Summary
There is limited information on immunologic predictors of post-allogeneic stem cell transplant (alloSCT) outcome in chronic lymphocytic leukaemia (CLL), such as mixed T-cell chimaerism. We analysed 143 consecutive patients with relapsed/refractory CLL, transplanted from 2000–2012, to determine the prognostic relevance of mixed chimaerism post-alloSCT and the ability of post-transplant immunomodulation to treat relapse. 50% of patients at 3 months and 43% at 6 months post-alloSCT had mixed T-cell chimaerism; this was associated on 3 and 6 month landmark analysis with inferior progression-free survival (PFS) [HR 1.93, p=0.003 and HR 2.58, p<0.001] and survival [HR 1.66, p=0.05 and HR 2.17, p<0.001], independent of baseline patient characteristics and a lower rate of grade II-IV acute GHVD (16% vs 52%, p<0.001). 33 patients were treated with immunomodulation for relapse post-alloSCT (immunosuppression withdrawal, n=6, DLI, n=27); 17 achieved CR, which predicted superior PFS (53 vs 10 months, p<0.001) and survival (117 vs 30 months, p=0.006). Relapsed patients with mixed chimaerism had inferior response to immunomodulation; conversion to full donor chimaerism was highly correlated both with CR and with the development of severe acute GVHD, which was fatal in 3/8 patients. Novel therapeutic strategies are required for patients with mixed T-cell chimaerism post-alloSCT for CLL.
Keywords: Chronic Lymphocytic Leukaemia, Transplant, Mixed Chimaerism, Relapse, Survival
Introduction
Long-term progression-free survival (PFS) is achieved in approximately 36–43% of patients receiving reduced-intensity conditioned (RIC) alloSCT for CLL.(Brown, et al 2013, Dreger, et al 2010, Khouri, et al 2011, Sorror, et al 2008) AlloSCT has been demonstrated to overcome the adverse prognostic impact of high-risk genetic features, including del(17p)(Dreger, et al 2010, Schetelig, et al 2008) and TP53, NOTCH1 and SF3B1 mutations.(Dreger, et al 2013) There is evidence of a “graft-versus-leukaemia (GVL)” effect;(Ben-Bassat, et al 2007, Delgado, et al 2009, Nishida, et al 2009) higher relapse rates are seen after T-cell depleted transplants(Dreger, et al 2010) and donor lymphocyte infusions (DLI) can induce durable complete remissions.(Dreger, et al 2010, Khouri, et al 2011)
There is limited data on the importance of mixed T-cell chimaerism post-alloSCT in CLL. Mixed chimaerism results from a state of bi-directional tolerance and is associated with higher relapse rates in Hodgkin Lymphoma(Peggs, et al 2011, Peggs, et al 2004) and Follicular Lymphoma(Thomson, et al 2010), likely due to an ineffective GVL effect. Full donor chimaerism can be induced with DLI,(Peggs, et al 2011, Thomson, et al 2010) with a correspondingly lower-than-expected relapse risk. We have previously reported that mixed T-cell chimaerism (defined as ≤95% donor T-cells in peripheral blood) at 3 months is associated with higher relapse rate in a much smaller group of patients;(Khouri, et al 2007) several other small studies have also demonstrated that mixed T-cell chimaerism at earlier timepoints is associated with higher relapse risk.(Jones, et al 2010, Shaffer, et al 2013) Most of our patients received non-myeloablative conditioning and many received in-vivo T-cell depletion with alemtuzumab or anti-thymocyte globulin (ATG); both are associated with high-rates of mixed chimaerism. We sought to determine whether mixed T-cell chimaerism (hereafter referred to as mixed chimaerism) post-transplant is associated with higher relapse rates and inferior survival in a large cohort of CLL patients.
Methods
We retrospectively analysed data from 143 consecutive patients transplanted from 2000–2012. Patients with Richter Transformation or receiving 2nd or subsequent alloSCT were excluded. The study was approved by the MD Anderson Cancer Center Institutional Review Board.
Statistical analysis was performed using R 3.0.3, SPSS version 22 (IBM Corp, Armonk, New York) and GraphPad Prism 6 (La Jolla, California). Descriptive statistics were used to summarise baseline characteristics. Univariable analyses (UVA) for binary variables were compared using Student’s t test or Χ2 tests as appropriate and multivariable analysis (MVA) was performed using logistic regression.
Chimaerism analysis was routinely performed at 1, 3 and 6 months post-alloSCT on peripheral blood (with myeloid and T lineage cell sorting) and as clinically indicated, through analysis of DNA microsatellite polymorphisms by polymerase chain reaction.
To assess the effect of mixed chimaerism on outcome, landmark PFS and survival analyses were performed from the time of chimaerism assessment (3 months and 6 months post-transplant), using the Kaplan-Meier method. The effect of mixed chimaerism on PFS was assessed using the log-rank test. PFS was defined as time from transplant to death or clinical disease progression requiring additional therapy, including donor lymphocyte infusion (DLI). The use of DLI to treat mixed chimaerism or minimal residual disease, in the absence of clinical progression, was not considered to constitute an event in PFS analysis. MVA using Cox regression was performed with the addition of fixed pre-treatment patient and transplant characteristics as co-variates. Pre-treatment characteristics were included in the MVA model if p values in UVA were <0.1 in unadjusted analysis. Del(17p) was not included in MVA for the following reasons: FISH was only available during the later time period covered by this study; del(17p) has previously not been shown to be associated with survival outcomes after transplant.
Cumulative incidences of relapse and treatment-related mortality (TRM) were assessed according to the cumulative incidence method and treated as competing risks. Acute and chronic GVHD incidences were also assessed according to the cumulative incidence method, with death and relapse considered competing risks.
Stem cell source, preparative regimens and post-transplant immunosuppressive regimens
Characteristics of the transplants are shown in table 1. Most (n=128) patients received non-myeloablative conditioning, predominantly with fludarabine, cyclophosphamide and rituximab-based preparative regimens; 15 patients received more intensive, myeloablative conditioning. All-but-two patients received calcineurin inhibitor-based immunosuppression for GVHD prophylaxis;(Khouri, et al 2011) 86/143 patients received in vivo T-cell depletion with ATG (n=46) or alemtuzumab (n=40); this was stem cell source-specific: 81/90 (90%) patients receiving transplants from unrelated donors, mismatched-related donors or umbilical cord blood transplants received in vivo T-cell depletion.
Table 1.
Transplant characteristics.
| Characteristic | n (%) unless stated |
|---|---|
| Myeloablative conditioning: | 15 (10.5) |
| BEAM + rituximab. | 13 |
| Other | 2 |
| Non-myeloablative conditioning: | 128 (89.5) |
| FCR-based | 98 |
| FBR-based | 9 |
| Flu-Mel-based | 9 |
| TLI + rituximab + ATG | 6 |
| FC + 200cGy TBI | 3 |
| Other | 3 |
| In vivo T-cell depletion | 86 (60.1) |
| Alemtuzumab | 40 |
| ATG | 46 |
| Donor type | |
| Matched sibling | 53 (37.1) |
| Mismatched related donor | 7 (4.9) |
| Matched unrelated donor | 74 (51.7) |
| Mismatched unrelated donor/UCBT | 9 (6.3) |
| Stem cell source | |
| Peripheral blood progenitor cells | 109 (76) |
| Bone marrow | 25 (17.5) |
| Umbilical cord blood | 9 (6.5) |
| CD34+ progenitor dose (×106/kg), median (range), for patients with adult donor | 4.8 (0.8–19.9) |
| Median total nucleated cell dose (×107/kg) for UCBT | 2.0 (1.1–4.8) |
Abbreviations: UCBT, umbilical cord blood transplant. Abbreviations: ATG, anti-thymocyte globulin; BEAM, carmustine, etoposide, cytarabine and melphalan; FBR, fludarabine, bendamustine and rituximab; FC, fludarabine and cyclophosphamide; FCR, fludarabine, cyclophosphamide and rituximab; Flu-Mel, fludarabine and melphalan; TBI, total body irradiation. TLI, total lymphoid irradiation UCBT, umbilical cord blood transplantation;
Results
Pre-transplant patient characteristics are shown in table 2. Median follow-up in surviving patients was 66 months (range 37–155).
Table 2.
Baseline patient characteristics.
| Baseline Characteristic, N=144 | n (%) unless stated. |
|---|---|
| Male | 113 (79.0) |
| Age, median (range) | 57 (29–72) |
| Age <60 | 85 (59.4) |
| HCT-CI, median (range) | 2 (0–8). |
| HCT-CI ≥3 | 67 (46.9) |
| Karnofsky performance status, median (range) | 90 (60–100) |
| Fludarabine-refractory | 60 (42.0) |
| Complex karyotype, n=133 | 16 (11.2) |
| FISH, n=100 | |
| del(13q) | 16 (11.2) |
| Negative | 30 (21.0) |
| Trisomy 12 | 10 (7.0) |
| del(11q) | 14 (9.8) |
| del(17p) | 30 (21.0) |
| Median number of prior therapies (range) | 3 (1–10) |
| Refractory to most recent prior therapy | 65 (45.5) |
| Bulky lymphadenopathy (≥5cm), n=142 | 24 (16.8) |
| Baseline β2-microglobulin, g/L, median (range) | 3.1 (1.4–13.2) |
| Baseline β2-microglobulin ≥4.0g/L, n=137 | 32 (22.4) |
| Baseline hemoglobin (g/dl) | 11.6 (6.2–16.2) |
| Albumin (g/l) | 42 (27–51) |
| Most recent prior therapy: | |
| Alemtuzumab-based treatment | 26 (18) |
| Moderate-intensity chemoimmunotherapy | 46 (32) |
| High-intensity chemoimmunotherapy | 56 (39) |
| Targeted therapy/other | 3 (2) |
| Chemotherapy alone | 3 (2) |
| Monoclonal antibody alone | 9 (6) |
Abbreviations: FISH; fluorescent in situ hybridization HCT-CI; hematopoietic cell transplant comorbidity index.
Engraftment
Successful engraftment occurred in 137/139 evaluable patients. Median time to neutrophil engraftment (ANC >0.5 × 109/l) was 11 days (range 0–32) and median time to platelet engraftment (first day of an un-transfused platelet count >20 × 109/l for ≥3 days) was 9 days (range 0–106). Secondary graft loss occurred in 12 patients for the following reasons: donor cell rejection 8; drugs 1; viral infection 2; persistent disease 1. Six patients had a second transplant; 2 achieved long-term CR; the remaining 4 patients died from TRM. Of the 6 patients not receiving 2nd transplants, 2 died from sepsis/marrow failure; the remaining 4 patients had autologous re-constitution of haematopoiesis and received alternative treatment for their residual CLL.
Response rates
Response to most recent pre-transplant therapy was: CR in 6%, PR in 50%; 44% of patients were refractory. Best response post-transplant was CR in 80/133 evaluable patients (60%); 31 (23%) achieved PR. 10 patients were not evaluable due to early death.
Cumulative incidences of acute GVHD, chronic GVHD treatment-related mortality (TRM) and relapse
Cumulative incidence of acute grade II-IV GVHD was 37.7%; no new cases were observed beyond 5.7 months in the absence of DLI. Cumulative incidence of chronic GHVD was 51.7%. Cumulative incidences of TRM were 11.1% at day 100, 23.8% at 1 year and 33.7% at 5 years. Cumulative incidences of relapse (CIR) were 6.3% at 100 days, 26.0% at 1 year, 44.4% at 5 years. Only 2 relapses were seen beyond 5 years. Associations between baseline variables, cumulative incidences of TRM (CITRM) and relapse are shown in figure 1. On MVA, myeloablative conditioning was significantly associated with lower CIR (p=0.04), offset by a higher CITRM (p=0.03). Sibling donor transplants were associated with reduced CITRM (p=0.007). Mixed T-cell chimaerism at 3 and 6 months was associated with significantly increased CIR (p<0.001 and p=0.004, respectively), but no difference in CITRM (p=0.23 and p=0.77, respectively).
Figure 1.
Cumulative incidences of relapse and treatment-related mortality (TRM), according to: A; transplant from sibling donor or not; receiving a sibling donor transplant was associated with a significantly lower incidence of TRM (p=0.006); B, Myeloablative vs reduced-intensity conditioning; receiving myeloablative conditioning was associated with a significantly lower cumulative incidence of relapse (p=0.04), but a higher cumulative incidence of TRM (p=0.001); C, complex karyotype (CKT) vs not; patients with CKT showed a trend toward a higher incidence of relapse (p=0.07); D, receipt of alemtuzumab as the last treatment prior to transplant; there was a trend toward higher TRM (p=0.13) in patients receiving alemtuzumab as their last treatment prior to transplant; E, F; the presence or absence of mixed T-cell chimaerism at 6 and 3 months post-transplant; mixed chimaerism post-transplant at 6 and 3 months was associated with a higher incidence of relapse, (p=0.004 and 0.0005, respectively), but no difference in TRM.
Progression-free survival, survival and causes of death
Median PFS for the total cohort was 12.4 months (95% CI 8.3–16.5); 5-year PFS was 22.1% (95% CI 15.0–29.2%). Univariable associations between pre-transplant characteristics and PFS are shown in supplementary table 1 and figure 2. On MVA (supplementary table 2), receiving alemtuzumab as the last therapy prior to alloSCT [HR 1.92 (1.11–3.31), p=0.02] and complex metaphase karyotype [HR 2.04 (1.11–3.74), p=0.02] were significantly associated with inferior PFS. Pre-transplant β2-microglobulin (B2M) ≥4.0mg/l was associated with a trend toward inferior PFS [HR 1.53 (0.94–2.49), p=0.09], figure 2. Patients with complex metaphase karyotype (CKT), defined as ≥3 unrelated chromosomal abnormalities, were more likely refractory to their most recent prior therapy [12/16 (75%) vs 50/116 (43.1%), p=0.017]. Univariable associations between pre-transplant characteristics and survival are shown in supplementary table 3. On MVA (supplementary table 4), only B2M ≥4.0 [HR 1.78 (1.11–2.85), p=0.02] was significantly associated with survival. There was a trend toward superior survival in patients <60 years of age [HR 0.67 (0.43–1.04), p=0.08]. Median survival for the total cohort was 42.2 months (95% CI 21.4–63.0); 5 year survival was 42.6% (95% CI 34.2–51.0%). Primary causes of death were as follows: disease recurrence or Richter Transformation, n=33 (36.3%); acute or chronic GVHD, n=27 (29.7%); infection, n=15 (16.5%); second cancer, n=4 (4.4%); organ failure, n=3 (3.3%); other, n=9 (9.9%).
Figure 2.
A, B, PFS (A) and survival (B) according to the presence or absence of complex karyotype (CKT). CKT was associated with a high incidence of early disease progression but no survival difference; C, D; PFS (C) and survival (D) according to whether alemtuzumab was given as the most recent anti-leukaemic therapy prior to transplant or not. Use of alemtuzumab as the most recent pre-transplant therapy was associated with inferior PFS and survival; E, F PFS (E) and survival (F) according to baseline β2-microglobulin. Pre-transplant B2M ≥4.0mg/l was associated with inferior PFS and survival.
T-cell Chimaerism results, 3 and 6 month landmark PFS and survival analyses
At 3 months, 55/110 (50%) had mixed chimaerism. Mixed chimaerism was defined as ≤95% donor T-cells present by microsatellite polymorphism analysis performed on peripheral blood, enriched for CD3+ cells.(Khouri, et al 2007) Pre-transplant variables associated with mixed chimaerism on UVA are shown in supplementary table 5. On MVA, receiving alemtuzumab-based therapy for treatment of CLL as the last treatment prior to transplant was associated with a significantly higher likelihood of mixed chimaerism at 3 months [HR 3.69 (1.09–12.54), p=0.036]. B2M ≥4.0mg/l was associated with a trend toward increased likelihood of mixed chimaerism [HR 2.83 (0.97–8.27), p=0.06]. Pre-transplant variables associated with mixed chimaerism at 6 months on UVA are shown in supplementary table 6. On MVA, only receiving in vivo T-cell depletion as part of transplant conditioning was significantly associated with a higher incidence of mixed chimaerism at 6 months [HR 2.64 (1.06–6.59), p=0.04].
Univariable and multivariable associations between baseline characteristics, mixed chimaerism, PFS and survival from the 3 and 6 month landmarks are shown in supplementary tables 7–14. Mixed chimaerism at 3 months was strongly associated with inferior PFS [HR 1.93 (1.24–3.00, p=0.003] on UVA, Figure 3. At 3-months, on MVA, there were trends toward inferior PFS for patients with mixed chimaerism [HR1.74 (0.94–3.23), p=0.06] and in patients who received alemtuzumab-based therapy as their most recent treatment prior to alloSCT [HR 1.75 (0.95–3.23), p=0.07]. On MVA, the only variable significantly associated with survival was age <60 [HR 0.56 (0.34–0.93), p=0.02]. At 6 months, 38/84 (45%) patients had mixed chimaersm. On MVA, mixed chimaerism [HR 3.14 (1.77–5.58), p<0.001], receiving alemtuzumab as the most recent treatment prior to alloSCT [HR 2.23 (1.05–5.15), p=0.037] and being refractory to most recent pre-transplant treatment [HR 1.87 (1.04–3.36), p=0.037) were all significantly associated with inferior PFS from the 6-month landmark; receiving a sibling donor transplant was associated with superior PFS [HR 0.56 (0.33–0.97), p=0.037]. On MVA, from the 6-month landmark, mixed chimaerism [HR 3.81 (1.94–7.48), p<0.001], receiving alemtuzumab as the most recent pre-transplant therapy [HR 2.78 (1.25–6.17), p=0.012] and age ≥60 [HR 0.44 (1.35–4.76), p=0.02], were significantly associated with inferior survival.
Figure 3.
A. Landmark progression-free survival (PFS) according to presence or absence of mixed T-cell chimaerism at 3 months. B. Landmark survival according to the presence or absence of mixed T-cell chimaerism at 3 months. C. Landmark PFS according to presence or absence of mixed T-cell chimaerism at 6 months. D. Landmark survival according to presence of absence of mixed T-cell chimaerism at 6 months. The presence of mixed T-cell chimaerism at 3 and 6 months was associated with inferior PFS and survival.
Seventy-nine patients had chimaerism results at both 3 and 6 months; 9/41 (22%) patients with mixed chimaerism at 3 months achieved ≥95% donor T-cell chimaerism at 6 months; the remaining 32 patients had persistent mixed chimaerism. Of 38 patients with full donor T-cell chimaerism at 3 months, 35 remained full donor, while 3 developed mixed chimaerism. There was no policy to give DLI for persistent mixed chimaerism and only 3 patients received pre-emptive DLI for persistent mixed chimaerism in the absence of disease relapse, although immunosuppression could be withdrawn according to physician preference.
Mixed chimaerism at 6 months was associated with a significantly lower cumulative incidence of grade 2–4 acute GHVD (16.3% vs 51.9%, p<0.001).
Immunomanipulation
Thirty-three patients were treated for either persistent or progressive disease with immunomanipulation. Seven patients who had tacrolimus withdrawn prior to planned DLI did not receive DLI: 3 achieved prompt CR without aGVHD; 4 developed acute GHVD prior to planned DLI, all of whom subsequently achieved CR; 6 of 7 developed chronic GVHD. Twenty-six patients received a total of 65 DLI: median number of DLI received was 2 (range 1–5); median dose was 1.3×107/kg (range 1×104-1.6×108). Median time to first DLI was 389 days post-transplant (range 18–2036). 13 patients received DLI from unrelated donors and 13 from sibling donors (1 with single antigen mismatch). Per protocol, patients receiving DLI from unrelated donors received a lower initial dose (1×106/kg) than those receiving DLI from matched sibling donors (1×107/kg). Patients received dose-escalation by approximately 1 log every 6 weeks in the absence of GVHD or significant disease response, until the maximum dose of approximately 108/kg. 22 patients also received either rituximab (n=21), or ofatumumab (n=1), for 2 weekly doses prior to the first dose of DLI and 2 weekly doses post-DLI to enhance immunoreactivity.
Thirty patients with progressive disease did not receive immunotherapy, for the following reasons: receiving alternative therapy, most commonly chemoimmunotherapy (n=10), active GVHD (n=7), Richter Transformation (n=5), graft failure (n=5) and other (n=2).
Response to DLI and toxicity
Thirteen of 26 (50%) patients receiving DLI developed acute GVHD. 9/13 patients with unrelated and 4/13 with sibling donors developed acute GVHD, p=0.08. Post-DLI acute GVHD was fatal in 4 cases, all of whom received DLI from unrelated donors; 2 of these fatal cases developed after a single DLI at 106/kg and 2 after the third dose of 108/kg. The response to immune therapy was dichotomous. In addition to the 7 patients that achieved CR after tacrolimus withdrawal alone, 9/26 patients achieved CR after DLI. There was only one stable partial response. The remaining 16 patients had progressive disease or died from acute GVHD-related complications prior to evaluation of response.
Of the 16 patients who achieved CR with immunomodulatory therapy, 6 are in ongoing CR at a median of 90 months (range 66–149 months). 7 patients subsequently relapsed; one, who relapsed 11 years after achieving CR, remains alive; the other 6 died: 1 patient developed Richter Transformation but achieved CR with chemoimmunotherapy; 2 patients died from GVHD, while one has been lost-to-follow-up. 13 of the 17 patients not achieving CR have died, 2 from GVHD, 1 from infection and 10 from CLL; 4 remain alive with disease after salvage therapy.
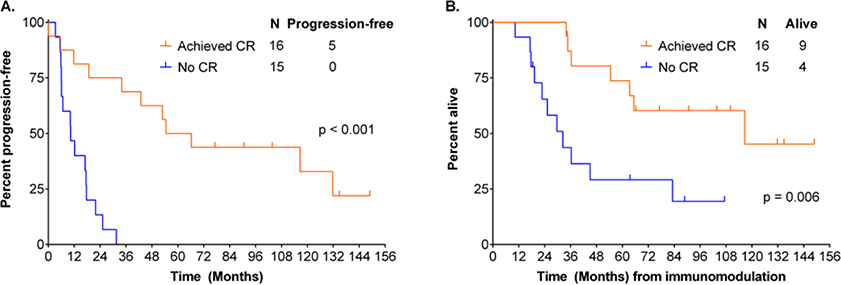
We performed landmark PFS analysis from the time of immunomodulation. Two patients were not evaluable due to early TRM. Evaluable patients achieving CR with immunotherapy had a superior subsequent PFS (median 60 months vs 10 months, p<0.001) and survival (median 117 months vs 32 months, p=0.006) to those who did not, (Figure 4). There was no difference in response according to whether patients had received in vivo T-cell depletion as part of transplant conditioning; 6/13 (46%) patients who had not received T-cell depletion achieved CR, while 10/18 (56%) patients who had received T-cell depletion responded (p=0.6). Patients with mixed chimaerism at the time of immunomodulatory therapy showed a trend toward inferior CR rate; 6/14 evaluable patients (43%) achieved CR compared to 9/12 (75%) patients with full donor chimaerism, p=0.1; in addition, patients with full donor chimaerism at the time of immunomodulatory therapy had a superior PFS from the date of first DLI or tacrolimus withdrawal (median 32 months vs 10 months, p=0.04), compared to those patients with mixed chimaerism. Of patients with mixed chimaerism at the time of immunomodulatory therapy, 8 of 14 converted to full donor chimaerism: 6/8 (75%) achieved CR, compared to 0/6 who did not convert to full donor chimaerism, p=0.005; 5/8 patients who converted to full donor chimaerism developed acute GVHD, which was fatal in 3 cases and 7/8 developed cGHVD. In contrast only 1 of 6 patients who had persistent mixed chimaerism developed acute and chronic GVHD; there was no TRM; all but one patient died from CLL.
Figure 4.
PFS (A) and survival (B) according to achieving CR or not post-immunotherapy; landmark analysis from the time of receiving immunomodulatory therapy. Achieving CR with immunotherapy for progressive disease post-transplant was associated with superior PFS and survival.
Discussion
AlloSCT produces long-term disease-free remission in high-risk CLL. Our data confirms the importance of immunologic control in achieving long-term disease control, given the high relapse risk conferred by mixed chimaerism and the achievement of durable CR in approximately half of all patients after immunosuppression withdrawal +/− DLI.
CKT and receiving alemtuzumab as the most recent therapy prior to transplant were associated with inferior PFS, but not inferior survival, indicating that some early relapses were successfully salvaged. Patients with CKT had a high incidence of early relapse, accounting for the inferior PFS, which may have related to inadequate pre-transplant disease control. Inferior PFS in patients receiving alemtuzumab for CLL treatment as their most recent treatment prior to transplant was due to a combination of both increased TRM and increased relapse risk, likely related to increased immunosuppression and an association with post-transplant mixed chimaerism and consequent failure of GVL. Pre-transplant B2M ≥4.0mg/l was associated with inferior survival. B2M level correlates with CLL protein synthesis(Vilpo, et al 1999) and markers of proliferation.(Rossi, et al 2008) Thus, high B2M level may indicate inadequate pre-transplant disease control and contribute to poorer post-transplant outcomes;(Gentile, et al 2009) this has also been seen in several non-transplant settings.(Hallek, et al 2010, Hallek, et al 1996, Tam, et al 2008, Tsimberidou, et al 2007, Wierda, et al 2009) Additionally, high levels of B2M may be associated with immune dysfunction through impaired ability to generate monocyte-derived dendritic cells;(Xie, et al 2003) it is thus possible that elevated B2M may contribute to failure of graft-vs-leukaemia (GVL)-mediated disease control.
T-cell donor chimaerism post-transplant of ≤95% at both 3 and 6 months is associated with increased risk of relapse, likely due to failure of GVL effect, resulting in excess mortality, predominantly early post-transplant. Lower incidence of aGVHD in patients with mixed chimaerism did not result in lower TRM. Mixed chimaerism was more frequently seen in patients who received in vivo T-cell depletion as part of their transplant conditioning and in patients who received alemtuzumab as their most recent pre-transplant anti-leukaemic therapy. Given the association between mixed chimaerism and inferior survival, anti-T-cell antibodies should be used only with caution in the setting of alloSCT for CLL.
Pre-emptive DLI effectively reduces relapse incidence in HL(Peggs, et al 2011) and FL.(Thomson, et al 2010) It is unclear from our data whether early, targeted intervention with immunosuppression reduction and/or DLI will reduce the incidence of disease relapse in patients with mixed chimaerism and enhance long-term outcomes, as this was not routinely performed. Theoretically, pre-emptive DLI for mixed chimaerism may be more effective than DLI given to treat clinical relapse, but this needs to be confirmed in a prospective study; the optimal timing of DLI would also need to be elucidated. Any enhanced disease control from early post-transplant immunomodulation would need to be balanced against increased risk of acute GVHD.
When DLI was given for relapse, the most important predictor of superior response rate and PFS was the presence of full donor T-cell chimaerism; nonetheless, a sub-group of patients with mixed chimaerism did achieve CR. Response was always associated with conversion to full-donor chimaerism; however, this was associated with severe acute GVHD, fatal in 3 cases. All of the patients who developed fatal GVHD had received DLI from unrelated donors. The lower response rates to DLI in patients with mixed chimaerism at the time of relapse and high rates of severe GVHD associated with conversion to full donor T-cell chimaerism indicate that novel approaches are likely required to manage patients with persistent mixed chimaerism post-transplant, particularly those who received alloSCT from unrelated donors. For those patients requiring DLI, enhancing the safety of DLI is a priority, particularly after unrelated donor transplant, where acute GVHD risk is higher. Transduction of donor T-cells with a an iCaspase-9 “suicide gene” has the potential to enhance safety; in a small study, rapid T-cell apoptosis after addition of the dimerizing agent AP1903 was seen, with concurrent resolution of GVHD.(Di Stasi, et al 2011)
Minimal residual disease (MRD) analysis using sensitive, standardized methods was not available at our institution until 2008. However, knowledge of level of CLL MRD and change over time would likely refine assessment of relapse risk; patients with mixed chimaerism but with low and falling MRD levels may have lower relapse risk than a patient with high or rising levels of MRD.(Dreger, et al 2010, Farina, et al 2009, Logan, et al 2013). This information could be combined with an assessment of GVHD risk from DLI to inform clinical decisions. In the modern era, patients relapsing after allogeneic stem cell transplant have a relatively favorable prognosis, due to the efficacy of novel agents such as ibrutinib in this setting;(Rozovski, et al 2015) treatment with novel agents could potentially delay or obviate the need for DLI, particularly in patients with high risk of GVHD, such as those with unrelated donors. Maintenance therapy with ibrutinib is currently being explored in a multi-center study; ibrutinib drives TH1 differentiation in T-cells by inhibition of interleukin 2-inducible kinase (ITK)(Dubovsky, et al 2013) and has also been shown to reduce cGVHD in a murine model.(Dubovsky, et al 2014) The direct anti-leukaemic activity of ibrutinib may also allow time for effective GVL to become established and potentially delay the need for DLI. Finally, the precise role of allogeneic stem cell transplant in patients with high-risk CLL, given the availability and success of novel therapies, such as ibrutinib(Byrd, et al 2013) and venetoclax(Roberts, et al 2016) remains to be defined and is the subject of much debate.(Dreger, et al 2014)
Supplementary Material
Acknowledgements
PAT designed research, collected and analyzed data and wrote the paper; FS analyzed data and wrote the paper; EJS and CMH designed research, provided clinical care to patients and wrote the paper, CL designed research, collected data and co-wrote the paper; MJK, WGW, SMO, ZE, KR, MQ, NS, SP, UP, PA, PK and RC designed research, provided clinical care to patients and co-wrote the paper.
Research support: supported in part by M.D. Anderson Cancer Center Core Grant P30 CA016672.
Footnotes
Competing interests: none
References
- Ben-Bassat I, Raanani P & Gale RP (2007) Graft-versus-leukemia in chronic lymphocytic leukemia. Bone Marrow Transplant, 39, 441–446. [DOI] [PubMed] [Google Scholar]
- Brown JR, Kim HT, Armand P, Cutler C, Fisher DC, Ho V, Koreth J, Ritz J, Wu C, Antin JH, Soiffer RJ, Gribben JG & Alyea EP (2013) Long-term follow-up of reduced-intensity allogeneic stem cell transplantation for chronic lymphocytic leukemia: prognostic model to predict outcome. Leukemia, 27, 362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, Grant B, Sharman JP, Coleman M, Wierda WG, Jones JA, Zhao W, Heerema NA, Johnson AJ, Sukbuntherng J, Chang BY, Clow F, Hedrick E, Buggy JJ, James DF & O’Brien S (2013) Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med, 369, 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado J, Milligan DW & Dreger P (2009) Allogeneic hematopoietic cell transplantation for chronic lymphocytic leukemia: ready for prime time? Blood, 114, 2581–2588. [DOI] [PubMed] [Google Scholar]
- Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, Straathof K, Liu E, Durett AG, Grilley B, Liu H, Cruz CR, Savoldo B, Gee AP, Schindler J, Krance RA, Heslop HE, Spencer DM, Rooney CM & Brenner MK (2011) Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med, 365, 1673–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreger P, Dohner H, Ritgen M, Bottcher S, Busch R, Dietrich S, Bunjes D, Cohen S, Schubert J, Hegenbart U, Beelen D, Zeis M, Stadler M, Hasenkamp J, Uharek L, Scheid C, Humpe A, Zenz T, Winkler D, Hallek M, Kneba M, Schmitz N, Stilgenbauer S & German CLLSG (2010) Allogeneic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: long-term clinical and MRD results of the German CLL Study Group CLL3X trial. Blood, 116, 2438–2447. [DOI] [PubMed] [Google Scholar]
- Dreger P, Schetelig J, Andersen N, Corradini P, van Gelder M, Gribben J, Kimby E, Michallet M, Moreno C, Stilgenbauer S, Montserrat E, European Research Initiative on, C.L.L., the European Society for, B. & Marrow, T. (2014) Managing high-risk CLL during transition to a new treatment era: stem cell transplantation or novel agents? Blood, 124, 3841–3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreger P, Schnaiter A, Zenz T, Bottcher S, Rossi M, Paschka P, Buhler A, Dietrich S, Busch R, Ritgen M, Bunjes D, Zeis M, Stadler M, Uharek L, Scheid C, Hegenbart U, Hallek M, Kneba M, Schmitz N, Dohner H & Stilgenbauer S (2013) TP53, SF3B1, and NOTCH1 mutations and outcome of allotransplantation for chronic lymphocytic leukemia: six-year follow-up of the GCLLSG CLL3X trial. Blood, 121, 3284–3288. [DOI] [PubMed] [Google Scholar]
- Dubovsky JA, Beckwith KA, Natarajan G, Woyach JA, Jaglowski S, Zhong Y, Hessler JD, Liu TM, Chang BY, Larkin KM, Stefanovski MR, Chappell DL, Frissora FW, Smith LL, Smucker KA, Flynn JM, Jones JA, Andritsos LA, Maddocks K, Lehman AM, Furman R, Sharman J, Mishra A, Caligiuri MA, Satoskar AR, Buggy JJ, Muthusamy N, Johnson AJ & Byrd JC (2013) Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood, 122, 2539–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovsky JA, Flynn R, Du J, Harrington BK, Zhong Y, Kaffenberger B, Yang C, Towns WH, Lehman A, Johnson AJ, Muthusamy N, Devine SM, Jaglowski S, Serody JS, Murphy WJ, Munn DH, Luznik L, Hill GR, Wong HK, MacDonald KK, Maillard I, Koreth J, Elias L, Cutler C, Soiffer RJ, Antin JH, Ritz J, Panoskaltsis-Mortari A, Byrd JC & Blazar BR (2014) Ibrutinib treatment ameliorates murine chronic graft-versus-host disease. J Clin Invest, 124, 4867–4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina L, Carniti C, Dodero A, Vendramin A, Raganato A, Spina F, Patriarca F, Narni F, Benedetti F, Olivieri A & Corradini P (2009) Qualitative and quantitative polymerase chain reaction monitoring of minimal residual disease in relapsed chronic lymphocytic leukemia: early assessment can predict long-term outcome after reduced intensity allogeneic transplantation. Haematologica, 94, 654–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile M, Cutrona G, Neri A, Molica S, Ferrarini M & Morabito F (2009) Predictive value of beta2-microglobulin (beta2-m) levels in chronic lymphocytic leukemia since Binet A stages. Haematologica, 94, 887–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, Hensel M, Hopfinger G, Hess G, von Grunhagen U, Bergmann M, Catalano J, Zinzani PL, Caligaris-Cappio F, Seymour JF, Berrebi A, Jager U, Cazin B, Trneny M, Westermann A, Wendtner CM, Eichhorst BF, Staib P, Buhler A, Winkler D, Zenz T, Bottcher S, Ritgen M, Mendila M, Kneba M, Dohner H & Stilgenbauer S (2010) Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet, 376, 1164–1174. [DOI] [PubMed] [Google Scholar]
- Hallek M, Wanders L, Ostwald M, Busch R, Senekowitsch R, Stern S, Schick HD, Kuhn-Hallek I & Emmerich B (1996) Serum beta(2)-microglobulin and serum thymidine kinase are independent predictors of progression-free survival in chronic lymphocytic leukemia and immunocytoma. Leuk Lymphoma, 22, 439–447. [DOI] [PubMed] [Google Scholar]
- Jones CD, Arai S, Lowsky R, Tyan DB, Zehnder JL & Miklos DB (2010) Complete donor T-cell engraftment 30 days after allogeneic transplantation predicts molecular remission in high-risk chronic lymphocytic leukaemia. Br J Haematol, 150, 637–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khouri IF, Bassett R, Poindexter N, O’Brien S, Bueso-Ramos CE, Hsu Y, Ferrajoli A, Keating MJ, Champlin R & Fernandez-Vina M (2011) Nonmyeloablative allogeneic stem cell transplantation in relapsed/refractory chronic lymphocytic leukemia: long-term follow-up, prognostic factors, and effect of human leukocyte histocompatibility antigen subtype on outcome. Cancer, 117, 4679–4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khouri IF, Saliba RM, Admirand J, O’Brien S, Lee M-S, Korbling M, Samuels BI, Giralt S, Lima DM, Keating MJ, Champlin RE & Bueso-Ramos C (2007) Graft-versus-leukaemia effect after non-myeloablative haematopoietic transplantation can overcome the unfavourable expression of ZAP-70 in refractory chronic lymphocytic leukaemia. British journal of haematology, 137, 355–363. [DOI] [PubMed] [Google Scholar]
- Logan AC, Zhang B, Narasimhan B, Carlton V, Zheng J, Moorhead M, Krampf MR, Jones CD, Waqar AN, Faham M, Zehnder JL & Miklos DB (2013) Minimal residual disease quantification using consensus primers and high-throughput IGH sequencing predicts post-transplant relapse in chronic lymphocytic leukemia. Leukemia, 27, 1659–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida T, Hudecek M, Kostic A, Bleakley M, Warren EH, Maloney D, Storb R & Riddell SR (2009) Development of tumor-reactive T cells after nonmyeloablative allogeneic hematopoietic stem cell transplant for chronic lymphocytic leukemia. Clin Cancer Res, 15, 4759–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peggs KS, Kayani I, Edwards N, Kottaridis P, Goldstone AH, Linch DC, Hough R, Morris EC, Fielding A, Chakraverty R, Thomson KJ & Mackinnon S (2011) Donor lymphocyte infusions modulate relapse risk in mixed chimeras and induce durable salvage in relapsed patients after T-cell-depleted allogeneic transplantation for Hodgkin’s lymphoma. J Clin Oncol, 29, 971–978. [DOI] [PubMed] [Google Scholar]
- Peggs KS, Thomson K, Hart DP, Geary J, Morris EC, Yong K, Goldstone AH, Linch DC & Mackinnon S (2004) Dose-escalated donor lymphocyte infusions following reduced intensity transplantation: toxicity, chimerism, and disease responses. Blood, 103, 1548–1556. [DOI] [PubMed] [Google Scholar]
- Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, Kipps TJ, Anderson MA, Brown JR, Gressick L, Wong S, Dunbar M, Zhu M, Desai MB, Cerri E, Heitner Enschede S, Humerickhouse RA, Wierda WG & Seymour JF (2016) Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med, 374, 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi D, Zucchetto A, Rossi FM, Capello D, Cerri M, Deambrogi C, Cresta S, Rasi S, De Paoli L, Bodoni CL, Bulian P, Del Poeta G, Ladetto M, Gattei V & Gaidano G (2008) CD49d expression is an independent risk factor of progressive disease in early stage chronic lymphocytic leukemia. Haematologica, 93, 1575–1579. [DOI] [PubMed] [Google Scholar]
- Rozovski U, Benjamini O, Jain P, Thompson PA, Wierda WG, O’Brien S, Burger JA, Ferrajoli A, Faderl S, Shpall E, Hosing C, Khouri IF, Champlin R, Keating MJ & Estrov Z (2015) Outcomes of Patients With Chronic Lymphocytic Leukemia and Richter’s Transformation After Transplantation Failure. J Clin Oncol, 33, 1557–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schetelig J, van Biezen A, Brand R, Caballero D, Martino R, Itala M, Garcia-Marco JA, Volin L, Schmitz N, Schwerdtfeger R, Ganser A, Onida F, Mohr B, Stilgenbauer S, Bornhauser M, de Witte T & Dreger P (2008) Allogeneic hematopoietic stem-cell transplantation for chronic lymphocytic leukemia with 17p deletion: a retrospective European Group for Blood and Marrow Transplantation analysis. J Clin Oncol, 26, 5094–5100. [DOI] [PubMed] [Google Scholar]
- Shaffer BC, Modric M, Stetler-Stevenson M, Arthur DC, Steinberg SM, Liewehr DJ, Fowler DH, Gale RP, Bishop MR & Pavletic SZ (2013) Rapid complete donor lymphoid chimerism and graft-versus-leukemia effect are important in early control of chronic lymphocytic leukemia. Exp Hematol, 41, 772–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorror ML, Storer BE, Sandmaier BM, Maris M, Shizuru J, Maziarz R, Agura E, Chauncey TR, Pulsipher MA, McSweeney PA, Wade JC, Bruno B, Langston A, Radich J, Niederwieser D, Blume KG, Storb R & Maloney DG (2008) Five-year follow-up of patients with advanced chronic lymphocytic leukemia treated with allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. J Clin Oncol, 26, 4912–4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam CS, O’Brien S, Wierda W, Kantarjian H, Wen S, Do KA, Thomas DA, Cortes J, Lerner S & Keating MJ (2008) Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood, 112, 975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson KJ, Morris EC, Milligan D, Parker AN, Hunter AE, Cook G, Bloor AJ, Clark F, Kazmi M, Linch DC, Chakraverty R, Peggs KS & Mackinnon S (2010) T-cell-depleted reduced-intensity transplantation followed by donor leukocyte infusions to promote graft-versus-lymphoma activity results in excellent long-term survival in patients with multiply relapsed follicular lymphoma. J Clin Oncol, 28, 3695–3700. [DOI] [PubMed] [Google Scholar]
- Tsimberidou AM, Wen S, O’Brien S, McLaughlin P, Wierda WG, Ferrajoli A, Faderl S, Manning J, Lerner S, Mai CV, Rodriguez AM, Hess M, Do KA, Freireich EJ, Kantarjian HM, Medeiros LJ & Keating MJ (2007) Assessment of chronic lymphocytic leukemia and small lymphocytic lymphoma by absolute lymphocyte counts in 2,126 patients: 20 years of experience at the University of Texas M.D. Anderson Cancer Center. J Clin Oncol, 25, 4648–4656. [DOI] [PubMed] [Google Scholar]
- Vilpo J, Vilpo L, Hurme M & Vuorinen P (1999) Induction of beta-2-microglobulin release in vitro by chronic lymphocytic leukaemia cells: relation to total protein synthesis. Leuk Res, 23, 913–920. [DOI] [PubMed] [Google Scholar]
- Wierda WG, O’Brien S, Wang X, Faderl S, Ferrajoli A, Do KA, Garcia-Manero G, Cortes J, Thomas D, Koller C, Burger J, Lerner S, Kantarjian H & Keating M (2009) Characteristics associated with important clinical end points in patients with chronic lymphocytic leukemia at initial treatment. J Clin Oncol, 27, 1637–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Wang Y, Freeman ME 3rd, Barlogie B & Yi Q (2003) Beta 2-microglobulin as a negative regulator of the immune system: high concentrations of the protein inhibit in vitro generation of functional dendritic cells. Blood, 101, 4005–4012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.