Abstract
Background
The ability to detect one’s own memory capacity and develop strategies based on daily contexts is important for daily activities. The Contextual Memory Test (CMT) assesses self-awareness, self-efficacy, self-perception/evaluation of performance, recall, and strategy use that are associated with daily contexts, and could be a potentially suitable measurement for assessing memory and meta-memory in older adults with and without cognitive impairment. Nevertheless, the test-retest reliability and minimal detectable change (MDC) remain unknown in these individuals.
Objective
The purpose of this study was to examine test-retest reliability and calculate MDC of the CMT in healthy older adults and those with mild cognitive impairment (MCI).
Methods
Eighty-three participants completed the CMT twice with a one-month interval. Test-retest reliability was examined using intraclass correlation coefficient (ICC) in all seven domains of the CMT and the recognition subtest. The standard error of measurement (SEM) and MDC were calculated. The Bland-Altman analysis was performed to evaluate the degree of agreement between measurements.
Results
The ICC of five domains (self-awareness, self-perception/evaluation of performance, immediate/delayed/total recall) and the recognition subtest were good to excellent (ICC = 0.63–0.94) in healthy and MCI participants and the MDC% were less than 30% The ICC of the other two domains (self-efficacy and total strategy use, TSS) were low (ICC = 0.07–0.59) and the MDC% exceeded 30%. The Bland-Altman analysis showed generally better performance in the 2nd than the 1st measurement in most CMT domains.
Conclusions
Our results revealed sufficient test-retest reliability and acceptable MDC in most CMT domains in healthy and MCI participants. Only the self-efficacy and TSS domains demonstrated low ICC and large MDC. Possible practice effects were found between repeated measurements. Clinicians should be cautious when evaluating self-efficacy and strategy use using the CMT in older adults. Further improvements are needed for these two domains.
Introduction
Meta-memory refers to one’s own knowledge and awareness about his/her own memory performance [1]. It is an important underlying component of most activities of daily living [2, 3]. To perform various daily activities, individuals need to have good insights of their memory and predict whether they would be able to complete the desired tasks and if not, which strategies should be taken to successfully complete the desired tasks. These processes heavily relied on memory and meta-memory function including self-awareness, self-efficacy, and prediction/estimation of one’ own performance [1]. Nevertheless, memory and meta-memory often decline with advancing age [4]. Studies have demonstrated that older adults with subjective memory impairments (SCI) or mild cognitive impairment (MCI) had greater chances of transferring from SCI/MCI to dementia if they had impaired meta-memory function [5, 6]. Accurately assessing and monitoring memory and meta-memory changes in older adults is thus critical for early identification of at-risk individuals to prevent memory and meta-memory decline.
A variety of measurements have been developed to assess and monitor memory and meta-memory changes. Common memory assessments include the Wechsler Memory Scale [7] and Memory Assessment Scale [8]. Although these measurements provide information about memory function, they do not address implications of memory deficits on daily life. Furthermore, most memory assessments examined memory capacity using names, digits and texts (e.g., word-pairs) that do not involve activities of daily living [9]. As a result, outcomes of these measurements may not necessarily reflect actual performance in daily life.
Common meta-memory assessments such as the Memory Functioning Questionnaire (MFQ) [10] and Meta-memory in Adulthood (MIA) [11] primarily examined individuals’ subjective awareness/efficacy of memory, frequency of forgetting things and strategy use in basic and/or complex activities of daily living. However, one major drawback of these measurements was the large numbers of items in the questionnaires (e.g., 108 items in MIA and 64 items in MFQ) and the considerable amount of time to complete the tests. Although the short-form versions of these measurements have been developed, they tend to focus on only one or some domains of the original assessments (e.g., self-efficacy domain of MFQ) [12, 13]. Furthermore, despite evaluation of strategy use, most meta-memory assessments did not assess whether individuals could recognize daily living contexts and use context cues to develop strategies to remember/recall items involving daily activities. The ability to spontaneously recognize familiar daily contexts and develop according strategies is crucial for independent living [14, 15]. Understanding if older adults, particularly those with MCI, still retain this ability will facilitate design of appropriate treatment plans to prevent memory or meta-memory deterioration and improve independent living in the early phase of cognitive decline.
To address the above issues, the Contextual Memory Test (CMT) was primarily developed to assess memory and meta-memory related to daily contexts [16–18]. The CMT aims to determine if individuals can spontaneously detect daily contexts and use contextual strategies to remember/recall associated items, and if providing context cues will improve individuals’ memory and meta-memory performance related to daily contexts. The CMT was developed based on theoretical frameworks of memory processing including the stage model and the context-dependent theory [19]. The stage model hypothesizes that memory processing involves three different stages, which are encoding, storage and retrial and memory can be enhanced through manipulation of these three stages [19]. For example, presenting the item to be remembered within a particular context is likely to induce an association strategy between the items and the context, which may facilitate encoding and storage of memory. In this way, the context will be used as a cue or strategy to activate the stored memory, and subsequently enhancing recall of this item in the context. This context-dependent memory is highly involved in activities of daily living because most essential daily tasks, such as grooming, bathing, or dining are embedded in a particular daily scene. The purpose of the CMT is to assist healthcare professionals in identifying individuals that may have impaired context-related memory, for example the inability to recognize the context cue or the inability to use the contextual information as a strategy to recall items in everyday tasks [16]. Furthermore, the CMT can be used to assess context-related memory/meta-memory changes over time, which may help healthcare professionals to evaluate patients’ improvement after treatments [16].
The CMT consists of 16 questions and two picture cards. It has seven domains addressing memory and meta-memory function including (1) self-efficacy, (2) self-perception and evaluation of performance, (3) immediate recall, (4) delayed recall, (5) total recall, (6) total strategy use, and (7) general self-awareness, and an additional recognition subtest. Specifically the items to remember/recall are listed in the two picture cards and each card involves a common daily context, one is the morning theme and the other is the restaurant theme. For example, items that usually involve morning routines such as the tooth brush, comb and razor are listed in the morning picture card and items that usually involve in the restaurant context such as a waitress, receipts and menus are listed in the restaurant picture card.
The cross-culture validation, construct and discriminative validity of CMT have been examined in several previous studies. Josman et al. (2000) [18] evaluated the construct validity of the CMT to discriminate between three age groups (i.e., young, middle aged and older adults) and the applicability of the CMT in an Israeli population. They found significant differences in the CMT outcomes between these three groups, indicating good discriminative validity. In addition, this study also demonstrated that the CMT could be properly used in the Israeli participants. This finding further indicated the CMT may be appropriate for use in different population across regions. The discriminative validity of CMT has also been examined in healthy older adults and those with Alzheimer’s disease (AD) [17]. Significant differences were identified in the CMT outcomes between these two groups of participants, suggesting good discriminative validity.
Although the construct and discriminative validity have been examined, the reliability of the CMT, for example the test-retest reliability has not fully explored in healthy older adults and those with mild cognitive impairment. Previous studies primarily determined the reliability of CMT in individuals with neurological impairments including stroke, hematoma and head trauma [16], and they found good test-retest reliability in the recall, self-prediction of performance and total strategy use domains. A comprehensive evaluation of test-retest reliability including the relative reliability (i.e., intra-class correlation coefficient, ICC), absolute reliability (i.e., the standard error of measurement, SEM) and minimal detectable change (MDC) of all CMT domains and the agreement between repeated measurements are important pre-requisite for using the CMT as a screening tool for identifying individuals with potential memory and meta-memory impairment and an assessment tool for evaluating memory and meta-memory changes after treatments in these individuals.
The purpose of this study was to determine the test-retest reliability and the MDC of the CMT in older adults with and without MCI. The findings of this study can inform clinicians/researchers of the reproducibility and measurement errors of the CMT in healthy and MCI older adults and enhance usability of CMT in clinical/research settings.
Materials and methods
Participants
This study was a prospective trial with a repeated testing design. Participants were assessed by the CMT twice, with a one-month interval in between tests. Participants were enrolled from community centers, senior daycare centers, and retirement homes in northern Taiwan. The inclusion criteria were (1) age greater than 50 years old and (2) adequate global cognitive function to follow instruction (the Mini-Mental State Examination scores≥17). The exclusion criteria were (1) having hemi-neglect or visual agnosia, (2) depression symptoms (i.e., the Geriatric Depression Scale-short form ≥ 5) [20], (3) anxiety symptoms (the Geriatric Anxiety Inventory-short form (GAI-SF)-five items≥3 (GAI-SF score range: 1–5) [21], and (4) diagnosis of dementia (e.g., Alzheimer’s disease or vascular dementia). The Montreal cognitive assessment (MOCA) was used to identify participants with mild cognitive impairment. The one-point educational adjustment (addition) was applied for participants with less than 12 years of education [22]. Participants with MOCA scores ≥ 26 were classified as healthy participants and participants with MOCA scores ≥ 17 and < 26 were classified as MCI participants [22, 23]. All participants provided written informed consents and the study procedures were approved by the Institutional Review Board of Fu Jen catholic university, Taiwan. All study procedures followed the declaration of Helsinki.
Procedures
Participants undertook the CMT twice, with a one-month interval. The raters administered the CMT following the procedures in the CMT manual [16]. The raters were trained by the principal investigators of this study to ensure they performed CMT correctly in a standardized manner. Participants’ demographics and clinical information was collected prior to the 1st measurement.
Measurements
Structures and procedures
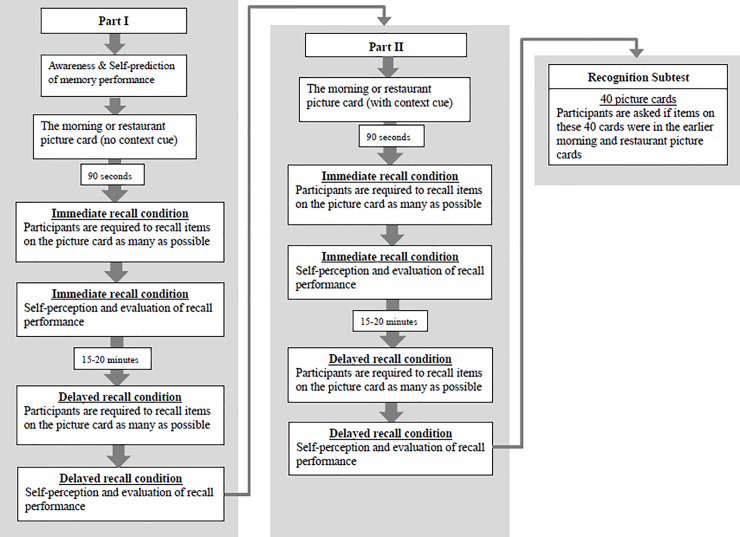
The CMT consists of 16 questions and two picture cards [16]. Each picture card contains 20 line drawings of items related to the morning routines or the restaurant theme (i.e., the morning version and the restaurant version). This design allows evaluation of participants’ ability to recognize the daily context and use the context as a strategy to remember/recall items. Participants were asked to remember and recall the items on the picture cards as many as possible. Fig 1 shows the structure and procedure of the CMT.
Fig 1. The structure and procedure of the CMT.
The CMT have two parts (Part I and Part II) in the immediate and delayed recall conditions, and an additional recognition subtest. The immediate recall condition always starts first followed by the delayed recall condition. The order of the CMT is Part I (immediate recall), Part I (delayed recall), Part II (immediate recall), Part II (delayed recall), and the recognition subtest. The context cue was provided only in Part II based on the standard instruction on the CMT manual. Participants were asked to think of what a person usually does when getting up in the morning and getting ready to leave the house (morning version) or think of a restaurant scene and the sequences of events that occur when a person is at the restaurant (restaurant version).
Part I began by asking participants standard questions about their awareness of memory function (question 1, 2, and 5–9), and their ability to predict memory performance (question 3). Then, the morning or restaurant picture card was shown to the participants and 90 seconds were given for participants to remember the 20 items on the picture card. After 90 seconds, participants were asked to recall the items as many as possible (immediate recall). The evaluator wrote down the numbers of items correctly recalled by the participants. After completing the picture card test, participants were asked how they felt about the picture card test including the difficulty of the test and their performance during the test (question 10–12) and estimated the numbers of items they had recalled (question 13). In addition, the evaluator assessed if the participants used any strategies to remember/recall those 20 items (question 15–16). Higher strategy use scores indicated that participants could spontaneously recognize the daily context and used it as the primary strategy to remember/recall items.
After an interval of 15 to 20 minutes, participants were asked to recall the items on the same picture card as many as possible (delayed recall). The evaluator asked again about participants’ perception (question 10–12) and estimation of performance (question 13) in the delayed recall condition.
In Part II, participants were given information about the context of the picture card. The purpose of Part II was to examine if participants could utilize the context cue as the primary strategy to remember/recall associated items. Part II began with the picture card test and followed by self-perception and evaluation (question 10–12), estimation of memory performance (question 13) and evaluation of strategy use (question 15–16) in both immediate and delayed recall conditions.
Part I was always performed first followed by a one-to-two hour break, and then Part II was delivered. Either the morning or restaurant version was used in Part I or Part II. For example, if the morning picture card was used for Part I, the restaurant picture card would be used for Part II. In this study, the order of morning and restaurant versions was randomized for each participant. This order was kept consistent for each participant in the 1st and 2nd measurements.
If participants failed to perform Part I and Part II, an additional subtest, which was the recognition subtest, would be administered. In the recognition subtest, 40 object cards were presented one at a time to the participants. Participants were asked if the presented object was in the earlier picture card test. The evaluators recorded the numbers of objects correctly identified by the participants.
In this study, Part I, Part II and the recognition subtest were all performed to comprehensively examine the reliability and MDC of all CMT domains. Participants were assessed in a quiet room to minimize interferences that may affect their cognitive responses. They were also required to relax and rest in the 15–20 minutes break between immediate recall and delayed recall conditions and in the one-to-two hour break between Part I and Part II.
Scoring
The CMT was scored independently in seven domains, which were the (1) self-efficacy domain including the prediction discrepancy and estimation discrepancy, (2) self-perception and evaluation domain (i.e., perceives task as difficult), (3) immediate recall (IR) (i.e., the numbers of items correctly recalled during the immediate recall condition), (4) delayed recall (DR) (i.e., the numbers of items correctly recalled during the delayed recall condition), (5) total recall (TR) (i.e., the numbers of total items correctly recalled in both immediate and delayed recall conditions), (6) total strategy use (TSS), and (7) general self-awareness (GA), as well as the recognition subtest (morning and restaurant versions). All domains were scored in Part I and Part II except the GA and prediction discrepancy, which were administered once in Part I. The scoring procedures followed the instruction on the CMT manual [16].
Statistical analysis
The ICC value represents the relative reliability. It was calculated based on the two-way random model and the absolute agreement (ICC2,1) [24]. An ICC value greater than 0.8 indicates excellent relative reliability; between 0.61 and 0.8 indicates good reliability; between 0.41 and 0.6 indicates moderate reliability and lower than 0.4 indicates poor reliability [25].
The SEM value represents the absolute reliability, which was the degree to which repeated measurements vary for individuals [26]. A smaller SEM indicates a better absolute reliability. The MDC is the minimal amounts of changes required to be considered as a real change and exceed the random errors [27]. The MDC95 was calculated in this study. It is a commonly used index that computed the MDC based on the 95% confidence interval (see the formula below). We calculated the percent of MDC95 (i.e., MDC%) because it was independent of the units of measurements. The MDC% was calculated by dividing the MDC95 by the maximum scores of each domain and multiplying it by 100 [28]. The MDC% less than 30 was considered an acceptable random measurement error based on recommendations from the literatures [28, 29]. The SEM and MDC95 were calculated based on the formula below,
where SD1 is the standard deviation of outcomes in the 1st assessment. z-value is the 95% confidence interval of a normal distribution (i.e., 1.96).
In addition, we also performed the Bland-Altman analysis to examine the agreement between repeated measurements [30]. The 95% limits of agreement (LOA) were calculated for each CMT domain, which were the mean differences (MD) of the two measurements ± 1.96SD. The LOA indicates the possible range of mean differences between two measurements for most individuals. In addition, a one-sample-test was used to examine if the mean differences (MD) between two measurements significantly deviated from 0, which suggested potential systematic bias [31]. The MD was the scores of the 2nd measurement subtracted the scores of the 1st measurement. A positive value indicated higher scores in the 2nd than the 1st measurement.
We conducted a sample size calculation based on a desired reliability coefficient of 0.8 reported in the previous reliability studies [16–18] and a minimum acceptable coefficient of 0.6 [25]. With an alpha value of 0.05, a power of 0.8 and two testing sessions, a minimum sample size of 39 was required for each group [32]. In addition to the reliability tests, we also performed an independent t test to examine whether there were differences in the age and educational levels between the healthy and MCI groups.
Results
Eighty-three participants were enrolled. All of them completed the two assessments. Among them, forty-four were healthy participants and thirty-nine were MCI participants. The mean age of healthy participants was 66.5 years old and their averaged MOCA scores were 28.14. The mean age of MCI participants was 69.44 years old and their averaged MOCA scores were 22.49. The age was similar between the two groups (t = -1.37, P = 0.18). The education level was higher in the healthy than MCI participants (t = 4.48, P<0.001). Table 1 shows the demographics and clinical characteristics of all participants.
Table 1. Demographics and clinical characteristics of healthy and MCI participants (N = 83).
| Characteristic | Healthy (n = 44) | MCI (n = 39) |
|---|---|---|
| Age (years) | 66.5 (8.99) | 69.44 (10.56) |
| Gender (male/female), n | 9/35 | 13/26 |
| Education (years) | 14 (3.44) | 9.58 (5.25) |
| GDS | 0.75 (1.06) | 1.38 (1.35) |
| GAI | 0.45 (0.9) | 0.33 (0.77) |
| MOCA | 28.14 (1.41) | 22.49 (2.84) |
| MMSE | 29.5 (0.73) | 27.49 (2.44) |
Value is presented as mean(standard deviation); MCI, Mild cognitive impairment; GDS, Geriatric Depression Scale; GAI, Geriatric Anxiety Inventory-Short Form; MOCA, Montreal Cognitive Assessment; MMSE, Mini-Mental State Examination.
Table 2 summarizes the ICC, SEM and MDC values of all CMT domains in Part I, Part II and the recognition subtest in healthy participants. Most ICC values were between 0.63 and 0.92, indicating good to excellent reliability except the self-efficacy (estimation discrepancy, Part I and II) and TSS (Part II) domains. The ICC values of the self-efficacy domain (estimation discrepancy, Part I and II) and TSS domain (Part II) were below 0.6 (range: 0.24–0.59). The SEM values were between 0.34 and 2.75. Only the MDC% values of the self-efficacy domain (prediction discrepancy) and TSS domain (Part II) were above 30% (32.22% and 35.24%).
Table 2. Results of the test-retest reliability and MDC of the CMT in healthy participants (n = 44).
| Part I. No context cue | Part II. Context cue | |||||
|---|---|---|---|---|---|---|
| ICC (95% CI) | SEM | MDC95 (MDC%) | ICC (95% CI) | SEM | MDC95 (MDC%) | |
| Self-efficacy | ||||||
| Prediction discrepancy | 0.71 (0.47, 0.84) | 2.32 | 6.44 (32.22%)* | |||
| Estimation discrepancy-IR | 0.29* (-0.31, 0.61) | 1.98 | 5.48 (27.4%) | 0.24* (-0.39, 0.59) | 1.83 | 5.06 (25.3%) |
| Estimation discrepancy-DR | 0.59* (0.26, 0.78) | 1.18 | 3.28 (16.41%) | 0.39* (-0.11, 0.67) | 1.81 | 5.03 (25.14%) |
| Self-perception and evaluation | ||||||
| Perceives task as difficult-IR | 0.78 (0.59, 0.88) | 0.67 | 1.86 (15.54%) | 0.92 (0.86, 0.96) | 0.34 | 0.96 (7.96%) |
| Perceives task as difficult-DR | 0.84 (0.71, 0.91) | 0.55 | 1.52 (12.65%) | 0.84 (0.71, 0.92) | 0.53 | 1.46 (12.19%) |
| Immediate recall (IR) | 0.79 (0.62, 0.89) | 1.09 | 3.28 (15.13%) | 0.67 (0.39. 0.82) | 1.24 | 3.45 (17.24%) |
| Delayed recall (DR) | 0.74 (0.52, 0.86) | 1.37 | 3.79 (18.97%) | 0.69 (0.42, 0.83) | 1.82 | 5.06 (25.28%) |
| Total recall (TR) | 0.81 (0.66, 0.9) | 2.05 | 5.69 (14.24%) | 0.72 (0.5, 0.85) | 2.75 | 7.63 (19.08%) |
| Total strategy use (TSS) | 0.63 (0.31, 0.8) | 1.06 | 2.94 (24.54%) | 0.41* (-0.08, 0.68) | 1.53 | 4.23 (35.24%)* |
| General awareness | 0.68 (0.41, 0.83) | 0.95 | 2.63 (9.05%) | |||
| Recognition-morning | 0.85 (0.71, 0.92) | 0.39 | 1.08 (5.44%) | |||
| Recognition-restaurant | 0.76 (0.56, 0.87) | 0.52 | 1.45 (7.26%) | |||
ICC, intraclass correlation coefficient; CI, confidence interval; SEM, standard error of measurement; MDC95, the minimal detectable change calculated based on 95% confidence interval.
*ICC< 0.6 or MDC % >30%.
Table 3 summarizes the ICC, SEM and MDC values of all CMT domains in Part I, Part II and the recognition subtest in MCI participants. Most ICC values were between 0.73 and 0.94, indicating good to excellent reliability except the self-efficacy domain (the estimation discrepancy, Part I and II) and TSS domain (Part II). The ICC values of the self-efficacy and TSS domains were below 0.6 (range: 0.07–0.48). The SEM values were between 0.42 and 2.12. Only MDC% values of TSS domain (Part II) were above 30% (35.14%).
Table 3. Results of the test-retest reliability and MDC of the CMT in MCI participants (n = 39).
| Part I. No context cue | Part II. Context cue | |||||
|---|---|---|---|---|---|---|
| ICC (95% CI) | SEM | MDC95 (MDC%) | ICC (95% CI) | SEM | MDC95 (MDC%) | |
| Self-efficacy | ||||||
| Prediction discrepancy | 0.73 (0.48, 0.86) | 2.11 | 5.84 (29.21%) | |||
| Estimation discrepancy-IR | 0.46* (-0.03, 0.72) | 1.98 | 5.47 (27.37%) | 0.07* (-0.76, 0.51) | 1.58 | 4.38 (21.89%) |
| Estimation discrepancy-DR | 0.42* (-0.11, 0.7) | 2.08 | 5.77 (28.83%) | 0.21* (-0.49, 0.58) | 2.01 | 5.57 (27.83%) |
| Self-perception and evaluation | ||||||
| Perceives task as difficult-IR | 0.93 (0.86, 0.96) | 0.55 | 1.53 (12.76%) | 0.94 (0.88, 0.97) | 0.42 | 1.17 (9.76%) |
| Perceives task as difficult-DR | 0.9 (0.81, 0.95) | 0.66 | 1.83 (15.22%) | 0.91 (0.83, 0.95) | 0.55 | 1.54 (12.8%) |
| Immediate recall (IR) | 0.91 (0.83, 0.95) | 1.08 | 3 (15.01%) | 0.88 (0.77, 0.94) | 1.1 | 3.04 (15.18%) |
| Delayed recall (DR) | 0.87 (0.75, 0.93) | 1.36 | 3.79 (18.93%) | 0.91 (0.82, 0.95) | 1.03 | 2.87 (14.33%) |
| Total recall (TR) | 0.91 (0.84, 0.95) | 2.12 | 5.88 (14.7%) | 0.91 (0.86, 0.96) | 1.9 | 5.28 (13.19%) |
| Total strategy use (TSS) | 0.76 (0.54, 0.87) | 0.93 | 2.59 (21.54%) | 0.48* (0.01,0.73) | 1.52 | 4.22 (35.14%)* |
| General awareness | 0.93 (0.87,0.96) | 0.75 | 2.08 (7.17%) | |||
| Recognition-morning | 0.84 (0.7, 0.92) | 1.14 | 3.16 (15.79%) | |||
| Recognition- restaurant | 0.78 (0.57, 0.88) | 1.38 | 3.83 (19.13%) | |||
ICC, intraclass correlation coefficient; CI, confidence interval; SEM, standard error of measurement; MDC95, the minimal detectable change calculated based on 95% confidence interval; MCI, mild cognitive impairment.
*ICC<0.6 or MDC %>30%.
Table 4 shows the Bland-Altman analysis results. The MD of the two measurements significantly deviated from 0 in most CMT domains, including the self-efficacy (estimation discrepancy, healthy, P = 0.03; prediction discrepancy, MCI, P = 0.01), self-perception and evaluation (MCI, P <0.001–0.007), IR, DR, TR (P<0.001), TSS (MCI, P<0.001–0.05) and the recognition subtest (healthy, morning, P = 0.02; MCI, restaurant, P = 0.004). Less prediction/estimation discrepancy, reduction of feeling of task as difficult and greater recall, recognition and TSS scores were found in the 2nd than the 1st measurement in healthy and MCI participants.
Table 4. Results of the Bland-Altman analysis in healthy and MCI participants.
| Part I. No context cue | Part II. Context cue | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthy | MCI | Healthy | MCI | |||||||||
| MD (SD) | 95% LOA | P | MD (SD) | 95% LOA | P | MD (SD) | 95% LOA | P | MD (SD) | 95% LOA | P | |
| Self-efficacy | ||||||||||||
| Prediction discrepancy | -1 (3.64) | 6.15, -8.15 | 0.08 | -1.58 (3.67) | 5.61, -8.79 | 0.01* | ||||||
| Estimation discrepancy-IR | -0.23 (3) | 5.66, -6.11 | 0.62 | -0.58 (3.1) | 5.49, -6.67 | 0.24 | -0.38 (2.68) | 4.88, -5.65 | 0.35 | -0.1 (2.76) | 5.31, -5.51 | 0.82 |
| Estimation discrepancy-DR | -0.77 (2.27) | 3.68, -5.22 | 0.03* | -0.44 (3.04) | 5.53, -6.4 | 0.38 | -0.45 (2.67) | 4.78, -5.69 | 0.27 | -0.97 (3.51) | 5.91, -7.86 | 0.09 |
| Self-perception and evaluation | ||||||||||||
| Perceives task as difficult-IR | -0.23 (1.16) | 2.04, -2.5 | 0.2 | -0.67 (1.01) | 1.31, -2.64 | <0.001* | -0.14 (0.63) | 1.1, -1.38 | 0.16 | -0.38 (0.85) | 1.27, -2.04 | 0.007* |
| Perceives task as difficult-DR | -0.36 (0.99) | 1.58, -2.3 | 0.2 | -0.56 (1.16) | 1.72, -2.85 | 0.004* | -0.18 (0.92) | 1.63, -1.99 | 0.2 | -0.26 (1.01) | 1.64, -2.25 | 0.12 |
| Immediate recall (IR) | 1.59 (1.83) | 5.19, -2 | <0.001* | 2.05 (1.99) | 5.97, -1.87 | <0.001* | 1.59 (2.06) | 5.63, -2.45 | <0.001* | 1.46 (2.28) | 5.91, -3.01 | <0.001* |
| Delayed recall (DR) | 2.2 (2.28) | 6.67, -2.26 | <0.001* | 1.64 (2.64) | 6.82, -3.53 | <0.001* | 1.15 (2.76) | 6.57, -4.25 | <0.001* | 1.51 (2.21) | 5.85, -2.82 | <0.001* |
| Total recall (TR) | 3.79 (3.46) | 10.59, -3 | <0.001* | 3.69 (4.01) | 11.55, -4.16 | <0.001* | 2.75 (4.33) | 11.24, -5.74 | <0.001* | 2.97 (3.71) | 10.26,- 4.31 | <0.001* |
| Total strategy use (TSS) | -0.23 (1.66) | 3.04, -3.5 | 0.37 | 0.33 (1.57) | 3.43, -2.76 | 0.2 | 0.45 (4.33) | 4.61, -3.7 | <0.001* | 0.74 (2.28) | 5.21, -3.72 | 0.05* |
| General awareness | 0.18(1.74) | 3.6, -3.23 | 0.49 | 0.15 (1.46) | 3.02, -2.71 | 0.52 | ||||||
| Recognition-morning | 0.26 (0.66) | 1.56, -1.04 | 0.02* | 0.47 (2.23) | 4.86, -3.91 | 0.2 | ||||||
| Recognition-restaurant | 0.22 (0.79) | 1.77, -1.33 | 0.08 | 1.23 (2.48) | 6.11, -3.64 | 0.004* | ||||||
MD, mean differences, the values of the 2nd test subtract the value of the 1st test; SD, standard deviation; LOA, limits of agreement; MCI, mild cognitive impairment.
*P<0.05.
Discussion
To the best of our knowledge, this is the first study to comprehensively examine the test-retest reliability and MDC of all CMT domains in healthy and MCI older adults. We found that memory and meta-memory function can be reliably assessed by most of the CMT domains in healthy and MCI participants except the TSS (Part II) and self-efficacy (Part I and II) domains. The ICC value was low and the MDC% was large in the TSS and self-efficacy domains in both healthy and MCI participants. The educational level was higher in healthy than MCI participants. Our study expanded findings of previous studies by showing that memory and meta-memory performance can be reliably assessed by most CMT domains in older adults with and without cognitive impairment. The ICC values reported in our study were also comparable to those of previous studies in individuals with neurological impairment (ICC = 0.73–0.81) [16–18]. This result indicates that the CMT can be used to assess memory and meta-memory function in not only individuals with brain injuries but also those with mild cognitive impairment.
Furthermore, we identified similar patterns of responses in the healthy and MCI participants, where the low test-retest reliability (i.e., ICC value) and large measurement errors (i.e., MDC%) were found in the TSS (Part II) and self-efficacy domains (Part I & II). This result indicated that use of memory strategies and self-efficacy might not be reliably assessed by the CMT in older adults irrespective of their educational levels or whether they had cognitive impairment. Two possible reasons may explain the insufficient test-retest reliability of these two domains. First, scoring of TSS in the context cue condition depended on the types of strategies adopted by the participants. The scores would be higher if participants used strategies fully or partially related to the contexts (i.e., morning/restaurant). Nevertheless, studies have shown that the familiarity to the contexts determined if episodic memory strategies would be used [33, 34]. If individuals are familiar with the association of items and contexts, they will have greater chances to trigger episodic memory and used context cues as the primary strategy to recall items [35, 36]. It may be possible that some of the items on the morning/restaurant picture cards are not embedded in participants’ usual morning /restaurant routines; consequently, providing context cues to these participants may not necessarily activate their episodic memory related to the morning/restaurant contexts and facilitate contextual strategy use. Instead, these participants may adopt various types of memory strategies, such as the sematic memory strategy (i.e., remembering the facts of the items), location or visualization of items, and caused high variances in measurements [37].
Similarly, self-efficacy domains including the prediction and estimation of performance may also rely on the prior knowledge of the contexts [38–40]. Participants may predict or estimate their performance more precisely and consistently if items on the picture cards are involved in their usual morning/restaurant routines and already built in their episodic memory [39]. In contrast, participants’ prediction or estimation performance may be less stable with greater random errors when they are unfamiliar with the association between the items and morning/restaurant contexts, thus resulting in a higher MDC value.
To minimize the potential impact of unfamiliarity of items/contexts on assessing strategy-use, we recommend expansion of current items on the morning/restaurant cards and individually select items that are relevant to each individual’s morning/restaurant routines [41]. For example, evaluators could ask individuals about their morning/restaurant routines to determine which items should be included in the picture card test. This will ensure that the items to remember/recall are coherent with the contexts, thus enabling true evaluation of individuals’ ability to utilize contextual strategies [42]. In addition, we recommended adding questions to examine the degrees of familiarity of items/contexts to the participants [39]. Questions such as “How familiar were these items to you?” or “Were these items involved in your usual morning/restaurant routines?” can be added to the CMT to determine if individuals’ strategy use and self-efficacy are affected by the unfamiliarity or solely by their memory/meta-memory dysfunction [43, 44]. Future studies could evaluate the impact of familiarity of activities of daily living items/contexts on contextual strategy uses and determine if individualization of items/contexts can help to improve test-retest reliability in the TSS and self-efficacy domains.
Second, it may also be possible that participants may have used contextual strategies but were unware of it or unable to verbalize it, which may affect TSS scores and result in higher variability and unstable measurements [45, 46]. To minimize this potential confounding effect, we recommended evaluators to analyze the order of recalled items along with the TSS scores to determine if participants had used contextual strategies. This will ensure that the TSS domain was scored appropriately.
We also identified large MDC% in the TSS and self-efficacy domains, indicating potentially greater random errors in these two domains. The relatively greater random errors in these two domains could be due to the inherent dynamic feature of meta-memory monitoring process. The meta-memory monitoring process, such as prediction or estimation of current memory performance is a dynamic process and may be affected by daily events and memory/meta-memory state of each person [47, 48]. Studies have also shown that day-to-day fluctuation of arousal, mental or environmental demands affected participants’ sense of control over their memory performance and the strategy they use to compensate for the unstable memory performance [48, 49]. Thus, it may be plausible that the fluctuated sense of control over memory performance led to the random errors in the self-efficacy and TSS domains and caused large MDC%. To prevent misinterpretation of CMT results in older adults, the MDC values reported in this study can be considered as a reference to determine if the observed changes overtime reflect real performance changes. In addition, we also recommend assessing these two domains at the same time of the day (e.g., morning or afternoon) in the same environment to minimize the influence of arousal levels/environments on measurements [47].
In addition to the assessment of ICC values, we also performed the Bland-Altman analysis to evaluate the agreement between two measurements. We found generally better performance (i.e., higher scores and fewer prediction/estimation errors) in the 2nd than the 1st measurement in most CMT domains in both groups of participants. This finding indicates that there may be potentially practice effects of CMT in healthy and MCI participants [50, 51]. Participants may have remembered the items/contexts or learned the contextual strategy in the 1st test and therefore improved their memory and meta-memory performance in the 2nd test. This result was consistent with the findings of practice effects of cognitive tests on learning and memory in the literatures for healthy and cognitively-impaired adults [51, 52]. To minimize the potential practice effects of CMT, we recommended using the dual-baseline approach. The dual-baseline approach has been suggested to be a useful approach to reduce practice effects of cognitive measurements [53, 54]. The CMT test can be administered twice in the same person within a few days or weeks. If significantly prominent improvement occurs from the 1st to the 2nd measurement, the 2nd measurement may serve as the baseline for subsequent analysis [50, 55]. Future studies could examine the best appropriate interval of two assessments for minimizing practice effects.
Seven limitations should be considered. First, although we have demonstrated that the CMT is appropriate for assessing memory and meta-memory in healthy and MCI participants, the usability of CMT in older adults with more severe cognitive impairment, such as dementia remains unknown. Second, we did not evaluate the validity of CMT, such as the concurrent validity in older adults although it has been established in individuals with brain injuries [16]. Future studies could include moderate-to-severe cognitively impaired older adults and examine the concurrent validity of CMT by comparing the CMT to other standardized memory/meta-memory assessments. Third, the cutoff value of the MDC% (i.e., 30%) was selected based on the recommendation in the literatures [28, 29]. However, we also provided the raw value of the MDC and the MDC% of each CMT domain. Future studies could use these raw values to determine their own cutoff points based on the study purposes. Fourth, the sample size of the study was relatively small although it was comparable to that of previous CMT studies [16–18]. Future study could recruit a larger sample of healthy and MCI participants to validate findings of this study. Fifth, we used the MOCA to identify whether participants had MCI; however, we did not assess the potential etiology of MCI using other assessments such as neurophysiological tests or brain imaging (e.g., Magnetic resonance imaging, MRI). Future study could examine the etiology of MCI participants to determine whether different etiology of MCI would affect the test-retest reliability of the CMT in these individuals. Sixth, the educational level was higher in the healthy than MCI participants although similar patterns of responses (i.e., low ICC and large MDC% of the TSS and self-efficacy domain) were found between the two groups. Future study could recruit MCI participants with different educational levels (e.g., high and low) to determine if the educational level would affect the test-retest reliability of CMT in MCI participants. Seventh, participants were evaluated in a quiet room and were required to relax during the break between the immediate and delayed recall condition as well as between Part I and Part II. This was to minimize the potential interference from the environment or conditions. Our results also showed good test-retest reliability in the immediate and delayed recall CMT domains, indicating that the procedures we performed may be useful. However, it is still possible that there were other environmental/conditional interferences that we did not control. Future studies are warranted to determine the best testing environments/conditions for performing the CMT in healthy and MCI participants.
Conclusions
Most memory and meta-memory tests do not directly assess memory/meta-memory performance related to daily contexts [7–13]. The CMT is specifically designed for evaluating awareness, self-perception and evaluation, self-efficacy, recall and strategy-use involving common daily contexts and therefore can be a potentially useful screening tool for identifying individuals that may have memory or meta-memory impairment in daily contexts and an assessment tool for evaluating memory and meta-memory changes involving daily tasks after treatments [16–18]. The CMT can also be used in conjunction with other memory or meta-memory assessments to provide a comprehensive evaluation of individuals’ cognitive ability related to activities of daily living.
Our study revealed that most CMT domains had sufficient test-retest reliability in healthy and MCI older adults. Only two domains (i.e., TSS and self-efficacy) had low test-retest reliability and large random errors, which could be due to participants’ unfamiliarity with the association between the items and contexts and the dynamic feature of meta-memory. We also identified potential practice effects of CMT in healthy and MCI participants. Clinicians/therapists should be cautious when explaining CMT scores between repeated measurements. Future studies are warranted to examine if individual selection of items based on each person’s familiar morning/restaurant routines along with the dual-baseline approach could help to improve test-retest reliability and reduce measurement errors of the CMT. This will enhance the usability of CMT in diagnosis of memory/meta-memory impairment and prognosis of memory/meta-memory recovery associated with daily contexts in the elderly.
Supporting information
(DOCX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by Chang Gung Memorial Hospital (CMRPD1F0411-413, CORPD1J0011, BMRP553; Recipient: CYW), Healthy Aging Research Center, Chang Gung University from the Featured Areas Research Center Program within the Framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan (EMRPD1I0451;Recipient: CYW), and the Ministry of Science and Technology (MOST 108-2314-B-182-040-MY3; Recipient: CYW) in Taiwan. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Flavell JH. Metacognition and cognitive monitoring: A new area of cognitive–developmental inquiry. Am Psychol. 1979;34:906–11. 10.1037/0003-066X.34.10.906 [DOI] [Google Scholar]
- 2.Dodge HH, Kadowaki T, Hayakawa T, Yamakawa M, Sekikawa A, Ueshima H. Cognitive Impairment as a Strong Predictor of Incident Disability in Specific ADL–IADL Tasks Among Community-Dwelling Elders: The Azuchi Study. The Gerontologist. 2005;45:222–30. 10.1093/geront/45.2.222 [DOI] [PubMed] [Google Scholar]
- 3.Hughes TF, Chang C-CH, Bilt JV, Snitz BE, Ganguli M. Mild cognitive deficits and everyday functioning among older adults in the community: the Monongahela-Youghiogheny Healthy Aging Team study. Am J Geriatr Psychiatry. 2012;20:836–44. 10.1097/JGP.0b013e3182423961 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isingrini M, Perrotin A, Souchay C. Aging, metamemory regulation and executive functioning. Prog Brain Res. 2008;169:377–92. 10.1016/S0079-6123(07)00024-6 . [DOI] [PubMed] [Google Scholar]
- 5.Petersen RC. Mild cognitive impairment: transition between aging and Alzheimer's disease. Neurologia. 2000;15:93–101. . [PubMed] [Google Scholar]
- 6.Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia—meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119:252–65. 10.1111/j.1600-0447.2008.01326.x . [DOI] [PubMed] [Google Scholar]
- 7.Wechsler D. Wechsler Memory Scale-Revised. Psychological Corporation; 1987. [Google Scholar]
- 8.Williams JM, Williams K, Gillard E. The Memory Assessment Scales (MAS): A new clinical memory battery. Arch Clin Neuropsychol. 1991;6:234–5. 10.1093/arclin/6.3.234a [DOI] [Google Scholar]
- 9.Franzen S, van den Berg E, Goudsmit M, Jurgens CK, van de Wiel L, Kalkisim Y, et al. A Systematic Review of Neuropsychological Tests for the Assessment of Dementia in Non-Western, Low-Educated or Illiterate Populations. J Int Neuropsychol Soc. 2019:1–21. 10.1017/s1355617719000894 . [DOI] [PubMed] [Google Scholar]
- 10.Gilewski MJ, Zelinski EM, Schaie KW. The Memory Functioning Questionnaire for assessment of memory complaints in adulthood and old age. Psychol Aging. 1990;5:482–90. 10.1037//0882-7974.5.4.482 . [DOI] [PubMed] [Google Scholar]
- 11.Dixon RA, Hultsch DF, Hertzog C. The Metamemory in Adulthood (MIA) questionnaire. Psychopharmacol Bull. 1988;24:671–88. . [PubMed] [Google Scholar]
- 12.Zelinski EM, Gilewski MJ. A 10-item Rasch modeled memory self-efficacy scale. Aging Ment Health. 2004;8:293–306. 10.1080/13607860410001709665 . [DOI] [PubMed] [Google Scholar]
- 13.McDonough IM, McDougall GJ, LaRocca M, Dalmida SG, Arheart KL. Refining the metamemory in adulthood questionnaire: a 20-item version of change and capacity designed for research and clinical settings. Aging Ment Health. 2019:1–10. 10.1080/13607863.2019.1594160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thivierge S, Simard M, Jean L, Grandmaison E. Errorless learning and spaced retrieval techniques to relearn instrumental activities of daily living in mild Alzheimer's disease: A case report study. Neuropsychiatr Dis Treat. 2008;4:987–99. 10.2147/ndt.s3684 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irish M, Lawlor BA, Coen RF, O'Mara SM. Everyday episodic memory in amnestic mild cognitive impairment: a preliminary investigation. BMC Neurosci. 2011;12:80 10.1186/1471-2202-12-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toglia JP. Contextual Memory Test Manual. Tucson, AZ: Therapy Skill Builders; 1993. [Google Scholar]
- 17.Gil N, Josman N. Memory and metamemory performance in Alzheimer’s disease and healthy elderly: The Contextual Memory Test (CMT). Aging Clin Exp Res. 2001;13:309–15. 10.1007/BF03353427 [DOI] [PubMed] [Google Scholar]
- 18.Josman N, Hartman-Maeir A. Cross-cultural assessment of the Contextual Memory Test (CMT). Occup Ther Int. 2000;7:246–58. 10.1002/oti.126 [DOI] [Google Scholar]
- 19.Sternberg RJ. Introduction to Optimizing Learning in College: Tips From Cognitive Psychology. Perspect Psychol Sci. 2016;11:651 10.1177/1745691616672227 [DOI] [PubMed] [Google Scholar]
- 20.Wancata J, Alexandrowicz R, Marquart B, Weiss M, Friedrich F. The criterion validity of the Geriatric Depression Scale: a systematic review. Acta Psychiatr Scand. 2006;114:398–410. 10.1111/j.1600-0447.2006.00888.x [DOI] [PubMed] [Google Scholar]
- 21.Byrne GJ, Pachana NA. Development and validation of a short form of the Geriatric Anxiety Inventory–the GAI-SF. Int Psychogeriatr. 2011;23:125–31. 10.1017/S1041610210001237 [DOI] [PubMed] [Google Scholar]
- 22.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9. 10.1111/j.1532-5415.2005.53221.x . [DOI] [PubMed] [Google Scholar]
- 23.Trzepacz PT, Hochstetler H, Wang S, Walker B, Saykin AJ, for the Alzheimer’s Disease Neuroimaging I. Relationship between the Montreal Cognitive Assessment and Mini-mental State Examination for assessment of mild cognitive impairment in older adults. BMC Geriatr. 2015;15:107 10.1186/s12877-015-0103-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med. 2016;15:155–63. 10.1016/j.jcm.2016.02.012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. . [PubMed] [Google Scholar]
- 26.Harvill LM. Standard error of measurement. Educ Meas Issues Pract. 1991;10:33–41. [Google Scholar]
- 27.Haley SM, Fragala-Pinkham MA. Interpreting Change Scores of Tests and Measures Used in Physical Therapy. Phys Ther. 2006;86:735–43. 10.1093/ptj/86.5.735 [DOI] [PubMed] [Google Scholar]
- 28.Chiu E-C, Yip P-K, Woo P, Lin Y-T. Test-retest reliability and minimal detectable change of the Cognitive Abilities Screening Instrument in patients with dementia. PLOS ONE. 2019;14:e0216450 10.1371/journal.pone.0216450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiu E-C, Wu W-C, Chou C-X, Yu M-Y, Hung J-W. Test-Retest Reliability and Minimal Detectable Change of the Test of Visual Perceptual Skills-Third Edition in Patients With Stroke. Arch Phys Med Rehabil. 2016;97:1917–23. 10.1016/j.apmr.2016.04.023 [DOI] [PubMed] [Google Scholar]
- 30.Giavarina D. Understanding Bland Altman analysis. Biochem Med (Zagreb). 2015;25:141–51. 10.11613/BM.2015.015 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox B, Henwood T, Neville C, Keogh J. Relative and absolute reliability of functional performance measures for adults with dementia living in residential aged care. Int Psychogeriatr. 2014;26:1659–67. 10.1017/S1041610214001124 [DOI] [PubMed] [Google Scholar]
- 32.Walter SD, Eliasziw M, Donner A. Sample size and optimal designs for reliability studies. Stat Med. 1998;17:101–10. [DOI] [PubMed] [Google Scholar]
- 33.Perfect TJ. Does Context Discriminate Recollection from Familiarity in Recognition Memory? Q J Exp Psychol A. 1996;49:797–813. 10.1080/713755644 [DOI] [PubMed] [Google Scholar]
- 34.Dalton P. The role of stimulus familiarity in context-dependent recognition. Mem Cogn. 1993;21:223–34. 10.3758/BF03202735 [DOI] [PubMed] [Google Scholar]
- 35.Ranganath C. Binding Items and Contexts: The Cognitive Neuroscience of Episodic Memory. Curr Dir Psychol Sci. 2010;19:131–7. 10.1177/0963721410368805 [DOI] [Google Scholar]
- 36.Levy-Gigi E, Vakil E. The dual effect of context on memory of related and unrelated themes: Discrimination at encoding and cue at retrieval. Memory. 2012;20:728–41. 10.1080/09658211.2012.701632 [DOI] [PubMed] [Google Scholar]
- 37.Schellings G, Van Hout-Wolters B. Measuring strategy use with self-report instruments: theoretical and empirical considerations. Metacogn Learn. 2011;6:83–90. 10.1007/s11409-011-9081-9 [DOI] [Google Scholar]
- 38.Toth JP, Daniels KA, Solinger LA. What you know can hurt you: effects of age and prior knowledge on the accuracy of judgments of learning. Psychol Aging. 2011;26:919–31. 10.1037/a0023379 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shanks LL, Serra MJ. Domain familiarity as a cue for judgments of learning. Psychon Bull Rev. 2014;21:445–53. 10.3758/s13423-013-0513-1 [DOI] [PubMed] [Google Scholar]
- 40.Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, et al. Schemas and Memory Consolidation. Science. 2007;316:76–82. 10.1126/science.1135935 [DOI] [PubMed] [Google Scholar]
- 41.Lee ML, Dey AK. Providing good memory cues for people with episodic memory impairment. Proceedings of the 9th international ACM SIGACCESS conference on Computers and accessibility; Tempe, Arizona, USA: Association for Computing Machinery; 2007. p. 131–8.
- 42.El Haj M, Kessels RPC. Context memory in Alzheimer's disease. Dement Geriatr Cogn Dis Extra. 2013;3:342–50. 10.1159/000354187 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hockley WE. The effects of environmental context on recognition memory and claims of remembering. J Exp Psychol Learn Mem Cogn. 2008;34:1412–29. 10.1037/a0013016 [DOI] [PubMed] [Google Scholar]
- 44.Ally BA, Gold CA, Budson AE. An evaluation of recollection and familiarity in Alzheimer's disease and mild cognitive impairment using receiver operating characteristics. Brain Cogn. 2009;69:504–13. 10.1016/j.bandc.2008.11.003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dunlosky J, Hertzog C. Measuring strategy production during associative learning: the relative utility of concurrent versus retrospective reports. Mem Cognit. 2001;29:247–53. 10.3758/bf03194918 . [DOI] [PubMed] [Google Scholar]
- 46.Gross AL, Rebok GW. Memory training and strategy use in older adults: results from the ACTIVE study. Psychol Aging. 2011;26:503–17. 10.1037/a0022687 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hourihan KL, Benjamin AS. State-based metacognition: How time of day affects the accuracy of metamemory. Memory. 2014;22:553–8. 10.1080/09658211.2013.804091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sliwinski MJ, Smyth JM, Hofer SM, Stawski RS. Intraindividual coupling of daily stress and cognition. Psychol Aging. 2006;21:545–57. 10.1037/0882-7974.21.3.545 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verhaeghen P, Hertzog C, Sliwinski MJ, Scott S. Boundary Conditions for Emotional Well-Being in AgingThe Importance of Daily Stress. Oxford University Press; 2014. [Google Scholar]
- 50.Bartels C, Wegrzyn M, Wiedl A, Ackermann V, Ehrenreich H. Practice effects in healthy adults: a longitudinal study on frequent repetitive cognitive testing. BMC Neurosci. 2010;11:118 10.1186/1471-2202-11-118 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gavett BE, Gurnani AS, Saurman JL, Chapman KR, Steinberg EG, Martin B, et al. Practice Effects on Story Memory and List Learning Tests in the Neuropsychological Assessment of Older Adults. PloS one. 2016;11:e0164492–e. 10.1371/journal.pone.0164492 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scharfen J, Jansen K, Holling H. Retest effects in working memory capacity tests: A meta-analysis. Psychon Bull Rev. 2018;25:2175–99. 10.3758/s13423-018-1461-6 . [DOI] [PubMed] [Google Scholar]
- 53.McCaffrey RJ, Westervelt HJ. Issues associated with repeated neuropsychological assessments. Neuropsychol Rev. 1995;5:203–21. 10.1007/BF02214762 . [DOI] [PubMed] [Google Scholar]
- 54.McCaffrey RJ, Kevin Duff., Holly James Westervelt. Practitioner's guide to evaluating change with neuropsychological assessment instruments. Dordrecht, Netherlands: Kluwer Academic Publishers; 2000. [Google Scholar]
- 55.Falleti MG, Maruff P, Collie A, Darby DG. Practice Effects Associated with the Repeated Assessment of Cognitive Function Using the CogState Battery at 10-minute, One Week and One Month Test-retest Intervals. J Clin Exp Neuropsychol. 2006;28:1095–112. 10.1080/13803390500205718 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.