Abstract
Ultra-processed food consumption has been associated with several health outcomes such as obesity, hypertension, cardiovascular disease and cancer. The deleterious nutrient profile of these products, and the presence of food additives, neoformed contaminants and contact materials such as phthalates and bisphenol may be some of the potential pathways through which ultra-processed food influences disease outcomes. The aim of this study was to examine the association between dietary contribution of ultra-processed foods and urinary biomarker concentrations of parent compounds or their metabolites including Di(2-ethylhexyl) phthalate (ΣDEHP), Di-isononyl phthalate (ΣDiNP), Monocarboxynonyl phthalate (mCNP), Mono (3-carboxypropyl) phthalate (mCPP), Monobenzyl phthalate (mBzP), Bisphenol A (BPA), Bisphenol F (BPF) and Bisphenol S (BPS), in the US. Participants from the cross-sectional 2009–2016 National Health and Nutrition Examination Survey, aged 6+ years, with urinary measures and with one 24-hour dietary recall were included in the study. Ultra-processed foods were identified based on the NOVA classification system, a four-group food classification based on the extent and purpose of industrial food processing. Linear regression was used to compare average urinary creatinine-standardized concentrations across quintiles of energy contribution of ultra-processed foods. Models incorporated survey sample weights and were adjusted for different sociodemographic and life-style variables. Adjusted geometric means of ΣDiNP, mCNP, mCPP, mBzP and BPF increased monotonically from the lowest to the highest quintile of ultra-processed food consumption. As both phthalates/bisphenol and ultra-processed foods have been previously associated with insulin resistance, diabetes, general/abdominal obesity and hypertension, our results suggest the possibility of contact materials in ultra-processed foods as one link between ultra-processed food and these health outcomes. Future studies could confirm findings and further explore these mechanisms of action.
Introduction
Ultra-processed foods are defined by NOVA (not an acronym) classification, as industrial formulations of food-derived substances (such as oils, fats, sugars, starch, protein isolates) that contain little or no whole food and often include flavorings, colorings, emulsifiers and other cosmetic additives [1]. Over the past decades, the consumption of ultra-processed foods has increased worldwide [2–8]. Prospective studies have linked ultra-processed food intake with a higher risk of overweight, obesity [9, 10], hypertension [11], dyslipidaemia [12], overall and breast cancer [13], cardiovascular diseases [14], diabetes [15] and all- cause mortality [16–18].
Several mechanisms may potentially explain these associations. Ultra-processed foods have a higher content in total fat, saturated fat, added sugar, energy density, and salt, together with a lower fibre, vitamin and mineral density, as compared to non-ultra-processed foods. Their consumption results in an overall deterioration of the nutritional quality of the diet [1, 19]. The convenience and hyperpalatability of ultra-processed foods, simultaneously lowers consumption of healthy non-ultra-processed foods such as fruit and vegetables [19]. Ultra-processed foods may also affect glycaemic responses and satiety [20] and create a gut microbial environment that promotes inflammatory disease [21]. Cosmetic additives frequently added to ultra-processed foods (such as glutamates, emulsifiers, sulfites and carrageenan) or several compounds that are neoformed during their processing (such as acrylamide or acrolein) could also promote disease [14]. A recent inpatient ad libitum cross-over randomized controlled trial conducted by the US National Institute of Health demonstrated that individuals consumed 508 more kcal/day and gained an average of 0.8 kg of weight during the 2-week ultra-processed diet (> 80% energy from ultra-processed foods) and lost 0.9 kg during the 2-week non-ultra-processed diet. The fact that diets were matched for total calories, macronutrients and fiber, suggests that mechanisms other than the dietary nutrient profile, like quicker eating time or reduced signs of satiety, might explain these results [22].
While not directly related with the food per se, the packaging of ultra-processed foods might also help explain the health effects of these products [14]. Ultra-processed foods are frequently packaged in materials that are a source of endocrine disrupting chemicals such as phthalates and bisphenol, associated with adverse health outcomes especially in pregnancy [23, 24]. A large body of cross-sectional studies have specifically linked Bisphenol A (BPA) exposure with higher risk of diabetes, general/abdominal obesity, and hypertension [25] and phthalates with diabetes [26] and insulin resistance [27].
Though contained in many consumer products, food is a ubiquitous source of phthalates and bisphenols attributed mainly to food production, processing, and packaging practices; food storage conditions and, also animal feeding practices [24]. These chemicals are not bound to the polymer matrix chemically and are known to migrate from food contact materials (plastics, paper, metal, glass, and printing inks) that protect food from physical damage and microbial spoilage [24]. The long-shelf life and ready-to-eat characteristics of ultra-processed foods entails that these substances are likely to leach into the food product, making ultra-processed foods a potential delivery vehicle for phthalates and bisphenols in humans. This leakage could be more severe in ready-to-eat or take-away food often heated or served warm in paper, cardboard or plastic containers [24, 28, 29].
The objective of our study was to examine the association between dietary contribution of ultra-processed food and exposure to Di(2-ethylhexyl) (ΣDEHP), Di-isononyl (ΣDiNP), Monocarboxynonyl (mCNP), Mono(3-carboxypropyl) (mCPP) and Monobenzyl (mBzP) phthalates, and Bisphenol A, F and S (BPA, BPF and BPS, respectively), in a US population aged 6 years and older.
Very few studies have explored this topic. To our knowledge, only one other study has assessed the link between ultra-processed food consumption and phthalates/bisphenol [30]. The authors of this study found a positive association between ultra-processed foods and urinary concentrations of Monocarboxynonyl (mCNP), Mono(3-carboxypropyl) (mCPP), and mono-(carboxyisoctyl) (MCOP) but not mono-benzyl (MBzP), Di(2-ethylhexyl) (ΣDEHP), or bisphenols. This study included data from NHANES cycle 2013–14 and was most likely underpowered to detect associations. The current study addresses this gap by including data from 2009 to 2016. We also examined the departure from linear relationship between percent of calories from ultra-processed foods and urinary concentrations of phthalate or bisphenol biomarkers. In addition, several sensitivity analyses were carried out to test the robustness of associations.
Material and methods
Data source, population and sampling
We used nationally representative data from National Health and Nutrition Examination Survey (NHANES) 2009–2016 (four 2-year cycles). NHANES is a continuous, nationally representative, cross-sectional survey of non-institutionalized, civilian US residents conducted by The Centers for Disease Control and Prevention [31]. Participants were recruited using a four-stage sample design based on the selection of counties, blocks, households, and the number of people within households.
The survey included an interview conducted in the home and a subsequent health examination performed at a mobile examination center (MEC) that included blood and urine collection. All NHANES participants who were examined at MECs were eligible for two 24-hour dietary recall interviews: the first one collected in-person in the MEC [32] and the second by telephone, 3 to 10 days later [33]. Dietary interviews were conducted by trained interviewers using the validated [34–36] US Department of Agriculture Automated Multiple-Pass Method [37]. Proxy-assisted interviews were conducted with children 6–11 years old; participants ≥ 12 years old completed the dietary interview for themselves.
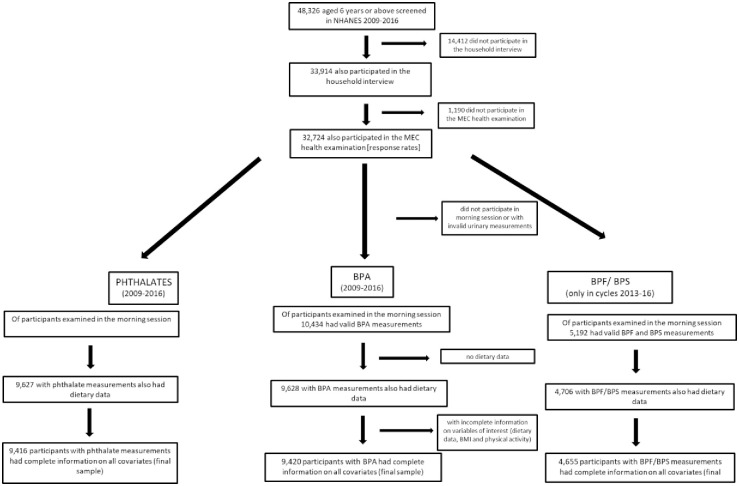
Our analytical sample comprised individuals aged 6 years or older (urinary concentrations of phthalate or bisphenol biomarkers were not measured in < 6 year old children), who provided a urine sample for phthalate or bisphenol analysis, completed a 24-hr dietary recall survey and had complete information on all variables of interest. This, resulted in a final sample size of 9,416 participants for phthalate analysis and 9,420 for Bisphenol A. The final sample size for Bisphenol F and S analyses was 4,655, as these 2 urinary concentrations were only measured in cycles 2013–2016 [38] (Fig 1).
Fig 1. Study flowchart.
The National Center for Health Statistics Research Ethics Review Board approved the study protocol. All participants provided written informed consent; parents or guardians provided consent for participants < 18 years of age.
Urinary chemical measurement
Due to their quick metabolism and consequent short half-lives (<24 h), exposures to phthalates and bisphenols are best characterized in urine (compared with blood) [39, 40]. Our study focused on the phthalate and bisphenol biomarkers (expressed in ng/mL) measured in all 4 studied cycles including Mono(2-ethylhexyl) (mEHP), Mono(2-ethyl-5-hydroxyhexyl) (mEHHP), Mono(2-ethyl-5-oxohexyl) (mEOHP), Mono(2-ethyl-5-carboxypentyl) (mECPP), Mono-isononyl (mNP/miNP), Monocarboxyoctyl (mCOP), Monocarboxynonyl (mCNP), Mono(3-carboxypropyl) (mCPP), Monobenzyl (mBzP), Monoethyl (mEP), Mono-n-butyl (mnBP), Mono-isobutyl (miBP), Bisphenol A (BPA) and its replacements Bisphenol S (BPS) and Bisphenol F (BPF) (S1 Table).
Urine specimens were collected in spot urine samples at the MEC and processed, stored under appropriate frozen (–20°C) conditions, and shipped to the Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention for analysis. Chemical analytes were quantified in urine using solid-phase extraction coupled online with high-performance liquid chromatography and tandem mass spectrometry and expressed as wet weights (ng/mL) [41, 42]. The limits of detection (LOD) ranged from 0.2 to 1.2 ng/mL for the phthalates [41] and from 0.1 to 0.4 ng/mL for bisphenols [42]. Where LOD varied across study cycles, we assumed the maximal LOD for each phthalate and bisphenol in our analysis to facilitate aggregation of data across study cycles [43].
For the sample of 9,416, 2858 individuals were below the lower detection limit (LLOD) for mEHP (0.8 ng/mL), 34 individuals for mEHHP (0.4 ng/mL), 40 individuals for mEOHP (0.2 ng/mL), 16 individuals for mECPP (0.4 ng/mL), 5533 for mNP/miNP (0.9 ng/mL), 20 for mCOP (0.3 ng/mL), 121 for mCNP (0.2 ng/mL), 808 for mCPP (0.4 ng/mL), 158 for mBzP (0.3 ng/mL), 18 for mEP (1.2 ng/mL), 200 for mBP/mnBP (0.4 ng/mL) and 130 for miBP (0.8 ng/mL). For the sample of 9,420, 637 individuals were below the LLOD for BPA (0.4 ng/mL). For the sample of 4655, 466 individuals were below the LLOD for BPS (0.1 ng/mL) and 2,093 for BPF (0.2 ng/mL). In NHANES, urinary phthalate and bisphenol measurements below the limits of detection of the used method were replaced with 1/√2 fraction of the detection limit. Individual concentrations (expressed in ng/mL) were rescaled in ηmol/mL by dividing each one by its molar mass. We calculated molar sums, representing classes of chemicals or parent compounds, by summing individual metabolite concentrations [44]: ΣDEHP (sum of di(2-ethylhexyl) phthalate metabolites: MEHP, MEHHP, MEOHP, and MECPP), and ΣDiNP (sum of Di-isononyl phthalate metabolites: mNP/miNP, and mCOP). In order to correct for urine dilution, urinary concentrations were normalized by urinary creatinine (and expressed in ηmol/g creatinine) [45]. This was done by dividing each individual concentration value (expressed in nmol/mL) by the corresponding urinary creatinine value (expressed in g/mL). Creatinine was measured using Beckman Synchron CX3 Clinical Analyser at the University of Minnesota [46].
Food classification according to level of processing
During the dietary interview, participants were prompted to list all foods and beverages consumed the day prior to the interview (in a 24-hr period, from midnight to midnight). All recorded food items (Food Codes) were classified according to NOVA, a food classification based on the extent and purpose of industrial food processing. NOVA includes 4 groups: “unprocessed or minimally processed foods” (such as fresh, dry or frozen fruits or vegetables; packaged grains and pulses; grits, flakes or flours made from corn, wheat or cassava; pasta, fresh or dry, made from flours and water; eggs; fresh or frozen meat and fish and fresh or pasteurized milk); “processed culinary ingredients” (including sugar, oils, fats, salt, and other substances extracted from foods and used in kitchens to season and cook unprocessed or minimally processed foods and to make culinary preparations), “processed foods” (including canned foods, sugar-coated dry fruits, salted meat products, cheeses and freshly made unpackaged breads, and other ready-to-consume products manufactured with the addition of salt or sugar or other substances of culinary use to unprocessed or minimally processed foods), and “ultra-processed foods”.
The NOVA group of ultra-processed foods of particular interest in this study, includes soft drinks, sweet or savory packaged snacks, confectionery and industrialized desserts, mass-produced packaged breads and buns, poultry and fish nuggets and other reconstituted meat products, instant noodles and soups, and many other ready-to-consume formulations of several ingredients. Besides salt, sugar, oils, and fats, these ingredients include food substances not commonly used in culinary preparations, such as modified starches, hydrogenated oils, protein isolates and classes of additives whose purpose is to imitate sensorial qualities of unprocessed or minimally processed foods and their culinary preparations, or to disguise undesirable qualities of the final product. These additives include colorants, flavorings, non-sugar sweeteners, emulsifiers, humectants, sequestrants, and firming, bulking, de-foaming, anti-caking and glazing agents. Unprocessed or minimally processed foods represent a small proportion of or are even absent from the list of ingredients of ultra-processed foods. A detailed definition of each NOVA food group and examples of food items classified in each group are shown elsewhere [47].
For all food items (Food Codes) judged to be a handmade recipe, the classification was applied to the underlying ingredients (Standard Reference Codes -SR Codes-) obtained from the USDA Food and Nutrient Database for Dietary Studies (FNDDS) [FNDDS] as further explained in previously published papers [48, 49]. Food items were sorted into mutually exclusive food subgroups within unprocessed or minimally processed foods (n = 11), processed culinary ingredients (n = 4), processed foods (n = 4) and ultra-processed foods (n = 18) [48].
To account for the propensity of phthalates and bisphenols to be concentrated in ready-to-eat or take-away food often heated or served warm in paper, cardboard or plastic containers [24, 28, 29], ultra-processed food subgroups were sorted into two groups: (1) Ready-to-heat/ Frozen meals (Frozen and shelf-stable plate meals; Pizza; French fries and other potato products; Sandwiches and hamburgers on bun); (2) Other ultra-processed foods (Breads; Cakes, cookies and pies; Salty snacks; Soft drinks, carbonated; Fruit drinks; Breakfast cereals; Sauces, dressings and gravies; Reconstituted meat or fish products; Sweet snacks; Ice cream and ice pops; Milk-based drinks; Desserts; Instant and canned soups; Other ultra-processed food).
Dietary assessment
USDA’s Food and Nutrient Database for Dietary Studies 5.0, 2011–2012, 2013–2014 and 2015–2016 [50] were used to code dietary intake data and to calculate Food Code energy and total fat energy intakes (kcal). For handmade recipes, we calculated the underlying ingredient (SR Code) energy and total fat values using variables from both FNDDS databases [50] and USDA National Nutrient Database for Standard Reference, Release 24, 26 and 28 [51]. The dietary recall interview also asked about the source from which each food was obtained. Fast food was defined as food obtained from restaurants without waiter/waitress service, or from pizza restaurants regardless of waiter/ waitress service.
For these analyses, we estimated 24-hr total energy intake, and energy intakes derived from ultra-processed foods, from total fat, from fat in ultra-processed foods, and from fast food, for every participant. We additionally estimated 24-hr total energy intake derived from both Ready-to-heat and Other ultra-processed food NOVA subgroups. The gram intake derived from ultra-processed foods was also estimated for sensitivity analysis.
Covariates
Potential confounders were identified from the literature [24, 30]. Socio-demographic covariates included sex, age, race/ethnicity, family income and cycle. Age was grouped into three categories (6–11 years, 12–19 years, 20 years of age and over) [31]. Race/ethnicity was categorized as Mexican- American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black and Other Races including Multi-Racial. With respect to family income, ratio of family income to poverty was established and categorized based on Supplemental Nutrition Assistance Program (SNAP) eligibility as 0.00–1.30, >1.30–3.50, and 3.50 and above [31]. Cycle included the following categories: 2009–10, 2011–12, 2013–14 and 2015–16.
BMI was calculated by dividing measured weight by height squared (kg/m2) [52] and used as a categorical covariate (underweight, normo-weight, overweight and obesity). Among adults, BMI values of <20 were classified as underweight, ≥25 as overweight and ≥30 kg/m2 as obesity according to World Health Organization criteria [53]. Among children, cutoff criteria were based on the Centers for Disease Control and Prevention's sex-specific 2000 BMI-for-age-sex growth charts for the United States: Underweight (BMI < 5th percentile), Normal weight (BMI 5th to < 85th percentiles), Overweight (BMI 85th to < 95th percentiles) and Obese (BMI ≥ 95th percentile) [54].
Physical activity was categorized into three intensity levels–light (<150 minutes per week of moderate intensity equivalent activity), moderate (150 to 300 minutes per week of moderate-intensity equivalent activity) and vigorous (>300 minutes per week of moderate- intensity equivalent activity) [55]. In individuals 12 + years of age, MET (metabolic equivalent of task)-minutes per week were calculated based on reported frequency and duration of physical activity in a typical week. In children<12 years of age, minutes per week were calculated based on response to question “Days physically active during past week (at least 60 minutes)”.
Smoking status was categorized as current smoker and non-smoker. Energy intake above recommended levels was coded as yes/ no according to sex–age–physical activity levels [56]. Total fat intake (% of total energy intake) was used as continuous or categorized as >30% (yes/no) [57]. Fat in ultra-processed food derived total energy intake (% of total energy intake) was categorized as > median value (18.7%) (yes/no) and into quintiles. Fast food intake (% of total energy intake) was used as continuous.
Data analysis
All available day 1 dietary intake data for each participant were utilized. First, we evaluated the mean dietary contribution of ultra-processed food (% of total energy) and urinary phthalate and bisphenol concentrations, overall and across socio-demographic, life- style and dietary characteristics of respondents using linear regression. Test of linear trend was performed for ordinal variables and Wald test with Bonferroni inequality adjustment for multiple comparisons was used for non-ordinal categorical variables or in the absence of a statistically significant linear trend. As urinary biomarker concentrations (both in ηmol/ ml and standardized in ηmol/g creatinine) had skewed distributions, these variables were log transformed (using natural logarithms) and geometric means were presented.
The average biomarker urinary concentrations were compared across quintiles of the dietary contribution of ultra-processed foods (% of total energy intake) using linear regression models. For each phthalate and bisphenol biomarker, four models were explored: 1) crude (in ηmol/ml); 2) standardized (in ηmol/g creatinine); 3) standardized (in ηmol/g creatinine) and adjusted for socio-demographic variables (sex, age group, race/ethnicity, ratio of family income to poverty, cycle); and 4) standardized (in ηmol/g creatinine) and adjusted for socio-demographic variables (sex, age group, race/ethnicity, ratio of family income to poverty, cycle), energy intake above recommended (yes, no), BMI (categorical), physical activity (categorical) and current smoking (yes, no). From these regression models, we estimated: a) geometric means of urinary chemical concentrations across quintiles of the energy contribution of ultra-processed foods as e(constant + β), where β are each of the estimated regression coefficients. On the basis of the multivariable regression models, margins were estimated at the means of all covariates; b) percent difference in urinary chemical concentrations comparing first and fifth quintile of the energy contribution of ultra-processed foods as (e(β4)– 1) × 100% with 95% CIs estimated as (e(β4 ± critical value × SE)– 1) × 100%, where β4 and SE are the estimated regression coefficient and standard error for the fifth quintile, respectively. Tests of linear trend were also performed to evaluate the effect of quintiles as a single continuous variable.
Thereafter, we used the restricted cubic spline in the multivariable linear regression models with five knots (5th, 27.5th, 50th, 72.5th, and 95th) following Harrell´s recommendations [58], to examine the shape of the dose-response relationship curve between percent of calorie from ultra-processed foods and urinary chemical concentrations [59].
To test the robustness of the associations, the following sensitivity tests were performed:
Using % of total gram intake of ultra-processed foods instead of % of total energy intake.
Previous studies have suggested that foods high in fat may be more contaminated by phthalates and BPA that are more lipophilic [60, 61]. For this reason and to test the robustness of the associations, we conducted sensitivity analyses (using the multivariable socio-demographic and life-style adjusted model) also adjusting for total fat intake (% of total energy intake) (continuous). We also carried out fully adjusted secondary analysis using as exposure variable the quintiles of fat in ultra-processed food derived total energy intake (% of total energy intake).
As studies have suggested that fast food may be a unique dietary source of ΣDiNP and mBzP [62, 63], we carried out sensitivity analysis also adjusting for fast food intake (% of total energy intake).
Because of lack of consensus on the most appropriate method to adjust for urinary dilution, the use of different methods for urinary dilution adjustment has been recommended [40]. For this reason, sensitivity analyses were also carried out adjusting for creatinine concentration (milligrams per deciliter) while using crude concentration measures (ηmol/mL) as suggested by Barr et al. [64].
Effect modification by sex, age group and data collection cycle were tested by including a one-by-one multiplicative interaction term (tested both as continuous and as dummy variable) in the multivariable socio-demographic and life-style adjusted model. Analyses were stratified according to statistically significant interaction variables and cycle.
We also examined the association between dietary contribution of Ready-to-heat and Other ultra-processed foods (each categorized into tertiles) and phthalate/bisphenol levels using adjusted models. Finally, we performed secondary analyses to test the association between quintiles of dietary contribution of each of the remaining three NOVA groups (minimally processed foods, processed culinary ingredients and processed foods) and urinary concentrations.
NHANES survey sample weights were used in all analyses to account for differential probabilities of selection for the individual domains, nonresponse to survey instruments, and differences between the final sample and the total US population. The Taylor series linearization variance approximation procedure was used for variance estimation in all analysis to account for the complex sample design and the sample weights [31]. Because we combined four survey cycles, new sample weights were calculated for each participant according to the analytical guidelines [31]. Statistical hypotheses were tested using a two-tailed p<0.05 level of significance. Data were analyzed using Stata statistical software package version 14.
Results
Overall dietary contribution of ultra-processed foods was 58% and decreased with age and cycle. Intakes were higher among non-Hispanic white and black (and lower among other race) and lower among those in the highest income level. Ultra-processed food consumption varied according to BMI status and was higher among smokers, and among individuals with energy and total fat intake above recommended levels (Table 1).
Table 1. Dietary contribution of ultra-processed foods and phthalate/bisphenol standardized levels (ηmol/g creatinine) according to characteristics of respondents.
US population aged 6 and above (NHANES 2009–2016).
| Dietary contribution of ultra-processed foods (% total energy intake) (n = 9,416) | PHTHALATES (ηmol/g creatinine) | BISPHENOL (ηmol/g creatinine)c | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ΣDEHP | ΣDiNP | mCNP | mCPP | mBzP | BPA | BPF | BPS | |||
| (n = 9,416) | (n = 9,416) | (n = 9,416) | (n = 9,416) | (n = 9,416) | (n = 9,420) | (n = 4,655) | (n = 4,655) | |||
| mean (SE) | GMa (GSE) | GM (GSE) | GM (GSE) | GM (GSE) | GM (GSE) | GM (GSE) | GM (GSE) | GM (GSE) | ||
| Gender | Men | 58.4 (0.4) | 87.8 (1.0) | 49.7 (1.0) | 7.1 (1.0) | 8.5 (1.0) | 17.5 (1.0) | 5.9 (1.0) | 2.1 (1.1) | 1.6 (1.1) |
| Women | 58.2 (0.5) | 103.6 (1.0)£ | 56.5 (1.0)£ | 8.0 (1.0)£ | 9.3 (1.0)£ | 22.2 (1.0)£ | 7.0 (1.0)£ | 2.4 (1.0)£ | 1.9 (1.0) | |
| Age groups (years) | 6 to 11 | 68.2 (0.5) | 176.5 (1.0) | 78.9 (1.1) | 11.7 (1.0) | 16.6 (1.0) | 50.8 (1.0) | 8.8 (1.0) | 2.3 (1.1)A | 1.9 (1.0) |
| 12 to 19 | 66.9 (0.7) | 90.5 (1.0) | 52.1 (1.1) | 6.8 (1.0) | 8.4 (1.1) | 23.8 (1.0) | 5.6 (1.0) | 2.0 (1.1)A | 1.2 (1.0) | |
| 20 or above | 55.9 (0.4)* | 90.3 (1.0)* | 51.0 (1.0)* | 7.3 (1.0)* | 8.4 (1.0)* | 17.5 (1.0)* | 6.4 (1.0)* | 2.3 (1.0)A | 1.9 (1.0)* | |
| Race/ethnicityb | Mexican American | 56.8 (0.6)A | 112.9 (1.1)B | 47.7 (1.1)AB | 6.6 (1.0)A | 7.7 (1.0)A | 20.8 (1.1)AB | 6.4 (1.0)A | 1.7 (1.1)A | 2.3 (1.1)B |
| Other Hispanic | 53.5 (0.9)B | 101.0 (1.0)AB | 58.9 (1.1)B | 7.0 (1.0)A | 8.9 (1.1)AB | 18.9 (1.1)AB | 6.4 (1.1)A | 1.7 (1.1)A | 2.3 (1.1)B | |
| Non-Hispanic White | 59.6 (0.5)C | 94.2 (1.0)A | 57.2 (1.1)B | 8.1 (1.0)B | 9.7 (1.0)B | 19.8 (1.0)AB | 6.5 (1.0)A | 2.5 (1.1)B | 1.6 (1.0)A | |
| Non-Hispanic Black | 61.4 (0.8)C | 84.8 (1.0)C | 41.6 (1.1)A | 6.4 (1.0)A | 7.1 (1.1)A | 21.5 (1.0)B | 6.4 (1.0)A | 2.1 (1.1)AB | 2.0 (1.0)B | |
| Other Race (including Multi-Racial) | 48.6 (1.0)D | 99.4 (1.0)AB | 43.8 (1.1)A | 6.1 (1.0)A | 7.4 (1.1)A | 17.0 (1.1)A | 5.9 (1.0)A | 2.0 (1.1)AB | 1.8 (1.1)AB | |
| Income to povertyb | 0.00–1.30 | 60.5 (0.7)C | 103.5 (1.0)B | 49.4 (1.0)A | 6.9 (1.0)A | 8.9 (1.0)AB | 26.9 (1.0)A | 7.0 (1.0)B | 2.0 (1.0)A | 2.0 (1.1)B |
| >1.30–3.50 | 59.5 (0.7)BC | 92.7 (1.0)A | 50.0 (1.0)A | 7.4 (1.0)B | 8.7 (1.0)AB | 21.4 (1.0)B | 6.7 (1.0)B | 2.4 (1.1)A | 1.8 (1.1)AB | |
| >3.50 and above | 56.3 (0.6)A | 92.7 (1.0)A | 60.3 (1.1)B | 8.1 (1.0)C | 9.3 (1.1)B | 15.3 (1.0)C | 6.0 (1.0)A | 2.4 (1.1)A | 1.6 (1.1)A | |
| missing | 56.2 (1.2)AB | 98.9 (1.1)AB | 45.0 (1.1)A | 7.1 (1.1)ABC | 7.6 (1.1)A | 19.6 (1.1)B | 6.2 (1.1)AB | 1.9 (1.1)A | 2.1 (1.1)B | |
| Cycle | 2009–10 | 58.3 (0.8) | 156.1 (1.1) | 47.5 (1.1) | 8.9 (1.0) | 12.7 (1.1) | 26.3 (1.1) | 8.5 (1.0) | _ | _ |
| 2011–12 | 60.1 (0.9) | 107.7 (1.0) | 77.3 (1.1) | 8.4 (1.0) | 13.5 (1.1) | 20.0 (1.0) | 7.5 (1.0) | _ | _ | |
| 2013–14 | 58.5 (0.8) | 77.8 (1.0) | 73.1 (1.0) | 8.3 (1.0) | 8.5 (1.1) | 17.4 (1.0) | 5.7 (1.0) | 2.7 (1.1) | 1.7 (1.1) | |
| 2015–16 | 56.4 (0.7)* | 65.4 (1.0)* | 30.1 (1.1)* | 5.2 (1.0)* | 4.5 (1.1)* | 17.0 (1.1)* | 4.8 (1.0)* | 1.9 (1.0)£ | 1.9 (1.1) | |
| BMIb | underweight | 57.0 (1.5)AB | 92.7 (1.1) | 53.9 (1.1)A | 7.6 (1.1)A | 9.0 (1.1) | 20.4 (1.1) | 7.2 (1.1)A | 3.0 (1.2)A | 1.8 (1.1)A |
| normoweight | 59.3 (0.6)B | 102.9 (1.0) | 53.8 (1.0)A | 7.8 (1.0)A | 9.6 (1.0) | 22.0 (1.0) | 6.6 (1.0)A | 2.1 (1.1)A | 1.7 (1.1)A | |
| overweight | 56.3 (0.6)A | 91.1 (1.0) | 52.1 (1.1)A | 7.3 (1.0)A | 8.6 (1.0) | 17.1 (1.0) | 6.3 (1.0)A | 2.3 (1.1)A | 1.7 (1.1)A | |
| obesity | 59.2 (0.5)B | 92.9 (1.0)* | 53.1 (1.0)A | 7.4 (1.0)A | 8.5 (1.0)* | 20.2 (1.0)* | 6.4 (1.0)A | 2.3 (1.1)A | 1.9 (1.1)A | |
| Physical activityb | Low | 58.5 (0.6) | 92.3 (1.0)A | 49.7 (1.0)A | 6.8 (1.0) | 8.1 (1.0) | 19.2 (1.0)A | 6.2 (1.0)A | 2.3 (1.1)A | 1.8 (1.1)A |
| Medium | 58.3 (0.8) | 102.7 (1.0)B | 58.2 (1.1)B | 8.2 (1.0) | 9.8 (1.1) | 19.9 (1.0)A | 6.6 (1.0)A | 2.2 (1.1)A | 1.8 (1.1)A | |
| High | 58.2 (0.5) | 95.5 (1.0)AB | 53.8 (1.0)AB | 7.8 (1.0)* | 9.2 (1.0)* | 20.2 (1.0)A | 6.6 (1.0)A | 2.3 (1.1)A | 1.8 (1.0)A | |
| Current smoker | no | 58.0 (0.4) | 96.8 (1.0) | 54.9 (1.0) | 7.8 (1.0) | 9.1 (1.0) | 19.4 (1.0) | 6.3 (1.0) | 2.2 (1.0) | 1.7 (1.0) |
| yes | 59.7 (0.9)£ | 89.3 (1.0)£ | 44.5 (1.1)£ | 6.4 (1.0)£ | 8.1 (1.0)£ | 22.1 (1.0)£ | 6.9 (1.0)£ | 2.8 (1.1)£ | 2.0 (1.1)£ | |
| Energy above recommended | no | 57.1 (0.5) | 92.4 (1.0) | 52.2 (1.0) | 7.3 (1.0) | 8.7 (1.0) | 19.5 (1.0) | 6.3 (1.0) | 2.2 (1.1) | 1.7 (1.0) |
| yes | 60.3 (0.5)£ | 100.6 (1.0)£ | 54.4 (1.0) | 7.8 (1.0)£ | 9.2 (1.0)£ | 20.3 (1.0) | 6.6 (1.0) | 2.3 (1.1) | 1.9 (1.0) | |
| Total fat intake (% of total energy intake) above >30% | no | 54.6 (0.6) | 97.8 (1.0) | 49.5 (1.0) | 7.1 (1.0) | 8.7 (1.0) | 20.2 (1.0) | 6.7 (1.0) | 2.1 (1.1) | 1.8 (1.1) |
| yes | 60.1 (0.4)£ | 94.4 (1.0) | 54.9 (1.0)£ | 7.7 (1.0)£ | 9.0 (1.0) | 19.6 (1.0) | 6.3 (1.0)£ | 2.3 (1.0) | 1.8 (1.0) | |
| Fat in UPF derived total energy intake (% of total energy intake) above median (18.7%) | no | 44.7 (0.4) | 94.2 (1.0) | 47.5 (1.0) | 7.0 (1.0) | 8.2 (1.0) | 18.9 (1.0) | 6.2 (1.0) | 2.0 (1.0) | 1.9 (1.0) |
| yes | 71.9 (0.3)£ | 96.8 (1.0) | 59.3 (1.0)£ | 8.0 (1.0)£ | 9.6 (1.0)£ | 20.7 (1.0)£ | 6.6 (1.0)£ | 2.5 (1.1)£ | 1.7 (1.0)£ | |
| Total | 58.3 (0.4) | 95.5 (1.0) | 53.1 (1.0) | 7.5 (1.0) | 8.9 (1.0) | 19.8 (1.0) | 6.4 (1.0) | 2.3 (1.0) | 1.8 (1.0) | |
aGM = Geometric means; GSE = Geometric standard error
bValues sharing a letter in the group label are not significantly different at the p<0.05 level (using Bonferroni inequality adjustment for multiple comparisons).
cBisphenol F and S analysis were only measured in 2013–2016 cycles
*Statistically significant linear trend (p<0.05)
£Statistically significant (p<0.05)
As can be seen in Table 1, phthalate/bisphenol concentrations were higher among women (except for BPS), decreased with age (except BPF which did not change and BPS which increased with age) and cycle (except for BPS) and varied across race/ethnicities (except for BPA) and income levels (except for BPF). Concentrations of some biomarkers also varied according to BMI status (decreased with BMI for ΣDEHP, mCPP and mBzP), physical activity (higher among middle physical activity level for ΣDEHP, ΣDiNP, mCNP and mCPP) and smoking status (higher among smokers for BPA, BPF, BPS and mBzP, and higher among non-smokers in remaining chemicals). For some biomarkers, levels varied according to excess total energy intake (positive association for ΣDEHP, mCNP and mCPP) and excess total fat intakes (positive association for ΣDiNP and mCNP, and inverse for BPS), and fat in ultra-processed foods derived total energy intake (positive association for all biomarkers, except for BPS which was inverse and ΣDEHP with no association).
Fully adjusted models showed a positive association between ultra-processed food quintiles and ΣDiNP, mCNP, mCPP, mBzP, and BPF concentration levels. Conversely, a lack of association was observed for ΣDEHP and BPA, and an inverse association for BPS (Table 2). Compared to the lowest ultra-processed food consumers (first quintile), the highest quintile had 23.4% (95% CI: 7.9% to 41.2%) higher levels of ΣDiNP, 14.6% (95% CI: 4.4% to 25.8%) higher levels of mCNP, 11.5% (95% CI: 0.2% to 24.1%) higher levels of mCPP, 10.7% (95% CI: -0.6% to 23.3%) higher levels of mBzP, 6.2% (95% CI: -2.7% to 15.9%) higher levels of BPA, and 33.8% (95% CI: 11.7% to 60.3%) higher levels of BPF. On the other hand, the highest quintile of ultra-processed food had 25.1% (95% CI: 12.0 to 36.2%) lower levels of BPS. The tendencies remained stable when ultra-processed food was expressed as % of total gram intake instead of % of total energy though the association with mCNP, mCPP and BPS lost statistical significance and the association with BPA gained significance (S2 Table).
Table 2. Phthalate/Bisphenol levels according to the quintiles of the dietary contribution of ultra-processed foods.
Subsample of US population aged 6 + years (NHANES 2009–2016).
| Quintile of dietary contribution of ultra-processed foods (% of total energy intake)a | |||||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | p for trend | ||
| ΣDEHP (GMb) | Crude (ηmol/mL) | 0.08 | 0.09 | 0.09 | 0.09 | 0.10 | <0.001 |
| Standardized (ηmol/g creatinine) | 93.3 | 96.2 | 91.9 | 96.5 | 100.0 | 0.075 | |
| Adjusted for socio-demographic variables (ηmol/g creat)c | 97.9 | 96.5 | 91.7 | 95.5 | 96.0 | 0.493 | |
| Adjusted for socio-demographic + other variables (ηmol/g creat)d | 98.4 | 96.4 | 91.5 | 95.3 | 96.0 | 0.425 | |
| ΣDiNP (GM) | Crude (ηmol/mL) | 0.04 | 0.05 | 0.05 | 0.06 | 0.06 | <0.001 |
| Standardized (ηmol/g creatinine) | 44.5 | 52.1 | 51.3 | 58.0 | 61.0 | <0.001 | |
| Adjusted for socio-demographic variables (ηmol/g creat)c | 47.0 | 52.5 | 50.7 | 58.3 | 57.7 | 0.001 | |
| Adjusted for socio-demographic + other variables (ηmol/g creat)d | 46.9 | 52.4 | 50.6 | 58.2 | 57.9 | 0.001 | |
| mCNP (GM) | Crude (ηmol/mL) | 0.006 | 0.007 | 0.007 | 0.008 | 0.008 | <0.001 |
| Standardized (ηmol/g creatinine) | 6.5 | 7.4 | 7.4 | 8.1 | 8.1 | <0.001 | |
| Adjusted for socio-demographic variables (ηmol/g creat)c | 6.9 | 7.5 | 7.3 | 8.1 | 7.8 | 0.001 | |
| Adjusted for socio-demographic + other variables (ηmol/g creat)d | 6.9 | 7.4 | 7.3 | 8.1 | 7.9 | 0.001 | |
| mCPP (GM) | Crude (ηmol/mL) | 0.007 | 0.008 | 0.008 | 0.009 | 0.011 | <0.001 |
| Standardized (ηmol/g creatinine) | 7.8 | 8.9 | 8.2 | 9.7 | 10.1 | <0.001 | |
| Adjusted for socio-demographic variables (ηmol/g creat)c | 8.4 | 9.1 | 8.1 | 9.5 | 9.4 | 0.039 | |
| Adjusted for socio-demographic + other variables (ηmol/g creat)d | 8.4 | 9.1 | 8.1 | 9.5 | 9.4 | 0.035 | |
| mBzP (GM) | Crude (ηmol/mL) | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 | <0.001 |
| Standardized (ηmol/g creatinine) | 17.3 | 17.8 | 19.1 | 21.6 | 24.1 | <0.001 | |
| Adjusted for socio-demographic variables (ηmol/g creat)c | 19.1 | 18.7 | 19.3 | 20.6 | 21.5 | 0.007 | |
| Adjusted for socio-demographic + other variables (ηmol/g creat)d | 19.2 | 18.8 | 19.3 | 20.5 | 21.3 | 0.017 | |
| BPA (GM) | Crude (ηmol/mL) | 0.005 | 0.006 | 0.006 | 0.007 | 0.007 | <0.001 |
| Standardized (ηmol/g creatinine) | 6.1 | 6.1 | 6.3 | 6.7 | 6.9 | 0.001 | |
| Adjusted for socio-demographic variables (ηmol/g creat)c | 6.3 | 6.2 | 6.3 | 6.7 | 6.7 | 0.046 | |
| Adjusted for socio-demographic + other variables (ηmol/g creat)d | 6.3 | 6.2 | 6.3 | 6.7 | 6.7 | 0.056 | |
| BPF (GM) | Crude (ηmol/mL) | 0.002 | 0.002 | 0.002 | 0.003 | 0.003 | <0.001 |
| Standardized (ηmol/g creatinine) | 1.8 | 2.3 | 2.3 | 2.5 | 2.4 | 0.001 | |
| Adjusted for socio-demographic variables (ηmol/g creat)c | 1.8 | 2.3 | 2.3 | 2.6 | 2.4 | 0.003 | |
| Adjusted for socio-demographic + other variables (ηmol/g creat)d | 1.8 | 2.3 | 2.3 | 2.6 | 2.4 | 0.004 | |
| BPS (GM) | Crude (ηmol/mL) | 0.0020 | 0.0017 | 0.0017 | 0.0017 | 0.0018 | 0.108 |
| Standardized (ηmol/g creatinine) | 2.2 | 1.8 | 1.8 | 1.7 | 1.6 | <0.001 | |
| Adjusted for socio-demographic variables (ηmol/g creat)c | 2.1 | 1.8 | 1.8 | 1.7 | 1.6 | 0.002 | |
| Adjusted for socio-demographic + other variables (ηmol/g creat)d | 2.1 | 1.8 | 1.8 | 1.7 | 1.6 | 0.001 | |
aFor all phthalates: Mean (range) dietary contribution of ultra-processed foods per quintile: 1st = 27.1 (0 to 39.5); 2nd = 46.8 (39.5 to 53.3); 3rd = 59.3 (53.3 to 65.2); 4th = 71.1 (65.2 to 77.7); 5th = 87.3 (77.7 to 100)
bGeometric means (GM) presented in all cases
cAdjusted for cycle, sex, age group (6 to 11, 12 to 19, +20), race/ethnicity (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, Other Race), ratio of family income to poverty (Supplemental Nutrition Assistance Program 0.00–1.30, >1.30–3.50 and >3.50 and over).
dAdjusted for cycle, sex, age group, race/ethnicity, and income, in addition to energy intake above recommended levels (y/n), BMI (underweight, normoweight, overweight, obesity), physical activity (low, medium, high) and current smoking (y/n).
Dose-response curve between dietary contribution of ultra-processed food and urinary biomarker concentrations using restricted cubic splines are displayed in S1 Fig. There was evidence of a dose-response association with no departure from linearity (p>0.05 for linearity) for mCPP and ΣDiNP.
In sensitivity analyses, additional adjustment for total fat intake did not change the main effects, though the positive association with mCPP lost statistical significance (S3 Table). When adjusting for fast food intake, the association with ΣDiNP and mCPP became non-significant. Further adjustment for creatinine concentration (as covariate), did not change the main effects though the association with BPA became significant. The strength of the association with urinary concentrations remained virtually the same when using quintiles of fat in ultra-processed food derived total energy intake (% of total energy intake) except for mBzP which became non-significant (S4 Table).
The association between ultra-processed food and urinary concentrations were not modified by cycle, age or sex, except ΣDiNP and BPA. The association of ultra-processed food intake with ΣDiNP was stronger in children than in adults and did not reach statistical significance among adolescents (p for interaction = 0.08). A positive non-significant association between ultra-processed food and BPA was found in both men and women, though the association was stronger in men (p for interaction = 0.02) (S5 Table).
Though exposure to phthalates and bisphenol has declined since 2009 (except for BPS), the trends of association between quintiles of UPF and phthalates and bisphenol concentrations in each cycle mirror the associations observed across all cycles (S6 Table).
We observed a monotonic increase of ΣDiNP, mCNP, mCPP, mBzP (p for trend ≤ 0.001) and BPA (p for trend = 0.042) with tertiles of Ready-to-heat ultra-processed foods. Non-Ready-to-heat ultra-processed foods were positively associated with BPF concentration (p for trend = 0.005) and inversely associated with BPS (p for trend ≤ 0.001) (Table 3).
Table 3. Phthalate/Bisphenol levels according to tertiles of dietary contribution of ultra-processed ready-to-heat and all remaining subgroupsa.
Subsample of US population aged 6 + years (NHANES 2009–2016).
| PHTHALATES (ηmol/g creatinine) | BISPHENOL (ηmol/g creatinine) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ΣDEHP | ΣDiNP | mCNP | mCPP | mBzP | BPA | BPF | BPS | |||
| Tertiles of dietary contribution of ultra-processed food subgroups (% of total energy intake) | Ready-to-heat food subgroupa | T1 | 94.6 | 47.1 | 7.1 | 8.4 | 19.2 | 6.3 | 2.1 | 1.9 |
| T2 | 99.6 | 58.0 | 7.4 | 9.0 | 20.3 | 6.4 | 2.7 | 1.6 | ||
| T3 | 95.5 | 62.4 | 8.2 | 9.8 | 20.6 | 6.7 | 2.3 | 1.7 | ||
| p for trend | 0.644 | <0.001 | <0.001 | <0.001 | <0.001 | 0.042 | 0.164 | 0.056 | ||
| Remaining subgroupsb | T1 | 97.6 | 53.6 | 7.4 | 9.0 | 19.9 | 6.3 | 1.9 | 2.0 | |
| T2 | 94.6 | 53.4 | 7.7 | 9.0 | 19.1 | 6.6 | 2.5 | 1.7 | ||
| T3 | 94.4 | 52.2 | 7.5 | 8.7 | 20.4 | 6.4 | 2.4 | 1.6 | ||
| p for trend | 0.208 | 0.573 | 0.586 | 0.364 | 0.487 | 0.525 | 0.005 | <0.001 | ||
All models adjusted for sex, age group (6 to 11, 12 to 19, +20), race/ethnicity (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, Other Race), ratio of family income to poverty (Supplemental Nutrition Assistance Program 0.00–1.30, >1.30–3.50 and >3.50 and over)
aFor all phthalates: Mean (range) dietary contribution of ready-to-heat ultra-processed foods per tertile: 1st = 0 (0 to 0); 2nd = 6.5 (0.1 to 11.4); 3rd = 30.7 (11.4 to 100)
bFor all phthalates: Mean (range) dietary contribution of remaining ultra-processed foods per tertile: 1st = 25.9 (0 to 38.7); 2nd = 47.3 (38.7 to 55.8); 3rd = 68.9 (55.8 to 100)
An inverse association was observed between quintiles of the dietary contribution of minimally processed foods and ΣDiNP, mCNP, mCPP, mBzP, BPA and BPF, and a positive association with BPS (S7 Table). Quintiles of the dietary contribution of processed culinary ingredients were positively associated with ΣDEHP and BPS and inversely associated with ΣDiNP and BPF (S8 Table). A lack of association was observed between quintiles of the dietary contribution of processed foods and phthalate or bisphenol levels (S9 Table).
Discussion
In this cross-sectional study of the US population aged 6 + years, there was evidence of monotonic dose-response association between ultra-processed food consumption (expressed as % of total energy intake) and urinary concentration of ΣDiNP, mCNP, mCPP, mBzP and BPF. No association was observed with ΣDEHP, BPA and an inverse association was observed with BPS. The association with BPA gained significance when ultra-processed food was expressed as % of total gram intake. These associations were largely consistent across age and sex subpopulations and remained significant after adjusting for total fat intake. Though exposures to phthalates and bisphenol have declined since 2009 (except for BPS) probably reflecting the effect of legislative activity and the advocacy efforts of nongovernmental organizations on the use of phthalates in consumer products and consumer behavior [43], these associations were also consistent across cycles. A previous study restricted to data from NHANES 2013–14 also reported a positive association between ultra-processed food consumption and mCNP, mCPP and Mono-(carboxyisoctyl) phthalate and a lack of association with ∑DEHP and BPA, however it did not observe a significant positive association with MBzP or BPF or an inverse association with BPS [30].
The lack of association of ∑DEHP with ultra-processed food quintiles was also observed with quintiles of minimally processed foods and processed foods. However, we did find an association with quintiles of processed culinary ingredients, which may be explained by the lipophilic nature of ∑DEHP which tend to concentrate in fattier foods such as butter, cream, cooking oils and animal fats (processed culinary ingredients) [60, 65].
While a positive though non-significant association was observed between BPA and both ultra-processed foods and processed foods, we found an inverse association with quintiles of minimally processed foods, and a lack of association with processed culinary ingredients. These findings may be explained by the fact that canned food which are mainly processed and ultra-processed foods, are considered the predominant source of BPA [66]. Indeed, contamination of food with BPA is usually caused by contact with food packaging materials containing epoxy resins and polycarbonate. Epoxy resins are often used as internal coatings of cans to protect from rusting and corrosion and to prevent direct contact of food with metal can walls, and in metal lids for in glass food jars. BPA in polycarbonate containers and coatings can migrate into foods, during storage and processing at elevated temperatures [66, 67].
Due to concerns regarding the health effects of BPA, industries have sought for alternatives such as BPF and BPS [68]. In this study we observed a positive association between ultra-processed food consumption and BPF levels but a negative association with BPS concentration. In recent years, BPS has been used as a substitute for BPA in thermal papers, while high levels of BPS and low levels of BPA have been found in thermal register receipts. What is still unknown, however, is whether BPS has been used as a substitute for BPA in can coatings [68]. In at least one study, BPS was not detected in any of the canned food composite samples and was detected instead in samples prepared from meat and meat products, indicating that sources of BPS other than can coatings are possible [68].
Epidemiological evidence on food sources of ΣDiNP, mCNP and mBzP is scarce [60, 69]. In our study, ΣDiNP, mCNP and mBzP were positively associated with ultra-processed food consumption. Some studies have suggested that fast food consumption may be a unique source of ΣDiNP [62, 63] and mBzP exposure [63]. Interestingly, when we further adjusted for dietary contribution of fast food, the association between ultra-processed food and ΣDiNP lost statistical significance but not the association with mBzP.
There is some epidemiological evidence of association between consumption of meats and fatty foods such as dairy and MCPP levels [60]. Consistent with these results, we observed a positive association between ultra-processed food consumption and MCPP concentrations which became non-significant with further adjustment by total fat intake or fast food intake.
Our study further suggests that ΣDiNP, mCNP, mCPP, mBzP and BPA may be more concentrated in ready-to-eat ultra-processed foods often heated or served in paper, plastic or cardboard containers, while non-ready-to-heat ultra-processed foods were directly associated with BPF and inversely associated with BPS. Though little is known about the migration of phthalates and bisphenol from food packaging during heating, at least one study observed a correlation between migration of dibutyl phthalate (DBP) and heating time [29]. Another study concluded that paper and cardboard used in food packaging may contribute to the inadvertent exposure of consumers to endocrine-disrupting chemicals [28].
We observed that the association of ultra-processed food intake with ΣDiNP was stronger in children than in adults and did not reach statistical significance among adolescents. Similarly, Buckley reported a stronger association between ultra-processed food intake and MCOP among children as compared with adults or adolescents [30]. Differences in types of ultra-processed foods consumed or metabolism between age groups may explain these results.
In our study we observed a positive association between ultra-processed food and urinary concentration of most phthalates and bisphenol, suggesting that contamination by contact materials may be an additional pathway to explain the associations seen between ultra-processed food and various health outcomes, as previously suggested by Srour [14]. Indeed, BPA exposure has been linked with higher risk of diabetes, general/abdominal obesity and hypertension [25] and phthalates with diabetes [26] and insulin resistance [27].
There are several strengths to this study including the use of a large, nationally representative sample of the US population, increasing the external validity of results. The disaggregation of recipes into underlying ingredients enabled the calculation of more precise estimates of dietary contribution of ultra-processed foods. The use of dietary contribution of ultra-processed food as exposure should reduce bias introduced by non-differential calorie misreporting from all foods.
Some study limitations must be acknowledged. The observational nature and cross-sectional design of NHANES does not allow the inference of causal relationship between ultra-processed food consumption and urinary phthalate or bisphenol concentrations. However, given the short biologic half-lives (<24 hour) of both phthalates and bisphenol [39], both urine samples and dietary information represent exposures during approximately the same 24-hour period. While multiple or 24-h urine samples are the ideal, reliance on a single spot urine sample corresponds well with short elimination half-lives [40].
Though self-reported dietary data are liable to information bias, 24-hour recalls as used in this study have been shown to be the least-biased self-report instrument available [70]. Additionally, standardized methods for assessing population intakes in NHANES have been shown to produce accurate intake estimates [34–36]. Differential underreporting of ultra-processed food consumption driven by social desirability bias could lead to underestimation of ultra-processed food dietary contribution or dilute the association between ultra-processed food consumption and urinary concentrations. Though NHANES collects limited information indicative of food processing (i.e. place of meals or product brand names), this is not consistently provided for all food items, which could lead to modest under or overestimation of the dietary contribution of ultra-processed foods. Lastly, the observed associations may be influenced by residual confounders such as source of food (i.e. food away from home, fast food or vending machine), exposure to materials in contact with foodstuffs, oral contact materials other than food (i.e. toys), dermal contact, dust in the environment or occupational exposure [66].
In conclusion, our findings suggest that ultra-processed food consumption may be a source of exposure to ΣDiNP, mCNP, mCPP, mBzP and BPF in the US population. Future studies should seek to confirm our findings and extend the research to examine health outcomes. As both phthalates/bisphenol and ultra-processed food have been previously linked with insulin resistance, diabetes, general/abdominal obesity and hypertension, future longitudinal studies may help to better understand the mediating role of contact materials in the association between ultra-processed food consumption and these outcomes.
Supporting information
- Di(2-ethylhexyl) phthalate (nmol/g creatinine): Wald test for linear term p = 0.354; Wald test for all non-linear terms p = 0.4203).
- Di-isononyl phthalate (nmol/g creatinine): There was little evidence of non-linearity in the restricted cubic spline model (Wald test for linear term p = 0.005; Wald test for all non-linear terms p = 0.3945).
- Mono(carboxynonyl) Phthalate (nmol/g creatinine): There was evidence of non-linearity in the restricted cubic spline model (Wald test for linear term p = 0.031; Wald test for all non-linear terms p = 0.4646).
- Mono(3-carboxypropyl) phthalate (nmol/g creatinine): Wald test for linear term p = 0.056; Wald test for all non-linear terms p = 0.4721.
- Mono-benzyl phthalate (nmol/g creatinine): Wald test for linear term p = 0.276; Wald test for all non-linear terms p = 0.1786.
- BPA: Wald test for linear term p = 0.177; Wald test for all non-linear terms p = 0.3955.
- BPF: Wald test for linear term p = 0.493; Wald test for all non-linear terms p = 0.2131.
- BPS: Wald test for linear term p = 0.318; Wald test for all non-linear terms p = 0.7867.
(XLSX)
(XLSX)
Subsample of US population aged 6 + years (NHANES 2009–2016). Legend. aFor all phthalates: Mean (range) dietary contribution of processed foods (% of total gram intake) per quintile [n]: 1st = 7.2 (0 to 12.4)%; 2nd = 17.2 (12.4 to 22.1)%; 3rd = 27.8 (22.1 to 34.2)%; 4th = 42.0 (34.2 to 51.3)%; 5th = 68.2 (51.3 to 100)%. All analysis adjusted for cycle, sex, age group (6 to 11, 12 to 19, +20), race/ethnicity (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, Other Race), ratio of family income to poverty (Supplemental Nutrition Assistance Program 0.00–1.30, >1.30–3.50 and >3.50 and over) + energy intake above recommended levels (y/n), BMI (underweight, normoweight, overweight, obesity), physical activity (low, medium, high) and current smoking (y/n). Geometric mean (GM) presented in all cases.
(XLSX)
Subsample of US population aged 6 + years (NHANES 2009–2016). Legend: aAll analysis adjusted for cycle, sex, age group (6 to 11, 12 to 19, +20), race/ethnicity (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, Other Race), ratio of family income to poverty (Supplemental Nutrition Assistance Program 0.00–1.30, >1.30–3.50 and >3.50 and over), energy intake above recommended levels (y/n), BMI (underweight, normoweight, overweight, obesity), physical activity (low, medium, high) and current smoking (y/n). bGM = geometric mean. cAditionally adjusted for total fat intake (% of total energy intake). dAditionally adjusted for fast food intake (% of total energy intake). eAditionally adjusted for creatinine concentration (milligrams/dL) (Ln).
(XLSX)
Subsample of US population aged 6 + years (NHANES 2009–2016) (n = 9,416 for phthalates and n = 9,320 for bisphenol). Legend: aMean (range) dietary contribution of UPF derived total fat intake per quintile: 1st = 6.3 (0 to 10.2); 2nd = 13.3 (10.2 to 16.1); 3rd = 18.8 (16.1 to 21.6); 4th = 24.6 (21.6 to 28.1); 5th = 34.1 (28.1 to 58.6). sAll analysis adjusted for cycle, sex, age group (6 to 11, 12 to 19, +20), race/ethnicity (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, Other Race), ratio of family income to poverty (Supplemental Nutrition Assistance Program 0.00–1.30, >1.30–3.50 and >3.50 and over) + energy intake above recommended levels (y/n), BMI (underweight, normoweight, overweight, obesity), physical activity (low, medium, high) and current smoking (y/n). Geometric mean (GM) presented in all cases.
(XLSX)
Subsample of US population aged 6 + years (NHANES 2009–2016). Legend: aGM = geometric mean. All analysis were adjusted for cycle, sex, age group (6 to 11, 12 to 19, +20), race/ethnicity (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, Other Race), ratio of family income to poverty (Supplemental Nutrition Assistance Program 0.00–1.30, >1.30–3.50 and >3.50 and over), education (<12y, 12y, >12y), energy intake above recommended levels (y/n), BMI (underweight, normoweight, overweight, obesity), physical activity (low, medium, high) and current smoking (y/n).
(XLSX)
Subsample of US population aged 6 + years (NHANES 2009–2016). Legend: aMean (range) dietary contribution of ultra-processed foods per quintile: 2009–10: 1st = 27.9 (0 to 40.2); 2nd = 47.0 (40.3 to 53.3); 3rd = 59.2 (53.3 to 64.7); 4th = 70.9 (64.8 to 77.1); 5th = 86.3 (77.1 to 100). 2011–12: 1st = 28.7 (0 to 40.8); 2nd = 48.6 (40.8 to 55.6); 3rd = 61.5 (55.7 to 67.2); 4th = 73.0 (67.2 to 79.7); 5th = 89.0 (79.7 to 100). 2013–14: 1st = 27.4 (0 to 40.1); 2nd = 47.2 (40.1 to 54.1); 3rd = 59.1 (54.2 to 64.8); 4th = 71.3 (64.8 to 78.5); 5th = 87.8 (78.5 to 100). 2015–16: 1st = 24.8 (0 to 36.8); 2nd = 44.6 (36.8 to 51.6); 3rd = 57.4 (51.6 to 63.6); 4th = 69.5 (63.7 to 75.6); 5th = 86.0 (75.6 to 100). bGM = geometric mean. All analysis adjusted for cycle, sex, age group, race/ethnicity, and income, in addition to energy intake above recommended levels (y/n), BMI (underweight, normoweight, overweight, obesity), physical activity (low, medium, high) and current smoking (y/n).
(XLSX)
Subsample of US population aged 6 + years (NHANES 2009–2016). Legend: aFor all phthalates: Mean (range) dietary contribution of unprocessed/ minimally processed foods per quintile [n]: 1st = 5.8 (0 to 11.9)% [n = 1784]; 2nd = 16.7 (15.8 to 23.6)% [n = 1746]; 3rd = 25.7 (23.6 to 31.3) [n = 1773]; 4th = 36.2 (31.3 to 41.3) [n = 1928]; 5th = 55.4 (41.3 to 100) [n = 2185]. All analysis adjusted for cycle, sex, age group (6 to 11, 12 to 19, +20), race/ethnicity (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, Other Race), ratio of family income to poverty (Supplemental Nutrition Assistance Program 0.00–1.30, >1.30–3.50 and >3.50 and over) + energy intake above recommended levels (y/n), BMI (underweight, normoweight, overweight, obesity), physical activity (low, medium, high) and current smoking (y/n). Geometric mean (GM) presented in all cases.
(XLSX)
Subsample of US population aged 6 + years (NHANES 2009–2016). Legend: aFor all phthalates: Mean (range) dietary contribution of processed cunilary ingredients per quintile [n]: 1st = 0 (0 to 0)% [n = 2701]; 2nd = 0.8 (0.003 to 1.4)% [n = 1071]; 3rd = 2.5 (1.4 to 3.6)% [n = 1782]; 4th = 5.3 (3.6 to 7.3)% [n = 1891]; 5th = 12.5 (7.3 to 51.4)% [n = 1971]. All analysis adjusted for cycle, sex, age group (6 to 11, 12 to 19, +20), race/ethnicity (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, Other Race), ratio of family income to poverty (Supplemental Nutrition Assistance Program 0.00–1.30, >1.30–3.50 and >3.50 and over) + energy intake above recommended levels (y/n), BMI (underweight, normoweight, overweight, obesity), physical activity (low, medium, high) and current smoking (y/n). Geometric mean (GM) presented in all cases.
(XLSX)
Subsample of US population aged 6 + years (NHANES 2009–2016). Legend: aFor all phthalates: Mean (range) dietary contribution of processed foods per quintile [n]: 1st = 0 (0 to 0)% [n = 2280]; 2nd = 1.5 (0.003 to 3.6)% [n = 2038]; 3rd = 5.9 (3.6 to 8.6)% [n = 1915]; 4th = 12.2 (8.6 to 16.7)% [n = 1713]; 5th = 28.2 (16.7 to 88.3)% [n = 1470]. All analysis adjusted for cycle, sex, age group (6 to 11, 12 to 19, +20), race/ethnicity (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, Other Race), ratio of family income to poverty (Supplemental Nutrition Assistance Program 0.00–1.30, >1.30–3.50 and >3.50 and over) + energy intake above recommended levels (y/n), BMI (underweight, normoweight, overweight, obesity), physical activity (low, medium, high) and current smoking (y/n). Geometric mean (GM) presented in all cases.
(XLSX)
Abbreviations
- BBzP
Benzylbutyl phthalate
- BPA
Bisphenol A
- BPF
Bisphenol F
- BPS
Bisphenol S
- DEHP
Di(2-ethylhexyl) phthalate
- DiDP
Di-isodecyl phthalate
- DiNP
Di-isononyl phthalate
- DOP/DnOP
Di-n-octyl phthalate
Data Availability
All analyses used publicly available datasets downloadable from NHANES website (<https://wwwn.cdc.gov/nchs/nhanes/Default.aspx>).
Funding Statement
This research received funding from Fundação de Amparo à Pesquisa do Estado de São Paulo (Processo n° 2015/14900-9) to CAM and from Fundação de Amparo à Pesquisa do Estado de São Paulo (Processo FAPESP n° 2018/17972-9) to EMS.
References
- 1.Monteiro CA, Cannon G, Levy RB, Moubarac JC, Louzada ML, Rauber F, et al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr. 2019. April;22(5):936–941. 10.1017/S1368980018003762 Epub 2019 Feb 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monteiro CA, Moubarac JC, Cannon G, Ng SW, Popkin B. Ultraprocessed products are becoming dominant in the global food system. Obes Rev 2013;14(Suppl 2):21–8. 10.1111/obr.12107 [DOI] [PubMed] [Google Scholar]
- 3.Moodie R, Stuckler D, Monteiro C, et al. , Lancet NCD Action Group. Profits and pandemics: prevention of harmful effects of tobacco, alcohol, and ultra-processed food and drink industries. Lancet 2013; 381:670–9. 10.1016/S0140-6736(12)62089-3 [DOI] [PubMed] [Google Scholar]
- 4.Moubarac JC, Batal M, Martins AP, et al. Processed and ultraprocessed food products: consumption trends in Canada from 1938 to 2011. Can J Diet Pract Res 2014; 75:15–21. 10.3148/75.1.2014.15 [DOI] [PubMed] [Google Scholar]
- 5.Martins AP, Levy RB, Claro RM, Moubarac JC, Monteiro CA. Increased contribution of ultra-processed food products in the Brazilian diet (1987–2009). Rev Saude Publica 2013; 47:656–65. [DOI] [PubMed] [Google Scholar]
- 6.Juul F, Hemmingsson E. Trends in consumption of ultra-processed foods and obesity in Sweden between 1960 and 2010. Public Health Nutr 2015; 18:3096–107. 10.1017/S1368980015000506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.PAHO. Ultra-processed food and drink products in Latin America: Trends, impact on obesity, policy implications. 2015. http://iris.paho.org/xmlui/bitstream/handle/123456789/7699/9789275118641_eng.pdf?sequence=5&isAllowed=y&ua=1
- 8.Vandevijvere S, Jaacks LM, Monteiro CA, Moubarac JC, Girling-Butcher M, Lee AC, et al. Global trends in ultraprocessed food and drink product sales and their association with adult body mass index trajectories. Obes Rev. 2019. May 17 10.1111/obr.12860 [DOI] [PubMed] [Google Scholar]
- 9.Mendonça RD, Pimenta AM, Gea A, et al. Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-Up (SUN) cohort study. Am J Clin Nutr 2016; 104:1433–40. 10.3945/ajcn.116.135004 [DOI] [PubMed] [Google Scholar]
- 10.Canhada et al. Ultra-processed foods, incident overweight and obesity, and longitudinal changes in weight and waist circumference: the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). PHN 2019 [DOI] [PMC free article] [PubMed]
- 11.Mendonça RD, Lopes AC, Pimenta AM, Gea A, Martinez-Gonzalez MA, Bes-Rastrollo M. Ultra-Processed Food Consumption and the Incidence of Hypertension in a Mediterranean Cohort: The Seguimiento Universidad de Navarra Project. Am J Hypertens 2017; 30:358–66. 10.1093/ajh/hpw137 [DOI] [PubMed] [Google Scholar]
- 12.Rauber F, Campagnolo PD, Hoffman DJ, Vitolo MR. Consumption of ultra-processed food products and its effects on children’s lipid profiles: a longitudinal study. Nutr Metab Cardiovasc Dis 2015; 25:116–22. 10.1016/j.numecd.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 13.Fiolet T, Srour B, Sellem L, et al. Consumption of ultra-processed foods and cancer risk: results from NutriNet-Santé prospective cohort. BMJ 2018; 360:k322 10.1136/bmj.k322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srour B, Fezeu LK, Kesse-Guyot E, Allès B, Méjean C, Andrianasolo RM, et al. Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Santé). BMJ. 2019. May 29; 365:l1451 10.1136/bmj.l1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernard Srour, Léopold K. Fezeu, Emmanuelle Kesse-Guyot, Benjamin Allès, Charlotte Debras, Nathalie Druesne-Pecollo, et al. Ultra-processed food consumption and risk of type 2 diabetes 1 among participants of the NutriNet-Santé prospective Cohort (accepted for publication) [DOI] [PMC free article] [PubMed]
- 16.Rico-Campà A, Martínez-González MA, Alvarez-Alvarez I, Mendonça RD, de la Fuente-Arrillaga C, Gómez-Donoso C, et al. Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. BMJ. 2019. May 29; 365:l1949 10.1136/bmj.l1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim H, Hu EA, Rebholz CM. Ultra-processed food intake and mortality in the USA: results from the Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994). Public Health Nutr. 2019. July;22(10):1777–1785. 10.1017/S1368980018003890 Epub 2019 Feb 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanco-Rojo R, Sandoval-Insausti H, López-Garcia E, Graciani A, Ordovás JM, Banegas JR, et al. Consumption of Ultra-Processed Foods and Mortality: A National Prospective Cohort in Spain. Mayo Clin Proc. 2019; 94(11): 2178–2188. 10.1016/j.mayocp.2019.03.035 [DOI] [PubMed] [Google Scholar]
- 19.Monteiro, C.A., Cannon, G., Lawrence, M., Costa Louzada, M.L. and Pereira Machado, P. 2019. Ultra-processed foods, diet quality, and health using the NOVA classification system. Rome, FAO.
- 20.Fardet A (2016) Minimally processed foods are more satiating and less hyperglycemic than ultra-processed foods: a preliminary study with 98 ready-to-eat foods. Food Funct 7, 2338–2346. 10.1039/c6fo00107f [DOI] [PubMed] [Google Scholar]
- 21.Zinöcker MK & Lindseth IA (2018) The Western diet–microbiome–host interaction and its role in metabolic disease. Nutrients 10, E365 10.3390/nu10030365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall KD, Ayuketah A, Brychta R, Cai H, Cassimatis T, Chen KY, et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019. July 2;30(1):67–77. e3. 10.1016/j.cmet.2019.05.008 Epub 2019 May 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. EDC-2: the Endocrine Society’s second Scientific Statement on endocrine-disrupting chemicals. Endocr Rev 2015; 36(6): E1–E150. 10.1210/er.2015-1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pacyga DC, Sathyanarayana S, Strakovsky RS. Dietary Predictors of Phthalate and Bisphenol Exposures in Pregnant Women. Adv Nutr. 2019. May 30 10.1093/advances/nmz029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rancière F, Lyons JG, Loh VHY, et al. Bisphenol A and the risk of cardiometabolic disorders: a systematic review with meta-analysis of the epidemiological evidence. Environ Health 2015; 14:46 10.1186/s12940-015-0036-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radke EG, Galizia A, Thayer KA, Cooper GS. Phthalate exposure and metabolic effects: a systematic review of the human epidemiological evidence. Environ Int. 2019. November; 132:104768 10.1016/j.envint.2019.04.040 Epub 2019 Jun 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shoshtari-Yeganeh B, Zarean M, Mansourian M, Riahi R, Poursafa P, Teiri H, et al. Systematic review and meta-analysis on the association between phthalates exposure and insulin resistance. Environ Sci Pollut Res Int. 2019. April;26(10):9435–9442. 10.1007/s11356-019-04373-1 Epub 2019 Feb 8. [DOI] [PubMed] [Google Scholar]
- 28.Lopez-Espinosa MJ, Granada A, Araque P, Molina-Molina JM, Puertollano MC, Rivas A, et al. Oestrogenicity of paper and cardboard extracts used as food containers. Food Addit Contam. 2007. January;24(1):95–102. 10.1080/02652030600936375 [DOI] [PubMed] [Google Scholar]
- 29.Moreira MA, Andre LC, Cardeal ZL. Analysis of phthalate migration to food simulants in plastic containers during microwave operations. Int J Environ Res Public Health 2013; 11(1):507–26. 10.3390/ijerph110100507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buckley JP, Kim H, Wong E, Rebholz CM. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013–2014. Environ Int. 2019. October; 131:105057 10.1016/j.envint.2019.105057 Epub 2019 Aug 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Health and Nutrition Examination Survey: Analytic Guidelines, 2011–2014 and 2015–2016. December 14, 2018.
- 32.NHANES. MEC In-Person Dietary Interviewers Procedures Manual. January 2009a. http://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/DietaryInterviewers_Inperson.pdf
- 33.NHANES. Phone Follow-Up Dietary Interviewer Procedures Manual. September 2009b. http://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/Dietary_PFU_09.pdf.
- 34.Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, et al. The USDA Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr 2008; 88:324–332. 10.1093/ajcn/88.2.324 [DOI] [PubMed] [Google Scholar]
- 35.Blanton CA, Moshfegh AJ, Baer DJ, Kretsch MJ. The USDA Automated Multiple-Pass Method accurately estimates group total energy and nutrient intake. J Nutr 2006. October; 136(10):2594–9. 10.1093/jn/136.10.2594 [DOI] [PubMed] [Google Scholar]
- 36.Rumpler WV, Kramer M, Rhodes DG, Moshfegh AJ, Paul DR, Kramer M. Identifying sources of reporting error using measured food intake. Eur J Clin Nutr 2008; 62:544–52. 10.1038/sj.ejcn.1602742 [DOI] [PubMed] [Google Scholar]
- 37.Automated Multiple-Pass Method. United States Department of Agriculture. Agriculture Research Service. http://www.ars.usda.gov/ba/bhnrc/fsrg (accessed August 2019).
- 38.National Health and Nutrition Examination Survey. NHANES Response Rates and Population Totals. Response Rates (2009–2016). <https://wwwn.cdc.gov/nchs/nhanes/ResponseRates.aspx> (accessed August 2019).
- 39.Calafat AM, Longnecker MP, Koch HM, Swan SH, Hauser R, Goldman LR, et al. Optimal exposure biomarkers for nonpersistent chemicals in environmental epidemiology. Environ Health Perspect 2015;123(7): A166–8. 10.1289/ehp.1510041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johns LE, Cooper GS, Galizia A, Meeker JD. Exposure assessment issues in epidemiology studies of phthalates. Environ Int. 2015. December; 85:27–39. 10.1016/j.envint.2015.08.005 Epub 2015 Aug 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey. Laboratory Procedure Manual. Metabolites of phthalates and phthalate alternatives. NHANES 2015–2016.
- 42.Centers for Disease Control and Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey. Laboratory Procedure Manual. Personal Care and Consumer Product Chemicals and Metabolites: benzophenone-3, bisphenol A, bisphenol F, bisphenol S, 2,4-dichlorophenol, 2,5-dichlorophenol, methyl-, ethyl-, propyl-, and butyl parabens, triclosan, and triclocarban. NHANES 2015–2016.
- 43.Zota AR, Calafat AM, Woodruff TJ. 2014. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ Health Perspect 122:235–241, 10.1289/ehp.1306681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, et al. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environmental health perspectives. 2012; 120(5):739–45. 10.1289/ehp.1104139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polinski KJ, Dabelea D, Hamman RF, Adgate JL, Calafat AM, Ye X, et al. Distribution and predictors of urinary concentrations of phthalate metabolites and phenols among pregnant women in the Healthy Start Study. Environ Res. 2018. April; 162:308–317. 10.1016/j.envres.2018.01.025 Epub 2018 Feb 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laboratory Procedure Manual. Urinary Creatinine. University of Minnesota, January 2011. https://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/ALB_CR_F_met_creatinine.pdf (accessed on 12 February 2017).
- 47.Monteiro CA, Cannon G, Moubarac JC, Levy RB, Louzada ML, Jaime PC. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr 2018; 21:5–17. 10.1017/S1368980017000234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinez Steele E, Baraldi LG, Louzada ML, Moubarac JC, Mozaffarian D, Monteiro CA. Ultra-processed foods and added sugars in the US diet: evidence from a nationally representative cross-sectional study. BMJ Open. 2016. March 9;6(3):e009892 10.1136/bmjopen-2015-009892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Juul F, Martinez-Steele E, Parekh N, Monteiro CA, Chang VW. Ultraprocessed food consumption and excess weight among US adults. Br J Nutr 2018; 120:90–100. 10.1017/S0007114518001046 [DOI] [PubMed] [Google Scholar]
- 50.U.S. Department of Agriculture, Agricultural Research Service. USDA Food and Nutrient Database for Dietary Studies 5.0, 2011–2012, 2013–2014 and 2015–2016. Food Surveys Research Group Home Page, http://www.ars.usda.gov/ba/bhnrc/fsrg
- 51.USDA National Nutrient Database for Standard Reference, Release 24, 26 and 28. Version Current: May 2016. Internet: http://www.ars.usda.gov/ba/bhnrc/ndl.
- 52.Centers for Disease Control and Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey. Anthropometry procedures manuals: 2009–2010; 2011–2012; 2013–2014.
- 53.World Health Organization (2016) BMI classification. http://apps.who.int/bmi/index.jsp?introPage=intro_3.html (accessed July 2019).
- 54.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: Methods and development. National Center for Health Statistics. Vital Health Stat 11(246). 2002. https://www.cdc.gov/nchs/data/series/sr_11/sr11_246.pdf (accessed July 2019). [PubMed] [Google Scholar]
- 55.U.S. Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. Washington (DC): U.S. Department of Health and Human Services; 2008. ODPHP Publication No. U0036. http://www.health.gov/paguidelines. Accessed April 2018.
- 56.US Department of Health and Human Services & US Department of Agriculture (2015) 2015–2020 Dietary Guidelines for Americans, 8th ed http://health.gov/dietaryguidelines/2015/guidelines (accessed September 2017).
- 57.Fats and fatty acids in human nutrition. Report of an expert consultation. FAO Food and Nutrition Paper (91). Rome, 2010. [PubMed]
- 58.Harrell F. E. Jr. 2001. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer. [Google Scholar]
- 59.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010. April 30;29(9):1037–57. 10.1002/sim.3841 Epub 2010 Jan 19. [DOI] [PubMed] [Google Scholar]
- 60.Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S. Phthalates and diet: a review of the food monitoring and epidemiology data. Environ Health 2014;13(1):43 10.1186/1476-069X-13-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cao XL. Phthalate esters in foods: sources, occurrence, and analytical methods. Compr Rev Food Sci Food Saf 2010;9(1):21–43. [DOI] [PubMed] [Google Scholar]
- 62.Zota AR, Phillips CA, Mitro SD. Recent fast food consumption and bisphenol A and phthalates exposures among the U.S. population in NHANES, 2003–2010. Environ Health Perspect 2016;124(10):1521–8. 10.1289/ehp.1510803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watkins DJ, Eliot M, Sathyanarayana S, et al. Variability and Predictors of Urinary Concentrations of Phthalate Metabolites during Early Childhood. Environ Sci Technol. 2014; 48(15):8881–8890. 10.1021/es501744v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005. February;113(2):192–200. 10.1289/ehp.7337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.NTP (National Toxicology Program). 2016. Report on Carcinogens, Fourteenth Edition.; Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service. https://ntp.niehs.nih.gov/go/roc14 (EndNote XML)
- 66.Geens T, Aerts D, Berthot C, Bourguignon JP, Goeyens L, Lecomte P, et al. 2012. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem Toxicol 50:3725–3740. 10.1016/j.fct.2012.07.059 [DOI] [PubMed] [Google Scholar]
- 67.Cao X.L., Perez-Locas C., Dufresne G., Clement G., Popovica S., Beraldin F et al. 2011. Concentrations of bisphenol A in the composite food samples from the 2008 Canadian total diet study in Quebec City and dietary intake estimates. Food Addit. Contam. Part A. 28, 791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cao XL, Kosarac I, Popovic S, Zhou S, Smith D, Dabeka R. LC-MS/MS analysis of bisphenol S and five other bisphenols in total diet food samples. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2019. July 30:1–8. 10.1080/19440049.2019.1643042 [DOI] [PubMed] [Google Scholar]
- 69.ECHA (European Chemicals Agency). Evaluation of New Scientific Evidence Concerning DINP and DIDP: In Relation to Entry 52 of Annex XVII to REACH Regulation (EC) No 1907/2006. 2012. https://echa.europa.eu/documents/10162/31b4067e-de40-4044-93e8-9c9ff1960715
- 70.Prentice R.L., Mossavar-Rahmani Y., Huang Y., Van Horn L., Beresford S.A.A., Caan B., et al. , 2011. Evaluation and comparison of food records, recalls, and frequencies for energy and protein assessment by using recovery biomarkers. Am. J. Epidemiol. (5), 591–603 September 1 10.1093/aje/kwr140 [DOI] [PMC free article] [PubMed] [Google Scholar]