Abstract
Risk-reducing mastectomy (RRM) and salpingo-oophorectomy (RRSO) are increasingly used to reduce breast and ovarian cancer risk following BRCA1/BRCA2 testing. However, little is known about how genetic counseling influences decisions about these surgeries. Although previous studies have examined intentions prior to counseling, few have examined RRM and RRSO intentions in the critical window between genetic counseling and test result disclosure. Previous research has indicated that intentions at this time point predict subsequent uptake of surgery, suggesting that much decision-making has taken place prior to result disclosure. This period may be a critical time to better understand the drivers of prophylactic surgery intentions. The aim of this study was to examine predictors of RRM and RRSO intentions. We hypothesized that variables from the Health Belief Model would predict intentions, and we also examined the role of affective factors. Participants were 187 women, age 21–75, who received genetic counseling for hereditary breast and ovarian cancer. We utilized multiple logistic regression to identify independent predictors of intentions. 49.2% and 61.3% of participants reported intentions for RRM and RRSO, respectively. Variables associated with RRM intentions include: newly diagnosed with breast cancer (OR = 3.63, 95% CI = 1.20–11.04), perceived breast cancer risk (OR = 1.46, 95% CI = 1.17–1.81), perceived pros (OR = 1.79, 95% CI = 1.38–2.32) and cons of RRM (OR = 0.81, 95% CI = 0.65–0.996), and decision conflict (OR = 0.80, 95% CI = 0.66–0.98). Variables associated with RRSO intentions include: proband status (OR = 0.28, 95% CI = 0.09–0.89), perceived pros (OR = 1.35, 95% CI = 1.11–1.63) and cons of RRSO (OR = 0.72, 95% CI = 0.59–0.89), and ambiguity aversion (OR = 0.79, 95% CI = 0.65–0.95). These data provide support for the role of genetic counseling in fostering informed decisions about risk management, and suggest that the role of uncertainty should be explored further.
Keywords: Hereditary breast/ovarian cancer, Intentions, Risk-reducing mastectomy, Risk-reducing oophorectomy, Genetic counseling, Decision-making
Following genetic counseling for hereditary breast and ovarian cancer, health beliefs but not affect are associated with risk-reducing surgery intentions.
Implications
Practice: The results provide support for the role of genetic counseling in fostering informed decisions about risk management, and suggest that genetic counselors could be proactive in addressing concerns about prophylactic surgery, and facilitating appropriate follow-up and support for their patients.
Policy: The important role genetic counselors play in helping to foster informed risk management decisions suggests that all at-risk women need access to a genetic counselor.
Research: Research is needed to evaluate the role of ambiguity aversion and uncertainty following the receipt of genetic test results, to determine whether patients may require additional support.
INTRODUCTION
Genetic testing for pathogenic variants in BRCA1 and BRCA2 (BRCA) is central to the clinical care of women at high risk for hereditary breast and ovarian cancer (HBOC) [1]. Women who carry a pathogenic variant have up to a 72% lifetime risk of developing breast cancer and up to a 44% lifetime risk of developing ovarian cancer (including fallopian tube and primary peritoneal cancers), compared to a 12.4% and 1.3% lifetime risk, respectively, in the general population [2, 3]. The most effective options for reducing these extremely high cancer risks are risk-reducing mastectomy (RRM) and risk-reducing bilateral salpingo-oophorectomy (RRSO). RRM refers to the prophylactic removal of both breasts, or in newly diagnosed patients it may entail the removal of the affected breast for treatment, and removal of the unaffected breast for risk reduction. RRSO refers to the prophylactic removal of both ovaries and fallopian tubes. The National Comprehensive Cancer Network (NCCN) recommends that female BRCA mutation carriers have a RRSO after they have finished child bearing, and consider having a RRM [1].
RRSO reduces risk for ovarian cancer by over 80% [4] and when performed premenopausally may also reduce breast cancer risk, especially in BRCA2 carriers under age 50 [5]. Although this surgery is recommended by the NCCN, it is associated with significant side effects, including surgical menopause, increased risks for cardiovascular diseases, cognitive impairment, and osteoporosis [6]. RRM reduces the risk for breast cancer by about 90% [7, 8] and can also have psychological benefits, like reducing cancer-related distress [9]. However, this surgery can also have undesirable consequences, such as negative impacts on sexuality and body image [9, 10], and surgical complications [11]. Additionally, neither of these surgeries completely eliminates the risk for breast and ovarian cancer. Clearly, decision-making about risk-reducing surgery is complex, with multiple factors to weigh and trade-offs to consider [12, 13]. To provide the best care for women at risk for HBOC, it is critical for practitioners to better understand how women make these difficult risk management decisions.
Studies that have examined risk-reducing surgery intentions prior to genetic counseling have found that in addition to sociodemographic predictors, cognitive and affective factors such as perceived cancer risk, perceived benefits, cancer worry, distress, and uncertainty reduction predict RRM and RRSO intentions [14–20]. Much less is known about how intentions are impacted by genetic counseling since few studies have examined intentions during the window between genetic counseling and the receipt of the test result. This is a crucial window because previous research has indicated that intentions at this time point predict subsequent uptake of prophylactic surgery [21]. In contrast to pre-counseling intentions which may not reflect accurate comprehension of personal risk, the implications of a positive test result, or the risks and benefits of RRM/RRSO, post-counseling intentions are likely to be more informed. Learning the drivers of these decisions could help genetic counselors to better understand their patients’ decision processes, and tailor genetic counseling to facilitate informed decisions. Further, understanding the predictors of post-counseling intentions may help to identify women who could benefit from additional decision support. Previous research suggests that decision support is of particular benefit to those women who have not reached decisions about RRM and RRSO prior to receipt of test results [13].
The Health Belief Model (HBM) is a well-established framework to understand and predict the adoption of a wide range of preventive health behaviors [22–24]. The framework posits that the likelihood of an individual engaging in a preventive health behavior is influenced by their perceived susceptibility to and perceived seriousness of the disease, self-efficacy for engaging in the behavior, their beliefs about whether the advantages of engaging in the health behavior outweigh the disadvantages, and cues to action. In this study, we focus on perceived benefits, perceived barriers, and perceived susceptibility as key HBM constructs that we predicted would be associated with RRM and RRSO intentions after genetic counseling, but prior to the receipt of test results.
Although we expect the cognitive HBM constructs to predict risk-reducing surgery intentions, as noted above, previous studies have found that affect is a strong predictor of RRM and RRSO intentions prior to genetic counseling. Emotional factors may be particularly salient prior to the provision of extensive information during genetic counseling. The information and decision support provided during genetic counseling could reduce the role that negative affect plays in decision-making. However, clinical observations suggest that the discussion of the risks and benefits surrounding testing and surgery options during genetic counseling can lead to feelings of uncertainty and worry [25] which may manifest as delayed or deferred decisions about risk-reducing surgery [26]. Thus, we examined whether affective factors including distress and uncertainty predict RRM and RRSO intentions after accounting for HBM factors following completion of genetic counseling.
METHOD
Participants
Participants were women undergoing genetic testing for HBOC who completed a baseline survey prior to randomization in a randomized controlled trial of post-test decision support. Participants were recruited from the clinical cancer genetics programs at the Lombardi Comprehensive Cancer Center (Washington, DC), Ohio State University Comprehensive Cancer Center (Columbus, OH), and Virginia Piper Cancer Institute (Minneapolis, MN). Eligible participants were women age 21–75 who received in-person genetic counseling for HBOC but who had not yet received genetic test results. Participants included women who were either affected or unaffected with breast cancer, but excluded those diagnosed with other cancers, besides nonmelanoma skin cancer. We included women who were newly diagnosed with breast cancer (i.e., who had not yet initiated definitive breast cancer treatment) but excluded newly diagnosed women who had already scheduled a bilateral mastectomy. We included both probands and women who had relatives with a BRCA pathogenic variant. We excluded participants who had been diagnosed with bilateral or metastatic breast cancer, had a previous bilateral mastectomy, were candidates for testing for another hereditary cancer syndrome, or had previously used breast cancer chemoprevention. We also excluded women who could not communicate in English or lacked the cognitive capacity to provide informed consent.
Of 352 potentially eligible participants, 79 declined study contact, 86 who agreed to contact received their genetic test results before completing the baseline survey, and 187 (53.1%) completed the baseline survey prior to receiving test results. This report includes all individuals who completed a baseline survey regardless of whether they were ultimately randomized in the trial. Of the 187 women who completed a baseline survey, six were excluded because they were administered an incorrect version of the survey. For the RRM analysis, two were excluded due to missing data on the outcome variable. For the RRSO analysis, one participant was excluded due to missing data on the outcome variable and 12 participants who previously had their ovaries removed were ineligible.
Procedure
Genetic counselors at each study site assessed patient eligibility during their pretest counseling session, explained the study to potentially eligible patients, and asked if they were interested in participation. Participants who met eligibility criteria, and agreed to participate, were enrolled immediately following their genetic counseling appointment. These women were contacted by the study staff and given the option to complete the baseline questionnaire electronically or over the telephone. Electronic surveys were completed using the HIPAA-compliant REDCap data capture tool hosted at Georgetown University [27]. Participants completed an Institutional Review Board approved verbal or electronic consent form prior to completion of the baseline survey. Participants were required to complete the baseline survey after the pretest genetic counseling session but prior to result disclosure. Following the interview and receipt of participants’ test results, the research assistant randomized eligible participants to one of several decision support interventions. This paper focuses only on pre-randomization baseline data.
Measures
Control variables
Sociodemographics.
We assessed age, race/ethnicity, relationship status, education, employment, and Jewish ancestry.
Medical history.
We assessed personal and family cancer history and used this information to calculate lifetime breast cancer risk with the BRCAPRO model [28].
HBM variables
Perceived breast/ovarian cancer risk.
We measured quantitative perceived risk by asking participants to rate their risk on a scale from 0 (definitely will not get breast/ovarian cancer) to 100 (definitely will get breast/ovarian cancer). For participants previously diagnosed with breast cancer, the items asked about the likelihood of developing a new breast cancer. We have used this measure in prior research [15, 29].
Perceived pros and cons for RRM.
We measured six pros (Risk-reducing mastectomy would reduce my risk for developing breast cancer) and eight cons (I am worried that risk-reducing mastectomy would change the way I feel about my appearance) of RRM on a 4-point Likert scale ranging from “not at all important” to “very important.” This scale was adapted from a measure used in previous studies [21]. Cronbach’s alpha was 0.76 for the pros and 0.73 for the cons.
Perceived pros and cons for RRSO.
Adapted from a previous study [21], we measured six pros (Having my ovaries removed would reduce my worry about developing ovarian cancer) and seven cons (I don’t want to go on hormone replacement therapy) of RRSO on a 4-point Likert scale from “not at all important” to “very important.” Cronbach’s alpha was 0.61 for the pros and 0.75 for the cons.
Affective/uncertainty variables
Cancer distress.
We measured cancer distress using the Impact of Event Scale [30], a 15-item Likert-style scale, where higher scores indicate more distress. Reliability in the present study was 0.91.
Perceived stress.
We used the 4-item Perceived Stress Scale [31]. This measure asks participants how often they have felt or thought a certain way in the last month. Items are scored on a 5-point Likert scale from “never” to “very often.” Cronbach’s alpha in the present study was 0.78.
Decisional conflict.
We measured decisional conflict regarding the management of one’s breast cancer risk with the 10-item version of the Decisional Conflict Scale [32]. Items were scored on a weighted 3-point scale, where higher scores indicate greater decisional conflict. Participants were asked to respond to questions like “Do you feel sure about what to choose?” with “yes,” “unsure,” or “no.” Cronbach’s alpha in the present study was 0.87.
Perceived uncertainty.
We used Baty’s 12-item measure to evaluate perceived uncertainty about genetic counseling and testing [25]. Items were scored on a 5-point Likert scale, from “very certain” to “very uncertain,” where higher scores represented greater uncertainty. Consistent with previous research [25], we calculated perceived uncertainty using the average of five items that assessed uncertainty about health care, positive outcomes of testing, and coping with results. Cronbach’s alpha for in the present study was 0.72.
Ambiguity aversion.
We measured aversion to ambiguity regarding medical tests and treatment using the 6-item AA-Med Scale [33]. Participants were asked to rate statements like “I would not have confidence in a medical test or treatment if experts had conflicting opinions about it” on a 5-point Likert scale, from “strongly disagree” to “strongly agree,” where higher scores indicate greater ambiguity aversion. Cronbach’s alpha was 0.79.
Outcome variables
Surgery intentions.
We assessed participants’ intentions for RRM a with a single face-valid item, “If you were to learn that you have a mutation in the BRCA1 or BRCA2 gene, would you have a bilateral risk-reducing mastectomy to reduce your risk for breast cancer?” with options on a 5-point scale ranging from “I definitely would not” to “I definitely would.” Because responses to this item were not normally distributed, we dichotomized this variable, with those responding “I definitely would not/I probably would not/I am not really sure” categorized as “no intention for RRM,” and those answering “I probably would/I definitely would” categorized as “intention for RRM.” For RRSO we used a similar item, asking “If you were to learn that you have a mutation in the BRCA1 or BRCA2 gene, would you have your ovaries removed for risk reduction?” and we dichotomized the variable in the same way as RRM.
Statistical analysis
After characterizing the sample in terms of demographics, family history, and lifetime risk of breast cancer, we used chi-square and t-tests to examine bivariate associations with RRM and RRSO intentions. Next, we used multiple logistic regression to identify independent predictors of intentions for RRM and RRSO. Guided by our conceptual model, in the initial model, we included personal demographic and medical history variables with bivariate associations at the p < .10 level. In the next step, we included the HBM variables of perceived pros and cons of RRM/RRSO (i.e., perceived benefits and barriers) and perceived risk (perceived susceptibility). In the final model, we included affect-related variables, based on significant bivariate associations. For the small amount of missing data we employed mean substitution. All analyses were performed using Statistical Analysis Software (SAS) version 9.4, SAS Inc. (Cary, NC).
RESULTS
Participants were primarily non-Hispanic white (72.7%), employed full-time (64.8%), and did not have a known BRCA mutation in their family (83.8%). The average age of participants was 47.5 years (SD = 12.5) and women had an average lifetime risk of breast cancer of 14.5%.
RRM results
Of the 179 women in this analysis, 88 (49.2%) reported that they probably or definitely would obtain a RRM if they were found to have a BRCA mutation. Table 1 displays the bivariate associations between our baseline variables and RRM intentions. The following variables had bivariate associations (p < .10) with RRM intentions: being employed, no family history of ovarian cancer, being newly diagnosed with breast cancer, higher objective breast cancer risk, higher perceived breast cancer risk, greater perceived pros and lower perceived cons of RRM, lower perceived uncertainty, and lower decisional conflict.
Table 1.
Bivariate associations between categorical and continuous predictors and risk-reducing mastectomy (RRM) intentions
| Categorical predictors | Full sample N = 179 |
Intention for RRM N (%) |
No intention for RRM N (%) |
p |
|---|---|---|---|---|
| Married | ||||
| No | 56 | 32 (57.1%) | 24 (42.9%) | .148 |
| Yes/living together | 121 | 55 (45.5%) | 66 (54.5%) | |
| Education | ||||
| College or less | 95 | 45 (47.4%) | 50 (52.6%) | .610 |
| More than college | 84 | 43 (51.2%) | 41 (48.8%) | |
| Race | ||||
| Non-Hispanic white | 128 | 66 (51.6%) | 62 (48.4%) | .498 |
| Hispanic/non-white | 48 | 22 (45.8%) | 26 (54.2%) | |
| Employment | ||||
| <Full-time | 63 | 25 (39.7%) | 38 (60.3%) | .062 |
| Full-time | 116 | 63 (54.3%) | 53 (45.7%) | |
| Jewish decent | ||||
| Yes | 24 | 11 (45.8%) | 13 (54.2%) | .834 |
| No | 135 | 65 (48.15%) | 70 (51.85%) | |
| Breast cancer | ||||
| Unaffected | 92 | 40 (43.5%) | 52 (56.5%) | .001 |
| Affected | 41 | 15 (36.6%) | 26 (63.4%) | |
| Newly diagnosed | 46 | 33 (71.7%) | 13 (28.3%) | |
| Children | ||||
| Yes | 120 | 59 (49.2%) | 61 (50.8%) | .919 |
| No | 54 | 27 (50%) | 27 (50%) | |
| Proband status | ||||
| Relative | 29 | 13 (44.8%) | 16 (55.2%) | .610 |
| Proband | 150 | 75 (50%) | 75 (50%) | |
| Breast cancer family history | ||||
| Yes | 133 | 64 (48.1%) | 69 (51.9%) | .372 |
| No | 41 | 23 (56.1%) | 18 (43.9%) | |
| Ovarian cancer family history | ||||
| Yes | 40 | 15 (37.5%) | 25 (62.5%) | .067 |
| No | 134 | 73 (54.5%) | 61 (45.5%) | |
| Continuous predictors | Full sample M (SD) |
Intention for RRM M (SD) |
No intention for RRM M (SD) |
p |
| Age | 47.5 (12.5) | 46.3 (12.0) | 48.7 (12.9) | .192 |
| Lifetime breast cancer risk | 14.5 (7.5) | 15.6 (8.1) | 13.3 (6.8) | .053 |
| Pros of RRM | 18.95 (3.4) | 20.4 (2.1) | 17.6 (3.8) | <.0001 |
| Cons of RRM | 19.0 (4.8) | 18.0 (4.5) | 20.0 (5.0) | .006 |
| Perceived breast cancer risk | 41.4 (27.4) | 48.8 (27.0) | 34.3 (26.1) | .0004 |
| Perceived uncertainty | 2.2 (0.7) | 2.1 (0.7) | 2.3 (0.7) | .026 |
| Ambiguity aversion | 17.5 (4.7) | 16.9 (4.6) | 18.0 (4.6) | .119 |
| Cancer distress | 23.8 (17.0) | 24.6 (16.2) | 23.2 (17.7) | .580 |
| Perceived stress | 5.4 (3.0) | 5.4 (3.0) | 5.5 (3.0) | .752 |
| Decision conflict | 29.1 (24.2) | 23.1 (23.3) | 34.9 (23.8) | .001 |
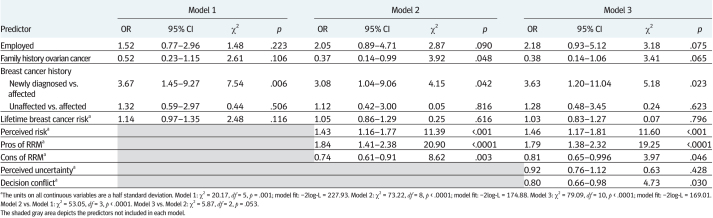
To evaluate the independent contribution of specific HBM and affective components, we utilized logistic regression with hierarchical variable entry. As seen in Table 2, we first entered background demographic and clinical variables that were associated with RRM intentions. These variables (employment, family history of ovarian cancer, and personal breast cancer history), in combination, were significantly associated with RRM intentions (χ2 (5, N = 179) = 20.17, p = .001).
Table 2.
Logistic regression models of risk-reducing mastectomy (RRM) intentions
In Model 2, we added the HBM variables of perceived pros, perceived cons, and perceived susceptibility. These variables, in combination, strongly predicted RRM intentions over and above the clinical and demographic variables already in the model (Δχ2 (3) = 53.05, p < .0001).
In the final model, we added all affective/uncertainty variables with p < .10 bivariate associations to RRM intentions (perceived uncertainty and decisional conflict). The combined contribution of perceived uncertainty and decision conflict approached statistical significance (Δχ2 (2) = 5.87, p = .053). In this final model, the following variables independently predicted RRM intentions: being newly diagnosed with breast cancer (OR = 3.63, 95% CI = 1.20–11.04), perceived risk of breast cancer (OR = 1.46, 95% CI = 1.17–1.81), perceived pros of RRM (OR = 1.79, 95% CI = 1.38–2.32), perceived cons of RRM (OR = 0.81, 95% CI = 0.65–0.996), and decision conflict (OR = 0.80, 95% CI = 0.66–0.98). Compared to women who were previously diagnosed with breast cancer, those who were newly diagnosed with breast cancer were more than three and a half times as likely to have high RRM intentions. Each half standard deviation increase in perceived breast cancer risk was associated with 46% increased odds of having high RRM intentions and each half standard deviation increase in perceived pros of RRM was associated with 79% increased odds of high RRM intentions. In contrast, each half standard deviation increase in perceived cons of RRM was associated with 19% decreased odds of high RRM intentions and each half standard deviation increase in decision conflict was associated with a 20% decrease in the odds of having high RRM intentions.
RRSO analysis
Of the 168 women included in this analysis, 103 (61.3%) responded that if they were found to have a BRCA mutation, they probably or definitely would obtain a RRSO. Table 3 displays the bivariate associations between our baseline predictors and RRSO intentions. The following variables exhibited p < .10 association with RRSO intentions: being non-Hispanic white, having a relative with a BRCA mutation, higher perceived ovarian cancer risk, greater pros and weaker cons of RRSO, and lower ambiguity aversion.
Table 3.
Bivariate associations between categorical and continuous predictors and risk-reducing salpingo-oophorectomy (RRSO) intentions
| Categorical predictors | Full sample N = 168 |
Intention for RRSO N (%) |
No intention for RRSO N (%) |
p |
|---|---|---|---|---|
| Married | ||||
| No | 54 | 32 (59.3%) | 22 (40.7%) | .739 |
| Yes/living together | 113 | 70 (61.95%) | 43 (38.05%) | |
| Education | ||||
| College or less | 85 | 57 (67.1%) | 28 (32.9%) | .122 |
| More than college | 83 | 46 (55.4%) | 37 (44.6%) | |
| Race | ||||
| Non-Hispanic white | 118 | 79 (66.95%) | 39 (33.05%) | .008 |
| Hispanic/non-white | 47 | 21 (44.7%) | 26 (55.3%) | |
| Employment | ||||
| <Full-time | 56 | 32 (57.1%) | 24 (42.6%) | .433 |
| Full-time | 112 | 71 (63.4%) | 41 (36.6%) | |
| Jewish decent | ||||
| Yes | 22 | 8 (36.4%) | 14 (63.6%) | .686 |
| No | 127 | 75 (59.1%) | 52 (40.9%) | |
| Breast cancer | ||||
| Unaffected | 89 | 61 (68.5%) | 28 (31.5%) | .119 |
| Affected | 35 | 18 (51.4%) | 17 (48.6%) | |
| Newly diagnosed | 44 | 24 (54.55%) | 20 (45.45%) | |
| Children | ||||
| Yes | 110 | 70 (63.6%) | 40 (36.4%) | .275 |
| No | 53 | 29 (54.7%) | 24 (45.3%) | |
| Proband status | ||||
| Relative | 28 | 23 (82.1%) | 5 (17.9%) | .013 |
| Proband | 140 | 80 (57.1%) | 60 (42.9%) | |
| Breast cancer family history | ||||
| Yes | 123 | 74 (60.2%) | 49 (39.8%) | .585 |
| No | 40 | 26 (65.0%) | 14 (35.0%) | |
| Ovarian cancer family history | ||||
| Yes | 37 | 25 (67.6%) | 12 (32.4%) | .377 |
| No | 126 | 75 (59.5%) | 51 (40.5%) | |
| Continuous predictors | Full sample M (SD) |
Intention for RRSO M (SD) |
No intention for RRSO M (SD) |
p |
| Age | 46.5 (12.0) | 47.1 (12.0) | 45.4 (11.6) | .399 |
| Lifetime breast cancer risk | 14.9 (7.6) | 15.4 (8.3) | 14.2 (6.5) | .325 |
| Pros of RRSO | 19.8 (3.0) | 20.2 (2.6) | 19.1 (3.5) | .020 |
| Cons of RRSO | 16.9 (4.5) | 15.8 (4.4) | 18.5 (4.3) | .0001 |
| Perceived ovarian cancer risk | 27.2 (23.6) | 30.6 (23.1) | 21.4 (23.4) | .013 |
| Perceived uncertainty | 2.19 (0.7) | 2.2 (0.7) | 2.2 (0.7) | .571 |
| Ambiguity aversion | 17.5 (4.7) | 16.6 (4.4) | 19.0 (4.9) | .002 |
| Cancer distress | 23.6 (17.1) | 22.9 (17.1) | 24.7 (17.2) | .505 |
| Perceived stress | 5.3 (3.0) | 5.4 (3.0) | 5.2 (3.0) | .708 |
| Decision conflict | 28.5 (24.3) | 26.5 (24.2) | 31.6 (24.2) | .187 |
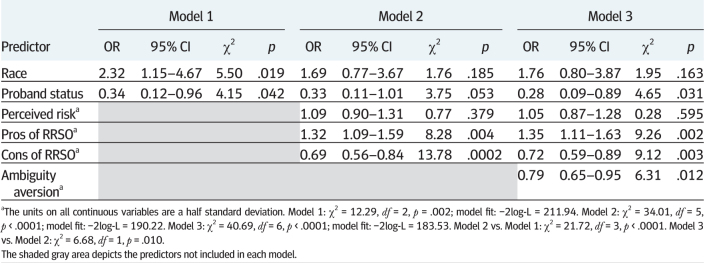
As displayed in Table 4, we used the same approach as for RRM to identify the independent contribution of specific HBM components. On the first step, the demographic and clinical variables that were associated (p < .10) with RRSO (race and proband status) were significantly associated with RRSO intentions (χ2 (2, N = 168) = 12.29, p = .002). In Model 2, the HBM variables (perceived pros, perceived cons, and perceived susceptibility) entered together were strong predictors of RRSO intentions over and above the variables already in the model (Δχ2 (3) = 21.72, p < .0001). Finally, ambiguity aversion, the only affect-related variable that was significantly associated with RRSO, significantly added to the model (Δχ2 (1) = 6.68, p = .010). The following variables were independent predictors of RRSO intentions in the final model: proband status (OR = 0.28, 95% CI = 0.09–0.89), perceived pros of RRSO (OR = 1.35, 95% CI = 1.11–1.63), perceived cons of RRSO (OR = 0.72, 95% CI = 0.59–0.89), and ambiguity aversion (OR = 0.79, 95% CI = 0.65–0.95). Those with a relative with a BRCA mutation were over 3.5 times more likely to have intentions for RRSO. Each half standard deviation increase in perceived pros of RRSO was associated with 35% increased odds of high RRSO intentions. Each half standard deviation increase in perceived cons of RRSO was associated with 28% decreased odds of high RRSO intentions and each half standard deviation increase in ambiguity aversion was associated with a 21% decrease in the odds of having high RRSO intentions.
Table 4.
Logistic regression models of risk-reducing salpingo-oophorectomy (RRSO) intentions
DISCUSSION
We evaluated intentions for RRM and RRSO following genetic counseling but prior to the receipt of genetic test results. We predicted that the HBM variables of perceived risk, and perceived pros and perceived cons of surgery would be important predictors following genetic counseling. We also tested whether affect and uncertainty were associated with intentions over and above the HBM variables. Consistent with our predictions, HBM variables were strong predictors of RRM and RRSO intentions even after controlling for sociodemographics and personal/family cancer history. However, the role of affect and uncertainty was less clear.
We found that 49.2% of participants reported that they probably or definitely would obtain a RRM if they received a positive BRCA test result and 61.3% reported that they would probably or definitely obtain a RRSO. These rates are higher than studies of pre-counseling risk-reducing surgery intentions [15, 34] and are comparable to the actual rates of RRM and RRSO reported in studies of BRCA mutation carriers. For example, in one study, at a mean of 5 years post-testing, 37% of BRCA mutation carriers had obtained RRM and 65% had obtained RRSO [35]. More recent studies have shown comparable but slightly higher rates of uptake [36]. This is consistent with our previous report documenting that post-counseling intentions were highly predictive of subsequent surgical decisions [21].
As expected, the HBM variables that we assessed in this study were important predictors of RRM and RRSO intentions. In particular, perceived benefits and barriers were strong predictors of RRM and RRSO intentions. These associations remained after controlling for relevant sociodemographic, objective risk, and cancer history variables. Recent conceptualizations of informed medical decision-making describe concordance between attitudes and decisions as a key component of informed decisions [37]. The fact that our participants’ intentions are broadly concordant with their preferences, along with previous research suggesting that patients have increased knowledge following genetic counseling [38], provides evidence that genetic counseling facilitates informed decisions about risk-reducing surgery.
In contrast to studies of pre-counseling intentions [15, 17, 39], distress and worry were not associated with risk-reducing surgery intentions. This could reflect the impact of genetic counseling on reducing distress, fostering more accurate risk comprehension, and providing information about the full range of management alternatives [40]. However, this finding must be considered in the context of previous studies that have identified reduction of worry as a key motivator of risk-reducing surgery [41, 42]. It is certainly possible that distress may reemerge as a motivator for surgery following the receipt of a positive test result.
Although distress was not related to risk-reducing surgery intentions, uncertainty in the form of decision conflict and ambiguity aversion emerged as an independent predictor of RRM and RRSO. Higher decisional conflict regarding RRM was associated with lower intentions for obtaining the surgery. Abundant research has documented that individuals with high levels of decisional conflict are less likely to behaviorally implement a health decision [43–45]. In this study, high decision conflict likely reflects that these participants are early in the decision process and have not reached a final decision. Those who have already decided to proceed with RRM in the event of a positive test result have lower decision conflict than those who are undecided or who favor screening. These results are consistent with our previous research in which those who rapidly reach a management decision maintain low decision conflict and high decision satisfaction in the short and longer term [13].
In terms of RRSO, participants who reported high ambiguity aversion also reported lower RRSO intentions. On the one hand, this finding might be surprising since RRSO is recommended for all BRCA mutation carriers and unequivocally reduces the risk of developing ovarian cancer [1, 4, 46]. Thus, one might expect that individuals who are high in ambiguity aversion would favor RRSO. However, the immediate period following genetic counseling and prior to receipt of genetic test results is a highly uncertain period and the decision to obtain an RRSO raises additional uncertainties. For example, there is ongoing uncertainty surrounding the probability of testing positive and the risk for developing ovarian cancer. The majority of women in the study do not have a family history of ovarian cancer. Thus, a positive result from genetic testing would be their only indicator of increased risk of this cancer, which may require some time to assimilate after initial uncertainty about the implications of risk information obtained from genetic testing. For premenopausal women, the uncertainty and decision conflict around RRSO may be related to how the surgery may affect quality of life (e.g., menopausal symptoms and sexual functioning) and how to manage the potential side effects of the surgery, including the appropriateness of hormone therapy. These data suggest that genetic counselors could be proactive in eliciting these concerns and facilitating appropriate follow-up and support for these patients.
This study has several limitations. First, as a cross-sectional analysis of intentions, it is important to extend these findings to prospectively evaluate predictors of uptake of these surgeries. Although intentions may change after participants receive their test results, previous research suggests that post-counseling attitudes are significant predictors of actual uptake of risk-reducing surgery [21]. Second, given the study’s 53% participation rate, caution must be taken in generalizing these findings. Third, this study includes participants who had recently been diagnosed with breast cancer, breast cancer survivors, and unaffected women. Further, we included both probands (i.e., first person in family to be tested) as well as individuals from families in which a pathogenic variant had previously been identified. The content of pretest genetic counseling, including discussions of risk-reducing surgery options, differs somewhat for these groups. Our sample size precluded examination of differences in predictors of risk-reducing surgery intentions across these groups, but this remains a question for future research. Fourth, although we focused on surgical intentions surrounding the identification of a BRCA pathogenic variant (by far the most commonly mutated genes in this population), about 85% of our participants had broader multigene panel testing. We did not evaluate prophylactic surgery intentions associated with genetic mutations other than BRCA1 and BRCA2. Additionally, due to participant burden considerations, we did not assess a number of variables that may have predicted intentions. For example, we did not assess physician recommendation for surgery despite prior evidence suggesting that it is a predictor of surgical decisions [47]. Finally, while we were guided in part by the HBM, we did not assess all HBM components and instead added an assessment of affective and decision-making factors that are not part of the HBM. A more complete assessment of HBM variables might have yielded additional associations that could have altered our conclusions.
Despite these limitations, the data reported here provide support for the role of genetic counseling in fostering informed risk management decisions. Participant attitudes were the strongest and most consistent predictors of both RRM and RRSO intentions. This contrasts with studies of pre-counseling intentions in which negative affect is a more important predictor [17]. It is certainly possible that affective factors will reemerge as important predictors among women who learn that they carry a mutation. However, it is reassuring that following genetic counseling, patients’ intentions appear to be broadly consistent with their preferences. Finally, these data suggest that the role of uncertainty should be further explored. Decision conflict and ambiguity aversion were inversely associated with intentions. Although it has already been established that additional decision support can reduce decision conflict and aid decision-making in the genetic testing context [13], future research should evaluate the role of ambiguity aversion and specific forms of uncertainty following the receipt of test results to determine whether additional support is required.
Acknowledgments
This study was supported by grants (R01 CA135179, R01 HG005055), the Survey, Recruitment and Biospecimen Collection Shared Resource of the Lombardi Comprehensive Cancer Center (P30-CA051008), and by the Jess and Mildred Fisher Center for Hereditary Cancer and Clinical Genomics Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Compliance with Ethical Standards
Conflict of Interest: Ms. M.K. Ladd, Ms. B.N. Peshkin, Ms. S. Baldinger, Dr. C. Isaacs, Ms. H. Segal, Ms. S. Philip, Ms. C. Phillips, Ms. K. Shane, Ms. A. Martin, Ms. V. Weinstein, Mr. R. Pilarski, Dr. J. Jeter, Mr. K. Sweet, Ms. B. Hatten, Ms. E.J. Wurtmann, Ms. S. Phippen, Ms. D. Bro, and Dr. M.D. Schwartz report no conflicts of interest. Ms. L. Senter has received honoraria from Ambry Genetics for a presentation at a sponsored conference unrelated to the content of this study. Ms. L. Senter is also a consultant for Astra Zeneca focused in the genetic testing and PARP inhibitor therapy in ovarian cancer patients; this topic is unrelated to the content of this study.
Authors’ Contributions: Ms. B.N. Peshkin, Dr. C. Isaacs and Dr. M.D. Schwartz conceived and designed the study. Ms. L. Senter and Ms. S. Baldinger contributed to the study design. Ms. L. Senter, Ms. S. Baldinger, Dr. C. Isaacs, Ms. C. Phillips, Ms. K. Shane, Ms. A. Martin, Ms. V. Weinstein, Mr. R. Pilarski, Dr. J. Jeter, Mr. K. Sweet, Ms. B. Hatten, Ms. E.J.Wurtmann., Ms. S. Philip and Ms. D. Bro recruited study participants. Ms. M.K. Ladd, Ms. H. Segal, and Ms. S. Philip collected data. Ms. M.K. Ladd and Dr. M.D. Schwartz performed data analysis. All authors contributed to the writing of this manuscript.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- 1. NCC Network. NCCN Clinical Practice Guidelines in Oncology. Genetic/Familial High-Risk Assessment: Breast and Ovarian. Version 2.2019. Available at https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessibility verified October 26, 2018.
- 2. Kuchenbaecker KB, Hopper JL, Barnes DR, et al. ; BRCA1 and BRCA2 Cohort Consortium Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317(23):2402–2416. [DOI] [PubMed] [Google Scholar]
- 3.Noone AM, Howlader N, Krapcho M, et al. (eds). SEER Cancer Statistics Review, 1975-2015, National Cancer Institute. Bethesda, MD, (https://seer.cancer.gov/csr/1975_2015/) based on November 2017 SEER data submission, posted to the SEER web site, April 2018. Accessibility verified October 26, 2018. [Google Scholar]
- 4. Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101(2):80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kotsopoulos J, Huzarski T, Gronwald J, et al. Bilateral oophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2016;109(1):djw177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parker WH, Jacoby V, Shoupe D, Rocca W. Effect of bilateral oophorectomy on women’s long-term health. Womens Health (Lond). 2009;5(5):565–576. [DOI] [PubMed] [Google Scholar]
- 7. Herrinton LJ, Barlow WE, Yu O, et al. Efficacy of prophylactic mastectomy in women with unilateral breast cancer: a cancer research network project. J Clin Oncol. 2005;23(19):4275–4286. [DOI] [PubMed] [Google Scholar]
- 8. Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE study group. J Clin Oncol. 2004;22(6):1055–1062. [DOI] [PubMed] [Google Scholar]
- 9. den Heijer M, Seynaeve C, Timman R, et al. Body image and psychological distress after prophylactic mastectomy and breast reconstruction in genetically predisposed women: a prospective long-term follow-up study. Eur J Cancer. 2012;48(9):1263–1268. [DOI] [PubMed] [Google Scholar]
- 10. Brandberg Y, Sandelin K, Erikson S, et al. Psychological reactions, quality of life, and body image after bilateral prophylactic mastectomy in women at high risk for breast cancer: a prospective 1-year follow-up study. J Clin Oncol. 2008;26(24):3943–3949. [DOI] [PubMed] [Google Scholar]
- 11. Skytte AB, Crüger D, Gerster M, et al. Breast cancer after bilateral risk-reducing mastectomy. Clin Genet. 2011;79(5):431–437. [DOI] [PubMed] [Google Scholar]
- 12. Schwartz MD, Peshkin BN, Tercyak KP, Taylor KL, Valdimarsdottir H. Decision making and decision support for hereditary breast-ovarian cancer susceptibility. Health Psychol. 2005;24(4S):S78–S84. [DOI] [PubMed] [Google Scholar]
- 13. Schwartz MD, Valdimarsdottir HB, DeMarco TA, et al. Randomized trial of a decision aid for BRCA1/BRCA2 mutation carriers: impact on measures of decision making and satisfaction. Health Psychol. 2009;28(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Howard AF, Balneaves LG, Bottorff JL. Women’s decision making about risk-reducing strategies in the context of hereditary breast and ovarian cancer: a systematic review. J Genet Couns. 2009;18(6):578–597. [DOI] [PubMed] [Google Scholar]
- 15. Tong A, Kelly S, Nusbaum R, et al. Intentions for risk-reducing surgery among high-risk women referred for BRCA1/BRCA2 genetic counseling. Psychooncology. 2015;24(1):33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Dijk S, Otten W, Zoeteweij MW, et al. Genetic counselling and the intention to undergo prophylactic mastectomy: effects of a breast cancer risk assessment. Br J Cancer. 2003;88(11):1675–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Driel CMG, Oosterwijk JC, Meijers-Heijboer EJ, et al. Psychological factors associated with the intention to choose for risk-reducing mastectomy in family cancer clinic attendees. Breast. 2016;30:66–72. [DOI] [PubMed] [Google Scholar]
- 18. Brain K, Gravell C, France E, Fiander A, Gray J. An exploratory qualitative study of women’s perceptions of risk management options for familial ovarian cancer: implications for informed decision making. Gynecol Oncol. 2004;92(3):905–913. [DOI] [PubMed] [Google Scholar]
- 19. Fang CY, Miller SM, Malick J, et al. Psychosocial correlates of intention to undergo prophylactic oophorectomy among women with a family history of ovarian cancer. Prev Med. 2003;37(5):424–431. [DOI] [PubMed] [Google Scholar]
- 20. Hurley KE, Miller SM, Costalas JW, Gillespie D, Daly MB. Anxiety/uncertainty reduction as a motivation for interest in prophylactic oophorectomy in women with a family history of ovarian cancer. J Womens Health Gend Based Med. 2001;10(2):189–199. [DOI] [PubMed] [Google Scholar]
- 21. O’Neill SC, Valdimarsdottir HB, Demarco TA, et al. BRCA1/2 test results impact risk management attitudes, intentions, and uptake. Breast Cancer Res Treat. 2010;124(3):755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Champion VL. Revised susceptibility, benefits, and barriers scale for mammography screening. Res Nurs Health. 1999;22(4):341–348. [DOI] [PubMed] [Google Scholar]
- 23. Skinner CS, Tiro J, Champion VL. The health belief model. In: Glanz K, Rimer BK, Viswanath K, eds. Health behavior: theory, research, and practice. 5th ed. San Francisco, CA: Jossey-Bass; 2015:75–94. [Google Scholar]
- 24. Tanner-Smith EE, Brown TN. Evaluating the health belief model: a critical review of studies predicting mammographic and pap screening. Soc Theory Health. 2010;8(1):95–125. [Google Scholar]
- 25. Baty BJ, Dudley WN, Musters A, Kinney AY. Uncertainty in BRCA1 cancer susceptibility testing. Am J Med Genet C Semin Med Genet. 2006;142C(4):241–250. [DOI] [PubMed] [Google Scholar]
- 26. Han PK, Moser RP, Klein WM. Perceived ambiguity about cancer prevention recommendations: relationship to perceptions of cancer preventability, risk, and worry. J Health Commun. 2006;11(suppl 1):51–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berry DA, Iversen ES Jr, Gudbjartsson DF, et al. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes. J Clin Oncol. 2002;20(11):2701–2712. [DOI] [PubMed] [Google Scholar]
- 29. King L, O’Neill SC, Spellman E, et al. Intentions for bilateral mastectomy among newly diagnosed breast cancer patients. J Surg Oncol. 2013;107(7):772–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom Med. 1979;41(3):209–218. [DOI] [PubMed] [Google Scholar]
- 31. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 32. O’Connor AM. Validation of a decision conflict scale. Med Decis Making. 1995;15(1):25–30. [DOI] [PubMed] [Google Scholar]
- 33. Han PK, Reeve BB, Moser RP, Klein WM. Aversion to ambiguity regarding medical tests and treatments: measurement, prevalence, and relationship to sociodemographic factors. J Health Commun. 2009;14(6):556–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Julian-Reynier C, Bouhnik AD, Mouret-Fourme E, et al. Time to prophylactic surgery in BRCA1/2 carriers depends on psychological and other characteristics. Genet Med. 2010;12(12):801–807. [DOI] [PubMed] [Google Scholar]
- 35. Schwartz MD, Isaacs C, Graves KD, et al. Long-term outcomes of BRCA1/BRCA2 testing: risk reduction and surveillance. Cancer. 2012;118(2):510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Garcia C, Wendt J, Lyon L, et al. Risk management options elected by women after testing positive for a BRCA mutation. Gynecol Oncol. 2014;132(2):428–433. [DOI] [PubMed] [Google Scholar]
- 37. Biesecker BB, Schwartz MD, Marteau TM. Enhancing informed choice to undergo health screening: a systematic review. Am J Health Behav. 2013;37(3):351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Braithwaite D, Emery J, Walter F, Prevost AT, Sutton S. Psychological impact of genetic counseling for familial cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2004;96(2):122–133. [DOI] [PubMed] [Google Scholar]
- 39. Meiser B, Butow P, Friedlander M, et al. Intention to undergo prophylactic bilateral mastectomy in women at increased risk of developing hereditary breast cancer. J Clin Oncol. 2000;18(11):2250–2257. [DOI] [PubMed] [Google Scholar]
- 40. Nelson HD, Pappas M, Zakher B, Mitchell JP, Okinaka-Hu L, Fu R. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: a systematic review to update the U.S. preventive services task force recommendation. Ann Intern Med. 2014;160(4):255–266. [DOI] [PubMed] [Google Scholar]
- 41. Fry A, Rush R, Busby-Earle C, Cull A. Deciding about prophylactic oophorectomy: what is important to women at increased risk of ovarian cancer?Prev Med. 2001;33(6):578–585. [DOI] [PubMed] [Google Scholar]
- 42. Litton JK, Westin SN, Ready K, et al. Perception of screening and risk-reduction surgeries in patients tested for a BRCA deleterious mutation. Cancer. 2010;115(8):1598–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Anderson CJ. The psychology of doing nothing: forms of decision avoidance result from reason and emotion. Psychol Bull. 2003;129(1):139–167. [DOI] [PubMed] [Google Scholar]
- 44. Knops AM, Goossens A, Ubbink DT, Legemate DA, Stalpers LJ, Bossuyt PM. Interpreting patient decisional conflict scores: behavior and emotions in decisions about treatment. Med Decis Making. 2013;33(1):78–84. [DOI] [PubMed] [Google Scholar]
- 45. O’Connor AM. User Manual - Decisional Conflict Scale Available at https://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_decisional_conflict.pdf. Accessibility verified February 2, 2018.
- 46. Tschernichovsky R, Goodman A. Risk-reducing strategies for ovarian cancer in BRCA mutation carriers: a balancing act. Oncologist. 2017;22(4):450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schwartz MD, Lerman C, Brogan B, et al. Impact of BRCA1/BRCA2 counseling and testing on newly diagnosed breast cancer patients. J Clin Oncol. 2004;22(10):1823–1829. [DOI] [PubMed] [Google Scholar]