protein genes ANGPT2 and VEGFA; and transcriptional factor genes WT1, LMX1B and PAX2.6 ZHX2 is one of the most potent transcriptional repressors of WT1,6,7 which makes it highly likely to be involved in the pathogenesis of FSGS. The majority of ZHX proteins in podocytes in vivo are located outside the nucleus, mostly tethered to the cell membrane as hetero- or homo-dimers.6 Loss of ZHX hetero- or homo-dimerization, shown by us6–8 and other groups,9–13 results in the entry of ZHX proteins into the nucleus, followed by changes in the expression of target genes.6–8
A major limiting factor in the study of ZHX proteins has been that cultured human and mouse podocytes, unlike in vivo podocytes, express nearly half of all ZHX proteins in the nucleus,6, 7 causing major baseline differences in podocyte gene and protein expression. This makes it virtually impossible to study ZHX – mediated, human disease relevant biological processes in vitro, which is the major reason for the predominantly in vivo approach in this study.
We previously studied the role of ZHX3 in human and experimental glomerular diseases. In the current study, we focus on the major role of podocyte ZHX2 and its synergy with its heterodimeric partners ZHX1 and ZHX3. ZHX2 mediates important biological processes outside the kidney. ZHX2 is the major transcriptional repressor of α-fetoprotein expression in adult mice.14 BALB/cJ mice, but not 25 other mouse strains studied, have a mouse endogenous retrovirus in the first intron of the Zhx2 gene, which results in a predominantly non-functional transcript, causing Zhx2 downregulation and high α-fetoprotein protein levels in adult BALB/cJ mice.14, 15
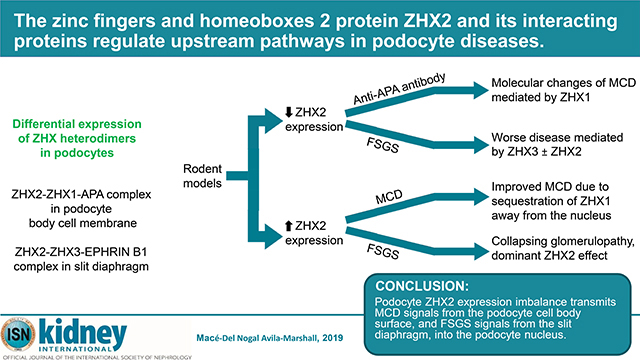
Because of the predominant cell membrane localization of ZHX proteins in podocytes in vivo, interaction with transmembrane proteins has been contemplated. ZHX2 is known to interact with EPHRIN B1,16,17 which is also expressed in the podocyte slit diaphragm.18 In the current study, we explore the candidacy of Aminopeptidase A (APA), a type 2 transmembrane protein product of the ENPEP gene, present all over the podocyte surface19 and interacts with actin via its cytoplasmic tail.20 Anti-APA antibodies induce proteinuria in some mouse strains,19,21 and are also present in γ2-nephrotoxic serum (NTS).22 This paper describes a novel paradigm for upstream podocyte gene regulation based on reduced overall podocyte ZHX2 expression with an altered ZHX2 - APA interaction on the podocyte body cell membrane, and altered ZHX2 – EPHRIN B1 interaction in the slit diaphragm in MCD and FSGS, respectively. Increased podocyte nuclear ZHX1 mediates changes in MCD, whereas increased podocyte nuclear ZHX3 or ZHX2 induce FSGS related changes.
RESULTS
Expression and distribution of ZHX proteins in human MCD and FSGS
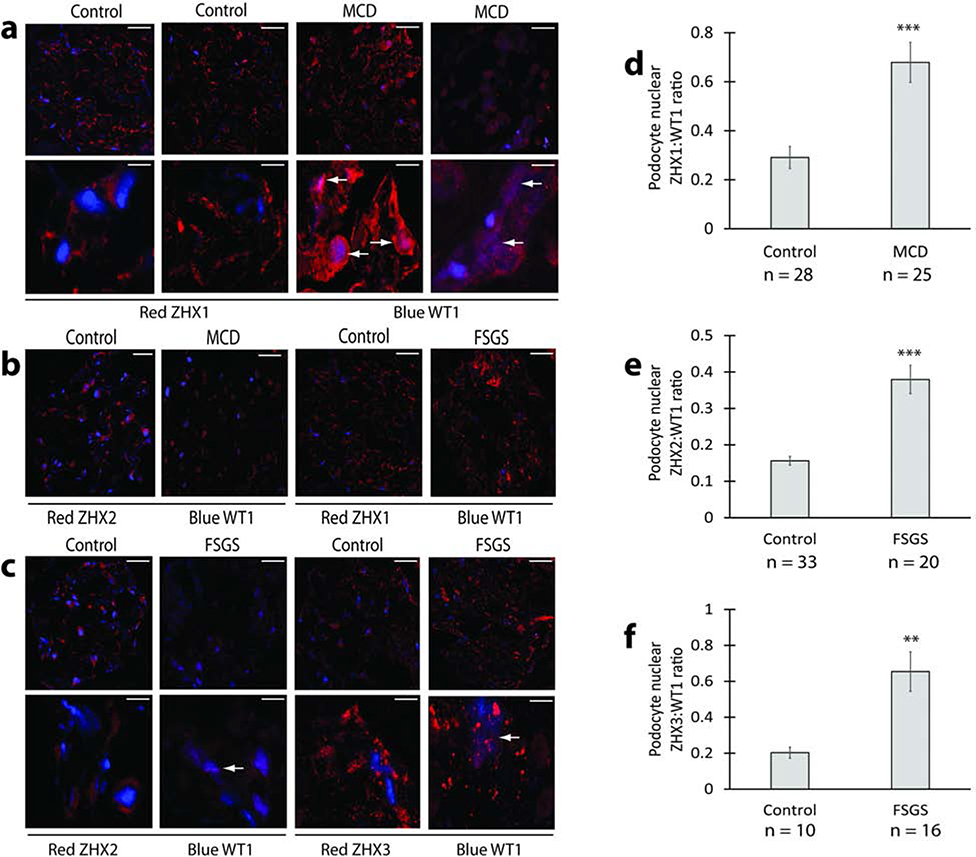
We used confocal imaging to assess for changes in expression and redistribution of ZHX proteins in human kidney disease biopsies (Figure 1, Supplementary Figure 1), using normal adult age and sex matched pre-implantation biopsies as control. The most prominent change in MCD patients was the significantly increased expression of ZHX1 in podocyte nuclei (Figure 1a, d), with overall low ZHX2 expression in podocytes (Figure 1b). In FSGS biopsies, some podocyte nuclei showed increased ZHX2 expression (Figure 1c, e), while others had increased nuclear ZHX3 expression (Figure 1c, f). ZHX1 was not significantly altered in FSGS biopsies (Figure 1b).
Figure 1: ZHX proteins in human glomerular disease.
(a) Representative confocal images from MCD patients and control human kidney showing increased overlap (white arrows) of podocyte ZHX1 (red) with the podocyte nucleus (blue) in MCD patients. (b) Representative confocal images showing general reduction of ZHX2 expression in MCD glomeruli and absence of major changes in glomerular ZHX1 expression in FSGS. (c) Representative confocal images from FSGS patients and control human kidney showing increased overlap (white arrows) of podocyte ZHX2 and ZHX3 (both red) with the podocyte nucleus (blue) in FSGS patients. (d) Morphometric quantification of increased podocyte nuclear ZHX1 expression as a ZHX1:WT1 ratio in MCD patients represented in panel a. (e) Morphometric quantification of increased podocyte nuclear ZHX2 expression as ZHX2:WT1 ratio in FSGS patients shown in panel c. (f) Morphometric quantification of increased podocyte nuclear ZHX3 expression as ZHX3:WT1 ratio in FSGS patients represented in panel c. Scale bars: 9.5 μm (a, c top panels, panel b); 6.0 μm (a and c bottom panels). ** P < 0.01; *** P < 0.001.
Podocyte Zhx2 deficient mouse models
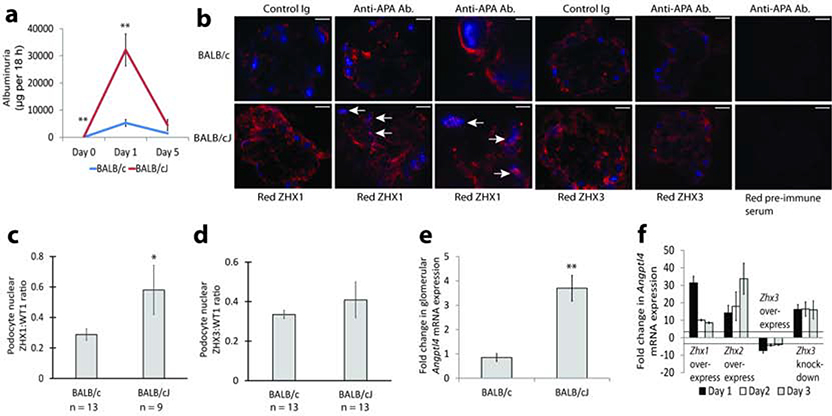
We tested whether downregulation of Zhx2 expression noted in the liver of BALB/cJ mice14,15 has parallel expression changes in podocytes in this strain. Dynabead isolated glomeruli from BALB/cJ mice had 5 – 7 fold lower mRNA expression of Zhx2 compared to BALB/c and C57BL/6 mice (Figure 2a). This was confirmed by confocal imaging, in which podocyte expression of ZHX2 was reduced, and that of ZHX1 and ZHX3 increased (Figure 2b to 2e). We also generated podocyte specific Zhx2 deficient mice (Zhx2 flox/flox;NPHS2 cre+/+), and confirmed moderate to severe depletion of glomerular ZHX2 protein expression (Figure 2f, Supplementary figure 2a). Zhx2 flox/flox;NPHS2 cre+/+ mice did not have albuminuria compared to Zhx2 flox/flox controls (Supplementary figure 2b). Next, we induced Adriamycin nephrosis in BALB/cJ and BALB/c mice using a threshold nephritogenic dose. BALB/cJ mice had significantly higher albuminuria than BALB/c mice (Figure 2g), suggesting that the Zhx2 deficient state predisposes to a more severe disease. BALB/cJ mice sacrificed at peak albuminuria on Day 14 of Adriamycin nephrosis had higher podocyte nuclear ZHX3, but not ZHX1, than BALB/c mice (Figure 2h, i).
Figure 2: Zhx2 deficient mice and Adriamycin Nephrosis.
(a) Results of real time PCR studies showing significantly lower Zhx2 mRNA expression in BALB/cJ mouse glomeruli compared to BALB/c and C57BL/6 glomeruli. (b) Confocal images of BALB/c and BALB/cJ mouse glomeruli showing reduced Zhx2, and increased ZHX1 and ZHX3, expression in BALB/cJ mice. (c) Morphometric quantification of ZHX2 expression in confocal images in panel b. (d) Morphometric quantification of ZHX1 expression in confocal images in panel b. (e) Morphometric quantification of ZHX3 expression in confocal images in panel b. (f) Partial depletion of glomerular ZHX2 in Zhx2 flox flox, NPHS2 cre+/+ mice by confocal imaging. (g) Albuminuria after induction of low dose Adriamycin nephrosis in BALB/c and BALB/cJ mice (n = 8 mice per group). (h) Podocyte nuclear expression of ZHX3 (arrows in left and amplified middle panels) and ZHX1 (right panels) on Day 14 of adriamycin nephrosis in BALB/c and BALB/cJ mice. (i) Morphometric quantification of increased podocyte nuclear ZHX3 expression as ZHX3:WT1 ratio in BALB/cJ mice represented in panel e. * P<0.05; ** P < 0.01; *** P<0.001. Scale bars: 9.1 μm (b), 10.2 μm (f), 12.5 μm (h left and right panels), 7.9 μm (h middle panel).
Podocyte specific overexpression of Zhx2 in transgenic rats worsened FSGS and improved experimental MCD
Zhx2 was overexpressed in podocytes under the control of the native human NPHS2 promoter (Figure 3a) to generate 3 transgenic Sprague Dawley rat based lines with varying increase in glomerular Zhx2 mRNA expression (mean increase, TG 14, 13.7 %; TG 142, 50.7%; TG 144, 309.8%) (Figure 3b). Transgenic rats were morphologically normal by light microscopy (TG 144 shown as an example, Supplementary Figure 2c, left panel). None of the transgenic rat lines had proteinuria (Figure 3c). Confocal imaging revealed increased peripheral podocyte expression of ZHX2 protein overlapping partly with Nephrin, but without increased overlap with the nuclear marker WT1, and unchanged ZHX1 expression (Figure 3d). As expected, no overlap with endothelial marker VWF was noted. Consistent with the extra-nuclear sequestration of transgene expressed Zhx2, no significant change in glomerular gene expression of a panel of 51 genes was noted (Supplementary Figure 2d).
Figure 3: Selective transgenic overexpression of Zhx2 in the rat podocyte, and its effects on glomerular disease.
(a) Construct used to generate 3 transgenic rat lines. (b) Fold increase in total glomerular Zhx2 expression in 3 month old male transgenic rats from different lines (n = 5 templates per group) compared to wild type littermates (horizontal bar). (c) Proteinuria in 3 month old male transgenic rats from 3 lines and wild type littermates (n = 8 rats per group). (d) Confocal characterization of the TG 144 line using immunostaining for ZHX proteins, podocyte markers Nephrin and WT1, and endothelial marker Von Willebrand factor (VWF). (e) Proteinuria after induction of adriamycin nephrosis (7.5 mg / Kg) in 4 months old male transgenic rats and wild type littermates (n = 8 rats per group). TG 144 rats died on day 9 or 10 from severe collapsing glomerulopathy in pilot studies, and had to be euthanized on Day 7 in this study. (f) Histological characterization of adriamycin injected wild type and transgenic rats using PAS. Black arrows show glomerular tuft collapse, green arrows proliferating epithelial cells, red arrows segmental scarring, and blue arrows podocyte hypertrophy. (g) Confocal images of glomeruli from wild type and TG 144 adriamycin treated rats euthanized on Day 7 to study the relative podocyte nuclear and non-nuclear expression of ZHX proteins (red). The podocyte nuclear marker WT1 is stained blue. (h) Buffalo Mna rats without and with the TG 144 transgene were assessed for proteinuria at age 8 months (n = 5 rats per group). (i) Proteinuria after induction of medium dose puromycin nephrosis (10 mg per 100 gram body weight i/v) in transgenic rats and wild type littermates (n = 4 rats per group). * P<0.05; ** P<0.01; *** P<0.001. Scale bars: 9.5 μm (d), 13.3 μm (f), 7.2 μm (g first and third panels), 4.6 μm (g second and fourth panels), 7.5 μm (g right panel).
To study the significance of increased podocyte nuclear ZHX2 staining noted in some FSGS patient biopsies, we induced adriamycin nephrosis in wild type and Zhx2 transgenic rats. TG 144 and TG 142 rats had significantly higher proteinuria than wild type rats following induction of adriamycin nephrosis, a model mimicking FSGS, whereas proteinuria in TG 14 rats was similar to wild type rats (Figure 3e). Since TG 144 rats died between days 9 and 10 after induction of adriamycin nephrosis in pilot studies, we studied this group and the corresponding control group on Day 7. Renal histology in TG 144 rats on Day 7 revealed extensive glomerular collapse (Figure 3f, Supplementary Figure 2e), whereas TG 142 rats developed more severe FSGS like lesions than wild type rats (Supplementary Figure 2f). No collapsing phenotype was noted in TG 142 or TG 14 rats. Confocal imaging of the kidney from TG 144 and wild type rats on Day 7 revealed equivalent levels of podocyte nuclear localization of ZHX3 but substantially higher nuclear presence of ZHX2 in the TG 144 compared to the wild type group (Figure 3g, Supplementary Figure 2g), suggesting thereby a role of podocyte nuclear ZHX2 in the pathogenesis of the collapsing glomerulopathy phenotype. Confocal imaging revealed a marginal increase in podocyte nuclear ZHX2 in TG 142 rats compared to wild type or TG 14 rats (Supplementary Figure 2h). To study effects of chronic podocyte Zhx2 overexpression on FSGS, the TG 144 transgene was backcrossed into the Buffalo Mna rat background for 10 generations, then interbred and aged. At age 7.5 – 8 months, podocyte Zhx2 overexpressing Buffalo Mna rats had significantly more proteinuria (Figure 3h) and worse histological changes (Supplementary Figure 2c) than their Buffalo Mna counterparts without the transgene.
To study potential effects of podocyte ZHX2 upregulation on MCD, we induced the single intravenous dose puromycin aminonucleoside nephrosis (PAN) model that mimics some aspects of MCD. Peak proteinuria in TG 142 and TG 144 was significantly lower than in wild type rats, with many more time points being lower in TG 142 rather than TG 144 (Figure 3i), suggesting sequestration of ZHX1 by ZHX2 in the periphery in this model. Proteinuria in TG 14 rats increased and peaked virtually identical to wild type rats, but the decline beyond peak proteinuria was closer to TG 142 and TG 144. This study showed a non-linear anti-proteinuric effect of transgenic increased podocyte Zhx2 expression most prominent in TG 142 rats, and proved that even mild increase in podocyte Zhx2 expression as present in TG 14 rats could influence recovery from peak proteinuria.
Distribution of ZHX proteins in the podocyte
Confocal imaging of rodent glomeruli showed co-localization of podocyte ZHX2 with APA, a protein present all over the surface including the podocyte body, and Nephrin, a protein restricted to the slit diaphragm in foot processes (Figure 4a). ZHX1 had major overlap with APA and minor overlap with Nephrin (Figure 4b), whereas ZHX3 had major overlap with Nephrin and minor overlap with APA (Figure 4c). This suggested that ZHX2 could form heterodimers with ZHX1 in the cell body, and with ZHX3 in the slit diaphragm. None of the studies showed major overlap of ZHX proteins with the podocyte nuclear marker WT1, confirming previously reported predominant peripheral (non-nuclear) localization in podocytes.6 By contrast, all 3 ZHX proteins were prominently expressed in tubular cell nuclei (Supplementary Figure 3a).
Figure 4: Podocyte ZHX proteins distribution and ZHX associated transmembrane proteins.
(a) Confocal images of rodent glomeruli for ZHX2, slit diaphragm protein Nephrin and podocyte surface protein APA showing partial co-localization of ZHX2 with both Nephrin and APA (magenta, white arrows). Only minor overlap with podocyte nuclear marker WT1 is noted. (b) Confocal images of mouse glomeruli for ZHX1, Nephrin, APA and WT1 showing major co-localization of ZHX1 with podocyte surface protein APA (magenta, white arrows), and only minor co-localization with Nephrin and WT1. (c) Confocal images of mouse glomeruli for ZHX3, Nephrin, APA and WT1 showing major co-localization of ZHX3 with slit diaphragm protein Nephrin (magenta, white arrows), and minor overlap with APA and WT1. (d) Co-immunoprecipitation (co-IP) and Western blot studies using rat glomerular protein extracts showing ZHX2 (green arrow) in both anti-ZHX2 and anti-APA IPs. Anti-APA IP contains two bands of intact APA, including the transmembrane protein (upper band, red arrow) and the endoplasmic reticulum fraction (lower band, black arrow). By contrast, only the upper APA band was noted in the anti-ZHX2 IP, suggesting that ZHX2 associated with APA exclusively at the cell membrane. (e) Changes in glomerular APA expression in human MCD (n = 3 patients). (f) Changes in glomerular EPHRIN B1 expression in human FSGS (n = 3 patients). (g) Densitometry of data presented in panel e. (h) Densitometry of data presented in panel h. ** P < 0.01; *** p < 0.001. Scale bars: 12.5 μm (a-c), 9.5 μm (e).
Association of ZHX proteins with transmembrane proteins in the podocyte
In line with the previously published ZHX2-EPHRIN B1 association16,17 and EPHRIN B1 podocyte distribution,18 EPHRIN B1 was co-localized with the slit diaphragm protein Nephrin by confocal imaging (Supplementary Figure 3b). Co-immuoprecipitation (Co-IP) and Western blot studies using rat glomerular protein extracts revealed the presence of ZHX2 in anti-APA IP and APA in anti-ZHX2 IP (Figure 4d). The anti-ZHX2 IP selectively contained the transmembrane upper APA band, suggesting that the APA-ZHX2 association occurred only at the cell membrane and not in the endoplasmic reticulum fraction (lower band of anti-APA IP). Confirmatory studies using overexpression of tagged ZHX2 and APA cytoplasmic tail constructs showed reciprocal co-IP between the two proteins (Supplementary Figure 3c). We were also able to co-IP ZHX1 with APA and ZHX3 with EPHRIN B1 (Supplementary Figure 3d, 3e). We also studied changes in the expression of APA and EPHRIN B1 in human biopsies by confocal imaging (Figure 4e, f). APA expression was significantly reduced in MCD (Figure 4e, g), and EPHRIN B1 expression marginally increased in FSGS (Figure 4f, h).
Effect of Zhx2, APA and Ephrin B1 deficient states on experimental kidney disease
Western blot and confocal imaging of Efnb1 flox/flox;NPHS2 cre+ mice revealed moderate to severe depletion of glomerular protein expression (Supplementary Figure 4a, 4b), whereas Enpep −/− mice had complete depletion of glomerular APA expression (Supplementary Figure 4a, 4c). In addition, Enpep −/− mice in the C57/BL6 background (Supplementary Figure 4d) and podocyte specific Efnb1 flox/flox;NPHS2 cre+ mice (Supplementary Figure 4e), had no albuminuria compared to wild type littermates. Dual gene deficient F1 offspring of Enpep −/− and BALB/cJ mice (Supplementary Figure 4f) were generated. With aging, albuminuria increased in single gene deficient mice and remained unchanged in dual gene deficient mice.
Mice lacking APA or EPHRIN B1 in podocytes expressed ZHX proteins (Supplementary Figure 5a, 5b), suggesting that there are alternative putative binding partners for ZHX proteins in these cells. To test the relative significance of the interaction of ZHX2 with APA and EPHRIN B1 compared to putative alternative binding partners, we induced two models of generalized albuminuria using lipopolysaccharide (LPS, Supplementary Figure 5c, mild albuminuria) and γ2-NTS (Figure 5a, severe albuminuria), and adriamycin nephrosis (shown to Figure 2g), a model of FSGS, in these single gene deficient or control mice using the minimum nephritogenic dose concept.23 All three gene deficient mice had developed higher albuminuria compared to controls, suggesting thereby that interaction of ZHX1 and ZHX3 with APA and EPHRIN B1, respectively, and ZHX2 with putative alternative ZHX binding partners is less protective under pathological conditions.
Figure 5: Experimental glomerular disease in Enpep and Zhx2 deficient mice.
(a) Induction of albuminuria in Enpep −/− and Enpep +/+ mice using γ−2 nephrotoxic serum (n = 7 mice per group). (b) Changes in glomerular mRNA expression on Day 14 of adriamycin nephrosis in BALB/c and BALB/cJ mice (n = 9 mice per group), including prototype genes often altered in FSGS (NPHS2) and MCD (ANGPTL4). Three-fold change (black lines) was considered significant. (c) Albuminuria after injection of anti-EPHRIN B1 antibody or control pre-immune serum in BALB/cJ mice (red arrow) on Day 7 of adriamycin nephrosis (n = 6 mice per group). * P<0.05; ** P<0.01.
BALB/cJ mice sacrificed at peak albuminuria on Day 14 of Adriamycin nephrosis (Figure 2g) had increased podocyte nuclear ZHX3 but not ZHX1 (Figure 2h, i), suggesting major slit diaphragm mediated effects in this model of FSGS. Glomerular mRNA expression of Ephrin B1 and NPHS2, another slit diaphragm related gene often altered in FSGS, were increased in BALB/cJ mice on Day 14 of Adriamycin nephrosis, whereas expression of Enpep and Angptl4, a gene upregulated in MCD, were unchanged (Figure 5b). In another follow up study, BALB/cJ mice with adriamycin nephrosis injected with either rabbit anti-EPHRIN B1 antibody or pre-immune serum on Day 7, developed lower albuminuria in the anti-EPHRIN B1 treated group (Figure 5c), suggesting that blocking the extracellular domains of EPHRIN B1 at the slit diaphragm during disease could potentially stabilize ZHX protein interactions at its cytoplasmic tail. By contrast, the same antibody had no effect on albuminuria when injected into BALB/c or BALB/cJ mice in the absence of superimposed disease (Supplementary Figure 5d).
Targeting APA in the Zhx2 deficient state induces ZHX1 mediated Angptl4 upregulation
Since monoclonal anti-APA antibodies are known to induce albuminuria in mice, we tested for, and found significantly higher albuminuria in BALB/cJ compared to BALB/c mice (Figure 6a). In keeping with the major podocyte body surface localization of ZHX1, we noted a major presence of ZHX1 in podocyte nuclei in BALB/cJ mice (Figure 6b, c). No major changes in ZHX3 distribution were noted (Figure 6b, d). The MCD related gene Angptl4 was selectively upregulated in BALB/cJ mice (Figure 6e), suggesting thereby that ZHX1 could be responsible for Angptl4 upregulation. Overexpression of Zhx1 and Zhx2 in cultured podocytes induced upregulation, and overexpression of Zhx3 caused downregulation of Angptl4 mRNA expression (Figure 6f). Knockdown of Zhx3 resulted in upregulation of Angptl4 (Figure 6f). To test if ZHX1 is directly responsible for upregulating ANGPTL4, we looked for, and located a consensus CdxA binding motif previously shown to bind other ZHX proteins in the Cathepsin L promoter6 relatively close to the transcriptional start site in human, rat and mouse Angptl4 promoters (Supplementary Figure 6a). Mutation of the human ANGPTL4 promoter at this motif (Supplementary Figure 6b) significantly reduced promoter activity (Supplementary Figure 6c). Co-transfection of Zhx3 expression constructs with the wild type (Supplementary Figure 6d), but not the mutated promoter (Supplementary Figure 6e), reduced ANGPTL4 promoter-reporter activity. Co-transfection of Zhx1 expression constructs with the wild type (Supplementary Figure 6f), but not the mutated promoter construct (Supplementary Figure 6g), increased Angptl4 promoter-reporter activity. This suggests that ANGPTL4 upregulation in MCD required a ZHX2 hypomorph state, and was mediated by relocation of ZHX1 from the podocyte body cell membrane to the nucleus.
Figure 6: Regulation of MCD mediator Angptl4 expression by ZHX proteins.
(a) Albuminuria after injecting anti-APA antibodies into BALB/c and BALB/cJ mice (n = 7 mice per group). (b) Confocal images showing podocyte nuclear expression of ZHX1 and ZHX3 proteins (red) 24 hours after injection of anti-APA antibodies or control IgG. Blue color is podocyte nuclear marker WT1. (c) Morphometric quantification of images in panel b showing increased podocyte nuclear ZHX1 expression as ZHX1:WT1 ratio in BALB/cJ mice injected with anti-APA antibodies. (d) Morphometric quantification of images in panel b showing unchanged podocyte nuclear ZHX3 expression as ZHX3:WT1 ratio in BALB/cJ mice injected with anti-APA antibodies. (e) Changes in glomerular Angptl4 mRNA expression 24 hours after injecting anti-APA antibodies compared to control IgG in BALB/cJ and BALB/c mice (n = 6 templates / group). (f) Angptl4 mRNA expression after transfecting Zhx1, Zhx2 and Zhx3 expression constructs, and SiRNA mediated knockdown of Zhx3 in cultured mouse podocytes (n = 6 templates / group). Three-fold change in expression (lines) was taken as significant. * P < 0.05, ** P<0.01. Scale bars: 11.4 μm (b 2 left and 3 right panels), 7.2 μm (b third panel).
DISCUSSION
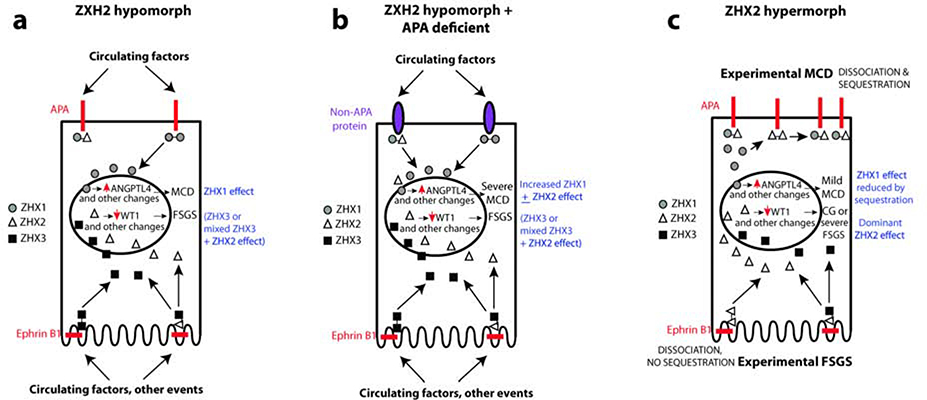
This study describes a novel paradigm by which extracellular signals interact with the podocyte surface and get transmitted into the nucleus based on the interaction between ZHX proteins and their binding partners (Figure 7). The system relies on the predominantly peripheral localization of ZHX proteins unique to podocytes among kidney epithelia. This peripheral localization is dependent on the level of expression, the ability to form heterodimers and interaction with transmembrane proteins, all of which likely neutralize or block two nuclear localization signals in each ZHX protein. In keeping with the redundancy in biological systems, ZHX proteins in podocytes likely have transmembrane partners other than APA and EPHRIN B1, since they maintain their non-nuclear localization in gene deficient mice and do not develop albuminuria. Similarly, rodents with low or high podocyte ZHX2 expression maintain a non-nuclear distribution of ZHX proteins potentially by forming peripheral homodimers and do not develop proteinuria. The biology of ZHX proteins changes when these alternative interactions are faced with disease inducing signals, such as adriamycin nephrosis, PAN, γ−2NTS, anti-APA antibody, and LPS models. Under these conditions, these alternative interactions appear to be less effective in retaining ZHX proteins in the periphery. Given the relative enrichment of ZHX1 in the podocyte body, and ZHX3 in the slit diaphragm, the downstream effects likely depend on what area of the podocyte was targeted by the disease process. Consequently, disease processes affecting the body would result in predominantly ZHX1, and sometimes ZHX1 and ZHX2 mediated effects, and those affecting the slit diaphragm would have ZHX3 or ZHX2 or combination effects. As a corollary, ANGPTL4 upregulation in MCD is likely a podocyte body surface phenomenon mediated mostly by ZHX1, whereas FSGS is more likely to be mediated through the slit diaphragm via ZHX3 and ZHX2. The presence of ZHX2 at both locations, its ability to affect both MCD (upregulation of ANGPTL4) and FSGS (potent downregulation of WT1) related genes suggest that podocyte nuclear ZHX2 could have dose dependent effects in different diseases. Indeed, high podocyte nuclear ZHX2 expression in podocyte specific Zhx2 overexpressing TG 144 rats during the adriamycin study was associated with a collapsing glomerulopathy – like phenotype.
Figure 7: Schematic representation of podocyte ZHX changes in MCD and FSGS.
(a) In a Zhx2 hypomorph state, higher ZHX1:ZHX2 ratio in the podocyte body cell membrane, and ZHX3:ZHX2 ratio in the slit diaphragm alters stability of the ZHX protein complex at the cytoplasmc aspect of APA and EPHRIN B1, respectively. Exposure to disease inducing stimuli or circulating proteins at the podocyte body cell membrane (MCD) or slit diaphragm (FSGS) induces dissociation of ZHX proteins from these transmembrane partners, followed by entry of ZHX1 (MCD) and ZHX3 alone or in combination with ZHX2 (FSGS) into podocyte nuclei. ZHX1 induces Angptl4 upregulation, whereas the ZHX3 + ZHX2 induces FSGS - related changes. (b) In a dual Zhx2 hypomorph and APA deficient state, ZHX proteins in the podocyte body cell membrane bind to alternative putative transmembrane proteins with lower affinity. Following exposure to MCD inducing stimuli, there is greater entry of ZHX1 + ZHX2 into podocyte nuclei, both of which are capable of inducing Angptl4 upregulation, causing more severe MCD. (c) In the experimental Zhx2 hypermorph state, transmembrane protein bound ZHX2 homodimers are increased. During experimental MCD, these homodimers in the podocyte body dissociate and re-associate with ZHX1 cleaved from ZHX1-ZHX2 heterodimers to sequester some ZHX1 in the periphery, resulting in less severe ZHX1 mediated MCD-like changes. In experimental FSGS, ZHX2 homodimers in the slit diaphragm dissociate and migrate into the nucleus to induce changes similar to collapsing glomerulopathy (CG) or severe FSGS in a dose-dependent manner.
Data from human kidney biopsies shows reduction of ZHX2 expression, and not complete absence, indicating that Zhx2 hypomorph mice more accurately reflect the disease state than complete knockouts. Normal podocyte ZHX2 expression is important as a defense mechanism against FSGS, as illustrated by worse disease in both Zhx2 deficient and overexpressing rodents compared to controls. Lower levels of PAN-related proteinuria in Zhx2 transgenic rats compared to wild type controls suggests that excessive ZHX2 in the periphery in transgenic rats may sequester ZHX1 and prevents its migration into the nucleus.
The current study shows that anti-APA monoclonal antibody model, established nearly 3 decades ago,19 is strongly influenced by the Zhx2 deficient state. ANGPTL4 upregulation in this paradigm provides the first clear mechanism for upregulation of ANGPTL4 by ZHX1 in the ZHX2 deficient state in MCD. Since ZHX3 binding to DNA at the CdxA binding motif was previously shown in the Cathepsin L promoter,6 we confirmed its ability to regulate the human ANGPTL4 promoter at a consensus CdxA binding site also showed that ZHX1 could induce upregulation of the human ANGPTL4 promoter via interaction at the same site.
Future directions would include exploration of changes in the expression and distribution of ZHX proteins in diabetic nephropathy. It is possible that aberrant glycation may directly disrupt podocyte ZHX heterodimers or ZHX associations with transmembrane proteins without requiring genomic abnormalities, producing a potpourri of downstream effects. Similar disruption of protein interactions is possible during the recurrence of primary glomerular diseases in the renal allograft, and could also account for some aspects of nephrotoxicity of immunosuppressive medications in transplant patients.
MATERIALS AND METHODS
Confocal imaging and antibodies used
We conducted confocal imaging on a Zeiss Pascal 5 confocal laser microscope. All secondary antibodies were purchased from Jackson labs. Previously published primary antibodies include rabbit anti-ZHX3 (4457–2B5)6,7, mouse anti-FLAG tag antibody, mouse anti-HSV tag antibody, and rat anti-mouse APA monoclonal antibodies ASD37 and ASD4119–21. We immunized host animals with recombinant peptides to raise the following custom polyclonal antibodies (Proteintech Laboratories Chicago, immunogen and antibody name/s notated): Rabbit anti-mouse ZHX2 (amino acids 15 to 150; 6965–1B3, 6965–2B4), Sheep anti-rat ZHX1 (amino acids 820 to 873; 8161-C2), Sheep anti-rat ZHX1 (amino acids 16 to 307; 8161-N5); Rabbit antirat APA (amino acids 44 to 942; 4091–2F), Rabbit anti-rat EPHRIN B1 (amino acids 25 to 239; 8605-R2B6), Guinea Pig anti-mouse WT1 (amino acids 1 to 271 of mouse glomerular WT1; 6786–49F). The following primary antibodies were purchased: mouse anti-WT1 (Santa Cruz # sc-7385); guinea pig anti-Nephrin (Fitzgerald # 20R-NP002); Sheep anti-VWF (Fitzgerald # 20R-VS001); Mouse anti-ZHX2 (Abnova, Taiwan # H00022882-A01; used for Western blot and confocal imaging), Rabbit anti-ZHX3 (Santa Cruz # sc-292339).
Densitometry analysis
Confocal microscopy images were used to assess densitometry using ImageJ software version 1.52a (NIH). Briefly, kidney sections were stained for podocyte proteins WT1 and ZHX transcriptional factors. Areas of advanced segmental sclerosis were not assessed. Podocyte nuclei were selected with a freeform tools and integrated intensity was measured for both channel (blue for podocyte nucleus marker WT1, red for transcription factor). The same method was done to measure three different background intensity. We then calculated the corrected total fluorescence (CTCF) of the selected area using the following formula (CTCF = Integrated Density – (Area of selected cell X Mean fluorescence of background readings)). For each podocyte nucleus, a ratio between red over blue channel fluorescence was calculated. For assessment of specific proteins present in whole glomeruli, a square of 400 by 400 pixels (for human glomeruli) or 200 by 200 pixels (for mouse glomeruli) was used to assess integrated intensity. The square was used 10 to 20 times (depending of the species and the size of the glomeruli) for fluorescence measurement. Finally, data from different images were pooled and statistics were done using Student’s t-test.
Co-immunoprecipitation and Western blot
Protocols for immunoprecipitation (IP) and Western blot are previously published.22,23,24 With the exception of Tris-Tricine gels used to study the cytoplasmic tail of APA, all Western blot gels were Tris Glycine. In any reaction, IP and co-IP studies were conducted simultaneously, and serve as positive controls for each other.
Glomerular isolation
Rat glomeruli were isolated by sieving22. For mouse glomerular isolation using dynabeads, the collagenase treatment aspect of the published protocol25 was modified to obtain higher quality RNA. For 12 dynabead perfused mouse kidneys, the collagenase mix comprised of 6 ml of HBSS containing 2 mg per ml collagenase and 200 units per ml of DNase. The incubation time with this collagenase mix was reduced to 10 minutes at 37°C, and the tube was strongly agitated.
Primer and probe sequences
Sequences of primers, probes and oligonucleotides for all studies including genotyping are shown in Supplementary Table 1. Other primers and Taqman probes were previously published.2,6,7
Animal studies
All animal studies conducted were approved by the IACUC at Rush University or the University of Alabama at Birmingham. Mouse models were obtained from the following sources: BALB/cJ (Jackson labs # 000651), BALB/c (Harlan Labs, Envigo, strain BALB/cAnNHsd), NPHS2-Cre (129S6.Cg-Tg(NPHS2-cre)295Lbh/BroJ; Jackson labs # 008523), Enpep −/− mice (Aminopeptidase A knockout mice) in mixed 129/C57BL/6 background (kind gift from Drs. Max Cooper and Wadih Arap), Ephrin B1 flox mice (129S-Efnb1tm1Sor /J; Jackson labs # 007664), C57BL/6 (Charles River # 027). Enpep −/− mice were backcrossed 10 generations into the C57BL/6 background. Mouse sperm with a floxed Zhx2 construct (zhx2tm1a(KOMP)Wtsi) was obtained from the UC Davis KOMP repository, and floxed mice generated at the UAB Kaul Center for Genetics. Podocyte specific Efnb1 and Zhx2 deficient mice were generated by breeding the NPHS2-cre+/+ mouse with the respective floxed mice, genotyped and the protein expression characterized by Western blot of dynabead isolated glomeruli and confocal imaging of kidney sections.
Rat models were obtained from the following sources: Buffalo Mna (MTA with Kyoto University, Japan), Sprague Dawley (Charles River # SAS SD)
Unless otherwise specified, male rodents were used for all studies. Whole sera were heat inactivated to destroy complement activity. All in vivo studies that explore potential protein-protein interactions were conducted using the minimum nephritogenic dose concept.23 The following animal models were induced: low dose mouse adriamycin nephrosis (intravenous adriamycin 8 mg/Kg); mouse adriamycin nephrosis with anti-EPHRIN B1 antibody or control serum (Adriamycin 10 mg/Kg i/v on day 0, antibody or control serum 200 μl i/v on Day 7); mouse LPS study (14 week old female mice, LPS 10 mg/Kg i/p); γ2-NTS study in mice (sheep anti-rat glomerular γ2-NTS 0.75 mg i/v); mouse anti-EPHRIN B1 antibody study (anti-EPHRIN B1 antibody or control serum 100 μl i/v on day 0); anti-APA antibody studies in mice (1mg each of ASD37 + ASD41 or 2 mg of isotype matched rat Ig i/v); rat adriamycin nephrosis (adriamycin 7.5 mg/Kg i/v); rat puromycin aminonucleoside nephrosis (puromycin aminonucleoside 100 mg / Kg i/v). General methods for conducting animal studies, timed urine collections (18 hours) in metabolic cages for rats and mice in the absence of food, and assays for proteinuria and albuminuria are previously described.2,6,7,22,23,24
Generation of podocyte-specific Zhx2 overexpressing rats
The full length human NPHS2 promoter construct2 was amplified by PCR using primers K468 and K511 in the presence of DMSO and inserted into the vector pTRE-tight-MP22 between the SacI and BamHI sites. Next, open reading frame of rat Zhx2 excluding the last 12 base pairs was amplified by PCR from Sprague Dawley rat glomerular cDNA and inserted along with a stop codon between the BamHI and SalI sites using primers K911 and K889. The resulting plasmid was digested with XhoI and the larger fragment was microinjected into fertilized Sprague Dawley eggs (University of Michigan) and implanted into pseudopregnant host Sprague Dawley females. The resulting offspring were genotyped using Taqman genomic DNA real time PCR using construct specific (H510, H511, P150) and control genomic prolactin (H448, H449, P131) primer and probe combinations. Three founder lines, TG14, TG142 and TG144 were established, and heterozygous rats interbred to obtain homozygous offspring. Colonies were refreshed with wild type Sprague Dawley rats every 3–4 generations.
Promoter – reporter studies
Consensus CdxA binding motifs, previously shown to bind ZHX3, were identified in human, rat and mouse Angptl4 promoters using TFSEARCH with a cutoff > 0.85. A 2 Kb fragment of the human ANGPTL4 promoter was PCR amplified with primers K834 and K835, using BAC clone RP11–886P16 as a template, and cloned into pSEAP2-basic between the HindIII and XhoI sites (clone 7678). The consensus motif closest to the transcriptional start site was mutated by site directed mutagenesis (TAT to CGG) to generate a mutated construct (clone m7678). HEK293 cells were transfected (n = 3 transfections per group) to study relative activity of the wild type and mutated CdxA binding motif by measuring SEAP activity in cell supernatant after 48 hours, and normalized to β-gal – luciferase expression. Expression constructs of rat Zhx3 (clone 6088) and mouse Zhx1 (clone 7231) in pcDNA3.1-TOPO were co-transfected separately with clones 7678 and m7678, and normalized SEAP activity measured as above after 48 hours.
Sources of human kidney biopsies
Human kidney biopsies for confocal imaging were obtained from the Instituto Nacional De Cardiologia in Mexico City via IRB approved protocols CONACYT 34751M, CONACYT 11–05, and DPAGA-UNAM IN-201902.
Statistical analysis
Values in all graphs are mean + s. e. m. For difference in proteinuria, albuminuria or gene expression involving 2 groups, we used the unpaired Student’s t test in Microsoft Excel 2013.
Supplementary Material
TRANSLATIONAL STATEMENT.
The transmission of extracellular signals from the cell membrane into the nucleus during the development of podocyte disease is not well understood. In this study, we show that transcriptional factors of the ZHX family that are normally localized to the cell membrane in a ZHX2 - dependent manner enter the nucleus in different combinations in primary glomerular diseases like MCD and FSGS to induce downstream changes. Stabilization of ZHX2 in the cell membrane directly or through its transmembrane partners could be important in the future treatment of these diseases.
ACKNOWLEDGEMENTS
Supported by NIH grants R01DK109713, R01DK111102, R01DK101637, R01DK090035 and R01DK077073 to SSC, K01DK096127 to LCC; AHA Scientist Development Grant 16SDG27500017 to CM; and VA CDA-2 – 1 IK2 BX001942 to CBM. The authors thank Guangxing Bai for technical assistance in making the NPHS2 promoter - Zhx2 transgenic construct under the direction of SSC, and early promoter – reporter studies. SSC thanks Karel J. M. Assmann, formerly Professor of Pathology at Nijmegen, for useful discussions on the anti-APA antibody model. SSC thanks Max Cooper, formerly at the University of Alabama at Birmingham, for useful discussions on Aminopeptidase A.
ABBREVIATIONS
- ZHX
Zinc Fingers and Homeoboxes
- APA
Aminopeptidase A
- Angptl4
Angiopoietin-like 4
- ENPEP
Gene for Aminopeptidase A (Glutamyl Aminopeptidase)
- MCD
Minimal Change Disease
- FSGS
Focal and Segmental Glomerulosclerosis
- CG
Non-HIV Collapsing Glomerulopathy
- NPHS1
gene for Nephrin
- NEPH1
gene for Neph1
- ZO1
gene for Zona Occludens 1
- CD2AP
gene for CD2 associated protein
- ACTN4
gene for α-Actinin 4
- COL4A3
gene for Collagen Type IV Alpha 3 chain
- COL4A4
gene for Collagen Type IV Alpha 4 chain
- NID1
gene for Nidogen 1
- PODXL
gene for Podocalyxin
- DAG1
gene for Dystroglycan 1
- ITGB1
gene for Integrin subunit Beta 1
- ANGPT2
gene for Angiopoietin 2
- VEGFA
gene for Vascular Endothelial Growth Factor A
- WT1
gene for Wilms Tumor 1
- LMX1B
gene for LIM Homeobox transcriptional Factor 1 Beta
- PAX2
gene for Paired Box 2
- MYC
gene for MYC Proto-Oncogene, BHLH Transcription Factor
Footnotes
DISCLOSURE
SSC is founder and president of GDTHERAPY LLC, and holds patents on kidney therapeutics related to recombinant human Angptl4, sialylation of proteins and cytokine depletion. All other authors declare no competing interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Chugh SS, Clement LC, and Macé C. New insights into human minimal change disease: Lessons from animal models. Am J Kid Dis. 2012;59:284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clement LC, Avila-Casado C, Macé C, et al. Podocyte – secreted Angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med. 2011;17:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shkreli M, Sarin KY, Pech MF, et al. Reversible cell-cycle entry in adult kidney podocytes through regulated control of telomerase and Wnt signaling. Nat Med. 2011;18(1):111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chugh SS and Clement LC. Telomerase at the center of collapsing glomerulopathy. Nat Med. 2012;18:26–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu G, Clement L, Kanwar YS, et al. ZHX proteins regulate podocyte gene expression during the development of nephrotic syndrome. J. Biol. Chem 2006;281:39681–39692. [DOI] [PubMed] [Google Scholar]
- 7.Clement L, Liu G, Perez-Torres I, et al. Early changes in gene expression that influence the course of primary glomerular disease. Kidney Int. 2007;72:337–347. [DOI] [PubMed] [Google Scholar]
- 8.Chugh SS. Transcriptional regulation of podocyte disease. Transl Res. 2007;149:237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawata H, Yamada K, Shou Z, et al. The mouse zinc-fingers and homeoboxes (ZHX) family; ZHX2 forms a heterodimer with ZHX3. Gene. 2003;323:133–140. [DOI] [PubMed] [Google Scholar]
- 10.Hirano S, Yamada K, Kawata H, et al. Rat zinc-fingers and homeoboxes 1 (ZHX1), a nuclear factor- YA-interacting nuclear protein, forms a homodimer. Gene. 2002;290(1–2):107–114. [DOI] [PubMed] [Google Scholar]
- 11.Yamada K, Kawata H, Shou Z, et al. Analysis of zinc-fingers and homeoboxes (ZHX)-1-interacting proteins: molecular cloning and characterization of a member of the ZHX family, ZHX3. Biochem J. 2003;373(Pt 1):167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada K, Kawata H, Matsuura K, et al. (2002) Functional analysis and the molecular dissection of zinc-fingers and homeoboxes 1 (ZHX1). Biochem Biophys Res Commun. 2002;297(2):368–374. [DOI] [PubMed] [Google Scholar]
- 13.Kawata H, Yamada K, Shou Z, et al. Zinc-fingers and homeoboxes (ZHX) 2, a novel member of the ZHX family, functions as a transcriptional repressor. Biochem J. 2003;373(Pt 3):747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perincheri S, Dingle RW, Peterson ML, et al. Hereditary persistence of alpha-fetoprotein and H19 expression in liver of BALB/cJ mice is due to a retrovirus insertion in the Zhx2 gene. Proc Natl Acad Sci USA. 2005;102:396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perincheri S, Peyton DK, Glenn M, et al. Characterization of the ETnII-alpha endogenous retroviral element in the BALB/cJ Zhx2 (Afr1 ) allele. Mamm Genome. 2008;19:26–31. [DOI] [PubMed] [Google Scholar]
- 16.Lu Q, Sun EE, Klein RS, et al. Ephrin-B reverse signaling is mediated by a novel PDZ-RGS protein and selectively inhibits G protein-coupled chemoattraction. Cell. 2001;105:69–79. [DOI] [PubMed] [Google Scholar]
- 17.Wu C, Qiu R, Wang J, et al. ZHX2 Interacts with Ephrin-B and regulates neural progenitor maintenance in the developing cerebral cortex. J Neurosci. 2009;29:7404–7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto T, Karasawa T, Saito A, et al. Ephrin-B1 localizes at the slit diaphragm of the glomerular podocyte. Kidney Int. 207;72(8):954–964. [DOI] [PubMed] [Google Scholar]
- 19.Assmann KJ, van Son JP, Dijkman HB, et al. A nephritogenic rat monoclonal antibody to mouse aminopeptidase A. Induction of massive albuminuria after a single intravenous injection. J Exp Med. 1992;175:623–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mentzel S, de Leeuw EP, van Son JP, et al. Characterization of a 43 kD protein associated to aminopeptidase A from murine kidney. Biol Chem Hoppe Seyler. 1994;375(9):623–627. [DOI] [PubMed] [Google Scholar]
- 21.Gerlofs-Nijland ME, Assmann KJ, Dijkman HB, et al. Albuminuria in mice after injection of antibodies against aminopeptidase A: role of angiotensin II. J Am Soc Nephrol. 2001;12(12):2711–2720. [DOI] [PubMed] [Google Scholar]
- 22.Chugh S, Yuan H, Haydar S, et al. Aminopeptidase A: A nephritogenic target antigen of nephrotoxic serum. Kidney Int 2001;59:601–613. [DOI] [PubMed] [Google Scholar]
- 23.Liu G, Kaw B, Kurfis J, et al. Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability. J Clin Invest. 2003;112:209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clement LC, Macé C, Avila-Casado C, et al. Circulating Angiopoietin-like 4 links proteinuria with hypertriglyceridemia in nephrotic syndrome. Nat. Med 2014;20:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takemoto M, Asker N, Gerhardt H, et al. A new method for large scale isolation of kidney glomeruli from mice. Am. J. Pathol 2002;161:799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.