Abstract
After nearly disappearing, invasive lymphangiography not only has resurged, but new approaches have been developed to guide lymphatic interventions. At the same time, noninvasive lymphatic imaging is playing a larger role in the evaluation of lymphatic pathologies. Lymphangioscintigraphy, computed tomography lymphangiography, and magnetic resonance lymphangiography are increasingly being used as alternatives to invasive diagnostic lymphangiography. The purpose of this article is to review current invasive and noninvasive lymphatic imaging techniques.
Keywords: lymphangioscintigraphy, CT lymphangiography, MR lymphangiography, lymphangiography, interventional radiology
First described in 1952 by a vascular surgeon, 1 invasive pedal lymphangiography was routinely used to diagnose lymphatic pathologies such as lymphangiopathies, benign lymph node disease, metastatic disease, and lymphoma. 2 With advancements in computed tomography (CT) and ultrasonography, pedal lymphangiography nearly became obsolete. However, in 1998 Dr. Constantine Cope, an interventional radiologist, described how pedal lymphangiography could be used to ultimately perform thoracic duct embolization. 3 Despite a resurgence in pedal lymphangiography for this indication, it remained technically challenging and time consuming. It was not until intranodal lymphangiography was described in pediatric 4 and adult 5 patients in 2011 and 2012, respectively, that invasive lymphangiography gained popularity as a precursor for lymphatic interventions. In addition to intranodal lymphangiography, transvenous retrograde, 6 7 8 9 percutaneous direct retrograde, 10 11 12 transhepatic, 13 14 15 and mesenteric lymphangiography 16 have also been described in case reports or series. These different invasive lymphangiography approaches have, in turn, enabled the treatment of lymphatic leakages at locations other than the thorax. 17 18 19 20 21
Simultaneous to advancements in invasive lymphangiography, noninvasive imaging modalities have played a larger role in evaluating the lymphatic system. Lymphangioscintigraphy, CT lymphangiography, and magnetic resonance (MR) lymphangiography are increasingly being used as alternatives to invasive diagnostic lymphangiography. 22 23 24
Lymphangioscintigraphy
Lymphangioscintigraphy demonstrates pathways and patterns of lymphatic drainage. It is one of the primary imaging modalities used to diagnose lymphedema and can also be performed to assess the effect of lymphedema treatments. 22 More recently, whole body lymphangioscintigraphy without and with SPECT/CT has been used to aid in the diagnosis of lymphatic pathologies that do not cause lymphedema, such as plastic bronchitis ( Fig. 1 ), iatrogenic leaks, or those associated with complex congenital heart disease. 25 26
Fig. 1.
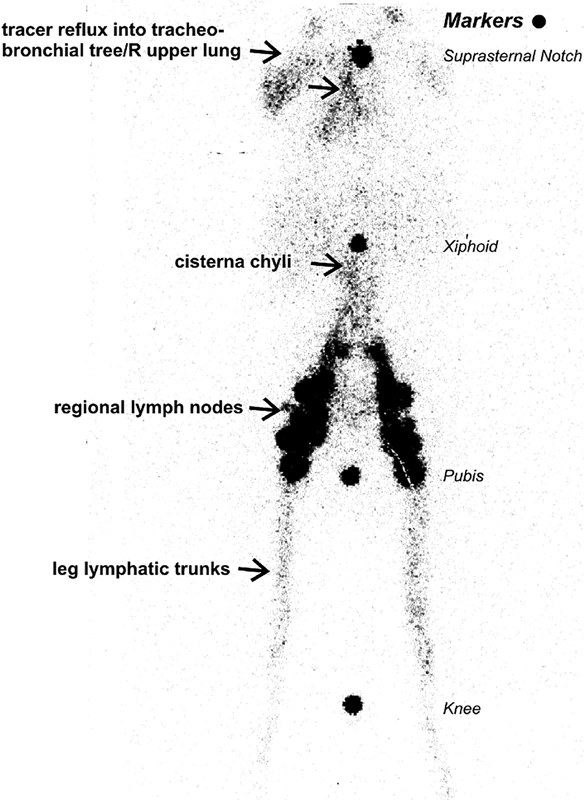
Bilateral lower extremity Tc99m sulfur colloid lymphangioscintigraphy. Note prompt cephalad tracer transport from foot injection sites into peripheral and central lymphatic trunks and regional inguinal and retroperitoneal lymph nodes up to the level of the cisterna chyli with demonstrable retrograde reflux of tracer (arrow) into the tracheobronchial tree and right upper lung field. Radioactive markers placed at key anatomic landmarks—knee, pubis, xiphoid, and suprasternal notch. (Reprinted with permission from Parikh et al. 25 )
Lymphangioscintigraphy is a minimally invasive study with insignificant patient exposure to ionizing radiation and without known adverse effects. The radiotracer used is Tc99m filtered sulfur colloid (particle size > 50 nm), which is readily available, inexpensive, and has an excellent safety profile. In adult patients, 1.0 mCi (0.1 mL) Tc99m sulfur colloid is injected intradermally into the interdigital web space between the first and second digits of the hands and/or feet. Therefore, when only bilateral upper or lower extremities are injected, the total adult activity is 2.0 mCi, but when bilateral upper and lower extremities are injected, the total adult activity is 4.0 mCi. Pediatric activity is calculated based on patient weight (0.028 mCi/kg) but should not exceed 2.0 mCi. Bilateral upper or lower extremities are typically injected simultaneously, so it requires two operators. Bilateral upper or lower extremities are injected even when one extremity is normal to compare tracer ascent.
A dual-head gamma camera is used with low-energy high-resolution collimators with energy setting 140 KeV 20% window. Following injection, whole body imaging is performed starting at the extremities and progressing to the chest at 10 to 13 cm/min. Images are obtained at 1 and 3 hours. Delayed imaging can be obtained as needed. Immediate imaging demonstrates injection sites and initial ascent of the tracer along the lymphatic trunks. Subsequent imaging shows clearance from the lymphatic trunks and collection into regional lymph nodes. Postprocessing includes image contrast adjustment to a level of optimal visualization of the lymphatic system, given the very small amount of tracer travelling and to avoid blooming artifact from the injection sites.
Lymphangioscintigraphy is hampered by low spatial resolution and lack of anatomic landmarks. SPECT/CT can be acquired as needed for anatomic localization of tracer distribution. Intradermal injection generally offers fairly rapid transport of the tracer, yet not rapid enough for dynamic imaging acquisition. Inability to see dynamic ascent of the tracer is another drawback precluding exact comparison between the two extremities. 22 27 The quantitative method has been described as having better sensitivity than qualitative assessment. 28 29 However, the quantitative method is not well established and not widely used in clinical practice.
Computed Tomography Lymphangiography
Multidetector CT alone has not been routinely used in clinical practice for dedicated lymphatic imaging due to radiation exposure and inability to characterize the anatomy and function of the lymphatic system without intralymphatic administration of contrast media. CT lymphangiography can be performed after injecting ethiodized oil via intranodal lymphangiography, or after injecting iodized water-soluble contrast via transabdominal access of the cisterna chyli.
CT lymphangiography after intranodal lymphangiography can be entirely performed in the CT suite or it can be staged. When performed entirely in the CT suite, an initial CT scan of the abdomen is performed to identify bilateral inguinal lymph nodes. Under CT guidance, a 20- to 23-gauge needle (depending on patient's body habitus) connected to tubing and a 3-mL syringe is used to access a target lymph node. After confirming appropriate needle position with CT, approximately 1 mL of water-soluble contrast (300 mg iodine/mL) is injected and CT repeated to confirm intranodal contrast injection. These steps are repeated on the contralateral side. Then a total of 2 mL of ethiodized oil is injected into bilateral accessed inguinal lymph nodes. Immediately after ethiodized oil injection, CT is performed to confirm nodal enhancement. Within 60 minutes, a CT scan from the mid-neck through the pelvis is performed. 30 31 When staged, intranodal lymphangiography is first performed in the angiography suite (as described later in section “Intranodal Lymphangiography”). The patient is then transferred to the CT suite and a CT scan from the mid-neck through the pelvis is performed within 60 minutes of completing intranodal lymphangiography.
CT lymphangiography after transabdominal access of the cisterna chyli involves accessing the cisterna chyli under CT guidance and then injecting water-soluble contrast to opacify the cisterna chyli and thoracic duct. 32
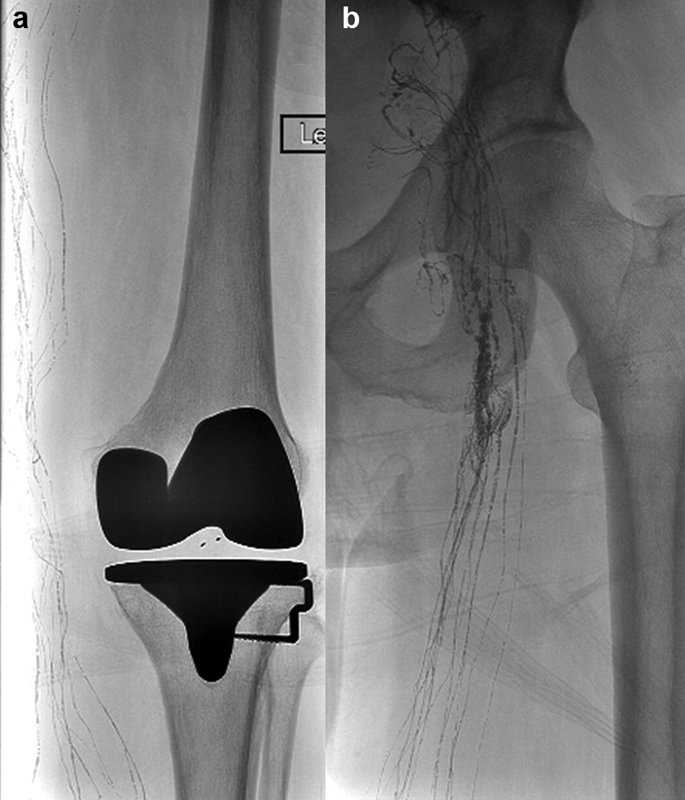
CT lymphangiography is more robust than MR lymphangiography in patients with metal implants and resulting metal artifact (e.g., from spinal implants and aortic stents; Fig. 2 ), and also allows faster image acquisition for patients who cannot tolerate a full MR imaging examination. Additionally, CT-guided interventions such as thoracic duct disruption can be performed immediately after CT lymphangiography. 32 33
Fig. 2.
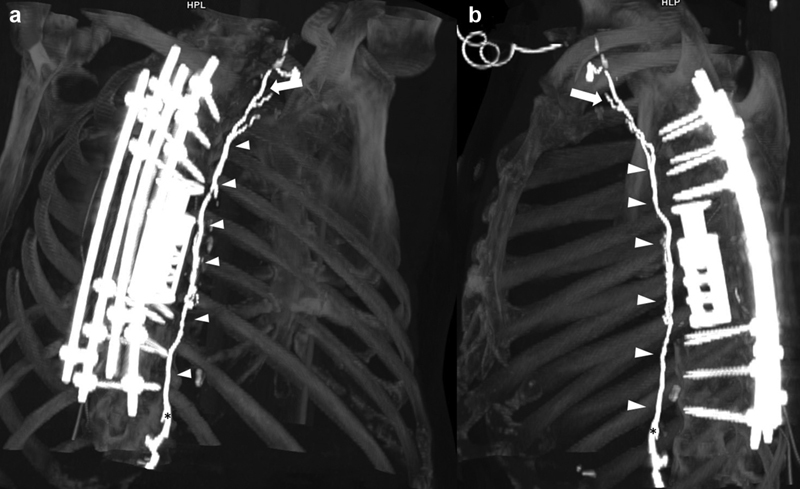
Volume-rendered reconstruction of a CT lymphangiography in ( a ) paracoronal and ( b ) sagittal orientation. The entire length of the thoracic duct (arrowheads) is clearly visualized from the cervical segment (arrow) to the cisterna chyli (asterisk) despite extensive spinal metal implants that would impair MR lymphangiography.
Magnetic Resonance Lymphangiography
Magnetic resonance lymphangiography protocols enable very good visualization of the lymphatic system. Similar to ultrasonography and CT, MR lymphangiography allows lymph node visualization. Additionally, MR lymphangiography allows visualization of lymphatic vessels, cisterna chyli, and thoracic duct. As a result, MR lymphangiography is increasingly being used as a noninvasive imaging alternative in lieu of invasive diagnostic lymphangiography. 34 35 There are two main MR lymphangiography protocols. Protocol selection is based on study indication and the need to obtain hemodynamic information. Table 1 describes the technical parameters for MR lymphangiography acquisition.
Table 1. Technical parameters for MR lymphangiography acquisition.
| MRL technique | Slice thickness (mm) | Slice overlap (%) | Voxel size (mm 3 ) | Phase encoding direction coronal | Phase encoding direction sagittal | TR/TE (ms) | Flip angle | FOV (mm) | Turbo factor/ETL | Bandwidth (Hz) |
|---|---|---|---|---|---|---|---|---|---|---|
| Noncontrast | 1 | 0 | 1.2 × 1.2 × 1.5 | Right to left | a-p | 2,000/97 | 1,408 | 400 | 270 | 501 |
| Dynamic | 1 | 0 | 1 × 1.3 × 1–1.3 | Right to left | a-p | 9.4/5.4 | 10 | 500 | 2 | 63 |
Abbreviations: a-p, anterior to posterior; ETL, echo train length; FOV, field of view; Hz, hertz; mm, millimeters; TE, echo time; TR, repetition time.
Non–Contrast-Enhanced MR Lymphangiography
Non–contrast-enhanced MR lymphangiography can be performed on 1.5-T or 3-T clinical imaging machines. Patients are imaged in the supine position. ECG leads are placed on the chest for cardiac gating, and anterior elements of phased-array coils are then placed over the chest, abdomen, and pelvis. Respiratory gating monitors are utilized for all sequences to reduce respiratory motion artifact.
Anatomic images are acquired using a steady-state balanced gradient series in the axial plane with coverage from the lower neck through the femoral heads using no angulation. Thoracic duct images are obtained using coronal and sagittal heavy T2 three-dimensional (3D) volume sequence using no angulation.
Z-axis coverage from the lower neck through the femoral heads is obtained in two stations with at least 20 mm overlap; in the sagittal plane, coverage in the lateral direction extends from midclavicular line to midclavicular line; in the coronal plane, coverage in the anterior-to-posterior direction includes the anterior chest wall through the mid-thoracic vertebral bodies ( Fig. 3 ). Technical parameters are described in Table 1 .
Fig. 3.
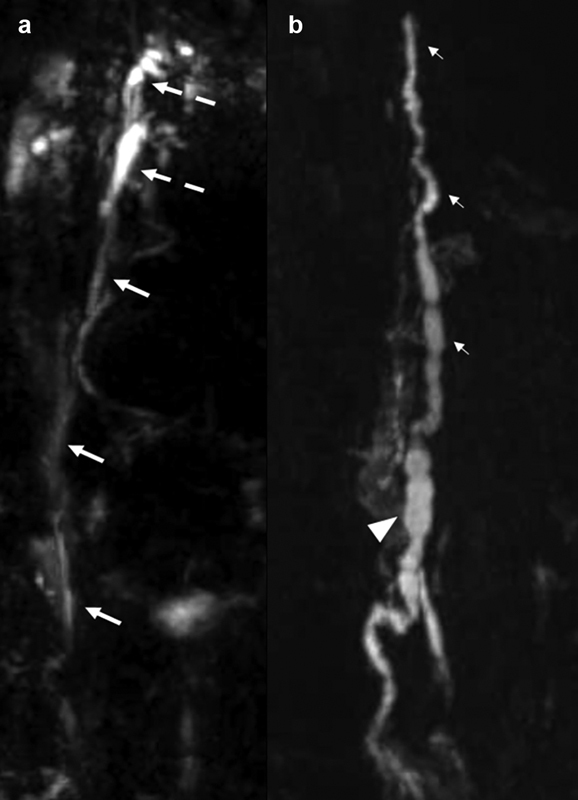
Coronal heavily T2-weighted non–contrast-enhanced MR lymphangiogram shows ( a ) the upper portion of the thoracic duct (arrows) with a duplicated cervical portion (broken arrows) and ( b ) the lower portion of the thoracic duct (small arrows) and the cisterna chyli (arrowhead).
Limitations of non-enhanced MR lymphangiography include lack of hemodynamic information and metal artifact especially in patients who have undergone orthopaedic and/or thoracic surgery. The presence of large pleural effusions which may be related to a lymphatic disorder can degrade image quality both with respiratory artifact and obscuration of portions of the duct located in close proximity. Smaller lymphatic vessels, especially in the extremities, are not well visualized using non-contrast MR lymphangiography.
Dynamic Contrast-Enhanced MR Lymphangiography
Dynamic contrast-enhanced MR lymphangiography was initially described by Krishnamurthy et al, 36 and is performed by direct intranodal injection of diluted gadolinium (1:1 dilution with normal saline) into the inguinal lymph nodes. Ultrasound-guided lymph node access is obtained (as described later in section “Intranodal Lymphangiography”). Correct needle position is confirmed sonographically by injection of either saline or ultrasound contrast media. Initial survey images are used to confirm positioning and coverage, which should extend from the middle of the neck to the inguinal region, or, in obese patients, from the middle of the neck through the lower abdomen. Non-contrast and contrast-enhanced fat-suppressed images are obtained using a breath-hold 3D spoiled gradient echo sequence. Five to 10 mL of diluted gadolinium is then slowly infused (∼1 mL/3 minutes). During contrast administration, imaging is obtained every 2 minutes using a spoiled gradient echo sequence until contrast material is seen in the retroperitoneal lymphatics. Subsequent imaging every 30 seconds to 1 minute occurs with filling of the central duct. Following this, anatomic imaging can be obtained with a short-tau inversion recovery (STIR) sequence. 36
Dynamic contrast-enhanced MR lymphangiography of the extremities can be obtained after subdermal injection of gadolinium-containing contrast media ( Fig. 4 ). For the subdermal injection, 8 mL of gadobenate dimeglumine is diluted with 1 mL of 1% lidocaine and 1 mL of sodium bicarbonate resulting in a 10-mL solution. One mL of this solution is injected subdermally along the dorsum of the hand or foot using a 25-gauge needle in the four interdigital spaces. Following the subdermal injections, imaging is obtained at 5, 10, 20, 25, and 65 minutes. In addition, 10 mL of intravenous ferumoxytol is administered for venographic imaging of the extremity of interest and corresponding central veins. Venous imaging is integral to MR lymphangiography. The central veins should be assessed for patency since veno-occlusive disease can directly or indirectly lead to lymphatic obstruction. The variable location of the lymphovenous junction should also be identified if retrograde transvenous thoracic duct catheterization is planned. Axial bright blood images of the pelvis or chest, as applicable, are acquired to assess the central veins and the inguinal or axillary region, respectively.
Fig. 4.
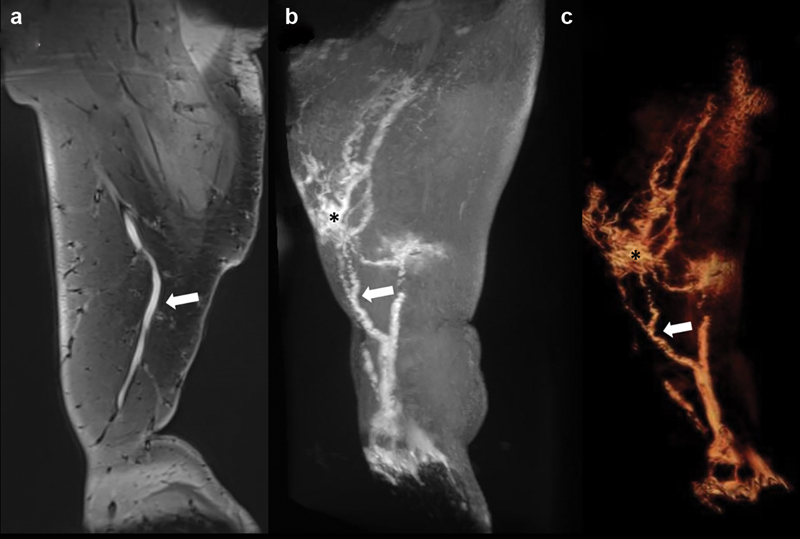
Dynamic contrast-enhanced MR lymphangiography of the right lower extremity demonstrates on ( a ) coronal spoiled gradient echo, ( b ) maximum intensity projection, and ( c ) 3D volume rendering a dominant lymphatic vessel from the foot (white arrow) with branching vessels and multiple foci of lymphatic ectasia (asterisk).
Anatomic and MR lymphangiography–specific sequences should be viewed simultaneously on a Picture Archive and Communication System (PACS) workstation and dedicated 3D postprocessing platform to relate anatomic structures to the lymphatic system and venous system. T2-weighted sagittal and coronal images provide the most coverage for the least amount of time when compared with axial images, and can be reformatted into an axial plane for direct correlation with the anatomic images. Interactive off-axis assessment using maximum intensity projection can be very helpful for visualization of the thoracic duct and the lymphovenous junction. Selective editing of the volumetric datasets with removal of the high-signal spinal fluid and pleural fluid allows for improved visualization of the central duct on postprocessing software platforms for 3D volume-rendered images.
Invasive Lymphangiography
Invasive lymphangiography is considered the gold standard for diagnostic imaging of the central and peripheral lymphatic systems. Invasive lymphangiography provides high spatial resolution anatomic imaging and allows for hemodynamic flow evaluation along the lymphatic system. However, it is limited by the need for local technical expertise. In the past, the lymphatic system was accessed via pedal lymphatic vessels (pedal lymphangiography). 1 37 Currently, it is most frequently accessed via inguinal lymph nodes (intranodal lymphangiography), although retrograde transvenous access of the thoracic duct, direct percutaneous retrograde access of the cervical portion of the thoracic duct, transhepatic access of intrahepatic lymphatic vessels, and mesenteric lymph node access have also been described. 4 5 6 7 8 9 10 11 12 13 14 15 16
Pedal Lymphangiography
The first step in pedal lymphangiography is injecting a vital blue dye, usually isosulfan blue 1% or methylene blue, at the interdigital web spaces to identify the location of pedal lymphatic vessels. 1 mL of the blue dye is aspirated into a tuberculin syringe. After wiping the planned injection site with an alcohol wipe, 0.5 mL blue dye is administered subcutaneously into two of the interdigital spaces of each foot between the first and second toes and either between the second and third or the third and fourth toes. If local anesthesia is desired at the blue dye injection sites, methylene blue can be mixed with lidocaine and administered concomitantly. However, isosulfan blue cannot be mixed with local anesthetics because the dye would immediately precipitate; so when using isosulfan blue, administration of local anesthetic must precede dye injection. The maximum dose of isosulfan blue that can be administered per study is 3 mL. Approximately 15 to 30 minutes after subcutaneous dye injection, the blue dye binds to interstitial proteins, the dye-protein complexes are absorbed by lymphatic vessels, and the vessels are visualized as blue subdermal streaks ( Fig. 5a ). Dye absorption may be accelerated by active or passive movements of the extremity such as walking or alternating between dorsiflexion and plantarflexion. Because isosulfan blue interferes with pulse oximetry readings, patients may exhibit spurious oxygen desaturations; these spurious readings peak around 30 minutes after injection and typically resolve by 4 hours. An arterial blood gas would confirm if the arterial partial pressure of oxygen is truly decreased. On the other hand, the arterial blood gas may demonstrate a spurious methemoglobin elevation so cooximetry may be needed to verify methemoglobin level. Within 24 hours, up to 10% of the subcutaneously administered dose of isosulfan blue is excreted unchanged in urine. Urine turns green as the excreted blue dye mixes with yellow urine ( Fig. 6 ). Isosulfan blue is contraindicated in patients with known hypersensitivity to triphenylmethane.
Fig. 5.
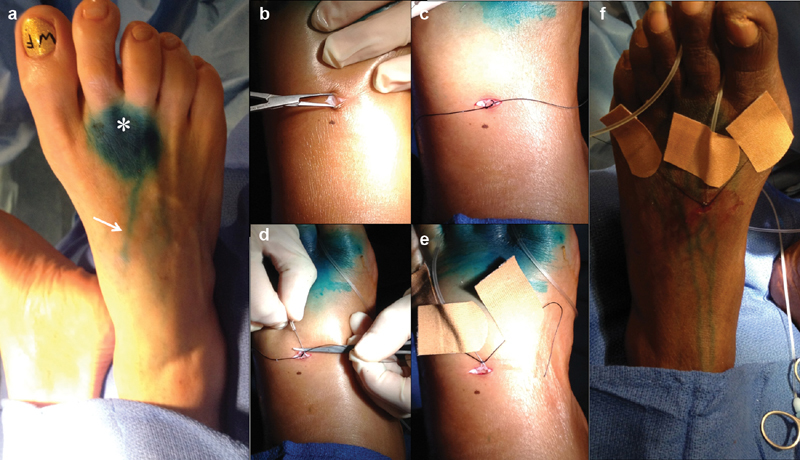
Photos depicting the steps of pedal lymphangiography: ( a ) appearance of the dorsum of the foot after injection of Lymphazurin blue (*, injection site; white arrow, dye in lymphatic vessel), ( b ) transverse incision on the dorsum of the foot and blunt dissection, ( c ) isolation of a pedal lymphatic vessel with silk tie, ( d ) access of a pedal lymphatic vessel with a 30-gauge lymphangiography needle, ( e ) securement of the lymphangiography needle in the lymphatic vessel by tying with the silk tie and of the silk tie to the foot with band aids, and ( f ) securement of the lymphangiography needle tubing around the toes.
Fig. 6.
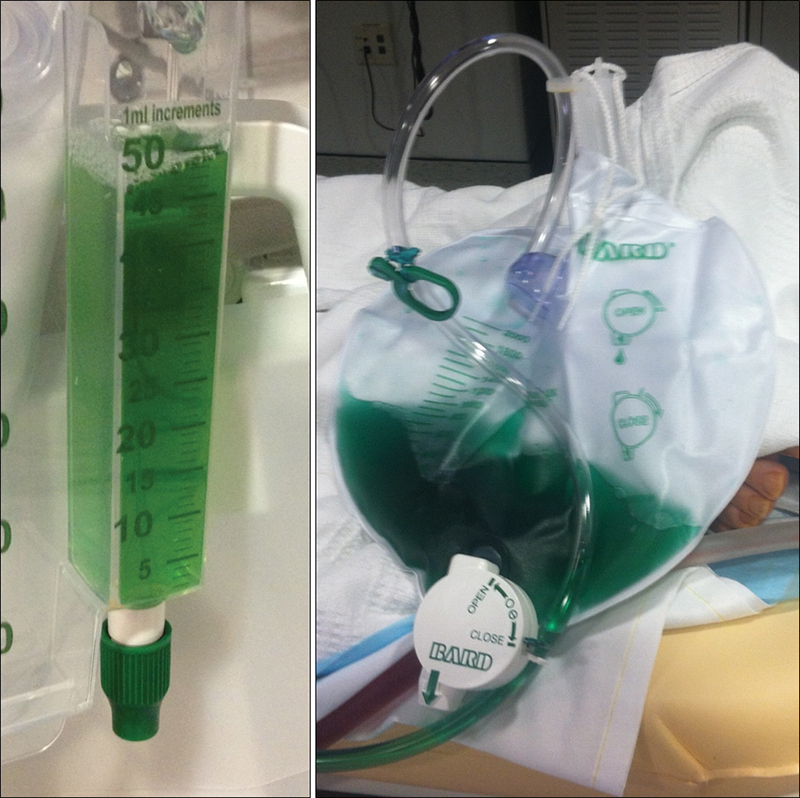
Excretion of green urine during and after pedal lymphangiography due to mixing of renally excreted isosulfan blue with yellow urine.
The second step is accessing a pedal lymphatic vessel on the dorsum of the foot. Both feet are prepped and draped in sterile fashion. A 1-cm superficial transverse incision is made overlying a stained lymphatic vessel ( Fig. 5b ). The lymphatic vessel is isolated with careful blunt dissection of the dermis and subcutaneous tissue using a mosquito clamp. A silk tie is loosely tied around the vessel ( Fig. 5c ). The vessel is accessed with 30 G lymphangiography catheter (Cook, Bloomington, IN; Fig. 5d ). Gentle hand injection of saline through the lymphangiography needle confirms that the needle is intraluminal if vessel engorgement and no leakage is observed. The needle is secured to the lymphatic vessel by tying the silk tie around the needle and vessel ( Fig. 5e ). The ends of the silk tie are taped to the dorsum of the foot using band aids or wound closure strips and the lymphangiography catheter is carefully placed between the toes without tension or slack to avoid needle dislodgement ( Fig. 5f ).
The third step is injecting ethiodized oil into the lymphatic system. Ethiodized oil (Lipiodol, Guerbet, Princeton, NJ) is iodized poppy seed oil containing 480 mg/mL iodine. Because of its high iodine concentration, ethiodized oil is also injected intramuscularly to prevent iodine-deficiency disorders. Administration of ethiodized oil is contraindicated in patients with hyperthyroidism or who are breast-feeding (can cause hypothyroidism in the neonate). Injecting bilateral feet concomitantly increases the speed of opacification of pelvic and retroperitoneal lymphatics. Although a lymphangiography injector ( Fig. 7 ) was traditionally used for pedal lymphangiography, a power injector can be used with the following settings: 8 to 12 mL/hour injection rate (faster rates for larger lymphatic vessels and small or no leakage upon saline testing), 10 mL total volume per extremity, and 150 psi pressure.
Fig. 7.

Traditional lymphangiography contrast injector.
The fourth step is tracking the progression of ethiodized oil in the lymphatic system. Spot radiographs are obtained every 15 to 20 minutes ( Fig. 8 ) until the cisterna chyli or cervical portion of the thoracic duct is visualized, as these structures can be targeted to gain access into the thoracic duct. The cisterna chyli can have four structural variations: fusiform, beadlike, conical, and ampullaceous ( Fig. 9a ). Besides structural variability, it is important to keep in mind that ethiodized oil remains nondependent in the cisterna chyli; so, only the most anterior portion of the cisterna is opacified, thus appearing smaller than it truly is. Sometimes, pedal lymphangiography alone reveals the leaking point ( Fig. 9b ). At procedure completion, lymphangiography catheters are removed, the incisions on the dorsum of the feet is sutured with absorbable subcuticular or nonabsorbable interrupted suture, and sterile dressings applied.
Fig. 8.
Spot radiographs of the left lower extremity showing ethiodized oil opacifying ( a ) proximal calf and distal thigh lymphatic vessels and ( b ) proximal thigh, inguinal, and pelvic lymphatic vessels and lymph nodes.
Fig. 9.
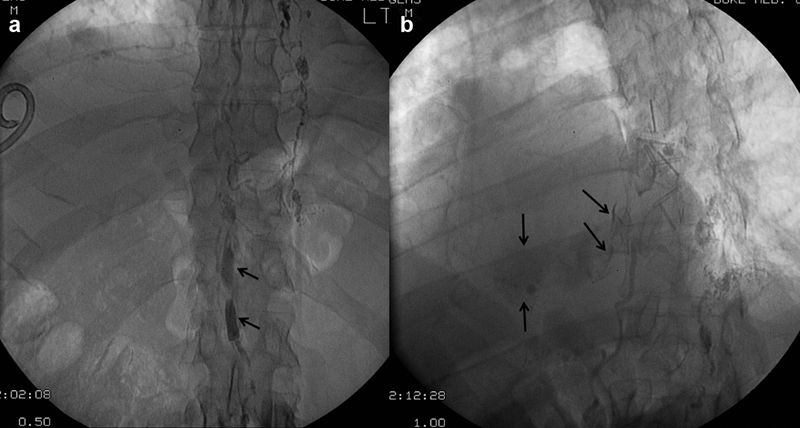
Spot radiographs of the ( a ) upper abdomen showing ethiodized oil opacifying an ampullaceous cisterna chyli (arrows) and ( b ) lower thorax showing extravasation of ethiodized oil (arrows) into the right hemithorax.
When pedal lymphangiography was used as a diagnostic study, special attention was given to the first phase of lymph node uptake—filling—as lymph node structure is best assessed then. Now that it is used to opacify the lymphatic system, it is important to keep in mind that the second phase of lymph node uptake—storage—starts 2 to 24 hours after ethiodized oil injection and lasts for 5 to 9 months. This information should be relayed to diagnostic radiologists so that they are not alarmed upon identifying opacified lymph nodes on radiographs or CT scans obtained months after the procedure. In contrast, lymphatic vessels remain opacified only within 24 hours of injection. Vessel opacification beyond 24 hours suggests underlying nodal or channel lymphatic pathology.
Intranodal Lymphangiography
Intranodal lymphangiography was initially described in pediatric patients. 4 The technique was prompted by difficulties in performing pedal lymphangiography in the pediatric population, given their small pedal lymphatic vessels. Shortly thereafter, it was described in adults. 5 Advantages of intranodal lymphangiography over pedal lymphangiography include that contrast progresses faster into the cisterna chyli and thoracic duct, especially with the use of sequential pneumatic compression devices, 38 and that it only requires basic interventional radiology skills.
Bilateral inguinal areas are evaluated with ultrasound to identify and mark at the skin any visible lymph nodes. Under direct ultrasound visualization of the target lymph node in longitudinal orientation, the tip of a 25-G needle (or spinal needle) attached to short connection tubing is advanced into the nodal cortex or medulla ( Fig. 10a ). Ideally, correct needle position is achieved in a single pass without advancing the needle beyond the posterior cortex and then retracting to the correct position as multiple needle passes and through and through passes lead to contrast extravasation. Correct needle position is confirmed under ultrasound by confirming that the needle tip is not extranodal, and fluoroscopically by injecting a small volume of contrast that opacifies the lymph node in a reticular pattern with smooth edges ( Fig. 11a ). Contrast extravasation is characterized fluoroscopically by a dense radiopaque lobular pattern with sharp edges ( Fig. 11b, c ). Once correct needle position is confirmed, the needle may be secured with wound closure strips or topical skin adhesive. However, attempts at securing the needle may result in needle dislodgement. Contrast injection then commences. We hand-inject 7 to 10 mL ethiodized oil per side at a rate of 0.5 to 1 mL/5 minute ( Fig. 12 ). Others use an insufflator by intermittently increasing the pressure to 3 atm or use a pediatric infusion pump. Whichever the method, overinjection must be avoided, as it results in extravasation along the needle tract. As contrast injection progresses, the lymph node will engorge ( Fig. 10b ) which may also result in needle displacement and consequent contrast extravasation. We monitor needle stability during contrast injection every 5 minutes by visual examination of needle position at the skin (lack of inward or outward migration), and quick fluoroscopic checks demonstrating stable tip position (compared with a referenced spot radiograph obtained immediately after initial confirmation of correct needle placement). If extravasation is identified, the needle is repositioned under ultrasonography or a different lymph node is accessed. Once ethiodized oil injection is completed, the short connection tubing is connected to a 250-mL normal saline bag in a pressure bag dripping slowly to promote central progression of ethiodized oil.
Fig. 10.
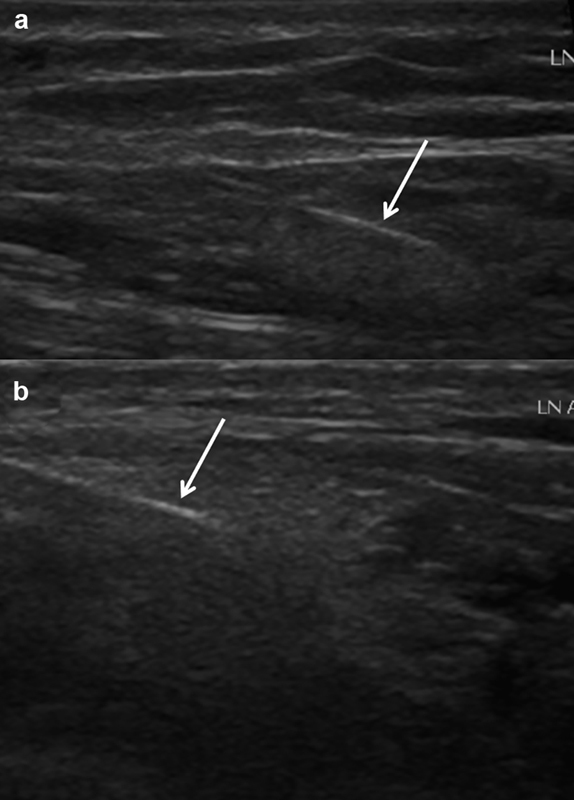
Ultrasound images of a right inguinal lymph node showing the 25-gauge needle (white arrow) in the hilum ( a ) prior to and ( b ) after ethiodized oil injection.
Fig. 11.
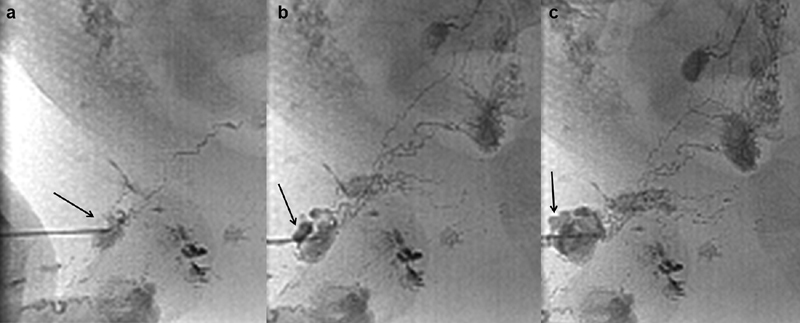
Spot radiographs of the right inguinal region of a pediatric patient during intranodal lymphangiography showing ( a ) needle access into a right inguinal lymph node opacified by ethiodized oil in a reticular pattern (arrow), ( b ) lobular dense focus of extravasation (arrow) developing at the needle entry site, and ( c ) worsening lobular focus of extravasation (arrow) at the needle entry site.
Fig. 12.
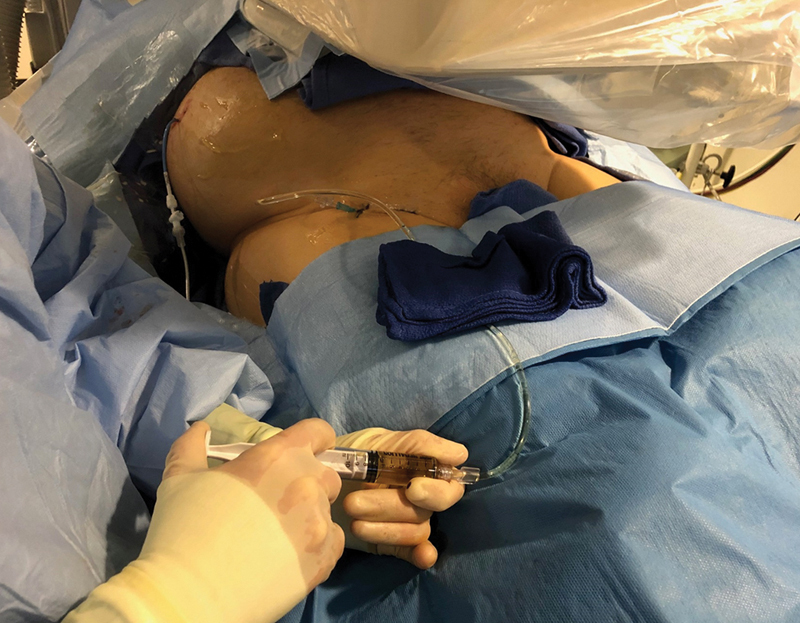
Photo demonstrating hand injection of ethiodized oil during inguinal intranodal lymphangiography.
Patients with spontaneous systemic venous to arterial shunts (e.g., patent foramen ovale with right-to-left shunting, pulmonary arteriovenous malformation) confirmed by echocardiography with contrast (bubble study) are at risk of nontarget embolization of ethiodized oil into the arterial circulation and consequent stroke. Pediatric patients with these shunts may benefit from intranodal lymphangiography with water-soluble contrast. 39 However, our experience in adults is that water-soluble contrast does not successfully opacify the lymphatic system cephalad to the pelvis ( Fig. 13 ); so, closure or embolization of the shunt should be performed prior to lymphangiography.
Fig. 13.
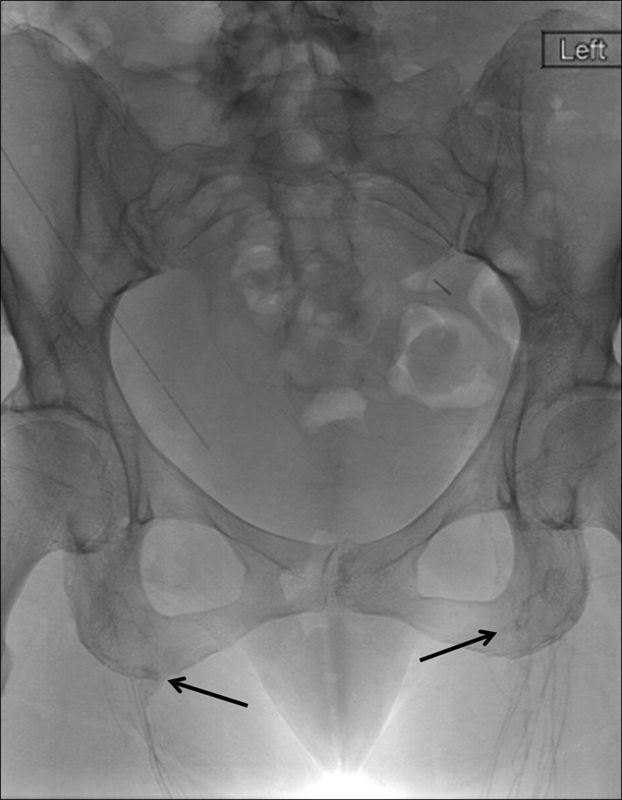
Spot radiograph of the pelvis showing water-soluble contrast opacifying lymphatic vessels and lymph nodes in bilateral proximal thighs and inguinal regions (arrows) and lack of proximal opacification of pelvic lymph nodes and vessels.
Antegrade Thoracic Lymphangiography
Once the cisterna chyli is identified fluoroscopically with pedal or intranodal lymphangiography, the cisterna chyli may be accessed transabdominally to perform antegrade thoracic lymphangiography. The right upper abdominal quadrant is prepared and draped in sterile fashion. Ultrasound evaluation is performed to identify the gallbladder and mark its margins on the skin to avoid transcholecystic puncture, as this may result in biliary peritonitis. Under fluoroscopic guidance, a 21- to 22-gauge Chiba needle of a length appropriate to patient body habitus is advanced from a skin entry site approximately 1 cm to the right and 1 cm inferior to the targeted cisterna chyli entry site. The needle is advanced in slight superomedial orientation into the cisterna chyli. Alternatively, the Chiba needle may be slightly bent approximately 1 cm from the tip and bulls-eye access of the cisterna chyli obtained with the angled tip-oriented cephalad. When the needle tip enters the cisterna chyli, the opacified cisterna chyli blanches. Correct needle tip position is confirmed fluoroscopically with orthogonal views. A microwire is advanced through the Chiba needle into the cisterna chyli and thoracic duct ( Fig. 14 ). The needle is removed, and an AccuStick introducer system (Boston Scientific, Marlborough, MA) is advanced over the microwire. After removing the metal cannula and inner dilator, water-soluble contrast is hand injected to obtain a thoracic lymphangiogram that can be used for diagnostic purposes and guidance for subsequent microcatheterization of the thoracic duct.
Fig. 14.
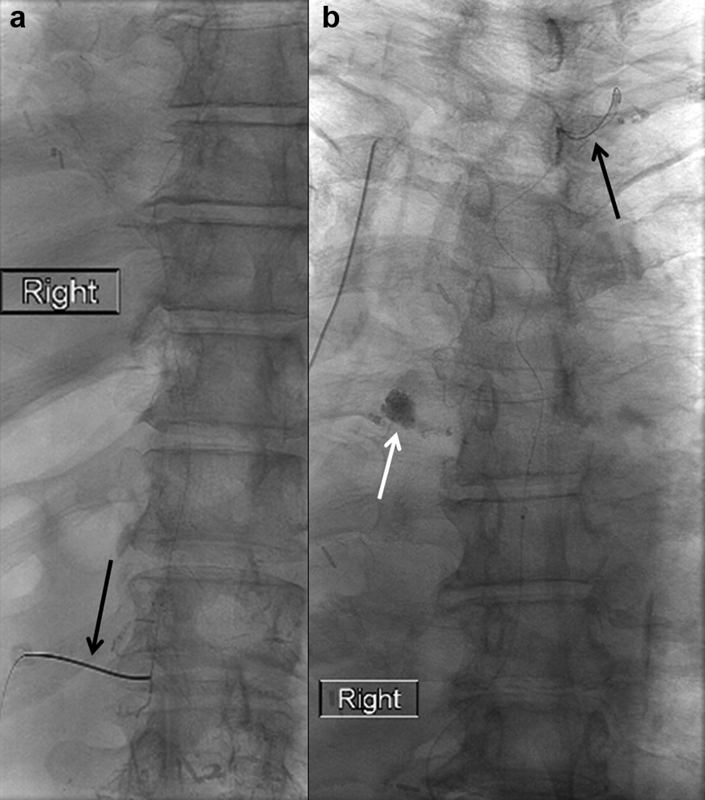
Spot radiographs of ( a ) the upper abdomen and lower thorax showing a 22-gauge Chiba needle (arrow) accessing a small fusiform cisterna chyli and ( b ) the thorax showing a microwire coursing through the thoracic duct all the way to the cervical portion of the thoracic duct (black arrow) and extravasation of contrast into the right hemithorax (white arrow).
Retrograde Transvenous Lymphangiography
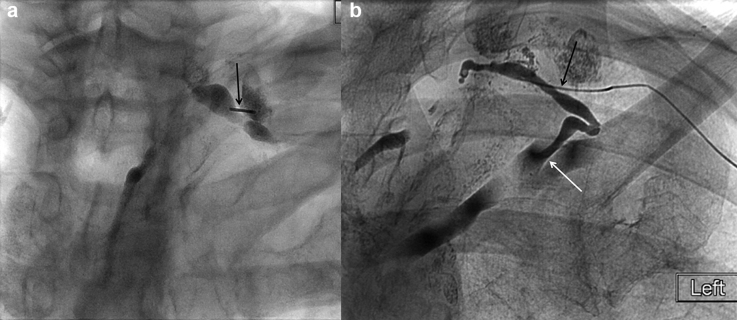
Retrograde transvenous lymphangiography has been described in case reports. 6 7 8 9 A left upper extremity vein is accessed under ultrasound guidance at the level of the upper arm. The basilic or brachial vein is preferred as the cephalic vein arch causes loss of catheter pushability and torquability. A vascular sheath is placed and an angled or curved catheter is used to select the lymphovenous junction. We use a 5 Fr × 65 cm RIM catheter (Cook, Bloomington, IN) to select the lymphovenous junction, and catheterize the thoracic duct using a variety of microwires and a microcatheter ( Fig. 15 ).
Fig. 15.
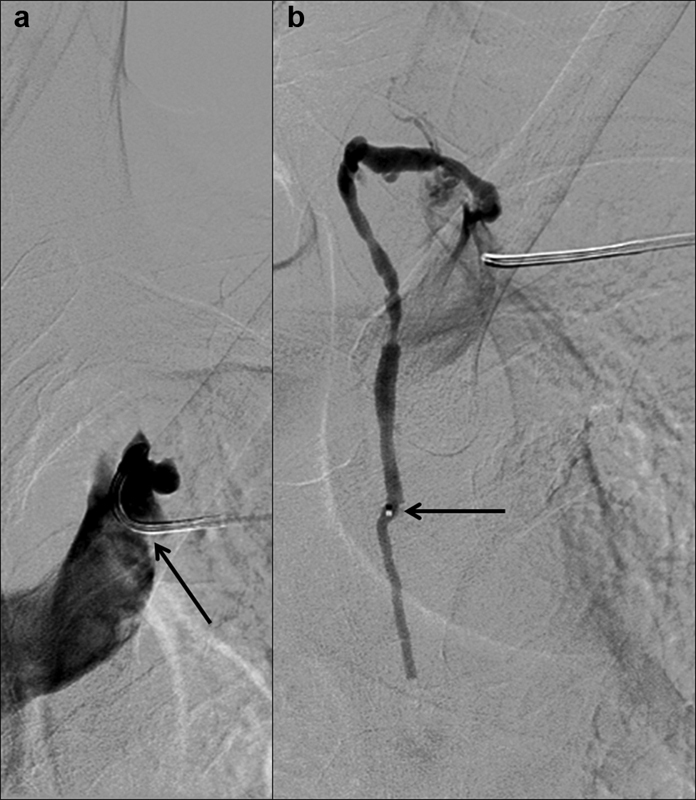
Digital subtraction ( a ) central venogram showing a 5-Fr RIM catheter (arrow) selecting the lymphovenous junction and ( b ) thoracic lymphangiogram through a microcatheter (arrow) advanced in retrograde fashion into the thoracic duct.
Performing retrograde transvenous lymphangiography without preceding pedal or intranodal lymphangiography to opacify the lymphovenous junction is extremely challenging given the variability in number and location(s) of lymphovenous junction(s). Thus, being able to visualize an opacified lymphovenous junction(s) makes this technique easier. However, advancing wires and catheters across the lymphovenous junction valve and other terminal valves remains problematic and may cause failure.
Retrograde Direct Percutaneous Lymphangiography
Retrograde direct percutaneous access of the cervical portion of the thoracic duct has been described both under fluoroscopic 10 11 and ultrasound 12 guidance. A micropuncture needle is advanced into the cervical portion of the thoracic duct ( Figs. 16 and 17a ). A microwire is advanced through the needle ( Fig. 18a ) and the needle exchanged for the micropuncture dilator system ( Fig. 17b ). Iodinated water-soluble contrast is hand injected to obtain a thoracic lymphangiogram that can be used for diagnostic purposes and guidance for subsequent microcatheterization of the thoracic duct ( Fig. 18b ).
Fig. 16.
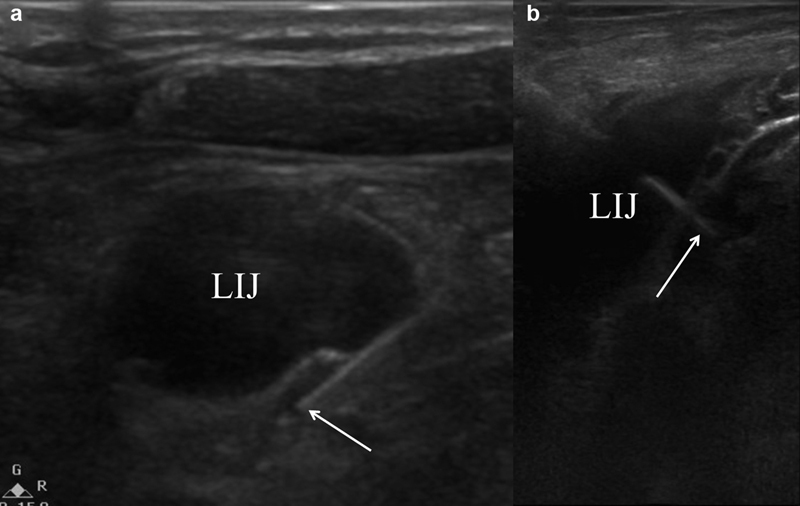
Ultrasound images showing a micropuncture needle (arrow) accessing the cervical portion of the thoracic duct ( a ) adjacent to the left internal jugular vein (LIJ) and ( b ) through the left internal jugular vein.
Fig. 17.
Spot radiographs of the left supraclavicular region showing ( a ) a micropuncture needle (arrow) accessing the cervical portion of the thoracic duct which is opacified by ethiodized oil from preceding intranodal lymphangiography, and ( b ) a 3-Fr catheter from a micropuncture kit (black arrow) in the cervical portion of the thoracic duct used to inject water-soluble contrast which reveals the lymphovenous junction (white arrow).
Fig. 18.
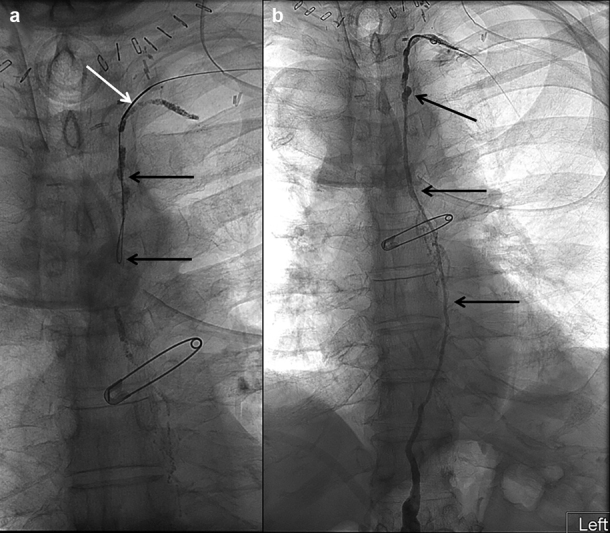
Spot radiographs of the thorax showing ( a ) a microwire (black arrows) advanced in retrograde fashion through a micropuncture needle (white arrow) accessing the cervical portion of the thoracic duct and ( b ) retrograde water-soluble thoracic lymphangiogram (arrows).
An advantage of retrograde transvenous and direct percutaneous lymphangiography is that these approaches enable evaluation of lymphatic pathologies in the thorax or abdomen ( Fig. 19 ). If not enough contrast refluxes into the abdominal lymphatic bed under evaluation, balloon occlusion lymphangiography from the thoracic duct may be performed to increase the volume of contrast refluxing into abdominal lymphatics and improve visualization. 40 Also, in patients who have previously undergone complete surgical thoracic duct ligation and have persistent chylothorax from a leak above the level of the surgical clips, the retrograde approaches allow access into the thoracic duct.
Fig. 19.
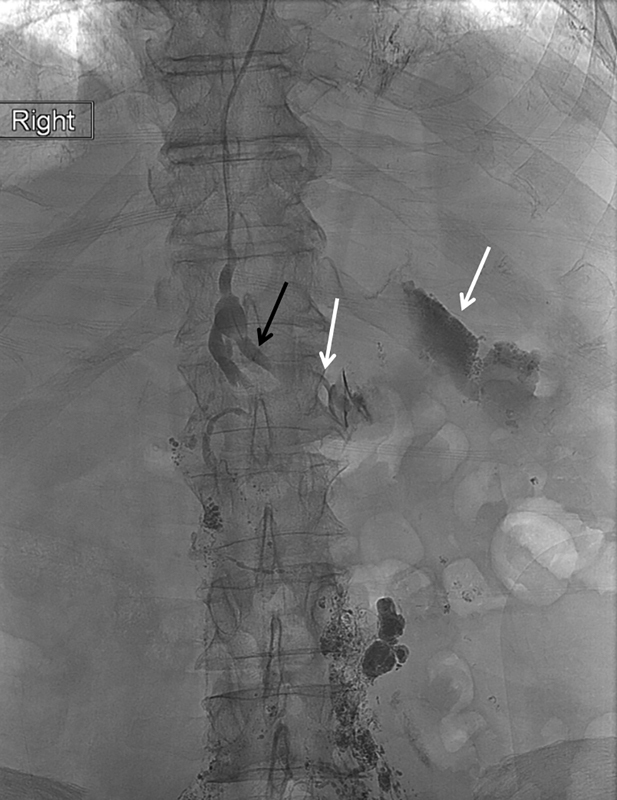
Spot radiograph of the upper abdomen during retrograde direct percutaneous lymphangiography with the tip of the microcatheter in the left lumbar trunk (black arrow) showing extravasation of contrast into the left upper quadrant (white arrows).
Transhepatic Lymphangiography
Transhepatic lymphangiography has been used to identify iatrogenic chylous ascites resulting from hepatic surgery (hepatic lymphorrhea) 13 14 as well as abnormal reflux into the duodenal lymphatics resulting in protein-losing enteropathy. 15 The procedure is performed under ultrasound guidance similar to a biliary drain placement except that the tip of the needle is advanced just outside the hyperechoic wall of the portal vein. Under fluoroscopy, contrast is gently injected to confirm periportal lymphatic needle placement ( Fig. 20a ). In fact, it is not uncommon to opacify periportal lymphatic vessels during percutaneous biliary drain placement ( Fig. 20b ).
Fig. 20.
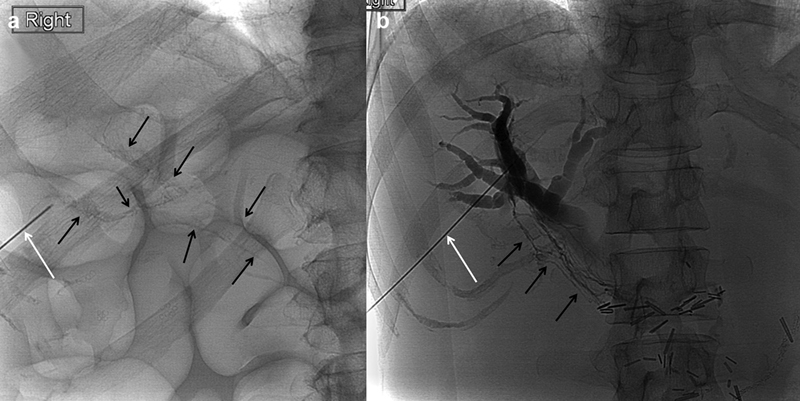
Spot radiographs of the right upper abdominal quadrant during ( a ) transhepatic lymphangiogram and ( b ) percutaneous biliary drain placement showing contrast injection through a Chiba needle (white arrow) opacifying periportal lymphatic vessels (black arrows).
Mesenteric Lymphangiography
Mesenteric lymphangiography has been described in a case report in which a patient with iatrogenic chylous ascites underwent exploratory laparotomy to identify mesenteric lymph nodes. Two of these nodes were accessed under ultrasound guidance and intranodal lymphangiography was performed revealing an iatrogenic leak in the aortocaval region on delayed spot radiography and nonenhanced CT. 16
Refinement of noninvasive and invasive lymphatic imaging techniques has resulted in improved diagnosis and treatment of lymphatic pathologies. In turn, this has opened a new frontier in vascular and interventional radiology.
Footnotes
Conflict of Interest None declared.
References
- 1.Kinmonth J B, Taylor G W, Harper R K.Lymphangiography; a technique for its clinical use in the lower limb BMJ 19551(4919):940–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuchs W A. Boston: Little, Brown and Company; 1983. Technique and complications of lymphography; pp. 1979–2007. [Google Scholar]
- 3.Cope C. Diagnosis and treatment of postoperative chyle leakage via percutaneous transabdominal catheterization of the cisterna chyli: a preliminary study. J Vasc Interv Radiol. 1998;9(05):727–734. doi: 10.1016/s1051-0443(98)70382-3. [DOI] [PubMed] [Google Scholar]
- 4.Rajebi M R, Chaudry G, Padua H M et al. Intranodal lymphangiography: feasibility and preliminary experience in children. J Vasc Interv Radiol. 2011;22(09):1300–1305. doi: 10.1016/j.jvir.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Nadolski G J, Itkin M. Feasibility of ultrasound-guided intranodal lymphangiogram for thoracic duct embolization. J Vasc Interv Radiol. 2012;23(05):613–616. doi: 10.1016/j.jvir.2012.01.078. [DOI] [PubMed] [Google Scholar]
- 6.Koike Y, Hirai C, Nishimura J, Moriya N, Katsumata Y. Percutaneous transvenous embolization of the thoracic duct in the treatment of chylothorax in two patients. J Vasc Interv Radiol. 2013;24(01):135–137. doi: 10.1016/j.jvir.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Mittleider D, Dykes T A, Cicuto K P, Amberson S M, Leusner C R.Retrograde cannulation of the thoracic duct and embolization of the cisterna chyli in the treatment of chylous ascites J Vasc Interv Radiol 200819(2, Pt 1):285–290. [DOI] [PubMed] [Google Scholar]
- 8.Arslan B, Masrani A, Tasse J C, Stenson K, Turba ÜC. Superselective retrograde lymphatic duct embolization for management of postoperative lymphatic leak. Diagn Interv Radiol. 2017;23(05):379–380. doi: 10.5152/dir.2017.16514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kariya S, Nakatani M, Ueno Y et al. Transvenous retrograde thoracic ductography: initial experience with 13 consecutive cases. Cardiovasc Intervent Radiol. 2018;41(03):406–414. doi: 10.1007/s00270-017-1814-y. [DOI] [PubMed] [Google Scholar]
- 10.Pieper C C, Schild H H. Direct cervical puncture for retrograde thoracic duct embolization in a postoperative cervical lymphatic fistula. J Vasc Interv Radiol. 2015;26(09):1405–1408. doi: 10.1016/j.jvir.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Reis S P, MacFarlane J, Anene A, Pillai A K. Direct percutaneous access to the cervical portion of the thoracic duct, an alternative to traditional access through the cisterna chyli. J Vasc Interv Radiol. 2015;26(12):1902–1904. doi: 10.1016/j.jvir.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 12.Guevara C J, Rialon K L, Ramaswamy R S, Kim S K, Darcy M D. US-guided, direct puncture retrograde thoracic duct access, lymphangiography, and embolization: feasibility and efficacy. J Vasc Interv Radiol. 2016;27(12):1890–1896. doi: 10.1016/j.jvir.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 13.Guez D, Nadolski G J, Pukenas B A, Itkin M. Transhepatic lymphatic embolization of intractable hepatic lymphorrhea. J Vasc Interv Radiol. 2014;25(01):149–150. doi: 10.1016/j.jvir.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto S, Mori H, Tada I. Successful demonstration of post-operative lymphatic fistula by percutaneous transhepatic lymphography. Clin Radiol. 2000;55(06):485–486. doi: 10.1053/crad.2000.0123. [DOI] [PubMed] [Google Scholar]
- 15.Itkin M, Piccoli D A, Nadolski G et al. Protein-losing enteropathy in patients with congenital heart disease. J Am Coll Cardiol. 2017;69(24):2929–2937. doi: 10.1016/j.jacc.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 16.Lee H, Kim S J, Hur S et al. The feasibility of mesenteric intranodal lymphangiography: its clinical application for refractory postoperative chylous ascites. J Vasc Interv Radiol. 2018;29(09):1290–1292. doi: 10.1016/j.jvir.2018.01.789. [DOI] [PubMed] [Google Scholar]
- 17.Chen E, Itkin M. Thoracic duct embolization for chylous leaks. Semin Intervent Radiol. 2011;28(01):63–74. doi: 10.1055/s-0031-1273941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itkin M. Magnetic resonance lymphangiography and lymphatic embolization in the treatment of pulmonary complication of lymphatic malformation. Semin Intervent Radiol. 2017;34(03):294–300. doi: 10.1055/s-0037-1604301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itkin M, Chen E H. Thoracic duct embolization. Semin Intervent Radiol. 2011;28(02):261–266. doi: 10.1055/s-0031-1280676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawasaki R, Sugimoto K, Fujii M et al. Therapeutic effectiveness of diagnostic lymphangiography for refractory postoperative chylothorax and chylous ascites: correlation with radiologic findings and preceding medical treatment. AJR Am J Roentgenol. 2013;201(03):659–666. doi: 10.2214/AJR.12.10008. [DOI] [PubMed] [Google Scholar]
- 21.Lai F C, Chen L, Tu Y R, Lin M, Li X. Prevention of chylothorax complicating extensive esophageal resection by mass ligation of thoracic duct: a random control study. Ann Thorac Surg. 2011;91(06):1770–1774. doi: 10.1016/j.athoracsur.2011.02.070. [DOI] [PubMed] [Google Scholar]
- 22.Witte C L, Witte M H, Unger E C et al. Advances in imaging of lymph flow disorders. Radiographics. 2000;20(06):1697–1719. doi: 10.1148/radiographics.20.6.g00nv141697. [DOI] [PubMed] [Google Scholar]
- 23.Feigen M, Crocker E F, Read J, Crandon A J. The value of lymphoscintigraphy, lymphangiography and computer tomography scanning in the preoperative assessment of lymph nodes involved by pelvic malignant conditions. Surg Gynecol Obstet. 1987;165(02):107–110. [PubMed] [Google Scholar]
- 24.Dori Y. Novel lymphatic imaging techniques. Tech Vasc Interv Radiol. 2016;19(04):255–261. doi: 10.1053/j.tvir.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Parikh K, Witte M H, Samson R et al. Successful treatment of plastic bronchitis with low fat diet and subsequent thoracic duct ligation in child with Fontan physiology. Lymphology. 2012;45(02):47–52. [PubMed] [Google Scholar]
- 26.Kuo P H, Barber B J, Kylat R I et al. Whole-body lymphangioscintigraphy and SPECT/CT in children with lymphatic complications after surgery for complex congenital heart disease. Lymphology. 2019;52(04):157–165. [PubMed] [Google Scholar]
- 27.Szuba A, Shin W S, Strauss H W, Rockson S. The third circulation: radionuclide lymphoscintigraphy in the evaluation of lymphedema. J Nucl Med. 2003;44(01):43–57. [PubMed] [Google Scholar]
- 28.Weissleder H, Weissleder R. Lymphedema: evaluation of qualitative and quantitative lymphoscintigraphy in 238 patients. Radiology. 1988;167(03):729–735. doi: 10.1148/radiology.167.3.3363131. [DOI] [PubMed] [Google Scholar]
- 29.Szuba A, Strauss W, Sirsikar S P, Rockson S G. Quantitative radionuclide lymphoscintigraphy predicts outcome of manual lymphatic therapy in breast cancer-related lymphedema of the upper extremity. Nucl Med Commun. 2002;23(12):1171–1175. doi: 10.1097/00006231-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Dong J, Xin J, Shen W et al. CT lymphangiography (CTL) in primary intestinal lymphangiectasia (PIL): a comparative study with intraoperative enteroscopy (IOE) Acad Radiol. 2019;26(02):275–281. doi: 10.1016/j.acra.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 31.Pimpalwar S, Annam A, Chinnadurai P, Hernandez A. Imaging of the thoracic duct using C-arm CT lymphangiography (CTL) following ultrasound guided inguinal nodal injection in children with right to left shunts. J Vasc Interv Radiol. 2013;24:S29. [Google Scholar]
- 32.Schoellnast H, Maybody M, Getrajdman G I, Bains M S, Finley D J, Solomon S B. Computed tomography-guided access to the cisterna chyli: introduction of a technique for direct lymphangiography to evaluate and treat chylothorax. Cardiovasc Intervent Radiol. 2011;34 02:S240–S244. doi: 10.1007/s00270-010-9851-9. [DOI] [PubMed] [Google Scholar]
- 33.Litherland B, Given M, Lyon S. Percutaneous radiological management of high-output chylothorax with CT-guided needle disruption. J Med Imaging Radiat Oncol. 2008;52(02):164–167. doi: 10.1111/j.1440-1673.2008.01935.x. [DOI] [PubMed] [Google Scholar]
- 34.Kim E Y, Hwang H S, Lee H Y et al. Anatomic and functional evaluation of central lymphatics with noninvasive magnetic resonance lymphangiography. Medicine (Baltimore) 2016;95(12):e3109. doi: 10.1097/MD.0000000000003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan E H, Russell J L, Williams W G, Van Arsdell G S, Coles J G, McCrindle B W. Postoperative chylothorax after cardiothoracic surgery in children. Ann Thorac Surg. 2005;80(05):1864–1870. doi: 10.1016/j.athoracsur.2005.04.048. [DOI] [PubMed] [Google Scholar]
- 36.Krishnamurthy R, Hernandez A, Kavuk S, Annam A, Pimpalwar S. Imaging the central conducting lymphatics: initial experience with dynamic MR lymphangiography. Radiology. 2015;274(03):871–878. doi: 10.1148/radiol.14131399. [DOI] [PubMed] [Google Scholar]
- 37.Guermazi A, Brice P, Hennequin C, Sarfati E.Lymphography: an old technique retains its usefulness Radiographics 200323061541–1558., discussion 1559–1560 [DOI] [PubMed] [Google Scholar]
- 38.Meisinger Q C, O'Brien S, Itkin M, Nadolski G J. Use of sequential pneumatic compression devices to facilitate propagation of contrast during intranodal lymphangiography. J Vasc Interv Radiol. 2017;28(11):1544–1547. doi: 10.1016/j.jvir.2017.07.035. [DOI] [PubMed] [Google Scholar]
- 39.Alomari M H, Lillis A, Kerr C, Newburger J W, Quinonez L, Alomari A I. The use of non-ionic contrast agent for lymphangiography and embolization of the thoracic duct. Cardiovasc Intervent Radiol. 2019;42(03):481–483. doi: 10.1007/s00270-018-2125-7. [DOI] [PubMed] [Google Scholar]
- 40.Hussain J S, Srinivasa R N, Srinivasa R N, Patel A, Gemmete J J, Chick J FB. Balloon-occluded retrograde lymphangiography and embolization of a posttraumatic lymphoenteric fistula. J Vasc Interv Radiol. 2018;29(07):1032–1033. doi: 10.1016/j.jvir.2017.10.034. [DOI] [PubMed] [Google Scholar]