Abstract
Lymphangiography as a diagnostic procedure dates back to the 1950s and was widely performed for several decades until being supplanted by other advanced imaging techniques. With the advent of thoracic duct embolization to treat chylothorax, Constantin Cope ushered in a transition from lymphangiography as a diagnostic procedure to a precursor for lymphatic intervention. Subsequently, technical modifications and applications of lymphatic embolization to other medical conditions have greatly expanded the scope and application of lymphangiography and lymphatic intervention. Although there is increasing familiarity with lymphatic interventions, few interventionalists have performed a high enough volume to be aware of potential complications and their management. Potential complications of lymphangiography and those encountered while performing lymphatic interventions are discussed along with approaches to minimize their risk and management strategies should they occur.
Keywords: lymphangiography, complication, nontarget embolization, oil embolization, chylothorax, interventional radiology
Lymphatic vessels were discovered in the 17th century through autopsy and animal vivisection. 1 In 1955, Kinmonth et al published the initial description of the pedal lymphangiography technique to successfully visualize lower extremity lymphatic vessels. His technique involved carefully dissecting the dorsum of the foot, directly cannulating a lymphatic vessel, and slowly injecting contrast media with concomitant radiographs. 2 Over the next 25 years, pedal lymphangiography would routinely be performed as a diagnostic modality in patients with lymphedema, lymphoma, or other primary malignancies to stage malignancies, evaluate chemotherapeutic responses, differentiate inflammatory from neoplastic processes, and many other indications. 3 The potential of lymphangiography, in addition to its limitations and potential complications, was extensively studied, as experience with this modality became widespread. 4 However, by the 1980s and 1990s, major improvements in noninvasive imaging modalities including ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI) supplanted traditional diagnostic lymphangiography. Over time, the intricate knowledge of pedal lymphangiography was deemphasized as lymphangiograms were performed less frequently. This led to a waning emphasis on lymphatics, as interventional radiologists lost the necessary experience and skill in performing and interpreting these studies.
In 1999, Constantin Cope renewed interest in pedal lymphangiography and the lymphatic circulation as a whole by pioneering a new interventional frontier with a prospective trial of thoracic duct embolization for chylothorax. 5 He reported that thoracic duct embolization achieved an astounding clinical success rate exceeding 70% in a retrospective analysis of 42 patients with chylothorax. 6 Dr. Max Itkin further expanded on these initial studies by reporting on 109 patients with traumatic chylothorax, achieving similarly promising results. 7
Despite the favorable results of lymphangiography and thoracic duct embolization for chylothorax, widespread adoption was slowed down by the sporadic need and several technical challenges. Pedal lymphangiography involved extensive training to develop competence, lengthy procedural times. Pedal lymphangiography faced difficulty in finding appropriate infusion pumps for the slow administration of ethiodized oil. The need for pedal lymphangiography was obviated with the onset of intranodal lymphangiography, described initially in children in 2011, and then in adults in 2012. Infusion pumps became unnecessary, procedural lengths became shorter, and technical challenges became simpler—intranodal lymphangiography could be performed with routinely available equipment. 8 9 In the ensuing years, many studies have further reinforced the acceptance of lymphangiography and thoracic duct embolization for chylothorax, including large series from multiple institutions, American College of Radiology Appropriateness Criteria for Chylothorax Treatment Planning, and a recent meta-analysis of over 400 patients with a pooled clinical success rate exceeding 80%. 10 11 12 13 The scope of lymphatic intervention has now expanded to successful treatment of chylous ascites, pediatric chylothorax, plastic bronchitis, protein losing enteropathy, lymphoceles, and thoracic duct stent-graft placement. 14 15 16 17 18
With more lymphatic procedures being performed across more centers, it is important to revisit the known and possible complications of lymphangiography, lymphatic access, and lymphatic embolization. Management of these complications and strategies to minimize their risk will both be emphasized.
Complications of Lymphangiography
By the 1960s, lymphangiography was more widely performed. Initial reports of adult and pediatric lymphangiography included such complications as impaired wound healing, blue dye hypersensitivity reactions, inadvertent venous administration of ethiodized oil, and varied pulmonary complications. 2 19 A survey of 83 physicians comprising 32,000 lymphangiograms was reported by Koehler, revealing 104 pulmonary complications (not including pulmonary oil embolization), 97 hypersensitivity reactions (both to blue dye and ethiodized oil), 18 deaths, 9 cerebral disorders, and 6 hypotensive crises. 4 Complications occurred between hours and up to 21 days postprocedure. Unfortunately, the ethiodized oil dose was not reported and no clinical data were available for the fatalities. Similar results were noted in a review of 7,641 lymphangiograms, compiling 66 complications. 20 Pulmonary complications were present in 31 patients, 17 patients had complications related to the dorsal foot incision, 7 had hypersensitivity to the blue dye, and 11 patients had other complications including a fatality.
As nodal lymphangiography has supplanted pedal lymphangiography, wound infections of dorsal foot incisions and hypersensitivity due to blue dye are no longer encountered. The most commonly encountered complication includes extravasation, which can be readily managed. Should extravasation of ethiodized oil occur during pedal or nodal lymphangiography, the access needle should be repositioned within the vessel. The extravasation can be treated with local wound care and managed expectantly should any symptoms arise ( Figs. 1 and 2 ). Only rarely will extravasation in the dorsum of the foot or groin result in a major complication. While fever, nausea, vomiting, and transient pain have historically been noted, these side effects were successfully managed symptomatically. 21 Potential major complications include a spectrum of pulmonary complications as well as cerebral and visceral oil embolization injuries, which will each be discussed separately.
Fig. 1.
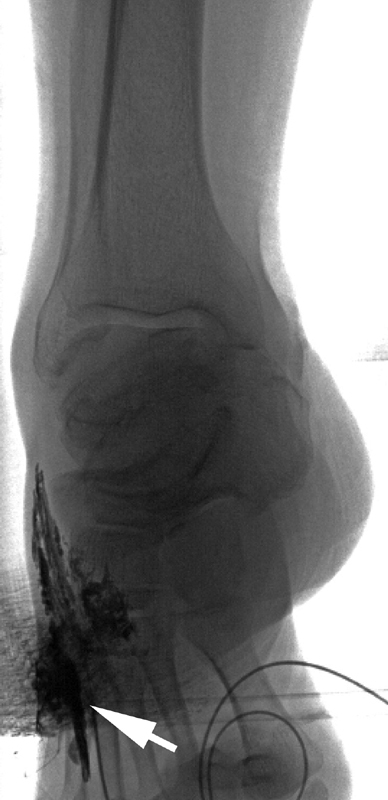
A 54-year-old man with chylothorax had a radiograph of the right foot taken during attempted pedal lymphangiography. Contrast extravasation (white arrow) is seen along the dorsolateral aspect of foot. This was asymptomatic and did not require additional care. Access was obtained in the left foot.
Fig. 2.
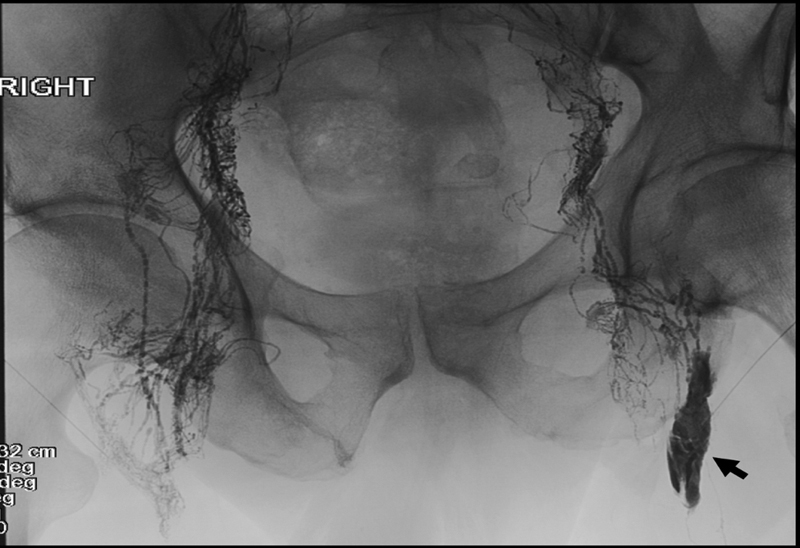
A 62-year-old man with traumatic chylothorax following esophagectomy. Spot radiograph of the pelvis after bilateral intranodal lymphangiography. Contrast extravasation (black arrow) is seen in the left inguinal region. This was asymptomatic and did not require additional care.
Pulmonary complications stem from the flow of the thoracic duct. The thoracic duct most commonly empties into the left venous angle, ultimately allowing lymphatically injected ethiodized oil to reach the pulmonary circulation through the venous system, causing pulmonary oil embolism ( Fig. 3 ). 22 Bron et al expanded on the incidence of pulmonary oil embolism in lymphangiography while limiting ethiodized oil to 20-mL injections. A majority, 55% of patients, developed oil pulmonary embolism. 23 Patients with radiologic evidence of lymphatic obstruction were at higher risk (81%) of suffering from oil embolism in comparison to patients without lymphatic obstruction (31%) and were the only patients to develop severe symptoms. 23 However, a spectrum of more serious respiratory complications included pulmonary infarction (0.25%), lipid pneumonia (0.04%), pulmonary edema (0.03%), and hemoptysis (0.03%). 4 Uncommonly, Löffler's syndrome and acute respiratory distress syndrome have also been reported. 24 25 These rarer pulmonary complications are attributed to an inflammatory response to ethiodized oil when it accumulates in the small pulmonary vasculature at a rate exceeding the lungs capacity to metabolize it. 21 22 23 24 25 Supportive care with supplementary oxygen, steroids, and antibiotics resolves most symptoms within 2 weeks.
Fig. 3.
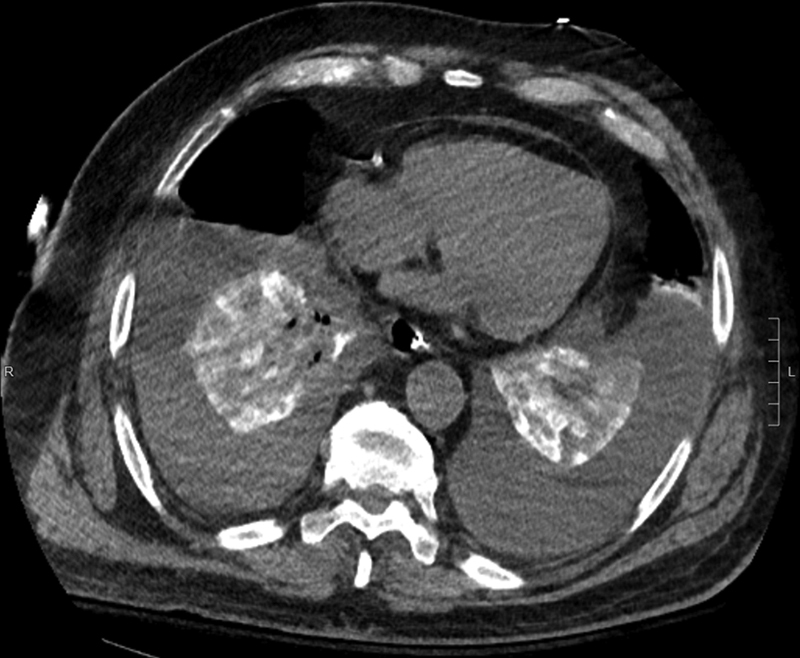
A 73-year-old man status-post partial left nephrectomy complicated by chylous ascites who underwent lymphangiography and embolization of the leaking left retroperitoneal branch. One day after embolization, the patient developed shortness of breath; a chest CT (axial image) reveals bilaterally collapsed lungs with bright pulmonary oil emboli in the lung bases as well as bilateral large volume pleural effusions.
Cerebral oil embolization usually presents with seizure, confusion, lethargy, or other mental status change within minutes to hours after completion of lymphangiography. 26 27 28 29 30 31 Notably, the migration of ethiodized oil to the cerebral circulation has not been documented radiographically during lymphangiography, but rather noted only on CT or MRI studies performed after clinical symptoms became apparent. Proposed mechanisms include right-to-left shunts (though many patients have had patent foramen ovale and pulmonary arteriovenous shunts excluded after the fact), loss of the filtering capability of the pulmonary capillaries due to overload of ethiodized oil, and lymphovenous shunts to the pulmonary veins. 26 27 28 29 30 31 Cerebral oil embolization has similarly been documented with transarterial chemoembolization and hysterosalpingography using ethiodized oil. 32 33 34 35 Ethiodized oil may lead to neurotoxicity due to vascular occlusion, blood–brain barrier disruption, or direct toxicity. Supportive care in a stroke intensive care unit is recommended to maximize the chance of recovery. Mixed outcomes have been noted, with elderly and cancer patients having worse outcomes.
Several case reports of visceral oil embolization, particularly in the liver and kidney, are present in the literature. 26 27 28 36 37 38 39 40 In one report of 17,000 lymphangiograms, hepatic oil embolization occurred in 36 patients (0.19%), while in another review of greater than 5,000 lymphangiograms, it occurred in (0.24%) of cases. 36 37 40 The hepatic parenchymal oil was primarily distributed through the portal tree. Most patients were asymptomatic only with transient laboratory changes ( Fig. 4 ). Generally, the potential for hepatic oil embolization occurs in the setting of retroperitoneal lymphatic obstruction; it has not been observed in the setting of normal lymphangiography. Likewise, renal oil embolization is rarely reported and generally occurred in patients with lymphoma who had mediastinal masses. 26 27 28 Although the pathogenesis is not completely known, theories suggest there may be peritumoral microscopic arteriovenous fistulas which may have associations with superior vena caval obstruction. Supportive care was provided and the outcome in each case was dependent on the underlying malignancy rather than the presence of visceral oil embolization.
Fig. 4.
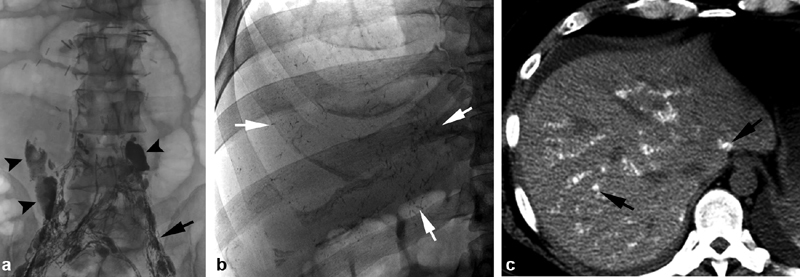
A 41-year-old man status-post retroperitoneal lymphadenectomy with persistent chylous ascites. ( a ) Multiple surgical clips overlying the spinal column. Intranodal lymphangiography reveals amorphous leakage of contrast material bilaterally (black arrowheads) at L4 and L5 levels, which is different in shape from lymph nodes (black arrow). No cephalad progression through the retroperitoneal lymphatics was present. ( b ) Spot radiographs of the abdomen taken after no cephalad progression of ethiodized oil was noted. White arrows reveal areas of intrahepatic oil, in a branching pattern. ( c ) Computed tomography of the abdomen after the spot radiographs revealed intrahepatic oil. A portal venous distribution of ethiodized oil is present (black arrow). No change in liver function tests was present and the patient remained asymptomatic.
There are several considerations to decrease complication rates during lymphangiography. Ensuring that injected ethiodized oil is flowing in the lymphatic system, rather than extravasating into the soft tissues or inadvertently flowing into the venous system, is paramount; this should be monitored with intermittent spot radiographs or fluoroscopy ( Fig. 5 ) Additionally, minimizing the amount of ethiodized oil necessary to perform a technically successful procedure will avoid many of the complications. A review of 522 lymphangiograms was stratified by volume of administered ethiodized oil, showing that patients who received less than 18 mL of ethiodized oil had a 13% risk of complication—between 18 and 20 mL a 24% risk, and more than 20 mL a 48% risk. 21 Lymphatic embolization can frequently be successfully performed with 15 mL of ethiodized oil or less. Moreover, the risk-to-benefit ratio of lymphangiography should be considered, especially in patients with known pulmonary diseases, right-to-left shunts, caval occlusions, bulky lymphatic masses, congenital lymphedema, or those undergoing active radiation treatment. Postprocedure monitoring will identify major complications, which can be managed expectantly and will usually require supportive care to resolution.
Fig. 5.
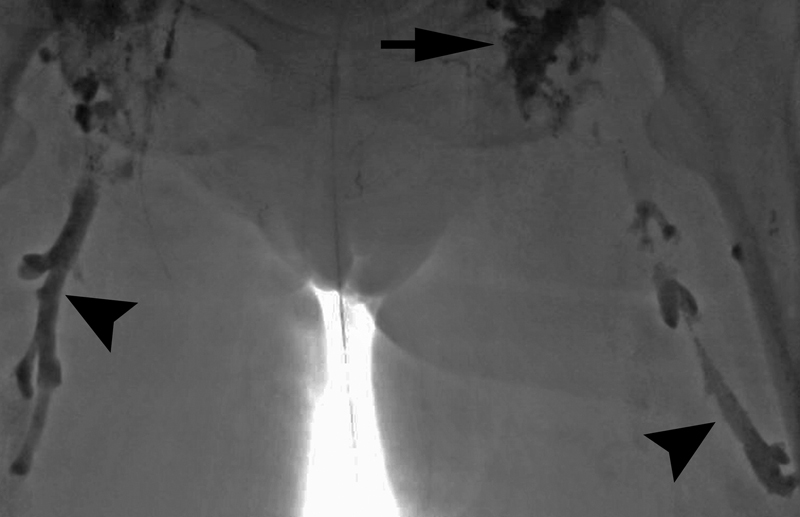
A 71-year-old woman with nontraumatic chylothorax undergoing intranodal lymphangiography (black arrow) had spontaneous lymphovenous channels. The bilateral femoral veins filled with ethiodized oil (black arrowheads). Bilateral retrograde femoral venous access was gained and the ethiodized oil was aspirated with multisidehole catheters.
Central Lymphatic Access Complications
Central lymphatic access can be performed from either an anterograde percutaneous transabdominal approach or from one of two retrograde approaches. From the perspective of contemporary lymphatic intervention, the percutaneous transabdominal approach has been most commonly used, dating to its initial description by Cope in 1998. 41 In the last decade, case reports and case series have described retrograde transvenous and transcervical access to the central lymphatics. 17 42 43 44 Each approach has unique advantages and risks, which will be further discussed.
Percutaneous transabdominal puncture is performed after lymphangiography opacifies either the cisterna chyli or another targetable retroperitoneal lymphatic vessel overlying the upper lumbar or lower thoracic vertebral bodies. Typically, a 21- or 22-gauge Chiba needle is used to directly puncture the skin to the target, aiming slightly right of midline to carefully avoid the colon. Often multiple passes are required to successfully achieve entry into the lymphatic channel, which is then probed with a centrally advanced microwire before the needle is exchanged for a microcatheter. A study cataloging which structures are transgressed with this puncture found that the most commonly penetrated structures, in descending order, were the liver, diaphragmatic crus, pancreas, portal vein or its branches, duodenum, inferior vena cava, colon, left renal vein, and other structures. 45 A review of the largest series of retrospective thoracic duct embolization analysis reported a single perihepatic hemorrhage and a single periaortic hematoma, with neither requiring treatment. 6 7 10 11 13 Solitary cases of bile peritonitis and pancreatitis have also been noted ( Fig. 6 ). 45 Several authors have reported that sheared guide wires could be sheared in the retroperitoneum and were asymptomatic. 10 11 46 While intra-abdominal structures are unquestionably traversed with small-gauge needles and microcatheters, it remains well tolerated and often clinically inconsequential. However, if hemodynamic instability or abdominal pain persists or worsens in the days postprocedure, or unexplained laboratory abnormalities develop, there should be a low threshold for abdominal CT imaging.
Fig. 6.
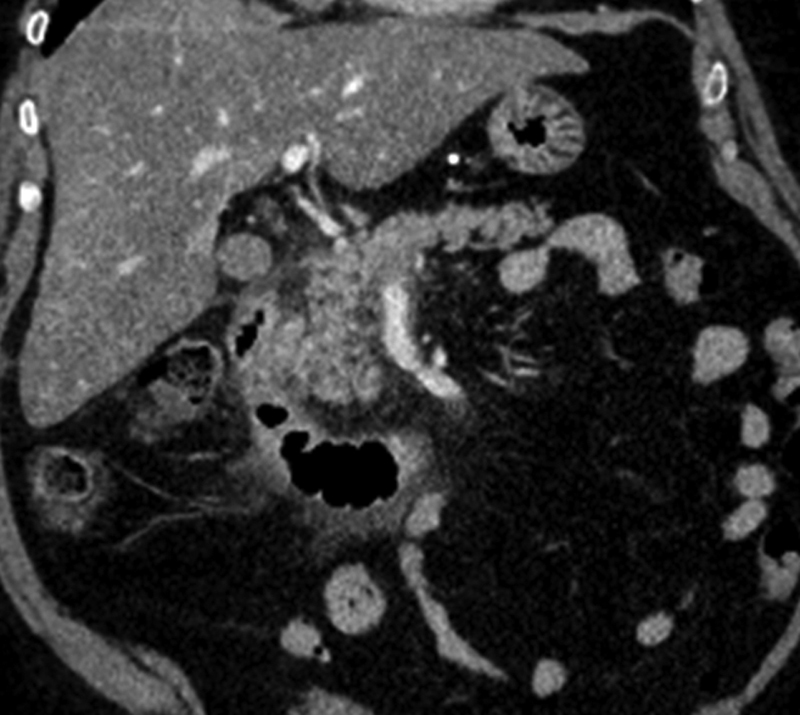
A 61-year-old woman with plastic bronchitis who underwent thoracic duct embolization via transabdominal antegrade access of the cisterna chyli. Postprocedurally, the patient developed severe abdominal pain, elevated amylase (219 U/L), and lipase (111 U/L). Abdominal computed tomography (coronal image) shows peripancreatic stranding, consistent with acute pancreatitis.
The percutaneous, transabdominal approach is particularly useful for traumatic and iatrogenic lymphatic injuries. Lymphangiography visualizes the lymphatics along the axial skeleton from the groin to the neck, is diagnostic for leaks, and has a therapeutic benefit. Prudent precautions should therefore be applied to minimize complication risks. Given that multiple transabdominal punctures through various structures may be required to achieve successful lymphatic access, coagulopathy should be corrected, and antiplatelet medications should be held if possible.
In contrast to transabdominal approach which provides anterograde, ascending access through the central lymphatics, transvenous and transcervical access establish a retrograde, descending access. Transvenous access is most commonly established through a left upper arm approach using a 5-Fr reverse curve catheter in the left subclavian vein to cross the terminal valves of the thoracic duct. Transcervical access is usually established with a 22-gauge needle. Access is performed under fluoroscopic guidance, ultrasonographic guidance, or both to directly puncture the cervical portion of the thoracic duct, pass a microwire, and navigate toward the cisterna chyli. Both retrograde approaches have been used to successfully study the central lymphatics and treat various lymphatic disorders, albeit in case reports and small case series. 17 42 43 44 Given the limited experience and bias of positive reporting, it remains difficult to draw conclusions regarding efficacy and complications for transvenous and transcervical accesses.
Advantages of the retrograde approaches include the potential to forego lymphangiography and achieve faster access to the central lymphatics. Additionally, retrograde access avoids transabdominal puncture in coagulopathic patients, may be easier in patients with larger abdomens, and is an opportunity for central lymphatic access when a retroperitoneal target is not visualized by lymphangiography. Retrograde approaches can be performed as an adjunct to lymphangiography or transabdominal access; are useful in nontraumatic lymphatic disorders; and allow for sheath placement to facilitate lymphatic drainage, balloon placement, or stenting. Challenges to retrograde access include a thick and short neck, a tortuous or plexiform termination of the thoracic duct, and potential anatomic anomalies through the course of the thoracic duct or at its termination. Moreover, navigating across a transected thoracic duct in the setting of a traumatic chylothorax or controlling the embolization, particularly when using glue, may be difficult ( Fig. 7 ).
Fig. 7.
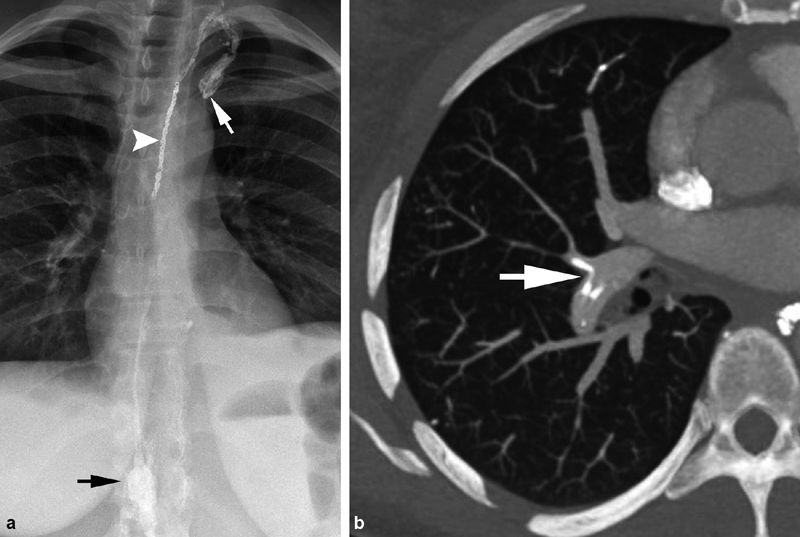
A 45-year-old woman with idiopathic chylothorax. ( a ) Following retrograde transvenous embolization of the thoracic duct with coils (white arrowhead) and glue, the glue migrated beyond the coil pack as the catheter was withdrawn, extending into the subclavian vein (white arrow). Black arrow—cisterna chyli. ( b ) The patient developed dyspnea 3 days postprocedure, and computed tomography of the chest was performed revealing multifocal glue segmental and subsegmental pulmonary emboli (white arrow).
Lymphatic Embolization Complications
In the past 20 years, the variety of emboli agents available has continued to increase, as has the frequency and territories in which embolization is performed. Most embolizations are arterial or venous, where there is widespread familiarity and knowledge of complications with the various coils, plugs, particles, and liquid agents. Moreover, many embolics were designed to be employed in blood, where platelets, fibrin, and the coagulation cascade are present in abundance and facilitate occlusion. In contrast, lymphatic embolizations are rarely performed at most medical centers. In addition, the lymphatic vessel flow dynamics are much slower than those of arteries or veins, and embolics behave differently in lymph. For instance, coils occlude less effectively in lymph due to the paucity of coagulation factors.
In the initial prospective trial of thoracic duct embolization, fibered platinum microcoils alone were used to achieve success. 5 In subsequent publications and in current practice, mechanical occlusion with coils was combined with a liquid agent, most commonly n -butyl-2-cyanoacrylate (nBCA; Trufill; Codman and Shurtleff, Raynham, MA). 6 7 10 11 13 Abdominal and pelvic lymphatic leaks are often embolized using nBCA alone through either direct access or a transnodal route. 14 18
While the use of the liquid emboli agents further diversifies potential embolization approaches and treatable conditions, experience is necessary to minimize complications. Uncommonly, asymptomatic and symptomatic pulmonary glue emboli have been described during lymphatic embolization, usually occurring when few or no coils were used. Proper priming of the system with 5% dextrose is necessary (usually < 1 mL) as is the appropriate dilution of the glue to ethiodized oil (1:1) for short-segment embolizations or more dilute for long-segment embolizations. Finally, the path of access must be considered; embolization approaching the access point is recommended with care to prevent overembolization ( Fig. 8 ). Care should also be taken when withdrawing the catheter as glue can potentially enter any structure that was traversed ( Fig. 9 ).
Fig. 8.
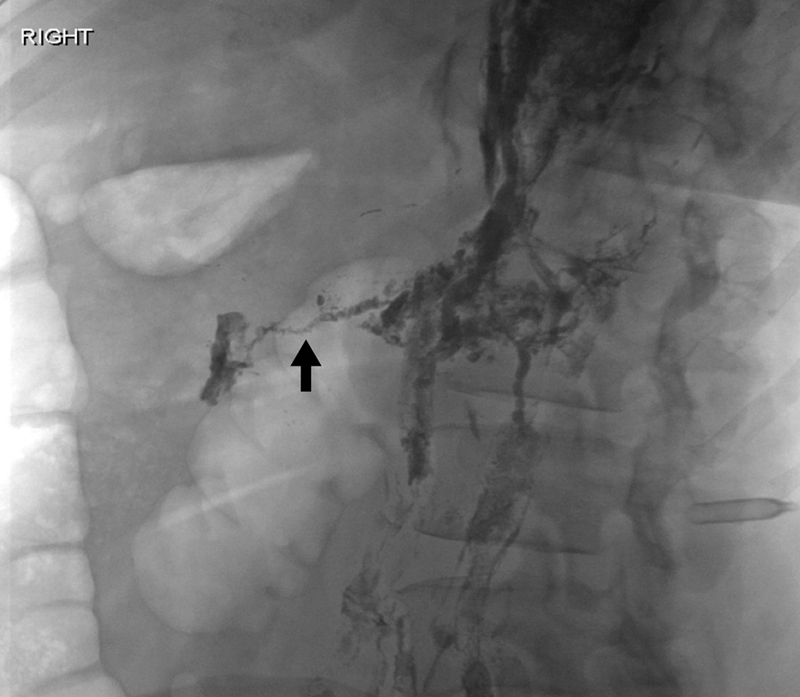
A 54-year-old woman with chylothorax underwent successful embolization of the thoracic duct. Oblique abdominal radiograph reveals a glue tract from the retroperitoneal lymphatics into the surrounding tissue (black arrow). This likely occurred from overembolization with glue and insufficient negative suction on the microcatheter as it was withdrawn.
Fig. 9.
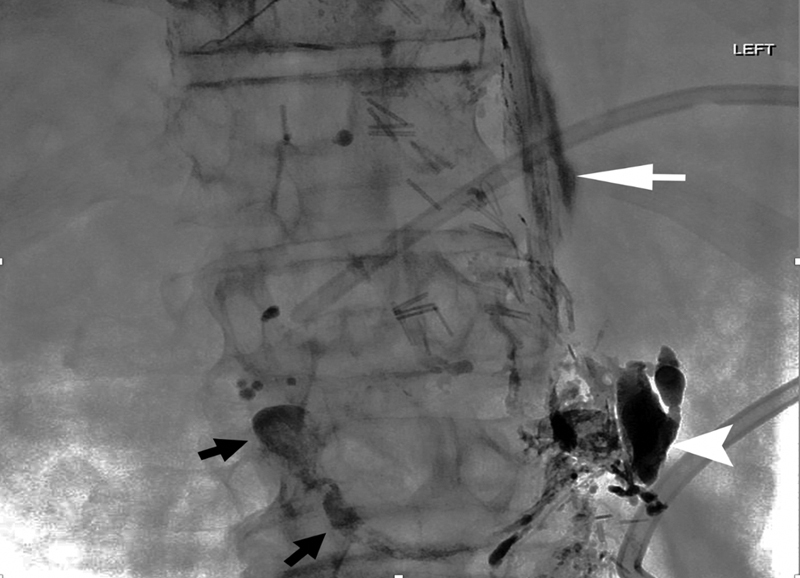
A 62-year-old woman with chylous ascites. While undergoing glue embolization, a left renal vein had been inadvertently accessed (near white arrowhead) and a glue cast extended into the inferior vena cava (black arrows). Extravasation of contrast along the spine (white arrow) occurred while attempting needle access into the lymphatics.
Nontarget embolization with glue is most often asymptomatic and well tolerated, generally requiring no therapy. Should pulmonary glue emboli be symptomatic, the patient should be heparinized and snare retrieval of glue casts can be attempted ( Fig. 10 ). If necessary, pulmonary glue embolectomy can be considered.
Fig. 10.
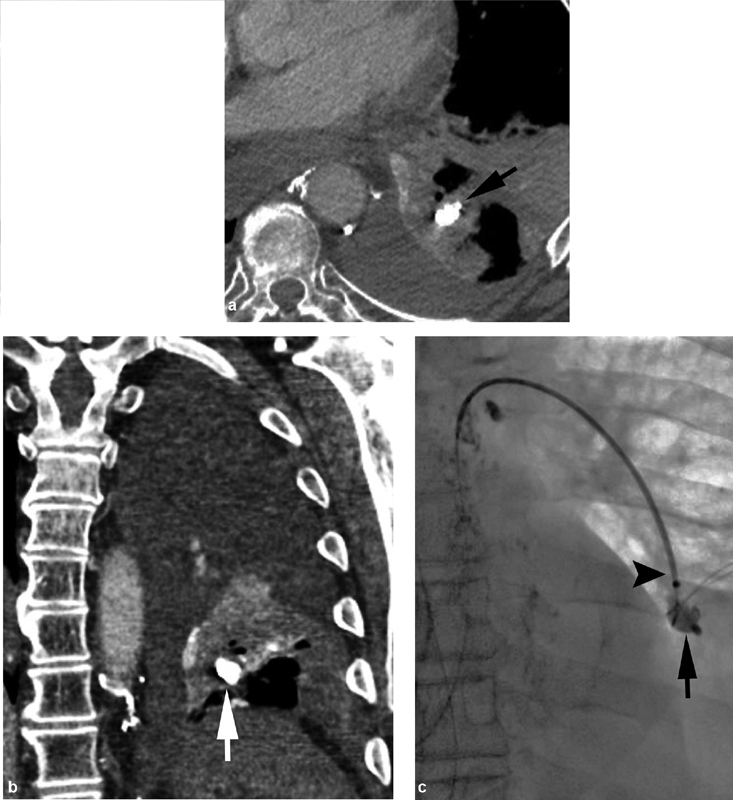
A 61-year-old man with chylothorax underwent successful thoracic duct embolization. The patient had persistent dyspnea, and axial ( a ) and coronal ( b ) computed tomographic imaging revealed a glue embolus (black arrow) in the lower left lobe. ( c ) Successful snare (black arrowhead) retrieval of a left lower lobe glue embolus (black arrow) was performed.
Conclusion
Lymphangiography and lymphatic interventions have a respectably high safety profile. To reduce risk to patients, it is important to avoid conventional lymphangiography in patients with contraindications, use the minimal necessary volume of ethiodized oil, correct patient coagulopathy, and perform informed technique during glue embolization.
Conflict of Interest The authors have no relevant disclosures. There was no grant funding or financial support for this manuscript.
Notes
All authors have read and contributed to this manuscript.
References
- 1.Aalami O O, Allen D B, Organ C H., Jr Chylous ascites: a collective review. Surgery. 2000;128(05):761–778. doi: 10.1067/msy.2000.109502. [DOI] [PubMed] [Google Scholar]
- 2.Kinmonth J B, Taylor G W, Harper R K.Lymphangiography; a technique for its clinical use in the lower limb BMJ 19551(4919):940–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallace S, Jackson L, Schaffer B et al. Lymphangiograms: theri diagnostic and therapeutic potential. Radiology. 1961;76:179–199. doi: 10.1148/76.2.179. [DOI] [PubMed] [Google Scholar]
- 4.Koehler P R. Complications of lymphography. Lymphology. 1968;1(04):116–120. [PubMed] [Google Scholar]
- 5.Cope C, Salem R, Kaiser L R. Management of chylothorax by percutaneous catheterization and embolization of the thoracic duct: prospective trial. J Vasc Interv Radiol. 1999;10(09):1248–1254. doi: 10.1016/s1051-0443(99)70227-7. [DOI] [PubMed] [Google Scholar]
- 6.Cope C, Kaiser L R. Management of unremitting chylothorax by percutaneous embolization and blockage of retroperitoneal lymphatic vessels in 42 patients. J Vasc Interv Radiol. 2002;13(11):1139–1148. doi: 10.1016/s1051-0443(07)61956-3. [DOI] [PubMed] [Google Scholar]
- 7.Itkin M, Kucharczuk J C, Kwak A, Trerotola S O, Kaiser L R.Nonoperative thoracic duct embolization for traumatic thoracic duct leak: experience in 109 patients J Thorac Cardiovasc Surg 201013903584–589., discussion 589–590 [DOI] [PubMed] [Google Scholar]
- 8.Rajebi M R, Chaudry G, Padua H M et al. Intranodal lymphangiography: feasibility and preliminary experience in children. J Vasc Interv Radiol. 2011;22(09):1300–1305. doi: 10.1016/j.jvir.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Nadolski G J, Itkin M. Feasibility of ultrasound-guided intranodal lymphangiogram for thoracic duct embolization. J Vasc Interv Radiol. 2012;23(05):613–616. doi: 10.1016/j.jvir.2012.01.078. [DOI] [PubMed] [Google Scholar]
- 10.Pamarthi V, Stecker M S, Schenker M P et al. Thoracic duct embolization and disruption for treatment of chylous effusions: experience with 105 patients. J Vasc Interv Radiol. 2014;25(09):1398–1404. doi: 10.1016/j.jvir.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 11.Yannes M, Shin D, McCluskey K, Varma R, Santos E. Comparative analysis of intranodal lymphangiography with percutaneous intervention for postsurgical chylous effusions. J Vasc Interv Radiol. 2017;28(05):704–711. doi: 10.1016/j.jvir.2016.12.1209. [DOI] [PubMed] [Google Scholar]
- 12.Majdalany B S, Murrey D A, Jr, Kapoor B Set al. ACR Appropriateness Criteria ® Chylothorax treatment planning J Am Coll Radiol 201714(5S):S118–S126. [DOI] [PubMed] [Google Scholar]
- 13.Kim P H, Tsauo J, Shin J H. Lymphatic interventions for chylothorax: a systematic review and meta-analysis. J Vasc Interv Radiol. 2018;29(02):194–2.02E6. doi: 10.1016/j.jvir.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Majdalany B S, Khayat M, Downing T et al. Lymphatic interventions for isolated, iatrogenic chylous ascites: a multi-institution experience. Eur J Radiol. 2018;109:41–47. doi: 10.1016/j.ejrad.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 15.Majdalany B S, Saad W A, Chick J FB, Khaja M S, Cooper K J, Srinivasa R N. Pediatric lymphangiography, thoracic duct embolization and thoracic duct disruption: a single-institution experience in 11 children with chylothorax. Pediatr Radiol. 2018;48(02):235–240. doi: 10.1007/s00247-017-3988-5. [DOI] [PubMed] [Google Scholar]
- 16.Dori Y, Keller M S, Rome J J et al. Percutaneous lymphatic embolization of abnormal pulmonary lymphatic flow as treatment of plastic bronchitis in patients with congenital heart disease. Circulation. 2016;133(12):1160–1170. doi: 10.1161/CIRCULATIONAHA.115.019710. [DOI] [PubMed] [Google Scholar]
- 17.Majdalany B S, Khayat M, Sanogo M L, Saad W E, Khaja M S. Direct trans-cervical endolymphatic thoracic duct stent-graft for plastic bronchitis. Lymphology. 2018;51(03):97–101. [PubMed] [Google Scholar]
- 18.Itkin M, Piccoli D A, Nadolski G et al. Protein-losing enteropathy in patients with congenital heart disease. J Am Coll Cardiol. 2017;69(24):2929–2937. doi: 10.1016/j.jacc.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 19.Altman D, Shaver W, Viamonte M., Jr Lymphangiography in children. Am J Dis Child. 1962;104:335–341. doi: 10.1001/archpedi.1962.02080030337003. [DOI] [PubMed] [Google Scholar]
- 20.Hessel S J, Adams D F, Abrams H L. Complications of angiography. Radiology. 1981;138(02):273–281. doi: 10.1148/radiology.138.2.7455105. [DOI] [PubMed] [Google Scholar]
- 21.Dolan P A. Lymphography: complications encountered in 522 examinations. Radiology. 1966;86(05):876–880. doi: 10.1148/86.5.876. [DOI] [PubMed] [Google Scholar]
- 22.Koehler P R, Meyers W A, Skelley J F, Schaffer B. Body distribution of Ethiodol following lymphangiography. Radiology. 1964;82:866–871. doi: 10.1148/82.5.866. [DOI] [PubMed] [Google Scholar]
- 23.Bron K M, Baum S, Abrams H L. Oil embolism in lymphangiography. Incidence, manifestations, and mechanism. Radiology. 1963;80:194–202. doi: 10.1148/80.2.194. [DOI] [PubMed] [Google Scholar]
- 24.Bray D A, Brown C H, III, Herdt J R, DeVita V T. Löffler's syndrome as a complication of bipedal lymphangiography. JAMA. 1970;214(02):369–371. [PubMed] [Google Scholar]
- 25.Silvestri R C, Huseby J S, Rughani I, Thorning D, Culver B H. Respiratory distress syndrome from lymphangiography contrast medium. Am Rev Respir Dis. 1980;122(04):543–549. doi: 10.1164/arrd.1980.122.4.543. [DOI] [PubMed] [Google Scholar]
- 26.Moskowitz G, Chen P, Adams D F. Lipid embolization to the kidney and brain after lymphangiography. Radiology. 1972;102(02):327–328. doi: 10.1148/102.2.327. [DOI] [PubMed] [Google Scholar]
- 27.Kusumoto S, Imamura A, Watanabe K. Case report: the incidental lipid embolization to the brain and kidney after lymphography in a patient with malignant lymphoma: CT findings. Clin Radiol. 1991;44(04):279–280. doi: 10.1016/s0009-9260(05)80199-0. [DOI] [PubMed] [Google Scholar]
- 28.Winterer J T, Blum U, Boos S, Konstantinides S, Langer M. Cerebral and renal embolization after lymphography in a patient with non-Hodgkin lymphoma: case report. Radiology. 1999;210(02):381–383. doi: 10.1148/radiology.210.2.r99fe09381. [DOI] [PubMed] [Google Scholar]
- 29.Kirschen M P, Dori Y, Itkin M, Licht D J, Ichord R, Vossough A. Cerebral lipiodol embolism after lymphatic embolization for plastic bronchitis. J Pediatr. 2016;176:200–203. doi: 10.1016/j.jpeds.2016.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheybani A, Gaba R C, Minocha J. Cerebral embolization of ethiodized oil following intranodal lymphangiography. Semin Intervent Radiol. 2015;32(01):10–13. doi: 10.1055/s-0034-1396957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geeroms B, Demaerel P, Wauters J, Verschakelen J, Maleux G. Devastating cerebral Lipiodol® embolization related to therapeutic lymphangiography for refractory chylothorax in a patient with Behçet's disease. Vasa. 2018;47(05):427–430. doi: 10.1024/0301-1526/a000715. [DOI] [PubMed] [Google Scholar]
- 32.Chu H J, Lee C W, Yeh S J, Tsai L K, Tang S C, Jeng J S. Cerebral lipiodol embolism in hepatocellular carcinoma patients treated with transarterial embolizations/chemoembolization. PLoS One. 2015;10(06):e0129367. doi: 10.1371/journal.pone.0129367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi C S, Kim K H, Seo G S et al. Cerebral and pulmonary embolisms after transcatheter arterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2008;14(30):4834–4837. doi: 10.3748/wjg.14.4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uzun O, Findik S, Danaci M, Katar D, Erkan L. Pulmonary and cerebral oil embolism after hysterosalpingography with oil soluble contrast medium. Respirology. 2004;9(01):134–136. doi: 10.1111/j.1440-1843.2003.00524.x. [DOI] [PubMed] [Google Scholar]
- 35.Yoo K M, Yoo B G, Kim K S, Lee S U, Han B H. Cerebral lipiodol embolism during transcatheter arterial chemoembolization. Neurology. 2004;63(01):181–183. doi: 10.1212/01.wnl.0000132645.23611.2d. [DOI] [PubMed] [Google Scholar]
- 36.Bruneton J N, Le Treut A, Abbes M, Occelli J P, Aubanel D. Hepatic oil embolization following lymphangiography. A report of 12 cases. Lymphology. 1982;15(02):70–73. [PubMed] [Google Scholar]
- 37.Chavez C M, Picard J D, Davis D. Liver opacification following lymphangiography: Pathogenesis and clinical significance. Surgery. 1968;63(04):564–570. [PubMed] [Google Scholar]
- 38.Bodie J F, Linton D S., Jr Hepatic oil embolization as a complication of lymphangiography. Radiology. 1971;99(02):317–318. doi: 10.1148/99.2.317. [DOI] [PubMed] [Google Scholar]
- 39.Hecht H, Berdon W, Baker D. Hepatic oil embolization following lymphangiography in a child with neuroblastoma. Am J Roentgenol Radium Ther Nucl Med. 1968;104(04):860–864. doi: 10.2214/ajr.104.4.860. [DOI] [PubMed] [Google Scholar]
- 40.Chavez C M, Berrong L G, Evers C G. Hepatic oil embolization after lymphangiography: role of the systeimcoportal lymphaticovenous anastomosis. Am J Surg. 1965;110:456–460. doi: 10.1016/0002-9610(65)90091-7. [DOI] [PubMed] [Google Scholar]
- 41.Cope C. Diagnosis and treatment of postoperative chyle leakage via percutaneous transabdominal catheterization of the cisterna chyli: a preliminary study. J Vasc Interv Radiol. 1998;9(05):727–734. doi: 10.1016/s1051-0443(98)70382-3. [DOI] [PubMed] [Google Scholar]
- 42.Mittleider D, Dykes T A, Cicuto K P, Amberson S M, Leusner C R.Retrograde cannulation of the thoracic duct and embolization of the cisterna chyli in the treatment of chylous ascites J Vasc Interv Radiol 200819(2, Pt 1):285–290. [DOI] [PubMed] [Google Scholar]
- 43.Guevara C J, Rialon K L, Ramaswamy R S, Kim S K, Darcy M D. US-guided, direct puncture retrograde thoracic duct access, lymphangiography, and embolization: Feasibility and efficacy. J Vasc Interv Radiol. 2016;27(12):1890–1896. doi: 10.1016/j.jvir.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 44.Kariya S, Nakatani M, Ueno Y et al. Transvenous retrograde thoracic ductography: Initial experience with 13 consecutive cases. Cardiovasc Intervent Radiol. 2018;41(03):406–414. doi: 10.1007/s00270-017-1814-y. [DOI] [PubMed] [Google Scholar]
- 45.Schild H H, Pieper C C. Where have all the punctures gone? An analysis of thoracic duct embolizations. J Vasc Interv Radiol. 2020;31(01):74–79. doi: 10.1016/j.jvir.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 46.Bundy J J, Srinivasa R N, Srinivasa R N, Gemmete J J, Hage A N, Chick J FB. Vascular and lymphatic complications after thoracic duct cannulation. J Vasc Surg Venous Lymphat Disord. 2018;6(06):730–736. doi: 10.1016/j.jvsv.2018.05.023. [DOI] [PubMed] [Google Scholar]


