Abstract
Objective:
Diabetic ketoacidosis (DKA) is a common emergency department (ED) presentation of both new-onset and established diabetes mellitus (DM). β-hydroxybutyrate (BOHB) provides a direct measure of the pathophysiologic derangement in DKA as compared to the non-specific measurements of blood pH and bicarbonate. Our objective was to characterize the relationship between BOHB and DKA.
Methods:
This is a cross-sectional retrospective study of pediatric patients with DM presenting to an urban pediatric ED between January 1, 2016 and September 30, 2018. Analyses were performed on each patient’s initial, simultaneous BOHB and pH. Diagnostic test characteristics of BOHB were calculated and logistic regression was performed to investigate the effects of age and other key clinical factors.
Results:
Among 594 patients with DM, median age 12.3 years (IQR 8.7, 15.9), 176 (29.6%) presented in DKA. The inclusion of age, transfer status, and new-onset in the statistical model did not improve the prediction of DKA beyond BOHB alone. BOHB demonstrated strong discrimination for DKA with an area under the curve of 0.95 (95% CI 0.93, 0.97). A BOHB value of 5.3 mmol/L predicted DKA with optimal accuracy (90.6% of patients were correctly classified). The sensitivity, specificity, positive and negative predictive values of this cut-point were 76.7% (95%CI 69.8%,82.7%), 96.4% (94.2%,98.0%), 90.0% (84.0%,94.3%), and 90.8% (87.7%,93.3%), respectively.
Conclusions:
BOHB accurately predicts DKA in children and adolescents. More importantly, as plasma BOHB is the ideal biochemical marker of DKA, BOHB may provide a more optimal definition of DKA for management decisions and treatment targets.
Keywords: β-hydroxybutyrate, Diabetes mellitus, Diabetic ketoacidosis
Introduction:
Diabetes mellitus (DM) is an increasingly common disease of childhood affecting approximately 500,000 children <15 years old worldwide1, and diabetic ketoacidosis (DKA) is a frequent and potentially life threatening complication of both established and new-onset diabetes mellitus (NODM).2–5 In the United States, a recent publication from the Type 1 Diabetes Exchange Registry showed that as many as 4% of children and adolescents with established type 1 DM report having at least one episode of DKA in the last 3 months.4 The frequency of type 2 DM in the pediatric age range is increasing, and 6% to 11% of youth with type 2 DM present with DKA.2,6
DKA occurs when absolute or relative insulin deficiency and elevated counterregulatory hormone concentrations cause unregulated production of ketoacids (ß-hydroxybutyric acid and acetoacetic acid).7 With progressive metabolic decompensation, the ratio of the concentration of β-hydroxybutyrate (BOHB) to acetoacetate in blood changes from 1:1 to, on average, 3:1.8,9 The production of BOHB and acetoacetate is accompanied by an equimolar production of hydrogen ions resulting in acidemia. A commonly used definition for the diagnosis of DKA in children includes three biochemical criteria: hyperglycemia (blood glucose > 200 mg/dL (18 mg/dL = 1 mmol/L) or 11 mmol/L), acidemia (venous pH < 7.3 or plasma bicarbonate (HCO3) < 15 mmol/L), and ketonemia or moderate or large ketonuria.10 The severity of acidemia in a patient with diabetes, as measured by plasma HCO3 concentraton and venous pH, can be affected by other factors that perturb acid-base balance such as the degree of respiratory and renal compensation, hyperchloremic nonanion gap acidosis attributable to renal loss of ketones11, metabolic alkalosis caused by vomiting with loss of hydrogen ions12, and elevated blood lactate concentration from poor tissue perfusion13, all common in patients with DKA. The plasma HCO3 value is less suscepetible than pH to abrupt changes in ventilation, but like pH is nonspecific. In addition, in the early stages of metabolic decompensation, ketosis may occur without acidemia if physiologic buffering is effective. Measurement of the concentration of blood BOHB, the predominant ketone present in decompensated type 1 DM, is increasingly recognized as a useful biochemical parameter for the diagnosis and management of DKA; however, there is conflicting guidance from expert bodies on how ketone measurements should be used in the management of ketoacidosis.14,15
In emergency departments (ED), measurement of venous pH and plasma HCO3 concentration to identify DKA is standard practice owing to their general availability in high-resource healthcare settings; however, routine measurement of blood BOHB, either using capillary point-of-care (POC) devices or standard laboratory measurements, is becoming increasingly widespread. As BOHB is a more specific measure of the metabolic state underlying DKA, the objective of this study was to determine the test performance of plasma BOHB among children and adolescents being evaluated for DKA in the pediatric emergency department. If plasma BOHB accurately predicts DKA based on current definitions, this knowledge will set the stage for considering BOHB as the primary determinant of DKA and singular target for DKA treatment strategies.
Materials and Methods:
Study Design and Setting:
We conducted a retrospective cross-sectional study of patients seen in a tertiary referral children’s hospital from January 1, 2016 to September 30, 2018. The study was approved by the Institutional Review Board at Boston Children’s Hospital.
Selection of Participants:
We identified patients aged 1–18 years with DM presenting to the ED who had a venous blood gas and plasma BOHB concentration measured within 30 minutes of one another. In May 2014, prior to the study period, the electronic medical record order-set for all patients with hyperglycemia presenting to the ED was changed to include routine measurement of plasma BOHB concentration. All patient encounters were individually reviewed to confirm that the patient had diabetes, to determine if the patient’s presentation was consistent with new-onset or established diabetes, and to assess if the patient had been transferred from an outside facility.
Outcomes:
DKA was defined according to the International Society for Pediatric and Adolescent Diabetes (ISPAD) as: 1) plasma glucose >200 mg/dL; and 2) either venous pH < 7.30 or plasma HCO3 <15 mmol/L.10 Analyses were performed on the initial set of laboratory values for each patient encounter.
Laboratory Measures:
Venous pH and plasma HCO3 were analyzed using electrochemical methods on a Radiometer ABL830 blood gas analyzer (Radiometer America, Brea CA). Plasma glucose was analyzed using an enzymatic method (hexokinase) on the Roche Cobas c501 using Roche reagents. Plasma BOHB was analyzed using an enzymatic method (Stanbio Liquicolor Assay, EKF Diagnostics, Boerne TX) on the Roche Cobas c501 analyzer (Roche Diagnostics, Indianapolis IN). BOHB measurements were reported as low as 0.05 mmol/L. Values greater than 4.5 mmol/L were diluted to achieve measurements up to 18 mmol/L.
Statistical Analyses:
The demographic and clinical characteristics of patients with and without DKA were compared using the Pearson chi square test.
To assess our primary aim, we characterized the relationship between BOHB and DKA using area under the receiver operator characteristic curve (AUC). We calculated test characteristics (sensitivity, specificity, positive and negative predictive values, percent correctly classified and positive and negative likelihood ratios) across a range of BOHB values. To test whether age, transfer status, and NODM added predictive ability beyond that of BOHB alone in the prediction of DKA we compared the AUC between these two models (BOHB alone versus BHOB, age, transfer status and NODM).
As a secondary analysis, we addressed the possibility that patients who had been transferred from an outside hospital prior to obtaining the patient’s initial set of laboratory values in the ED may exhibit different degrees of ketonemia relative to acidemia if therapy had been initiated before these measurements were obtained in our ED. We therefore excluded the transferred patients and characterized the relationship between BOHB and DKA using AUC. Also, after excluding the transferred patients, we tested whether age and NODM added predictive ability beyond that of BOHB alone in the prediction of DKA by comparing the AUC between these two models.
The following additional exploratory analyses were conducted. First, because young children with type 1 DM are more prone to ketosis than older children and adolescents,16 we assessed differences in the ability of BOHB to predict DKA by age (categorized into three age groups: <7 years, 7–12 years, and ≥13 years) by estimating a logistic regression model with DKA as the dependent variable and BOHB, age group, and the BOHB-by-age group interaction as the independent variables. Significant interaction effects would indicate that the association between BOHB and DKA varied by age group. Second, we tested the moderating effect of new-onset (vs. established diabetes) status by estimating a logistic regression model with DKA as the dependent variable and BOHB, onset status (new vs. established), and the BOHB-by-onset group interaction as the independent variables. Both of these models were repeated while including transfer status as a covariate in the model, and again after excluding the transferred patients.
Lastly, we calculated test characteristics (as defined above) for the subset of patients with moderate or severe DKA as defined by either venous pH <7.20 or plasma HCO3 <10 mmol/L.10
All tests were two-tailed and alpha was set at 0.05. Analyses were performed using the software package STATA, version 14.2 (College Station, Texas).
Results:
Characteristics of Study Subjects:
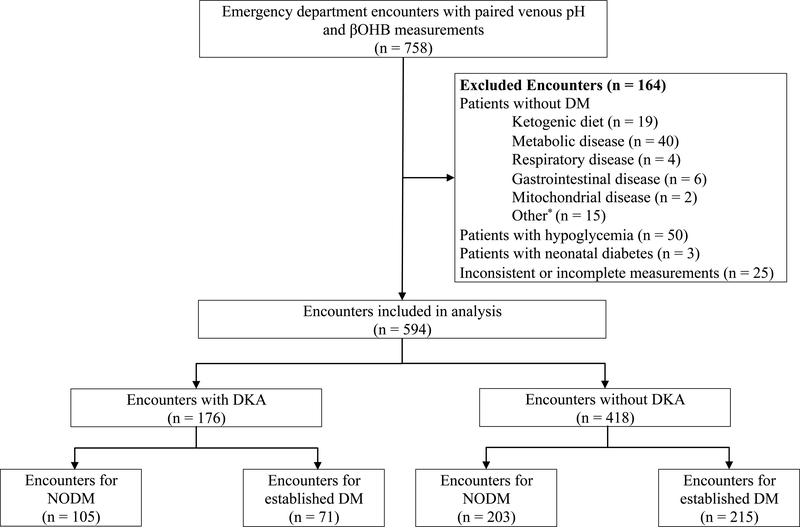
A total of 758 encounters had paired laboratory values. After excluding ineligible encounters, 594 patient encounters were included in the analyses (Figure 1). Among 594 ED encounters, 176 patients (29.6%) presented in DKA. Eighty-five patients (14.3%) met criteria for moderate or severe DKA. The median age of the study sample was 12.3 years (IQR 8.7, 15.9). There were no statistically significant differences between patients who presented with DKA and those who did not on the basis of sex, age, race, ethnicity, or insurance status (Table 1). Patients who presented with DKA were more likely to have been transferred from an outside facility (24% vs. 15%), to be NODM (60% vs. 49%), and to be admitted to the hospital from the ED (94% vs 69%).
Figure 1.
Flow diagram of study encounter selection.
*Other reasons for exclusion included Russell Silver syndrome, supraventricular tachycardia, and neurologic compromise not related to DKA.
Table 1.
Demographic and clinical characteristics of Emergency Department patients with and without diabetic ketoacidosis (DKA)
| Characteristic, n (%) | No DKA n=418 | DKA n=176 |
|---|---|---|
|
| ||
| Female | 201 (48) | 95 (54) |
| Age (years) | ||
| 1–6 | 75 (18) | 28 (16) |
| 7–12 | 155 (37) | 68 (39) |
| 13–18 | 188 (45) | 80 (45) |
| Race | ||
| White | 257 (65) | 103 (63) |
| Black | 62 (16) | 28 (17) |
| Other | 78 (20) | 33 (20) |
| Ethnicity | ||
| Not Hispanic | 301 (85) | 120 (81) |
| Hispanic | 54 (15) | 28 (19) |
| Insurance Status | ||
| Private | 256 (62) | 106 (60) |
| Public | 160 (38) | 70 (40) |
| Transferred from another hospital | 61 (15) | 42 (24)* |
| New-onset diabetes mellitus | 203 (49) | 105 (60)* |
p<0.05
Main Results:
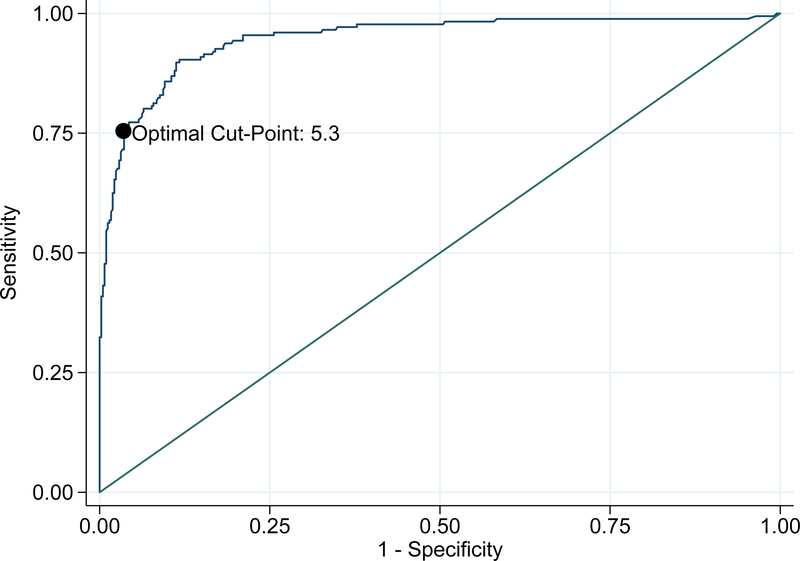
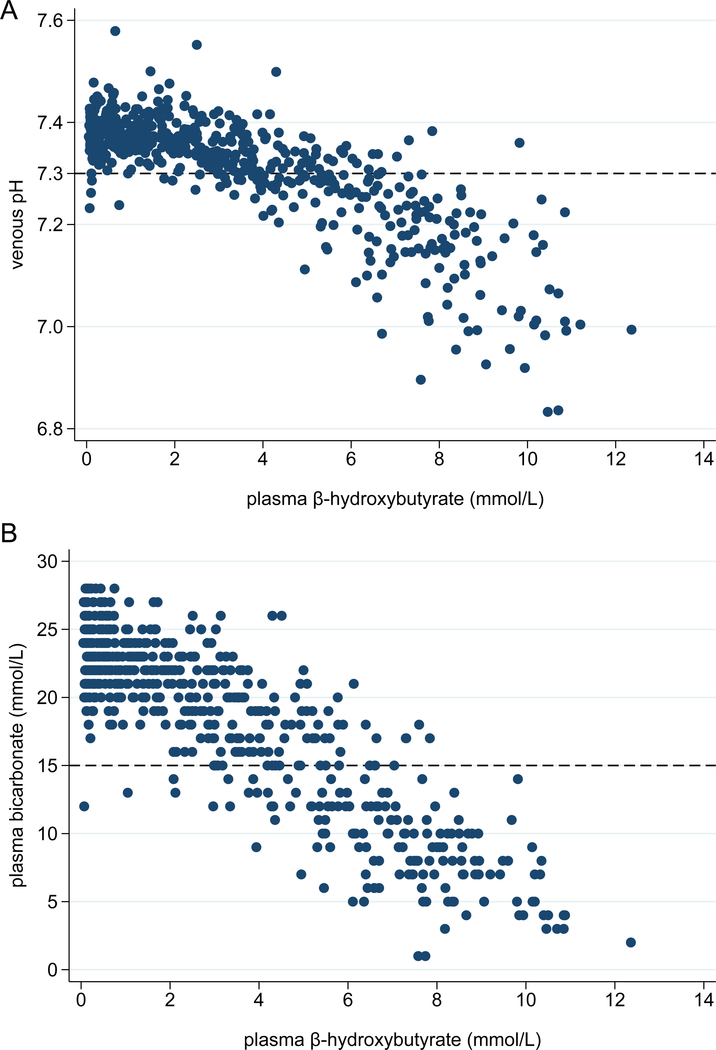
BOHB demonstrated strong discrimination for DKA with an AUC of 0.95 in Figure 2 (95% CI 0.93, 0.97). Table 2 shows test characteristics across a range of integer BOHB values. Optimal accuracy, defined as the maximum overall proportion of patients correctly classified, was achieved using a plasma BOHB value of 5.3 mmol/L as a cut-point, with 90.6% of patients correctly classified. The sensitivity, specificity, positive and negative predictive values of this cut-point were 76.7% (95% CI 69.8%, 82.7%), 96.4% (94.2%, 98.0%), 90.0% (84.0%, 94.3%), and 90.8% (87.7%, 93.3%), respectively. Scatterplots of BOHB versus venous pH and plasma HCO3 shown in Figures 3A and 3B, respectively, demonstrate a clear relationship between ketonemia and acidosis.
Figure 2.
Receiver-operator characteristic curve of plasma β -hydroxybutyrate predicting diabetic ketoacidosis among pediatric emergency department patients with hyperglycemia.
Optimal cut-point was defined as the maximum overall proportion of patients correctly classified.
Table 2.
Test characteristics across a range of cut-points of plasma β-hydroxybutyrate (BOHB) in the prediction of diabetic ketoacidosis among pediatric emergency department patients.
| BOHB Cut-point (mmol/L) | Sensitivity (%) | Specificity (%) | Positive Predictive Value (%) | Negative Predictive Value (%) | Correctly Classified (%) | LR+ | LR− |
|---|---|---|---|---|---|---|---|
|
| |||||||
| ≥ 1 | 98.3 | 47.6 | 44.1 | 98.5 | 62.6 | 1.88 | 0.04 |
| ≥ 2 | 96.6 | 65.3 | 54.3 | 97.9 | 74.6 | 2.78 | 0.05 |
| ≥ 3 | 94.3 | 79.9 | 66.4 | 97.1 | 84.2 | 4.69 | 0.07 |
| ≥ 4 | 86.9 | 89.0 | 77.3 | 94.2 | 88.4 | 7.90 | 0.15 |
| ≥ 5 | 77.3 | 94.5 | 85.5 | 90.8 | 89.4 | 14.04 | 0.24 |
| ≥ 6 | 64.8 | 97.9 | 92.6 | 86.7 | 88.1 | 30.08 | 0.36 |
| ≥ 7 | 48.3 | 99.0 | 95.5 | 81.8 | 84.0 | 50.47 | 0.52 |
| ≥ 8 | 30.7 | 100.0 | 100.0 | 77.4 | 79.5 | --- | 0.69 |
Likelihood ratio positive (LR+); Likelihood ratio negative (LR-)s
Figure 3.
(Panel A) Plasma β-hydroxybutyrate and venous pH among all patients. (Panel B) Plasma β-hydroxybutyrate and plasma bicarbonate levels among all patients.
Dashed lines at pH 7.3 and bicarbonate 15 mmol/L represent the biochemical criteria for DKA.
The inclusion of age, transfer status, and NODM did not add predictive ability beyond that of BOHB alone for identifying DKA (p=0.103). In the subsample of patients who were not transferred from another hospital, the model with BOHB, age, and NODM was statistically superior to that of BOHB alone (p=0.046); however, the magnitude of the difference was not clinically meaningful (AUC 0.965 versus 0.952, respectively).
There was no evidence that the effect of BOHB was moderated by age group (omnibus test of age group-by-BOHB interaction terms: Χ2(2)=1.93, p=0.381). The result was unchanged after statistical adjustment for transfer status (Χ2(2)=1.91, p=0.385), and with transferred patients (n=103) excluded from the model (Χ2(2)=2.88, p=0.237). Similarly, there was no evidence that the effect of BOHB was moderated by new-onset status (test of NODM-by-BOHB interaction term: odds ratio (95% confidence interval) = 1.37 (0.96, 1.97)). The result was unchanged after statistical adjustment for transfer status (OR (95% CI) = 1.38 (0.96, 1.99)), and with transferred patients (n=103) excluded from the model (OR (95% CI) = 1.34 (0.90, 2.00)).
Among the subgroup of patients with moderate or severe DKA (n = 85), 88.5% were correctly classified using the cut-point of 5.3 mmol/L. In this subset, the sensitivity, specificity, positive and negative predictive values were 97.7% (95% CI 91.8%, 99.7%), 87.0% (83.8%, 89.8%), 55.7% (47.4%, 63.8%), and 99.6% (98.4, 100.0%), respectively. A cut-point of BOHB ≥ 3.0 mmol/L, as suggested by the ISPAD guidelines, when used to classify patients with moderate or severe DKA had a sensitivity, specificity, postitive and negative predictive value of 100.0% (95% CI 95.8%, 100.0%), 67.7% (63.5%,71.8%), 34.1% (28.3%, 40.4%), and 100.0% (98.9, 100.0%), respectively.
Discussion:
This study has shown that among nearly 600 children and adolescents with DM who presented to an ED with hyperglycemia, plasma BOHB accurately predicted DKA with an AUC of 0.95, and a value of ≥ 5.3 mmol/L provided the optimal threshold for diagnosing DKA.
Current diagnostic algorithms for DKA antedated the ability to conventiently and accurately measure blood BOHB concentrations and therefore rely on nonspecific measures of acid-base status and imprecise qualitative urine ketone concentrations. Blood BOHB concentration is a more precise measure of the pathophysiologic disturbance that leads to DKA. The addition of plasma BOHB to the diagnostic criteria of DKA has the potential to improve the accuracy and efficiency of DKA diagnosis and management. In addition to providing a single superior measure of DKA severity, point-of-care measurement might streamline triage practice.
In our model, a BOHB of ≥ 3 mmol/L, suggested by the most recent ISPAD guidelines10, had a positive predictive value of only 66.4% and correctly classified 84% of patients. Considering only patients with moderate or severe DKA, a BOHB of ≥3 mmol/L had a sensitivity and specificity of 100% and 67.7%, respectively; whereas a BOHB of 5.3 mmol/L has a sensitivity of 97.7% and specificity of 87.0%. Using a cut-point of 5.3 mmol/L to define moderate or severe DKA correctly classified 88.5% of patients and, most importantly, had a negative predictive value of 99.6%. Thus, clinicians can have confidence that using a BOHB cut-point of 5.3 mmol/L would correctly recognize the vast majority of patients with moderate or severe DKA.
There is only one published study that evaluated blood BOHB measurement for the diagnosis of DKA in children. Sheikh-Ali et al. retrospectively analyzed encounters in 129 children with DM (mean age 10.8 years; 86 with DKA) and determined that serum BOHB values of 3.0 mmol/L and 4.4 mmol/L corresponded with serum HCO3 of 18 mEq/L and 15 mEq/L, respectively. Diagnostic discordance between the BOHB cutoff and at least one of the conventional diagnostic criteria (serum HCO3, venous pH or plasma glucose) was observed in 15% of children with DKA. When a BOHB level of 3.0 mmol/L was used to define DKA, 25% of children classified as not having DKA had a venous pH ≤ 7.3 (note that pH data were available for only 36 (28%) of subjects).9
Studies in adults presenting to an ED have shown that a blood BOHB level is a significantly more reliable diagnostic marker for DKA than urine ketone concentrations.17,18 In addition, Arora et al. directly compared POC blood BOHB measurements to urine dipstick ketone measurements in a prospective study of 516 adults with hyperglycemia (54 in DKA): the sensitivity of both tests was 98.1%, whereas the specificity of blood BOHB for DKA diagnosis was significantly greater than that of urine ketones (78.6% vs. 35.1%).
Children have a lower extracellular buffering capacity than adults26 and young children with type 1 DM are more prone to ketosis than older children and adolescents.16 Our secondary analyses included an assessment of age-related differences in the ability of BOHB to predict DKA; however, we found no evidence that age group moderated the effect of BOHB.
Reliance on BOHB as the primary measure for DKA has the potential to facilitiate more accurate and efficient diabetes management. Outpatient capillary BOHB measurement with POC devices has been shown to reduce ED visits and hospitalizations.19,20 Additionally, POC measurement may have valuable implications for triage and initiation of efficient care in the ED. Several studies have also documented the usefulness of capillary BOHB measurement for the diagnosis and management of DKA and shown reduced length of stay and cost of treatment.17,21–23
Although superior to urine ketone measurement, POC capillary BOHB measurement has important limitations. In particular, when compared to laboratory measurements of BOHB, POC measurements are less accurate at high BOHB levels. Depending on the device used, loss of linearity and accuracy begins at a BOHB level of ≥5.0 mmol/L to 6.0 mmol/L.14,21,24,25
Depending on the clinical context and patient factors, clinicians may choose a more sensitive or specific BOHB cut-off value. Our study established these test characteristics for a range of plasma BOHB values (Table 2) allowing the clinician to choose a cut-off that balances sensitivity and specificity to suit the clinical situation and best inform management decisions.
This study has a few key limitations. It was performed at a single center with a local referral pattern. Nonetheless, there is no reason to believe that the population of patients seeking emergency care for hyperglycemia at this tertiary care children’s hospital would differ significantly from other populations of patients with diabetes in their relation between ketonemia and acidemia. Furthermore, we compared “simultaneous” laboratory values of BOHB and venous blood gas collected within a 30 minute interval, regardless of order; we suspect the majority of blood samples are collected during the same phlebotomy procedure, but we did not perform a sensitivity analysis across this time interval to understand if this short interval might alter the correlations.
Functionally, the identification of DKA among patients with hyperglycemia triggers a specific protocolized management pathway in the ED— typically including the administration of continuous intravenous insulin along with standardized fluid and electrolye replacement. Existing protocols that rely on pH and HCO3 to define DKA are affected by the patient’s ability to modulate acid-base status through respiratory and renal compensation. We intentionally measured the value of BOHB in the framework of a currently accepted definition of DKA, but it is important to recognize the pathophysiologic process is a continuum of metabolic derangement; therefore future management strategies may use BOHB as the most relevant biomarker of successful treatment as it specifically reflects the severity of the metabolic decompensation resulting from insulin deficiency.
In summary, the widespread use of automated chemistry analyzers now makes it possible for virtually any laboratory to rapidly perform measurements of plasma or serum BOHB concentration. We suggest that the addition of blood BOHB to measurements of acid-base status for refining the diagnostic criteria for DKA is rational as it directly reflects the cause of metabolic decompensation and is not susceptible to additional perturbations of acid-base balance. Prospective evaluation of blood BOHB in other patient populations is needed and may support its use as the primary measure of DKA.
Acknowledgments
Funding Source This project was supported by grant number T32-HS000063 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. Drs. Millington and Tremblay were also supported by NIH Grant T32-DK007699.
Abbreviations
- AUC
area under the curve
- BOHB
β-hydroxybutyrate
- DKA
Diabetic ketoacidosis
- DM
diabetes mellitus
- ED
Emergency Department
- HCO3
plasma bicarbonate
- ISPAD
International Society for Pediatric and Adolescent Diabetes
- NODM
new-onset diabetes mellitus
- POC
point-of-care
Footnotes
Conflict of Interest The authors have no potential conflicts of interest relevant to this article to disclose.
Financial Disclosure The authors have no financial relationships relevant to this article to disclose.
References
- 1.Patterson CC, Guariguata L, Dahlquist G, Soltész G, Ogle G, Silink M. Diabetes in the young – a global view and worldwide estimates of numbers of children with type 1 diabetes. Diabetes Res Clin Pract. 2014;103(2):161–175. doi: 10.1016/j.diabres.2013.11.005 [DOI] [PubMed] [Google Scholar]
- 2.Dabelea D, Rewers A, Stafford JM, et al. Trends in the Prevalence of Ketoacidosis at Diabetes Diagnosis: The SEARCH for Diabetes in Youth Study. Pediatrics. 2014;133(4):e938–e945. doi: 10.1542/peds.2013-2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Usher-Smith JA, Thompson M, Ercole A, Walter FM. Variation between countries in the frequency of diabetic ketoacidosis at first presentation of type 1 diabetes in children: A systematic review. Diabetologia. 2012;55(11):2878–2894. doi: 10.1007/s00125-012-2690-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foster NC, Beck RW, Miller KM, et al. State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016–2018. Diabetes Technol Ther. 2019;21(2):dia.2018.0384. doi: 10.1089/dia.2018.0384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maahs DM, Hermann JM, Holman N, et al. Rates of diabetic ketoacidosis: International comparison with 49,859 pediatric patients with type 1 diabetes from England, Wales, the U.S., Austria, and Germany. Diabetes Care. 2015;38(10):1876–1882. doi: 10.2337/dc15-0780 [DOI] [PubMed] [Google Scholar]
- 6.Klingensmith GJ, Connor CG, Ruedy KJ, et al. Presentation of youth with type 2 diabetes in the Pediatric Diabetes Consortium. Pediatr Diabetes. 2016;17(4):266–273. doi: 10.1111/pedi.12281 [DOI] [PubMed] [Google Scholar]
- 7.Miles J ;Rizza RA ; Haymond MW ; Gerich JE Effects of Acute Insulin Deficiency on Glucose and Ketone Body Turnover in Man: Evidence for the Primacy of Overproduction of Glucose and Ketone Bodies in the Genesis of Diabetic Ketoacidosis. Diabetes. 1980;29(November):926–930. [DOI] [PubMed] [Google Scholar]
- 8.Laffel L Ketone bodies: A review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev. 1999;15(6):412–426. http://doi.wiley.com/10.1002/%28SICI%291520-7560%28199911/12%2915%3A6%3C412%3A%3AAID-DMRR72%3E3.0.CO%3B2-8. [DOI] [PubMed] [Google Scholar]
- 9.Sheikh-Ali M, Karon BS, Basu A, et al. Can serum β-hydroxybutyrate be used to diagnose diabetic ketoacidosis? Diabetes Care. 2008;31(4):643–647. doi: 10.2337/dc07-1683 [DOI] [PubMed] [Google Scholar]
- 10.Wolfsdorf JI, Glaser N, Agus M, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. 2018;19:155–177. doi: 10.1111/pedi.12701 [DOI] [PubMed] [Google Scholar]
- 11.Adrogué HJ ; Wilson H ; Boyd AE ; Suki WN ; Eknoyan G Plasma Acid-Base Patterns in Diabetic Ketoacidosis. N Engl J Med. 1982;306(7):424–426. doi: 10.11s11/j.1369-1600.2011.00418.x [DOI] [PubMed] [Google Scholar]
- 12.Lim K, Walsh C. Diabetic ketoalkalosis: a readily misdiagnosed entity. Br Med J. 1976;2(6026):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox K, Cocchi MN, Salciccioli JD, Carney E, Howell M, Donnino MW. Prevalence and significance of lactic acidosis in diabetic ketoacidosis. J Crit Care. 2012;27(2):132–137. doi: 10.1016/j.jcrc.2011.07.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Misra S, Oliver NS. Utility of ketone measurement in the prevention, diagnosis and management of diabetic ketoacidosis. Diabet Med. 2015;32(1):14–23. doi: 10.1111/dme.12604 [DOI] [PubMed] [Google Scholar]
- 15.Wolfsdorf JI. The International Society of Pediatric and Adolescent Diabetes guidelines for management of diabetic ketoacidosis: Do the guidelines need to be modified? Pediatr Diabetes. 2014;15(4):277–286. doi: 10.1111/pedi.12154 [DOI] [PubMed] [Google Scholar]
- 16.Wadwa RP, Chase HP, Raghinaru D, et al. Ketone production in children with type 1 diabetes, ages 4–14 years, with and without nocturnal insulin pump suspension. Pediatr Diabetes. 2017;18(6):422–427. doi: 10.1111/pedi.12410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taboulet P, Haas L, Porcher R, et al. Urinary acetoacetate or capillary β-hydroxybutyrate for the diagnosis of ketoacidosis in the Emergency Department setting. Eur J Emerg Med. 2004;11(5):251–258. doi: 10.1097/00063110-200410000-00003 [DOI] [PubMed] [Google Scholar]
- 18.Taboulet P, Deconinck N, Thurel A, et al. Correlation between urine ketones (acetoacetate) and capillary blood ketones (3-beta-hydroxybutyrate) in hyperglycaemic patients. Diabetes Metab. 2007;33(2):135–139. doi: 10.1016/j.diabet.2006.11.006 [DOI] [PubMed] [Google Scholar]
- 19.Weber C, Kocher S, Neeser K, Joshi SR. Prevention of diabetic ketoacidosis and self-monitoring of ketone bodies: an overview. Curr Med Res Opin. 2009;25(5):1197–1207. doi: 10.1185/03007990902863105 [DOI] [PubMed] [Google Scholar]
- 20.Laffel LM, Wentzell K, Loughlin C, Tovar A, Moltz K, Brink S. Sick day management using blood 3-hydroxybutyrate (3-OHB) compared with urine ketone monitoring reduces hospital visits in young people with T1DM: a randomized clinical trial. Diabet Med. 2006;23(3):278–284. doi: 10.1111/j.1464-5491.2005.01771.x [DOI] [PubMed] [Google Scholar]
- 21.Rewers A, McFann K, Chase HP. Bedside Monitoring of Blood β -Hydroxybutyrate Levels in the Management of Diabetic Ketoacidosis in Children. Diabetes Technol Ther. 2006;8(6):671–676. doi: 10.1089/dia.2006.8.671 [DOI] [PubMed] [Google Scholar]
- 22.Naunheim R, Jang TJ, Banet G, Richmond A, McGill J. Point-of-care Test Identifies Diabetic Ketoacidosis at Triage. Acad Emerg Med. 2006;13(6):683–685. doi: 10.1197/j.aem.2006.01.020 [DOI] [PubMed] [Google Scholar]
- 23.Klocker AA, Phelan H, Twigg SM, Craig ME. Blood β-hydroxybutyrate vs. urine acetoacetate testing for the prevention and management of ketoacidosis in Type 1 diabetes: A systematic review. Diabet Med. 2013;30(7):818–824. doi: 10.1111/dme.12136 [DOI] [PubMed] [Google Scholar]
- 24.Yu HYE, Agus M, Kellogg MD. Clinical utility of Abbott Precision Xceed Pro® ketone meter in diabetic patients. Pediatr Diabetes. 2011;12(7):649–655. doi: 10.1111/j.1399-5448.2011.00768.x [DOI] [PubMed] [Google Scholar]
- 25.Ham MR, Okada P, White PC. Bedside ketone determination in diabetic children with hyperglycemia and ketosis in the acute care setting. Pediatr Diabetes. 2004;5(1):39–43. doi: 10.1111/j.1399-543X.2004.00032.x [DOI] [PubMed] [Google Scholar]
- 26.Cassels DE, Morse M. Arterial blood gases and acid-base balance in normal children. J Clin Invest. 1953;32(9):824–836. doi: 10.1172/JCI102799 [DOI] [PMC free article] [PubMed] [Google Scholar]