Abstract
Lymphedema of the extremities related to oncologic therapies such as cancer surgery, radiation therapy, and chemotherapy is a major long-term cause of morbidity for cancer patients. Both nonsurgical and surgical management strategies have been developed. The goals of these therapies are to achieve volume reduction of the affected extremity, a reduction in patient symptoms, and a reduction in associated morbidities such as recurrent soft-tissue infections. In this article, we review both nonsurgical and surgical management strategies. Traditional surgical therapy has focused on more ablative techniques such as the Charles procedure and suction-assisted lipectomy/liposuction. However, newer more physiologic surgical methods such as lymphovenous anastomoses and vascularized lymph node transfers have become a more common treatment modality for the management of this complex problem.
Keywords: lymphedema, lymphovenous bypass, vascularized lymph node transfer, interventional radiology
Lymphedema is a disease process resulting from a buildup of protein-rich extracellular fluid within the interstitial tissues. This arises from an imbalance between lymph production and the transport of lymph fluid back into the systemic circulation. Subsequently, the excess lymph can lead to discomfort, inflammation, adipose tissue hypertrophy, and ultimately soft-tissue fibrosis resulting in a decline in quality of life, disfigurement, decreased mobility, and decreased extremity function. 1
Lymphedema affects an estimated 90 to 250 million people worldwide. 2 Primary lymphedema results from genetic or developmental anomalies. Secondary lymphedema results from obesity, trauma, infection, malignancy, or radiation to the lymphatic system. 3 The majority of secondary lymphedema cases worldwide are caused by a parasitic roundworm infection of the lymphatic system called Wuchereria bancrofti. However, in developed countries, the leading causes of lymphedema are related to oncologic therapy. These include breast cancers, gynecologic malignancies, melanoma, sarcoma, and urologic and prostate cancer ( Fig. 1 ). 1 Lymphedema secondary to breast cancer therapy is the most common. This is typically a result of axillary lymph node dissection and radiotherapy. Obstruction and damage to the lymphatic system results in a series of events leading to lymphedema. Approximately 29 to 49% of patients undergoing axillary lymph node dissection can develop lymphedema and 5 to 7% after sentinel lymph node removal. 1 4 However, the onset of disease and the severity of disease can be highly variable depending on the type of breast cancer treatment received. On average, lymphedema will develop 12 to 30 months after surgery but can present as late as 48 months after ablative surgery. 5 Independent risk factors for the development of lymphedema include obesity, infection, and genetic factors. 6
Fig. 1.

(Left) Stage II breast cancer–related lymphedema of the right upper extremity. (Right) Uterine cancer–related lymphedema of the left lower extremity.
Lymphedema is a life-long condition that has a significant impact on quality of life. Patients often complain of pain, tightness, heaviness, and swelling, as well as recurrent bouts of cellulitis in the affected extremity and an inability to find proper clothing. There is also a significant financial burden. One study estimated that the cost of breast cancer treatment in patients with lymphedema was nearly double of that of nonlymphedema patients—$23,167 versus $14,877, respectively. 7 Lymphedema has also been associated with significant psychosocial effects such as social isolation, depression, and anger. 8
To date, there are no curative treatments for lymphedema. However, current therapies, both surgical and nonsurgical, have been shown to be effective in reducing the morbidity associated with the disease. This review will provide an overview of conventional therapies as well as recent surgical advances to address this debilitating disease.
Diagnosis of Lymphedema
Common symptoms of lymphedema include swelling, tightness, heaviness, thickened tissues, recurrent infections, and pain in the affected limb. To diagnose lymphedema, one must rule out other causes of extremity swelling such as congestive heart failure, renal disease, infection, recurrent malignancy, or vascular insufficiency. A thorough history and physical examination are essential, as primary and secondary lymphedema can often be distinguished by patient history alone. Evaluation of the extremity should note limb size variation and comparison to the unaffected limb, scar location, and general skin condition. Several methods of volumetric analysis of the extremity exist and are discussed below. Various diagnostic guidelines for lymphedema exist and generally include limb circumference differences of 2 cm, limb volume differences of 200 mL, or 5% volume difference between limbs. 9 10
Once a clinical diagnosis has been made, additional testing can be performed to determine both the extent of disease and the functional status of the lymphatic system. Lymphoscintigraphy, which utilizes the radionuclide technetium-99 as a tracer molecule taken up by the lymphatics, was previously viewed as the “gold standard” for lymphedema imaging. The tracer would be injected into the dermis of the foot or hand and resulting images, taken at various time points over a period of up to 6 hours, could demonstrate dynamic flow, drainage of lymph into nodal basins, and areas of lymphatic blockage or backflow. However, radionuclide-based techniques result in poor image resolution and provide a set of static images. Additionally, lymphoscintigraphy is time consuming and rarely aids in surgical planning. 11
More recently, magnetic resonance lymphangiography (MRL) has been developed to provide high-resolution images of the lymphatic system. MRL provides detailed characterization of the soft tissues, including fibrotic changes seen in late-stage lymphedema, fluid contribution to the excess limb volume, as well as calculating limb circumference and overall limb volumes. Injection of ferumoxytol at the time of the MRL can distinguish between lymphatic channels and veins. MRL has become a valuable tool for evaluating the functional status of a patient's lymphatics, including soft-tissue quality and the presence of nodal basins, which assists the surgeon in operative planning. The presence of significant soft-tissue fibrosis and nonfunctional lymphatics would often preclude a patient from any physiologic procedure. 11 Use of this modality comes with the caveat that not all centers have been able to reproduce the high-quality images published by a limited number of authors.
Indocyanine green (ICG) lymphangiography is commonly used for real-time visualization of lymphatic flow. Dye is injected in a subdermal plane in the distal extremity and binds to interstitial protein molecules which, when excited in the near-infrared spectrum, fluoresce under infrared light and allow visualization of draining lymphatic channels and dermal reflux. ICG can reveal the condition of lymphatic vessels, their contractility, as well as valvular competence and its diffusion pattern can assist in grading the severity of lymphedema. A linear pattern of ICG flow along the extremity is considered normal, whereas a “splash” pattern or “stardust” pattern indicates reflux of dye from deeper lymphatic transport channels back into the subdermal lymphatic plexus as a result of occlusion of the more proximal lymphatic channels. ICG lymphangiography can be performed in the clinic setting as a diagnostic tool, as well as in the operating room where it is used to define the location of those lymphatic channels requiring surgical intervention.
Lymphedema Staging
Lymphedema staging systems are important tools in classifying the severity of disease and, ultimately, defining the appropriate treatment modality. The most widely used staging system was proposed by the International Society of Lymphology. It classifies lymphedema into four clinical stages:
Stage 0: Latent or subclinical condition where swelling is not present despite impaired lymphatic transport. It may exist months or years before overt edema occurs.
Stage I: Early accumulation of fluid relatively high in protein content (i.e., compared with venous edema). Edema subsides with limb elevation. Pitting can be present.
Stage II:
• Early—Pitting is present which does not resolve with elevation alone.
• Late—Tissue fibrosis develops, and pitting may or may not be elicited.
Stage III : Lymphostatic elephantiasis with absence of pitting. Trophic skin changes, lipodystrophy, and warty skin overgrowth develop. Stage III is the most severe form of lymphedema and is commonly associated with the filarial cause of secondary lymphedema.
Within each stage, severity based on volume excess (when compared with the unaffected limb) may be subclassified as minimal (<20% volume excess), moderate (20–40% volume excess), and severe (>40% volume excess).
MD Anderson devised a classification scheme using ICG lymphangiography to assist with surgical planning in lymphedema of the arm ( Fig. 2 ) 12 :
Fig. 2.

MD Anderson lymphedema classification based on indocyanine green lymphangiographic findings. ( a ) Stage 1: many patent lymphatic vessels, with minimal, patchy dermal backflow. ( b ) Stage 2: moderate number of patent lymphatic vessels, with segmental dermal backflow. ( c ) Stage 3: few patent lymphatic vessels, with extensive dermal backflow involving the entire arm. ( d ) Stage 4: no patent lymphatic vessels seen, with severe dermal backflow involving the entire arm and extending to the dorsum of the hand.
Stage I: Many patent lymphatic vessels, with minimal, patchy dermal backflow.
Stage II: Moderate number of patent lymphatic vessels, with segmental dermal backflow.
Stage III: Few patent lymphatic vessels, with extensive dermal backflow involving the entire arm.
Stage IV: No patent lymphatic vessels seen, with severe dermal backflow involving the entire arm and extending to the dorsum of the hand.
Stage V: No movement of dye proximal to injection sites.
These staging systems are important for classifying degree of lymphedema, defining disease severity, and guiding treatment discussions between patients and clinicians.
Monitoring Lymphedema
Volumetric measurements of affected limbs and their comparison with the nonaffected limb allow for quantitative measurement of disease progression or improvement. The most common methods include arm tape circumference measurements, water displacement, and perometer measurements.
Circumference measurements with a measuring tape are the most frequently used technique due to the low expense and ease of use. These measurements are taken at bony landmarks or established locations on the limb by an experienced clinician ( Fig. 3 ). 13 Accuracy of the measurements can be influenced by where the measurements are taken along the limb, the tension placed on the measuring tape and the timing of the measurement. This factors can affect intra- and inter-rater reliability. It is ideal to perform as many measurements as feasible at defined 4- to 10-cm intervals. A modified cone equation is then used to calculate the limb volume based on circumferential limb measurements.
Fig. 3.

Certified lymphedema therapist performing circumferential tape measurements on a patient with breast cancer–related right upper extremity lymphedema.
Water displacement is often viewed as the gold standard for volumetric measurements and involves submerging the affected limb in a container with a preset amount of water. The water displaced represents the total volume of the limb that was submerged. This technique can be difficult to use, as it is often difficult to identify a landmark to submerge the limb during each measurement. The devices used are also large and can be impractical in a clinic setting. For these reasons, water displacement measurements are more commonly used in research settings as opposed to clinical practice. 14
Finally, perometry uses infrared optoelectronic technology to detect changes in limb volume. It uses 360 degrees of infrared light to take surface measurements of the affected limb in 0.5-cm increments ( Fig. 4 ). Volume is then calculated from this information allowing for precise and rapid limb measurement. Potential disadvantages of this technique include equipment expense and machine size. 15 Perometry has been shown to have better inter- and intra-rater reliability than measuring tape-based circumference measurements. 16
Fig. 4.

Certified lymphedema therapist performing limb measurements with perometry on the left lower limb.
Management Strategies
The goal of lymphedema treatment is limb volume reduction and improvement in patient symptoms, such as recurrent infections. Interventions can be categorized as either conservative/nonsurgical or surgical. Surgical treatments can be further subclassified into ablative and physiologic procedures. Traditional surgical therapy has focused on more ablative techniques such as the Charles procedure and suction-assisted lipectomy/liposuction. The newer more physiologic surgical methods include lymphovenous anastomoses and vascularized lymph node transfers (VLNTs). Most lymphedema patients are managed nonoperatively. Conservative management has been shown to reduce limb volumes by as much as 60% in several case series. 17 Surgical intervention is warranted in appropriate patients who do not respond to initial conservative management.
Pharmacologic Management
Currently, there are no data to support the use of medications in the routine treatment of lymphedema. Diuretics have been anecdotally used in the initial treatment of lymphedema but are not recommended long term. Several studies have evaluated the use of diuretics compared with placebo and found no difference in outcomes. Furthermore, some authors believe that diuretics may worsen the lymphedema by concentrating protein in the extracellular space. 18 Coumarin, vitamin E, and pentoxifylline have all been evaluated in randomized clinical trials with no evidence to suggest that they are useful for treating or preventing lymphedema. 19 20 There has been a temporary benefit observed to corticosteroid use in lymphedema patients; however, the steroids were usually administered for a differing medical reason and the incidental effect on the patient's lymphedema was not sustained past 1 month. 21 Antibiotics are commonly used to treat episodes of cellulitis and are recommended prophylactically for patients with more than three episodes of cellulitis per year. The inflammation associated with recurrent cellulitis has been linked to increasing levels of soft-tissue fibrosis and disease progression. 22 Anti-inflammatories have been investigated but have not shown to be effective in reducing limb volumes in lymphedema.
Complete Decongestive Therapy
Conservative measures are generally considered first-line interventions for secondary lymphedema. Complete decongestive therapy (CDT) is the mainstay of initial lymphedema management and includes manual lymphatic drainage (MLD), daily bandaging, exercise, and skin care. The original studies on CDT reported an estimated reduction in arm volume of 43% compared with 11% with compression alone. 23
CDT is divided into two phases: Phase 1 is an intensive, certified therapist–led volume reduction phase which includes MLD, wrapping the affected limb with compression bandages, edema-reducing exercises, and skin care education.
Performed by the therapist, MLD involves slow, light repetitive stroking and circular massage movements of the limb in specific sequences to clear congestion and redirect lymphatic fluid. These sessions are performed for 60 minutes two to three times a week immediately prior to bandaging. Wrapping the limb with a low-stretch bandage is the main component of this intensive phase to achieve rapid volume reduction in patients with severe pitting edema. This low-stretch bandage is wrapped in multiple layers after covering the affected limb with foam padding ( Fig. 5 ). The bandages exert a higher pressure on the limb during activity and a constant, lower, even pressure at rest and are worn for 23 hours a day during this initial treatment phase. Exercises are an important component of CDT, as it increases the rate of return of lymphatic fluid to the venous circulation by a factor of three to four times. These exercises are performed with low-stretch compression in place so that muscle contraction against the compression augments lymph transport.
Fig. 5.

Certified lymphedema therapist applying short stretch compression bandages to patient's left lower extremity.
Phase 2 is the maintenance phase and is mostly patient directed. Once maximal limb volume reduction has been achieved from the intensive phase, patients will start to use a compression garment during the day and low-stretch bandages at night ( Fig. 6 ). Compression garments deliver anywhere between 20 and 60 mm Hg of pressure with different grading depending on the garment and manufacturer. The aim of this phase is to minimize recurrence of edema, maintain stable limb volume reduction, and educate the patient on infection-reducing lifestyle changes. 24 Often during the maintenance phase, patients will utilize pneumatic compression devices to stimulate the flow of lymph fluid toward functional lymphatics. Each device consists of multiple chambers, and the sequential inflation of the chambers propagates along the affected extremity. Compression garments are removed and the device is placed over the affected extremity and will be used for 20 to 40 minutes at a time. 24 25
Fig. 6.

Patient with breast cancer–related right upper extremity lymphedema wearing a custom measured and sewn compression garment.
CDT is both time consuming and labor intensive and adds a significant cost beyond that of compression garments alone. Several recent studies, although underpowered, showed no difference between CDT and less intensive methods. A large, randomized controlled trial of 100 patients showed no significant difference in quality of life or volume reduction despite a small volume difference in CDT patients. 26 Currently, several larger studies are underway to further delineate the indications for compression and MLD in lymphedema treatment.
Surgical Management
Operative interventions for the treatment of lymphedema have existed since the early 1900s, albeit without notable success. One of the earliest methods involved threading a silk suture in the subcutaneous plane of the affected extremity in an attempt to bridge the area of obstruction and establish a conduit for lymphatic fluid to egress out of the affected extremity. 27 More recent surgical techniques for lymphedema management can broadly be categorized as ablative or physiologic. In ablative surgery, the edematous and fibrotic soft tissues are removed by either direct surgical excision or liposuction. Physiologic surgeries attempt to recreate ectopic lymphatic drainage pathways or provide an alternate route for lymph flow out of the affected limb. One such strategy has been the creation of a shunt between the congested lymphatic channel and the venous system. The other technique relies on the surgical transplantation of a vascularized lymph node flap from an unaffected area of the body to the affected extremity. 28
Ablative Surgical Techniques
Ablative surgical techniques involve the removal of excess edematous and fibrotic tissue between the deep fascial layer and the dermis. The earliest and most radical ablative procedure is the Charles procedure and was first described in 1912. With the Charles procedure, all of the fibrotic skin and subcutaneous tissue above the deep fascia of the affected extremity is removed and the resulting wound is covered with a skin graft. The procedure is extremely morbid and disfiguring and is now reserved only for the most severe cases of lymphedema ( Fig. 7 ). 29 In patients with more localized soft-tissue disease, such as massive localized lymphedema most commonly associated with morbid obesity, a simple wedge excision may be used to remove the affected area.
Fig. 7.

The Charles procedure. ( a ) Patient's left lower extremity with active lymphedema-associated cellulitis. ( b ) Excision of skin and subcutaneous tissue to the level of muscle fascia. The fascia is then skin grafted using the resected skin as a donor site. ( c ) A 52-year-old patient at initial preoperative consultation with bilateral lower extremity lymphedema secondary to obesity. ( d ) Patient's left lower extremity 1 year postoperatively with wounds healed. The final contour can be disfiguring and there is worsened edema of the foot.
More recently, suction-assisted protein lipectomy or liposuction has been used as an ablative method to remove the diseased fat from the affected extremity. The hypertrophy of the subcutaneous fatty tissue is a side effect of lymphedema and further aggravates the excess limb volume. This buildup of fatty tissue is not responsive to CDT and thus liposuction is useful in patients with a nonpitting lymphedematous limb. This volume-reducing technique is less morbid than direct excision procedures. External scarring is minimal. However, because liposuction does not affect the disrupted fluid dynamics of the extremity, the fluid component of the disease is not addressed and a compression garment must be worn continuously following the procedure to control excess fluid accumulation and maintain volume reduction. 30 In properly selected patients, liposuction can provide excellent long-term results with both a decrease in limb volume and decreased infection rates ( Fig. 8 ). 31
Fig. 8.
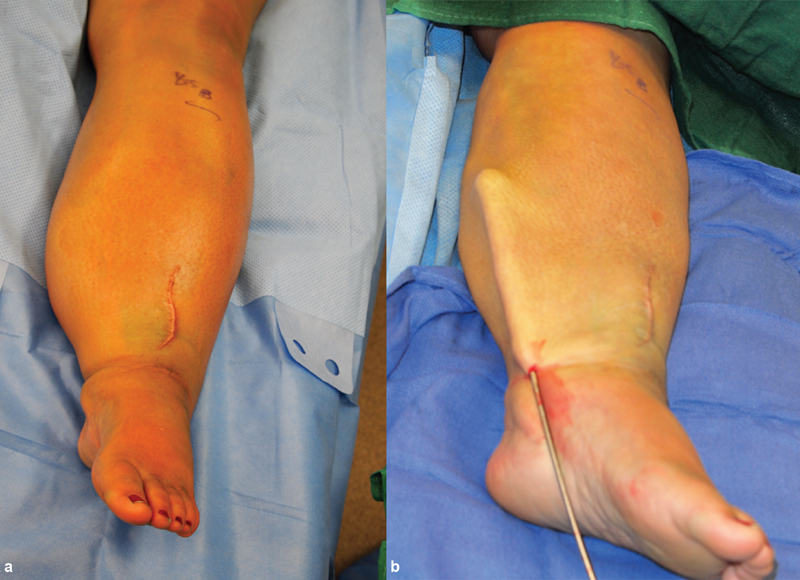
( a ) A 46-year-old woman with left lower extremity lymphedema related to cervical cancer, obturator node dissection, and radiotherapy. She had previously undergone a vascularized omental lymph node transfer to her lower extremity, but continued to have increased extremity volume related to soft-tissue fibrosis. ( b ) Significant volume reduction achieved with liposuction.
Currently, it is believed that excisional ablative methods should be reserved for more advanced stages of disease where there have been permanent architectural changes in the soft tissue. Once fibrosis and lipodystrophy have accumulated in the affected limb, correcting the fluid accumulation will not address the fat-related volume excess. Liposuction is increasingly used either before or after physiologic lymphedema-reducing procedures to optimize limb volume reduction.
Physiologic Surgical Methods
Physiologic methods are aimed at reducing the lymphatic fluid burden by improving lymphatic circulation. As microsurgical techniques have become more sophisticated over the past two decades, a new wave of surgical options for lymphedema was developed. The most commonly performed procedures include lymphovenous anastomosis (LVA) and VLNT. These surgeries address the impaired lymphatic outflow by either creating an alternate outflow pathway for the lymph fluid or introducing new healthy nodal tissue from a distant autologous source to the affected limb as a vascularized free tissue transfer.
Lymphovenous Anastomosis
Lymphovenous anastomosis is a surgical procedure where the lymphatic vessels in a lymphedematous extremity are connected to nearby veins and venules. This anastomosis allows the clearance of lymphatic fluid from the affected limb by utilizing the venous system, in effect creating an alternate clearance route. LVA was first described in the 1960s in canines and later applied to lower extremity lymphedema in human patients. 32 33 34 O'Brien reported good results using LVA in the upper extremity in breast cancer patients as early as 1977. 35 Long-term follow -up of these patients demonstrated an average volume reduction of 26% and a 58% reduction in cellulitis incidence. 33 There has been increased interest in this surgical technique, as improvements in equipment and imaging techniques have allowed this procedure to be performed more easily. Intraoperative ICG lymphography has improved a surgeon's ability to identify lymphatic vessels appropriate for LVA. Better operating microscopes and microsurgical instruments have allowed for the development of “supermicrosurgery” techniques, whereby anastomoses are performed at the capillary level with vessel diameters of 0.3 to 0.8 mm.
LVAs are utilized in patients with early-stage disease (MD Anderson Stage I or II), where some functionality of their lymphatic system remains, as the intrinsic pump function of the lymphatics is necessary to generate pressures exceeding those of the venous system to ensure flow of lymph into the venous circulation. 12 In the operating room, ICG is injected intradermally and areas of lymphatic obstruction are noted. Functional channels are identified proximal to sites of obstruction, as noted by areas of dermal reflux, and marked to guide sites for surgical exploration ( Fig. 9a ). Lymphazurin blue dye can also be injected distally in the extremity to aid in the visual identification of lymphatic vessels ( Fig. 9b ). At selected cutaneous sites, several small incisions are made and lymphatic channels are carefully identified and anastomosed with 11–0 or 12–0 suture to subdermal venules of similar caliber ( Fig. 9c ). After anastomosis, the lymphazurin dye and/or ICG dye can usually be seen traversing the LVA site ( Fig. 9d ).
Fig. 9.

Example of a lymphovenous bypass anastomosis. ( a ) Lymph channels are identified and marked using subcutaneous injection of indocyanine green dye distally in the hand and detection using an infrared camera. Veins are also marked with a vein finder. Skin incisions are 2–3 cm in length. ( b ) Isosulfan blue dye injected 2 cm distal to the incision can be used to assist in the visual identification of lymph channels. ( c ) An example of two end-to-end anastomoses of lymph vessel to vein using 11–0 nylon suture. ( d ) Patency of the anastomosis can be confirmed when indocyanine green dye can be seen traversing the anastomosis.
Numerous studies have shown improved subjective symptoms, decreased cellulitis rates, and up to a 61% reduction in limb volume in early-stage lymphedema following LVA surgery at 1-year postoperatively. Patient satisfaction rates are as high as 90%. Additionally, studies have shown a reduction in the use of compressive garments postoperatively. 12 36 37 38 One significant benefit of this procedure is that it involves very little surgical site morbidity. Incisions are localized to the affected limb and are typically 2 to 3 cm in length ( Fig. 10 ). Patients are typically discharged on the same day of surgery.
Fig. 10.

(Left) A 54-year-old woman with grade II lymphedema in her left arm following left mastectomy and radiotherapy. Her left arm is 32% larger than her normal right arm. (Right) Five lymphovenous bypasses were performed in this patient and at 3 months there has been an 84% reduction in volume differential.
Prophylactic Lymphovenous Anastomosis
Given the progressive and irreversible nature of lymphedema as a disease process, more attention has been paid to preventative techniques. More recent advances have led to a technique known as the lymphatic microsurgical preventive healing approach (LYMPHA), first described in Italy. This procedure involves the anastomosis of disrupted upper extremity lymphatics in the axilla with a nearby vein at the time of axillary lymph node dissection to facilitate lymph drainage into the venous system. In one study, of the 74 patients who were treated with LYMPHA following an axillary dissection, only 4% developed lymphedema after 4 years. 39 A more recent systematic review of the literature demonstrated a pooled lymphedema incidence rate of 14.1% in axillary lymph node dissection patients versus 2.1% in patients who underwent axillary lymph node dissection with LYMPHA. If regional lymph node radiation therapy was administered following axillary dissection, lymphedema rates increased to 33.4% compared with only 10.3% in patient who underwent LYMPHA. 40 Recently, LYMPHA has been performed in the lower extremity after inguinal lymph node dissection and shown to be safe and reliable. 41 Though data are limited, early results suggest that LYMPHA should be considered in at-risk patients.
Vascularized Lymph Node Transfer
Vascularized lymph node transfer involves the transfer of functional lymph nodes from an unaffected part of the body to an extremity affected by lymphedema to restore physiologic lymphatic flow. The blood supply of the transferred lymph nodes is anastomosed to nearby vessels in the affected limb to maintain nodal perfusion. Although not fully understood, the mechanisms of action of VLNTs are thought to be twofold: (1) The transplanted lymph nodes secrete lymphatic growth factors such as vascular endothelial growth factor C (VEGF-C) 42 which induces lymphangiogenesis. The new lymphatic collateral pathways join adjacent lymph nodes to generate new lymphatic outflow for the extremity. (2) The newly transplanted lymph nodes act as a sponge to absorb lymphatic fluid and direct it into the venous network.
Various donor sites have been described for harvesting of vascularized lymph node flaps, including the inguinal, thoracic, submental, supraclavicular, mesenteric, and omental lymph node basins. 43 44 45 46 47 48 Each of these flaps have their own advantages and disadvantages. The donor site is typically chosen based on surgeon preference, scar location, risks of donor site lymphedema, and the amount of lymphatic tissue required.
A known risk of VLNTs is the potential for iatrogenic secondary lymphedema at the donor site. Secondary iatrogenic lymphedema has been reported after groin, lateral thoracic, and supraclavicular lymph node harvest. One method to prevent this complication is to use a reverse mapping technique to identify and protect lymph nodes that preferentially drain that extremity. This involves injecting technetium-99 radiotracer distally in the limb and ICG more proximally in the groin for inguinal or axilla for lateral thoracic lymph node harvest, respectively. Intraoperatively, lymph nodes identified by ICG are also examined by a gamma probe. Any nodes that are “hot” and elicit radiotracer uptake are spared, as these nodes preferentially drain the donor extremity. 49
A more recent strategy to decrease donor limb lymphedema is harvest of intra-abdominal lymph nodes from either the omentum or jejunal mesentery. Vascularized omental lymph node flaps ( Fig. 11 ) are based on the gastroepiploic blood supply compared with vascularized jejunal lymph node flaps which incorporate a vascular arcade along the periphery of the mesentery ( Fig. 12 ). 50 To date, there have been no reported cases of intra-abdominal lymphedema related to harvesting of either of these flaps.
Fig. 11.

Omental flap harvest and inset. ( a ) Patient with right upper extremity lymphedema after mastectomy and axillary lymph node dissection. Axilla with significant scarring and retraction of skin. ( b ) Omental flap to the right axilla after scar excision.
Fig. 12.

Free vascularized mesenteric lymph node harvest and inset. ( a ) Mesenteric lymph nodes (arrow) are identified via palpation and transillumination. These nodes are identified and a mesenteric artery and vein are also identified to vascularize the flap. ( b ) Average flap size is ∼3 cm in diameter. The mesentery defect is repaired to prevent an internal bowel hernia. ( c ) The flap is anastomosed to vessels at the recipient site in the distal arm. The arrow indicates a side-to-end anastomosis of the mesenteric artery to the radial artery. The mesenteric vein was anastomosed to the cephalic vein. ( d ) Often, primary closure can be achieved over these flaps after undermining and removal of excess subcutaneous tissue.
Once a flap has been chosen, a decision must be made on where to place the lymph nodes. For the upper extremity, potential recipient sites include the axilla, elbow, or wrist. Lower extremity recipient sites include the groin, posterior knee, or the ankle. There are many factors used to determine recipient site including location of the lymphedema, recipient vessel availability, previous surgical scars, and surgeon experience. 3
Women who are seeking breast reconstruction and lymphedema treatment may be candidates for a simultaneous microsurgical breast reconstruction and VLNT. This approach involves the harvest of lymph nodes near the superficial epigastric vessels on the side contralateral to the deep inferior epigastric vessels used to revascularize the abdominal tissues during breast reconstruction. The superficial epigastric vessels are then anastomosed to vessels in the axilla to allow for additional venous drainage from the lymph node basin. 51
Regardless of flap choice or recipient location, VLNT results have been promising. The most recent systemic review identified 10 high-quality studies including 185 patients. The use of VLNT in these patients was to treat moderate- to advanced-stage lymphedema. The weighted average for limb reduction was 39.5% compared with the unaffected limb. VLNT was demonstrated to be generally safe, but the most common complications were cellulitis, lymphocele, donor site pain, and seroma. The studies that mentioned quality-of-life outcomes showed improved function, appearance, and mood. 36 VLNT offers an exciting new horizon for physiologic treatment of lymphedema. For the more difficult cases, combining VLNT and LVA may optimize the chances for improvement in lymphedema, as these two approaches work via different mechanisms.
Conclusion
Lymphedema remains an extremely challenging disease process, the pathogenesis of which remains poorly understood. There is no routine lymphedema. The treatment of lymphedema requires a multimodal approach combing medical and surgical therapies. Not uncommonly, several of these techniques, LVB, VLNT, and liposuction, can be combined to optimize the result of each patient. The obstacles in successfully managing lymphedema remain formidable. However, clinical research efforts are continuing to show significant promise which could lead to more complete, sustainable treatment strategies in the future.
Footnotes
Conflict of Interest The authors have nothing to disclose.
References
- 1.Warren A G, Brorson H, Borud L J, Slavin S A. Lymphedema: a comprehensive review. Ann Plast Surg. 2007;59(04):464–472. doi: 10.1097/01.sap.0000257149.42922.7e. [DOI] [PubMed] [Google Scholar]
- 2.Rockson S G, Rivera K K. Estimating the population burden of lymphedema. Ann N Y Acad Sci. 2008;1131:147–154. doi: 10.1196/annals.1413.014. [DOI] [PubMed] [Google Scholar]
- 3.Allen R J, Jr, Cheng M H. Lymphedema surgery: patient selection and an overview of surgical techniques. J Surg Oncol. 2016;113(08):923–931. doi: 10.1002/jso.24170. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin S A, Wright M J, Morris K T et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: patient perceptions and precautionary behaviors. J Clin Oncol. 2008;26(32):5220–5226. doi: 10.1200/JCO.2008.16.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDuff S GR, Mina A I, Brunelle C L et al. Timing of lymphedema after treatment for breast cancer: when are patients most at risk? Int J Radiat Oncol Biol Phys. 2019;103(01):62–70. doi: 10.1016/j.ijrobp.2018.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrek J A, Senie R T, Peters M, Rosen P P. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001;92(06):1368–1377. doi: 10.1002/1097-0142(20010915)92:6<1368::aid-cncr1459>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Shih Y C, Xu Y, Cormier J N et al. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: a 2-year follow-up study. J Clin Oncol. 2009;27(12):2007–2014. doi: 10.1200/JCO.2008.18.3517. [DOI] [PubMed] [Google Scholar]
- 8.Fu M R, Ridner S H, Hu S H, Stewart B R, Cormier J N, Armer J M. Psychosocial impact of lymphedema: a systematic review of literature from 2004 to 2011. Psychooncology. 2013;22(07):1466–1484. doi: 10.1002/pon.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czerniec S A, Ward L C, Kilbreath S L. Breast cancer-related arm lymphedema: fluctuation over six months and the effect of the weather. Lymphat Res Biol. 2016;14(03):148–155. doi: 10.1089/lrb.2015.0030. [DOI] [PubMed] [Google Scholar]
- 10.Fu M R. Breast cancer-related lymphedema: symptoms, diagnosis, risk reduction, and management. World J Clin Oncol. 2014;5(03):241–247. doi: 10.5306/wjco.v5.i3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang D W, Masia J, Garza R, III, Skoracki R, Neligan P C.Lymphedema: surgical and medical therapy Plast Reconstr Surg 2016138(3, Suppl):209S–218S. [DOI] [PubMed] [Google Scholar]
- 12.Chang D W, Suami H, Skoracki R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast Reconstr Surg. 2013;132(05):1305–1314. doi: 10.1097/PRS.0b013e3182a4d626. [DOI] [PubMed] [Google Scholar]
- 13.Taylor R, Jayasinghe U W, Koelmeyer L, Ung O, Boyages J. Reliability and validity of arm volume measurements for assessment of lymphedema. Phys Ther. 2006;86(02):205–214. [PubMed] [Google Scholar]
- 14.Beek M A, te Slaa A, van der Laan L et al. Reliability of the inverse water volumetry method to measure the volume of the upper limb. Lymphat Res Biol. 2015;13(02):126–130. doi: 10.1089/lrb.2015.0011. [DOI] [PubMed] [Google Scholar]
- 15.Ohberg F, Zachrisson A, Holmner-Rocklöv Å. Three-dimensional camera system for measuring arm volume in women with lymphedema following breast cancer treatment. Lymphat Res Biol. 2014;12(04):267–274. doi: 10.1089/lrb.2014.0026. [DOI] [PubMed] [Google Scholar]
- 16.Deltombe T, Jamart J, Recloux S et al. Reliability and limits of agreement of circumferential, water displacement, and optoelectronic volumetry in the measurement of upper limb lymphedema. Lymphology. 2007;40(01):26–34. [PubMed] [Google Scholar]
- 17.Maclellan R A, Couto R A, Sullivan J E, Grant F D, Slavin S A, Greene A K. Management of primary and secondary lymphedema: analysis of 225 referrals to a center. Ann Plast Surg. 2015;75(02):197–200. doi: 10.1097/SAP.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 18.Cemal Y, Pusic A, Mehrara B J. Preventative measures for lymphedema: separating fact from fiction. J Am Coll Surg. 2011;213(04):543–551. doi: 10.1016/j.jamcollsurg.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loprinzi C L, Kugler J W, Sloan J A et al. Lack of effect of coumarin in women with lymphedema after treatment for breast cancer. N Engl J Med. 1999;340(05):346–350. doi: 10.1056/NEJM199902043400503. [DOI] [PubMed] [Google Scholar]
- 20.Gothard L, Cornes P, Earl J et al. Double-blind placebo-controlled randomised trial of vitamin E and pentoxifylline in patients with chronic arm lymphoedema and fibrosis after surgery and radiotherapy for breast cancer. Radiother Oncol. 2004;73(02):133–139. doi: 10.1016/j.radonc.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Kim J G, Bae S O, Seo K S. A comparison of the effectiveness of complex decongestive physiotherapy and stellate ganglion block with triamcinolone administration in breast cancer-related lymphedema patients. Support Care Cancer. 2015;23(08):2305–2310. doi: 10.1007/s00520-014-2593-5. [DOI] [PubMed] [Google Scholar]
- 22.Kligman L, Wong R K, Johnston M, Laetsch N S. The treatment of lymphedema related to breast cancer: a systematic review and evidence summary. Support Care Cancer. 2004;12(06):421–431. doi: 10.1007/s00520-004-0627-0. [DOI] [PubMed] [Google Scholar]
- 23.Moseley A L, Carati C J, Piller N B. A systematic review of common conservative therapies for arm lymphoedema secondary to breast cancer treatment. Ann Oncol. 2007;18(04):639–646. doi: 10.1093/annonc/mdl182. [DOI] [PubMed] [Google Scholar]
- 24.Schaverien M V, Moeller J A, Cleveland S D. Nonoperative treatment of lymphedema. Semin Plast Surg. 2018;32(01):17–21. doi: 10.1055/s-0038-1635119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris S R, Schmitz K H, Campbell K L, McNeely M L.Clinical practice guidelines for breast cancer rehabilitation: syntheses of guideline recommendations and qualitative appraisals Cancer 2012118(8, Suppl):2312–2324. [DOI] [PubMed] [Google Scholar]
- 26.Dayes I S, Levine M N, Julian J A et al. Lymphedema in women with breast cancer: characteristics of patients screened for a randomized trial. Breast Cancer Res Treat. 2008;110(02):337–342. doi: 10.1007/s10549-007-9727-0. [DOI] [PubMed] [Google Scholar]
- 27.Campisi C, Boccardo F. Lymphedema and microsurgery. Microsurgery. 2002;22(02):74–80. doi: 10.1002/micr.21728. [DOI] [PubMed] [Google Scholar]
- 28.Garza R, III, Skoracki R, Hock K, Povoski S P. A comprehensive overview on the surgical management of secondary lymphedema of the upper and lower extremities related to prior oncologic therapies. BMC Cancer. 2017;17(01):468. doi: 10.1186/s12885-017-3444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dumanian G A, Futrell J W. The Charles procedure: misquoted and misunderstood since 1950. Plast Reconstr Surg. 1996;98(07):1258–1263. doi: 10.1097/00006534-199612000-00022. [DOI] [PubMed] [Google Scholar]
- 30.Schaverien M V, Munnoch D A, Brorson H. Liposuction treatment of lymphedema. Semin Plast Surg. 2018;32(01):42–47. doi: 10.1055/s-0038-1635116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brorson H. Liposuction in lymphedema treatment. J Reconstr Microsurg. 2016;32(01):56–65. doi: 10.1055/s-0035-1549158. [DOI] [PubMed] [Google Scholar]
- 32.Jacobson J H, II, Suarez E L. Microvascular surgery. Dis Chest. 1962;41:220–224. doi: 10.1378/chest.41.2.220. [DOI] [PubMed] [Google Scholar]
- 33.O'Brien B M, Mellow C G, Khazanchi R K, Dvir E, Kumar V, Pederson W C. Long-term results after microlymphaticovenous anastomoses for the treatment of obstructive lymphedema. Plast Reconstr Surg. 1990;85(04):562–572. doi: 10.1097/00006534-199004000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Yamada S, Yamagishi T. Light and electron microscopical studies on the structure of lymphatic sinus in lymph nodes. Nagoya Med J. 1961;7:7–16. [PubMed] [Google Scholar]
- 35.O'Brien B M, Sykes P, Threlfall G N, Browning F S. Microlymphaticovenous anastomoses for obstructive lymphedema. Plast Reconstr Surg. 1977;60(02):197–211. doi: 10.1097/00006534-197708000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Carl H M, Walia G, Bello R et al. Systematic review of the surgical treatment of extremity lymphedema. J Reconstr Microsurg. 2017;33(06):412–425. doi: 10.1055/s-0037-1599100. [DOI] [PubMed] [Google Scholar]
- 37.Cormier J N, Rourke L, Crosby M, Chang D, Armer J. The surgical treatment of lymphedema: a systematic review of the contemporary literature (2004-2010) Ann Surg Oncol. 2012;19(02):642–651. doi: 10.1245/s10434-011-2017-4. [DOI] [PubMed] [Google Scholar]
- 38.Campisi C, Bellini C, Campisi C, Accogli S, Bonioli E, Boccardo F. Microsurgery for lymphedema: clinical research and long-term results. Microsurgery. 2010;30(04):256–260. doi: 10.1002/micr.20737. [DOI] [PubMed] [Google Scholar]
- 39.Boccardo F, Casabona F, De Cian F et al. Lymphatic microsurgical preventing healing approach (LYMPHA) for primary surgical prevention of breast cancer-related lymphedema: over 4 years follow-up. Microsurgery. 2014;34(06):421–424. doi: 10.1002/micr.22254. [DOI] [PubMed] [Google Scholar]
- 40.Johnson A R, Kimball S, Epstein Set al. Lymphedema incidence after axillary lymph node dissection: quantifying the impact of radiation and the lymphatic microsurgical preventive healing approach Ann Plast Surg 201982(4S, Suppl 3):S234–S241. [DOI] [PubMed] [Google Scholar]
- 41.Boccardo F, Valenzano M, Costantini S et al. LYMPHA technique to prevent secondary lower limb lymphedema. Ann Surg Oncol. 2016;23(11):3558–3563. doi: 10.1245/s10434-016-5282-4. [DOI] [PubMed] [Google Scholar]
- 42.Viitanen T P, Visuri M T, Hartiala P et al. Lymphatic vessel function and lymphatic growth factor secretion after microvascular lymph node transfer in lymphedema patients. Plast Reconstr Surg Glob Open. 2013;1(02):1–9. doi: 10.1097/GOX.0b013e318293a532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaverien M V, Badash I, Patel K M, Selber J C, Cheng M H. Vascularized lymph node transfer for lymphedema. Semin Plast Surg. 2018;32(01):28–35. doi: 10.1055/s-0038-1632401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forte A J, Cinotto G, Boczar D, Huayllani M T, McLaughlin S A. Omental lymph node transfer for lymphedema patients: a systematic review. Cureus. 2019;11(11):e6227. doi: 10.7759/cureus.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng M H, Chen S C, Henry S L, Tan B K, Lin M C, Huang J J. Vascularized groin lymph node flap transfer for postmastectomy upper limb lymphedema: flap anatomy, recipient sites, and outcomes. Plast Reconstr Surg. 2013;131(06):1286–1298. doi: 10.1097/PRS.0b013e31828bd3b3. [DOI] [PubMed] [Google Scholar]
- 46.Cheng M H, Huang J J, Nguyen D H et al. A novel approach to the treatment of lower extremity lymphedema by transferring a vascularized submental lymph node flap to the ankle. Gynecol Oncol. 2012;126(01):93–98. doi: 10.1016/j.ygyno.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 47.Schaverien M V, Hofstetter W L, Selber J C. Vascularized jejunal mesenteric lymph node transfer for lymphedema: a novel approach. Plast Reconstr Surg. 2018;141(03):468e–469e. doi: 10.1097/PRS.0000000000004162. [DOI] [PubMed] [Google Scholar]
- 48.Barreiro G C, Baptista R R, Kasai K E et al. Lymph fasciocutaneous lateral thoracic artery flap: anatomical study and clinical use. J Reconstr Microsurg. 2014;30(06):389–396. doi: 10.1055/s-0034-1372482. [DOI] [PubMed] [Google Scholar]
- 49.Dayan J H, Dayan E, Smith M L. Reverse lymphatic mapping: a new technique for maximizing safety in vascularized lymph node transfer. Plast Reconstr Surg. 2015;135(01):277–285. doi: 10.1097/PRS.0000000000000822. [DOI] [PubMed] [Google Scholar]
- 50.Coriddi M, Skoracki R, Eiferman D. Vascularized jejunal mesenteric lymph node transfer for treatment of extremity lymphedema. Microsurgery. 2017;37(02):177–178. doi: 10.1002/micr.30037. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen A T, Chang E I, Suami H, Chang D W. An algorithmic approach to simultaneous vascularized lymph node transfer with microvascular breast reconstruction. Ann Surg Oncol. 2015;22(09):2919–2924. doi: 10.1245/s10434-015-4408-4. [DOI] [PubMed] [Google Scholar]


