Abstract
Lymphatic malformations are low-flow vascular malformations that are typically apparent in the pediatric population and can cause significant functional limitations and effects on quality of life. While surgical resection has historically been the mainstay of therapy, percutaneous sclerotherapy has garnered increasing popularity due to its efficacy and low complication rates. The role of interventional radiology in the multidisciplinary management of these often complex malformations requires thorough understanding of the disease process. This article will review the pathophysiology, clinical presentation, imaging workup, and management options of lymphatic malformations. Special attention will be devoted to available sclerosants, the mammalian target of rapamycin inhibitor sirolimus, and complex lymphatic anomalies.
Keywords: lymphatic malformation, sclerotherapy, sirolimus
Lymphatic malformations (LMs) are congenital low-flow vascular malformations caused by abnormal embryogenesis of lymphatic channels which rarely communicate with the normal lymphatic system and occur in approximately 1 in 2,000 to 4,000 live births. 1 The updated 2014 International Society for the Study of Vascular Anomalies (ISSVA) Vascular Anomalies Classification divides common (or cystic) LMs as macrocystic, microcystic, or mixed. 2 Although no clear size parameters exist, macrocystic lesions are generally defined as those larger than 1 to 2 cm. 3 The most common locations of LMs are those areas already enriched with lymphatic tissue: the head and neck and axillary regions as well as the mediastinum, groin, and retroperitoneum. 2 4 5 Special attention should also be paid to LMs involving the airway and orbit as rapid expansion of lesions in these locations in the setting of intralesional hemorrhage can cause mass effect with significant morbidity and mortality. 6
LMs are also associated with several syndromes such as Klippel–Trenaunay–Weber syndrome (KTS), CLOVES (congenital lipomatous overgrowth, vascular malformations, epidermal nevi, spinal/skeletal abnormalities) syndrome, Turner's syndrome, and Proteus' syndrome. 7 8 Although a thorough description of all syndromes associated with LMs is beyond the scope of this article, it is important for interventional radiologists to be aware of these syndromes so that they provide necessary, comprehensive, multidisciplinary care that is commonly required to treat patients. 9 10
The purpose of this article is to describe the clinical presentation, diagnostic workup, and treatment of LMs, with an emphasis on common macrocystic, microcystic, and mixed types. Complex lymphatic anomalies will also be discussed briefly.
Cell Signaling Pathways
Several mutations have been implicated in the development of LMs. The phosphoinositide-3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) signaling pathway is an important part of the normal cell cycle, and activating mutations in the PI3K catalytic subunit-α (PIK3CA) have been associated with sporadic LMs as well as in patients with CLOVES and KTS. 3 7 11 The PI3K/mTOR pathway is a target for systemic sirolimus therapy (see discussion later), which has become a vital therapeutic component for many patients with simple and complex LMs.
Additionally, RASA1 mutations have been associated with capillary, arteriovenous, and LMs. 12 Vascular endothelial growth factor receptor-3 (VEGFR3), when activated by vascular endothelial growth factor C (VEGFC), is associated with lymphangiogenesis, and VEGFC overexpression has been demonstrated in Milroy's disease. 7 Additional implicated genes include Ang2, Lyve1, and Nrp2. 6 Ongoing research is being done to continue to elucidate more specific cell-signaling targets for systemic treatment of LMs. 13
Clinical Presentation and Workup
LMs present as ballotable soft-tissue masses that are present at birth and grow proportionally with the child. The overlying skin can have a bluish appearance but commonly appears normal. The presence of cutaneous vesicles or angiokeratomas is common in microcystic lesions and increase the risk of superinfection. 14 15 Although these vesicles predominantly contain clear fluid, the presence of chylous fluid suggests intra-abdominal involvement, as its components are absorbed from the intestinal tract. 14 16 LMs are technically purely cystic without a soft-tissue component, despite the morphologic appearance of some microcystic LMs, but overgrowth of adjacent soft tissues and osseous structures is common. 17 When intralesional hemorrhage or superinfection occurs, rapid enlargement and skin changes can occur, which is of importance in the setting of airway and intraorbital lesions. LMs are commonly very firm in times of acute inflammation or intralesional hemorrhage. Even in the absence of acute expansion, airway lesions can cause significant difficulties with oral nutrition, dyspnea, sleep apnea, and speech, while intraorbital lesions can affect optic nerve and extraocular muscular function. 7 Inflammatory expansion can also occur during systemic infectious processes such as upper respiratory viral infections. 3
Sporadic LMs are not typically associated with consumptive processes, although a small case series demonstrated lymphocytopenia in patients with large microcystic LMs. 3 18 Complex and syndromic malformations can be associated with elevated D-dimer and thrombocytopenia (Kasabach–Merritt phenomenon), especially KLA. 16 19 KLA and kaposiform hemangioendothelioma (KHE) patients may have elevated angiopoietin-2 serum levels at presentation, which decreases after sirolimus treatment. 20
Imaging
Ultrasound
Macrocystic LMs appear as multiloculated cystic lesions on ultrasound and do not demonstrate flow on color Doppler except within intervening septae. Multiple wall interfaces cause microcystic lesions to appear hyperechoic and potentially more solid. 15 Intralesional hemorrhage can alter the ultrasonographic appearance with increased echogenicity or solid echogenic thrombus. 21 Superinfected LMs also can demonstrate increased echogenicity as well as surrounding hyperemia and thickening of the septae. 21 22 Differentiating an abscess from an infected LM can be challenging via imaging, and often requires clinical follow-up to solidify the diagnosis. Additionally, prenatal ultrasound in the second and third trimesters can often diagnose LMs and prompt further workup, such as fetal magnetic resonance imaging (MRI). 7
Computed Tomography
Computed tomography (CT) has a limited role in the evaluation of LMs, but its short acquisition time is useful in the setting of hemorrhage with rapid enlargement and resultant compression. 6 LMs appear as well defined, lobulated, fluid density structures on CT and can demonstrate fluid–fluid levels depending on intralesional composition. 23 Mild septal and capsular enhancement is common. Intralesional contents have a hyperdense appearance if intralesional hemorrhage is present.
Magnetic Resonance Imaging
Macrocystic LMs appear as multicystic T1 hypointense, T2 hyperintense lesions on MRI which can demonstrate minimal septal and capsular enhancement following contrast administration. Microcystic LMs can appear solid and hyperintense on fluid-sensitive sequences with near homogeneous enhancement ( Fig. 1a, b ). 23 24 MRI is superior in delineating the extent of involvement of fascial and anatomic planes. As with other modalities, the presence of proteinaceous debris and hemorrhage can alter the MRI appearance of LMs. Marked post–contrast enhancement can occur in the setting of active inflammation. Fetal MRI is also important in prenatal evaluation and directs further intervention including delivery. 7
Fig. 1.
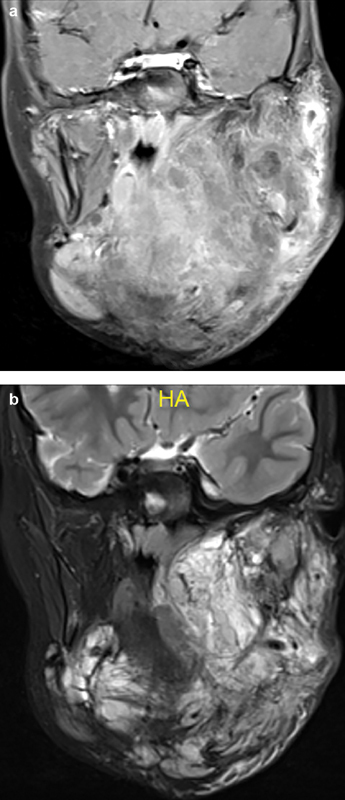
Coronal T1 postcontrast ( a ) and T2 ( b ) MRI in a 6-year-old female with a transspatial microcystic lymphatic malformation show increased T2 signal throughout with a contrast enhancement pattern suggestive of a confluent, solid/semisolid mass due to the microcystic nature of the malformation.
Treatment
The primary goal in the management of LMs is to improve function (most notably in head/neck LMs), decrease the risk of infection, and minimize symptoms with aesthetic preservation. Acute life-threatening situations from rapid lesion enlargement require immediate intervention. In the nonacute setting, evaluation within a multispecialty vascular anomalies clinic is recommended, as many of these lesions commonly require minimally invasive, systemic, and surgical treatments to achieve treatment goals.
Sclerotherapy
Percutaneous sclerotherapy involves the instillation of a sclerosing agent which damages the lymphatic endothelium and promotes fibrosis and scarring within the malformation. Multiple sclerosing agents are available and include doxycycline, bleomycin, sodium tetradecyl (STS) foam, OK432 (Picibanil), and ethanol, although doxycycline and bleomycin are the most used drugs to treat LMs. No randomized control trial has been performed to compare outcomes of surgical excision versus percutaneous sclerotherapy, but retrospective data suggest similar results. 25 26 Sclerotherapy has become increasingly popular in recent decades in the initial management of LM due to its safety, efficacy, and cost profile compared with surgery. 27 28
For macrocystic LMs ( Fig. 2a–i ), access is typically obtained under ultrasound guidance, and cyst aspiration is performed. If large enough, a pigtail catheter is often used for access into the largest, noncommunicating macrocysts. Multiple access sites are often required for thorough treatment. Aspirated fluid should be sent for cytology at initial intervention, as several cystic neoplasms can mimic LMs such as teratomas, rhabdomyosarcomas, and synovial sarcomas. 3 21 Once the cyst has been aspirated, cystography is performed under fluoroscopy to exclude communication with the systemic venous vasculature. Next, sclerosant is instilled to partially fill the cyst. Typically, 50 to 75% of the aspirated volume is instilled in the cyst. If the cyst is large enough, a pigtail catheter can be used to obtain access and perform multiday treatment, based on the chosen sclerosant. 14
Fig. 2.

A neonate with a large, transspatial, head and neck macrocystic lymphatic malformation (LM). Coronal T1 postcontrast ( a ) and coronal T2 ( b ) MRI show the classic appearance of septal/peripheral contrast enhancement and high T2 signal throughout the LM. Ultrasound images ( c, d ) show anechoic fluid collections with thin intervening septae with no internal Doppler signal. Treatment entailed ultrasound-guided need access (red arrows) into the macrocysts ( e ) and placement of a pigtail drainage catheter (red arrows) ( f ). ( g ) Digitally subtracted angiography to ensure that there is no outflow into the systemic venous system. In this case, a 6-Fr pigtail catheter ( h ) was used to instill the doxycycline, which was left to dwell for 45 minutes. Coronal T2 MRI image ( i ) shows reduction in size of the LM after 3 sclerotherapy treatments.
Microcystic LMs are more difficult to treat via sclerotherapy and often require more treatments than macrocystic LMs. When multiple treatments are required to treat macrocystic or microcystic LMs, these are commonly spaced 6 to 8 weeks apart. Multiple sclerosing agents including STS foam, doxycycline, bleomycin, and OK432 have been used in the percutaneous management of microcystic LMs and are described in more detail later. 6
Patient comfort and lesion characteristics such as size and location often necessitate general anesthesia for percutaneous sclerotherapy in neonates and children. 14 Older patients may tolerate moderate sedation based on the location of the LM. Antibiotic prophylaxis prior to percutaneous access is common to minimize the risk of lesion superinfection but is not required. 6 Systemic steroids such as dexamethasone can also be administered to reduce postprocedural swelling in large macrocystic LMs or those involving the airway and orbit. 29 Complications of percutaneous sclerotherapy include skin ulceration, superinfection, scarring, and nerve damage. Compartment syndrome can also occur when treating large lesions in the extremity. 29 A recent systematic review of percutaneous sclerotherapy by de Maria et al reported overall rates of permanent morbidity and mortality of 1.2% and local transient complication, such as skin injury, of 14.0%. 30 Higher complication rates have been reported in the management of microcystic lesions. 30
Doxycycline
Doxycycline is a tetracycline antibiotic that is both inexpensive and easily accessible. It is also a safe and effective sclerosant option for macrocystic LMs. 31 Its use as a sclerosing agent was first reported in 1995 by Molitch et al, and although its exact mechanism is unknown, it causes matrix metalloproteinase and VEGF inhibition. 32 33
Multiple studies have touted doxycycline as superior to multiple other sclerosing agents with success rates of 79 to 100%. 30 33 34 35 Side effects include hemolytic anemia and metabolic acidosis in neonates as well as tooth discoloration and skin ulceration/necrosis if extravasation occurs. 6 Due to the side effect profile, many institutions limit single-session doses to 150 mg in neonates, 300 mg in infants <12 months and 1,200 mg in all other patients with typical concentrations ranging from 10 to 20 mg/mL reconstituted in a combination of sterile water and iodinated contrast. 3 6 Because doxycycline can cause significant pain for several hours following instillation, patients should be managed aggressively periprocedurally including with narcotic pain medications and nonsteroidal anti-inflammatory medications. 14
When lesions are large enough to accommodate pigtail catheters ( Fig. 3a–e ), doxycycline can be injected and aspirated after 4-hour dwell time for 2 days and then reinstilled on the third day prior to drain removal. 3 6 34 36 Other institutions allow the doxycycline to dwell for 45 to 60 minutes or less and remove the drain on the same day or instill the doxycycline and leave it to dwell without aspiration. 3 33 37 No studies have been performed to show superiority of one technique over another.
Fig. 3.

A 3 year-old-male presented with a right lower extremity macrocystic lymphatic malformation. Coronal T1 postcontrast ( a ), T2 ( b ), and axial T2 ( c ) MRI images show multiple macrocysts with peripheral/septal enhancement and high T2 signal. These lesions were treated with multiple doxycycline sclerotherapy procedures. ( d ) The classic sonographic appearance of doxycycline at the time of instillation/sclerotherapy. T2 axial MR ( e ) shows decreased size (as compared with c ) of the macrocysts following multiple sclerotherapy treatments.
Bleomycin
Bleomycin is an antibiotic derivative and antineoplastic agent predominantly utilized in the treatment of carcinomas and lymphomas and elicits DNA synthesis disruption as well as endothelial damage when directly instilled into LMs. 38 Major advantages of bleomycin are its utility in treating microcystic lesions and its minimal postprocedural swelling, which makes it an ideal agent in the treatment of airway, floor of mouth, and orbital lesions ( Fig. 4a–c ). 29 39 Prolonged edema has been reported following sclerotherapy of microcystic floor of mouth LMs. 39 Bleomycin can be administered in a liquid or foam medium and is typically reconstituted in normal saline then mixed with 25% human serum albumin and air in a ratio of 1:1:4. 40 Foamed bleomycin offers increased endothelial surface contact and allows for smaller per treatment doses. 41
Fig. 4.

A 5-year-old female presented with acute onset of right-sided proptosis. Coronal T2 MR ( a ) shows a retroorbital, intra- and extraconal lymphatic malformation (red arrow). This lesion was treated with bleomycin sclerotherapy. ( b ) A 25-gauge needle (red arrow) within the largest microcyst. Fluid was aspirated prior to instillation of 15 U of bleomycin in 2 mL of normal saline. Coronal T2 MR following two sclerotherapy treatments ( c ) shows marked reduction in size of the macrocysts with resolution of proptosis.
Special care is taken in the treatment of delicate areas involving the airway. Depending on location and risk of airway obstruction, sclerotherapy procedures are performed under general anesthesia with endotracheal intubation. While low-risk lesions can be treated with moderate sedation without a secured airway or general anesthesia with laryngeal mask airway, equipment for emergent endotracheal intubation and/or tracheostomy should be readily available. 42 Multiple staged treatments should also be considered to minimize postprocedural edema. 42 Following sclerotherapy of airway lesions, the patient should be observed in the intensive care setting. Intravenous dexamethasone can mitigate postprocedural swelling. When endotracheal intubation is required, evaluation for risk of airway obstruction postextubation should be frequently monitored and, although controversial, an air leak test can be utilized to predict extubation success. 43
Orbital lesions also require special technical considerations. Fluoroscopic evaluation following access should be performed to confirm that the lesion does not drain into the superior ophthalmic vein or cavernous sinus. 44 Increased orbital pressure from postprocedural swelling can result in blindness, and pre- and postsclerotherapy dexamethasone can decrease that risk. 44 45
Its side effect profile is most well studied in the setting of systemic chemotherapy and organs without bleomycin hydrolase, a bleomycin-inactivating enzyme, such as the lungs and skin are most susceptible to toxicity-related effects. 46 The most common pulmonary presentation is bleomycin-induced pneumonitis, which can progress to pulmonary fibrosis, while other entities including bronchiolitis obliterans have also been reported. 46 Risk factors are related to cumulative dose, impaired renal function, and advanced age. 47 48 Cumulative doses exceeding 300 mg have been associated with rates of up to 8.5% of pulmonary toxicity. 39 49 A common institutional dose policy is 0.5 to 1 mg/kg with a single-session maximum dose of 15 mg. To date, no percutaneous sclerotherapy-associated cases of pulmonary fibrosis have been reported, although a single case of acute lung toxicity from bleomycin treatment of a vascular malformation has been reported. 50 51 52 A small prospective study of pediatric patients with microcystic LMs undergoing percutaneous bleomycin sclerotherapy showed no significant changes in chest radiography and pulmonary function tests 6 and 12 months following treatment, and abnormalities in diffusing capacity of the lungs for carbon monoxide and vital capacity have been described as most sensitive and specific for bleomycin-induced damage, respectively. 39 Importantly, because of the rarity of pulmonary complications associated with bleomycin sclerotherapy (with adherence to dose limits), it is not common practice to obtain preprocedure chest X-rays or pulmonary function tests.
Hypersensitivity-type and idiosyncratic reactions to bleomycin have also been reported in chemotherapy literature, can occur within hours of administration, and are not related to dose. 39 Typical symptoms include hyperpyrexia, chills, hypotension, and respiratory distress. 53 Dermatologic manifestations of toxicity include hyperpigmentation and self-limited flagellate erythema. 40
Sodium Tetradecyl Sulfate
STS (Sotradecol; AngioDynamics, Queensbury, NY) is a detergent that emulsifies membrane lipoproteins. 38 This results in increased membrane permeability and may increase efficacy when used in combination with additional sclerosants. 29 38 STS foam is created by combining the detergent with ethiodized oil (Ethiodol; Savage Laboratories, Melville, NY) and air, typically with a 1:2:3 ratio of ethiodized oil to STS to air. 3 Side effects include hemolysis and increased infection risk, but overall systemic and neurologic side effects are reportedly lower than ethanol. 14 29 38 STS has been shown to be less efficacious than doxycycline in the treatment of LMs, and subsequently its use to treat LMs is limited. 34
OK-432
OK-432 (Picibanil) is a strain of group A Streptococcus pyogenes pretreated with benzylpenicillin G and heat which triggers an inflammatory response and fibrosis when injected into LMs. 6 38 54 OK432 is efficacious in treating both macrocystic and microcystic lesions. 54 55 Side effects include fever, pain, edema, and rarely anaphylaxis, and systemic side effects are reported at higher rates than other sclerosants. 30 38 Since institutional review board approval is needed to use OK-432, and its efficacy is not superior to other sclerosants; its use in the United States is limited. 56
Other Sclerosants
Several other sclerosing agents warrant comment. Absolute ethanol, which causes dehydration of endothelial cells, is effective but has a high rate of complications, most commonly skin ulceration, as well as an extensive side effect profile including respiratory depression, arrhythmias, and seizures. 38 57 Because of its poor safety profile, it is typically not used in the treatment of LMs. Pingyangmycin is structurally similar to bleomycin, is used predominantly in China, and is associated with low complication rates. 57 Its use in the United States is limited.
Ablation
Radiofrequency ablation (RFA) is an emerging therapy for LMs, especially microcystic and superficial lesions with two potential approaches based on lesion characteristics. High-frequency mode can be utilized to treat deeper tissues, while low-frequency mode is superior in treating thin, more superficial components. 58 RFA causes minimal nontarget thermal damage and is ideal for airway, oral cavity, and other delicately located lesions. 7 59
While early results have demonstrated cryoablation as a safe and efficacious treatment option in venous malformations, its use in LMs has not been evaluated and requires further study. 60 61
Laser Therapy
Laser therapy is another treatment option for superficial cutaneous LMs. Carbon dioxide lasers emit light that is converted to vaporizing heat upon contact with the skin and seals blood and lymphatic vessels. CO 2 lasers can penetrate to the mid-dermis and are useful in the management of cutaneous microcystic lesions. 62 Treatment with CO 2 lasers requires general anesthesia and can result in scarring. 7 63 Neodymium:yttrium-aluminum-garnet (Nd:YAG) lasers have had particular success in debulking oral cavity lesions. 63 Use of pulsed dye laser has also been described, but its penetration depth is less than that of Nd:YAG lasers, limiting its utility to more superficial cutaneous LMs. 63
Surgery
Surgical management of LMs has been the historic gold standard with a goal of complete excision. However, because LMs can involve multiple fascial planes, total surgical resection is often not possible, especially in microcystic lesions. 5 Preservation of vascular and neural structures is prioritized as well minimizing blood loss. 5 64 Preoperative considerations include the need for multiple staged surgeries as well as securing the airway and enteral nutrition with tracheostomy and/or gastrostomy tubes prior to treatment. 5 Recurrence is common and can present with vesicle formation within scar tissue, especially when complete excision is not possible. Surgical resection is commonly utilized as a complementary procedure following incomplete response after sclerotherapy or for resection of residual fibrotic scar tissue after the lesion has shrunk in response to sclerotherapy. 3 Complications from surgery include hemorrhage, nerve damage, infection, seroma, lymphatic leak, postoperative pain, and lesion recurrence. 29 65
Medical Management
Sirolimus
The mTOR is a serine/threonine protein kinase involved in protein synthesis, cell motility, angiogenesis, and cellular growth and has been implicated in the development of vascular malformations and LMs. 66 67 Sirolimus (rapamycin) is an mTOR inhibitor with antiproliferative and immunosuppressant properties and has been shown to decrease available VEGF receptor 3, which is involved in lymphangiogenesis. 66 It has shown early promise in the management of LMs and other vascular anomalies since its first use for which indication was reported in 2011, especially extensive and difficult to treat microcystic LMs. 66 67
Sirolimus is usually initiated at a dose of 0.8 mg/m 2 twice daily and requires frequent laboratory assessment for dose titration. In a systematic review, 95% of patients with LMs demonstrated response to sirolimus. 66 In a cohort of six patients with diffuse microcystic LM, capillary lymphaticovenous malformation, and KHE treated with systemic sirolimus, all patients showed clinical improvement. 67 Sirolimus has also been shown to be effective in cervicofacial LMs, and early results suggest that macrocystic LMs may respond better than microcystic and mixed lesions. 68 Symptomatic improvement has also been shown in GLA and GSD after sirolimus. 69
Sirolimus is associated with several potentially severe side effects. Immunosuppressive qualities of sirolimus increase infection risk, and cellulitis has been described in the treatment of LMs. 68 Other adverse effects include blood and bone marrow toxicity including anemia and thrombocytopenia, gastrointestinal issues, dermatologic manifestations like rash and mucositis, glucose intolerance, and hypercholesterolemia. 29 70 71 Although multiple retrospective studies and reviews have described the safety and efficacy of sirolimus, further investigation is necessary to establish its role in the management of LMs, and it should be administered under the supervision of an experienced clinician. The emergence of sirolimus as an important component in the management of LM underscores the importance of multidisciplinary management of these lesions.
Complex Lymphatic Anomalies
In addition to macrocystic, microcystic, and mixed LMs, the ISSVA also describes several other entities referred to as complex lymphatic anomalies. The complex lymphatic anomalies include generalized lymphatic anomaly (GLAD), Gorham-Stout disease (GSD), and Kaposiform lymphangiomatosis (KLA). GLA describes multifocal LMs and can involve both superficial soft tissues as well as thoracic and abdominal organs. 2 GLA also commonly demonstrates osseous involvement from persistently enlarged lymphatics and can cause lytic lesions of multiple bones, usually within the appendicular skeleton with preservation of the overlying cortex. 2 16 GSD is a related but separate entity with thin lymphatic-like channels which also affects the skeletal system and is associated with cortical bone loss and has been referred to as the “vanishing bone disease.” 16 KLA presents similarly with GLA but with a predilection for the mediastinum and cystic splenic lesions. 16 19 KLA is histologically distinct from other complex lymphatic anomaly subtypes with characteristic sheets of spindled lymphatic endothelial cells, and it is associated with respiratory distress from pericardial and pleural effusions and worse clinical outcomes. 19 72 73 Primary lymphedema is another congenital LM caused by dysgenesis of the lymphatic system and is associated with several genetic mutations. 2 Although congenital, primary lymphedema typically presents clinically around puberty with lower extremity edema, may be hormonally sensitive, and includes entities such as Milroy's disease. 74 75 Congenital conducting lymphatic anomalies are associated with lymphangiectasia and lymphatic dysmotility with resultant stasis and obstruction. 16 19
Although interventional radiology currently plays a limited role in the treatment of complex lymphatic anomalies, diagnosis and characterization of extent of these anomalies often relies on multimodality imaging techniques. Radiography can demonstrate osseous abnormalities from GSD and GLA. While progressive osseous resorption and cortical loss are features of GSD, appendicular skeletal involvement is more common in GLA. 76 The role of radiography in the diagnosis of LMs is otherwise limited. CT demonstrates thoracic involvement in GLA and KLA as increased soft-tissue density along interlobular septae and bronchovascular bundles, within the mediastinum, and along the pleura. 73 GSD, GLA, and KLA can all affect the spine, most commonly the lumbar spine, with T1 hypointense fatty marrow replacement and T2 hyperintensity at affected levels on MRI. 77 Associated infiltrative soft-tissue components appear similar to common LMs with T2 hyperintensity. 73 Magnetic resonance lymphangiography (MRL) is also helpful in establishing the extent and involvement of complex lesions. 78 The technique for MRL has been described extensively by prior authors and commonly involves interventional radiologists.
Conclusion
The diagnosis and management of LMs is often complex and requires multidisciplinary care. Although the role of percutaneous sclerotherapy as primary treatment has increased, systemic medication and surgery are often complementary therapeutic options. As therapies continue to evolve, a thorough understanding of the disease process, cell-signaling pathways, and medications/sclerosants used to treat LMs is paramount for interventional radiologists.
Conflict of Interest There are no conflicts of interest to disclose.
Notes
No funding was received for this study. The data have not been submitted for presentation at any meeting.
References
- 1.Kennedy T L, Whitaker M, Pellitteri P, Wood W E.Cystic hygroma/lymphangioma: a rational approach to management Laryngoscope 2001111(11, Pt 1):1929–1937. [DOI] [PubMed] [Google Scholar]
- 2.Wassef M, Blei F, Adams D et al. Vascular anomalies classification: recommendations from the international society for the study of vascular anomalies. Pediatrics. 2015;136(01):e203–e214. doi: 10.1542/peds.2014-3673. [DOI] [PubMed] [Google Scholar]
- 3.Hawkins C M, Chewning R H. Diagnosis and management of extracranial vascular malformations in children: arteriovenous malformations, venous malformations, and lymphatic malformations. Semin Roentgenol. 2019;54(04):337–348. doi: 10.1053/j.ro.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Perkins J A, Manning S C, Tempero R Met al. Lymphatic malformations: review of current treatment Otolaryngol Head Neck Surg 201014206795–803., 803.e1 [DOI] [PubMed] [Google Scholar]
- 5.Elluru R G, Balakrishnan K, Padua H M. Lymphatic malformations: diagnosis and management. Semin Pediatr Surg. 2014;23(04):178–185. doi: 10.1053/j.sempedsurg.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Cahill A M, Nijs E L. Pediatric vascular malformations: pathophysiology, diagnosis, and the role of interventional radiology. Cardiovasc Intervent Radiol. 2011;34(04):691–704. doi: 10.1007/s00270-011-0123-0. [DOI] [PubMed] [Google Scholar]
- 7.Defnet A M, Bagrodia N, Hernandez S L, Gwilliam N, Kandel J J. Pediatric lymphatic malformations: evolving understanding and therapeutic options. Pediatr Surg Int. 2016;32(05):425–433. doi: 10.1007/s00383-016-3867-4. [DOI] [PubMed] [Google Scholar]
- 8.Puig S, Casati B, Staudenherz A, Paya K. Vascular low-flow malformations in children: current concepts for classification, diagnosis and therapy. Eur J Radiol. 2005;53(01):35–45. doi: 10.1016/j.ejrad.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 9.Bertino F, Braithwaite K A, Hawkins C M et al. congenital limb overgrowth syndromes associated with vascular anomalies. Radiographics. 2019;39(02):491–515. doi: 10.1148/rg.2019180136. [DOI] [PubMed] [Google Scholar]
- 10.Bertino F, Chaudry G. overgrowth syndromes associated with vascular anomalies. Semin Roentgenol. 2019;54(04):349–358. doi: 10.1053/j.ro.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Keppler-Noreuil K M, Parker V E, Darling T N, Martinez-Agosto J A. Somatic overgrowth disorders of the PI3K/AKT/mTOR pathway & therapeutic strategies. Am J Med Genet C Semin Med Genet. 2016;172(04):402–421. doi: 10.1002/ajmg.c.31531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michelini S, Paolacci S, Manara E et al. Genetic tests in lymphatic vascular malformations and lymphedema. J Med Genet. 2018;55(04):222–232. doi: 10.1136/jmedgenet-2017-105064. [DOI] [PubMed] [Google Scholar]
- 13.di Blasio L, Puliafito A, Gagliardi P A et al. PI3K/mTOR inhibition promotes the regression of experimental vascular malformations driven by PIK3CA-activating mutations. Cell Death Dis. 2018;9(02):45. doi: 10.1038/s41419-017-0064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burrows P E. Endovascular treatment of slow-flow vascular malformations. Tech Vasc Interv Radiol. 2013;16(01):12–21. doi: 10.1053/j.tvir.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Dubois J, Alison M. Vascular anomalies: what a radiologist needs to know. Pediatr Radiol. 2010;40(06):895–905. doi: 10.1007/s00247-010-1621-y. [DOI] [PubMed] [Google Scholar]
- 16.Trenor C C, III, Chaudry G. Complex lymphatic anomalies. Semin Pediatr Surg. 2014;23(04):186–190. doi: 10.1053/j.sempedsurg.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Paltiel H J, Burrows P E, Kozakewich H P, Zurakowski D, Mulliken J B. Soft-tissue vascular anomalies: utility of US for diagnosis. Radiology. 2000;214(03):747–754. doi: 10.1148/radiology.214.3.r00mr21747. [DOI] [PubMed] [Google Scholar]
- 18.Tempero R M, Hannibal M, Finn L S, Manning S C, Cunningham M L, Perkins J A. Lymphocytopenia in children with lymphatic malformation. Arch Otolaryngol Head Neck Surg. 2006;132(01):93–97. doi: 10.1001/archotol.132.1.93. [DOI] [PubMed] [Google Scholar]
- 19.Goyal P, Alomari A I, Kozakewich H P et al. Imaging features of kaposiform lymphangiomatosis. Pediatr Radiol. 2016;46(09):1282–1290. doi: 10.1007/s00247-016-3611-1. [DOI] [PubMed] [Google Scholar]
- 20.Le Cras T D, Mobberley-Schuman P S, Broering M, Fei L, Trenor C C, III, Adams D M. Angiopoietins as serum biomarkers for lymphatic anomalies. Angiogenesis. 2017;20(01):163–173. doi: 10.1007/s10456-016-9537-2. [DOI] [PubMed] [Google Scholar]
- 21.White C L, Olivieri B, Restrepo R, McKeon B, Karakas S P, Lee E Y. Low-flow vascular malformation pitfalls: from clinical examination to practical imaging evaluation--Part 1, Lymphatic malformation mimickers. AJR Am J Roentgenol. 2016;206(05):940–951. doi: 10.2214/AJR.15.15793. [DOI] [PubMed] [Google Scholar]
- 22.Sadick M, Müller-Wille R, Wildgruber M, Wohlgemuth W A. Vascular anomalies (Part I): classification and diagnostics of vascular anomalies. RoFo Fortschr Geb Rontgenstr Nuklearmed. 2018;190(09):825–835. doi: 10.1055/a-0620-8925. [DOI] [PubMed] [Google Scholar]
- 23.Francavilla M L, White C L, Oliveri B, Lee E Y, Restrepo R. Intraabdominal lymphatic malformations: pearls and pitfalls of diagnosis and differential diagnoses in pediatric patients. AJR Am J Roentgenol. 2017;208(03):637–649. doi: 10.2214/AJR.16.17008. [DOI] [PubMed] [Google Scholar]
- 24.Merrow A C, Gupta A, Patel M N, Adams D M. 2014 Revised classification of vascular lesions from the international society for the study of vascular anomalies: radiologic-pathologic update. Radiographics. 2016;36(05):1494–1516. doi: 10.1148/rg.2016150197. [DOI] [PubMed] [Google Scholar]
- 25.Balakrishnan K, Bauman N, Chun R H et al. Standardized outcome and reporting measures in pediatric head and neck lymphatic malformations. Otolaryngol Head Neck Surg. 2015;152(05):948–953. doi: 10.1177/0194599815577602. [DOI] [PubMed] [Google Scholar]
- 26.Adams M T, Saltzman B, Perkins J A. Head and neck lymphatic malformation treatment: a systematic review. Otolaryngol Head Neck Surg. 2012;147(04):627–639. doi: 10.1177/0194599812453552. [DOI] [PubMed] [Google Scholar]
- 27.Ono Y, Osuga K, Takura T et al. Cost-effectiveness analysis of percutaneous sclerotherapy for venous malformations. J Vasc Interv Radiol. 2016;27(06):831–837. doi: 10.1016/j.jvir.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 28.Mathur N N, Rana I, Bothra R, Dhawan R, Kathuria G, Pradhan T. Bleomycin sclerotherapy in congenital lymphatic and vascular malformations of head and neck. Int J Pediatr Otorhinolaryngol. 2005;69(01):75–80. doi: 10.1016/j.ijporl.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Acord M, Srinivasan A S, Cahill A M. percutaneous treatment of lymphatic malformations. Tech Vasc Interv Radiol. 2016;19(04):305–311. doi: 10.1053/j.tvir.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 30.De Maria L, De Sanctis P, Balakrishnan K, Tollefson M, Brinjikji W. Sclerotherapy for lymphatic malformations of head and neck: systematic review and meta-analysis. J Vasc Surg Venous Lymphat Disord. 2020;8(01):154–164. doi: 10.1016/j.jvsv.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Burrows P E, Mitri R K, Alomari Aet al. Percutaneous sclerotherapy of lymphatic malformations with doxycycline Lymphat Res Biol 20086(3-4):209–216. [DOI] [PubMed] [Google Scholar]
- 32.Molitch H I, Unger E C, Witte C L, vanSonnenberg E. Percutaneous sclerotherapy of lymphangiomas. Radiology. 1995;194(02):343–347. doi: 10.1148/radiology.194.2.7529933. [DOI] [PubMed] [Google Scholar]
- 33.Farnoosh S, Don D, Koempel J, Panossian A, Anselmo D, Stanley P. Efficacy of doxycycline and sodium tetradecyl sulfate sclerotherapy in pediatric head and neck lymphatic malformations. Int J Pediatr Otorhinolaryngol. 2015;79(06):883–887. doi: 10.1016/j.ijporl.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 34.Thomas D M, Wieck M M, Grant C N et al. Doxycycline sclerotherapy is superior in the treatment of pediatric lymphatic malformations. J Vasc Interv Radiol. 2016;27(12):1846–1856. doi: 10.1016/j.jvir.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Shergill A, John P, Amaral J G. Doxycycline sclerotherapy in children with lymphatic malformations: outcomes, complications and clinical efficacy. Pediatr Radiol. 2012;42(09):1080–1088. doi: 10.1007/s00247-012-2406-2. [DOI] [PubMed] [Google Scholar]
- 36.Chaudry G, Burrows P E, Padua H M, Dillon B J, Fishman S J, Alomari A I. Sclerotherapy of abdominal lymphatic malformations with doxycycline. J Vasc Interv Radiol. 2011;22(10):1431–1435. doi: 10.1016/j.jvir.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 37.Tu J H, Do H M, Patel V, Yeom K W, Teng J MC. Sclerotherapy for lymphatic malformations of the head and neck in the pediatric population. J Neurointerv Surg. 2017;9(10):1023–1026. doi: 10.1136/neurintsurg-2016-012660. [DOI] [PubMed] [Google Scholar]
- 38.Bagrodia N, Defnet A M, Kandel J J. Management of lymphatic malformations in children. Curr Opin Pediatr. 2015;27(03):356–363. doi: 10.1097/MOP.0000000000000209. [DOI] [PubMed] [Google Scholar]
- 39.Chaudry G, Guevara C J, Rialon K L et al. Safety and efficacy of bleomycin sclerotherapy for microcystic lymphatic malformation. Cardiovasc Intervent Radiol. 2014;37(06):1476–1481. doi: 10.1007/s00270-014-0932-z. [DOI] [PubMed] [Google Scholar]
- 40.Azene E, Mitchell S, Radvany M, Agrawal N, Eisele D, Weiss C. Foamed bleomycin sclerosis of airway venous malformations: The role of interspecialty collaboration. Laryngoscope. 2016;126(12):2726–2732. doi: 10.1002/lary.26077. [DOI] [PubMed] [Google Scholar]
- 41.Ul Haq F, Mitchell S E, Tekes A, Weiss C R. Bleomycin foam treatment of venous malformations: a promising agent for effective treatment with minimal swelling. J Vasc Interv Radiol. 2015;26(10):1484–1493. doi: 10.1016/j.jvir.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Chen A W, Wang T, Huang Y Y, Liu S H. Multistage sclerotherapy for extensive lymphatic malformations with airway involvement in infant: a protocol to prevent tracheotomy. J Oral Maxillofac Surg. 2017;75(09):1882–1890. doi: 10.1016/j.joms.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Wratney A T, Benjamin D K, Jr, Slonim A D, He J, Hamel D S, Cheifetz I M. The endotracheal tube air leak test does not predict extubation outcome in critically ill pediatric patients. Pediatr Crit Care Med. 2008;9(05):490–496. doi: 10.1097/PCC.0b013e3181849901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanif A M, Saunders J A, Hawkins C M, Wojno T H, Kim H J. Use of percutaneous bleomycin sclerotherapy for orbital lymphatic malformations. Orbit. 2019;38(01):30–36. doi: 10.1080/01676830.2018.1480636. [DOI] [PubMed] [Google Scholar]
- 45.MacIntosh P W, Yoon M K, Fay A.Complications of intralesional bleomycin in the treatment of orbital lymphatic malformations Semin Ophthalmol 201429(5-6):450–455. [DOI] [PubMed] [Google Scholar]
- 46.Sleijfer S. Bleomycin-induced pneumonitis. Chest. 2001;120(02):617–624. doi: 10.1378/chest.120.2.617. [DOI] [PubMed] [Google Scholar]
- 47.Liu T, De Los Santos F G, Phan S H. The bleomycin model of pulmonary fibrosis. Methods Mol Biol. 2017;1627:27–42. doi: 10.1007/978-1-4939-7113-8_2. [DOI] [PubMed] [Google Scholar]
- 48.Roden A C, Camus P. Iatrogenic pulmonary lesions. Semin Diagn Pathol. 2018;35(04):260–271. doi: 10.1053/j.semdp.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 49.O'Sullivan J M, Huddart R A, Norman A R, Nicholls J, Dearnaley D P, Horwich A. Predicting the risk of bleomycin lung toxicity in patients with germ-cell tumours. Ann Oncol. 2003;14(01):91–96. doi: 10.1093/annonc/mdg020. [DOI] [PubMed] [Google Scholar]
- 50.Muir T, Kirsten M, Fourie P, Dippenaar N, Ionescu G O. Intralesional bleomycin injection (IBI) treatment for haemangiomas and congenital vascular malformations. Pediatr Surg Int. 2004;19(12):766–773. doi: 10.1007/s00383-003-1058-6. [DOI] [PubMed] [Google Scholar]
- 51.Méndez-Echevarría A, Fernandez-Prieto A, de la Serna O et al. acute lung toxicity after intralesional bleomycin sclerotherapy. Pediatrics. 2018;141(01):e20161787. doi: 10.1542/peds.2016-1787. [DOI] [PubMed] [Google Scholar]
- 52.Atwa K, Abuhasna S, Shihab Z, Hashaykeh N, Hasan R. Acute pulmonary toxicity following intralesional administration of bleomycin for a lymphovenous malformation. Pediatr Pulmonol. 2010;45(02):192–196. doi: 10.1002/ppul.21139. [DOI] [PubMed] [Google Scholar]
- 53.Lam M S. The need for routine bleomycin test dosing in the 21st century. Ann Pharmacother. 2005;39(11):1897–1902. doi: 10.1345/aph.1G235. [DOI] [PubMed] [Google Scholar]
- 54.Poldervaart M T, Breugem C C, Speleman L, Pasmans S. Treatment of lymphatic malformations with OK-432 (Picibanil): review of the literature. J Craniofac Surg. 2009;20(04):1159–1162. doi: 10.1097/SCS.0b013e3181abb249. [DOI] [PubMed] [Google Scholar]
- 55.Ghaffarpour N, Petrini B, Svensson L A, Boman K, Wester T, Claesson G. Patients with lymphatic malformations who receive the immunostimulant OK-432 experience excellent long-term outcomes. Acta Paediatr. 2015;104(11):1169–1173. doi: 10.1111/apa.13086. [DOI] [PubMed] [Google Scholar]
- 56.Motz K M, Nickley K B, Bedwell J R et al. OK432 versus doxycycline for treatment of macrocystic lymphatic malformations. Ann Otol Rhinol Laryngol. 2014;123(02):81–88. doi: 10.1177/0003489414523561. [DOI] [PubMed] [Google Scholar]
- 57.Horbach S E, Lokhorst M M, Saeed P, de Goüyon Matignon de Pontouraude C M, Rothová A, van der Horst C M. Sclerotherapy for low-flow vascular malformations of the head and neck: a systematic review of sclerosing agents. J Plast Reconstr Aesthet Surg. 2016;69(03):295–304. doi: 10.1016/j.bjps.2015.10.045. [DOI] [PubMed] [Google Scholar]
- 58.Kim S W, Kavanagh K, Orbach D B, Alomari A I, Mulliken J B, Rahbar R. Long-term outcome of radiofrequency ablation for intraoral microcystic lymphatic malformation. Arch Otolaryngol Head Neck Surg. 2011;137(12):1247–1250. doi: 10.1001/archoto.2011.199. [DOI] [PubMed] [Google Scholar]
- 59.Lisan Q, Villepelet A, Parodi M et al. Value of radiofrequency ablation in the management of retropharyngeal lymphatic malformation. Int J Pediatr Otorhinolaryngol. 2016;83:37–40. doi: 10.1016/j.ijporl.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 60.Ramaswamy R S, Tiwari T, Darcy M D et al. Cryoablation of low-flow vascular malformations. Diagn Interv Radiol. 2019;25(03):225–230. doi: 10.5152/dir.2019.18278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thompson S M, Callstrom M R, McKusick M A, Woodrum D A. Initial results of image-guided percutaneous ablation as second-line treatment for symptomatic vascular anomalies. Cardiovasc Intervent Radiol. 2015;38(05):1171–1178. doi: 10.1007/s00270-015-1079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Savas J A, Ledon J, Franca K, Chacon A, Zaiac M, Nouri K. Carbon dioxide laser for the treatment of microcystic lymphatic malformations (lymphangioma circumscriptum): a systematic review. Dermatol Surg. 2013;39(08):1147–1157. doi: 10.1111/dsu.12220. [DOI] [PubMed] [Google Scholar]
- 63.França K, Chacon A, Ledon J, Savas J, Izakovic J, Nouri K. Lasers for cutaneous congenital vascular lesions: a comprehensive overview and update. Lasers Med Sci. 2013;28(04):1197–1204. doi: 10.1007/s10103-012-1220-2. [DOI] [PubMed] [Google Scholar]
- 64.Mulliken J B, Fishman S J, Burrows P E. Vascular anomalies. Curr Probl Surg. 2000;37(08):517–584. doi: 10.1016/s0011-3840(00)80013-1. [DOI] [PubMed] [Google Scholar]
- 65.Alqahtani A, Nguyen L T, Flageole H, Shaw K, Laberge J M. 25 years' experience with lymphangiomas in children. J Pediatr Surg. 1999;34(07):1164–1168. doi: 10.1016/s0022-3468(99)90590-0. [DOI] [PubMed] [Google Scholar]
- 66.Wiegand S, Wichmann G, Dietz A. Treatment of lymphatic malformations with the mTOR inhibitor sirolimus: a systematic review. Lymphat Res Biol. 2018;16(04):330–339. doi: 10.1089/lrb.2017.0062. [DOI] [PubMed] [Google Scholar]
- 67.Hammill A M, Wentzel M, Gupta A et al. Sirolimus for the treatment of complicated vascular anomalies in children. Pediatr Blood Cancer. 2011;57(06):1018–1024. doi: 10.1002/pbc.23124. [DOI] [PubMed] [Google Scholar]
- 68.Strychowsky J E, Rahbar R, O'Hare M J, Irace A L, Padua H, Trenor C C., III Sirolimus as treatment for 19 patients with refractory cervicofacial lymphatic malformation. Laryngoscope. 2018;128(01):269–276. doi: 10.1002/lary.26780. [DOI] [PubMed] [Google Scholar]
- 69.Ricci K W, Hammill A M, Mobberley-Schuman P et al. Efficacy of systemic sirolimus in the treatment of generalized lymphatic anomaly and Gorham-Stout disease. Pediatr Blood Cancer. 2019;66(05):e27614. doi: 10.1002/pbc.27614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McCormack F X, Inoue Y, Moss J et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364(17):1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arriola Apelo S I, Lamming D W. Rapamycin: an InhibiTOR of aging emerges from the soil of Easter Island. J Gerontol A Biol Sci Med Sci. 2016;71(07):841–849. doi: 10.1093/gerona/glw090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Croteau S E, Kozakewich H P, Perez-Atayde A R et al. Kaposiform lymphangiomatosis: a distinct aggressive lymphatic anomaly. J Pediatr. 2014;164(02):383–388. doi: 10.1016/j.jpeds.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ozeki M, Fujino A, Matsuoka K, Nosaka S, Kuroda T, Fukao T. Clinical features and prognosis of generalized lymphatic anomaly, Kaposiform lymphangiomatosis, and Gorham-Stout disease. Pediatr Blood Cancer. 2016;63(05):832–838. doi: 10.1002/pbc.25914. [DOI] [PubMed] [Google Scholar]
- 74.Steiner J E, Drolet B A. Classification of vascular anomalies: an update. Semin Intervent Radiol. 2017;34(03):225–232. doi: 10.1055/s-0037-1604295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grada A A, Phillips T J. Lymphedema: pathophysiology and clinical manifestations. J Am Acad Dermatol. 2017;77(06):1009–1020. doi: 10.1016/j.jaad.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 76.Lala S, Mulliken J B, Alomari A I, Fishman S J, Kozakewich H P, Chaudry G. Gorham-Stout disease and generalized lymphatic anomaly--clinical, radiologic, and histologic differentiation. Skeletal Radiol. 2013;42(07):917–924. doi: 10.1007/s00256-012-1565-4. [DOI] [PubMed] [Google Scholar]
- 77.Kato H, Ozeki M, Fukao T, Matsuo M. MR imaging findings of vertebral involvement in Gorham-Stout disease, generalized lymphatic anomaly, and kaposiform lymphangiomatosis. Jpn J Radiol. 2017;35(10):606–612. doi: 10.1007/s11604-017-0674-3. [DOI] [PubMed] [Google Scholar]
- 78.Chavhan G B, Amaral J G, Temple M, Itkin M. MR lymphangiography in children: technique and potential applications. Radiographics. 2017;37(06):1775–1790. doi: 10.1148/rg.2017170014. [DOI] [PubMed] [Google Scholar]


