Abstract
γc cytokine receptor signaling is required for the development of all lymphocytes. Why γc signaling plays such an essential role is not fully understood, but induction of the serine/threonine kinase Pim1 is considered a major downstream event of γc as Pim1 prevents apoptosis and increases metabolic activity. Consequently, we asked whether Pim1 overexpression would suffice to restore lymphocyte development in γc-deficient mice. By analyzing Pim1-transgenic γc-deficient mice (Pim1TgγcKO), we show that Pim1 promoted T-cell development and survival in the absence of γc. Interestingly, such effects were largely limited to CD4+ lineage αβ T cells as CD4+ T-cell numbers improved to near normal levels but CD8+ T cells remained severely lymphopenic. Notably, Pim1 overexpression failed to promote development and survival of any T-lineage cells other than αβ T cells, as we observed complete lack of γδ, NKT, FoxP3+ T regulatory cells and TCRβ+ CD8αα IELs in Pim1TgγcKO mice. Collectively, these results uncover distinct requirements for γc signaling between CD4+ αβ T cells and all other T-lineage cells, and they identify Pim1 as a novel effector molecule sufficient to drive CD4+ αβ T-cell development and survival in the absence of γc cytokine receptor signaling.
Keywords: apoptosis, cytokines, homeostasis, thymopoiesis
Introduction
All T-lineage lymphocytes depend on two non-redundant signals for their development and differentiation in the thymus. One signal is mediated by the T-cell antigen receptor (TCR) which induces thymocyte differentiation [1, 2], the other signal is mediated by cytokines of the common γ-chain (γc) cytokine family which is proposed to be essential for cell survival [3]. In the absence of either one of these signals, T-cell development in the thymus is critically impaired [4–7].
The developmental requirements for TCR signals are rather well-defined. TCR signals terminate expression of recombination activating genes (RAG) and fix the specificity of the TCR [8]. TCR signals also upregulate expression of the TCR itself and induce expression of anti-apoptotic molecules and cytokine receptors [8, 9]. In contrast, the role of γc signaling remains less understood. γc signals are primarily considered as survival factors, but recent data also suggested new roles for γc beyond its pro-survival function. For example, CD4/CD8 lineage specification of αβ T cells in the thymus and terminal differentiation of NKT-cells have been attributed as γc signaling effects distinct from its pro-survival effect [10, 11]. Thus, the role of γc signaling in T-lineage cell development and differentiation needs further clarification.
γc is a 64 kDa transmembrane protein that is the central signaling component for a series of cytokines, including interleukin-2 (IL-2), IL-4, IL-7, IL-9, IL-15 and IL-21 [3]. In T cells, the major targets of γc signaling are primarily anti-apoptotic molecules. In recent years, yet another role of γc as a pro-metabolic signal has gained much attention. As such, absent γc signaling was found to cause cellular atrophy with lower metabolic activities and reduced cell size [9, 12]. Mechanistically, γc signaling activated Akt and the mammalian target of rapamycin, resulting in glucose transporter-1 (Glut-1) upregulation and ribosomal S6 kinase activation to increase glucose consumption and anabolic processes, respectively [13–15]. Thus, the pro-survival function of γc is likely a combined effect of anti-apoptotic and pro-metabolic activities. Hence, replacing γc’s survival function with molecules from the anti-apoptotic arm of γc signaling alone is probably insufficient.
In this regard, the serine/threonine kinase Pim1 provides an attractive solution to assess γc requirement in vivo, because Pim1 exerts both anti-apoptotic and pro-metabolic activities. Pim1 is a proto-oncogene originally identified as a pro-viral insertion site of the Moloney Murine Leukemia Virus (MoMuLV). Overexpression of Pim1 conferred growth factor independent cell survival and proliferation both in vitro and in vivo [16, 17]. Moreover, earlier studies with an Eμ enhancer driven transgenic Pim1 mouse demonstrated that the Pim1 transgene was expressed in all lymphoid lineage cells [18], and that it increased overall thymocyte numbers in cytokine signaling-deficient mice [16, 17]. In agreement with such effects, Pim1 had been identified as an immediate downstream effector of γc cytokine signaling [19]. Specifically, Pim1 expression was induced upon γc cytokine signaling in T cells and prevented programmed cell death by inactivating the pro-apoptotic factors Bad and PTP-U2S [20–22]. Additionally, Pim1 also upregulated metabolism by promoting glycolysis and activating the translational regulator, eukaryotic initiation factor 4E (eIF-4E) [23–25]. Thus, Pim1 is uniquely positioned downstream of γc to induce both anti-apoptotic and pro-metabolic signals for T-cell survival.
In this study, we introduced an Eμ enhancer driven transgenic Pim1 [18] into γc-deficient mice to restore both arms of γc pro-survival function. In such Pim1TgγcKO mice, we found that most T-lineage cells, including γδ T-cells, NKT-cells, FoxP3+ T regulatory (Treg) cells and CD8αα intraepithelial lymphocytes (IELs) still failed to develop and survive. On the other hand, Pim1 greatly promoted αβ T-cell development in the thymus and improved peripheral αβ T-cell numbers. Specifically, CD4+ αβ T-cell but not CD8+ T-cell numbers were restored to near wildtype T-cell numbers, and such γc independent CD4+ T-cells were functionally mature as they upregulated CD40L by TCR stimulation and could produce pro-inflammatory cytokines upon in vitro differentiation. These results suggest that CD4+ T-cells are unique among T-lineage cells in that they are independent of γc signals in their differentiation and homeostasis - if pro-survival signals are provided. Collectively, these results unveil novel requirements for γc signaling in T-lineage cell specification and differentiation that are distinct from its pro-survival effects.
Results
Pim1 promotes thymocyte development and increases T-cell numbers
Thymocytes and resting T-cells do not express detectable levels of Pim1 unless signaled by TCR or cytokines [16, 19]. However, Eμ enhancer driven Pim1Tg mice express Pim1 in all lymphocytes and independently of signaling [18, 19, 21, 26] (Supporting Information Fig. 1A, B). In such Pim1Tg mice, we found that ectopic Pim1 expression did not affect thymocyte differentiation (Fig. 1A), but that it significantly increased overall thymocyte numbers (Fig. 1B). Increased cell numbers were not associated with aberrant differentiation of immature CD4, CD8 double negative (DN) thymocytes as we did not find significant differences in DN1-DN4 stage differentiation (Fig. 1C and Supporting Information Fig. 1C). Also, Pim1Tg positive selection was comparable with WT mice (Fig. 1D). Thus, transgenic Pim1 improved total thymocyte numbers without affecting thymocyte differentiation or selection.
Figure 1.
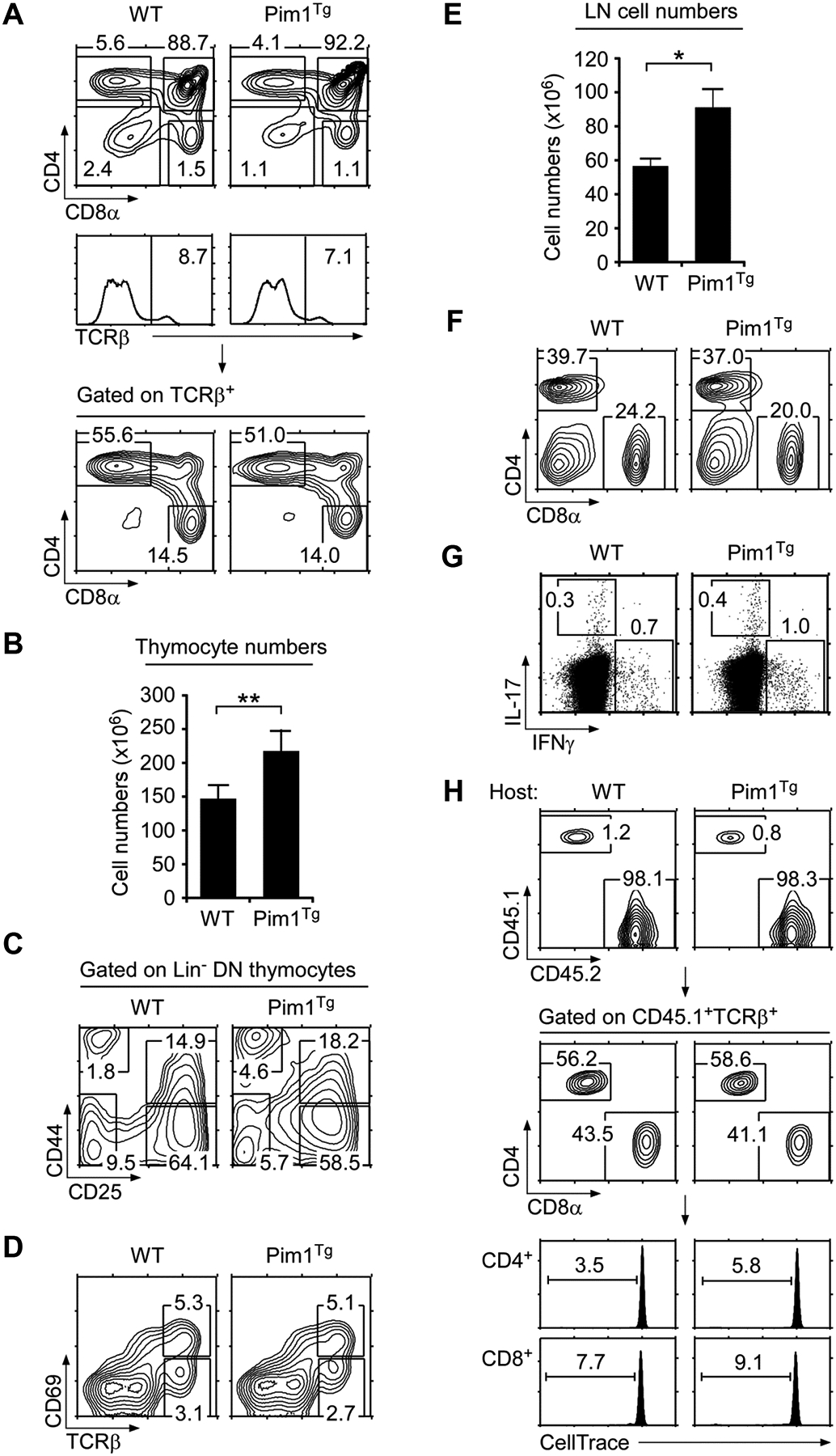
(A) Thymocyte profiles of WT and Pim1Tg mice. Contour plots show CD4/CD8 profiles of total (top) and TCRβ+ gated thymocytes (bottom). Gating strategy is shown in histograms (middle). Numbers indicate percentages of gated cells. Data shown are representative of eight independent experiments.
(B) Thymocyte numbers in WT and Pim1Tg mice. Data are shown as mean +/− SEM of 11 WT and 8 Pim1Tg mice.
(C) DN thymocyte differentiation. Lineage marker negative (Lin−) DN thymocytes were assessed for DN1–DN4 differentiation by CD44/CD25 expression.
(D) Thymocyte selection in WT and Pim1Tg mice. Whole thymocytes were stained for CD69 and TCRβ expression. Data shown are representative of eight independent experiments.
(E) LN cell numbers of WT and Pim1Tg mice. Data are shown as mean +/− SEM of 8 WT and 7 Pim1Tg mice.
(F) LN cell profiles of WT and Pim1Tg mice. CD4/CD8 contour plots are representative of eight independent experiments.
(G) Cytokine expression in CD4+ LN T-cells. Freshly isolated CD4+ T-cells were stimulated for 3 hours with PMA + ionomycin in the presence of brefeldin A and assessed for intracellular IL-17 and IFN-γ expression. Data are representative of three independent experiments.
(H) T-cell adoptive transfer into WT or Pim1Tg hosts. WT (CD45.1) T-cells labeled with the CFSE analog “CellTrace™ Violet” were tail vein injected and analyzed 6 days later from LN of WT (CD45.2) or Pim1Tg (CD45.2) host mice. Data shown are representative of two experiments. (B, D) Data shown are from one experiment representative of eight experiments performed. *p<0.05, **p<0.01, two-tailed Student’s t test.
To assess whether Pim1 also improved peripheral T-cell numbers, next we analyzed LN cells in WT and Pim1Tg mice. Pim1 significantly increased both CD4+ and CD8+ LNT numbers (Fig. 1E, F). Importantly, T-cell numbers increased in the absence of T cell activation, as Pim1Tg T cells did not upregulate CD69 (Supporting Information Fig. 1D) and freshly isolated Pim1Tg CD4+ Tcells did not express pro-inflammatory cytokines (Fig. 1G and Supporting Information Fig. 1E). Such effects were intrinsic to Pim1Tg T-cells, as adoptively transferred wildtype (WT) T-cells did not show increased proliferation in Pim1Tg hosts compared with control WT host mice (Fig. 1H). Thus, Pim1 expands the size of the peripheral T-cell pool, and it likely does it so by providing survival through inactivation of pro-apoptotic Bad [19], but without direct upregulation of anti-apoptotic molecule mRNA expression (Supporting Information Fig. 1F). Collectively, Pim1 is a potent pro-survival factor that promotes thymopoiesis and peripheral T-cell homeostasis.
Transgenic Pim1 promotes thymocyte development in γcKO mice
To assess the extent to which Pim1 overexpression can replace γc signaling, we generated Pim1TgγcKO mice. γcKO mice do not generate meaningful number of thymocytes [4, 5]. Pim1TgγcKO mice, however, had significantly increased thymocyte numbers compared with γcKO mice (Fig. 2A). Transgenic Bcl-2 also improved thymocyte numbers in γcKO mice, but its effect was much weaker than Pim1 (Fig. 2A). Nevertheless, thymocyte numbers in Pim1TgγcKO mice remained significantly lower than those in WT mice (Fig. 2A). Thus, Pim1 can partially substitute but cannot entirely replace γc signaling during thymopoiesis.
Figure 2.
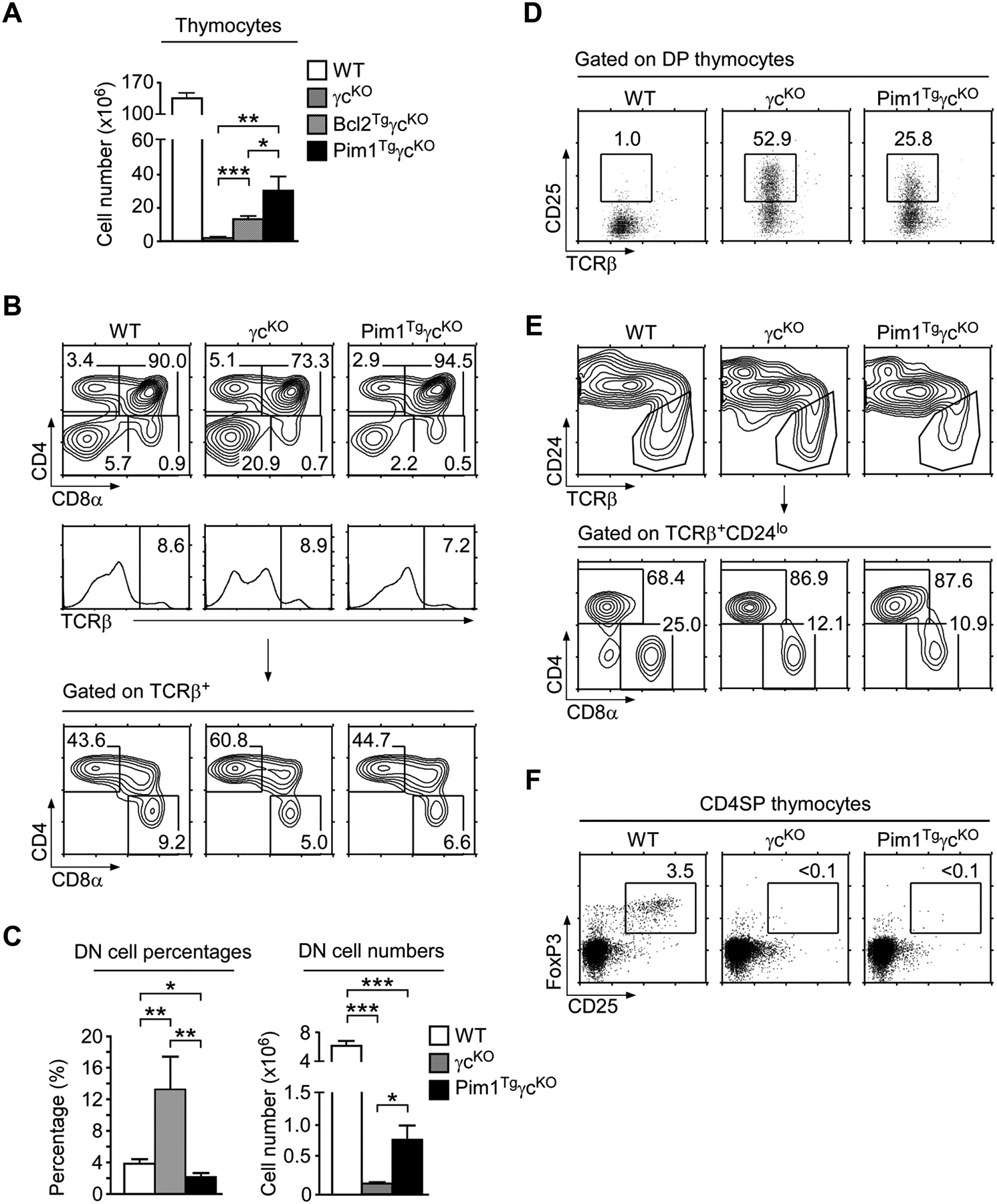
(A) Thymocyte numbers in WT, γcKO, Bcl2TgγcKO, Pim1TgγcKO mice. Data are shown as mean +/− SEM from 12 WT, 7 γcKO, 9 Bcl2TgγcKO, and 9 Pim1TgγcKO mice.
(B) Thymocyte profiles of WT, γcKO, and Pim1TgγcKO mice. Data shown are representative of seven independent experiments.
(C) Percentages and numbers of DN thymocytes. Data are shown as mean +/− SEM of 12 WT, 7 γcKO, and 9 Pim1TgγcKO mice.
(D) Surface CD25 expression on gated DP thymocytes. Data shown are representative of three independent experiments.
(E) Mature thymocyte profiles of WT, γcKO, and Pim1TgγcKO mice. Total thymocytes were analyzed for CD24 and TCRβ expression (top). Gated TCRβ+CD24lo mature cells were plotted for CD4 versus CD8 expression (bottom). Data shown are representative of seven independent experiments.
(F) Treg-cell development in Pim1TgγcKO thymus. Gated CD4SP thymocytes were assessed for intracellular FoxP3 and surface CD25 expression. Data shown are representative of three independent experiments. (A, C) Data shown are pooled from seven experiments performed. *p<0.05, **p<0.01, ***p<0.001, two-tailed Student’s t test.
To further understand Pim1’s effect on γcKO thymocytes, we analyzed individual thymocyte subsets in Pim1TgγcKO mice. Remarkably, unlike the Bcl2Tg (Supporting Information Fig. 2A), we found that Pim1Tg greatly relieved the developmental arrest of immature DN cells that was prominent in γcKO thymocytes (Fig. 2B top and Fig. 2C). Particularly, DN cell percentages were restored to normal levels and DN thymocyte numbers significantly improved compared with those in γcKO mice (Fig. 2C). Moreover, CD25 expression on DP thymocytes, which indicates impaired proliferation and differentiation of DN cells [27], was significantly reduced in Pim1TgγcKO mice (Fig. 2D). Thus, Pim1 improved both cell numbers and thymocyte differentiation.
In mature thymocytes, Pim1 overexpression increased cell numbers (Supporting Information Fig. 2B). But percentages and numbers of TCRβ+ CD8SP cells in Pim1TgγcKO thymocytes were still reduced compared with WT thymocytes (Fig. 2B bottom and Supporting Information Fig. 2C). Such skewed CD4/CD8 lineage ratio was further confirmed when gated on the most mature TCRβhiCD24lo thymocyte subset. Absent γc cytokine signaling preferentially impaired CD8SP thymocyte development (Fig. 2E), with a concomitant increase in CD4/CD8 ratio regardless of the absence or presence of Pim1 transgene (Fig. 2E bottom and Supporting Information Fig. 2D). Thus, we conclude that CD8SP thymocyte development requires specific signals downstream of γc that cannot be replaced by Pim1.
In addition to αβ T-cells, other T-lineage cells also require γc signals for their generation in the thymus. CD25+FoxP3+ regulatory CD4+ T-cell development is critically dependent on γc cytokines, specifically IL-2. Consequently, Treg cells are absent in γcKO mice. But, while CD4SP thymocyte numbers were greatly improved, CD4+ FoxP3+ Treg cells were still completely absent in Pim1TgγcKO mice (Fig. 2F). These results document that, unlike regular CD4+ αβ T cells, CD4+ Treg-cell development requires lineage specifying signals independent of pro-survival signals. Along this line, thymic NKT-cells, which are dependent on IL-15, and thymic γδ T-cells, which require IL-7, also failed to develop in Pim1TgγcKO mice (Supporting Information Fig. 2E, F). Collectively, these results suggest that, possibly with the exception of CD4SP thymocytes, development of all T-cell subsets in the thymus requires lineage specifying signals through the γc that cannot be replaced by anti-apoptotic and pro-metabolic activities of transgenic Pim1.
Radiation bone marrow chimera analysis
To further demonstrate that increased thymopoiesis in Pim1TgγcKO mice is cell intrinsic to Pim1 expression, we created 1:1 mixed bone marrow chimera with γcKO and Pim1TgγcKO bone marrow cells. Seven weeks after injection into RAG2KO hosts, chimeric mice were analyzed for T-cell reconstitution in thymus and peripheral tissues. γc-deficient thymocytes have a pronounced developmental block at the DN2 stage (Fig. 3A). In chimeric mice, we found that γcKO bone marrow-derived thymocytes (identified by CD45.1+/2+ congenic markers) were still developmentally arrested in DN cells, specifically at the DN2 stage (Fig. 3B left). However in the same mice, the development of Pim1TgγcKO bone marrow-derived thymocytes (identified by CD45.1−/2+ congenic markers) proceeded normally through the DN compartment and effectively generated both CD4SP and CD8SP mature thymocytes (Fig. 3B middle). Strikingly, the vast majority of chimeric thymocytes were reconstituted from Pim1TgγcKO, and not γcKO derived cells, suggesting that Pim1 provides a survival advantage to developing thymocytes under competing conditions (Fig. 3B top). Along this line, peripheral T-cells were also mostly reconstituted from Pim1TgγcKO derived cells, and only few γcKO T-cells survived in the absence of transgenic Pim1 (Fig. 3C). Importantly, survival of Pim1TgγcKO T-cells was independent of T-cell activation as CD69 expression was comparable to γcKO T-cells (Fig. 3C). Collectively, these results indicate that Pim1 promotes thymopoiesis and T-cell survival in a cell-intrinsic manner.
Figure 3.
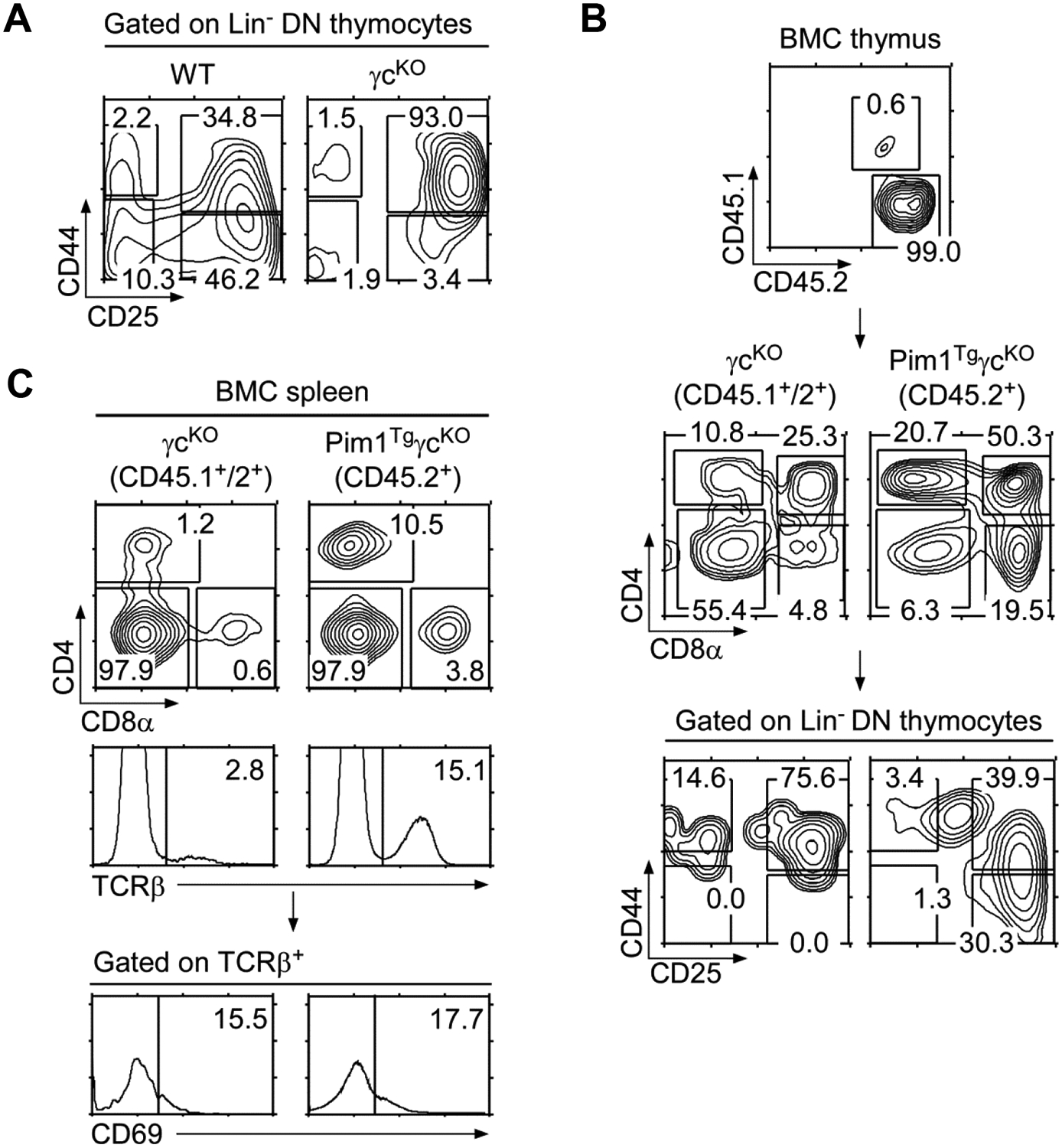
(A) DN thymocyte differentiation in γcKO mice. Lineage marker negative (Lin−) DN thymocytes were assessed for DN1–DN4 differentiation by CD44/CD25 expression.
(B) Thymocyte analysis of γcKO and Pim1TgγcKO bone marrow chimeric (BMC) mice. CD45.1/CD45.2 chimeric distribution in lethally irradiated RAG2KO (CD45.2) recipient mice reconstituted with 1:1 mixture of γcKO and Pim1TgγcKO bone marrow cells (top). Contour plot show CD4/CD8 profile of total thymocytes (middle) and CD44/CD25 expression on Lin− DN thymocytes (bottom). Data shown are representative results from five bone marrow chimeras.
(C) Spleen cell analysis of bone marrow chimeric mice. CD45.1/CD45.2 plot shows chimeric distribution in splenocytes (top). Cells were further assessed for their CD4/CD8 profile and TCRβ expression, followed by CD69 expression on TCRβ+ gated spleen cells. Data shown are representative of three experiments.
Pim1 restores CD4+ αβ T-cell numbers in the absence of γc signaling
To further assess the effect of Pim1 on T-cell survival, next, we analyzed Pim1TgγcKO LN cells (Fig. 4A). Compared with γcKO LN, Pim1TgγcKO LN contained both increased percentages and numbers of TCRβ+ T-cells (Fig. 4A and Supporting Information Fig. 3A). Moreover, we observed a dramatic increase in CD8+ T-cell percentages compared with γcKO LN cells (Fig. 4A). Such increase was specific to LN cells because transgenic Pim1 did not increase CD8SP percentages in thymocytes (Fig. 2B bottom). Thus, Pim1 improves peripheral survival of CD8+ T-cells but does not promote their generation in the thymus in the absence of γc signaling.
Figure 4.
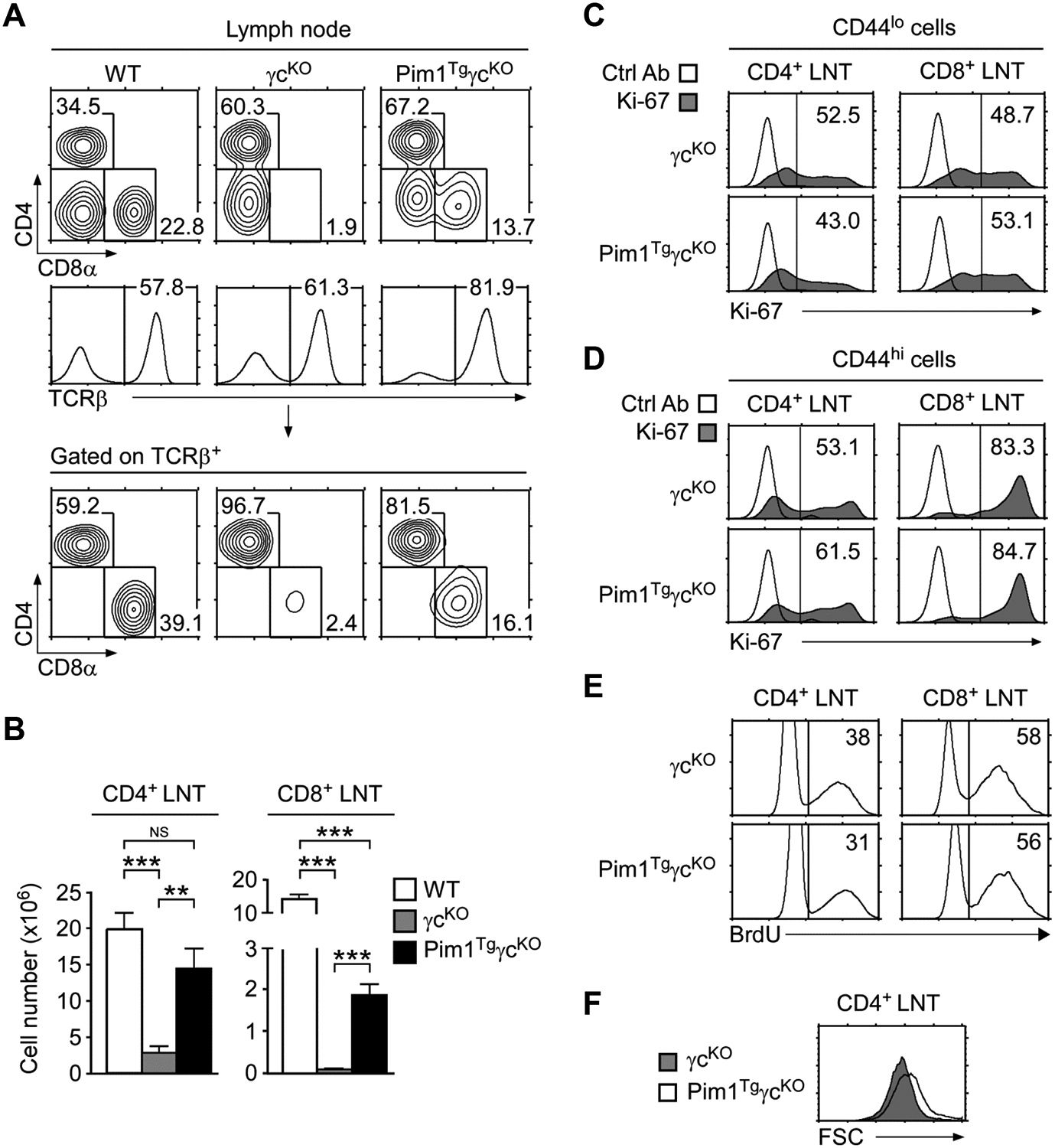
(A) LN cell profiles of WT, γcKO, and Pim1TgγcKO mice. Contour plots show CD4/CD8 profiles of total LN cells (top) and TCRβ+ LN cells (bottom). Gating strategy and percentages of TCRβ+ cells are shown in histograms (middle). Data shown are representative of seven independent experiments.
(B) CD4+ and CD8+ LN T-cell numbers. Data are shown as mean +/− SEM of 11 WT, 6 γcKO, and 8 Pim1TgγcKO mice, and are pooled from seven experiments performed. *p<0.05, **p<0.01, ***p<0.001, two-tailed Student’s t test.
(C) Ki-67 expression in CD44lo LN T-cells. Data shown are representative of three independent experiments.
(D) Ki-67 expression in CD44hi LN T-cells. Data shown are representative of three independent experiments.
(E) BrdU labeling of LN T-cells. LN T-cells were fixed and permeabilized before intra-nuclear staining with anti-BrdU antibodies. Data shown are representative of three mice of each genotype.
(F) Cell size of cultured γcKO and Pim1TgγcKO CD4+ LN T-cells. Freshly isolated LN T-cells were rested in medium for 36 hours. Cell size was assessed by determining FSC values at the end of the culture. Data shown are representative of three independent experiments.
Despite increased survival, Pim1 failed to restore the peripheral CD8+ LN T-cell pool as Pim1TgγcKO CD8+ LN T-cell numbers were still severely reduced compared with those in WT mice (Fig. 4B right). In striking contrast, we observed a pronounced increase in CD4+ LN T-cell numbers (Fig. 4B left). In fact, transgenic Pim1 restored CD4+ T-cell numbers in Pim1TgγcKO mice close to the levels in WT mice. Notably, such increased cellularity was not because of increased proliferation. Both intra-nuclear Ki-67 staining and in vivo BrdU labeling did not show any differences between γcKO and Pim1TgγcKO LN T-cells (Fig. 4C, D, E), suggesting that Pim1 did not affect cell cycling or proliferation. Instead, we found that Pim1TgγcKO T-cells were metabolically more active and more resistant to apoptosis than γcKO T-cells, because cell size of CD69neg resting T-cells were larger and caspase-3 activity was significantly lower in Pim1TgγcKO mice compared with that in γcKO mice (Fig. 4F and Supporting Information Fig. 3B, C). Thus, Pim1 increases peripheral T-cell numbers by promoting cell survival.
Next we wished to assess whether Pim1 also improves survival of other T-lineage cells. We analyzed T-cell subpopulations in Pim1TgγcKO LN and spleen, but found that neither γδ T-cells, CD25+FoxP3+ Treg-cells, or NKT-cells were recovered (Fig. 5A, B, C). Also, CD8α+ IELs were drastically reduced and the IL-15-dependent CD8αα IEL population was completely absent (Fig. 5D), suggesting a non-redundant role of γc cytokines in generation and maintenance of these cells. We also failed to observe any γδ T-cells in the IEL population (Fig. 5E). Altogether, Pim1 was sufficient to restore peripheral CD4+ αβ T-cell numbers and to improve CD8+ T-cell survival in the absence of γc. However, it was insufficient to restore other T-lineage cells, including γδ T-cells, NKT-cells, CD8αα IELs, and FoxP3+ Treg-cells. Thus, CD4+ T-cells are unique in that Pim1-mediated survival effect was sufficient to meet their γc signaling requirement.
Figure 5.
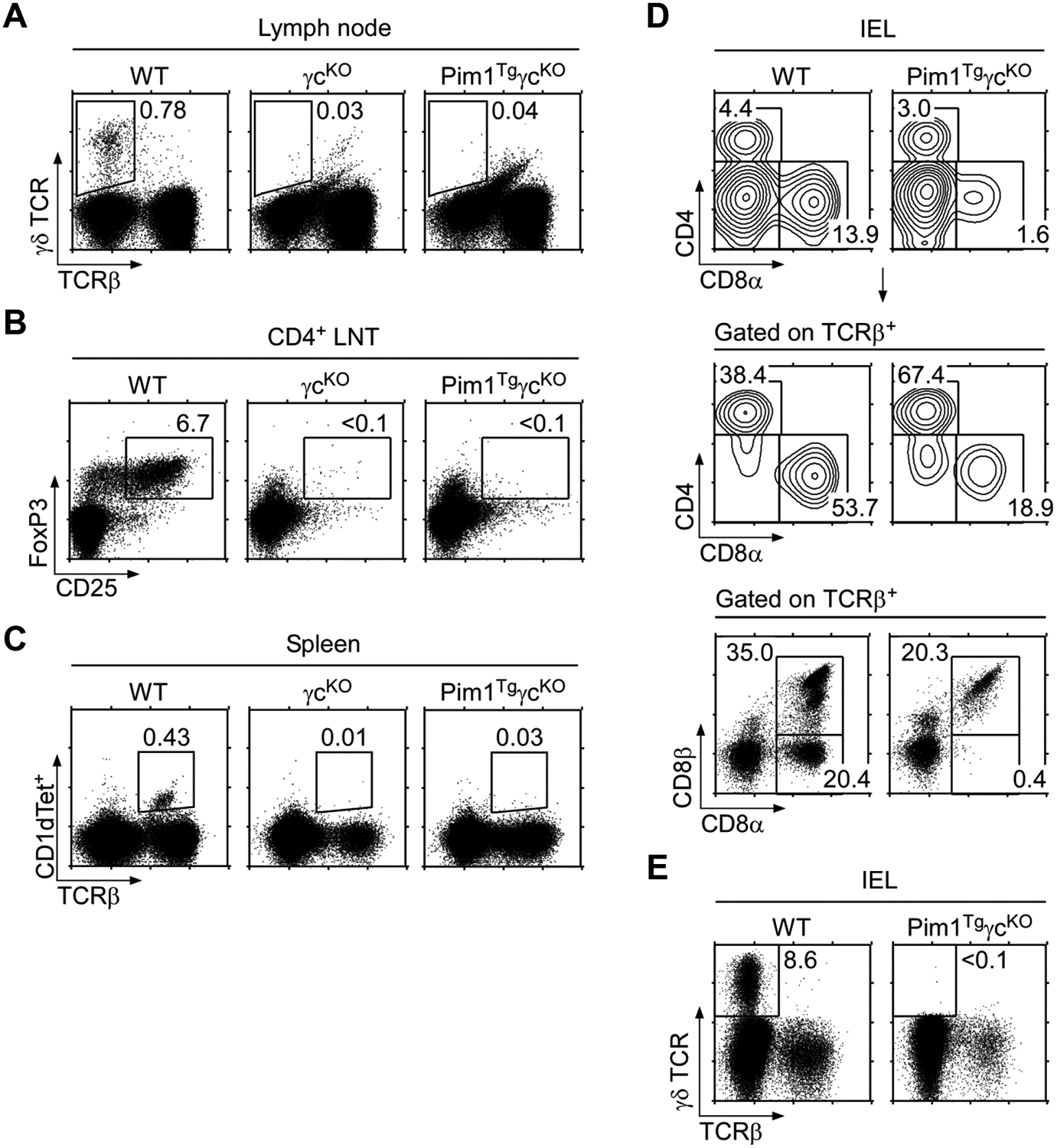
(A) LN γδ T-cells in WT, γcKO, and Pim1TgγcKO mice. Data shown are representative of three independent experiments.
(B) LN Treg cells in WT, γcKO, and Pim1TgγcKO mice. Data shown are representative of three independent experiments.
(C) Spleen NKT-cells in WT, γcKO, and Pim1TgγcKO mice. Total splenocytes were assessed for TCRβ expression and CD1d-tetramer staining to identify NKT-cells. Data shown are representative of three independent experiments.
(D) IEL analysis in WT and Pim1TgγcKO mice. Purified IELs from WT and Pim1TgγcKO mice were assessed for CD4/CD8α expression (top) and for the presence of TCRβ+ CD8 T-cells (middle). TCRβ+ cells were further analyzed for CD8α/CD8β expression (bottom). Data shown are representative of two independent experiments.
(E) γδ T-cell analysis in WT and Pim1TgγcKO IELs. Purified IELs were surface stained for γδ and αβ TCR to identify γδ T-cells. Data shown are representative of two independent experiments.
T-cell survival in the absence of γc cytokine signaling
To understand the extent to which Pim1 can replace the γc requirement, we analyzed Pim1TgγcKO LN T-cells in further detail. We found that all LN T-cells had downregulated IL-7Rα and CD103 expression which resembles an activated/memory phenotype (Fig. 6A). In agreement, most Pim1TgγcKO CD4+ and CD8+ T cells expressed high levels of the memory marker CD44 (Fig. 6B). Thus, Pim1 promotes T-cell survival in the absence of γc, but it fails to maintain a naïve T-cell pool. Interestingly, surface CD8 protein levels on Pim1TgγcKO CD8+ T-cells were significantly lower than on WT CD8+ T-cells (Fig. 6C). Since in vivo CD8 surface and mRNA levels are determined by IL-7 signaling [28], reduced CD8 surface and mRNA levels suggested that Pim1 cannot replace the CD8 regulatory arm of γc signaling (Fig. 6C and Supporting Information Fig. 3D). Along this line, we found that expression of the CD8 lineage specifying factor Runx3, but not Runx1, was significantly reduced in Pim1TgγcKO CD8+ T-cells (Supporting Information Fig. 3D). Taken together, these data indicate that Pim1 is limited in its ability to replace in vivo effects of γc signaling, and that additional γc signaling pathways are necessary to maintain CD8+ T-cell homeostasis.
Figure 6.
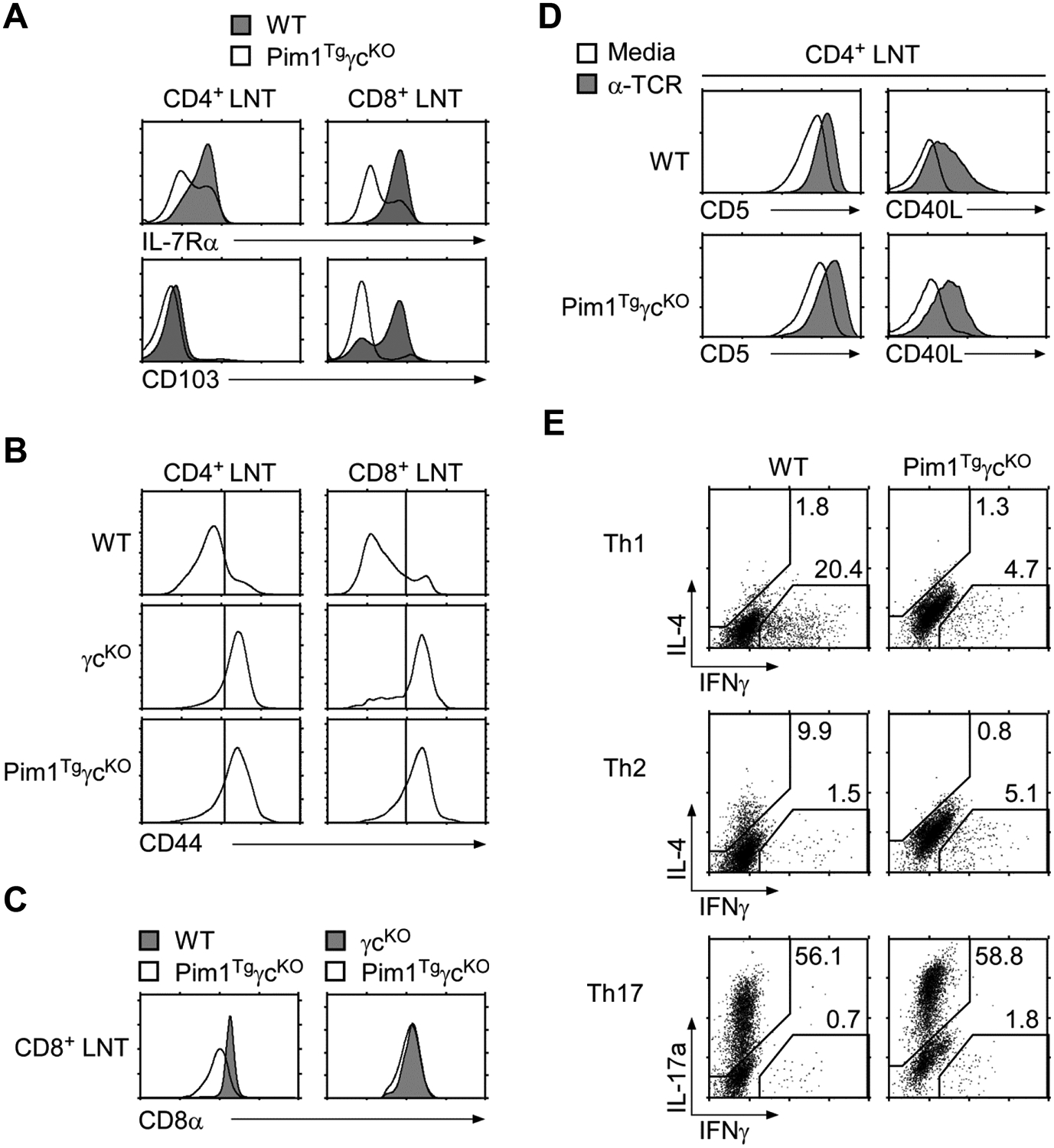
(A) IL-7Rα and CD103 expression on WT and Pim1TgγcKO CD8+ T-cells. Freshly isolated LN T-cells were assessed for IL-7Rα and CD103 expression. Data shown are representative of three independent experiments.
(B) Surface CD44 expression on freshly isolated WT, γcKO, and Pim1TgγcKO LN T-cells. Data shown are representative of seven independent experiments.
(C) CD8 coreceptor levels on WT, γcKO, and Pim1TgγcKO CD8+ T-cells. Surface CD8α expression was assessed on Pim1TgγcKO CD8+ T-cells and compared with either those of WT (left) or γcKO CD8+ T-cells (right). Data shown are representative of seven experiments.
(D) Surface CD5 and CD40L expression on TCR stimulated WT and Pim1TgγcKO CD4+ LN T-cells. Data shown are representative of two independent experiments.
(E) In vitro T-helper cell differentiation of Pim1TgγcKO CD4+ T-cells. CD4+ T-cells from WT and Pim1TgγcKO mice were stimulated under Th1, Th2, and Th17 skewing conditions and assessed for IL-4, IFN-γ, and IL-17a expression by intracellular staining. Data shown are representative of two independent experiments.
Impaired CD4+ T-helper function in the absence of γc
To test whether γc signaling is required for T-helper function, next we analyzed surface CD40L expression on activated Pim1TgγcKO CD4+ T-cells. Overnight TCR stimulation upregulated CD5 and CD40L expression on both WT and Pim1TgγcKO CD4+ T-cells (Fig. 6D). CD40L expression was CD4+ T-cell specific since activated CD8+ T-cells failed to express CD40L (Supporting Information Fig. 3E). These results indicate that CD4+ T-helper function can be acquired in the absence of γc. On the other hand, T-helper lineage differentiation was dependent on γc signaling. Stimulation of Pim1TgγcKO CD4+ T-cells under Th1 or Th2 cell differentiating conditions failed to produce Th1 or Th2 cells based on intracellular IFNγ and IL-4 expression, respectively (Fig. 6E). However, IL-17a producing Th17-cell differentiation, which is mediated by the non-γc cytokines IL-6 and TGF-β, was intact in Pim1TgγcKO CD4+ T-cells (Fig. 6E bottom). Of note, we observed consistently higher levels of IL-17 expression in Pim1TgγcKO CD4+ T-cells compared with that in WT cells. These results are in agreement with the observation that blocking IL-2 signaling impairs Th17 differentiation [29], which is disabled in Pim1TgγcKO cells. Collectively, here we documented that Pim1 permits survival and functional maturation of CD4+ T-cells in the absence of γc, but that lineage differentiation in the periphery still required γc signals that could not be replaced by Pim1.
Discussion
To understand the role of γc signaling in T-lineage cells, here we aimed to reconstitute γc deficiency by overexpressing Pim1. Using Pim1TgγcKO mice, we specifically asked whether Pim1 would be sufficient to replace γc requirement in T-cell development and survival. While Pim1 improved CD4+ αβ T-cell development and restored peripheral CD4+ T-cell numbers, it failed to do so for other T-lineage cells, including CD8+ T-cells, CD4+ Treg cells, NKT-cells, CD8αα IELs, and γδ T-cells. Thus, in contrast to all other T-lineage cells, CD4+ T-cells are unique to require γc signaling primarily for pro-survival purposes and to be γc-independent in their lineage specification and differentiation.
Classically, γc cytokines had been considered essential for T-cell development because of their pro-survival effects. γc signaling induces expression of anti-apoptotic molecules such as Bcl-2 and Mcl-1 [12, 30], and it inhibits pro-apoptotic factors such as Bax, Bad, and Bim [31–33]. Accordingly, Bax-deficiency significantly restored thymopoiesis in IL-7 receptor-deficient mice, and Bcl-2 overexpression improved T-cell development in γc-deficient mice [34–36]. However, anti-apoptotic effects alone are insufficient to fully account for γc requirement in T-cell development. Also, the Bcl-2 effect on increased thymocyte numbers itself is conflicting, with studies arguing for improved differentiation versus mere increase of developmentally arrested thymocyte numbers in Bcl-2 transgenic mice [16, 35–37]. Thus, the survival function of γc is presumably more complex than solely providing anti-apoptotic signals. In this regard, recent studies showed that trophic effects of γc signaling are also critical components of its survival function. In fact, pro-metabolic activities were found to be important also for CD4+ T-cell differentiation [38, 39] and for determining CD8+ cytotoxic T-cell fate [40, 41]. Thus, pro-metabolic activity is another important arm of the γc cytokine signaling pathway.
The Pim1 kinase epitomizes the full range of γc survival effects as it induces both anti-apoptotic and pro-metabolic pathways. Pim1 inactivates Bad to prevent apoptosis, and it activates 4E-BP1 and S6 kinase to upregulate metabolism [19, 23, 42]. In resting T cells, Pim1 is expressed below detectable levels, but IL-7 stimulation in vitro potently induces Pim1 expression [19]. Consequently, Pim1 transgenic mice displayed enhanced T cell survival and metabolic activity, although interpreting these results comes with the limitation of ectopic overexpression as usually observed in transgenic mouse models [16, 19]. In the current study, we found that such Pim1 mediated survival effects significantly improved CD4+ T-cell development in the absence of γc, but that these survival signals were not sufficient to restore development of other T-lineage cells. Therefore, γc downstream effects in addition to or in parallel to a pro-survival function must be necessary for the development and survival of non-CD4 T lineage cells. In thymic NKT-cell development, for example, IL-15 signaling is essential and γc-deficient mice lack mature NKT-cells [43]. Specifically, IL-15 signaling is important because it induces expression of the T-box family transcription factor T-bet [10]. This case exemplifies a γc requirement that is distinct to its survival effect. Along this line, we recently showed that CD8+ T-cell development requires intrathymic γc cytokine signals for lineage commitment as IL-7 signaling induced Runx3 expression to specify CD8 lineage choice [11, 44]. Whether γc signaling is also required to induce expression of nuclear factors that specify CD8αα IEL, FoxP3+ Treg cells, and γδ T-cell lineage differentiations is not clear. However, the failure to replace their development with transgenic Pim1 suggests that these T-lineage cells might be indeed dependent on γc-mediated lineage specification signals. Altogether, these data support a model of T-cell development where all T-lineage cells require γc cytokine signals, not only for survival, but also for lineage commitment and differentiation with the exception of CD4+ T-cells.
Why CD4+ αβ T-cell differentiation would be independent of γc is an intriguing question. We think that the kinetic signaling model of T-cell development might provide the best molecular explanation for this observation [45]. Accordingly, expression of the CD4 lineage specifying nuclear factor ThPOK is induced by persistent TCR signals whereas the CD8 lineage specifying factor Runx3 is induced by intrathymic γc cytokines [11, 44, 46]. Thus, in contrast to CD8 lineage choice, absent γc signals would not affect CD4 lineage choice or differentiation [11]. However, because ThPOK is induced by TCR signals and not by γc cytokine signals, we consider that TCR and pro-survival signals are presumably all that is required for CD4+ T-cell generation and maintenance. In support of this idea, we further documented that Pim1TgγcKO CD4+ T-cells, which were generated in the absence of γc, were functionally mature. We found that they upregulated CD40L expression upon TCR signaling and were thus capable of providing B cell help [47]. At the same time, Pim1TgγcKO CD4+ T cells failed to differentiate into either Th1 or Th2 cells in vitro. This was even more remarkable as they were mostly CD44hi activated/memory phenotype cells and they also responded normally to TCR stimulation. These results demonstrated that Th1/Th2 cytokine production and T helper cell differentiation require γc signaling independent of Pim1. Along this line, it was interesting that inflammatory Th17 differentiation was intact, if not enhanced, in the absence of γc which, however, can be explained by the negative effect of IL-2 signaling on IL-17 expression. Of note, because Pim1TgγcKO mice lack FoxP3+ Treg cells and since Pim1TgγcKO CD4+ T-cells could be induced to differentiate into inflammatory T-cells, it was surprising that we did not find any signs of autoimmunity in Pim1TgγcKO mice. The in vivo immune response of these mice is currently under investigation.
Collectively, the present study establishes pro-survival effects as the only factor downstream of γc signaling that is required for CD4+ T-cell development. Such characteristics set these cells apart from other T-lineage cells which presumably also require lineage specification signals downstream of γc signaling. We expect that further functional studies of γc-deficient CD4+ T-cells, together with genetic reconstitution of other select γc downstream pathways, such as constitutively active Akt or STAT5, will help decipher the detailed molecular pathways in T-lineage cell development and maintenance.
Materials and methods
Mice
CD45.1+ or CD45.2+ C57BL/6 and γc-deficient mice were obtained from the Jackson Laboratory. Human Bcl-2 transgenic mice were provided by Dr. Alfred Singer (Natl. Cancer Inst., Bethesda, MD) [48]. Pim1 transgenic mice have been described [18], and were provided by Dr. Anton Berns (The Netherlands Cancer Institute, Amsterdam, The Netherlands). Animal experiments were approved by the Natl. Cancer Inst. Animal Care and Use Committee, and all mice were cared for in accordance with National Institutes of Health guidelines.
Flow cytometry
Cells were stained and analyzed on LSRII, ARIAII or FACSCalibur flow cytometers (Becton Dickinson). Dead cells were excluded by forward light scatter gating and propidium iodide staining. Antibodies with the following specificities were used for staining: CD8β, CD44, HSA, IL-7Rα, FoxP3, Ki-67 (eBioscience); CD4, CD8α, TCRβ, CD103, γc, human CD3, IL-4, IL-17 (Becton Dickinson); γδ TCR, IFNγ (Biolegend). For intracellular cytokine staining, in vitro differentiated cells were restimulated for 3 hrs with PMA and ionomycin with the addition of brefeldin A (eBioscience). Cells were fixed and permeabilized with IC fixation buffer (eBioscience). For nuclear FoxP3 staining, cells were first surface stained and then fixed and permeabilized using FoxP3 intracellular staining buffer set according to the manufacturer’s instructions (eBioscience). Active caspase-3 was assayed using a CaspGLOW active caspase-3 kit following the manufacturer’s instructions (eBioscience).
Preparation of IELs
Intestines were harvested and washed using 2% FBS in HBSS. After slicing into smaller pieces, intestines were washed using 2% FBS in HBSS and stirred for 20 min at 37° C in 10% FBS in HBSS with 1 mM DTT. Tissue suspensions were then filtered, centrifuged, and washed once with PBS before preparing a Percoll (Sigma) gradient. Percoll layers were formed at concentrations of 80%, 40%, and 20%, with the cells being mixed in 20% Percoll. The gradient was then centrifuged at 500g for 25 minutes, and cells were harvested from the interface between the 40% and 80% Percoll layers for further analysis.
Mixed bone marrow chimera analysis
Radiation bone marrow chimeras (BMC) were generated by reconstructing irradiated (600 Rad) RAG2KO recipient mice with a total of 15 X 106 T-cell depleted bone marrow donor cells, mixed at 1:1 ratio of γcKO and Pim1TgγcKO cells. Chimeric mice were analyzed 7 weeks after reconstitution.
BrdU cell proliferation assay
Cell proliferation was measured by BrdU (5-bromodeoxyuridine) incorporation. B6, γcKO or Pim1TgγcKO mice were given intraperitoneal injections of BrdU dissolved in PBS (1 mg per mouse) and analyzed 3 days later. Thymocytes were first stained for surface markers, and then fixed and permeabilized with Cytofix/Cytoperm and Cytofix/Cytoperm Plus for intra-nuclear anti-BrdU staining according to the manufacturer’s protocol (Becton Dickinson).
In vitro T-helper cell differentiation
LN T-cells were depleted of B-cells with anti-mouse IgG magnetic beads and further depleted of CD8+ cells with anti-CD8 antibodies followed by anti-rat IgG magnetic beads (Qiagen). Isolated CD4+ LN T-cells were stimulated with standard T-helper cell differentiating cytokine cocktails: Th0, media alone; Th1, 10 ng/mL IL-12 (Peprotech), 10 μg/mL α-IL-4 (eBioscience); Th2, 20 ng/mL IL-4 (Peprotech), 10 μg/mL α-IFN-γ (eBioscience); Th17, 10 μg/mL α-IL-4, 10 μg/mL α-IFN-γ, 30 ng/mL IL-6 (BD Pharmingen), 5 ng/mL TGF-β (Peprotech), and incubated in tissue culture plates coated with α-CD3 and α-CD28 (1 μg/mL) for 5 days.
Immunoblotting
Freshly isolated thymocytes and LN cells were lysed in CelLytic-M lysis reagent (Sigma) for 30 min on ice. Cell lysate was cleared from cellular debris by centrifugation, and supernatant was resolved by SDS-PAGE in 4–12% Bis-Tris acrylamide gels (Invitrogen) under reducing conditions. Upon electrotransfer of proteins onto PVDF membranes (Invitrogen), blots were blocked with 2% BSA in TBS and incubated with rabbit anti-Pim1 polyclonal antibodies (Cell Signaling Tech) followed by horseradish peroxidase (HRP)-conjugated anti-rabbit (GE Healthcare) or HRP-conjugated anti-β-actin antibodies (Santa Cruz Biotechnology). Reactivity was detected by enhanced chemiluminescence (Perkin Elmer).
Quantitative reverse transcription PCR
CD8+ LN T-cells were electronically sorted from WT and Pim1TgγcKO lymph nodes. Total RNA was immediately isolated with the RNeasy kit (Qiagen). RNA was reverse transcribed into cDNA by oligo(dT) priming with the QuantiTect reverse transcription kit (Qiagen). Quantitative RT-PCR (qRT-PCR) was performed with an ABI PRISM 7900HT and the QuantiTect SYBR green detection system (Qiagen). The following primers were used for PCR: CD8α (Forward: 5’-AAGTGTTGGGGTCCGTTTCG-3’; Reverse: 5’-AATCTTCTGGTCTCTGGGGCTG-3’); Runx3 (Forward: 5’-GCGACATGGCTTCCAACAGC-3’; Reverse: 5’-CTTAGCGCGCCGCTGTTCTCGC-3’); Runx1 (Forward: 5’-AGCTAGTGCGCACCGACAGC-3’; Reverse: 5’-CCCCCAGTGCCACCACCTTG-3’); Hprt (Forward: 5’-GCGATGATGAACCAGGTTATGA-3’; Reverse: 5’-ACAATGTGATGGCCTCCCAT-3’).
Statistical analysis
Data are shown as mean +/− SEM. Two-tailed Student’s t test was used to calculate P-values for all experiments. A value of P ≤ 0.05 was considered statistically significant. *P<0.05, ** P<0.01, *** P< .001.
Supplementary Material
Acknowledgements:
We are grateful for Drs. A. Singer and R. Etzensperger for critical review of the manuscript. This study was supported by the Intramural Research Program of the US National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Gascoigne NR and Palmer E, Signaling in thymic selection. Curr Opin Immunol 2011. 23: 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kisielow P, Teh HS, Bluthmann H and von Boehmer H, Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature 1988. 335: 730–733. [DOI] [PubMed] [Google Scholar]
- 3.Rochman Y, Spolski R and Leonard WJ, New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol 2009. 9: 480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao X, Shores EW, Hu-Li J, Anver MR, Kelsall BL, Russell SM, Drago J, and et al. , Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity 1995. 2: 223–238. [DOI] [PubMed] [Google Scholar]
- 5.DiSanto JP, Muller W, Guy-Grand D, Fischer A and Rajewsky K, Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc Natl Acad Sci U S A 1995. 92: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mombaerts P, Clarke AR, Rudnicki MA, Iacomini J, Itohara S, Lafaille JJ, Wang L, and et al. , Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature 1992. 360: 225–231. [DOI] [PubMed] [Google Scholar]
- 7.Philpott KL, Viney JL, Kay G, Rastan S, Gardiner EM, Chae S, Hayday AC and Owen MJ, Lymphoid development in mice congenitally lacking T cell receptor alpha beta-expressing cells. Science 1992. 256: 1448–1452. [DOI] [PubMed] [Google Scholar]
- 8.Starr TK, Jameson SC and Hogquist KA, Positive and negative selection of T cells. Annu Rev Immunol 2003. 21: 139–176. [DOI] [PubMed] [Google Scholar]
- 9.Yu Q, Park JH, Doan LL, Erman B, Feigenbaum L and Singer A, Cytokine signal transduction is suppressed in preselection double-positive thymocytes and restored by positive selection. J Exp Med 2006. 203: 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordy LE, Bezbradica JS, Flyak AI, Spencer CT, Dunkle A, Sun J, Stanic AK, and et al. , IL-15 regulates homeostasis and terminal maturation of NKT cells. J Immunol 2011. 187: 6335–6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JH, Adoro S, Guinter T, Erman B, Alag AS, Catalfamo M, Kimura MY, and et al. , Signaling by intrathymic cytokines, not T cell antigen receptors, specifies CD8 lineage choice and promotes the differentiation of cytotoxic-lineage T cells. Nat Immunol 2010. 11: 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rathmell JC, Farkash EA, Gao W and Thompson CB, IL-7 enhances the survival and maintains the size of naive T cells. J Immunol 2001. 167: 6869–6876. [DOI] [PubMed] [Google Scholar]
- 13.Powell JD, Pollizzi KN, Heikamp EB and Horton MR, Regulation of Immune Responses by mTOR. Annu Rev Immunol 2012. 30: 39–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wieman HL, Wofford JA and Rathmell JC, Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell 2007. 18: 1437–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wofford JA, Wieman HL, Jacobs SR, Zhao Y and Rathmell JC, IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood 2008. 111: 2101–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs H, Krimpenfort P, Haks M, Allen J, Blom B, Demolliere C, Kruisbeek A, and et al. , PIM1 reconstitutes thymus cellularity in interleukin 7- and common gamma chain-mutant mice and permits thymocyte maturation in Rag- but not CD3gamma-deficient mice. J Exp Med 1999. 190: 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nosaka T, Kawashima T, Misawa K, Ikuta K, Mui AL and Kitamura T, STAT5 as a molecular regulator of proliferation, differentiation and apoptosis in hematopoietic cells. EMBO J 1999. 18: 4754–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Lohuizen M, Verbeek S, Krimpenfort P, Domen J, Saris C, Radaszkiewicz T and Berns A, Predisposition to lymphomagenesis in pim-1 transgenic mice: cooperation with c-myc and N-myc in murine leukemia virus-induced tumors. Cell 1989. 56: 673–682. [DOI] [PubMed] [Google Scholar]
- 19.Fox CJ, Hammerman PS and Thompson CB, The Pim kinases control rapamycin-resistant T cell survival and activation. J Exp Med 2005. 201: 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aho TL, Sandholm J, Peltola KJ, Mankonen HP, Lilly M and Koskinen PJ, Pim-1 kinase promotes inactivation of the pro-apoptotic Bad protein by phosphorylating it on the Ser112 gatekeeper site. FEBS Lett 2004. 571: 43–49. [DOI] [PubMed] [Google Scholar]
- 21.Bachmann M and Moroy T, The serine/threonine kinase Pim-1. Int J Biochem Cell Biol 2005. 37: 726–730. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Bhattacharya N, Meyer MK, Seimiya H, Tsuruo T, Tonani JA and Magnuson NS, Pim-1 negatively regulates the activity of PTP-U2S phosphatase and influences terminal differentiation and apoptosis of monoblastoid leukemia cells. Arch Biochem Biophys 2001. 390: 9–18. [DOI] [PubMed] [Google Scholar]
- 23.Beharry Z, Mahajan S, Zemskova M, Lin YW, Tholanikunnel BG, Xia Z, Smith CD and Kraft AS, The Pim protein kinases regulate energy metabolism and cell growth. Proc Natl Acad Sci U S A 2011. 108: 528–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox CJ, Hammerman PS, Cinalli RM, Master SR, Chodosh LA and Thompson CB, The serine/threonine kinase Pim-2 is a transcriptionally regulated apoptotic inhibitor. Genes Dev 2003. 17: 1841–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng C, Knebel A, Morrice NA, Li X, Barringer K, Li J, Jakes S, and et al. , Pim kinase substrate identification and specificity. J Biochem 2007. 141: 353–362. [DOI] [PubMed] [Google Scholar]
- 26.Saris CJ, Domen J and Berns A, The pim-1 oncogene encodes two related protein-serine/threonine kinases by alternative initiation at AUG and CUG. EMBO J 1991. 10: 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crompton T, Moore M, MacDonald HR and Malissen B, Double-negative thymocyte subsets in CD3 zeta chain-deficient mice: absence of HSA+CD44-CD25- cells. Eur J Immunol 1994. 24: 1903–1907. [DOI] [PubMed] [Google Scholar]
- 28.Park JH, Adoro S, Lucas PJ, Sarafova SD, Alag AS, Doan LL, Erman B, and et al. , ‘Coreceptor tuning’: cytokine signals transcriptionally tailor CD8 coreceptor expression to the self-specificity of the TCR. Nat Immunol 2007. 8: 1049–1059. [DOI] [PubMed] [Google Scholar]
- 29.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, and et al. , Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 2007. 26: 371–381. [DOI] [PubMed] [Google Scholar]
- 30.Dunkle A, Dzhagalov I and He YW, Cytokine-dependent and cytokine-independent roles for Mcl-1: genetic evidence for multiple mechanisms by which Mcl-1 promotes survival in primary T lymphocytes. Cell Death Dis 2011. 2: e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim K, Lee CK, Sayers TJ, Muegge K and Durum SK, The trophic action of IL-7 on pro-T cells: inhibition of apoptosis of pro-T1, -T2, and -T3 cells correlates with Bcl-2 and Bax levels and is independent of Fas and p53 pathways. J Immunol 1998. 160: 5735–5741. [PubMed] [Google Scholar]
- 32.Li WQ, Guszczynski T, Hixon JA and Durum SK, Interleukin-7 regulates Bim proapoptotic activity in peripheral T-cell survival. Mol Cell Biol 2010. 30: 590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li WQ, Jiang Q, Khaled AR, Keller JR and Durum SK, Interleukin-7 inactivates the pro-apoptotic protein Bad promoting T cell survival. J Biol Chem 2004. 279: 29160–29166. [DOI] [PubMed] [Google Scholar]
- 34.Khaled AR, Li WQ, Huang J, Fry TJ, Khaled AS, Mackall CL, Muegge K, and et al. , Bax deficiency partially corrects interleukin-7 receptor alpha deficiency. Immunity 2002. 17: 561–573. [DOI] [PubMed] [Google Scholar]
- 35.Kondo M, Akashi K, Domen J, Sugamura K and Weissman IL, Bcl-2 rescues T lymphopoiesis, but not B or NK cell development, in common gamma chain-deficient mice. Immunity 1997. 7: 155–162. [DOI] [PubMed] [Google Scholar]
- 36.Rodewald HR, Waskow C and Haller C, Essential requirement for c-kit and common gamma chain in thymocyte development cannot be overruled by enforced expression of Bcl-2. J Exp Med 2001. 193: 1431–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakajima H and Leonard WJ, Role of Bcl-2 in alpha beta T cell development in mice deficient in the common cytokine receptor gamma-chain: the requirement for Bcl-2 differs depending on the TCR/MHC affinity. J Immunol 1999. 162: 782–790. [PubMed] [Google Scholar]
- 38.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, and et al. , The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol 2011. 12: 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerriets VA and Rathmell JC, Metabolic pathways in T cell fate and function. Trends Immunol 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finlay D and Cantrell DA, Metabolism, migration and memory in cytotoxic T cells. Nat Rev Immunol 2011. 11: 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macintyre AN, Finlay D, Preston G, Sinclair LV, Waugh CM, Tamas P, Feijoo C, and et al. , Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity 2011. 34: 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macdonald A, Campbell DG, Toth R, McLauchlan H, Hastie CJ and Arthur JS, Pim kinases phosphorylate multiple sites on Bad and promote 14-3-3 binding and dissociation from Bcl-XL. BMC Cell Biol 2006. 7: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boesteanu A, Silva AD, Nakajima H, Leonard WJ, Peschon JJ and Joyce S, Distinct roles for signals relayed through the common cytokine receptor gamma chain and interleukin 7 receptor alpha chain in natural T cell development. J Exp Med 1997. 186: 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCaughtry TM, Etzensperger R, Alag A, Tai X, Kurtulus S, Park JH, Grinberg A, and et al. , Conditional deletion of cytokine receptor chains reveals that IL-7 and IL-15 specify CD8 cytotoxic lineage fate in the thymus. J Exp Med 2012. 209: 2263–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singer A, Adoro S and Park JH, Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat Rev Immunol 2008. 8: 788–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He X, Park K, Wang H, Zhang Y, Hua X, Li Y and Kappes DJ, CD4-CD8 lineage commitment is regulated by a silencer element at the ThPOK transcription-factor locus. Immunity 2008. 28: 346–358. [DOI] [PubMed] [Google Scholar]
- 47.van Kooten C and Banchereau J, CD40-CD40 ligand. J Leukoc Biol 2000. 67: 2–17. [DOI] [PubMed] [Google Scholar]
- 48.Sentman CL, Shutter JR, Hockenbery D, Kanagawa O and Korsmeyer SJ, bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell 1991. 67: 879–888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


