Abstract
Patient: Female, 46-year-old
Final Diagnosis: COVID provoked thromboembolism
Symptoms: Cough • dyspnea
Medication:—
Clinical Procedure: —
Specialty: Infectious Diseases • General and Internal Medicine
Objective:
Unknown ethiology
Background:
Coronavirus disease 2019 (COVID-19) occurs because of a novel enveloped ribonucleic acid coronavirus called severe acute respiratory distress syndrome coronavirus-2 (SARS-CoV-2). One of the major reported complications of COVID-19 includes both arterial and venous thromboembolism (VTE). Here we describe a case of COVID-19 provoked pulmonary embolism in a young patient already receiving prophylactic treatment for VTE.
Case Report:
A 46-year-old female with past medical history of diabetes mellites, hypertension, and asthma presented in the emergency department (ED) with dyspnea requiring 6 liters per minute of oxygen on presentation. Her main complaints were cough and vomiting. In the ED, hypoxemia worsened, and she ultimately required endotracheal intubation. Labs were suggestive of diabetic ketoacidosis (DKA) and showed increase in all inflammatory markers and absolute lymphocytopenia. Chest X-ray showed bilateral diffuse patchy airspace opacities. Standard DKA management was started. She was also started on ceftriaxone, azithromycin, hydroxychloroquine, and subcutaneous heparin (5000 U every 8 h) for VTE prophylaxis. SARS-Cov2 reverse transcription-polymerase chain reaction returned positive. Ceftriaxone and azithromycin were discontinued the very next day because of low suspicion of bacterial infection while hydroxychloroquine was completed for 5 days. On the third day of admission, the patient self-extubated and was immediately placed on nonrebreather with spO2 in low 90s. On the fourth day of admission, D-dimer came back 4.74 mg/L, which was elevated from a prior value, so computed tomography angiography of the lungs was done, which disclosed multiple emboli in the lungs. She was started on therapeutic doses of enoxaparin sodium, which was continued through her admission. She was switched to Apixaban on discharge.
Conclusions:
The finding of the case suggested that low-molecular-weight heparin prophylaxis may not be sufficient to prevent VTE in COVID-19 pneumonia. Some of these patients may benefit from receiving prophylactic half doses or full doses of anticoagulants.
MeSH Keywords: COVID-19, Pulmonary Embolism, Venous Thromboembolism
Background
Coronavirus disease 2019 (COVID-19), which is caused by the severe acute respiratory syndrome coronavirus 2 (SARSCoV-2), is a novel enveloped ribonucleic acid beta coronavirus believed to have its origin from bats and is thought to have originated in the meat markets of Wuhan, China. According to the new data, almost 5 517 028 confirmed cases are present worldwide, and it has been declared a pandemic by the World Health Organization [1]. So far, multiple complications of COVID-19 have been observed, including both arterial and venous thromboembolism, leading to deep vein thrombosis, pulmonary embolism (PE), and ischemic stroke [2,3]. Precise knowledge pertinent to the pathogenesis of such complications as well as COVID-19 have hardly been described [4] and is being actively discussed by many researchers and clinicians. Here, we describe a case of COVID-19 that provoked PE in a young patient already receiving prophylactic treatment of venous thromboembolism (VTE).
Case Report
A 46-year-old nonpregnant female with past medical history of diabetes mellitus, hypertension, and asthma presented in the Emergency Department (ED) with dyspnea, requiring 6 liters per minute of oxygen on admission. The patient was complaining of cough and had a few episodes of vomiting. In the ED, her respiratory failure with hypoxemia worsened; it was initially managed with nonrebreather but she ultimately required endotracheal intubation. Labs were consistent with diabetic ketoacidosis (DKA). Chest X-ray showed bilateral diffuse patchy airspace opacities, which may represent multifocal or viral pneumonia (Figure 1). Standard DKA management was started. She was also started on ceftriaxone, azithromycin, hydroxychloroquine, and subcutaneous heparin (5000 U every 8 h) for VTE prophylaxis. Upon admission she was afebrile with Tmax 37.3°C, blood pressure 151/87, heart rate 110, respiratory rate 28. Labs showed C-reactive protein (CRP) 392.2 mg/L, lactic acid 1.1 mmol/L, serum sodium 135 mmol/L, serum potassium 5.2 mmol/L, glucose 590 mg/dL, anion gap 18 mmol/L, blood urea nitrogen 30 mg/dL, serum creatinine 1.04 mg/dL, lactate dehydrogenase 571, alanine aminotransferase 18, aspartate aminotransferase 38, white blood cells 10.1 with absolute lymphocyte count of 0.5, red blood cells 4.02, hemoglobin 10.9 mg/dL with mean corpuscular volume 90.3 fL, platelet 308, hematocrit 38.3%, D-dimer 3.68 mg/L, fibrinogen >800 mg/dL. Testing for influenza A, B, respiratory syncytial virus, pneumococcus urine antigen, and legionella urine antigen were negative. The SARS-CoV-2 was confirmed by real-time reverse transcription–polymerase chain reaction test from nasopharyngeal or oropharyngeal swabs. Ceftriaxone and azithromycin were discontinued the very next day because of low suspicion of bacterial infection, whereas hydroxychloroquine was completed for 5 days. On the third day of admission, the patient self-extubated and was immediately placed on nonrebreather with spO2 in low 90s. The next day, she remained on the nonrebreather at 15 L/min with spO2 ranging 95 to 98%. On the fourth day of admission, D-dimer came back 4.74 mg/L, which was elevated from the prior value. A computed tomography (CT) angiography of the lungs (Figures 2, 3) was done, which disclosed multiple emboli involving segmental and subsegmental pulmonary arteries in the left lower and upper lobes and the left lower lobe lobar artery and subsegmental emboli in the right upper lobe, and no large central emboli. There was probable associated right heart strain. The spleen was prominent in size and displayed few small hypoattenuation areas peripherally and its inferior region highly likely related to focal infarcts. Her PE was suspected to be provoked by COVID-19. She was placed on enoxaparin sodium 1 mg/kg every 12 h and was discharged on Apixaban 5 mg twice daily for at least 3 months of anticoagulant.
Figure 1.
Chest X-ray (anteroposterior view), showing bilateral lower diffuse patchy airspace opacities, which may represent multifocal or viral pneumonia.
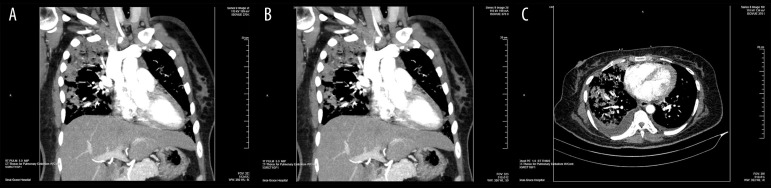
Figure 2.
(A–C) Computed tomography angiography demonstrating multiple emboli involving segmental and subsegmental pulmonary arteries in the left lower and upper lobes and the left lower lobe lobar artery and subsegmental emboli in the right upper lobe. No large central emboli.
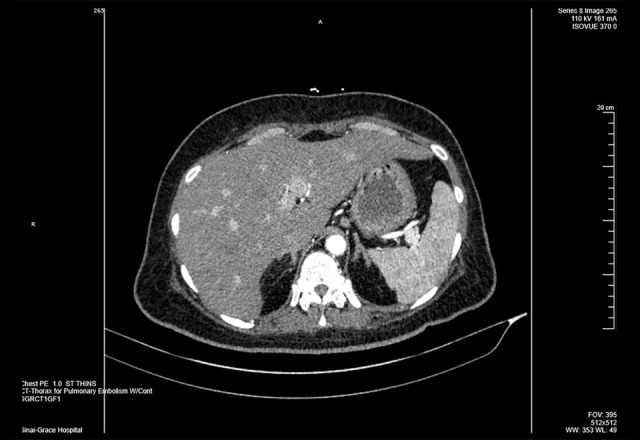
Figure 3.
Computed tomography displays spleen prominent in size and shows few small hypoattenuation areas peripherally and its inferior region highly likely related to focal infarcts. The liver is slightly large and shows focal hypoattenuation anterior–inferior left liver medial section near the fissure compatible with focal fat infiltration.
Discussion
Thrombotic complications are new emerging issues in patients infected with COVID-19, as very limited data are available on postmortem/autopsy findings of such patients that suggest thrombotic microangiopathy [5]. It is suspected to be more prevalent in COVID-19 patients, especially those needing admission in the intensive care unit. Moreover, the precise pathogenetic mechanism is unknown, but multiple etiologies are being suspected by experts in this field [6,7]. One mechanism of pathogenesis being considered is virus attaching to angiotensin-converting enzyme 2 (ACE-2) receptors in pulmonary epithelium and endothelium, leading to proinflammatory cytokine activation, which further activates the coagulation system, such as interleukin 6, a prominent inducer of tissue factor and hence activates thrombin generation. Another perspective involved the coagulation system being evolved as an effector pathway of the immune response. For instance, the end result of inflammation is thrombosis as in Behcet’s disease or vasculitis, and anticoagulants do not improve the outcome in these states. Instead, we treat the inflammatory process.
Moreover, hypoxia could be induced by transcription factor, which leads to a prothrombotic state (affects tissue factor and plasminogen activator inhibitor-1 genes). The patient in discussion developed PE despite being on a prophylactic dose of heparin, suggesting that only a prophylactic dose may not be sufficient to prevent VTE in COVID-19 patients with higher inflammatory markers. Various institutions have implemented their own protocols regarding anticoagulation and testing for VTE in COVID-19. Many institutions are giving double the dose than the prophylactic anticoagulation therapy in COVID-19 patients. Some institutions are giving full-dose anticoagulants to all COVID-19 patients, especially those with D-dimer levels three to five times higher than normal. It is not known if this is the right strategy, and we will need to look toward randomized controlled trials to seek definitive answers in this situation.
Conclusions
The findings of the case suggested that low-molecular-weight heparin prophylaxis may not be sufficient to prevent VTE in COVID-19 patients with proinflammatory state. Some of these patients may benefit from receiving a prophylactic half dose or full dose of anticoagulant. Nevertheless, in the presence of clinical signs or suspicion of VTE, compression ultrasound or echocardiography or CT PE should always be performed, irrespective of disease stage. Last, D-dimer is not a very specific marker for VTE, as it is generally increased in severe COVID-19 pneumonia, but it can still guide testing and anticoagulation strategy in patients with COVID-19 pneumonia on the medical floors as well those in the critical care unit.
References:
- 1.World Health Organization (WHO) Coronavirus disease (COVID-2019) situation reports. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports.
- 2.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–47. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poggiali E, Bastoni D, Ioannilli E, et al. Deep vein thrombosis and pulmonary embolism: Two complications of COVID-19 pneumonia? Eur J Case Rep Int Med. 2020;7(5):001646. doi: 10.12890/2020_001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study [published correction appears in Br Med J. 2020; 368: m1295] Br Med J. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox SE, Akmatbekov A, Harbert JL, et al. Pulmonary and cardiac pathology in covid-19: The first autopsy series from New Orleans. med. Rxiv. 2020:10. doi: 10.1016/S2213-2600(20)30243-5. https://www.medrxiv.org/content/10.1101/2020.04.06.20050575v1.full.pdf+html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–99. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. 2020;18(7):1559–61. doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]