Abstract
Objective The main purpose of this article is to define prognosis of pregnancies in epileptic women in Benin.
Methods This was a case–control study that included 54 epileptic women who had at least one pregnancy matched to 162 controls on age, pregnancy term, and monitoring center. Information about epilepsy, treatment, pregnancy, and childbirth were collected. A logistic regression with odds ratio (OR) calculation was used to study the association.
Results During pregnancy 22.22% of epileptic women experienced an increase in seizure frequency. Epileptics had more frequent miscarriages (OR: 1.84 [1.01–3.51]), more incidents during pregnancy (OR: 4.03 [1.04–15.60]), and were more often hospitalized (OR: 3.35 [1.46–7.69]) than women without epilepsy. They, more often, had premature children before 37 weeks of amenorrhea (OR: 2.10 [1.12–3.91]) and gave birth to low-birth-weight children (OR = 2.17 [1.00–4.76]).
Conclusion Occurrence of a pregnancy in an epileptic woman in Benin is at risk and requires multidisciplinary monitoring by both neurologist and obstetrician to reduce complications.
Keywords: epilepsy, pregnancy, prognosis, Africa
Introduction
Epilepsy is a stigmatizing condition sometimes considered as a real handicap with significant socioeconomic repercussions. 1 2 The occurrence of a pregnancy in an epileptic woman is considered as stress factor 3 that can also affect quality of life and has been often a subject of obsession both for the caregiver and the patient, as well as those around her. Several questions are related to the pregnancy outcome, epileptic seizures during pregnancy and their consequences, obstetric complications, condition of the new born at birth, antiepileptic drugs, malformations, and others. In light of many studies, seizures do not increase in frequency during pregnancy, 4 5 6 especially if the pregnancy is well planned with good seizure control. 7 But when these seizures occur, there is a risk of brain damage and cognitive deficits in children, 8 including risk for infant of gestational age, risk of preterm delivery, and risk of low-birth-weight infant. 9 Complications occurring during pregnancy and delivery in epileptics can be multiple and varied ranging from high risk of hospitalization, premature rupture of membranes, early uterine contractions, caesarean, and intensive care unit admission of neonates, to urinary infection, hypertension, breech presentation, and low birth weight. 10 11 12 On the other hand, pregnancy and delivery can be without complications. 5 6 Also, the teratogenic risk of antiepileptic drugs, 13 14 as their use during pregnancy increases the risk of fetal malformation and hemorrhage in the neonatal period, is known. 15 16
In Africa, some studies have been devoted to reproductive health in epileptic women, but very few have focused on the prognosis and pregnancy outcome among these women. Epilepsy can change fertility and pregnancy outcome. 17 So, in addition to sexual disorders experienced by women with epilepsy, 18 complications can occur during their reproductive life. 19 The roles of both epileptic discharges and antiepileptic drugs in the alteration of the hypothalamic–pituitary–ovarian axis and the resulting hormones have been discussed. 8
In Benin, prevalence of epilepsy range from 6 to 40‰ 20 21 with 3 to 7‰ epileptics among pregnant women. 22 In a context of available data on the pregnancy outcome among women with epilepsy in Benin, this study was conducted to determine the obstetric rate complications in epileptic women who had experienced pregnancy and identify its associated factors.
Methods
Setting
This study was performed in neurology and gynecology-obstetrics departments of Parakou University Teaching Hospital (CHUD-B/A) and the University Teaching Hospital Hubert Koutoukou Maga (CNHU/HKM) of Cotonou. Cotonou is the economical capital and the biggest city located in the south of Benin; Parakou is the third biggest city and located in the northern Benin.
Design of Study
This was a multicenter observational case–control study about epileptics conducted over a 5-year period, from January 1, 2013, to December 31, 2017.
Population
The study population consisted of women who had experienced at least one pregnancy and were followed in the departments of neurology and gynecology-obstetrics of CNHU/HKM and CHUD-B/A.
Inclusion Criteria
For the cases, we included epileptic women, in childbearing age, who have had at least one pregnancy and had given their consent for the study.
For the controls, we included nonepileptic women, of same age, who had been followed in the same period and in the same center.
For each case included were matched three controls according to the matching criteria.
Diagnosis of epileptics was confirmed by a neurologist.
Seizure frequency was defined as “improved” if there was a 50% of reduction, “worsened” if there was a 50% of increase, and “unchanged” in the other cases.
Noninclusion Criteria
Women with paroxysmal symptoms related to particular situations such as metabolic disorders, heart-related discomfort, or eclampsia seizures were not included if they were not diagnosed as epileptics.
Exclusion Criteria
Unreachable women and those who refused to participate in this study were excluded.
Sampling
Size : The sample size was calculated assuming an odds ratio (OR) between cases and controls of three and assuming a risk of first species 0.05 and a risk of second species 20% (power of 80%). Three controls per case with a frequency of complications of pregnancy and/or delivery of 20% were used as the basis of calculation. It was thus obtained using the Epitable software of Epi-Info version 6.04C, a minimum number of 46 cases and 138 controls.
Sampling technique : For the case, we conducted an exhaustive recruitment of epileptic women in childbearing age. This recruitment was based on the consultation records in the two neurology departments.
For the controls, we randomly selected them from the next 10 childbirth records of the respective maternity. In case of refusal, we chose the next. These were the controls that followed the case in the childbirth register.
Data Collection
Collection technique : Sociodemographic data, obstetrical and medico-surgical history, and data related to pregnancy, epilepsy, delivery, and condition of the newborn at delivery were collected during a face-to-face interview. The care records and, if necessary, the medical files were examined in the different centers. Any provoked paroxysmal phenomena during pregnancy related to hypertensive disorders, preeclampsia, or eclampsia were not accounted for seizure.
Statistics and Data Analysis
The collected data were verified and inserted using Epi data 3.1. Data were analyzed with Statistical Package for the Social Sciences (SPSS) software. Qualitative variables were described by using their frequency, proportion, and the quantitative variables by using mean (with one standard deviation) or the median (with the interquartile interval). The proportions were compared using chi-squared test or Fisher’s exact test according to the cases. Means were compared using Student’s t -test. To study stability of the association between epilepsy and various factors, all factors with significant p -value at 10% in bivariate analysis were simultaneously introduced in a logistic regression model by successive iterations type, step by step descending. The OR was determined with their 95% confidence interval (CI) to estimate the direction, strength, and stability of the association. The threshold of significance was at 5%.
Ethical Considerations
The study protocol was approved by the Local Ethical Committee of Biomedical Research (CLERB) of Parakou University. Before inclusion, oral and formal consents of each women included were obtained. The data confidentiality was ensured.
Results
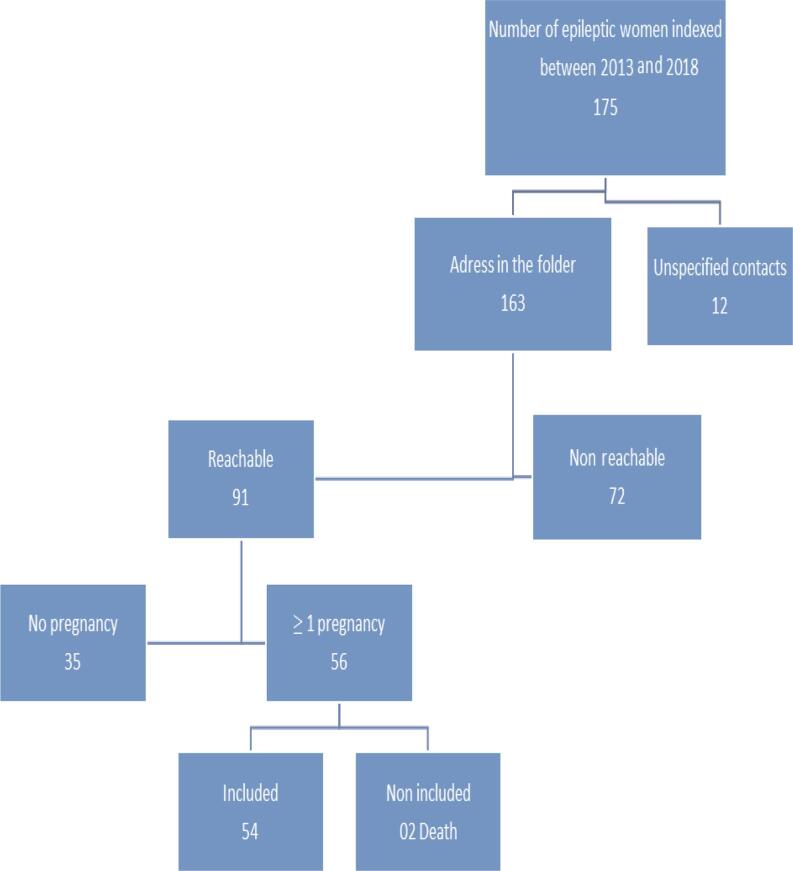
A total of 216 subjects were included, of which 54 cases and 162 controls. Figure 1 shows the flow diagram of the selection of epileptic patients.
Fig. 1.
Flowchart of women selection with epilepsy, Parakou 2019.
They were aged between 20 and 43 years with a mean age at 30.12 years; most of them had primary education and had monthly income below the guaranteed minimum wage. Table 1 summarizes the sociodemographic characteristics of the women included.
Table 1. Sociodemographic characteristics of epileptic and nonepileptic women, Parakou 2019.
| Case | Control | |||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| Abbreviations: CFA, (is the currency of Benin) Communauté Financière Africaine; FCFA, Franc Communauté Financière Africaine. | ||||
| Age groups (y) | ||||
| 20–25 | 12 | 22.22 | 26 | 16.05 |
| 25–30 | 16 | 29.63 | 79 | 48.77 |
| 30–35 | 12 | 22.22 | 35 | 21.60 |
| 35–40 | 8 | 14.81 | 19 | 11.73 |
| 40–45 | 6 | 11.11 | 3 | 1.85 |
| Level of education | ||||
| Not educated | 8 | 14.81 | 23 | 14.19 |
| Primary | 23 | 42.59 | 72 | 44.44 |
| Secondary | 17 | 31.48 | 46 | 28.40 |
| University | 6 | 11.11 | 21 | 12.96 |
| Profession | ||||
| Housewife | 9 | 16.67 | 24 | 14.815 |
| Retailer | 21 | 38.88 | 71 | 43.83 |
| Artisan (hairdresser, sewing, etc.) | 12 | 22.22 | 39 | 24.07 |
| Employee | 7 | 12.96 | 17 | 10.494 |
| Traders | 2 | 3.704 | 6 | 3.704 |
| Others | 3 | 5.56 | 5 | 3.086 |
| Monthly income ( FCFA) | ||||
| <40,000 | 29 | 53.70 | 77 | 47.53 |
| 40,000–59,999 | 12 | 22.22 | 49 | 30.25 |
| 60,000–100,000 | 5 | 9.26 | 18 | 11.11 |
| <10,0000 | 8 | 14.81 | 18 | 11.11 |
| Marital status | ||||
| Celibate | 9 | 16.67 | 11 | 6.79 |
| Married or in a relationship | 45 | 83.33 | 151 | 93.21 |
| Ethnic group | ||||
| Bariba | 8 | 14.81 | 26 | 16.05 |
| Dendi | 18 | 33.33 | 25 | 15.43 |
| Fon and related | 21 | 38.89 | 93 | 57.41 |
| Nagot and related | 7 | 12.96 | 18 | 11.11 |
Clinically, generalized epilepsy was predominant (74.07%) and presumed nonsymptomatic in 92.59% of epileptics. The age of diagnosis and the onset of the first seizure were generally before 18 years in, respectively, 53.70 and 62.96% of cases.
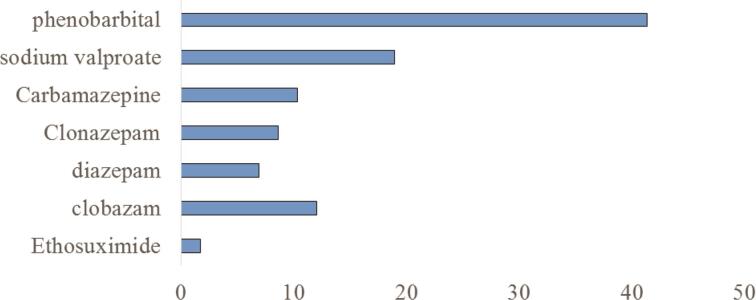
Regarding the neurological follow-up of epileptic women during pregnancy, most of them (57.41%) were not followed. During pregnancy, 22.22% of epileptics experienced an increase in seizure frequency, 59.26% were stable, and 18.52% had a decrease in seizure frequency. Most epileptics were on medication (87.04%): 76.60% on monotherapy, 7.41% under indigenous treatment, and 5.55% under mixed drug and indigenous treatment. Phenobarbital was the most used antiepileptic drug. The duration of treatment was less than or equal to 60 months in 66.67% of cases. Seven epileptics (12.96%) stopped their treatment before pregnancy and for 11 (20.37%) a therapeutic modification was done. Figure 2 summarizes antiepileptic treatment in epileptics.
Fig. 2.
Antiepileptic treatment in epileptic women (in percentage), Parakou 2019.
The most common complications during pregnancy with epileptic women were fever (38.89%), infections (38.89%), and seizures (29.63%) compared with nonepileptic women in whom the complications that occurred were fever (26.54%), infections (26.54%), and gestational hypertension (12.96%).
According to the past medical history, women with epilepsy had more miscarriage (40.74%) than those without epilepsy (27.16%). During childbirth, epileptic women had more delivery hemorrhage (3.70%), gave birth before term (51.85%), had longer time of delivery (40.74%), and had more caesarean (27.78%) and instrumental fetal extraction (3.70%) than nonepileptic women. There was more incident during childbirth in epileptics (9.26%) than in nonepileptics (2.47%). Table 2 summarizes the complications during delivery. At birth, 12.5% of children were resuscitated and one case (1.85%) of congenital malformation was noted with epileptics. Children of women with epilepsy did not had more complications compared with those without epilepsy and the number of livebirths was not significantly different ( Table 3 ).
Table 2. Complications during delivery in epileptic and nonepileptic women, Parakou 2019.
| Epileptics | Nonepileptics | |||
|---|---|---|---|---|
| n | % | n | % | |
| Delivery hemorrhage | 2 | 3.7 | 2 | 1.23 |
| Term of birth ≤ 37 wk | 28 | 51.85 | 55 | 33.95 |
| Duration of delivery (h) | ||||
| < 7 | 32 | 59.26 | 111 | 68.52 |
| ≥ 7 | 22 | 40.74 | 51 | 31.48 |
| Type of delivery | ||||
| Assisted | 3 | 5.56 | 10 | 6.17 |
| Caesarean | 15 | 27.78 | 37 | 22.84 |
| Childbirth triggered | 1 | 1.85 | 9 | 5.56 |
| Episiotomy | 3 | 5.56 | 10 | 6.17 |
| Instrumental extraction | 2 | 3.7 | 1 | 0.62 |
| Incident during childbirth | 5 | 9.26 | 4 | 2.47 |
Table 3. Childbirth and postnatal status in women with pregnancy according to their epileptic status, Parakou 2019.
| Women with epilepsy n (%) |
Women without epilepsy n (%) |
p -Value | |
|---|---|---|---|
| Neonatal resuscitation | 7 (12.96) | 9 (5.56) | 0.07 |
| Livebirth | 53 (98.15) | 158 (97.53) | 0.633 |
| Low birth weight | 2 (3.7) | 2 (1.23) | 0.243 |
| Congenital malformation | 1 (1.85) | 2 (1.23) | 0.737 |
| Psychomotor retardation | 2 (3.77) | 1 (0.63) | 0.154 |
| Child with epilepsy | 3 (5.56) | 2 (1.27) | 0.101 |
| Growth retardation | 3 (5.56) | 2 (1.27) | 0.101 |
In univariate analysis, age, p = 0.022; marital status, p = 0.03; triggering of delivery, p = 0.028 ; menarche, p = 0.02; seizure, p = 0.000; hospitalization, p = 0.004; and incident of childbirth, p = 0.04, were associated with epilepsy. After logistic regression, the real associated factors with epilepsy were menarche ( p = 0.026), hospitalization, seizures during delivery ( p = 0.000), delivery term, and marital status ( p = 0.04). Table 4 summarizes the associated factors in univariate and multivariate analyses in logistic regression model.
Table 4. Factors associated with epilepsy in univariate and multivariate analyses, Parakou 2019.
| Univariate analysis OR (95% Confidence interval) |
p -Value | Multivariate analysis OR (95% Confidence interval) |
p -Value | |
|---|---|---|---|---|
| Abbreviation: OR, odds ratio. | ||||
| Age (y) | 0.02 | 0.05 | ||
| 20–25 25–30 30–35 35–40 40–45 |
4.33 (0.92–20.32) 9.87 (2.23–43.66) 5.83 (1.25–27.02) 4.75 (0.94–23.84) 1 |
2.90 (0.47–17.52) 5.33 (1.00–29.07) 2.97 (0.52–16.94) 4.15 (0.65–26.20) 1 |
||
| Marital status Single Married or in relationship |
2.78 (1.07–7.14) 1 |
0.03 | 3.70 (1.23–11.11) 1 |
0.019 |
| Delivery hemorrhage No Yes |
2.77 (0.88–8.63) 1 |
0.079 | – | |
| Delivery term (wk) > 37 |
2.08 (1.12–4.00) 1 |
0.04 | 2.22 (1.07–4.62) 1 |
0.033 |
| Triggering of delivery Yes No |
0.32 (0.03–2.59) 1 |
0.06 | – | |
| Menarche (y) <15 ≥15 |
2.01 (1.07–3.77) 1 |
0.029 | 4.05 (1.56–10.52) 1 |
0.016 |
| Miscarriage Yes No |
1.84 (0.96–3.51) 1 |
0.063 | – | |
| Incident during childbirth Yes No |
4.0 (1.03–15.50) 1 |
0.04 | – | |
| Seizure during delivery Yes No |
4.03 (1.04–15.60) 1 |
0.045 | 5.88 (1.92–16.67) 1 |
0.02 |
| Hospitalization Yes No |
3.35 (1.46–7.68) 1 |
0.004 | 2.78 (1.01–7.69) 1 |
0.047 |
Discussion
Most of our epileptic women did not had neurological follow-up during pregnancy, while there is evidence that pregnancy planning and follow-up in women with epilepsy reduces the risk of seizures and changes in treatment during pregnancy. 7 Menarche is associated with epilepsy and the complications of pregnancy and childbirth are multiple and varied in our epileptic patients. The rate of malformation during pregnancy in our epileptic women is low compared with other authors. Mostacci et al in 2018 noted a 2.3% rate of malformation during pregnancy in epileptics. 23 The malformations are mainly attributable to treatment, especially polytherapy, more than to epilepsy itself; the most incriminated molecule is sodium valproate, 24 which also alters placental function in early pregnancy. 25 Most of our patients are on phenobarbital monotherapy for many reasons such as economical—since sodium valproate is very costly with regard to their standard of living—or nonavailability of other new antiepileptic drugs. So, this drop in the malformation rate could be justified. Also, systematic supplementation with folic acid at a dose of 5 mg per day in the first quarter of pregnancy in any pregnant woman in our country, including women with epilepsy, could contribute to the reduction in this rate of malformation. Low-dose folic acid supplementation in pregnant women, according to some authors, improves the Intellectual Quotient of children born to these women without significantly lowering the rate of intrauterine deaths. 26 Increased risk of preterm delivery identified in our study may be related to antiepileptic treatments or crises that occurred during pregnancy. Epileptic women on antiepileptic treatment have an increased risk of childbirth and delivery complications compared with women with nonuse of antiepileptic drugs. 27 Dadah et al reported a preterm birth rate of 41.8% in women with epilepsy who experienced pregnancy in Senegal. 18 The exacerbation of seizures in epileptic patients during pregnancy can range from 20 to 35% 28 29 and sometimes more. 18 These had probably multifactorial origin, ranging from the stress and psychosocial factors related to epilepsy, 18 to the fear of malformation, sleep disorders related to the state of pregnancy, 30 underdosing or discontinuation of antiepileptic drugs, and hormonal fluctuations during pregnancy, especially in the first quarter. 8 The organization of the health system and sensitization should be the way for prevention. The lack of information about the diseases and sociocultural considerations associated with epilepsy may explain the low rate of follow-up of pregnancies in epileptics. The mass media should create awareness especially in local languages and through community radios. Medical practitioners must involve their effort to share knowledge with people, especially their patients.
We did not observe intrauterine fetal death or perinatal death as observed in other studies, the risk being greater with combination therapy than monotherapy (relative risk [RR], 1.38; 95% CI, 1.14–1.66), parental history of major congenital malformation (RR, 1.92; 95% CI, 1.20–3.07), maternal age (RR, 1.06; 95% CI, 1.04–1.07), and the number of fetal intrauterine fetal death (RR, 1.09, 1.00–1.19) were associated to epilepsy. 31 For epileptic treatment, most epileptics were on benzodiazepines and phenobarbital, which are less effective than the newer antiepileptic drugs prescribed in western countries. The low purchasing power of populations and the permanent nonavailability of new antiepileptic drugs could explain it. This should further encourage planning for pregnancies in women with epilepsy, especially since many still take drugs not recommended for women of childbearing age. Nevertheless, some studies showed that the frequency of the obstetrical complications did not change between planned and unplanned pregnancy. 7
Strengths and Limitations : This study is the first to focus on the prognosis of pregnancies in epileptics in Benin. Nevertheless, its limits lie in the number of cases included focusing on the nonexistence of a national registry of epileptic women and children born to women with epilepsy in Benin. Also, the functional prognosis in the medium- and long term could not be evaluated. Subsequent studies with cohort follow-up of larger number of epileptic subjects will make it possible to evaluate the neurocognitive profile of these children born to women with epilepsy.
Conclusion
At the end of this study on the prognosis of pregnancy in women with epilepsy in Benin, it appears that pregnancies in epileptic women are not followed up and thus are at risk, with a high rate of premature delivery probably due to the use of some antiepileptic drug. It, therefore, requires increased control, and a coordinated multidisciplinary approach by a neurologist, gynecologist, and psychologist is essential to reduce complications.
Funding Statement
Funding None.
Footnotes
Conflict of Interest None declared.
References
- 1.Laganà A S, Triolo O, D’Amico V et al. Management of women with epilepsy: from preconception to post-partum. Arch Gynecol Obstet. 2016;293(03):493–503. doi: 10.1007/s00404-015-3968-7. [DOI] [PubMed] [Google Scholar]
- 2.Birbeck G, Chomba E, Atadzhanov M, Mbewe E, Haworth A. The social and economic impact of epilepsy in Zambia: a cross-sectional study. Lancet Neurol. 2007;6(01):39–44. doi: 10.1016/S1474-4422(06)70629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lunardi L L, Costa A L, Guerreiro C A, Souza E A.Quality of life in pregnant women with epilepsy versus women with epilepsy Arq Neuropsiquiatr 201169(2B)336–341. [DOI] [PubMed] [Google Scholar]
- 4.EURAP Study G roup. Seizure control and treatment in pregnancy: observations from the EURAP epilepsy pregnancy registry. Neurology. 2006;66(03):354–360. doi: 10.1212/01.wnl.0000195888.51845.80. [DOI] [PubMed] [Google Scholar]
- 5.Meador K J, Baker G A, Finnell R H et al. NEAD Study Group. In utero antiepileptic drug exposure: fetal death and malformations. Neurology. 2006;67(03):407–412. doi: 10.1212/01.wnl.0000227919.81208.b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.La Neve A, Boero G, Francavilla T, Plantamura M, De Agazio G, Specchio L M. Prospective, case-control study on the effect of pregnancy on seizure frequency in women with epilepsy. Neurol Sci. 2015;36(01):79–83. doi: 10.1007/s10072-014-1908-0. [DOI] [PubMed] [Google Scholar]
- 7.Abe K, Hamada H, Yamada T, Obata-Yasuoka M, Minakami H, Yoshikawa H. Impact of planning of pregnancy in women with epilepsy on seizure control during pregnancy and on maternal and neonatal outcomes. Seizure. 2014;23(02):112–116. doi: 10.1016/j.seizure.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Sazgar M. Treatment of women with epilepsy. Continuum (Minneap Minn) 2019;25(02):408–430. doi: 10.1212/CON.0000000000000713. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y H, Chiou H Y, Lin H C, Lin H L. Affect of seizures during gestation on pregnancy outcomes in women with epilepsy. Arch Neurol. 2009;66(08):979–984. doi: 10.1001/archneurol.2009.142. [DOI] [PubMed] [Google Scholar]
- 10.Block H S. Neurological complications of pregnancy. Curr Neurol Neurosci Rep. 2016;16(07):67. doi: 10.1007/s11910-016-0665-2. [DOI] [PubMed] [Google Scholar]
- 11.Laskowska M, Leszczyńska-Gorzelak B, Oleszczuk J. Pregnancy in women with epilepsy. Gynecol Obstet Invest. 2001;51(02):99–102. doi: 10.1159/000052902. [DOI] [PubMed] [Google Scholar]
- 12.Richmond J R, Krishnamoorthy P, Andermann E, Benjamin A. Epilepsy and pregnancy: an obstetric perspective. Am J Neurol Sci. 2015;36(01):79–83. doi: 10.1016/j.ajog.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Huber-Mollema Y, Oort F J, Lindhout D, Rodenburg R. Maternal epilepsy and behavioral development of the child: family factors do matter. Epilepsy Behav. 2019;94(94):222–232. doi: 10.1016/j.yebeh.2019.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Liverpool & Manchester Neurodevelopment Group . Mawer G, Briggs M, Baker G A. Pregnancy with epilepsy: obstetric and neonatal outcome of a controlled study. Seizure. 2010;19(02):112–119. doi: 10.1016/j.seizure.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauser W A, Annegers J F, Rocca W A. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc. 1996;71(06):576–586. doi: 10.4065/71.6.576. [DOI] [PubMed] [Google Scholar]
- 16.Borthen I, Eide M G, Veiby G, Daltveit A K, Gilhus N E. Complications during pregnancy in women with epilepsy: population-based cohort study. BJOG. 2009;116(13):1736–1742. doi: 10.1111/j.1471-0528.2009.02354.x. [DOI] [PubMed] [Google Scholar]
- 17.Kariuki J G, Joshi M D, Adam A M, Kwasa T O, Machoki M. Fertility rate of epileptic women at Kenyatta National Hospital. East Afr Med J. 2008;85(07):341–346. doi: 10.4314/eamj.v85i7.9646. [DOI] [PubMed] [Google Scholar]
- 18.Dadah S M, Ndiaye M, Diop M S et al. [Epilepsy and reproductive health: Senegalese cohort] Rev Neurol (Paris) 2014;170(10):608–613. doi: 10.1016/j.neurol.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Maiga Y, Napon C, Kuate Tegueu C et al. [Epilepsy and women’s life: particularities of their management. Literature review] Mali Med. 2010;25(03):1–9. [PubMed] [Google Scholar]
- 20.Adjien K C, Goudjinou G, Gnonlonfoun D, Adoukonou T, Agbetou M, Houinato D. Prevalence of personality disorders in epileptics in the commune of DJIDJA (department of ZOU) in Benin. Le Bénin Médical. 2008;39/40:54. [Google Scholar]
- 21.Adoukonou T, Djagoun E, Tognon-Tchegnonsi E, Sego-sounon D, Kouna-Ndouongo P, Houinato D. Prevalence of epilepsy in adults at Tourou, in northern Benin. Med Sante Trop. 2013;23(01):83–88. doi: 10.1684/mst.2013.0158. [DOI] [PubMed] [Google Scholar]
- 22.Yemadje L P, Houinato D, Quet F, Druet-Cabanac M, Preux P M. Understanding the differences in prevalence of epilepsy in tropical regions. Epilepsia. 2011;52(08):1376–1381. doi: 10.1111/j.1528-1167.2011.03099.x. [DOI] [PubMed] [Google Scholar]
- 23.Mostacci B, Esposto R, Lello S, Bisulli F, Licchetta L, Tinuper P. Estrogen-related seizure exacerbation following hormone therapy for assisted reproduction in women with epilepsy. Seizure. 2018;61:200–202. doi: 10.1016/j.seizure.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 24.Battino D, Tomson T, Crean S, Fahrbach K, Probst C. Management of epilepsy during pregnancy. Drugs. 2007;67(18):2727–2746. doi: 10.2165/00003495-200767180-00007. [DOI] [PubMed] [Google Scholar]
- 25.Tetro N, Imbar T, Wohl D et al. The effects of valproic acid on early pregnancy human placentas: pilot ex vivo analysis in cultured placental villi. Epilepsia. 2019;60(05):e47–e51. doi: 10.1111/epi.14721. [DOI] [PubMed] [Google Scholar]
- 26.Meador K J, Baker G A, Browning N et al. NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12(03):244–252. doi: 10.1016/S1474-4422(12)70323-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borthen I, Eide M G, Daltveit A K, Gilhus N E. Obstetric outcome in women with epilepsy: a hospital-based, retrospective study. BJOG. 2011;118(08):956–965. doi: 10.1111/j.1471-0528.2011.03004.x. [DOI] [PubMed] [Google Scholar]
- 28.Sabers A, Ohman I, Christensen J, Tomson T. Oral contraceptives reduce lamotrigine plasma levels. Neurology. 2003;61(04):570–571. doi: 10.1212/01.wnl.0000076485.09353.7a. [DOI] [PubMed] [Google Scholar]
- 29.Harden C L, Hopp J, Ting T Y et al. American Academy of Neurology; American Epilepsy Society. Practice parameter update: management issues for women with epilepsy–focus on pregnancy (an evidence-based review): obstetrical complications and change in seizure frequency: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73(02):126–132. doi: 10.1212/WNL.0b013e3181a6b2f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harden C L, Meador K J, Pennell P B et al. American Academy of Neurology; American Epilepsy Society. Management issues for women with epilepsy-Focus on pregnancy (an evidence-based review): II. Teratogenesis and perinatal outcomes: Report of the Quality Standards Subcommittee and Therapeutics and Technology Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia. 2009;50(05):1237–1246. doi: 10.1111/j.1528-1167.2009.02129.x. [DOI] [PubMed] [Google Scholar]
- 31.Tomson T, Battino D, Bonizzoni E et al. EURAP Study Group. Antiepileptic drugs and intrauterine death: a prospective observational study from EURAP. Neurology. 2015;85(07):580–588. doi: 10.1212/WNL.0000000000001840. [DOI] [PubMed] [Google Scholar]