Abstract
Traumatic chylothorax occurs more often now than in historic reports. In part, this is due to the increased ability to perform more advanced and aggressive thoracic resections and cardiovascular surgeries as well as the improved mortality of cancer patients. If untreated, chylothorax can result in significant morbidity and mortality, particularly in patients with underlying malignancy. Thoracic duct embolization for chylothorax was the first successful lymphatic intervention and has been performed for over 20 years. An overview of the clinical and technical approach to thoracic duct embolization for traumatic chylothorax is presented in addition to a review of outcomes.
Keywords: chylothorax, thoracic duct embolization, iatrogenic, traumatic, postoperative, interventional radiology
Chylothorax is a condition in which chyle (lymphatic fluid containing protein, triglycerides, and lymphocytes) is present in the pleural space. 1 An unremitting chylothorax can result in protein depletion, immunocompromise, nutritional deficiency, and respiratory distress. 2 The underlying etiologies of chylothorax are divided into traumatic and nontraumatic, guiding therapeutic approach. 3 Historically, nontraumatic chylothorax was more common and was concerning for an undiagnosed malignancy, infection, or inflammatory process. 4 Traumatic chylothorax typically arises from injury to the thoracic duct either via penetrating trauma or as a postoperative/iatrogenic complication. As thoracic, cardiovascular, and oncologic surgical techniques have become more aggressive and more patients are able to undergo surgery, iatrogenic trauma has become the most common cause of chylothorax, accounting for approximately 54% of cases reported. 1 3 4 While medical management and surgical thoracic duct ligation (TDL) were the traditional mainstays of therapy, a significant proportion of patients suffered from the morbidity and mortality of prolonged conservative management or repeat operations. 5 6 In the late 1990s, thoracic duct embolization (TDE), an interventional radiology alternative, successfully cured greater than 70% of all chylothoraces and was particularly effective for traumatic chylothorax. 7 8
Anatomy and Etiologies
Lymphatic flow from the lower half of the body, including the organs below the diaphragm, converges on a central point, the cisterna chyli. The cisterna chyli is a saccular structure typically located at the L1–L2 level to the right of the abdominal aorta and behind the right diaphragmatic crus. 9 10 The cisterna chyli receives multiple lymphatic branches and gives rise to the dominant lymphatic channel, the thoracic duct, to the right of the aorta at the T12–L2 level. The thoracic duct enters the thoracic cavity via the aortic diaphragmatic hiatus, alongside the aorta, esophagus, and azygous vein. The thoracic duct then crosses from right to left at the T5–T6 level, ascends above the left brachiocephalic vein, and turns inferiorly to terminate into the left subclavian vein at the confluence of the jugular vein ( Fig. 1 ). Approximately 50% of people exhibit this standard anatomy, which drains up to 75% of their lymphatic fluid. 9 10
Fig. 1.

Overview of lymphatic flow in the body. The lower extremities, left upper extremity, left head, left hemithorax, and subdiaphragmatic organs drain into the cisterna chyli and transit through the thoracic duct emptying into the left venous angle, near the junction of the left internal jugular and subclavian veins. The right upper extremity, head, and hemithorax (drainage pattern shaded yellow) drain to the right lymphatic duct, near the junction between the right internal jugular and subclavian veins.
Given the intimate relationship of the thoracic duct with the aorta, esophagus, vertebral column, lungs, and other thoracic structures, it comes as no surprise that surgery or injury to any of these structures can damage the thoracic duct. 11 The incidence of chylothorax after cardiothoracic surgery is estimated to range between 2 and 5%. 12 13 Reported rates vary between cardiothoracic surgery subtypes. For patients undergoing McKeown's esophagectomy, rates of chylothorax range from 2.5 to 12% in patients with concurrent thoracic-duct ligation. 14 15 Conversely, for video-assisted thorascopic wedge resection, chylothorax occurred in as few as 0.2% of cases. 16 Referral patterns, operator experience, and technical approach will all affect the frequency with which a traumatic chylothorax occurs. However, the clinical impact is underscored by a retrospective study of 1,092 patients who underwent thoracic aneurysm repair. Findings showed that postoperative chylothorax resulted in prolonged hospital stay, increased risk of tracheostomy, heightened likelihood of graft infection, and prolonged ICU stays. 17 In general, all-cause chylothorax is estimated to occur in 1:6,000 hospital admissions. 4
Clinical Diagnosis and Management
Diagnosis of chylothorax is primarily achieved by fluid sampling, either from a chest tube or thoracentesis. Chylous fluid resembles buttermilk and is typically opaque or turbid in appearance ( Fig. 2 ). Pleural fluid concentration of triglycerides above 110 mg/dL or the presence of chylomicrons is diagnostic for chylothorax. 1 Conversely, a concentration below 55 mg/dL excludes chylothorax, unless the patient has been persistently nil per os in which case the fluid should be tested after feeding with a fatty diet. The rate of accumulation of fluid into the pleural space of 400 mL/day is an additional diagnostic clue which should raise suspicions in the postoperative period. 1
Fig. 2.
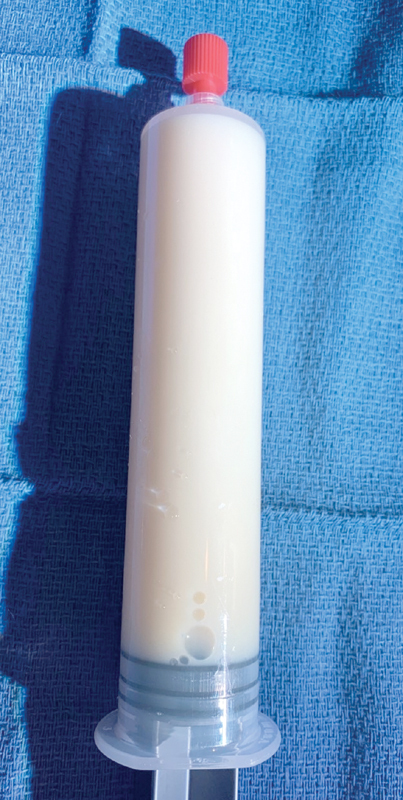
A 33-year-old man with chylothorax underwent diagnostic thoracentesis. Chyle is triglyceride rich, turbid, opaque, and resembles buttermilk.
After diagnosis of chylothorax is achieved, the patient can be managed medically with dietary modification and somatostatin or octreotide. The goal of conservative management is to decrease interstitial lymphatic flow and decrease the volume of chyle. 5 6 This approach is effective in 27% of nontraumatic cases and 50% of traumatic cases. 5 6 Chylothoraces which produce an output above 500 mL/day or which have persisted for longer than 2 weeks will likely require a procedure to achieve clinical success. 3
Invasive procedures used to treat chylothorax include surgical TDL, pleurodesis, and TDE. With an experienced operator, surgical TDL has high clinical success rate, though the potential morbidity and mortality in a debilitated patient may also be high. In particular, these patients are at increased risk for postoperative adhesions, infection, and poor wound healing with postoperative mortality in this subset of patients ranging from 4.5 to 50%. 13 A treatment algorithm for traumatic chylothorax is present in Fig. 3 .
Fig. 3.

Treatment algorithm for a known traumatic chylothorax.
Lymphangiography and Thoracic Duct Embolization
Thoracic duct embolization is the percutaneous alternative to TDL and was initially reported by Dr. Constantine Cope in the late 1990s. His initial work embolizing the thoracic duct of animals as a proof of concept allowed for the prospective trial in humans and subsequently lead to his retrospective report on 42 patients. 8 18 19 To perform the procedure, as initially described by Cope, pedal lymphangiography preceded percutaneous transabdominal access into the thoracic duct and eventual embolization. This procedure was tedious and technically challenging, slowing widespread adoption.
Presently, intranodal lymphangiography (ILN) has displaced pedal lymphangiography allowing for much shorter procedure times and increased technical success. 20 21 Performing ILN requires ultrasound-guided placement of a 25-gauge needle into inguinal lymph node ( Fig. 4a–4d ). Position is verified by injection of a small aliquot of contrast, before instilling ethiodized oil at a rate of 1 mL every 3 to 5 minutes. Using sequential compression devices (SCDs) further speeds the process and decreases procedure time. 22 Sequential spot radiographs are acquired of the pelvis, abdomen, and chest to delineate the lymphatic anatomy, find a targetable lymphatic for transabdominal access, and ascertain the source of the leakage.
Fig. 4.

Intranodal lymphangiography. ( a ) Ultrasound-guided access into a lymph node is performed, positioning the needle (white arrowhead) centrally within the lymph node (white arrow). Asterisk—blood vessel. ( b ) Bi-inguinal nodal lymphangiography. A manifold three-way stopcock (black arrowhead) allows for controlled injection from polycarbonate syringes through tubing which is connected to the needle (black arrow). ( c ) Nodal lymphangiogram with the needle (black arrow) in the lymph node produces a rapid result opacifying multiple lymphatic vessels (white arrow) and upstream lymph nodes.
With the patient positioned supine, a 21- or 22-gauge 15-cm Chiba needle with inner stylet is directed cranial with a slight bend at the tip, and then inserted at a 10- to 20-degree angle into the cisterna chyli or a prominent retroperitoneal lymphatic vessel ( Fig. 5a–d ). The needle should puncture the target, displacing contrast. Subsequently, the inner stylet should be removed and a 0.018-inch wire (our wire of choice is a V-18 Control; Boston Scientific, Natick, MA) should be used to probe the thoracic duct for cannulation. If unable to establish wire access, the inner stylet should be replaced and repeated attempts at access can be performed. If access is unsuccessful from the repeated needle probing, thoracic duct disruption (TDD) can be performed. If the microwire successfully advances cephalad into the thoracic duct and moves freely within the lumen, the needle may be exchanged for a microcatheter (our choice is a 2.4-Fr 110-cm Progreat; Terumo, Somerset, NJ). The microcatheter is inserted over the wire into the cisterna chyli and advanced into the thoracic duct over the wire. Stabilization of the microcatheter with back tension of the microwire during advancement is important to ensure cannulation. Transcatheter digital subtraction thoracic ductography can then be performed using iodinated contrast.
Fig. 5.

Establishing central lymphatic access in a 62-year-old male with left chylothorax. ( a ) The needle (white arrow) is angled ∼10 to 20 degrees cephalad. The image intensifier may be similarly angled to help facilitate targeting. ( b ) The needle (black arrowhead) approaches the retroperitoneal target (black arrow). ( c ) As the needle (black arrowhead) punctures the retroperitoneal lymphatic, the contrast (black arrow) is displaced cephalad. ( d ) After puncturing the target successfully and with the needle (white arrowhead) secured, the vessel can be probed with a microwire tip (white arrow) until it freely floats within the lumen toward the upper portions of the chest. The needle can now be exchanged for a microcatheter.
If there is a thoracic duct transection, injury, or extravasation of contrast, the microcatheter should be navigated across the injury if possible, or as close to the site of injury as possible. Tightly packed microcoils can be placed to serve as scaffolding for glue embolization using n- butyl cyanoacrylate (nBCA; Trufill; Codman and Shurtleff, Raynham, MA; Fig. 6a–c ). Care should be taken to properly prepare the microcatheter with 5% dextrose, dilute the nBCA with the proper amount of ethiodized oil, allow for appropriate polymerization, and prevent glue emboli into the pulmonary circulation or the soft tissue upon microcatheter retraction.
Fig. 6.

A 71-year-old man with high-volume right chylothorax following lung cancer resection undergoing thoracic duct embolization. ( a ) Digital subtraction lymphangiography from a needle (white arrowhead) puncturing the cisterna chyli (black arrow) reveals abrupt transection of the thoracic duct with extravasation (black arrowheads) into the right pleural space. ( b ) Multiple microcoils were tightly packed below the level of injury (between the black arrowheads). ( c ) Below the lowest microcoil (black arrowhead), glue was injected (black arrow).
Recently, transvenous and transcervical retrograde access into the thoracic duct have been described in case reports and small case series. Both approaches have been used to successfully gain access to the thoracic duct and perform TDE and can potentially be performed without lymphangiography or transabdominal punctures. 23 24 25 However, lymphatic anatomic anomalies and thoracic duct transection may pose challenges to achieving technical success. As more experience is gained and reported using these approaches, they may be further validated.
Outcomes
In 2002, Cope and Kaiser reported the results of adult TDE on 42 patients achieving greater than 70% clinical success. 8 Itkin et al reported their results on 109 adult patients with traumatic chylothorax in 2010, with more than 90% clinical success when TDE was performed and more than 70% clinical success when TDD was performed. 26 Subsequently, several large series from multiple medical centers have reported similarly promising results. 27 28 A 2018 meta-analysis comprising nine studies and over 400 patients revealed a more than 80% clinical success rate of TDE for chylothorax and a more than 50% clinical success rate of lymphangiography for chylothorax. 29 TDE for pediatric chylothorax (children ages ranging from 2 weeks to 17 years) has also been reported, albeit in smaller series compared with adult cohorts. Etiologies in the pediatric population included congenital chylothorax and postsurgical chylothorax following congenital heart surgery, among other causes. Technical success rates of 64 to 94% have been reported in children. 13 30 Reported acute complications in both adult and pediatric TDE are usually minor, self-limited, and range around 3%. 29 Uncommonly, symptomatic and asymptomatic pulmonary glue emboli have been reported. Long-term complications of TDE at 32 months include lower extremity swelling (8%), chronic abdominal swelling (6%), and chronic diarrhea (12%). 31
Conclusion
In both adults and children, lymphangiography and TDE for the management of traumatic chylothorax has emerged as the treatment modality of choice in the frequent instance of conservative management failure.
Conflict of Interest None declared.
Disclosures
All authors have read and contributed to this manuscript. The authors have no relevant disclosures. There was no grant funding or financial support for this manuscript.
References
- 1.Maldonado F, Hawkins F J, Daniels C E, Doerr C H, Decker P A, Ryu J H. Pleural fluid characteristics of chylothorax. Mayo Clin Proc. 2009;84(02):129–133. doi: 10.4065/84.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merigliano S, Molena D, Ruol A et al. Chylothorax complicating esophagectomy for cancer: a plea for early thoracic duct ligation. J Thorac Cardiovasc Surg. 2000;119(03):453–457. doi: 10.1016/s0022-5223(00)70123-1. [DOI] [PubMed] [Google Scholar]
- 3.Majdalany B S, Murrey D A, Jr, Kapoor B Set al. ACR Appropriateness Criteria ® chylothorax treatment planning J Am Coll Radiol 201714(5S)S118–S126. [DOI] [PubMed] [Google Scholar]
- 4.Doerr C H, Allen M S, Nichols F C, III, Ryu J H. Etiology of chylothorax in 203 patients. Mayo Clin Proc. 2005;80(07):867–870. doi: 10.4065/80.7.867. [DOI] [PubMed] [Google Scholar]
- 5.Maldonado F, Cartin-Ceba R, Hawkins F J, Ryu J H. Medical and surgical management of chylothorax and associated outcomes. Am J Med Sci. 2010;339(04):314–318. doi: 10.1097/MAJ.0b013e3181cdcd6c. [DOI] [PubMed] [Google Scholar]
- 6.Nair S K, Petko M, Hayward M P. Aetiology and management of chylothorax in adults. Eur J Cardiothorac Surg. 2007;32(02):362–369. doi: 10.1016/j.ejcts.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 7.Cope C, Salem R, Kaiser L R. Management of chylothorax by percutaneous catheterization and embolization of the thoracic duct: prospective trial. J Vasc Interv Radiol. 1999;10(09):1248–1254. doi: 10.1016/s1051-0443(99)70227-7. [DOI] [PubMed] [Google Scholar]
- 8.Cope C, Kaiser L R. Management of unremitting chylothorax by percutaneous embolization and blockage of retroperitoneal lymphatic vessels in 42 patients. J Vasc Interv Radiol. 2002;13(11):1139–1148. doi: 10.1016/s1051-0443(07)61956-3. [DOI] [PubMed] [Google Scholar]
- 9.Aalami O O, Allen D B, Organ C H., Jr Chylous ascites: a collective review. Surgery. 2000;128(05):761–778. doi: 10.1067/msy.2000.109502. [DOI] [PubMed] [Google Scholar]
- 10.Breslin J W, Yang Y, Scallan J P, Sweat R S, Adderley S P, Murfee W L. Lymphatic vessel network structure and physiology. Compr Physiol. 2018;9(01):207–299. doi: 10.1002/cphy.c180015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Platis I E, Nwogu C E. Chylothorax. Thorac Surg Clin. 2006;16(03):209–214. doi: 10.1016/j.thorsurg.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Nix J T, Albert M, Dugas J E, Wendt D L.Chylothorax and chylous ascites; a study of 302 selected cases Am J Gastroenterol 1957280140–53., discussion, 53–55 [PubMed] [Google Scholar]
- 13.Savla J J, Itkin M, Rossano J W, Dori Y. Post-operative chylothorax in patients with congenital heart disease. J Am Coll Cardiol. 2017;69(19):2410–2422. doi: 10.1016/j.jacc.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Oshikiri T, Takiguchi G, Miura S et al. thoracic duct resection during esophagectomy does not contribute to improved prognosis in esophageal squamous cell carcinoma: a propensity score matched-cohort study. Ann Surg Oncol. 2019;26(12):4053–4061. doi: 10.1245/s10434-019-07627-x. [DOI] [PubMed] [Google Scholar]
- 15.Bao T, Wang Y J, Li K K, Liu X H, Guo W.Short- and long-term outcomes of prophylactic thoracic duct ligation during thoracoscopic-laparoscopic McKeown esophagectomy for cancer: a propensity score matching analysis Surg Endosc 2019; [Epub ahead of print] 10.1007/s00464-019-07297-6 [DOI] [PubMed] [Google Scholar]
- 16.Hung W T, Hung M H, Wang M L, Cheng Y J, Hsu H H, Chen J S. Nonintubated thoracoscopic surgery for lung tumor: seven years' experience with 1,025 patients. Ann Thorac Surg. 2019;107(06):1607–1612. doi: 10.1016/j.athoracsur.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Wu D, Chesnokova A E, Akvan S et al. Postoperative chylothorax after thoracoabdominal aortic aneurysm repair. Semin Thorac Cardiovasc Surg. 2018;30(02):215–219. doi: 10.1053/j.semtcvs.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Cope C. Percutaneous thoracic duct cannulation: feasibility study in swine. J Vasc Interv Radiol. 1995;6(04):559–564. doi: 10.1016/s1051-0443(95)71134-4. [DOI] [PubMed] [Google Scholar]
- 19.Cope C. Percutaneous transabdominal embolization of thoracic duct lacerations in animals. J Vasc Interv Radiol. 1996;7(05):725–731. doi: 10.1016/s1051-0443(96)70840-0. [DOI] [PubMed] [Google Scholar]
- 20.Rajebi M R, Chaudry G, Padua H M et al. Intranodal lymphangiography: feasibility and preliminary experience in children. J Vasc Interv Radiol. 2011;22(09):1300–1305. doi: 10.1016/j.jvir.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Nadolski G J, Itkin M. Feasibility of ultrasound-guided intranodal lymphangiogram for thoracic duct embolization. J Vasc Interv Radiol. 2012;23(05):613–616. doi: 10.1016/j.jvir.2012.01.078. [DOI] [PubMed] [Google Scholar]
- 22.Meisinger Q C, O'Brien S, Itkin M, Nadolski G J. Use of sequential pneumatic compression devices to facilitate propagation of contrast during intranodal lymphangiography. J Vasc Interv Radiol. 2017;28(11):1544–1547. doi: 10.1016/j.jvir.2017.07.035. [DOI] [PubMed] [Google Scholar]
- 23.Mittleider D, Dykes T A, Cicuto K P, Amberson S M, Leusner C R.Retrograde cannulation of the thoracic duct and embolization of the cisterna chyli in the treatment of chylous ascites J Vasc Interv Radiol 200819(2, Pt 1):285–290. [DOI] [PubMed] [Google Scholar]
- 24.Guevara C J, Rialon K L, Ramaswamy R S, Kim S K, Darcy M D. US-guided, direct puncture retrograde thoracic duct access, lymphangiography, and embolization: feasibility and efficacy. J Vasc Interv Radiol. 2016;27(12):1890–1896. doi: 10.1016/j.jvir.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 25.Majdalany B S, Khayat M, Sanogo M L, Saad W E, Khaja M S. Direct trans-cervical endolymphatic thoracic duct stent-graft for plastic bronchitis. Lymphology. 2018;51(03):97–101. [PubMed] [Google Scholar]
- 26.Itkin M, Kucharczuk J C, Kwak A, Trerotola S O, Kaiser L R.Nonoperative thoracic duct embolization for traumatic thoracic duct leak: experience in 109 patients J Thorac Cardiovasc Surg 201013903584–589., discussion 589–590 [DOI] [PubMed] [Google Scholar]
- 27.Pamarthi V, Stecker M S, Schenker M P et al. Thoracic duct embolization and disruption for treatment of chylous effusions: experience with 105 patients. J Vasc Interv Radiol. 2014;25(09):1398–1404. doi: 10.1016/j.jvir.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 28.Yannes M, Shin D, McCluskey K, Varma R, Santos E. Comparative analysis of Intranodal Lymphangiography with percutaneous intervention for postsurgical chylous effusions. J Vasc Interv Radiol. 2017;28(05):704–711. doi: 10.1016/j.jvir.2016.12.1209. [DOI] [PubMed] [Google Scholar]
- 29.Kim P H, Tsauo J, Shin J H. Lymphatic interventions for chylothorax: a systematic review and meta-analysis. J Vasc Interv Radiol. 2018;29(02):194–2.02E6. doi: 10.1016/j.jvir.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Majdalany B S, Saad W A, Chick J FB, Khaja M S, Cooper K J, Srinivasa R N. Pediatric lymphangiography, thoracic duct embolization and thoracic duct disruption: a single-institution experience in 11 children with chylothorax. Pediatr Radiol. 2018;48(02):235–240. doi: 10.1007/s00247-017-3988-5. [DOI] [PubMed] [Google Scholar]
- 31.Laslett D, Trerotola S O, Itkin M. Delayed complications following technically successful thoracic duct embolization. J Vasc Interv Radiol. 2012;23(01):76–79. doi: 10.1016/j.jvir.2011.10.008. [DOI] [PubMed] [Google Scholar]


