Abstract
Chylothorax is a rare condition characterized by lymph accumulation in the pleural space. When it occurs independent of trauma, it is even more rare and difficult to treat as identification of lymphatic leak is unpredictable. In addition, treatment of this condition with conventional lymphangiography and thoracic duct embolization may not result in positive outcomes. As such, the role of contrast-enhanced dynamic magnetic resonance lymphangiography to guide treatment is key to maximizing success with the advantage of localizing the site of lymphatic leak. Herein, we summarize etiologies of nontraumatic chylothorax, offer an updated treatment algorithm to stratify affected patients and determine appropriate treatment options, and review procedural techniques critical to efficient and effective treatment.
Keywords: thoracic duct, chylous, chylothorax, lymphatic, interventional radiology, pleural space
Etiology
Chylothorax is characterized by chyle accumulating in the pleural space, and thoracentesis with evacuation of milky fluid is the definitive diagnostic test after suspecting pleural fluid following a thorough physical exam, screening laboratory tests, and initial imaging studies. Specifically, the fluid must have a triglyceride count above 200 mg/dL (assuming a regular diet) or more than 70% proportion of lymphocytes to total cell count to be definitively defined as chyle. The gold standard diagnostic test is the presence of chylomicrons on pleural fluid analysis.
Typically, chylothorax results from traumatic disruption of the thoracic duct within the thorax with subsequent accumulation in the pleural space facilitated by the intrathoracic pressure gradient during inspiration. This disruption can be iatrogenic from thoracic interventions or from trauma. On the other hand, nontraumatic chylothorax is usually secondary to mass effect on the thoracic duct prohibiting physiologic transport of lymph to the venous system and causing spontaneous rupture or direct lymphatic invasion from mediastinal pathology.
In approximately half of cases, the culprit of nontraumatic chylothorax is malignancy. The malignancy mostly associated with this condition is lymphoma. Additional, less common etiologies include congenital lymphatic disorders, autoimmune diseases including sarcoidosis, lupus, and Behcet's, and benign mediastinal tumors such as teratomas, thoracic aortic aneurysm, and infections, particularly tuberculosis. 1 2 3 4 5 In the case of congenital lymphatic diseases, entities such as Gorham's disease, Kaposiform lymphangiomatosis, and generalized lymphatic anomaly can cause chylothorax due to the aforementioned mechanisms or from the formation of high-output lymphatic malformations that overwhelm the capacity of the lymphatic vessels leading to rupture. 6
It is imperative to recognize concurrent chylous ascites in any case of chylothorax, as typically the latter is caused by the former via translocation of ascites through diaphragmatic fenestrations facilitated by negative intrathoracic pressure. If there is suspicion of this mechanism of chylous accumulation, dynamic contrast-enhanced magnetic resonance (MR) lymphangiography should be used if available for definitive identification of the leaking nidus. 7 8 9 This imaging technique is also useful for pretreatment evaluation of lymphatic conduction disorders, which result from abnormal lymphatic vessels, valves, or lymphatic volume/flow during fluid overload states (congestive heart failure, cirrhosis). These disorders can cause reversal of normal lymphatic flow, which may result in retrograde flow into the lungs and development of plastic bronchitis or pulmonary lymphatic perfusion syndrome ( Fig. 1 ). 10 Thoracic duct embolization is an effective treatment for these idiopathic conditions. 11
Fig. 1.
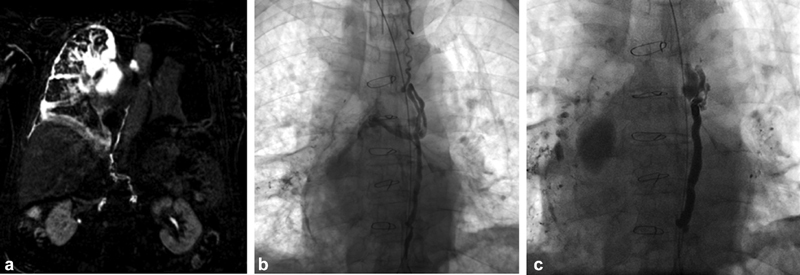
66-year-old male with pulmonary lymphatic perfusion syndrome manifesting with plastic bronchitis and chylothorax. (a) MR lymphangiography MIP reconstruction using heavily T2-weighted sequences demonstrating large lymphatic masses in the right hemithorax and peribronchial lymphatic flow. (b) Catheter lymphangiogram demonstrating transection of the central thoracic duct and filling of the right thoracic lymphatic masses. (c) Completion fluoroscopic image after coil and glue embolization of the thoracic duct.
Management
Contemporary management of nontraumatic chylothorax begins with a trial of conservative management. 12 13 14 This includes placing the patient on a low-fat diet or trial of nothing by mouth. If this fails, serial thoracentesis can be performed to bridge the patient to definitive therapy, which traditionally involved a combined surgical approach involving pleurodesis and thoracic duct ligation. 15 16 Now that thoracic duct embolization by interventional radiology is more widely available, it should be considered in some cases in lieu of surgical management, as it avoids a morbid surgical intervention and can detect nuanced anatomic derangements of the duct. 17 Thoracic duct embolization performed by interventional radiology precipitates collateralization of the lymphatics in the upper abdomen proximal to the embolization site allowing closure of the leak and rerouting of lymphatic flow into veins in the abdomen. 18
The first step in minimally invasive management of a nontraumatic chylothorax is to determine whether chylous ascites is present. If this is suspected on imaging or clinical exam, definitive confirmation of chylous ascites should be obtained by analysis of an abdominal fluid sample, preferably obtained at the same time as pleural fluid sampling to allow comparison of the two fluids. Embolization of the thoracic duct in cases of chylothorax resulting from chylous ascites can worsen both ascites and chylothorax. This occurs because occlusion of the normal outflow of lymphatic drainage through the thoracic duct diverts all lymph and chyle through the persistent leak in the abdomen which is then further translocated into the chest.
Preintervention assessment of the lymphatic system is essential for identifying lymphatic anatomy, flow patterns, and the source of a leak. 19 Contrast-enhanced dynamic MR lymphangiography simplifies the management of nontraumatic chylothorax, which is often more challenging than management of its traumatic counterpart. 7 8 9 As the initial diagnostic test, MR lymphangiography can exclude or confirm the presence of ascites and, if present, can aid in treating this condition. MR lymphangiography utilizes heavily T2-weighted sequences for identification of fluid leak, lymphatic anatomy, and pathologic mediastinal masses. Adding dynamic contrast and static postcontrast series to enhance the thoracic duct and lymphatic structures increases the likelihood of identifying these structures, the presence of lymphatic flow within them, and the precise site of lymphatic leak. 7 Performing contrast-enhanced MR lymphangiography can be challenging in that it is facilitated by relatively close proximity of the angiographic and MR suites to confirm needle placement. However, contrast-enhanced ultrasound has been demonstrated to be a valid localization tool for needle access of the inguinal lymph nodes prior to MR, which can be done wherever point-of-care ultrasound is available. 20
Findings of MR lymphangiography can be categorized into three groups. If contrast is seen leaking from the thoracic duct or there is reversal of pulmonary lymphatic flow, the thoracic duct should be embolized or reconstruction with stent grafts should be considered. Although this latter method could offer the benefit of restoring continuity and function of the thoracic duct, it has only been described in case reports and the application to a larger cohort of patients and long-term follow-up is necessary to characterize its efficacy. 21 If there are lymphatic masses within the retroperitoneum, direct access of the masses should be performed with subsequent glue embolization, which is sometimes referred to as interstitial embolization. In the same manner, if lymph flows from abdominal masses directly to the lungs or pleural space, direct access of these masses should be performed and treated with glue embolization. In the latter pair of scenarios, the intervention with direct glue embolization of lymphatic masses should be performed with the intention of maintaining patency of the thoracic duct.
Technique
When preprocedural planning is complete, the procedure should begin with intranodal lymphangiography to confirm MR findings. Intranodal lymphangiography is superior to traditional pedal access of lymphatics for thoracic duct lymphangiography, as it allows for a shorter interval between injection and opacification of the cisterna chyli and is more often technically successful. 22 A 25-gauge spinal needle is advanced into an inguinal lymph node at a shallow angle under ultrasound guidance, positioning the needle tip at the transitional zone between the nodal cortex and hilum. The needle should be attached to a 3-mL polycarbonate syringe via short extension tubing, the Stylet removed, and flushed with oil-based contrast prior to lymphatic access to reduce needle movement after positioning. The shallow angle will also aid in needle stabilization through the creation of a long subcutaneous tract. Contrast should be injected slowly, at a rate of 0.1 mL per minute. Efferent lymphatics or lymph node opacification should be confirmed. Then, contrast can be injected by attaching an angioplasty balloon inflator filled with lipiodol and maintaining the pressure at 3 mm Hg.
Contrast should reach the L3 level before discontinuing injection. Each lymph node can accept 6 to 12 mL of lipiodol to this end. If 12 mL of lipiodol is injected without visualization of the cisterna chyli, normal saline should be injected at 0.2 mL per minute to further propel the lipiodol through the pelvic lymphatics. Pneumatic sequential compression devices can be placed on the patient's lower legs, which significantly decreases the contrast transit time from the accessed node to the cisterna chyli. 23 After visualization of the cisterna chyli, depending on findings discovered on pretreatment contrast-enhanced MR lymphangiography discussed earlier, the thoracic duct or lymphatic masses are directly accessed using a 21- or 22-gauge needle under fluoroscopic guidance. Lipiodol or water-based iodinated contrast is injected to confirm needle placement followed by injection of N-butyl cyanoacrylate glue diluted with ethiodol at a proportion of 1:1 to 1:3 glue to ethiodol. The dilution factor should be tailored depending on the size of the lymphatic system being embolized with more dilute glue being able to propagate further and fill a larger system.
If the thoracic duct is patent, but there are aberrations in lymphatic flow or abnormal collaterals, thoracic duct embolization can be performed ( Fig. 2 ). This is also indicated if the thoracic duct is occluded causing a leak proximal to the level of the occlusion, which has been shown to be a common cause of nontraumatic chylothorax, and one in which embolization is highly effective. 5 In these cases, a 0.014- or 0.018-inch guidewire should be navigated through the lymphatics cranially, over which a 3F microcatheter can be advanced. Platinum microcoils can be delivered through the catheter just proximal to the abnormal lymphatic structure, followed by a 1:1 mixture of N-butyl cyanoacrylate and ethiodol.
Fig. 2.
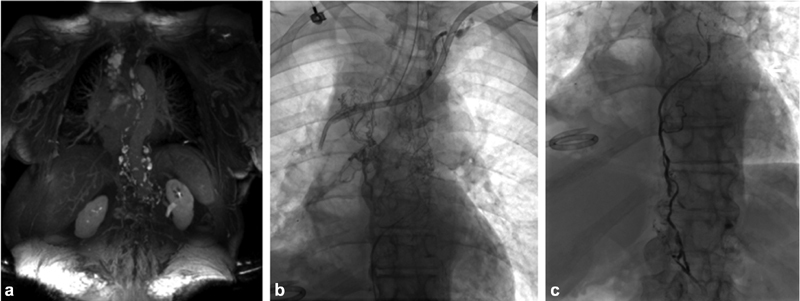
74-year-old female with remote history of triple negative breast cancer treated with chemoradiation and idiopathic right chylothorax accumulating 2 liters of fluid every two weeks. (a) MR dynamic lymphangiography MIP of TWIST sequences (Siemens, Erlangen, Germany) after lymphatic groin access and contrast administration demonstrates extensive lymphatic collateralization in the mediastinum and upper retroperitoneum without identification of a single thoracic duct. (b) Cisterna chyli accessed with a 22-gauge needle and lymphangiography performed. (c) Lymphangiogram after deployment of MVP-3Q vascular plug (Medtronic, Dublin, Ireland) and n-BCA glue embolization of the dominant lymphatic conduit.
Another potential option for the treatment of thoracic duct occlusion or an obvious leak on contrast-enhanced MR lymphangiography is thoracic duct reconstruction using stent grafting. This procedure is performed in a similar manner to embolization, with intranodal lymphangiography, and through-and-through access of the thoracic duct after snaring the wire within the left upper arm venous system after the antegrade wire access of the thoracic duct via the cisterna chyli. Then, a stent graft is deployed over the previously identified leak. 21 This concept has been used in a small number of patients with traumatic disruption of the thoracic duct and subsequent chylothorax; as such, it remains unknown but plausible that this may be effective in cases of nontraumatic chylothorax as long as through-and-through access of the thoracic duct is possible. Although this method could offer the benefit of restoring continuity of the thoracic duct, the overall efficacy and durability has not yet been established.
In cases of obvious thoracic duct outflow obstruction, surgical reanastomosis of the thoracic duct to the subclavian vein or other cervical venous branches has had successful outcomes in the pediatric population, including in nontraumatic etiologies of chylothorax. This technique requires a hybrid approach with interventional radiology and microvascular surgery, with access of the thoracic duct prior to cervical exploration for dye injection of the duct and identification of the thoracic duct during surgery. 24
Follow-up and Outcomes
Following intervention, thoracostomy tube output should be monitored, and once the output has decreased to or below 200 mL/24 hours, a low-fat diet can be resumed to increase lymphatic flow and confirm success with persistence of low output from the tubes, which can then be removed. If output is not decreasing, repeat lymphatic intervention can be attempted.
Nontraumatic chylothorax treatment is not as successful as its traumatic counterpart. Although the largest available published series predated the use of the intranodal lymphangiography and MR lymphangiography, the intention to treat successfully was 53% (18/34), 6 as in 30% of cases the cisterna chyli was not visualized or the thoracic duct could not be accessed. In the subset of technically successful embolizations, the cure rate increased to 68% (16/24). A recent meta-analysis demonstrates similar findings, with 50% clinical success in nontraumatic cases, as opposed to 93% clinical success in traumatic chylothorax. 25 With the advent of intranodal techniques and imaging assessment, cure rate would likely be higher, and future studies involving the algorithm and techniques outlined here are likely to demonstrate improved success in line with cure rates for traumatic chylothorax.
The literature on surgical/conservative management of nontraumatic chylothorax demonstrates poor cure rates, around 27%. 26 In light of these poor outcomes, even pedal lymphangiography techniques for thoracic duct interventions appear auspicious in comparison.
Summary
Nontraumatic chylothorax is a rare condition, stemming from a wide variety of disease states, and is more difficult to treat than traumatic chylothorax. Expectations for outcomes should be tempered, as the proportion of clinical success in the treatment of nontraumatic chylothorax is significantly lower than the success in the treatment of its traumatic counterpart. Despite this, through innovative imaging and procedural techniques, a treatment algorithm ( Fig. 3 ) is proposed that will likely result in improved treatment outcomes and a more auspicious era for affected patients.
Fig. 3.
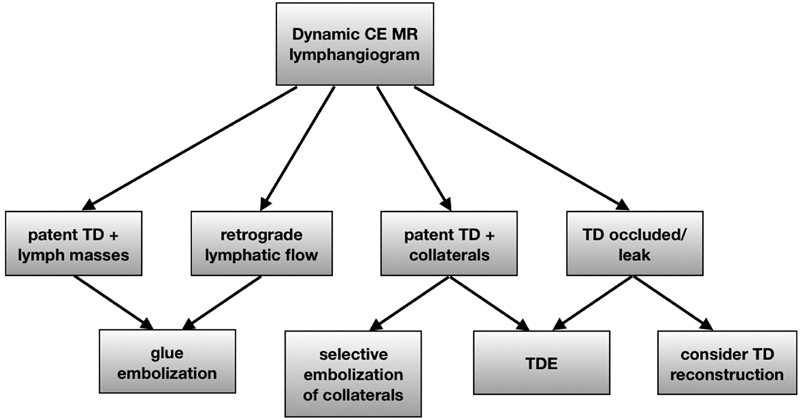
Treatment algorithm for non-traumatic chylothorax in the absence of chylous ascites. CE - contrast enhanced, TD-thoracic duct, TDE- thoracic duct embolization.
Footnotes
Conflict of Interest The authors report no conflict of interest.
References
- 1.Strausser J L, Flye M W. Management of nontraumatic chylothorax. Ann Thorac Surg. 1981;31(06):520–526. doi: 10.1016/s0003-4975(10)61342-2. [DOI] [PubMed] [Google Scholar]
- 2.Romero S. Nontraumatic chylothorax. Curr Opin Pulm Med. 2000;6(04):287–291. doi: 10.1097/00063198-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Valentine V G, Raffin T A. The management of chylothorax. Chest. 1992;102(02):586–591. doi: 10.1378/chest.102.2.586. [DOI] [PubMed] [Google Scholar]
- 4.Doerr C H, Allen M S, Nichols F C, III, Ryu J H. Etiology of chylothorax in 203 patients. Mayo Clin Proc. 2005;80(07):867–870. doi: 10.4065/80.7.867. [DOI] [PubMed] [Google Scholar]
- 5.Bhattarai B, Schmidt F, Devkota A et al. A case of chylothorax in a patient with sarcoidosis: a rare and potentially fatal complication. J Community Hosp Intern Med Perspect. 2015;5(04):28300. doi: 10.3402/jchimp.v5.28300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nadolski G J, Itkin M. Thoracic duct embolization for nontraumatic chylous effusion: experience in 34 patients. Chest. 2013;143(01):158–163. doi: 10.1378/chest.12-0526. [DOI] [PubMed] [Google Scholar]
- 7.Dori Y, Zviman M M, Itkin M. Dynamic contrast-enhanced MR lymphangiography: feasibility study in swine. Radiology. 2014;273(02):410–416. doi: 10.1148/radiol.14132616. [DOI] [PubMed] [Google Scholar]
- 8.Dori Y, Keller M S, Rychik J, Itkin M. Successful treatment of plastic bronchitis by selective lymphatic embolization in a Fontan patient. Pediatrics. 2014;134(02):e590–e595. doi: 10.1542/peds.2013-3723. [DOI] [PubMed] [Google Scholar]
- 9.Krishnamurthy R, Hernandez A, Kavuk S, Annam A, Pimpalwar S. Imaging the central conducting lymphatics: initial experience with dynamic MR lymphangiography. Radiology. 2015;274(03):871–878. doi: 10.1148/radiol.14131399. [DOI] [PubMed] [Google Scholar]
- 10.Dori Y, Keller M S, Rome J J et al. Percutaneous lymphatic embolization of abnormal pulmonary lymphatic flow as treatment of plastic bronchitis in patients with congenital heart disease. Circulation. 2016;133(12):1160–1170. doi: 10.1161/CIRCULATIONAHA.115.019710. [DOI] [PubMed] [Google Scholar]
- 11.Itkin M G, McCormack F X, Dori Y. Diagnosis and treatment of lymphatic plastic bronchitis in adults using advanced lymphatic imaging and percutaneous embolization. Ann Am Thorac Soc. 2016;13(10):1689–1696. doi: 10.1513/AnnalsATS.201604-292OC. [DOI] [PubMed] [Google Scholar]
- 12.Jarman P R, Whyte M K, Sabroe I, Hughes J M. Sarcoidosis presenting with chylothorax. Thorax. 1995;50(12):1324–1325. doi: 10.1136/thx.50.12.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Callaghan A M, Mead G M. Chylothorax in lymphoma: mechanisms and management. Ann Oncol. 1995;6(06):603–607. doi: 10.1093/oxfordjournals.annonc.a059251. [DOI] [PubMed] [Google Scholar]
- 14.al-Khayat M, Kenyon G S, Fawcett H V, Powell-Tuck J. Nutritional support in patients with low volume chylous fistula following radical neck dissection. J Laryngol Otol. 1991;105(12):1052–1056. doi: 10.1017/s0022215100118171. [DOI] [PubMed] [Google Scholar]
- 15.Sieczka E M, Harvey J C. Early thoracic duct ligation for postoperative chylothorax. J Surg Oncol. 1996;61(01):56–60. doi: 10.1002/(SICI)1096-9098(199601)61:1<56::AID-JSO12>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 16.Robinson C L. The management of chylothorax. Ann Thorac Surg. 1985;39(01):90–95. doi: 10.1016/s0003-4975(10)62531-3. [DOI] [PubMed] [Google Scholar]
- 17.Kerlan R K, Jr, Laberge J M. Intranodal lymphangiography: coming soon to a hospital near you. J Vasc Interv Radiol. 2012;23(05):617. doi: 10.1016/j.jvir.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Itkin M, Kucharczuk J C, Kwak A, Trerotola S O, Kaiser L R.Nonoperative thoracic duct embolization for traumatic thoracic duct leak: experience in 109 patients J Thorac Cardiovasc Surg 201013903584–589., discussion 589–590 [DOI] [PubMed] [Google Scholar]
- 19.Nadolski G, Itkin M. Thoracic duct embolization for the management of chylothoraces. Curr Opin Pulm Med. 2013;19(04):380–386. doi: 10.1097/MCP.0b013e3283610df2. [DOI] [PubMed] [Google Scholar]
- 20.Nadolski G J, Ponce-Dorrego M D, Darge K, Biko D M, Itkin M. Validation of the position of injection needles with contrast-enhanced ultrasound for dynamic contract-enhanced MR lymphangiography. J Vasc Interv Radiol. 2018;29(07):1028–1030. doi: 10.1016/j.jvir.2018.02.034. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasa R N, Chick J FB, Hage A N, Gemmete J J, Murrey D C, Srinivasa R N. Endolymphatic thoracic duct stent-graft reconstruction for chylothorax: approach, technical success, safety, and short-term outcomes. Ann Vasc Surg. 2018;48:97–103. doi: 10.1016/j.avsg.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 22.Nadolski G J, Itkin M. Feasibility of ultrasound-guided intranodal lymphangiogram for thoracic duct embolization. J Vasc Interv Radiol. 2012;23(05):613–616. doi: 10.1016/j.jvir.2012.01.078. [DOI] [PubMed] [Google Scholar]
- 23.Meisinger Q C, O'Brien S, Itkin M, Nadolski G J. Use of sequential pneumatic compression devices to facilitate propagation of contrast during intranodal lymphangiography. J Vasc Interv Radiol. 2017;28(11):1544–1547. doi: 10.1016/j.jvir.2017.07.035. [DOI] [PubMed] [Google Scholar]
- 24.Reisen B, Kovach S J, Levin L S et al. Thoracic duct-to-vein anastomosis for the management of thoracic duct outflow obstruction in newborns and infants: a CASE series. J Pediatr Surg. 2020;55(02):234–239. doi: 10.1016/j.jpedsurg.2019.10.029. [DOI] [PubMed] [Google Scholar]
- 25.Kim P H, Tsauo J, Shin J H. Lymphatic interventions for chylothorax: a systematic review and meta-analysis. J Vasc Interv Radiol. 2018;29(02):194–2.02E6. doi: 10.1016/j.jvir.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Maldonado F, Cartin-Ceba R, Hawkins F J, Ryu J H. Medical and surgical management of chylothorax and associated outcomes. Am J Med Sci. 2010;339(04):314–318. doi: 10.1097/MAJ.0b013e3181cdcd6c. [DOI] [PubMed] [Google Scholar]


