Abstract
The vertebrate anteroposterior (AP) axis forms through elongation of multiple tissues during embryonic development. Tight coordination among tissues that originate from different germ layers is required for proper elongation. Importantly, failure of both force-generating, tissue-autonomous mechanisms and inter-tissue coordination during AP axis formation leads to severe developmental anomalies such as body truncation and neural tube defects (NTDs). In living systems, shape changes require either the rearrangement of existing materials – such as individual cells, tissues, and the extracellular matrix (ECM) – or the local addition of new materials, i.e. anisotropic growth, through cell proliferation, cell growth, and matrix deposition. Elongation of the vertebrate body, which entails a substantial modification of the initial embryo shape, employs both mechanisms. Acting at different timescales, these mechanisms participate to AP axis formation prevalently during distinct phases of embryonic development. While tissue rearrangements through cell intercalation and migration power body elongation at early stages, when tissue geometry and architecture change rapidly, volumetric growth comes generally into play after tailbud formation. This structure contains proliferating neuromesodermal progenitors (NMPs) that differentiate both into the mesodermal and neuroectodermal lineages, thus sustaining elongation via posterior addition of new tissue. From a physical perspective, cell rearrangements depend on cell-generated forces and tissue material properties. Notably, the spatiotemporal variation of these mechanical parameters has been recently investigated in the context of vertebrate body elongation. Due to its accessibility to embryological manipulation, the rich literature on its genetic control, and recent studies demonstrating the feasibility of stress and rheological measurements during its development, AP axis formation represents an ideal model to shed light into the crosstalk between signaling and mechanics during tissue and organ morphogenesis.
Keywords: Axis elongation, vertebrate embryo, morphogenesis, tissue mechanics
Introduction
The vertebrate body is characterized by an AP axis that forms through highly coordinated elongation of different tissues at embryonic stages (Bénazéraf 2018, Bénazéraf et al. 2017, Bénazéraf & Pourquié 2013, Dray et al. 2013, McMillen & Holley 2015). While the specific cellular machineries may differ among model organisms, this process is invariably coupled to segmentation, the periodic organization of the AP axis in segments, i.e. the somites. Apparent as symmetric epithelial blocks enclosing a mesenchymal core, somites originate from the presomitic mesoderm (PSM) and give rise to the axial musculoskeletal system (Dubrulle & Pourquie 2004a). Importantly, the coordination of elongation speed and segmentation pace has been proposed to control segment number and final body length by defining the PSM lifetime (Gomez et al. 2008). Posterior axis elongation and segmentation depend on a shared system of signaling pathways, further increasing the complexity of their interdependence (Aulehla & Pourquié 2010, Bénazéraf & Pourquié 2013, Dubrulle & Pourquie 2004a). A gradient of FGF signaling, for example, controls elongation by maintaining high cell motility in the paraxial mesoderm (Bénazéraf et al. 2010, Delfini et al. 2005) and concurrently ensures correct segmentation by regulating gene oscillations downstream of the segmentation clock, which drive the rhythmic production of somites (Aulehla & Pourquié 2010, Dubrulle et al. 2001, Dubrulle & Pourquie 2004b).
Formation of the AP body axis spans several developmental stages, from the onset of gastrulation to late somitogenesis. In amniotes, PS extension represents the first morphogenetic event demarcating anatomically the embryo’s AP axis (Bénazéraf 2018, Bénazéraf & Pourquié 2013). In anamniote embryos, characterized by a spherical geometry, the axis starts to form with the anterior progression of the hypoblast along the overlying epiblast (Keller et al. 2003). Anterior elongation, which leads to formation of the head anlage, is driven by a combination of convergence and extension (CE) movements of the three germ layers and directed collective cell migration of the anterior-most axial mesoderm (Tada & Heisenberg 2012). Posterior elongation, which leads to the formation of trunk and tail, relies on tissue convergence of paraxial cells towards the body midline only at early stages. At later stages, unidirectional elongation of the posterior body occurs without thinning of the mediolateral axis and is sustained by addition of new cells generated in the tailbud (Bénazéraf & Pourquié 2013). After entering the paraxial mesoderm through a process of epithelial-to-mesenchymal transition (EMT), these cells display unique behaviors, such as lateral divergence and random cell motility (cell diffusion) (Bénazéraf et al. 2010, Kanki & Ho 1997, Marlow et al. 2004). More recently, tissue-scale mechanisms such as fluidification of the tailbud and solidification of the PSM have also been described in this context (Lawton et al. 2013, Mongera et al. 2018). Posterior body elongation thus entails a continuation of mechanisms deployed during gastrulation as well as novel/different mechanisms specific to caudal morphogenesis.
Importantly, while cells derived from all the germ-layers contribute to body extension, the mesoderm has been proposed as a major source of active force and a plethora of studies have since focused on this tissue (Keller et al. 2003). Early studies with Xenopus explants reported that axial mesodermal tissues generate a pushing force in the AP direction. This mechanism of force production is accompanied by tissue stiffening along the same direction (Keller et al. 2003, Moore et al. 1995). In chicken, a random cell motility gradient in the posterior paraxial mesoderm is required for elongation and surgical removal of this region causes posterior axis elongation to stall (Bénazéraf et al. 2010).
More recent studies have focused on the mechanical coupling between distinct tissues as a means to sustain proper body axis formation (Dray et al. 2013, Smutny et al. 2017, Xiong et al. 2018). The PSM compresses axial tissues, such as the notochord and neural tube, allowing the formation of a mechanical positive feedback loop that ensures the self-sustaining properties of posterior elongation (Xiong et al. 2018). In zebrafish, downregulating adhesion between the PSM and axial tissues has a dramatic effect on elongation and leads to buckled axial tissue (Dray et al. 2013). Moreover, adhesion-dependent friction forces at the interface between the axial mesoderm and the overlying neuroectoderm are important to guarantee proper anterior elongation and the correct positioning of the neural anlage (Smutny et al. 2017).
Tissue rearrangements and volumetric growth drive elongation at very different timescales. Thus, their differential deployment correlates with species- and stage-specific rates of embryonic morphological change. Cell rearrangements allow for rapid morphogenesis and dominate during early development. They entail the tight coordination of both active cellular forces and tissue material properties, which define how the system responds to the generated stresses (Stooke-Vaughan & Campàs 2018). At these stages the geometry of the embryo changes rapidly, at timescales shorter than the timescale of anisotropic growth driven by cell proliferation. The most prominent example of shape change driven by cell rearrangements is mesoderm CE, a process based on cell migration and coordinated neighbor exchanges among highly polarized cells. At the tissue-scale, this cell behavior results in concurrent extension and narrowing of the embryo along orthogonal directions and has been shown to regulate axis formation in all the vertebrate models analyzed (Keller et al. 2000, Tada & Heisenberg 2012).
On the contrary, tissue morphogenesis through volumetric growth — via cell proliferation, cell growth, and ECM deposition — requires longer periods of time (set by cell division rate, ECM synthesis rate, etc.) and usually characterizes later stages of development. In zebrafish, posterior body elongation is driven by volumetric growth only moderately, when notochord and spinal cord, but not the unsegmented region of the paraxial mesoderm, increase their mass after the 24-somite stage (Steventon et al. 2016). In chicken instead, posterior volumetric growth plays a more prominent role and is largely based on paraxial mesoderm and NT expansion from the tailbud, indicating differences in tissue-specific behaviors among vertebrates (Bénazéraf et al. 2017, Steventon et al. 2016). While in zebrafish volumetric growth begins at the axial level where caudal (tail) vertebrae will form, in chicken a volume increase in posterior elongating tissues is already detectable at cervical (neck) levels (Bénazéraf et al. 2017, Steventon et al. 2016).
Following the development of more precise mechanical measurements, the contribution of these different mechanisms to the formation of the vertebrate AP axis is now understood in more details. Concepts and tools from mechanics, which studies forces and material deformations, are becoming essential for a quantitative description of the biological mechanisms underlying vertebrate body elongation. In this review, we will first highlight morphogenetic mechanisms which rely on cell rearrangements without tissue growth. We will then discuss mechanisms based on tissue volume increase. Finally, we will review the tissue-scale mechanical measurements that have been used to quantitatively describe such morphogenetic processes.
1. Mechanisms based on cell rearrangements
Even though elongation of the embryonic body axis can last several days, the associated cellular rearrangements usually take place at much shorter timescales (minute/hour range). Convergent extension (CE) is a fundamental tissue-scale process that underlies the elongation of many embryonic structures, including the vertebrate AP axis (Bénazéraf 2018, Keller et al. 2000, Roszko et al. 2009, Tada & Heisenberg 2012). This process ultimately relies on the movement of cells towards the embryo midline and their displacement along the orthogonal direction. These movements can be driven by two distinct mechanisms: cell intercalation and cell migration, which can be used by the same cell population (Tada & Heisenberg 2012). A third mechanism based on biased cell diffusivity has been shown to drive extension, but not convergence, of the posterior body axis (Bénazéraf et al. 2010, Regev et al. 2017).
1.1. CE via cell intercalation
CE is a mechanism leading to the modification of a tissue’s aspect ratio in absence of volume changes. Simultaneous tissue narrowing and lengthening is a widely used morphogenetic module among metazoans and accounts for many of the changes in tissue shape occurring during embryogenesis (Keller et al. 2000). A fundamental cellular mechanism driving CE movements is cell intercalation, the polarized translocation of one individual cell between its neighbors (Huebner & Wallingford 2018). Importantly, defects in the correct implementation of this stereotypical cell behavior can lead to neural tube and skeletal malformations, such as spina bifida, scoliosis, and sacral agenesis in humans (Butler & Wallingford 2017, De Marco et al. 2014, Wallingford et al. 2013).
Two fundamental modes of cell intercalation have been described: intercalation by cell crawling and intercalation by junction shrinking (Huebner & Wallingford 2018, Shindo 2017). While displaying very distinct features, both mechanisms are based on the spatiotemporal control of actomyosin contractility and adhesion strength (Shindo 2017). Intercalation by cell crawling involves cell elongation and alignment along the axis of intercalation, the formation of polarized, lamellipodia-like protrusions, the establishment of stable contacts with neighboring cells, and the application of traction by the protrusions. This intercalation mode has been extensively analyzed in mesenchymal tissues such as the mesoderm, where this cell behavior resembles aspects of cell migration on a substrate. A classic example is notochord formation in frog (Adams et al. 1990, Keller et al. 2000, Keller et al. 1985), where axial mesodermal cells form bipolar actin-rich membrane protrusions and are characterized by cortical actomyosin contractions (Keller et al. 2000, Keller et al. 1992a, Kim & Davidson 2011). Importantly, defective protrusions caused by impaired Rho and Rac signaling lead to reduced CE movements during body elongation (Tahinci & Symes 2003). The second mode is driven by junctional remodeling and is typical of epithelial tissues during extension (Guillot & Lecuit 2013). Originally discovered in Drosophila during germband extension (Irvine & Wieschaus 1994), this cell behavior is driven by actomyosin contractile forces and causes the modification of cell-cell junction length. The shrinkage of a cell junction leads to the formation of new contacts via T1 transitions which in turn lead to tissue elongation and thinning (Bertet et al. 2004, Huebner & Wallingford 2018).
While these two modes have long been considered as acting in distinct cellular contexts, within tissues displaying radically different architectures (mesenchymal vs epithelial), recent studies highlighted the possibility of their coexistence during CE of the same tissue. In mouse, for example, cells of the neural plate intercalate by junctional shrinking apically and by protrusion-mediated traction basally (Williams et al. 2014). Moreover, notochord formation in Xenopus has been recently shown to involve cell junctional remodeling via actomyosin contraction in addition to protrusion-mediated intercalation (Shindo & Wallingford 2014).
Importantly, planar cell polarity (PCP) signaling has been involved in the regulation of cell intercalation in all the vertebrate models analyzed so far (Butler & Wallingford 2017, Heisenberg et al. 2000, Tada & Heisenberg 2012, Wallingford et al. 2000). Planar polarity refers to polarized asymmetries in the localization of molecular determinants within cells in the plane of the tissue sheet (Butler & Wallingford 2017). As for apical-basal polarity, planar cell polarity involves typically tissue-wide coordination of cell behavior. The PCP pathway was first identified in Drosophila through screens for regulators of the oriented and coordinated arrangements of bristles and hairs (Butler & Wallingford 2017, Goodrich & Strutt 2011, Peng & Axelrod 2012). Tissue patterning through planar polarity is controlled by a ‘core’ PCP pathway which includes, among others, Frizzled, Van Gogh, Dishevelled, Wnt5/11, and the Fat-Dachsous-Four-jointed (Ft-Ds-Fj) module (Butler & Wallingford 2017). Importantly, the major step in PCP is the localization of different subsets of core PCP proteins to opposite domains along the cell cortex. These molecular modules are mutually exclusive and, by interacting at cell-cell junctions, they generate a tissue-scale pattern. In addition to cell-cell communication, planar polarity within a tissue can be also coordinated by gradients of secreted ligands (Chu & Sokol 2016) and mechanical forces (Aw et al. 2016, Mitchell et al. 2007).
In the context of early morphogenesis and axis elongation, PCP proteins have been shown to regulate membrane protrusions (Wallingford et al. 2000), actomyosin contraction (Shindo & Wallingford 2014), cell-cell adhesion (Kraft et al. 2012), and cell-matrix-adhesion (Dohn et al. 2013, Goto et al. 2005). Forward genetic screens in zebrafish allowed the isolation of mutants for many components of the pathway, including Vangl2 (Jessen et al. 2002), Wnt11 (Heisenberg et al. 2000), Wnt5 (Rauch et al. 1997), and Gpc4 (Topczewski et al. 2001). Mutations in these genes cause defects in CE movements — but not cell fate specification — and lead to embryos with shortened AP body axis and wider mesoderm-derived structures and neural plate (Nishimura et al. 2012, Roszko et al. 2009).
Intercalation during anamniote axis elongation
While local cell rearrangements have been proposed as a plausible mechanism for driving CE movements already by Waddington (Waddington 1940), the actual process of cell intercalation was only described much later in a series of classical studies conducted in Xenopus laevis by Ray Keller and colleagues. Shown to power CE movements specifically during embryonic axial elongation, cell intercalation takes place both in mesodermal (Keller et al. 1989, Keller & Tibbetts 1989, Keller 1984, Shih & Keller 1992a, Shih & Keller 1992b) and neuroectodermal tissues (Elul et al. 1997, Keller et al. 1992b). Importantly, cell intercalation can occur along the embryo’s radial axis, normal to its surface, or along the mediolateral axis. Both contribute to AP body elongation, although radial intercalation is predominant in the first half of gastrulation, during epibolic movements, and mediolateral intercalation characterizes the stages between blastopore closure and neurulation (Keller et al. 2000). Using explants of dorsal tissue, CE movements driven by cell intercalation were shown to be active, tissue autonomous and independent of traction on the substrate (Keller & Danilchik 1988, Keller et al. 1985). Forces exerted locally by cells during intercalation lead to a pushing force in the AP direction, paralleled by the stiffening of the prospective body axis from gastrulation to neurulation. A mean stress of 5 Pa together with a 4-fold stiffening (from 20 Pa to 80 Pa) have been recorded in explants of dorsal tissues during this time period (Moore et al. 1995, Zhou et al. 2009, Zhou et al. 2015). Further studies of isolated tissues from these explants showed that paraxial mesoderm and neural plate are the major producers of elongation forces whereas notochord contribution to axial pushing is negligible (Zhou et al. 2015). Interestingly, stiffening due to mediolateral cell intercalation is anisotropic and reaches higher values in the AP direction (Moore et al. 1995). Governed by actomyosin contractility rather than by matrix assembly, tissue stiffness increases along with the pushing force produced, thus explaining the nearly constant elongation rate (Zhou et al. 2015). Interestingly, increasing the stiffness of the gel used as a force sensor leads to higher levels of recorded forces. These findings, in the context of the study of axis elongation, led to the concept of mechanical accommodation, i.e. the tight regulation of force production in response to changing mechanical resistance which allows for robust morphogenesis (Zhou et al. 2015). F-actin dynamics and myosin contractility, both at the protrusive leading edges and within the pericellular region of cells, provide the forces for crawling and intercalation (Kim & Davidson 2011, Skoglund et al. 2008). Moreover, protrusive activity of intercalating cells has been linked to proper assembly of the extracellular matrix (Davidson et al. 2008).
Radial and mediolateral cell intercalation also regulate tissue spreading during epiboly and CE movements during axis extension in zebrafish (Ellen et al. 1995, Warga & Kimmel 1990). Radial intercalation in the epiblast depends on E-Cadherin-mediated adhesion, thus impairing tissue spreading and axis extension (Kane et al. 2005). As in Xenopus embryos, the anterior dorsal domain, containing prechordal mesodermal cells, extends towards the animal pole through directed collective migration, while the posterior dorsal domain, which will give rise to the notochord, is characterized by intense mediolateral cell intercalation (Glickman et al. 2003, Roszko et al. 2009). Compared to axial tissues, CE movements in the paraxial mesoderm have been found to depend additionally on radial cell intercalation, which contributes to bias elongation in the AP direction (Yin et al. 2008). The threefold higher extension rate (but identical convergence rate) of axial mesodermal cells compared to PSM cells may be accounted for by the differences in the repertoire of cell behaviors exploited by the two tissues (Glickman et al. 2003, Yin et al. 2008). Several zebrafish mutants for the Wnt/PCP pathway display defects in the cell behaviors underlying CE movements and are characterized by shortened and wider body axes (Roszko et al. 2009). silberblick/wnt11 mutants exhibit defective mediolateral intercalation in the paraxial mesoderm and, as a direct consequence, abnormal extension of axial tissues leading to cyclopia and other defects of the head anlage (Heisenberg et al. 1996, Heisenberg et al. 2000). trilobite/vangl2 mutants display broader somites and truncated posterior body (Jessen et al. 2002). Importantly, interactions between Van Gogh like 2 and Glypican 4 — a proteoglycan potentiating non canonical Wnt signaling which is truncated in knypek mutants (Topczewski et al. 2001) — are required for proper radial intercalation in the paraxial mesoderm, as revealed by the analysis of double mutant embryos (Yin et al. 2008). The supracellular stresses associated to mediolateral (ML) cell intercalation have been recently measured in the zebrafish PSM (Mongera et al. 2018). These stresses display a ML orientation and a posterior-to-anterior increasing gradient, with values ranging from 25 and 50 Pa (Mongera et al. 2018). This increase in mediolateral constriction is also coupled to tissue stiffening (Serwane et al. 2017).
Intercalation during chicken primitive streak extension
In the chicken embryo, the AP axis becomes first visible with PS extension. The PS is a multilayered structure which marks the level where epiblast cells ingress to form the endoderm and mesoderm. The PS progressively extends anteriorly from the posterior part of the blastoderm during gastrulation. This extension is accompanied by two large counter-rotating circular flows called polonaise movements which are important for correct patterning of the AP body axis (Chuai & Weijer 2008). Oriented cell division in the PS was initially proposed as a mechanism driving its extension (Wei & Mikawa 2000). However, subsequent experiments showed that inhibition of cell division impairs only the counter-rotating flows, but not streak elongation (Cui et al. 2005). A concurrent hypothesis proposed that CE via mediolateral cell intercalation drives PS extension. Using multi-photon time-lapse microscopy, extensive cell-cell intercalation was recorded along the PS during its extension. Disruption of the Wnt/PCP pathway through morpholino electroporation and overexpression of dominant negative constructs arrested PS extension (Voiculescu et al. 2007). This also led to defects in mesendoderm internalization and to the accumulation of cells along the PS (Hardy et al. 2008). Subsequently, light-sheet microscopy provided maps of tissue expansion and contraction, along with cell-cell rearrangements at unprecedented resolution (Rozbicki et al. 2015). Medio-laterally oriented myosin cables in the posterior region of the blastoderm are involved in a pulling mechanism contracting the tissue towards the midline which aligns the cells. The contraction towards the midline was proposed to drive both cell ingression into the PS and cell intercalation along the AP axis which passively extends it. Importantly, this work highlighted the role of myosin I and V in directing PS extension. Indeed, inhibition of myosin II through blebbistatin treatment leads to no significant reduction of elongation speed (Rozbicki et al. 2015). Recently, a supracellular actomyosin ring has been identified at the margin between the embryo proper and the extraembryonic tissues (Saadaoui et al. 2018). This tensile structure as well as fluidity of the epiblast, actively maintained by cell division (Firmino et al. 2016), are proposed to account for tissue flow during gastrulation, including PS extension (Saadaoui et al. 2018).
Intercalation during neural plate CE in mouse
Proper neurulation requires CE movements within the neural plate (NP), the embryonic structure that gives rise to the neural tube. Importantly, mediolateral cell intercalation is thought to facilitate neural tube closure, and thus neurulation, by reducing the distance between the two symmetric neural folds (Wallingford & Harland 2001, Wallingford & Harland 2002). In amniotes, the NP is formed by a single-layered pseudostratified epithelium, whereas in amphibians it is constituted by multiple layers of deep mesenchymal cells (Williams et al. 2014). In mouse, oriented cell division does not contribute to elongation at early somite stages, as no bias in the orientation of the division plane has been found (Williams et al. 2014). Notably, convergence is modest compared to extension, suggesting that tissue volumetric growth may contribute to axial elongation in parallel to mediolateral intercalation (Williams et al. 2014). CE of the NP requires planar polarity, as mouse mutants for PCP core components such as Vangl2 and Ptk7 display morphological defects, including a decrease in CE index, abnormal cell shape, and craniorachischisis (Williams et al. 2014). In vivo analysis of NP morphogenesis indicated a role for these PCP proteins in controlling cell intercalation. Looptail/Vangl2 mutants display fewer, although still mediolaterally oriented, intercalation events, while genetic lesions in the Ptk7 locus lead to non-oriented neighbor exchanges, which nevertheless occur at normal rates (Williams et al. 2014). Both apical boundary rearrangements and basal protrusions drive mediolateral cell intercalation of NP cells in mouse (Williams et al. 2014). Vangl2 is shown to regulate apical neighbor exchange, while Ptk7 controls oriented protrusive activity in the basal domain (Williams et al. 2014).
1.2. Cell migration
A second mechanism driving shape changes independently of volumetric growth is cell migration. Cell migration contributes to axis elongation in large part by supporting convergent movements, as reported in zebrafish during dorsal migration of paraxial mesoderm progenitors (Sepich et al. 2005). However, different patterns of cell migration can also be found, as anteriorly directed collective crawling of prechordal plate cells (Montero et al. 2005, Ulrich et al. 2003, Ulrich et al. 2005). Importantly, if considered locally and not at the embryo- or organ-scale, cell migration leads to volumetric changes — that is, a volume increase of the target region and a correspondent volume decrease of the source region. This is the case of cell injection from the tailbud, which will be discussed later in this Review.
In Xenopus and zebrafish, mesodermal progenitor cells internalize around the entire circumference of the blastoderm margin and only then move dorsally to narrow and extend the body axis (Keller et al. 2003). While in amphibians CE movements of the mesendoderm are driven exclusively by cell intercalation, in zebrafish tissue convergence is initiated by cell migration (Keller et al. 2003, Roszko et al. 2009, Sepich et al. 2005). At the beginning of zebrafish gastrulation, mesodermal cells migrate anteriorly and they switch to dorsal-ward direction at mid-gastrulation (Sepich et al. 2005). The switch from animal-ward to dorsal-ward migration of mesodermal cells is independent of non-canonical Wnt, which is required for fast and more directed dorsal migration at later stages of gastrulation (Jessen et al. 2002, Sepich et al. 2005, Sepich et al. 2000). Importantly, correct balance of N-Cadherin-mediated adhesion is required for effective directed migration of the mesoderm (Warga & Kane 2007).
After internalization, prechordal plate (PPL) cells forming the anterior axial mesoderm actively migrate towards the animal pole without displaying any intercalation behavior. These directed collective movements, accompanied by the formation of pseudopod-like processes at the leading edge, are regulated by the Wnt/PCP pathway. PPL cells in silberblick/wnt11 mutants show reduced average velocity and persistence during their anterior migration. Moreover, the pseudopod-like processes that in wildtype embryos are aligned along the axis of migration, display a random orientation in the mutant (Ulrich et al. 2003). Importantly, after internalization, cells upregulate E-Cadherin, which is required for proper PPL migration along the overlying epiblast (Montero et al. 2005). E-Cadherin is a downstream effector of Wnt11 signaling, promoting collective migration through the control of cell cluster cohesion and inter-tissular coupling (Smutny et al. 2017, Ulrich et al. 2005). Membrane-to-cortex attachment, the mechanical coupling of membrane and actomyosin cortex, regulates the right balance between different protrusion types, i.e. blebs and actin-rich cell processes, in the context of PPL directed migration (Diz-Muñoz et al. 2010). Importantly, this directed migration is characterized by alternating run and tumbling phases, regulated by actin-rich protrusions powering traction and blebs allowing for exploration of the environment (Diz-Muñoz et al. 2016). Recently, Frizzled 7 has been shown to act as a permissive signal during PPL cell migration (Čapek et al. 2019).
1.3. Random cell motility/biased cell diffusion
While new cell addition from the tail bud is required on long timescales to sustain body elongation, at short timescales, elongation was proposed to be driven by a random cell motility gradient in the posterior PSM of the chicken embryo. Indeed, only bilateral ablation of the posterior PSM leads to an immediate reduction in the speed of axis elongation. Inhibiting cell proliferation or removing the axial progenitor domain in chick do not affect the immediate speed of axis elongation and only shows elongation defects in longer term (Bénazéraf et al. 2010). In both chicken and zebrafish, a posterior-to-anterior decreasing gradient in cell diffusivity in the PSM has been described (Bénazéraf et al. 2010, Lawton et al. 2013, Mongera et al. 2018). While tracks of individual cells in the posterior PSM show an apparent posterior migration, subtracting ECM motion from these tracks revealed their diffusive (random walk) behavior with respect to their local environment (Bénazéraf et al. 2010). Thus, the apparent posterior motion of the cells corresponds in fact to the posterior deformation of the tissue and not to directed cell migration. This AP profile in cell motility depends on a gradient of FGF signaling: blocking FGF signaling decreases cell diffusivity which, in turn, slows posterior body axis elongation (Bénazéraf et al. 2010). FGF was proposed to act as an effective temperature, imposing a biased diffusion to the cells (Bénazéraf et al. 2010). Importantly, cell density is low in the posterior PSM and increases towards the somites (Bénazéraf et al. 2017). Thus, due to the confinement of the motile cells of the posterior PSM by rigid boundaries, their active movement was proposed to result in the production of a posteriorly oriented force (pressure) driving the posterior elongation of embryonic tissues. These hypotheses have recently been modelled mathematically, showing that high motility can produce higher effective pressure that, opposed by confinement in the anterior PSM and lateral tissues, biases elongation posteriorly (Regev et al. 2017). In zebrafish, this high cell diffusivity in the posterior PSM has been recently linked to low physical constraints from the cellular microenvironment as well as larger and more frequent cell-cell contact fluctuations (Mongera et al. 2018). Analysis in the fish PSM showed caged behavior in the anterior part, consistent with cells being trapped in a solid-like material, and uncaged cell mixing in its posterior part, consistent with this region being fluid-like (Mongera et al. 2018).
2. Tissue growth-based mechanisms
While cell rearrangement and cell flow produce tissue shape changes that contribute to body axis elongation at short timescales, AP axis formation ultimately relies on the long-term accumulation of biomaterials, most notably through cell proliferation, cell growth, and addition of new cells from neighboring tissues, i.e. cell injection. Moreover, as tissues differentiate, specialized extracellular matrix and other non-cellular structures may also contribute to the increase in length. Notably, high cell diffusion/motility has also been proposed as a mechanism for increasing overall tissue volume without increasing cell number or cell size. Here we focus on the stages of body axis formation until the total number of somites is formed.
2.1. Cell Proliferation, growth, and cell injection
Cell proliferation alone does not lead to volume increase in the absence of cell growth, as evidenced by volumetric analysis in early cleavage stage embryos (Olivier et al. 2010, Xiong et al. 2018). The actual increase in cell mass requires biomaterial synthesis which depends on energy sources. In early embryos, cells feed on internal sources such as the egg yolk or external ones via the placenta (mammals). The great variety in size and availability of the initial energy/material source imposes differential constraints on the growth mode of embryos of different species (O’Farrell 2015). It was suggested that the extent of contribution by proliferation and growth to body axis elongation varies accordingly (Steventon et al. 2016). For example, zebrafish embryos complete elongation mostly with existing cells reorganized but little volume growth, presumably an adaptation to its fast development and early need for feeding (Stevenson et al. 2016). Importantly, the zebrafish harpy/emi1 mutants, where cells stop dividing during early gastrulation, undergo normal elongation (Riley et al. 2010, Zhang et al. 2008). In contrast, mouse and chick embryos show a much more significant volume increase in elongating tissues at comparable stages (Bénazéraf et al. 2017, Steventon et al. 2016), possibly because they have access to abundant nutritional supply. In birds, the paraxial mesoderm is the major contributor to volume increase along the axis. Such an increase is linked to a faster cell cycle (9 hours) compared to axial tissues (11 hours for the NT and 28 hours for the notochord) (Bénazéraf et al. 2017).
As the body axis elongates, embryonic tissues become produced by the tail bud, a posteriorly located territory containing the progenitors of the posterior body. The tailbud is usually viewed as a growth zone where cells divide and increase in size to generate tissue growth. The tail bud is conceptually similar to plant meristems (Beddington 1994, Beemster & Baskin 1998), although a major difference is that plant cells are not motile and thus morphogenesis is entirely controlled by patterned cell proliferation and growth in absence of cell rearrangements. Compared to cell reorganization mechanisms such as CE, proliferation and growth-based mechanisms are significantly slower as they are fundamentally limited in speed by the need of cells to produce essential building blocks, to replicate the genome and to assemble the cell cycle machinery (O’Farrell et al. 1989). This raises the question of how important of a role cell proliferation plays in animal axis elongation in relation to other mechanisms discussed earlier. Quantitative observations that dissect contributions of these mechanisms have only been made recently (Bénazéraf et al. 2017, Lawton et al. 2013, Steventon et al. 2016).
In zebrafish, the tailbud is formed by a dorsal medial (DM) zone, located in the epiblast, and a ventral progenitor zone (PZ), which constitutes the most posterior region of the paraxial mesoderm. While the DM zone contains neural, mesodermal, and bipotent neuromesodermal progenitors (NMPs), only mesodermal progenitors are found in the PZ (Kimelman 2016, Martin & Kimelman 2012). A dorsal-to-ventral flux of cells characterizes the tailbud and is accompanied by an order-to-disorder transition in cellular motion coupled to an epithelial-to-mesenchymal transition that correlates with the downregulation of cell surface cadherin 2 (Das et al. 2017, Goto et al. 2017, Lawton et al. 2013, Martin & Kimelman 2012). While cell tracks display high degrees of directional correlation when located in the epiblast, disordered motion arises as they translocate ventrally and eventually sort bilaterally to form the two PSM columns. This disorder motion, described originally in chicken(Bénazéraf et al. 2010) and likely conserved among vertebrates, gives rise to short-lived left-right anisotropies in cell fluxes that in time balance each other. Recent in silico and in vivo analyses have demonstrated that levels of order/disorder in cell motion must be tightly regulated, as their spatial misregulation can lead to both asymmetric elongation or slow posterior extension. Localized high order is maintained in the dorsal region, where cells move posteriorly before entering the PZ, and is necessary for rapid elongation. Localized low order is maintained in the PZ and is required to guarantee bilateral symmetry. Importantly, an excessive order would lead to self-sustaining monolateral vortices in the cell tracks and axial deformation (Das et al. 2017). Overly disordered cell motion in the epiblast of cdh2 mutants causes jam of cell tracks and a slow-down of axis elongation, which however occurs symmetrically. Increase of order in the PZ by impairing Wnt signaling, a major player in driving EMT in this tissue, causes asymmetric body axis (Das et al. 2017). This transition between order and disorder motion has been also used to infer differences in tissue fluidity in the tailbud. In particular, coherence of collective migration and flow rate have been analyzed during dorsal-to-ventral cell flows in the tailbud (Lawton et al. 2013). Flows in the DM have been found coherent, posteriorly directed, and fast, while in the PZ there is a decrease in the global order of the cell flow without corresponding changes in cell velocity. Importantly, defects in axis formation arise when only coherence of cell movements is affected. If reduction of coherence is coupled to decreased flow rate, defects can be rescued (Lawton et al. 2013).
In amniotes, the PSM cells involved in the control of later stages of axis elongation are first produced by the anterior PS and later on by the tail bud. The precursors of the PSM are initially located in the epiblast where they exhibit an epithelial organization. They undergo an EMT to enter the paraxial mesoderm where they become highly motile in response to FGF signaling. Fgf8 is only transcribed in the tail bud precursors and it stops being transcribed when cells become mesenchymal and enter the posterior PSM (Dubrulle & Pourquie 2004b). Thus, as cells mature, becoming located progressively more anteriorly in the PSM, their Fgf8 mRNA content decays, resulting in the formation of an mRNA gradient peaking in the tail bud. In mouse embryos, FGF-dependent cell ingression is required for axis elongation (Ciruna & Rossant 2001, Rossant et al. 1997). FGF signaling through FGFR1 orchestrates the EMT of mesendodermal precursors at the PS. In FGFR1 mutants, these cells accumulate within the PS as the downregulation of E-cadherin required for cell ingression is prevented (Ciruna & Rossant 2001). Fgf8 −/− embryos do not form mesoderm and endoderm as cells fail to move away from the PS after ingression leading to body axis truncation (Naiche et al. 2011, Sun et al. 2017). In the chicken embryo, in order to produce the sustained elongation movements necessary to generate the entire posterior body axis, new highly motile cells need to be constantly added at the posterior end of the PSM. Recent experiments have shown that the posterior PSM generates a mediolateral compression that pushes the axial organs posteriorly ensuring the posterior displacement of the precursor domain. This PSM cells’ activity establishes a mechanical positive feedback loop in which PSM cells random motility leads to a mediolateral compression of the axial tissues, pushing the progenitor domain posteriorly and favoring progenitor cells injection in the posterior PSM (Xiong et al. 2018).
2.2. Cell swelling
While cell proliferation can be assessed across the body axis by detecting cell cycle markers, the cell growth rate of many tissues in the elongating body axis has not been systematically measured. Elongation in plants is further supported by turgor pressure, which can be maintained at high levels through the cell wall (Hamant & Traas 2010). The most marked change is observed in the notochord, where cells undergo vacuolization to swell (Jurand 1962, Mookerjee 1953). In frogs, this cell size increase is accompanied by the deposition of an ECM sheath that wraps and contains the notochord, resulting in stiffening of the tissue and elongation (Adams et al. 1990). Similarly, in zebrafish, an outer cell layer forms an epithelial-like sheath containing an inner layer of cells that swell by making a large vacuole (Ellis et al. 2013). Therefore, the swelling of notochord cells not only adds new volume to the axis but also plays a mechanical role in straightening it and potentially the surrounding tissues. In amniotes however, the swelling of the notochord cells happen later in relation to body axis elongation and the notochord occupies a significantly smaller volume of the whole axis (Stemple 2005), suggesting a less important role of cell swelling in elongation compared to the anamniotes.
2.3. Non-cellular Growth
Another way to generate tissue growth is to deposit extracellular matrix and to increase intercellular space, thereby gaining new volume without creating new cells. How cell motility leads to tissue level expansion remains unclear. It is possible that it is related to the extracellular matrix (ECM) in the tissue (Rifes et al. 2007). The cell movements might generate pulling forces on the ECM network causing its remodeling to occupy more space. Alternatively, cell motility may be associated with other concurrent processes such as ECM secretion. The accumulation of certain ECM components such as hyaluronan could lead to tissue expansion through water absorption (Nakamura et al. 1993). This aspect of tissue growth is less understood as tools that assess non-cellular behaviors and components remain scarce compared to the cellular counterparts. Importantly, fibrillar fibronectin contributes to maintain cell polarity during epiboly but is dispensable for axis elongation (Rozario & Desimone 2009).
3. Tissue forces and mechanical properties
Role of mechanics in morphogenesis
Morphogenetic processes such as axis elongation, and their recapitulation during cancer ontogeny and tissue repair, require growth and collective cell movements (Lecaudey & Gilmour 2006, Stooke-Vaughan & Campàs 2018). In absence of growth, a condition typical of early morphogenesis, tissues modify their shape through coordinated cell rearrangements that are ultimately driven by cell-generated active stresses and depends on tissue material properties (Campàs 2016, Heisenberg & Bellaiche 2013, Stooke-Vaughan & Campàs 2018).
Stress production in embryonic tissues
During morphogenesis, embryonic tissues undergo important deformations. Such deformations are the result of active stress production within the deforming tissue or applied by neighboring tissues. Several mechanisms can lead to stress production. Cells can modify their osmotic pressure by producing glycosaminoglycans (Adams et al. 1990) or by the production ATP-dependent, ion-filled vacuoles (Ellis et al. 2013). In densely packed tissues, the contractile activity of the cell cytoskeleton (mostly through the actomyosin contractility) can generate a stress within the tissue owing to the mechanical continuity of the tissue. Furthermore, it has been hypothesized that motile cells can exert an active pressure by their movements (Bénazéraf et al. 2010).
While stresses are generated by cells, they can propagate at the tissue scale. Measuring the amplitude of such stresses is necessary to provide a quantitative description of morphogenetic processes. While force measurement techniques have been actively developed in vitro during the past decade, their applications in vertebrate embryos remain scarce (for a review of existing techniques, see (Sugimura et al. 2016)).
Recent techniques such as the development of FRET (Förster resonance energy transfer) tension sensors have only been recently applied to C. elegans (Krieg et al. 2014) and Drosophila embryos (Cai et al. 2014) but not yet to vertebrate embryos. Tension at intercellular junctions has been extensively studied in epithelia. While ablation and trapping of junction to measure relative value of tension within a tissue is now commonly investigated in Drosophila. It has also been applied at the tissue scale in zebrafish to show the anisotropy of tension during epiboly and tissue spreading (Behrndt et al. 2012, Campinho et al. 2013). However, it is worth mentioning that laser ablation does not provide absolute value of tension (unless viscosity can be precisely measured, which is rarely possible). Conversely, absolute values of cortical tension could be measured in the early mouse embryo (Maitre et al. 2012). However, this technique requires a direct access to the cell membrane and is therefore not applicable for thick tissues or large 3D structures.
In order to measure stress within a tissue, mechanical probes have been injected and their deformation provides absolute stress values. These probes can be oil drops for which the surface tension is known, therefore their deformation can give a measurement of the stress anisotropy (Campàs et al. 2014). The local variation of the curvature of the drop also yields estimates of the stress generation at the cell scale (Campàs et al. 2014, Lee et al. 2019, Mongera et al. 2018). Gel drops can also be used to measure compressive stress (Mohagheghian et al. 2018). However, to measure absolute values, the volume of the drop in a stress-free state should be measured which has not been carried out yet in embryos.
The stress generated by a whole tissue on its environment can also be measured. In this way, the stress generated by Xenopus dorsal explants during convergent extension has been measured by embedding them in calibrated gels (Zhou et al. 2015). The contraction force of Xenopus marginal zone has also been measured by means of a calibrated cantilever (Shook et al. 2018). A non-invasive technique has been developed to infer relative tensions and pressures within an epithelium based on the geometry of the junctions (Ishihara & Sugimura 2012) (Brodland et al. 2014). This technique has recently been applied in the zebrafish gastrula (Krens et al. 2017).
Mechanical properties of embryonic tissues
Mechanical properties of embryonic tissues control how they deform instantaneously and over time to different applied stresses. Embryonic tissues exhibit properties of complex materials at the intercept between the behaviors of solids and fluids. A solid deforms instantaneously and the magnitude of its deformation is set by its stiffness. Conversely, a fluid flows over time and the flow rate is controlled by its viscosity. Depending on the timescale, embryonic tissues’ response is solid-like or fluid-like. At short times (below a 1–5 min), embryonic tissues have solid properties, while at longer timescale (over 10 min), they might exhibit fluid properties (Forgacs et al. 1998, Phillips & Steinberg 1978). Tissues’ fluid properties might also arise only under a critical state of stress. In this case a minimal stress (yield stress) must be reached to trigger tissue flow (Mongera et al. 2018). However, depending on the tissues, solid properties can also be maintained on long times (Luu et al. 2011).
In order to measure these properties, different mechanical assays can be carried out. The tissue dynamical response during these assays reveals its mechanical properties, which can be quantified using rheological models. Such models predict the temporal evolution of the deformation or the stress and allow to fit the tissue response and extract mechanical parameters (see Figure 3 and Box 1).
Figure 3 |.

Rheological models quantifying different mechanical behaviors. Solid and fluid behaviors at long time can be quantified by different viscoelastic models. The deformation of a Kelvin-Voigt model saturates after a typical time when it undergoes a step of imposed stress while the tissue flows at a flow rate for a Maxwell model. In the case of linear mechanics, the relations between the maximum deformation (for solid materials) or the flow rate (for fluid materials) and the imposed stress are linear. In the special case of a fluid with a yield stress σy, the relation between flow rate and stress is also linear with an offset, which is the minimal stress that needs to be applied to start the flow.
Box 1: Quantifying mechanical properties.
In order to quantify the mechanical properties of embryonic tissues, their response to a constant deformation (stress relaxation test) or to a constant stress (creep test) needs to be measured over time. Several rheological models predicting such temporal responses can be used to fit experimental data and extract mechanical parameters. Rheological models comprise different combinations of spring and dashpot elements which determine the temporal evolution of a material’s response to applied stresses. Springs represent the elastic response, the resistance of the material to deformation, while dashpots represent the viscous response, the resistance of the material to flow.
The ratio of a viscous element (which unit is in Pa.s) over a spring element (which unit is in Pa) defines a characteristic time, i.e. the relaxation time scale, which can be used to estimate the solid and fluid regimes of a tissue. Simple models, such as the Maxwell model or Kelvin-Voigt model, comprise only one spring element and one viscous element, arranged in series or in parallel, respectively. As these models have only one timescale, more complex models (such as the Standard Linear Solid model) can be used to account for the different timescales of tissues. An extreme case is the power law model which is equivalent to an infinity of timescales which has been shown to fit well cytoplasm rheology (Balland et al. 2006, Fabry et al. 2001).
In embryonic tissues, both the solid and the fluid behaviors have been investigated, although the latter received a significant smaller attention. Regarding embryo axis formation, it is interesting to measure the embryonic solid properties to account for the ability of embryonic tissues to resist stresses from their environment and consolidate the growing axis. Tissue fluidity plays also an important role during tissue rearrangement and shape changes. We review here the different techniques used to measure solid and fluid properties in embryonic tissues only in vertebrates’ embryo at stages relevant to AP axis formation. In such studies, the biological functions and processes controlling the mechanical properties have often been investigated. However, the biological origins of mechanical properties are often complex to dissect (see Box 2).
Box 2: Biological origins of tissues’ mechanical properties.
Mechanical properties can be tuned through several biological processes. The solid response is a consequence of the crosslinked states of both the cell cytoskeleton and ECM, the cell-cell adhesion and cell-ECM adhesion, which all resist deformation. However, these structural components are also dynamically assembled and disassembled enabling stress relaxation in the system. Their turnover sets the different timescales of the tissue stress relaxation (Wyatt et al. 2016). The origin of stress relaxation timescales has been recently investigated in Drosophila embryos but it remains to be studied in vertebrates embryos (Clement et al. 2017).
Distinct cell- and tissue-level mechanical properties are often regulated by the same molecular players or are maintained through different processes that are nevertheless tightly entangled. It is, therefore, difficult to impair individual tissue material properties without affecting, at least partially, other aspects of the mechanical microenvironment. For instance, studying the impact of cell motility alone on tissue viscosity is challenging as modifying cell motility also impacts the cell cytoskeleton, thus affecting cell cortex elasticity and viscosity. Nevertheless, there are a few examples of mechanical properties that could be attributed to specific biological determinants:
Actomyosin contractility controls tissue stiffness during Xenopus gastrulation and ECM was shown to have a limited contribution (Zhou et al. 2009).
Although it has been shown in vitro that intermediate filaments have an important role in the tissue integrity by controlling its maximum strain (Harris et al. 2012), their role in vivo has not been investigated yet.
The control of intercellular junction tension and tissue surface tension has been extensively investigated in Drosophila and cellular aggregates. Junction tension is finely set by a balance between cell-cell adhesion and cortical contractility. The relative contributions of contractility (2/3) and adhesion (1/3) were measured in cellular aggregates (Krieg et al. 2008, Stirbat et al. 2013).
Tissue fluidity was shown to be controlled, in part, by adhesion dependent intercellular space and contractility-driven cell-cell contact fluctuations along the zebrafish AP axis (Mongera et al. 2018). During avian gastrulation, the epiblast undergoes an active fluidification which depends on cell-division mediated tissue rearrangement (Firmino et al. 2016). Localized fluidification of the zebrafish blastoderm, depending on reduction of cell-cell adhesion, has also been recently reported (Petridou et al. 2018).
Measurements of the embryonic solid properties
In order to measure tissue stiffness, strategies must be devised to measure the stress-deformation relation. Several kinds of approaches are used to impose a controlled stress or deformation. These measurements highlighted the very soft properties of embryonic tissues (typically from 20 to 400 Pa) compared to adult tissues (a few kPa).
Whole tissue explants have been compressed by a nanonewton force apparatus to carry out stress relaxation assay (Davidson & Keller 2007). In this way, the stiffness of Xenopus dorsal explants has been extensively measured (Zhou et al. 2009, Zhou et al. 2010). A slightly different technique of pushing on a tissue can be devised to perform this mechanical assay in vivo. The embryo can be locally compressed by indentation. Indentation has been performed using rod shaped indenters (Filas et al. 2015, Xu et al. 2010, Zamir & Taber 2004) and more recently by atomic force microscopy (AFM). AFM is largely used to measure mechanical properties at the cell scale. However, by means of a colloidal tip which allows to probe larger areas, tissue scale responses can also be analyzed. For example, head mesoderm stiffening has been recently measured in frog and found necessary for proper neural crest migration (Barriga et al. 2018). It has been shown that tissues might be under tension which can impact the stiffness measurement. In addition, non-linearities during indentation, dependency on the rate of stress application and the fact that tissues are not purely elastic make the force-displacement curve analysis more complex (Zamir & Taber 2004).
In addition to pushing techniques, pulling strategies have been developed. Uniaxial stretch using a force sensor have been used on the whole chicken embryo (Agero et al. 2010). Xenopus large explants have also been stretched (Agero et al. 2010, Moore et al. 1995, Shook et al. 2018) and specific tissues such as the chicken gut have been probed in this way (Chevalier et al. 2016, Nerurkar et al. 2017). Alternatively, embryonic tissues can be pulled at finer scale by aspiration. Xenopus gastrula have been aspirated in a microfluidic channel (von Dassow et al. 2010). Chicken heart and zebrafish neural explants were aspirated through micropipettes (Majkut et al. 2013, Porazinski et al. 2015). A recent technique allowed to measure in situ tissue mechanical properties by injecting ferrofluid droplets in zebrafish embryo to apply controlled stress. The temporal evolution could be fitted by a two-timescales model which provided a measurement of the tissue stiffness along the axis (Serwane et al. 2017). A gradient in tissue stiffness has been recently reported in the mouse limb bud combining the injection of magnetic beads and a 3D magnetic device producing a uniform magnetic field (Zhu et al. 2018).
In theory, these techniques should allow to fit the temporal evolution of stress relaxation tests or creep and extract the spring constant (see Box 1). In practice, these temporal responses are often poorly fitted and, in most of these studies, the deformation is defined by the displacement at a fixed time when most of the deformation was considered to be achieved. While this procedure enables comparison of apparent stiffness within the same system, it makes the comparison between different systems delicate.
A different approach to investigate tissue stiffness was devised using a contactless method called Brillouin microscopy. This method is based on Brillouin light scattering, which is the consequence of interaction of light with spontaneous acoustic waves traveling through the sample. Light wavelength undergoes a slight shift which depends on the tissue stiffness. Therefore, by precisely measuring the wavelength shift, relative variation of stiffness can be measured across the sample. After development of the technique on cells (Scarcelli et al. 2015), recent measurements have been carried out on murine embryos (Raghunathan et al. 2017). While this technique offers in principle some advantages compared to more invasive techniques, more work is necessary to clarify the structures whose mechanical responses can be analyzed.
Measurements of the embryonic fluid properties
Little attention has been dedicated to the measurement of tissue fluidity. One of the reasons is that most of the mechanical measurements have been carried out on short timescales. Another reason is that, as mentioned above, the temporal evolution of deformation has seldom been carefully fitted by rheological models. For instance, while pipette aspiration has been shown to be a powerful technique to measure long-time viscosity in vitro (Guevorkian et al. 2010), few examples of similar creep tests have been carried out in embryonic tissues. Using ferrofluid droplets actuated by a uniform and constant magnetic field an increase in viscosity has been reported (Serwane et al. 2017).
As embryonic tissues can be considered as fluids at long timescales, they are often characterized by a surface tension, the property of liquids which tends to minimize their surface area/volume ratio. Surface tension is a force by unit of length and originates from the balance of cell contractility and cell-cell adhesion (for a theoretical formalism of surface tension in biological tissues, see (Krieg et al. 2008)). Since D’Arcy Thompson, it has been hypothesized that surface tension could have an important morphogenetic role, particularly during cell sorting (for an historical review see (Heisenberg 2017)). However, recent in vivo investigations mitigated the role of surface tension in cell sorting (at least during zebrafish gastrulation) and highlighted the importance of osmolarity in maintaining proper surface tension of the nascent germ layers (Krens et al. 2017).
Tissue surface tension has been first measured by parallel plate compression of cellular aggregates (Foty et al. 1996) and later by pipette aspiration (Guevorkian et al. 2010). Surface tension measurement can be used to calculate tissue viscosity with complementary experiments of rounding or fusion of tissues (Gordon et al. 1972). Rounding and fusion experiments yield a surface tension/viscosity ratio. In this way tension measurement by parallel compression combined with fusion experiments yielded estimates of viscosity in zebrafish explants (Schötz et al. 2008).
Tissue fluidity is also spatiotemporally controlled during 3D in vivo morphogenesis. In zebrafish, proper posterior elongation requires both fluid behavior at the growing body end and solid behavior during subsequent differentiation of the tissue. This fluid-to-solid transition is paralleled by an increase in the yield stress, which is the minimal stress that needs to be applied to initiate tissue flow (Mongera et al. 2018).
Mechanical properties and force production have been a growing subject of interest for developmental biologists in the past years. A wide number of approaches, often previously developed on in vitro systems, are actively being adapted to embryonic tissues. Despite important efforts, embryos often exhibit noisier measurements. Such a large variability and the novelty of many techniques do not provide us with a clear picture of embryo mechanics yet. Furthermore, embryos are complex and active systems that might exhibit different responses depending on the magnitude and the timescale of the assay. For this reason, different techniques might lead to different results. As a result, in order to offer a more robust description of embryo mechanics, it appears critical to the authors that mechanical measurements be cross-validated using multiple techniques on the same system in the future.
Figure 1 |.
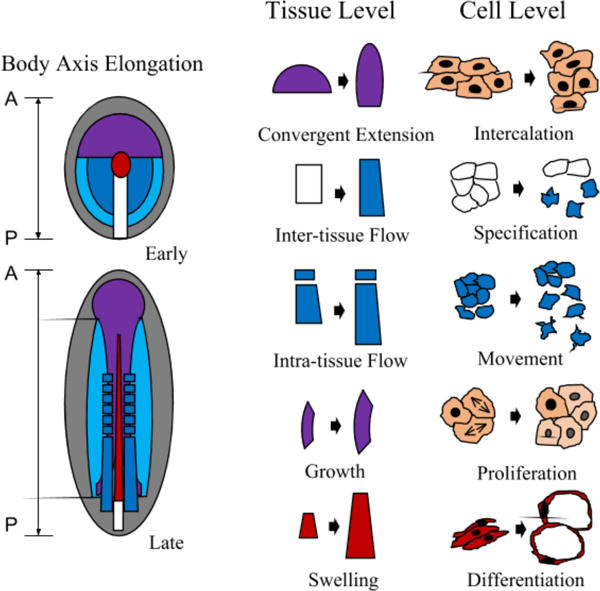
Summary of body axis elongation and driving mechanisms. Left: Generalized vertebrate embryo undergoing body axis formation. A-P indicates Anterior to Posterior. Colored regions are fate maps of different tissues composing the body axis (for simplicity, not all tissues are included). Red, axial mesoderm/notochord; purple, neural plate/neural tube; blue, paraxial mesoderm/somites; light blue, lateral mesoderm; white box, blastopore/primitive streak; grey, other tissues. Right: different mechanisms that drive elongation. Note that the mechanisms are prominent in but not exclusive to the illustrated tissues selected. The mechanisms listed here are based on a range of active cell behaviors. Passive and non-cellular mechanisms are not included (see text for further discussions).
Figure 2 |.
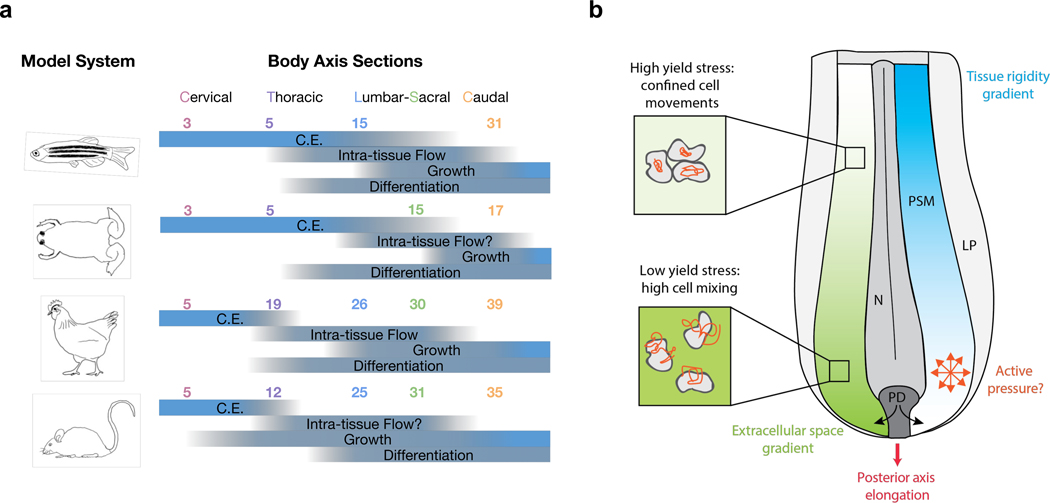
Axis elongation mechanisms across vertebrate species. a. Stage-dependent prominence of elongation mechanisms. The graph is composed based on our survey of literature that study these mechanisms in the corresponding stages and model systems. The listed processes are likely present throughout these stages in different tissues, the blue bars only indicate their relative importance in body axis elongation. The numbers indicate somite numbers that mark the approximate boundaries between body axis sections in different model systems. A “?” indicates insufficient information from our literature coverage. C.E. convergence and extension. b. Posterior axis elongation similarities in the chicken and the zebrafish. In both species, after earliest stages, elongation is associated with coupled anteroposterior gradients of extracellular space and cell motility. The high caudal motility is likely to exert an active pressure propelling the axis. The tissue rigidification towards the anterior end plays a role in maintaining the axis extended. The process is sustained by a continued injection of new cells from the progenitor domain (PD). N: neural tissue, PSM: presomitic mesoderm, LP: lateral plate.
Table 1 |.
Measured mechanical properties in vertebrates’ embryonic tissues during AP axis formation
| Technique | Measured tissue | Measured parameter | Value | Reference |
|---|---|---|---|---|
| Uniaxial compression | Xenopus dorsal explant | Apparent Young modulus after 180s | 20–80 Pa | (Zhou et al. 2009) |
| Indentation | Chicken brain | Shear modulus | 200 Pa | (Xu et al. 2010) |
| Xenopus head mesoderm | Apparent bulk modulus | 70–130 Pa | (Barriga et al. 2018) | |
| Uniaxial stretch | Xenopus marginal zone | Young modulus | 15–20 Pa | (Moore et al. 1995; Shook et al. 2018) |
| Chicken midline structures | Young modulus | 1.3 kPa | (Agero et al. 2010) | |
| Chicken gut | Young modulus | 1–20 kPa | (Chevalier et al. 2016; Nerurkar et al. 2017) | |
| Aspiration | Chicken heart (HH12) | Young modulus | 300 Pa | (Majkut et al. 2013) |
| Xenopus gastrula dorsal region | Young modulus | 30 Pa | (von Dassow et al. 2010) | |
| Zebrafish blastoderm | Viscosity | 500–1000 Pa.s | (Petridou et al. 2018) | |
| Ferrofluid droplets | Zebrafish posterior zone and PSM | Young modulus | 300 – 600 Pa | (Serwane et al. 2016) |
| Short-time and long-time viscosities | 0.5 – 5 kPa.s |
Table 2 |.
measured stress production in vertebrates’ embryonic tissues during AP axis formation
| Technique | Measured tissue | Measured parameter | Value | Reference |
|---|---|---|---|---|
| Oil drop | Mouse dental mesenchyme | Anisotropic component of normal stress | 1 kPa | (Campàs et al. 2014) |
| Gel drop | Zebrafish early blastula | Normal and shear compressive stress change | 50–800 Pa | (Mohagheghian et al. 2018) |
| Tissue embedded in gel | Xenopus dorsal explant | Extension stress | 5 Pa | (Zhou et al. 2015) |
| Cantilever | Xenopus marginal zone | Traction maximum force | 1–4 μN | (Shook et al. 2018) |
References
- Adams DS, Keller R, Koehl MA. 1990. The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development 110: 115–30 [DOI] [PubMed] [Google Scholar]
- Agero U, Glazier JA, Hosek M. 2010. Bulk elastic properties of chicken embryos during somitogenesis. Biomed Eng Online 9: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulehla A, Pourquié O. 2010. Signaling gradients during paraxial mesoderm development. Cold Spring Harb Perspect Biol 2: a000869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw WY, Heck BW, Joyce B, Devenport D. 2016. Transient Tissue-Scale Deformation Coordinates Alignment of Planar Cell Polarity Junctions in the Mammalian Skin. Curr. Biol 26: 2090–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balland M, Desprat N, Icard D, Féréol S, Asnacios A, et al. 2006. Power laws in microrheology experiments on living cells: Comparative analysis and modeling. Phys Rev E Stat Nonlin Soft Matter Phys 74: 021911 [DOI] [PubMed] [Google Scholar]
- Barriga EH, Franze K, Charras G, Mayor R. 2018. Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo. Nature 554: 523–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beddington RS. 1994. Induction of a second neural axis by the mouse node. Development 120: 613–20 [DOI] [PubMed] [Google Scholar]
- Beemster GT, Baskin TI. 1998. Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana. Plant Physiol. 116: 1515–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrndt M, Salbreux G, Campinho P, Hauschild R, Oswald F, et al. 2012. Forces driving epithelial spreading in zebrafish gastrulation. Science 338: 257–60 [DOI] [PubMed] [Google Scholar]
- Bénazéraf B. 2018. Dynamics and mechanisms of posterior axis elongation in the vertebrate embryo. Cellular and Molecular Life Sciences: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénazéraf B, Beaupeux M, Tchernookov M, Wallingford A, Salisbury T, et al. 2017. Multi-scale quantification of tissue behavior during amniote embryo axis elongation. Development 144: 4462–72 [DOI] [PubMed] [Google Scholar]
- Bénazéraf B, Francois P, Baker RE, Denans N, Little CD, Pourquié O. 2010. A random cell motility gradient downstream of FGF controls elongation of an amniote embryo. Nature 466: 248–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénazéraf B, Pourquié O. 2013. Formation and segmentation of the vertebrate body axis. Annu. Rev. Cell Dev. Biol 29: 1–26 [DOI] [PubMed] [Google Scholar]
- Bertet C, Sulak L, Lecuit T. 2004. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 429: 667–71 [DOI] [PubMed] [Google Scholar]
- Brodland GW, Veldhuis JH, Kim S, Perrone M, Mashburn D, Hutson MS. 2014. CellFIT: a cellular force-inference toolkit using curvilinear cell boundaries. PloS one 9: e99116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MT, Wallingford JB. 2017. Planar cell polarity in development and disease. Nat. Rev. Mol. Cell Biol 18: 375–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Chen S-C, Prasad M, He L, Wang X, et al. 2014. Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell 157: 1146–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campàs O. 2016. A toolbox to explore the mechanics of living embryonic tissues. Semin Cell Dev Biol 55: 119–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campàs O, Mammoto T, Hasso S, Sperling RA, O’Connell D, et al. 2014. Quantifying cell-generated mechanical forces within living embryonic tissues. Nat Methods 11: 183–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campinho P, Behrndt M, Ranft J, Risler T, Minc N, Heisenberg CP. 2013. Tension-oriented cell divisions limit anisotropic tissue tension in epithelial spreading during zebrafish epiboly. Nat Cell Biol 15: 1405–14 [DOI] [PubMed] [Google Scholar]
- Čapek D, Smutny M, Tichy A-M, Morri M, Janovjak H, Heisenberg C-P. 2019. Light-activated Frizzled7 reveals a permissive role of non-canonical Wnt signaling in mesendoderm cell migration. eLife Sciences 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier NR, Gazquez E, Bidault L, Guilbert T, Vias C, et al. 2016. How Tissue Mechanical Properties Affect Enteric Neural Crest Cell Migration. Sci Rep 6: 20927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C-W, Sokol SY. 2016. Wnt proteins can direct planar cell polarity in vertebrate ectoderm. eLife Sciences 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuai M, Weijer CJ. 2008. The mechanisms underlying primitive streak formation in the chick embryo. Curr. Top. Dev. Biol 81: 135–56 [DOI] [PubMed] [Google Scholar]
- Ciruna B, Rossant J. 2001. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev. Cell 1: 37–49 [DOI] [PubMed] [Google Scholar]
- Clement R, Dehapiot B, Collinet C, Lecuit T, Lenne PF. 2017. Viscoelastic Dissipation Stabilizes Cell Shape Changes during Tissue Morphogenesis. Curr Biol 27: 3132–42 e4 [DOI] [PubMed] [Google Scholar]
- Cui C, Yang X, Chuai M, Glazier JA, Weijer CJ. 2005. Analysis of tissue flow patterns during primitive streak formation in the chick embryo. Dev. Biol 284: 37–47 [DOI] [PubMed] [Google Scholar]
- Das D, Chatti V, Emonet T, Holley SA. 2017. Patterned Disordered Cell Motion Ensures Vertebral Column Symmetry. Dev. Cell 42: 170–80.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson L, Keller R. 2007. Measuring mechanical properties of embryos and embryonic tissues. Methods Cell Biol. 83: 425–39 [DOI] [PubMed] [Google Scholar]
- Davidson LA, Dzamba BD, Keller R, Desimone DW. 2008. Live imaging of cell protrusive activity, and extracellular matrix assembly and remodeling during morphogenesis in the frog, Xenopus laevis. Dev. Dyn 237: 2684–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco P, Merello E, Piatelli G, Cama A, Kibar Z, Capra V. 2014. Planar cell polarity gene mutations contribute to the etiology of human neural tube defects in our population. Birth Defects Res. Part A Clin. Mol. Teratol 100: 633–41 [DOI] [PubMed] [Google Scholar]
- Delfini M-C, Dubrulle J, Malapert P, Chal J, Pourquié O. 2005. Control of the segmentation process by graded MAPK/ERK activation in the chick embryo. Proc. Natl. Acad. Sci. U.S.A 102: 11343–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diz-Muñoz A, Krieg M, Bergert M, Ibarlucea-Benitez I, Muller DJ, et al. 2010. Control of directed cell migration in vivo by membrane-to-cortex attachment. PLoS Biol. 8: e1000544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diz-Muñoz A, Romanczuk P, Yu W, Bergert M, Ivanovitch K, et al. 2016. Steering cell migration by alternating blebs and actin-rich protrusions. BMC Biol. 14: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohn MR, Mundell NA, Sawyer LM, Dunlap JA, Jessen JR. 2013. Planar cell polarity proteins differentially regulate extracellular matrix organization and assembly during zebrafish gastrulation. Dev. Biol 383: 39–51 [DOI] [PubMed] [Google Scholar]
- Dray N, Lawton A, Nandi A, Jülich D, Emonet T, Holley SA. 2013. Cell-fibronectin interactions propel vertebrate trunk elongation via tissue mechanics. Curr. Biol 23: 1335–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrulle J, McGrew MJ, Pourquie O. 2001. FGF signaling controls somite boundary position and regulates segmentation clock control of spatiotemporal Hox gene activation. Cell 106: 219–32 [DOI] [PubMed] [Google Scholar]
- Dubrulle J, Pourquie O. 2004a. Coupling segmentation to axis formation. Development 131: 5783–93 [DOI] [PubMed] [Google Scholar]
- Dubrulle J, Pourquie O. 2004b. fgf8 mRNA decay establishes a gradient that couples axial elongation to patterning in the vertebrate embryo. Nature 427: 419–22 [DOI] [PubMed] [Google Scholar]
- Ellen Cretekos CJ, Helde KA. 1995. Cell Mixing During Early Epiboly in the Zebrafish Embryo. Developmental Genetics: 1–10 [DOI] [PubMed] [Google Scholar]
- Ellis K, Bagwell J, Bagnat M. 2013. Notochord vacuoles are lysosome-related organelles that function in axis and spine morphogenesis. J. Cell Biol 200: 667–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elul T, Koehl MA, Keller R. 1997. Cellular Mechanism Underlying Neural Convergent Extension in Xenopus laevis Embryos. 1–16 [DOI] [PubMed] [Google Scholar]
- Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, Fredberg JJ. 2001. Scaling the microrheology of living cells. Phys. Rev. Lett 87: 148102 [DOI] [PubMed] [Google Scholar]
- Filas BA, Xu G, Taber LA. 2015. Probing regional mechanical properties of embryonic tissue using microindentation and optical coherence tomography. Methods Mol Biol 1189: 3–16 [DOI] [PubMed] [Google Scholar]
- Firmino J, Rocancourt D, Saadaoui M, Moreau C, Gros J. 2016. Cell Division Drives Epithelial Cell Rearrangements during Gastrulation in Chick. Dev. Cell 36: 249–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgacs G, Foty RA, Shafrir Y, Steinberg MS. 1998. Viscoelastic properties of living embryonic tissues: a quantitative study. Biophys. J 74: 2227–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foty RA, Pfleger CM, Forgacs G, Steinberg MS. 1996. Surface tensions of embryonic tissues predict their mutual envelopment behavior. Development 122: 1611–20 [DOI] [PubMed] [Google Scholar]
- Glickman NS, Kimmel CB, Jones MA, Adams RJ. 2003. Shaping the zebrafish notochord. Development 130: 873–87 [DOI] [PubMed] [Google Scholar]
- Gomez C, Ozbudak EM, Wunderlich J, Baumann D, Lewis J, Pourquié O. 2008. Control of segment number in vertebrate embryos. Nature 454: 335–39 [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Strutt D. 2011. Principles of planar polarity in animal development. Development 138: 1877–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon R, Goel NS, Steinberg MS, Wiseman LL. 1972. A rheological mechanism sufficient to explain the kinetics of cell sorting. J Theor Biol 37: 43–73 [DOI] [PubMed] [Google Scholar]
- Goto H, Kimmey SC, Row RH, Matus DQ, Martin BL. 2017. FGF and canonical Wnt signaling cooperate to induce paraxial mesoderm from tailbud neuromesodermal progenitors through regulation of a two-step epithelial to mesenchymal transition. Development 144: 1412–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T, Davidson L, Asashima M, Keller R. 2005. Planar cell polarity genes regulate polarized extracellular matrix deposition during frog gastrulation. Current Biology 15: 787–93 [DOI] [PubMed] [Google Scholar]
- Guevorkian K, Colbert M-J, Durth M, Dufour S, Brochard-Wyart F. 2010. Aspiration of biological viscoelastic drops. Phys. Rev. Lett 104: 218101 [DOI] [PubMed] [Google Scholar]
- Guillot C, Lecuit T. 2013. Mechanics of Epithelial Tissue Homeostasis and Morphogenesis. 1–6 [DOI] [PubMed] [Google Scholar]
- Hamant O, Traas J. 2010. The mechanics behind plant development. New Phytol 185: 369–85 [DOI] [PubMed] [Google Scholar]
- Hardy KM, Garriock RJ, Yatskievych TA, D’Agostino SL, Antin PB, Krieg PA. 2008. Non-canonical Wnt signaling through Wnt5a/b and a novel Wnt11 gene, Wnt11b, regulates cell migration during avian gastrulation. Dev. Biol 320: 391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AR, Peter L, Bellis J, Baum B, Kabla AJ, Charras GT. 2012. Characterizing the mechanics of cultured cell monolayers. Proc Natl Acad Sci U S A 109: 16449–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg CP. 2017. D’Arcy Thompson’s ‘on Growth and form’: From soap bubbles to tissue self-organization. Mech Dev 145: 32–37 [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Bellaiche Y. 2013. Forces in tissue morphogenesis and patterning. Cell 153: 948–62 [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Brand M, Jiang YJ, Warga RM, Beuchle D, et al. 1996. Genes involved in forebrain development in the zebrafish, Danio rerio. Development 123: 191–203 [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saúde L, Concha ML, et al. 2000. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 405: 76–81 [DOI] [PubMed] [Google Scholar]
- Huebner RJ, Wallingford JB. 2018. Coming to Consensus: A Unifying Model Emerges for Convergent Extension. Dev. Cell 46: 389–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine KD, Wieschaus E. 1994. Cell intercalation during Drosophila germband extension and its regulation by pair-rule segmentation genes. Development 120: 827–41 [DOI] [PubMed] [Google Scholar]
- Ishihara S, Sugimura K. 2012. Bayesian inference of force dynamics during morphogenesis. J. Theor. Biol 313: 201–11 [DOI] [PubMed] [Google Scholar]
- Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, et al. 2002. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat. Cell Biol 4: 610–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurand A. 1962. The Development of the Notochord in Chick Embryos. 1–28 [PubMed] [Google Scholar]
- Kane DA, McFarland KN, Warga RM. 2005. Mutations in half baked/E-cadherin block cell behaviors that are necessary for teleost epiboly. Development 132: 1105–16 [DOI] [PubMed] [Google Scholar]
- Kanki JP, Ho RK. 1997. The development of the posterior body in zebrafish. Development 124: 881–93 [DOI] [PubMed] [Google Scholar]
- Keller R, Cooper MS, Danilchik M, Tibbetts P, Wilson PA. 1989. Cell intercalation during notochord development in Xenopus laevis. Journal of Experimental Zoology 251: 134–54 [DOI] [PubMed] [Google Scholar]
- Keller R, Danilchik M. 1988. Regional expression, pattern and timing of convergence and extension during gastrulation of Xenopus laevis. Development 103: 193–209 [DOI] [PubMed] [Google Scholar]
- Keller R, Davidson L, Edlund A, Elul T, Ezin M, et al. 2000. Mechanisms of convergence and extension by cell intercalation. Philosophical Transactions of the Royal Society B: Biological Sciences 355: 897–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R, Davidson LA, Shook DR. 2003. How we are shaped: the biomechanics of gastrulation. Differentiation 71: 171–205 [DOI] [PubMed] [Google Scholar]
- Keller R, Shih J, Domingo C. 1992a. The patterning and functioning of protrusive activity during convergence and extension of the Xenopus organiser. Dev. Suppl: 81–91 [PubMed] [Google Scholar]
- Keller R, Shih J, Sater A. 1992b. The cellular basis of the convergence and extension of the Xenopus neural plate. Developmental Dynamics 193: 199–217 [DOI] [PubMed] [Google Scholar]
- Keller R, Tibbetts P. 1989. Mediolateral cell intercalation in the dorsal, axial mesoderm of Xenopus laevis. Dev. Biol 131: 539–49 [DOI] [PubMed] [Google Scholar]
- Keller RE. 1984. The Cellular Basis of Gastrulation in Xenopus laevis: Active, Postinvolution Convergence and Extension by Mediolateral Interdigitation. Integr Comp Biol 24: 589–603 [Google Scholar]
- Keller RE, Danilchik M, Gimlich R, Shih J. 1985. The function and mechanism of convergent extension during gastrulation of Xenopus laevis. J Embryol Exp Morphol 89 Suppl: 185–209 [PubMed] [Google Scholar]
- Kim HY, Davidson LA. 2011. Punctuated actin contractions during convergent extension and their permissive regulation by the non-canonical Wnt-signaling pathway. J. Cell. Sci 124: 635–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D. 2016. Tales of Tails (and Trunks): Forming the Posterior Body in Vertebrate Embryos. Curr Top Dev Biol 116: 517–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft B, Berger CD, Wallkamm V, Steinbeisser H, Wedlich D. 2012. Wnt-11 and Fz7 reduce cell adhesion in convergent extension by sequestration of PAPC and C-cadherin. J. Cell Biol 198: 695–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krens SFG, Veldhuis JH, Barone V, Čapek D, Maître J-L, et al. 2017. Interstitial fluid osmolarity modulates the action of differential tissue surface tension in progenitor cell segregation during gastrulation. Development 144: 1798–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg M, Arboleda-Estudillo Y, Puech PH, Käfer J, Graner F, et al. 2008. Tensile forces govern germ-layer organization in zebrafish. Nat. Cell Biol 10: 429–36 [DOI] [PubMed] [Google Scholar]
- Krieg M, Dunn AR, Goodman MB. 2014. Mechanical control of the sense of touch by beta-spectrin. Nat Cell Biol 16: 224–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton AK, Nandi A, Stulberg MJ, Dray N, Sneddon MW, et al. 2013. Regulated tissue fluidity steers zebrafish body elongation. Development 140: 573–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecaudey V, Gilmour D. 2006. Organizing moving groups during morphogenesis. Curr Opin Cell Biol 18: 102–7 [DOI] [PubMed] [Google Scholar]
- Lee W, Kalashnikov N, Mok S, Halaoui R, Kuzmin E, et al. 2019. Dispersible hydrogel force sensors reveal patterns of solid mechanical stress in multicellular spheroid cultures. Nat Commun 10: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu O, David R, Ninomiya H, Winklbauer R. 2011. Large-scale mechanical properties of Xenopus embryonic epithelium. Proc. Natl. Acad. Sci. U.S.A 108: 4000–05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitre JL, Berthoumieux H, Krens SF, Salbreux G, Julicher F, et al. 2012. Adhesion functions in cell sorting by mechanically coupling the cortices of adhering cells. Science 338: 253–6 [DOI] [PubMed] [Google Scholar]
- Majkut S, Idema T, Swift J, Krieger C, Liu A, Discher DE. 2013. Heart-specific stiffening in early embryos parallels matrix and myosin expression to optimize beating. Curr. Biol 23: 2434–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow F, Gonzalez EM, Yin C, Rojo C, Solnica-Krezel L. 2004. No tail co-operates with non-canonical Wnt signaling to regulate posterior body morphogenesis in zebrafish. Development 131: 203–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BL, Kimelman D. 2012. Canonical Wnt signaling dynamically controls multiple stem cell fate decisions during vertebrate body formation. Dev Cell 22: 223–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen P, Holley SA. 2015. The tissue mechanics of vertebrate body elongation and segmentation. Curr. Opin. Genet. Dev 32: 106–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell B, Jacobs R, Li J, Chien S, Kintner C. 2007. A positive feedback mechanism governs the polarity and motion of motile cilia. Nature 447: 97–101 [DOI] [PubMed] [Google Scholar]
- Mohagheghian E, Luo J, Chen J, Chaudhary G, Chen J, et al. 2018. Quantifying compressive forces between living cell layers and within tissues using elastic round microgels. Nat Commun 9: 1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongera A, Rowghanian P, Gustafson HJ, Shelton E, Kealhofer DA, et al. 2018. A fluid-to-solid jamming transition underlies vertebrate body axis elongation. Nature 45: 1–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero J-A, Carvalho L, Wilsch-Bräuninger M, Kilian B, Mustafa C, Heisenberg C-P. 2005. Shield formation at the onset of zebrafish gastrulation. Development 132: 1187–98 [DOI] [PubMed] [Google Scholar]
- Mookerjee S. 1953. Experimental dissociation of cells from chick embryos. Nature 171: 796. [DOI] [PubMed] [Google Scholar]
- Moore SW, Keller RE, Koehl MA. 1995. The dorsal involuting marginal zone stiffens anisotropically during its convergent extension in the gastrula of Xenopus laevis. Development 121: 3131–40 [DOI] [PubMed] [Google Scholar]
- Naiche LA, Holder N, Lewandoski M. 2011. FGF4 and FGF8 comprise the wavefront activity that controls somitogenesis. Proc Natl Acad Sci U S A 108: 4018–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Hikida M, Nakano T, Ito S, Hamano T, Kinoshita S. 1993. Characterization of water retentive properties of hyaluronan. Cornea 12: 433–36 [DOI] [PubMed] [Google Scholar]
- Nerurkar NL, Mahadevan L, Tabin CJ. 2017. BMP signaling controls buckling forces to modulate looping morphogenesis of the gut. PNAS 114: 201700307–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Honda H, Takeichi M. 2012. Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell 149: 1084–97 [DOI] [PubMed] [Google Scholar]
- O’Farrell PH. 2015. Growing an Embryo from a Single Cell: A Hurdle in Animal Life. Cold Spring Harb Perspect Biol 7: a019042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Farrell PH, Edgar BA, Lakich D, Lehner CF. 1989. Directing cell division during development. 1–6 [DOI] [PubMed] [Google Scholar]
- Olivier N, Luengo-Oroz MA, Duloquin L, Faure E, Savy T, et al. 2010. Cell lineage reconstruction of early zebrafish embryos using label-free nonlinear microscopy. Science 329: 967–71 [DOI] [PubMed] [Google Scholar]
- Peng Y, Axelrod JD. 2012. Asymmetric protein localization in planar cell polarity: mechanisms, puzzles, and challenges. Curr. Top. Dev. Biol 101: 33–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petridou NI, Grigolon S, Salbreux G, Hannezo E, Heisenberg C-P. 2018. Fluidization-mediated tissue spreading by mitotic cell rounding and non-canonical Wnt signalling. Nat. Cell Biol 153: 948. [DOI] [PubMed] [Google Scholar]
- Phillips HM, Steinberg MS. 1978. Embryonic tissues as elasticoviscous liquids. I. Rapid and slow shape changes in centrifuged cell aggregates. J. Cell. Sci 30: 1–20 [DOI] [PubMed] [Google Scholar]
- Porazinski S, Wang H, Asaoka Y, Behrndt M, Miyamoto T, et al. 2015. YAP is essential for tissue tension to ensure vertebrate 3D body shape. Nature 521: 217–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunathan R, Zhang J, Wu C, Rippy J, Singh M, et al. 2017. Evaluating biomechanical properties of murine embryos using Brillouin microscopy and optical coherence tomography. J Biomed Opt 22: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch GJ, Hammerschmidt M, Blader P, Schauerte HE, Strähle U, et al. 1997. WNT5 Is Required for Tail Formation in the Zebrafish Embryo. Cold Spring Harb Symp Quant Biol 62: 227–34 [PubMed] [Google Scholar]
- Regev I, Guevorkian K, Pourquié O, Mahadevan L. 2017. Motility-gradient induced elongation of the vertebrate embryo. bioRxiv: 187443 [Google Scholar]
- Rifes P, Carvalho L, Lopes C, Andrade RP, Rodrigues G, et al. 2007. Redefining the role of ectoderm in somitogenesis: a player in the formation of the fibronectin matrix of presomitic mesoderm. Development 134: 3155–65 [DOI] [PubMed] [Google Scholar]
- Riley BB, Sweet EM, Heck R, Evans A, McFarland KN, et al. 2010. Characterization of harpy/Rca1/emi1 mutants: patterning in the absence of cell division. Dev. Dyn 239: 828–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J, Ciruna B, Partanen J. 1997. FGF Signaling in Mouse Gastrulation and Anteroposterior Patterning. Cold Spring Harb Symp Quant Biol 62: 127–33 [PubMed] [Google Scholar]
- Roszko I, Sawada A, Solnica-Krezel L. 2009. Regulation of convergence and extension movements during vertebrate gastrulation by the Wnt/PCP pathway. Semin. Cell Dev. Biol 20: 986–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozario T, Desimone DW. 2009. The physical state of fibronectin matrix differentially regulates morphogenetic movements in vivo. Dev. Biol 327: 386–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozbicki E, Chuai M, Karjalainen AI, Song F, Sang HM, et al. 2015. Myosin-II-mediated cell shape changes and cell intercalation contribute to primitive streak formation. Nat. Cell Biol 17: 397–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadaoui M, Corson F, Rocancourt D, Roussel J, Gros J. 2018. A tensile ring drives tissue flows to shape the gastrulating amniote embryo. bioRxiv: 412767 [DOI] [PubMed] [Google Scholar]
- Scarcelli G, Polacheck WJ, Nia HT, Patel K, Grodzinsky AJ, et al. 2015. Noncontact three-dimensional mapping of intracellular hydromechanical properties by Brillouin microscopy. Nat Methods 12: 1132–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schötz E-M, Burdine RD, Jülicher F, Steinberg MS, Heisenberg C-P, Foty RA. 2008. Quantitative differences in tissue surface tension influence zebrafish germ layer positioning. HFSP J 2: 42–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepich DS, Calmelet C, Kiskowski M, Krezel LS. 2005. Initiation of convergence and extension movements of lateral mesoderm during zebrafish gastrulation. Developmental Dynamics 234: 279–92 [DOI] [PubMed] [Google Scholar]
- Sepich DS, Myers DC, Short R, Topczewski J, Marlow F, Krezel LS. 2000. Role of the zebrafish trilobite locus in gastrulation movements of convergence and extension. genesis 27: 159–73 [DOI] [PubMed] [Google Scholar]
- Serwane F, Mongera A, Rowghanian P, Kealhofer DA, Lucio AA, et al. 2017. In vivo quantification of spatially varying mechanical properties in developing tissues. Nat Methods 14: 181–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih J, Keller R. 1992a. Cell motility driving mediolateral intercalation in explants of Xenopus laevis. Development 116: 901–14 [DOI] [PubMed] [Google Scholar]
- Shih J, Keller R. 1992b. Patterns of cell motility in the organizer and dorsal mesoderm of Xenopus laevis. Development 116: 915–30 [DOI] [PubMed] [Google Scholar]
- Shindo A. 2017. Models of convergent extension during morphogenesis. WIREs Dev Biol 7: e293–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo A, Wallingford JB. 2014. PCP and Septins Compartmentalize Cortical Actomyosin to Direct Collective Cell Movement. Science 343: 649–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook DR, Kasprowicz EM, Davidson LA, Keller R. 2018. Large, long range tensile forces drive convergence during Xenopus blastopore closure and body axis elongation. eLife Sciences 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoglund P, Rolo A, Chen X, Gumbiner BM, Keller R. 2008. Convergence and extension at gastrulation require a myosin IIB-dependent cortical actin network. Development 135: 2435–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smutny M, Ákos Z, Grigolon S, Shamipour S, Ruprecht V, et al. 2017. Friction forces position the neural anlage. Nat. Cell Biol 19: 306–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemple DL. 2005. Structure and function of the notochord: an essential organ for chordate development. Development 132: 2503–12 [DOI] [PubMed] [Google Scholar]
- Steventon B, Duarte F, Lagadec R, Mazan S, Nicolas J-F, Hirsinger E. 2016. Species-specific contribution of volumetric growth and tissue convergence to posterior body elongation in vertebrates. Development 143: 1732–41 [DOI] [PubMed] [Google Scholar]
- Stirbat TV, Mgharbel A, Bodennec S, Ferri K, Mertani HC, et al. 2013. Fine tuning of tissues’ viscosity and surface tension through contractility suggests a new role for α-catenin. PloS one 8: e52554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stooke-Vaughan GA, Campàs O. 2018. ScienceDirect Physical control of tissue morphogenesis across scales. Curr. Opin. Genet. Dev 51: 111–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura K, Lenne PF, Graner F. 2016. Measuring forces and stresses in situ in living tissues. Development 143: 186–96 [DOI] [PubMed] [Google Scholar]
- Sun Z, Amourda C, Shagirov M, Hara Y, Saunders TE, Toyama Y. 2017. Basolateral protrusion and apical contraction cooperatively drive Drosophila germ-band extension. Nat. Cell Biol 19: 375–83 [DOI] [PubMed] [Google Scholar]
- Tada M, Heisenberg C-P. 2012. Convergent extension: using collective cell migration and cell intercalation to shape embryos. Development 139: 3897–904 [DOI] [PubMed] [Google Scholar]
- Tahinci E, Symes K. 2003. Distinct functions of Rho and Rac are required for convergent extension during Xenopus gastrulation. Dev. Biol 259: 318–35 [DOI] [PubMed] [Google Scholar]
- Topczewski J, Sepich DS, Myers DC, Walker C, Amores A, et al. 2001. The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev. Cell 1: 251–64 [DOI] [PubMed] [Google Scholar]
- Ulrich F, Concha ML, Heid PJ, Voss E, Witzel S, et al. 2003. Slb/Wnt11 controls hypoblast cell migration and morphogenesis at the onset of zebrafish gastrulation. Development 130: 5375–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich F, Krieg M, Schotz EM, Link V, Castanon I, et al. 2005. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev Cell 9: 555–64 [DOI] [PubMed] [Google Scholar]
- Voiculescu O, Bertocchini F, Wolpert L, Keller RE, Stern CD. 2007. The amniote primitive streak is defined by epithelial cell intercalation before gastrulation. Nature 449: 1049–52 [DOI] [PubMed] [Google Scholar]
- von Dassow M, Strother JA, Davidson LA. 2010. Surprisingly simple mechanical behavior of a complex embryonic tissue. PloS one 5: e15359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington CH. 1940. Organisers & genes. [Google Scholar]
- Wallingford JB, Harland RM. 2001. Xenopus Dishevelled signaling regulates both neural and mesodermal convergent extension: parallel forces elongating the body axis. Development 128: 2581–92 [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Harland RM. 2002. Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development 129: 5815–25 [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Niswander LA, Shaw GM, Finnell RH. 2013. The continuing challenge of understanding, preventing, and treating neural tube defects. Science 339: 1222002–02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. 2000. Dishevelled controls cell polarity during Xenopus gastrulation. Nature 405: 81–5 [DOI] [PubMed] [Google Scholar]
- Warga RM, Kane DA. 2007. A role for N-cadherin in mesodermal morphogenesis during gastrulation. Dev Biol 310: 211–25 [DOI] [PubMed] [Google Scholar]
- Warga RM, Kimmel CB. 1990. Cell movements during epiboly and gastrulation in zebrafish. Development 108: 569–80 [DOI] [PubMed] [Google Scholar]
- Wei Y, Mikawa T. 2000. Formation of the avian primitive streak from spatially restricted blastoderm: evidence for polarized cell division in the elongating streak. Development 127: 87–96 [DOI] [PubMed] [Google Scholar]
- Williams M, Yen W, Lu X, Sutherland A. 2014. Distinct Apical and Basolateral Mechanisms Drive Planar Cell Polarity-Dependent Convergent Extension of the Mouse Neural Plate. Dev. Cell 29: 34–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt T, Baum B, Charras G. 2016. A question of time: tissue adaptation to mechanical forces. Curr Opin Cell Biol 38: 68–73 [DOI] [PubMed] [Google Scholar]
- Xiong F, Ma W, Bénazéraf B, Mahadevan L, Pourquie O. 2018. Mechanical Coupling Coordinates the Co-elongation of Axial and Paraxial Tissues in Avian Embryos. bioRxiv: 412866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Kemp PS, Hwu JA, Beagley AM, Bayly PV, Taber LA. 2010. Opening angles and material properties of the early embryonic chick brain. J Biomech Eng 132: 011005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C, Kiskowski M, Pouille P-A, Farge E, Solnica-Krezel L. 2008. Cooperation of polarized cell intercalations drives convergence and extension of presomitic mesoderm during zebrafish gastrulation. J. Cell Biol 180: 221–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir EA, Taber LA. 2004. On the effects of residual stress in microindentation tests of soft tissue structures. J Biomech Eng 126: 276–83 [DOI] [PubMed] [Google Scholar]
- Zhang L, Kendrick C, Julich D, Holley SA. 2008. Cell cycle progression is required for zebrafish somite morphogenesis but not segmentation clock function. Development 135: 2065–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Kim HY, Davidson LA. 2009. Actomyosin stiffens the vertebrate embryo during crucial stages of elongation and neural tube closure. Development 136: 677–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Kim HY, Wang JHC, Davidson LA. 2010. Macroscopic stiffening of embryonic tissues via microtubules, RhoGEF and the assembly of contractile bundles of actomyosin. Development 137: 2785–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Pal S, Maiti S, Davidson LA. 2015. Force production and mechanical accommodation during convergent extension. Development 142: 692–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Tao H, Samani M, Luo M, Wang X, et al. 2018. Three-dimensional tissue stiffness mapping in the mouse embryo supports durotaxis during early limb bud morphogenesis. bioRxiv: 412072 [Google Scholar]


