Abstract
Opioids play a critical role in acute postoperative pain management. Our objective was to develop machine learning models to predict postoperative opioid requirements in patients undergoing ambulatory surgery. To develop the models, we used a perioperative dataset of 13,700 patients (≥ 18 years) undergoing ambulatory surgery between the years 2016–2018. The data, comprising of patient, procedure and provider factors that could influence postoperative pain and opioid requirements, was randomly split into training (80%) and validation (20%) datasets. Machine learning models of different classes were developed to predict categorized levels of postoperative opioid requirements using the training dataset and then evaluated on the validation dataset. Prediction accuracy was used to differentiate model performances. The five types of models that were developed returned the following accuracies at two different stages of surgery: 1) Prior to surgery—Multinomial Logistic Regression: 71%, Naïve Bayes: 67%, Neural Network: 30%, Random Forest: 72%, Extreme Gradient Boost: 71% and 2) End of surgery—Multinomial Logistic Regression: 71%, Naïve Bayes: 63%, Neural Network: 32%, Random Forest: 72%, Extreme Gradient Boost: 70%. Analyzing the sensitivities of the best performing Random Forest model showed that the lower opioid requirements are predicted with better accuracy (89%) as compared with higher opioid requirements (43%). Feature importance (% relative importance) of model predictions showed that the type of procedure (15.4%), medical history (12.9%) and procedure duration (12.0%) were the top three features contributing to model predictions. Overall, the contribution of patient and procedure features towards model predictions were 65% and 35% respectively. Machine learning models could be used to predict postoperative opioid requirements in ambulatory surgery patients and could potentially assist in better management of their postoperative acute pain.
1. Introduction
Pain is a commonly reported symptom among patients after surgery [1,2]. However, the management of acute postoperative pain continues to be difficult for both the patients and the health care providers. Patients with unrelieved postoperative pain are associated with slower recovery, delayed ambulation and increased risks of infection and thromboembolism [3]. Further, patients with poorly controlled postoperative pain are at higher risk of developing chronic pain [4]. In addition to patient impact, there are also deleterious consequences of inadequate pain management for hospitals, including extended length of stay, increased risk of readmission, and increased cost of care [3].
Opioids are often used to manage postoperative pain [5]. Despite their widespread use to mitigate pain, opioid use is also associated with negative side effects including neurological effects, respiratory depression, gastrointestinal effects, and pruritus [6]. For these reason, opioid-sparing multimodal analgesic options are increasingly being adopted for optimal pain control in the perioperative setting [7]. Nevertheless, opioids still have a critical role in acute postoperative pain management especially for procedures where a primary neuraxial, regional or local infiltration is not possible.
Predicting postoperative pain levels and opioid requirements could facilitate proactive strategies that can optimize pain control to avoid underuse or overuse of opioids. Towards this, previous studies have retrospectively looked for predictors of postoperative pain and analgesic consumption, identifying four significant predictors including age, type of surgery, anxiety levels, and psychological distress [8]. Some of these studies have focused on specific types of surgeries and patient population to determine factors associated with postoperative opioid usage [9,10]. To date, a review of the published literature indicates the lack of rigorous research pertaining to the identification of perioperative predictive factors for acute postoperative pain and opioid requirements across a wide spectrum of surgeries. Furthermore, previous studies used traditional statistical methods as opposed to trying machine learning, potentially limiting their predictive abilities [11].
Recently, artificial intelligence methods such as machine learning have increasingly been used in the medical field to predict clinical events [12]. Machine learning is particularly suited to analyze large datasets, compute complex interactions, identify hidden patterns, and generate actionable predictions in clinical settings. In many cases, machine learning has been shown to be superior to traditional statistical techniques [13–18]. Machine learning models offer a promising method to predict pain levels and opioid requirements following surgery. However, the applications of machine learning in the context of opioids have been specific and limited in scope, with attempts to predict opioid overdose risk among Medicare beneficiaries with opioid prescriptions and inadequate pain management in patients suffering from depression as two examples [19,20].
In this study, we developed machine learning models to predict postoperative opioid requirements for a wide range of outpatient surgeries. The models were developed and validated using a large dataset comprised of patient, procedure and provider data. The models were built to predict postoperative opioid requirements prior to surgery using preoperative data and at the end of surgery using both preoperative and intraoperative data.
2. Materials and methods
Study setting
This study was approved by the University of Washington Institutional Review Board (IRB# STUDY00002256). Requirement for patient consent was waived. Our academic medical center performs approximately 18,000 adult surgical procedures annually with ambulatory surgeries comprising approximately 40–45% of the surgical volume.
Data sources
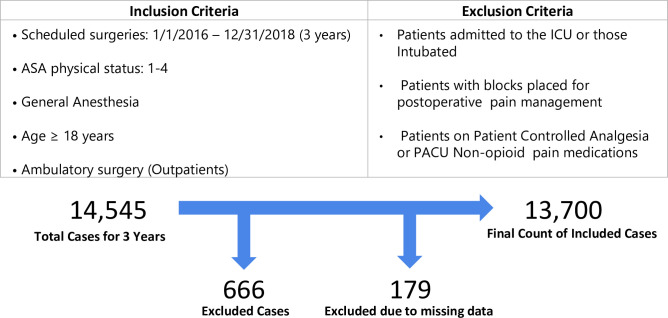
Patient and procedure information on ambulatory surgeries for 3 years (2016–2018) were extracted from our institution’s perioperative information management system data warehouse. Only adult (≥ 18 years of age) outpatients that received general anesthesia were included. Patients who were on patient-controlled analgesics or who received non-opioid analgesics in the post anesthesia care unit (PACU) were excluded. Additionally, patients who remained intubated, or had a peripheral nerve block placed for postoperative pain management were also excluded. The inclusion and exclusion criteria as well as the patient counts are presented in Fig 1. The choice of data variables for model development was largely based on prior literature that identified factors influencing postoperative pain levels or opioid consumption [8,21–24]. Patient and procedure specific parameters prior to and during surgery were considered to achieve the goal of developing models to predict postoperative opioid requirements both prior to and at the end of surgery. The summarized list of patient and procedure specific variables used for model development is outlined in Table 1.
Fig 1. Inclusion and exclusion criteria used to select the cohort for the study are shown.
The total count of patients, the number of exclusions and the final count of patients are shown.
Table 1. Parameters used in prediction models.
The parameters marked in green were used for postoperative opioid requirements prediction prior to surgery and parameters marked in blue were added to the prediction models to perform a postoperative opioid requirements prediction at the end of surgery.
| Patient specific parameters | Procedure specific parameters |
|---|---|
| Age | Surgical specialty |
| Gender | Procedure type (scheduled) |
| Body Mass index (BMI) | Procedure duration (estimated) |
| Race | Preoperative holding area opioids |
| ASA physical status | Preoperative holding area other drugs (Acetaminophen, Gabapentin, Celecoxib) |
| Medical history or anomalies | Preoperative pain levels |
| • Cardiac | Anesthesia method |
| • Pulmonary | • Endotracheal general anesthesia |
| • Renal | • Laryngeal mask airway |
| • Endocrine (diabetes) | • Total intravenous anesthesia |
| • Musculoskeletal | Inhalation agents (type and duration) |
| • Hepatic | Intraoperative opioids (MME) |
| • Neurological | Other intraoperative meds |
| • Cancer | • Acetaminophen |
| • Sleep apnea (Diagnosed or at risk) | • Ketamine |
| • Chronic pain | • Ketorolac |
| Social history | • Naloxone |
| • Smoking status | • Esmolol infusion |
| • Alcohol abuse | • Lidocaine infusion |
| • Drug abuse | • Propofol infusion |
| Psychiatric/Neurological issues | Patient position |
| • Anxiety | Local infiltration |
| • Depression | Input fluids (Crystalloids, colloids, blood) |
| • Post-traumatic stress disorder (PTSD) | Output fluids (Urine, blood loss, gastric output) |
| • Spinal cord injury | |
| Home medications | Providers |
| • On opioids | • Surgeon |
| • On non-opioid pain medications | • Anesthesiologist |
Data preparation
Several data preparation steps were performed prior to model development. Records with outlier data values and key missing data points were identified. They comprised only a small fraction of the total number of records (<1%, N = 179). Hence, they were simply excluded from model development.
Data elements relating to medical, social and psychiatric histories, were embedded in free text fields in the electronic medical record (EMR). Standard natural language processing techniques were used to generate modelling features from these free text data. Pain levels recorded in the EMR were a combination of patient reported numeric rating (0 –no pain to 10 most severe pain) or nurse assessed pain levels (none, mild, moderate and severe pain). For modeling purposes, numeric rating pain scores were normalized to pain levels (0: no pain, 1–3: mild pain, 4–6 moderate pain and 7–10 severe pain) [25].
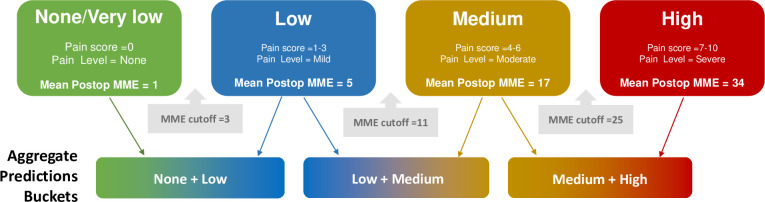
Administered opioids could be of different types and potencies. Therefore, Morphine Milligram Equivalent (MME) representation was used to consolidate the opioid doses into a single normalized value [26–28]. The MME conversion ratios used for the study are tabulated in S1 Table. Numeric MME values are less practical to interpret and act in a clinical setting than categories of opioid requirements that correspond to pain levels. Hence, we proceeded to categorize the MME values into four compartments of opioid requirement–None/very low, low, medium and high. This categorization was based on the average MME opioids used when the pain levels were none, mild, moderate and severe. The mean MME requirements for no, mild, moderate and severe pain levels are shown in Table 2. The average value of MME requirements for adjacent pain levels were used to determine the MME ranges of opioid requirement categories. MME ranges for the opioid requirement categories were: None/very low (0–3 MME), low (3–11 MME), medium (11–25 MME), and high (≥25 MME). The categorized postoperative opioid MME requirement served as the dependent variable for the predictive models.
Table 2. Mean postoperative MME requirements for different peak pain levels in PACU.
| Peak Pain in PACU | Mean Postoperative MME | MME ranges for opioid requirement categories |
|---|---|---|
| No pain (Pain score = 0) | 1 | None/very low: 0–3 |
| Mild (Pain score 1–3) | 5 | Low: 3–11 |
| Moderate (Pain score 4–6) | 17 | Medium: 11–25 |
| Severe (Pain score 7–10) | 34 | High: > 25 |
Procedure types proved to be useful in defining decision boundaries, but were too numerous for practical use without further processing. Hence, they were thus aggregated into a smaller list based on the location of surgical site and whether the procedure was open or closed. The recategorized procedure types and counts are in S2 Table.
Model development
The master dataset was randomly split into two groups comprised of a training dataset (80% of the records) and a “holdout” dataset (20% of the records) for unbiased validation. Model development and parameter tuning were each performed using the training dataset. Five models of different classes were developed to predict postoperative opioid requirements: Multinomial Regression, Naïve Bayesian, Neural Network, Random Forest and Extreme Gradient Boosting Trees. Models were developed to predict probabilities of postoperative opioid requirements for each procedure among the four categories (none/very low, low, medium and high). Models were trained to predict opioid requirements prior to surgery using only preoperative data and then a second time at the end of surgery using both preoperative and intraoperative data. This two-phase model development matched the expected user requirements: an initial estimate prior to surgery and a more informed estimate post-surgery both having utility in the clinical setting.
Model development was performed in R programming environment (R Foundation for Statistical Computing, Vienna, Austria) [29].
Model validation
Model validation was performed on the “holdout” dataset that was not used for training. Prediction accuracy over the holdout set was used to differentiate models. The output for model predictions were probabilities in each of the established four categories: none, low, medium, or high opioid requirement. Prediction accuracy could be computed based on the single category with the highest prediction probability and comparing that against the category in which the actual opioid requirement falls. However, this approach becomes overly strict especially when the model predicted probabilities in adjacent categories are similar. In consultation with our clinical partners we adopted an alternate, more balanced, approach to compute accuracy. Instead of simply selecting the category with the highest prediction probability the models were evaluated based on their ability to perform an aggregate prediction within two adjacent opioid requirement categories: None + Low, Low + Medium and Medium+ High. The aggregate prediction bucket with the highest combined prediction probability was determined to be the model prediction. The predicted aggregate bucket was compared to the bucket corresponding to the actual opioid requirement for estimating the model accuracy. The concept is shown in Fig 2. The model with the highest accuracy was chosen and further evaluated in terms of precision and recall. Further, prediction accuracies for different surgical specialties were also determined. To explain the model predictions in terms of feature importance, we used permutation method. The method works by randomly shuffling data one feature at a time for the entire dataset and calculating how much the prediction accuracy decreases when a feature is excluded. A larger change in prediction accuracy represents a greater importance of that feature.
Fig 2. The concept of using aggregate model prediction for model validation is shown.
The models predicted probabilities of postoperative opioid requirements in four buckets None/very low, Low, Medium and High. For model validation, the ability to predict within two adjacent buckets (None + Low, Low + Medium, Medium + High) was considered. The aggregate prediction bucket that had the highest combined prediction probability was considered as the model prediction. The predicted aggregate bucket was compared against the bucket corresponding to the actual opioid requirement for estimating the model accuracy.
3. Results
A total of 13,700 patients were included in the study. The patient and procedure characteristics are presented in Table 3. The mean ± SD age of the patients was 51±17 years with geriatric patients (>65 years of age) comprising 22% of the population. The mean BMI was 28.4 ± 7.2 with 35% of the patients obese (BMI > 30). Female patients were a higher fraction (58%) while racial demographics was predominantly white (83%). A significant portion of the patients suffered from depression (24%) or anxiety (26%). Among home medications, 52% of the patient cohort were on non-opioid pain medications and 23% on opioids. Chronic pain was diagnosed in 3.8% of the patients. The mean ± SD of the procedure duration was 75±56 minutes with the main surgical specialties being General (22%), ENT (18%) and Urology (15%). Table 3 also presents the characteristics of the training and testing data subsets. The patient and procedure factors were well matched between the two data subsets with none the factors significantly different between the datasets.
Table 3. Primary patient and procedure characteristics observed in the overall (N = 13,700), training (N = 10,960) and testing (N = 2740) datasets.
Proportions are presented for categorical variables while mean ± standard deviation (SD) are shown for continuous variables. The comparison of characteristics between the training and testing datasets is also presented.
| Overall (N = 13,700) | Train (N = 10,960) | Test (N = 2740) | diff | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Counts | Proportions /Mean ± SD | Counts | Proportions /Mean ± SD | Counts | Proportions /Mean ± SD | p-value | |
| Age (years) | 51 ± 17 | 52 ± 17 | 51 ± 17 | 0.43 | ||||
| • Geriatric (Age≥65y) | 3,352 | 24% | 2,683 | 24% | 669 | 24% | ||
| Sex: | ||||||||
| • Male | 5,699 | 42% | 4,556 | 42% | 1,143 | 42% | ||
| • Female | 8,001 | 58% | 6,404 | 58% | 1,597 | 58% | 0.91 | |
| Race: | ||||||||
| • White | 11,355 | 83% | 9,062 | 83% | 2,293 | 84% | 0.22 | |
| • African American | 640 | 5% | 527 | 5% | 113 | 4% | 0.14 | |
| • Asian | 1,040 | 7% | 845 | 8% | 195 | 7% | 0.31 | |
| • Other | 665 | 5% | 526 | 5% | 139 | 5% | 0.58 | |
| BMI (kg/m2) | 28.4 ± 7.2 | 28.4 ± 7.2 | 28.5 ± 7.2 | 0.67 | ||||
| • Obese (BMI>30) | 4,094 | 30% | 3,259 | 30% | 835 | 30% | ||
| ASA physical status | ||||||||
| • ASA ≥ 3 | 4,740 | 35% | 3,791 | 35% | 949 | 35% | 0.98 | |
| Medical history or anomalies | ||||||||
| • Cardiac | 5487 | 40% | 4,393 | 40% | 1094 | 40% | 0.90 | |
| • Pulmonary | 3,647 | 27% | 2,907 | 27% | 740 | 27% | 0.63 | |
| • Renal | 1,978 | 14% | 1,609 | 15% | 369 | 13% | 0.11 | |
| • Endocrine (diabetes) | 1,624 | 12% | 1,313 | 12% | 311 | 11% | 0.38 | |
| • Musculoskeletal | 6,874 | 50% | 5,536 | 51% | 1,338 | 49% | 0.12 | |
| • Hepatic | 647 | 5% | 511 | 5% | 136 | 5% | 0.54 | |
| • Neurological | 6,088 | 44% | 4,872 | 44% | 1,216 | 44% | 0.96 | |
| • Cancer | 4,902 | 36% | 3,898 | 36% | 1,004 | 37% | 0.30 | |
| • Sleep apnea (diagnosed or at risk) | 6,470 | 47% | 5,198 | 47% | 1,272 | 46% | 0.36 | |
| • Chronic pain | 516 | 4% | 409 | 4% | 107 | 4% | 0.71 | |
| Social history | ||||||||
| • Smoking status | 1,303 | 10% | 1,038 | 9% | 265 | 10% | 0.78 | |
| • Alcohol abuse | 1,692 | 12% | 1,355 | 12% | 337 | 12% | 0.95 | |
| • Drug abuse | 1,310 | 10% | 1,023 | 9% | 287 | 10% | 0.08 | |
| Psychiatric/Neurological issues | ||||||||
| • Anxiety | 3,521 | 26% | 2,781 | 25% | 740 | 27% | 0.08 | |
| • Depression | 3,342 | 24% | 2,642 | 24% | 700 | 26% | 0.12 | |
| • Post-traumatic stress disorder (PTSD) | 326 | 2.4% | 253 | 2.3% | 73 | 2.7% | 0.31 | |
| • Spinal cord injury | 255 | 1.9% | 204 | 1.9% | 51 | 1.9% | 1.00 | |
| Home medications | ||||||||
| • On opioids | 3,107 | 23% | 2,463 | 22% | 644 | 24% | 0.26 | |
| • On non-opioid pain medications | 7,116 | 52% | 5,682 | 52% | 1,434 | 52% | 0.66 | |
| Surgical specialty | ||||||||
| • General | 2,955 | 22% | 2,352 | 21% | 603 | 22% | 0.55 | |
| • Neurological | 676 | 5% | 544 | 5% | 132 | 5% | 0.79 | |
| • Orthopedic | 1,091 | 8% | 872 | 8% | 219 | 8% | 0.98 | |
| • Gynecology | 1,436 | 10% | 1,147 | 10% | 289 | 11% | 0.93 | |
| • ENT | 2,511 | 18% | 2,016 | 18% | 495 | 18% | 0.71 | |
| • Urology | 1,999 | 15% | 1,606 | 15% | 393 | 14% | 0.70 | |
| • Thoracic | 504 | 4% | 408 | 4% | 96 | 4% | 0.63 | |
| • Vascular | 167 | 1% | 140 | 1% | 27 | 1% | 0.25 | |
| • Plastic | 1,619 | 12% | 1,302 | 12% | 317 | 12% | 0.68 | |
| • Oral | 400 | 3% | 306 | 3% | 94 | 3% | 0.09 | |
| Surgery duration (min) | 75 ± 56 | 75 ± 56 | 75 ± 55 | 0.86 | ||||
BMI–Body Mass Index, ASA–American Society of Anesthesiologists, ENT–Ear Nose Throat.
Exploratory data analysis
Exploratory data analysis was performed to understand relationships between variables and to inform modeling steps. To have a basic understanding of the factors affecting opioid requirements, bivariate relationships between patient or procedure factors and postoperative opioid requirements were found. The statistically significant preoperative factors are shown in Fig 3. Longer duration procedures, patients on opioids and plastic surgeries were top factors that were related to higher opioid requirements. On the other hand, no preoperative pain, older age, urological surgeries were the top factors related to lower opioid requirements.
Fig 3. Bivariate relationships between preoperative features and postoperative MME opioid requirements are shown.
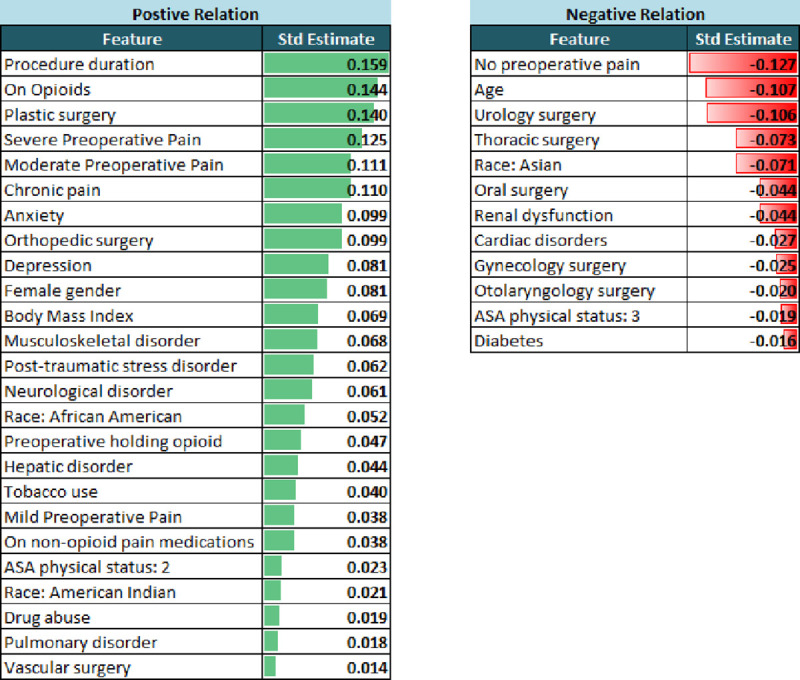
Only statistically significant (p < 0.05) relations are shown with standard estimate (Std Estimate) representing the strength of the relationship. The positive and negative relations are show in green and red colors respectively.
Model predictions
Models were validated on a hold-out dataset (N = 2740, 20% of total data). The prediction accuracies for all five models when including just the preoperative features and when adding intraoperative features are show in Table 4. Random Forest and Multinomial Regression models had the best accuracy. Adding the intraoperative features did not enhance the prediction accuracy of the models significantly. Table 5 presents detailed results for the best performing model, which was the Random Forest. Model accuracies for different surgical specialties are shown. Model accuracies varied for different surgical specialties. Oral and thoracic surgeries had the highest accuracies, though counts of these surgeries were comparatively small. General and plastic surgeries had lower accuracies. Since the model accuracies when predicting opioid requirements at the beginning and end of surgery were similar further analyses focused on the beginning of surgery stage. Table 6 presents the recall (sensitivity) and precision (positive predictive value) of random forest model predictions. Recall was highest when the model predicted to the “none+low” aggregate category. For the “low+medium” category recall was very poor. Precision was highest for “none+low” and “medium+high” categories while lowest for “low+medium”. Table 7 shows the confusion matrices for each category of opioid requirement with true positive, true negative, false positive and false negative counts and rates. Overall, the model performance was poorer when predicting higher opioid requirements. The model had most difficulty predicting the “low+medium” opioid requirement as compared with the other categories. Model performance metrics in predicting single categories is also presented in S3 and S4 Tables.
Table 4. Prediction accuracies of different models prior to surgery and at the end of surgery are presented.
The models utilized preoperative factors for predictions prior to surgery while the end of surgery predictions used both preoperative and intraoperative factors.
| Validation Data Set: N = 2740 | ||||
|---|---|---|---|---|
| Observed Opioid Requirements in Validation Data Set | ||||
| None | Low | Medium | High | |
| 1,290 (47%) | 409 (15%) | 536 (20%) | 505 (18%) | |
| Model | Accuracy | |||
| Prior to Surgery | End of Surgery | |||
| Multinomial Logistic Regression | 71% | 71% | ||
| Naïve Bayes | 67% | 63% | ||
| Neural Network | 30% | 32% | ||
| Random Forest | 72% | 72% | ||
| Extreme Gradient Boost | 71% | 70% | ||
Table 5. Detailed prediction accuracies of random forest model for different categories of surgeries and aggregate opioid requirements.
Prediction accuracies prior to and after surgery are shown.
| Surgical Specialty | Mean opioid requirement (MME) | Accuracy | |
|---|---|---|---|
| Beginning of surgery | End of surgery | ||
| General (N = 603) | 12.1 ± 17.8 | 67% | 70% |
| Gynecology (N = 289) | 11.0 ± 17.2 | 71% | 72% |
| Neuro (N = 132) | 12.6 ± 25.1 | 70% | 74% |
| Oral (N = 94) | 7.7 ± 17.1 | 87% | 86% |
| Orthopedic (N = 219) | 19.7 ± 21.2 | 74% | 70% |
| Otolaryngology (N = 495) | 11.3 ± 25.4 | 68% | 67% |
| Plastic (N = 317) | 20.0 ± 23.4 | 67% | 66% |
| Thoracic (N = 96) | 2.9 ± 8.4 | 95% | 94% |
| Urology (N = 393) | 6.8 ± 14.4 | 80% | 80% |
| Vascular (N = 27) | 16.2 ± 25.2 | 70% | 59% |
| Overall | 12.2 ± 20.7 | 72% | 72% |
Table 6. Recall and precision of random forest model predicting aggregate opioid requirements.
Prediction results prior to surgery are shown.
| Recall | Precision | ||||
|---|---|---|---|---|---|
| None + Low | Low + Medium | Medium + High | None + Low | Low + Medium | Medium + High |
| 88% | 5% | 41% | 72% | 50% | 73% |
| (N = 1699) | (N = 945) | (N = 1041) | (N = 1699) | (N = 945) | (N = 1041) |
Table 7. Confusion matrix for each category of opioid requirement with true positive, true negative, false positive and false negative counts are shown.
Also, presented are the True Positive Rate (TPR), False Negative Rate (FNR), False Positive Rate (FPR) and True Negative Rate (TNR). Positive Predictive Value (PPV), False Omission Rate (FOR), False Discovery Rate (FDR) and Negative Predictive Value (NPV) are also presented.
| Opioid category | Predicted | |||||||
| None + Low | Positive | Negative | Total | |||||
| Actual | Positive | 1500 | 199 | 1699 | TPR | 88% | FNR | 12% |
| Negative | 572 | 469 | 1041 | FPR | 55% | TNR | 45% | |
| Total | 2072 | 668 | 2740 | |||||
| PPV | FOR | |||||||
| 72% | 30% | |||||||
| FDR | NPV | |||||||
| 28% | 70% | |||||||
| Opioid category | Predicted | |||||||
| Low + Medium | Positive | Negative | Total | |||||
| Actual | Positive | 43 | 902 | 945 | TPR | 5% | FNR | 95% |
| Negative | 43 | 1752 | 1795 | FPR | 2% | TNR | 98% | |
| Total | 86 | 2654 | 2740 | |||||
| PPV | FOR | |||||||
| 50% | 34% | |||||||
| FDR | NPV | |||||||
| 50% | 66% | |||||||
| Opioid category | Predicted | |||||||
| Medium + High | Positive | Negative | Total | |||||
| Positive | 423 | 618 | 1041 | TPR | 41% | FNR | 59% | |
| Negative | 159 | 1540 | 1699 | FPR | 9% | TNR | 91% | |
| Total | 582 | 2158 | 2740 | |||||
| PPV | FOR | |||||||
| 73% | 29% | |||||||
| FDR | NPV | |||||||
| 27% | 71% | |||||||
Feature importance
Feature importance of the Random Forest model, determined through permutation method, is presented in Table 8. The average relative importance of different features contributing to model prediction is outlined. The type of procedure, patient’s medical history and procedure duration were the top three features contributing to model predictions. Overall, patient features contributed 65% while procedure features contributed 35% towards model predictions.
Table 8. Feature importance of Random Forest Model explaining the relative importance of various features contributing to predictions of opioid requirements.
Similar features are consolidated.
| Features | Relative importance |
|---|---|
| Procedure type | 15.4% |
| Medical History (Cardiac/Pulmonary/Neurological/Hepatic/Endocrine/Musculoskeletal/Renal/Cancer) | 12.9% |
| Procedure Duration | 12.0% |
| Age | 9.8% |
| Surgical specialty | 8.3% |
| Body Mass Index | 8.2% |
| Home and preoperative pain medications (Opioid/Non opioid) | 7.3% |
| Preoperative Pain Levels | 4.8% |
| ASA Physical Status | 4.2% |
| Race | 4.2% |
| Social History (Tobacco/Alcohol/Recreational Drug Use) | 4.1% |
| Psychiatric/Neurological issues (Anxiety/Depression/PTSD/Spinal Cord Injury) | 4.1% |
| History or risk for sleep apnea | 2.1% |
| Gender | 1.9% |
| History of Chronic Pain | 0.6% |
4. Discussion
Management of acute postoperative pain with opioids needs to be optimal to avoid the adverse effects of overdose and underdose. Towards this, we applied artificial intelligence methods specifically, machine learning in this instance to predict postoperative opioid requirements so that proactive planning could be enabled. We used a comprehensive and large perioperative dataset to develop and validate the models which were trained to make predictions preoperatively prior to surgery and at the end of surgery. Our study showed that machine learning models can predict postoperative opioid requirements with an accuracy around 70% when adjacent opioid requirement categories are aggregated. Among the models tried, Random Forest, Multinomial regression and Extreme Gradient Boost models performed better than Naïve Bayes and Neural Network. The differences between the performances of these higher performing models were only marginal.
Several key findings are noted while observing the model predictions. Surprisingly, the model accuracies were very similar prior to surgery and at the end of surgery; suggesting that intraoperative data (intraoperative opioids, other types of analgesics, inhalation agents, fluids, patient position, etc.) did not contribute to improving model accuracy. This may prove to be advantageous because a model that can preoperatively predict the postoperative opioid requirements without compromising accuracy could potentially enable proactive pain management strategies prior to surgery.
Model sensitivity (recall) and precision were only modest at best and that too only for the “none+low” category. The model had particular difficulty predicting whether a patient’s opioid requirement would fall in the “low+medium” category with a tendency to misclassify the requirement as “none+low”. The model performance was better when predicting “none+low” as compared with other categories. This may explain why model accuracies varied for different surgical specialties. The specialties that had higher opioid requirements tended to have lower model accuracies.
Feature importance for best performing Random Forest model predictions made prior to surgery reveals interesting observations (Table 8). The scheduled procedure type proved to be the most important feature in model predictions. Yet, patient specific factors—demographics, medical history, social history, and psychiatric issues together played a predominant role in determining the postoperative opioid requirements.
Despite including a comprehensive dataset of preoperative and intraoperative parameters, model accuracies in predicting postoperative opioid requirements were not over 72%. This suggests that additional factors influencing opioid requirements were potentially not included in the data used for training the models. A potential factor that we considered was provider practice pattern in ordering opioids for pain management. However, adding surgeon and anesthesiologist data into the model led to no notable improvement. Accuracy of machine learning models can be, in principle, improved with more data. Additional data for model training could be obtained either by extending the time range of the dataset or obtaining data from more institutions. However, here are downsides to each approach. By extending the time range, the risk for encountering changes in practice and documentation patterns over time increases potentially compromising data consistency. Similarly, institutional variations in case mix and practices can negatively affect consistency of multi-institutional data. In this particular project, we noted that adding two additional years of data yielded no improvement in accuracy.
The single center nature of data is a limitation of the study and whether the model performance can be replicated in other centers is unknown at this time. As a future step training and validating the model against standardized multicenter data such as those hosted by Multicenter Perioperative Outcomes Group (www.mpog.org) could be a way to validate the model across institutions. The second limitation of this project is that we focused only on outpatients. This was deliberate to avoid the confounding factors of patient-controlled analgesia, regional blocks for postoperative pain management and variable length of stay that are difficult to incorporate into the model. For this reason, we chose to keep the scope limited to outpatients in this first modeling effort.
In summary, machine learning models were able to predict postoperative opioid requirements in ambulatory surgery patients. Prediction accuracies remained unchanged even after adding intraoperative information to preoperative data. In general, model prediction sensitivities were greater in patients requiring lower amounts of opioids as compared with those requiring higher amounts. Translating such models into point of care tools could provide assistive intelligence to the perioperative care provider leading to improved management of postoperative acute pain.
Supporting information
(DOCX)
(DOCX)
Prediction accuracies prior to and after surgery are shown.
(DOCX)
Prediction recall and precision prior to surgery are shown.
(DOCX)
Acknowledgments
We would like to acknowledge the Center for Perioperative & Pain Initiatives in Quality Safety Outcome (PPiQSO) at the University of Washington for providing the perioperative data for this study.
Data Availability
The raw data used in this study are bound by institutional data use agreement and therefore can not be released to the public. To obtain release of study data please contact Center for Perioperative & Pain Initiatives in Quality Safety Outcome (PPiQSO) at ppiqso@uw.edu to establish a data release agreement.
Funding Statement
BGN owns equity in Perimatics LLC and serves as its advisor. This commercial affiliation did not play a role in the study. The University of Washington provided support in the form of salaries for some of the authors [BGN, JDL, CTF], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Similarly, Perimatics LLC provided support in the form of salaries for some of the authors [NV, RV, LB], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Lastly, VA Puget Sound Health System provided support in the form of salaries for the author [MH], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The other authors [AAN, MAV, JAL] were summer student interns and were unfunded. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Chung F, Un V, Su J. Postoperative symptoms 24 hours after ambulatory anaesthesia. Can J Anaesth 1996;43(11):1121–7. 10.1007/BF03011838 [DOI] [PubMed] [Google Scholar]
- 2.Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: Results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97(2):534–40. 10.1213/01.ane.0000068822.10113.9e [DOI] [PubMed] [Google Scholar]
- 3.Wells N, Pasero C, McCaffery M. Improving the quality of care through pain assessment and management In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Hand- book for Nurses, Vol I Rockville, MD: Agency for Healthcare Research and Quality; 2008:469–97. [PubMed] [Google Scholar]
- 4.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: Risk factors and prevention. Lancet. 2006;367(9522):1618–25. 10.1016/S0140-6736(06)68700-X [DOI] [PubMed] [Google Scholar]
- 5.Veterans Health Administration. VHA/DoD Clinical Practice Guideline for the Management of Postoperative Pain. https://www.healthquality.va.gov/guidelines/Pain/pop/pop_fulltext.pdf Published 2002. Accessed June 08, 2019.
- 6.Wheeler M, Oderda GM, Ashburn MA, Lipman AG. Adverse events associated with postoperative opioid analgesia: A systematic review. J Pain. 2002;3(3):159–80. 10.1054/jpai.2002.123652 [DOI] [PubMed] [Google Scholar]
- 7.Sullivan D, Lyons M, Montgomery R, Quinlan-Colwell A. Exploring Opioid-Sparing Multimodal Analgesia Options in Trauma: A Nursing Perspective. J Trauma Nurs. 2016;23(6):361–75. 10.1097/JTN.0000000000000250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ip HYV, Abrishami A, Peng PWH, Wong J, Chung F. Predictors of postoperative pain and analgesic consumption: A qualitative systematic review. Anesthesiology. 2009;111(3):657–77. 10.1097/ALN.0b013e3181aae87a [DOI] [PubMed] [Google Scholar]
- 9.Raiff D, Vaughan C, McGee A. Impact of intraoperative acetaminophen administration on postoperative opioid consumption in patients undergoing hip or knee replacement. Hosp Pharm. 2014;49(11):1022–32. 10.1310/hpj4911-1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burbridge MA, Stone SA, Jaffe RA. Acetaminophen Does Not Reduce Postoperative Opiate Consumption in Patients Undergoing Craniotomy for Cerebral Revascularization: A Randomized Control Trial. Cureus. 11(1):e3863 10.7759/cureus.3863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banka TR, Ruel A, Fields K, YaDeau J, Westrich G. Preoperative predictors of postoperative opioid usage, pain scores, and referral to a pain management service in total knee arthroplasty. HSS J. 2015;11(1):71–5. 10.1007/s11420-014-9418-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang F, Jiang Y, Zhi H, Dong Y, Li H, Ma S, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230–43. 10.1136/svn-2017-000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsich E, Gorodeski EZ, Blackstone EH, Ishwaran H, Lauer MS. Identifying important risk factors for survival in patient with systolic heart failure using random survival forests. Circ Cardiovasc Qual Outcomes. 2011;4(1):39–45. 10.1161/CIRCOUTCOMES.110.939371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorodeski EZ, Ishwaran H, Kogalur UB, Blackstone EH, Hsich E, Zhang ZM, et al. Use of hundreds of electrocardiographic biomarkers for prediction of mortality in postmenopausal women: The Women’s Health Initiative. Circ Cardiovasc Qual Outcomes. 2011;4(5):521–32. 10.1161/CIRCOUTCOMES.110.959023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen G, Kim S, Taylor JM, Wang Z, Lee O, Ramnath N, et al. Development and validation of a quantitative real-time polymerase chain reaction classifier for lung cancer prognosis. J Thorac Oncol. 2011;6(9):1481–7. 10.1097/JTO.0b013e31822918bd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amalakuhan B, Kiljanek L, Parvathaneni A, Hester M, Cheriyath P, Fischman D. A prediction model for COPD readmissions: catching up, catching our breath, and improving a national problem. J Community Hosp Intern Med Perspect. 2012;2(1):9915–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chirikov VV, Shaya FT, Onukwugha E, Mullins CD, dosReis S, Howell CD. Tree-based claims algorithm for measuring pretreatment quality of care in Medicare disabled hepatitis C patients. Med Care. 2017;55(12):e104–12. 10.1097/MLR.0000000000000405 [DOI] [PubMed] [Google Scholar]
- 18.Thottakkara P, Ozrazgat-Baslanti T, Hupf BB, Rashidi P, Pardalos P, Momcilovic P, et al. Application of machine learning techniques to high-dimensional clinical data to forecast postoperative complications. PLoS One. 2016;11(5):e0155705 10.1371/journal.pone.0155705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo-Ciganic WH, Huang JL, Zhang HH, Weiss JC, Wu Y, Kwoh CK, et al. Evaluation of Machine-Learning Algorithms for Predicting Opioid Overdose Risk Among Medicare Beneficiaries with Opioid Prescriptions. JAMA Netw Open. 2019;2(3):e190968 10.1001/jamanetworkopen.2019.0968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parthipan A, Banerjee I, Humphreys K, Asch SM, Curtin C, Carroll I, et al. Predicting inadequate postoperative pain management in depressed patients: A machine learning approach. PLoS ONE. 2019;14(2):e0210575 10.1371/journal.pone.0210575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Cosmo G, Congedo E, Lai C, Primieri P, Dottarelli A, Aceto P. Preoperative psychologic and demographic predictors of pain perception and tramadol consumption using intravenous patient-controlled analgesia. Clin J Pain. 2008;24(5):399–405. 10.1097/AJP.0b013e3181671a08 [DOI] [PubMed] [Google Scholar]
- 22.Taenzer P, Melzack R, Jeans ME. Influence of psychological factors on postoperative pain, mood and analgesic requirements. Pain.1986;24(3):331–42. 10.1016/0304-3959(86)90119-3 [DOI] [PubMed] [Google Scholar]
- 23.Kalkman CJ, Visser K, Moen J, Bonsel GJ, Grobbee DE, Moons KG. Preoperative prediction of severe postoperative pain. Pain. 2003;105(3):415–23. 10.1016/s0304-3959(03)00252-5 [DOI] [PubMed] [Google Scholar]
- 24.Healey M, Maher P, Hill D, Gebert R, Wein P. Factors associated with pain following operative laparoscopy: A prospective observational study. AustNZJ Obstet Gynaecol. 1998;38(1):80–4. [DOI] [PubMed] [Google Scholar]
- 25.National Institutes of Health, Warren Grant Magnuson Clinical Center (UW) Pain intensity instruments. 0–10 Numeric Rating Scale. Bethesda, MD: Warren Grant Magnuson Clinical Center; http://www.mvltca.net/Presentations/mvltca.pdf. Published 2003. Accessed June 11, 2019.
- 26.Watts R, Thiruvenkatarajan V, Calvert M, Newcombe G, van Wijk RM: The effect of perioperative esmolol on early postoperative pain: A systematic review and meta-analysis. J Anaesthesiol Clin Pharmacol. 2017;33(1):28–39. 10.4103/0970-9185.202182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chevalier P, Smulders M, Chavoshi S, Sostek M, LoCasale R. A description of clinical characteristics and treatment patterns observed within prescribed opioid users in Germany and the UK. Pain Manag. 2014;4(4):267–76. 10.2217/pmt.14.26 [DOI] [PubMed] [Google Scholar]
- 28.CDC. Morphine Milligram Equivalent table. CDC. https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-Aug-2017.pdf. Published August, 2017. Accessed June 11, 2019.
- 29.Lesmeister C. Mastering Machine Learning with R. 2nd ed Birmingham: PACKT Publishing; 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Prediction accuracies prior to and after surgery are shown.
(DOCX)
Prediction recall and precision prior to surgery are shown.
(DOCX)
Data Availability Statement
The raw data used in this study are bound by institutional data use agreement and therefore can not be released to the public. To obtain release of study data please contact Center for Perioperative & Pain Initiatives in Quality Safety Outcome (PPiQSO) at ppiqso@uw.edu to establish a data release agreement.