Abstract
Background
Prenatal exposures to ambient air pollution and traffic have been associated with adverse birth outcomes, and may also lead to an increased risk of obesity. Obesity risk may be reflected in changes in body composition in infancy.
Objective
To estimate associations between prenatal ambient air pollution and traffic exposure, and infant weight and adiposity in a Colorado-based prospective cohort study.
Methods
Participants were 1,125 mother-infant pairs with term births. Birth weight was recorded from medical records and body composition measures (fat mass, fat-free mass, and adiposity [percent fat mass]) were evaluated via air displacement plethysmography at birth (n=951) and at ~5 months (n=574). Maternal residential address was used to calculate distance to nearest roadway, traffic density, and ambient concentrations of fine particulate matter (PM2.5) and ozone (O3) via inverse-distance weighted interpolation of stationary monitoring data, averaged by trimester and throughout pregnancy. Adjusted linear regression models estimated associations between exposures and infant weight and body composition.
Results
Participants were urban residents and diverse in race/ethnicity and socioeconomic status. Average ambient air pollutant concentrations were generally low; the median, interquartile range (IQR), and range of third trimester concentrations were 7.3 μg/m3 (IQR: 1.3, range: 3.3–12.7) for PM2.5 and 46.3 ppb (IQR: 18.4, range: 21.7–63.2) for 8-hour maximum O3. Overall there were few associations between traffic and air pollution exposures and infant outcomes. Third trimester O3 was associated with greater adiposity at follow-up (2.2% per IQR, 95% CI 0.1, 4.3), and with greater rates of change in fat mass (1.8g/day, 95% CI 0.5, 3.2) and adiposity (2.1%/100 days, 95% CI 0.4, 3.7) from birth to follow-up.
Conclusions
We found limited evidence of an association between prenatal traffic and ambient air pollution exposure and infant body composition. Suggestive associations between prenatal ozone exposure and early postnatal changes in body composition merit further investigation.
Keywords: Air pollution, traffic, pregnancy, infant, adiposity
1. Introduction1
Environmental exposures during the prenatal period are believed to be important influences on child growth and metabolic disease risk later in life (1–4). Higher concentrations of certain ambient air pollutants during pregnancy, including particulate matter with a diameter of ≤2.5 micrometers (PM2.5) and ozone (O3), have been associated with lower infant birth weight (5–22). Low birth weight and subsequent rapid growth have, in turn, been linked to greater risk of obesity and cardio-metabolic disease in childhood and adulthood (23–26).
Motor vehicle traffic is one of many sources of particulate and gaseous air pollutants in the urban environment, and includes PM2.5, nitrogen oxides, and volatile organic compounds that contribute to ozone formation (27). A limited number of human studies indicate that prenatal exposure to traffic and ambient air pollution may influence infant growth and body composition, with consequences for child obesity risk and related metabolic health (28–33). The plausibility of this hypothesis is supported by findings that mice prenatally exposed to a real-world mixture of air pollutants demonstrated greater body weight, inflammation, oxidative stress, and dyslipidemia compared to mice exposed to the same mixture filtered to remove particulate matter (34). Moreover, combustion-derived black carbon particles have been detected on the fetal side of human placentae at delivery, with particle counts correlated with maternal residential black carbon exposure during pregnancy (35). An analogy may also be drawn to prenatal tobacco smoke exposure, which leads to lower weight at birth but is associated with greater risk of obesity later in life (36, 37).
Many previous studies of exposure to ambient air pollution or traffic and infant or child weight and adiposity have been conducted in relatively high exposure settings, such as the northeast US (6, 29, 30, 32), southern California (5, 12, 38, 39), and urban areas of China (40, 41). Particulate matter concentrations in the Denver metropolitan area are relatively low (42, 43), but pollutant levels vary by season and by proximity to major roadways (44–47). Previous studies in other major cities have shown that traffic-related spatial variables may explain a large percentage of the intra-urban variation in annual average fine particulate matter (48). However, the Mountain West region where Denver is located is relatively understudied, and the composition of PM2.5 varies both regionally and seasonally (49), suggesting that studies in diverse geographic areas are needed.
In addition, most previous studies of prenatal exposure to air pollution have used only birth weight as an outcome, while a few studies have examined infant adiposity via ponderal index (50) or skinfold thicknesses (33). Infant body composition (fat mass relative to fat-free mass) may be more important than weight in predicting the risk of excess adiposity later in life; for example, one large study demonstrated that ponderal index at birth (an indirect measure of adiposity) but not birth weight was positively associated with child fat-to-lean mass ratio at age 9–10 years old (51). Air displacement plethysmography is an accurate and non-invasive method to calculate body density and estimate adiposity (52, 53), and was used to evaluate body composition among infants in the Colorado-based Healthy Start cohort.
We aimed to estimate associations between prenatal exposure to traffic and ambient air pollution and infant weight and body composition at birth and at a 5-month follow-up, and to evaluate whether child sex may modify such associations. We hypothesized that prenatal exposure to traffic and ambient air pollutants would be associated with reduced weight and adiposity at birth, but greater adiposity later in infancy.
2. Subjects and Methods
2.1. Study population and eligibility
Pregnant women were recruited from outpatient obstetrics clinics at the University of Colorado Hospital prior to 24 completed weeks of gestation. Eligibility criteria were singleton pregnancies to women with no history of stillbirth or preterm birth at less than 25 weeks of gestation, and no chronic medical conditions including diabetes, cancer, asthma managed with steroid medications, or mental illness. A total of 1,410 pregnant women enrolled in the Healthy Start study (2009–2014) and 11 of these withdrew or were lost to follow-up prior to delivery. Study participants were invited to participate in four in-person research visits: two visits during pregnancy (at median gestational ages of 17 and 27 weeks), a third visit shortly after delivery, and a fourth visit when the child was approximately 5 months old.
Additional criteria for exclusion from this analysis were as follows: fetal demise (n=19), residence outside of the Denver, Colorado metropolitan area (defined here as Census 2010 Urbanized Areas within Adams, Arapahoe, Boulder, Broomfield, Denver, Douglas, and Jefferson counties [Supplemental Figure 1]; n=44), no address that could be geographically located (n=57), and preterm birth (n=78). From these 1,203 potentially eligible participants, we additionally excluded those missing the essential covariates of gestational age at birth (n=69) and child sex (n=9), leading to an eligible sample size of 1,125 mother-infant pairs for the analysis of term birth weight. For the analysis of adiposity at birth, participants were additionally excluded if they did not have body composition measurements within 3 days of birth (n=174), and therefore the sample size was 951 mother-infant pairs. For the analysis of adiposity at 5 months of age, 352 participants were missing body composition data at follow-up and 25 were missing information on exclusive breastfeeding, leading to a sample size of 574 mother-infant pairs.
2.2. Exposure assessment
Residential address was self-reported by participants via questionnaire at the time of enrollment in the Healthy Start study. Addresses were geocoded using ArcGIS Desktop 10.1, and all initially unmatched addresses were manually checked for errors. After this correction step, 96% of potentially eligible participants were successfully matched to geographic locations within the study area using the U.S. Census TIGER/Line shapefiles from 2016 and 2011.
Based on the geographic location of the enrollment address, two measures of traffic exposure (29) were calculated: (1) distance to the nearest major roadway, calculated separately for interstates/limited access highways and for other major and minor arterial and connector roads, and (2) traffic density on major roads (including interstates, highways and arterial and connector roads) within a specified buffer distance of the residence. Stationary monitors in the United States Environmental Protection Agency (US EPA) Air Quality System (AQS) were used to estimate outdoor exposure to PM2.5 and O3 near the residential address, as described in detail below. All layers were projected into the Universal Transverse Mercator coordinate system, Zone 13N for analysis.
2.2.1. Distance to nearest major roadway
For the distance to roadway measures, distance was calculated separately for interstates and limited access highways (according to Colorado Department of Transportation application of Federal Highway Administration functional classifications) and for other major and minor arterial and connector roads, using shapefiles obtained from the Colorado Department of Transportation. The ArcGIS “near” tool was used to determine the shortest Euclidean distance in meters from the geocoded enrollment residence to the closest point on the roadway. Distance to roadway was categorized both as quartiles and binary according to categories (<150m from a highway or <50m from another major roadway) employed in previous studies (7, 54).
2.2.2. Traffic density near residence
Circular buffers were created around each residential location with the following radii: 150m, 250m, 500m, and 1000m. The major roadway segments within each buffer were identified using ArcGIS Desktop 10.1. Buffer distances were selected based on previous literature and empirically determined distances of diffusion of traffic-related particles from roadways (55, 56). For a given participant, we applied the Colorado Department of Transportation’s estimate of average daily vehicle count for a given roadway segment for the year of the child’s birth. For each road segment, the contribution to traffic density was calculated as the average daily vehicle count multiplied by the length of the segment within the buffer (29). For each participant address, the total traffic density within the buffer (vehicles/day x meters of roadway) was calculated as the sum of contributions from each intersecting road segment with average daily vehicle count data available.
2.2.3. Inverse-distance-weighted average of PM2.5 concentrations
We identified and geocoded the locations of 10 stationary monitors measuring 24-hour average PM2.5 during the study period and located within 50km of at least one study participant (Supplemental Figure 1). Data were obtained from the Colorado Department of Public Health and Environment and the US EPA AQS Data Mart. For each monitor, daily PM2.5 values were averaged over each trimester for each conception date among study participants. Conception date was calculated as the date of delivery minus gestational age at birth in days. The three trimesters were defined as days 0–91, days 92–182, and days 183 to delivery. Daily values were also averaged over the full pregnancy (day 0 to delivery). Averages from a given monitor were included if at least 75% of expected values were non-missing (57, 58). Most monitors reported PM2.5 data every 3 days, but other monitors reported every 1 day or every 6 days; therefore the expected completeness varied by monitor. All monitors with non-missing data located within 50km of a residence were used to determine average PM2.5 exposure. Trimester or full pregnancy averages from each monitor were weighted in calculating the average exposure at a given residence according to the formula 1/distance-squared (59).
2.2.4. Inverse-distance-weighted average of O3 concentrations
We identified and geocoded the locations of 19 stationary monitors measuring hourly O3 during the study period and located within 50km of at least one study participant (Supplemental Figure 1). Hourly values were averaged over each 8-hour interval during a 24-hour period for each monitor, and daily 8-hour maximum values were averaged over each trimester and full pregnancy for each conception date among study participants, as described above for PM2.5. Averages from a given monitor were included if at least 75% of expected values were non-missing. All monitors with non-missing data within 50 km of the residence were used to determine inverse-distance weighted average O3 for each participant.
2.3. Outcome assessment
Birth weight was obtained from medical records. Infant fat mass, fat-free mass, and adiposity at birth and at approximately 5 months of age were measured by trained research staff at the Children’s Hospital of Colorado using the PEAPOD device (COSMED, Rome, Italy). The PEAPOD uses air displacement plethysmography to calculate body density, which is used to estimate fat mass and fat-free mass (52). Infants were unclothed and wore a tight spandex cap over their hair for body composition assessment. Body composition was assessed up to three times in each infant and the mean of the two closest measurements was used in this study. Adiposity was calculated as a percentage: fat mass divided by total body mass, x 100%. The mid-infancy adiposity assessment was added to the study protocol in 2010 and therefore does not include the earliest births in the study. For infants with body composition measured at both time points, we additionally calculated the rate of change in each body composition measure. For example, the rate of change in fat mass was calculated as: (fat mass at 5 months – fat mass at birth) / age in days at follow-up visit.
2.4. Other variables
Pregnant women enrolled in the Healthy Start study self-reported their age, race/ethnicity, education completed, and number of previous pregnancies (gravidity) via questionnaire at study enrollment. Maternal smoking during pregnancy was assessed via self-report at two study visits during pregnancy and again shortly after delivery. Maternal pre-pregnancy body mass index was calculated based on measured height at the first study visit, and pre-pregnancy weight obtained from the medical record (87%) or, if unavailable, from self-report at study enrollment (13%). Gestational weight gain was calculated as the difference between the last recorded weight during pregnancy and the pre-pregnancy weight. Infant sex and gestational age at birth were obtained from the delivery medical record. Census tract-level socioeconomic data including the median income and percentage of persons below the poverty level were obtained from the 2012–2016 American Community Survey, and participant addresses were identified as located within a given Census tract by joining spatial data in ArcGIS Desktop 10.1. At the 5-month follow-up visit, mothers reported via questionnaire whether they were currently feeding their infant breast milk and whether the infant had ever received any formula. This information was used to create a binary variable for exclusive breastfeeding up to the follow-up visit.
Hourly temperature data was recorded at 16 monitoring locations within the study area and hourly values were averaged over each 24-hour period. As described above for PM2.5 and O3, 24-hour averages were averaged over each trimester for each conception date among study participants. Averages from a given monitor were included if at least 75% of expected values were non-missing. Data from all monitors within 50 km of a residence were used to calculate distance-weighted average temperature for each participant for each trimester or across the full pregnancy.
2.5. Statistical analysis
The distributions of all variables were examined using histograms or frequency tables. Box plots were generated to examine the variability in ambient air pollution measures. The traffic density and distance to roadway variables were highly skewed and were categorized into quartiles for analysis to reduce the influence of outliers. Bivariate associations between covariates and outcomes, and covariates and exposures, were examined using box plots, scatter plots, linear regression and analysis of variance. Pairwise Spearman correlations were examined between all exposure variables.
The associations between prenatal air pollution and traffic exposures and infant weight and adiposity were evaluated using separate linear regression models, adjusted for potential confounders. Adjusted associations between continuous PM2.5 and O3 exposure variables and primary outcomes were additionally modeled using generalized additive models with spline transformations to evaluate the presence of non-linearity in each exposure-outcome relationship, and subsequently presented as quartiles of exposure if significant non-linearity was detected.
Potential confounders were selected based on previously identified predictors of infant weight and adiposity in this cohort (60, 61) and via the construction of directed acyclic graphs. All models were adjusted for the following covariates: maternal age (years), pre-pregnancy body mass index (kg/m2), gestational weight gain (kg), race/ethnicity (4 categories: non-Hispanic white, Hispanic, non-Hispanic African-American, and all others combined), maternal education (5 categories: less than high school, completed high school or equivalent, some college, 4-year college, graduate degree), smoking during pregnancy (any vs none), gravidity (any vs no previous pregnancies), infant sex, and percentage of persons in the Census tract with income below the poverty level (as quartiles).
Models for birth weight and adiposity at birth were additionally adjusted for gestational age at birth (days). Adiposity models were additionally adjusted for age in days at the body composition assessment, and models for adiposity at 5-month follow-up were adjusted for exclusive breastfeeding to the follow-up visit (yes/no). Models using average PM2.5 or O3 as an exposure were also adjusted for season of birth (indicator variable for winter, spring, summer, fall), year of birth, the other ambient air pollutant (PM2.5 or O3), and average temperature during the specified pregnancy period (quartiles). Effect modification by infant sex was evaluated by including a product interaction term between sex and the exposures in all fully adjusted models, and results were presented stratified by sex if any exposure-by-sex interaction term for a given exposure was significant at p<0.05 (indicating a departure from additivity) (62).
In addition to the primary outcomes of birth weight, adiposity at birth, and adiposity at 5-month follow-up, the following secondary outcomes were evaluated in linear regression models parallel to the ones described above: fat mass and fat-free mass at birth, fat mass and fat-free mass at 5-month follow-up, and the rates of change in fat mass, fat-free mass, and adiposity from birth to follow-up.
Because ambient air pollution exposure was not directly measured at the maternal residence but rather interpolated from a distance-weighted average of values recorded at fixed monitoring stations, we evaluated the potential for measurement error due to spatial heterogeneity in the study area by examining the agreement between trimester-long average concentrations recorded at fixed monitoring stations. Specifically, for each participant with at least 3 monitors contributing to a trimester-long weighted average exposure, the intra-class correlation coefficient (ICC) for that trimester was calculated among the three closest monitors. The ICC was interpreted as the degree of spatial homogeneity of each pollutant in the study area.
Analyses were conducted in R version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria) and SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
3. Results
Characteristics of the study participants did not differ notably between the subset with 5-month follow-up data (n=574) and the full sample (n=1,125) with the exception of birth year; this difference was expected because the follow-up visit was added to the protocol one year after the study start date, so births in 2010 were under-represented in the 5-month follow-up group (Table 1). Participants were racially and ethnically diverse and generally represented the composition of the east Denver-Aurora urban area, with 54% non-Hispanic white, 25% Hispanic, 15% African American, and 6% of participants from all other races and ethnic groups combined. Approximately 46% of participants completed a four-year college and/or graduate degree. Characteristics of the 1,125 participants included in this analysis did not differ from the 1,410 enrolled participants in the Healthy Start study, except for minor differences in characteristics related to the exclusion of preterm births (Supplemental Table 1).
Table 1.
Characteristics of eligible mothers and infants enrolled in the Healthy Start study, 2009–2014, and included in this analysis.
| Birthweight (N=1,125) | Adiposity at birth (n=951) | Adiposity at follow-up (n=574) | |
|---|---|---|---|
| Characteristic | Mean ± SD, Median (25th–75th percentile), or N (%) | ||
| Covariates | |||
| Maternal age (years) | 27.9 ± 6.2 | 27.9 ± 6.1 | 28.5 ± 6.1 |
| Pre-pregnancy body mass index (kg/m2) | 25.7 ± 6.2 | 25.7 ± 6.2 | 25.7 ± 6.3 |
| Normal weight/underweight (16.00–24.99) | 617 (55) | 520 (55) | 308 (54) |
| Overweight (25.00–29.99) | 282 (25) | 241 (25) | 155 (27) |
| Obese (30.00–65.41) | 226 (20) | 190 (20) | 111 (19) |
| Race/ethnicity | |||
| Non-Hispanic white | 604 (54) | 507 (53) | 318 (55) |
| Hispanic | 284 (25) | 239 (25) | 143 (25) |
| Non-Hispanic black | 170 (15) | 148 (16) | 80 (14) |
| All others | 67 (6) | 57 (6) | 33 (6) |
| Maternal education completed | |||
| Less than high school | 153 (14) | 128 (13) | 69 (12) |
| High school or equivalent | 204 (18) | 174 (18) | 84 (15) |
| Some college | 252 (22) | 215 (23) | 142 (25) |
| Four year college | 261 (23) | 220 (23) | 133 (23) |
| Graduate degree | 255 (23) | 214 (23) | 146 (25) |
| Year of birth | |||
| 2010 | 132 (12) | 123 (13) | 19 (3) |
| 2011 | 238 (21) | 205 (22) | 85 (15) |
| 2012 | 292 (26) | 243 (26) | 139 (24) |
| 2013 | 290 (26) | 241 (25) | 207 (36) |
| 2014 | 173 (15) | 139 (15) | 124 (22) |
| Season of birth | |||
| Winter | 249 (22) | 205 (22) | 138 (24) |
| Spring | 267 (24) | 231 (24) | 148 (26) |
| Summer | 338 (30) | 289 (30) | 147 (26) |
| Fall | 271 (24) | 226 (24) | 141 (25) |
| Maternal smoking during pregnancy (any) | 94 (8) | 80 (8) | 35 (6) |
| Previous pregnancies (any) | 737 (66) | 619 (65) | 374 (65) |
| Gestational weight gain (kg) | 13.9 ± 6.5 | 14.1 ± 6.4 | 13.7 ± 6.4 |
| Infant sex = male | 596 (53) | 492 (52) | 284 (49) |
| Gestational age at birth (days) | 277.1 ± 7.6 | 277.1 ± 7.6 | 277.3 ± 7.7 |
| Age at follow-up visit (days) | -- | -- | 155 ± 37 |
| Exclusively breastfed to follow-up visit | -- | -- | 218 (38) |
| Median income in Census tract (in $1000s) | 56.8 (44.8–83.6) | 56.7 (44.2–83.6) | 56.8 (44.1–84.1) |
| Persons below poverty level in Census tract (%) | 12.5 (6.8–23.1) | 12.8 (6.8–23.1) | 12.4 (6.6–22.5) |
| Outcomes | |||
| Birth weight (g) | 3286 ± 432 | 3290 ± 430 | 3280 ± 423 |
| Fat mass at birth (g) | -- | 293 ± 149 | 288 ± 140 |
| Fat free mass at birth (g) | -- | 2848 ± 330 | 2836 ± 324 |
| Adiposity at birth (%) | -- | 9.1 ± 3.9 | 9.0 ± 3.7 |
| Fat mass at follow-up visit (g) | -- | -- | 1677 ± 502 |
| Fat-free mass at follow-up visit (g) | -- | -- | 5146 ± 620 |
| Adiposity at follow-up visit (%) | -- | -- | 24.3 ± 5.4 |
| Rate of change in fat mass (g/day) | -- | -- | 9.3 ± 3.6 |
| Rate of change in fat-free mass (g/day) | -- | -- | 15.2 ± 3.1 |
| Rate of change in adiposity (%/100 days) | -- | -- | 10.4 ± 4.7 |
| Exposures | |||
| Trimester 1 average PM2.5 (μg/m3)a | 7.47 (6.80–8.13) | 7.48 (6.83–8.15) | 7.47 (6.81–8.09) |
| Trimester 2 average PM2.5 (μg/m3)b | 7.36 (6.76–8.06) | 7.36 (6.78–8.06) | 7.40 (6.78–8.04) |
| Trimester 3 average PM2.5 (μg/m3)c | 7.29 (6.74–8.06) | 7.29 (6.74–8.05) | 7.38 (6.75–8.21) |
| Full pregnancy average PM2.5 (μg/m3) | 7.45 (7.01–7.82) | 7.45 (7.02–7.83) | 7.46 (7.07–7.78) |
| Trimester 1 average 8-hr max O3 (ppb) | 41.9 (33.1–52.7) | 41.8 (32.9–52.6) | 44.1 (33.3–53.6) |
| Trimester 2 average 8-hr max O3 (ppb) | 43.6 (34.1–52.6) | 42.8 (33.9–52.3) | 43.4 (33.8–52.8) |
| Trimester 3 average 8-hr max O3 (ppb) | 46.3 (35.3–53.7) | 46.2 (35.5–53.7) | 44.7 (34.5–53.5) |
| Full pregnancy average 8-hr max O3 (ppb) | 44.0 (40.9–46.8) | 43.8 (40.8–46.8) | 44.0 (40.9–46.8) |
| Distance to highway (m) | 842 (396–1705) | 840 (396–1739) | 855 (410–1769) |
| Distance to non-highway major roadway (m) | 172 (72–321) | 171 (72–314) | 168 (70–312) |
| Traffic density <150m (vehicles*km/day) | 0 (0–2791) | 29 (0–2810) | 197 (0–2379) |
| Traffic density <250m (vehicles*km/day) | 3049 (0–9441) | 3276 (0–9494) | 3159 (0–9238) |
| Traffic density <500m (vehicles*km/day) | 23186 (9372–42615) | 23478 (8891–42485) | 22612 (8619–41102) |
| Traffic density <1000m (vehicles*km/day) | 111805 (63577–159922) | 112345 (64710–161232) | 111770 (62932–162629) |
Abbreviations: O3, ozone; PM2.5, particulate matter with diameter ≤ 2.5 micrometers.
Trimester 1 missing data due to >25% missing monitor values: 73 for birthweight, 61 for adiposity at birth, 40 for adiposity at follow-up.
Trimester 2 missing data due to >25% missing monitor values: 76 for birthweight, 64 for adiposity at birth, 33 for adiposity at follow-up.
Trimester 3 missing data due to >25% missing monitor values: 20 for birthweight, 17 for adiposity at birth, 10 for adiposity at follow-up.
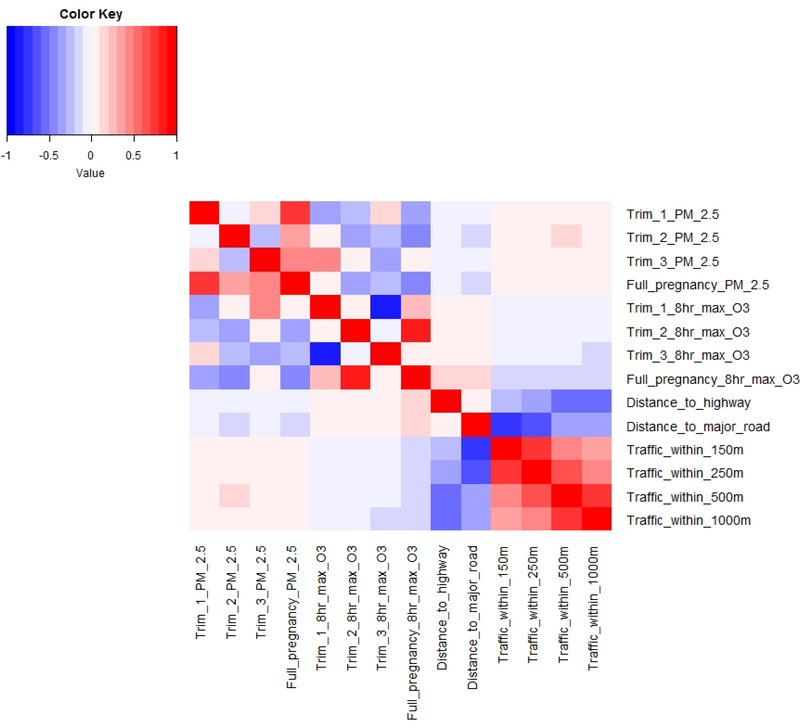
Trimester and full pregnancy average concentrations of ambient air pollutants at the maternal residence were generally low and particularly PM2.5 showed limited variability in the interquartile range (Table 1, Supplemental Figure 2). Exposures to ambient air pollutants and traffic did not differ meaningfully by analytic sub-group (Table 1). Spearman correlations between air pollution exposure metrics were generally as expected, given that seasons and trimesters of pregnancy last approximately 3 months and therefore intra-individual variability is expected to partially reflect seasonal differences in PM2.5 and O3 within the study area. There was a weak positive correlation between average daily PM2.5 in trimesters 1 and 3 (rs=0.19), while trimesters 1 and 2 were uncorrelated (rs= −0.05) and trimesters 2 and 3 were inversely correlated (rs= −0.27) (Figure 1). For average 8-hour maximum O3, trimesters 1 and 2 were largely uncorrelated (rs=0.07), trimesters 1 and 3 were strongly inversely correlated (rs= −0.82), likely reflecting the difference between summer and winter O3 levels, and trimesters 2 and 3 were uncorrelated (rs= −0.04). Average PM2.5 and O3 measures by trimester or across the full pregnancy were generally weakly correlated or uncorrelated with traffic density and distance to roadway measurements (rs from −0.17 to 0.15).
Figure 1.
Pairwise Spearman correlations between measures of prenatal exposure to traffic and ambient air pollution among 1,125 eligible mother-infant pairs.
Average PM2.5 exposures by trimester or throughout pregnancy were not associated with birth weight or adiposity in models pooled by infant sex (Table 2). Significant interaction between the exposure and infant sex was observed for first trimester PM2.5 and 5-month adiposity only. In female infants, first trimester PM2.5 was inversely associated with adiposity at the 5-month follow-up visit (−2.05% per IQR increase in PM2.5, 95% CI −3.31, −0.79), while in male infants the association was positive but non-significant (1.13% per IQR increase in PM2.5, 95% CI −0.15, 2.40). Similarly, among females only, first trimester PM2.5 was inversely associated with fat mass but not with fat-free mass at follow-up (Supplemental Table 2), and also inversely associated with the rate of change in fat mass and adiposity, but not fat-free mass, between birth and the follow-up visit (Supplemental Table 3).
Table 2.
Associations between average PM2.5 at the residential address during pregnancy and infant weight and adiposity outcomes.
| Birth weight (n=1,125)a | Adiposity at birth (n=951)b | Adiposity at 5-month follow-up (n=574)c | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Time interval of PM2.5 average | Difference (g) per IQRd and 95% CI | p-value for sex interaction | Difference (%) per IQRd and 95% CI | p-value for sex interaction | Difference (%) per IQRd and 95% CI | p-value for sex interaction | |||
| Both sexes | |||||||||
| Trimester 1 | 1.8 | (−41.6, 45.2) | 0.48 | 0.11 | (−0.37, 0.60) | 0.78 | −0.49 | (−1.35, 0.37) | 0.01 |
| Trimester 2 | 24.3 | (−13.0, 61.7) | 0.76 | 0.14 | (−0.27, 0.56) | 0.47 | −0.04 | (−0.82, 0.73) | 0.43 |
| Trimester 3 | −20.5 | (−57.0, 16.0) | 0.59 | −0.08 | (−0.47, 0.32) | 0.28 | 0.24 | (−0.43, 0.90) | 0.62 |
| Full pregnancy | −5.5 | (−49.0, 37.9) | 0.39 | 0.21 | (−0.28, 0.69) | 0.67 | 0.12 | (−0.81, 1.05) | 0.26 |
| Males only | |||||||||
| Trimester 1 | 13.0 | (−49.2, 75.2) | 0.27 | (−0.40, 0.94) | 1.13 | (−0.15, 2.40) | |||
| Trimester 2 | 3.9 | (−50.6, 58.3) | −0.03 | (−0.60, 0.54) | 0.26 | (−0.88, 1.41) | |||
| Trimester 3 | −19.6 | (−70.9, 31.7) | −0.17 | (−0.69, 0.35) | −0.24 | (−1.12, 0.63) | |||
| Full pregnancy | −19.1 | (−81.1, 42.9) | −0.14 | (−0.79, 0.52) | 1.23 | (−0.10, 2.56) | |||
| Females only | |||||||||
| Trimester 1 | −20.2 | (−82.3, 41.9) | −0.11 | (−0.84, 0.63) | −2.05 | (−3.31, −0.79) | |||
| Trimester 2 | 50.0 | (−3.4, 103.3) | 0.23 | (−0.41, 0.86) | 0.24 | (−0.88, 1.36) | |||
| Trimester 3 | −32.1 | (−85.6, 21.3) | 0.01 | (−0.62, 0.65) | 0.90 | (−0.17, 1.97) | |||
| Full pregnancy | −8.0 | (−69.8, 53.7) | 0.40 | (−0.33, 1.13) | −0.43 | (−1.83, 0.97) | |||
Abbreviations: CI, confidence interval; IQR, interquartile range; O3, ozone; PM2.5, particulate matter with diameter ≤ 2.5 micrometers.
Model 1: Adjusted for maternal age, pre-pregnancy body mass index, gestational weight gain, education, race/ethnicity, smoking during pregnancy, gravidity, infant sex, gestational age at birth, year of birth, season of birth, average temperature and average 8-hour maximum O3 concentration during the specified pregnancy period, and percentage of persons below the poverty level in the Census tract of residence.
Model 2: Adjusted for Model 1 variables and infant age in days at neonatal body composition measurement.
Model 3: Adjusted for Model 1 variables except gestational age at birth, and additionally adjusted for infant age in days at follow-up and exclusive breastfeeding.
IQRs for PM2.5: Trimester 1, 1.33 μg/m3; Trimester 2, 1.30 μg/m3; Trimester 3, 1.32 μg/m3; Full pregnancy, 0.82 μg/m3.
Average 8-hour maximum O3 was not associated with weight or adiposity at birth (Table 3). However, a positive association was observed between average 8-hour maximum O3 in the third trimester and adiposity at the 5-month follow-up (2.16% per IQR increase in O3, 95% CI 0.07, 4.26). There were no significant interactions with infant sex. While generalized additive models indicated some departures from linearity for O3 models, analysis of O3 as quartiles rather than continuous exposures did not reveal any strong non-linear trends (Supplemental Table 4). Secondary outcomes of fat mass and fat-free mass and rate of change in body composition were generally consistent with the main analysis: third trimester O3 was positively associated with fat mass but not fat-free mass at follow-up (Supplemental Table 5), and associated with a greater rate of change in fat mass and adiposity from birth to the 5-month follow-up visit (Supplemental Table 6).
Table 3.
Associations between average 8-hour daily maximum O3 at the residential address during pregnancy and infant weight and adiposity outcomes.
| Birth weight (n=1,125)a | Adiposity at birth (n=951)b | Adiposity at 5-month follow-up (n=574)c | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Time interval of O3 average | Difference (g) per IQRdand 95% CI | p-value for sex interaction | Difference (%) per IQRd and 95% CI | p-value for sex interaction | Difference (%) per IQRdand 95% CI | p-value for sex interaction | |||
| Both sexes | |||||||||
| Trimester 1 | 24.5 | (−100.7, 149.8) | 0.61 | 1.05 | (−0.36, 2.46) | 0.61 | −0.69 | (−3.11, 1.74) | 0.97 |
| Trimester 2 | −18.3 | (−140.5, 103.9) | 0.38 | 0.24 | (−1.11, 1.59) | 0.69 | 0.10 | (−2.33, 2.54) | 0.38 |
| Trimester 3 | 20.3 | (−88.0, 128.7) | 0.31 | 0.40 | (−0.80, 1.60) | 0.96 | 2.16 | (0.07, 4.26) | 0.53 |
| Full pregnancy | −1.0 | (−63.0, 61.0) | 0.81 | 0.58 | (−0.11, 1.26) | 0.75 | 0.31 | (−0.86, 1.47) | 0.73 |
Abbreviations: CI, confidence interval; IQR, interquartile range; O3, ozone; PM2.5, particulate matter with diameter ≤ 2.5 micrometers.
Model 1: Adjusted for maternal age, pre-pregnancy BMI, gestational weight gain, education, race/ethnicity, smoking during pregnancy, gravidity, infant sex, gestational age at birth, year of birth, season of birth, average temperature and average daily PM2.5 concentration during the specified pregnancy period, and percentage of persons below the poverty level in the Census tract of residence.
Model 2: Adjusted for Model 1 variables and infant age in days at neonatal body composition measurement.
Model 3: Adjusted for Model 1 variables except gestational age at birth, and additionally adjusted for infant age in days at follow-up and exclusive breastfeeding.
IQRs for O3: Trimester 1, 19.6 ppb; Trimester 2, 18.6 ppb; Trimester 3, 18.4 ppb; Full pregnancy, 5.9 ppb.
There were no consistent associations between traffic indicators and infant weight and adiposity outcomes. Residential distance to a highway was not associated with birth weight or adiposity outcomes, but mothers living closest to a non-major roadway had infants with slightly higher average birth weight (61.8g, 95% CI 0.7, 122.9) compared to those living farthest away (Table 4). Traffic density within 1000m of the residence was associated with lower adiposity at birth, among both sexes combined (0.77% lower adiposity in the highest vs. lowest quartiles of traffic, 95% CI −1.48, −0.07). In sex-stratified analyses, this association was more pronounced in males (1.37% lower adiposity in the highest vs. lowest quartiles, 95% CI −2.33, −0.42) and nearly null in females (Supplemental Tables 7–8). Overall, traffic density was not associated with fat mass or fat-free mass at birth or follow-up (Supplemental Table 9), nor with the rate of change in body composition measures from birth to 5-month follow-up (Supplemental Table 10). Mothers living within 50m of a non-highway major roadway had infants with a lower rate of change in fat-free mass from birth to follow-up (−0.55 g/day, 95% CI −1.10, −0.10) (Supplemental Table 10).
Table 4.
Measures of traffic exposure at the residential address during pregnancy and infant weight and adiposity.
| Birth weight (n=1,125)a | Adiposity at birth (n=951)b | Adiposity at 5-month follow-up (n=574)c | ||||
|---|---|---|---|---|---|---|
| Traffic measure | Difference (g) and 95% confidence interval | Difference (%) and 95% confidence interval | Difference (%) and 95% confidence interval | |||
| Distance to highway (m) | ||||||
| Q1: [2.74, 396] | 10.8 | (−52.7, 74.3) | −0.12 | (−0.81, 0.58) | 0.30 | (−0.97, 1.56) |
| Q2: (396, 842] | −35.5 | (−98.3, 27.3) | −0.59 | (−1.28, 0.09) | 1.20 | (−0.02, 2.43) |
| Q3: (842, 1706] | 45.7 | (−14.7, 106.1) | 0.07 | (−0.59, 0.74) | 0.43 | (−0.76, 1.62) |
| Q4: (1706, 4590] | Ref | -- | Ref | -- | Ref | -- |
| Binary <150m | −48.7 | (−134.3, 37.0) | 0.09 | (−0.86, 1.04) | 0.22 | (−1.57, 2.01) |
| Distance to other major roadway (m) | ||||||
| Q1: [0.05, 72] | 61.8 | (0.7, 122.9) | 0.07 | (−0.61, 0.75) | 0.30 | (−0.93, 1.52) |
| Q2: (72, 172] | 1.7 | (−59.2, 62.5) | −0.45 | (−1.13, 0.23) | 0.18 | (−1.02, 1.39) |
| Q3: (172, 321] | 43.7 | (−16.6, 103.9) | −0.28 | (−0.95, 0.39) | 0.72 | (−0.49, 1.94) |
| Q4: (321, 2015] | Ref | -- | Ref | -- | Ref | -- |
| Binary <50m | 42.1 | (−12.2, 96.4) | 0.28 | (−0.33, 0.88) | 0.15 | (−0.91, 1.22) |
| Traffic density <150m (vehicles*km/day) | ||||||
| Q1: 0 | Ref | -- | Ref | -- | Ref | -- |
| Q2: 0d | ||||||
| Q3: (27, 2791] | −.8 | (−61.9, 44.4) | −0.17 | (−0.75, 0.42) | −0.08 | (−1.10, 0.94) |
| Q4: (2791, 45896] | 4.8 | (−47.6, 57.2) | 0.21 | (−0.37, 0.79) | −0.11 | (−1.16, 0.93) |
| Traffic density <250m (vehicles*km/day) | ||||||
| Q1: 0 | Ref | -- | Ref | -- | Ref | -- |
| Q2: (7, 3049] | −2.9 | (−64.8, 59.0) | −0.35 | (−1.05, 0.34) | 0.11 | (−1.12, 1.34) |
| Q3: (3049, 9441] | 23.6 | (−35.0, 82.2) | −0.28 | (−0.92, 0.37) | 0.02 | (−1.13, 1.18) |
| Q4: (9441, 99851] | 32.5 | (−26.0, 90.9) | 0.15 | (−0.50, 0.80) | 0.05 | (−1.13, 1.23) |
| Traffic density <500m (vehicles*km/day) | ||||||
| Q1: [0, 9372] | Ref | -- | Ref | -- | Ref | -- |
| Q2: (9372, 23186] | −14.5 | (−74.6, 45.6) | −0.21 | (−0.88, 0.46) | −0.15 | (−1.33, 1.03) |
| Q3: (23186, 42615] | −22.2 | (−82.8, 38.3) | −0.36 | (−1.02, 0.30) | −0.39 | (−1.57, 0.79) |
| Q4: (42615, 291462] | 33.2 | (−29.7, 96.1) | 0.12 | (−0.57, 0.81) | 0.06 | (−1.17, 1.29) |
| Traffic density <1000m (vehicles*km/day) | ||||||
| Q1: [0, 63577] | Ref | -- | Ref | -- | Ref | -- |
| Q2: (63577, 111805] | 2.5 | (−58.0, 63.0) | −0.44 | (−1.11, 0.23) | 0.30 | (−0.90, 1.50) |
| Q3: (111805, 159922] | −22.4 | (−86.1, 41.2) | −0.78 | (−1.49, −0.08) | −0.58 | (−1.85, 0.68) |
| Q4: (159922, 736032] | −16.1 | (−80.8, 48.6) | −0.77 | (−1.48, −0.07) | 0.72 | (−0.53, 1.97) |
Model 1: Adjusted for maternal age, pre-pregnancy body mass index, gestational weight gain, education, race/ethnicity, smoking during pregnancy, gravidity, infant sex, gestational age at birth, and percentage of persons below the poverty level in the Census tract of residence.
Model 2: Adjusted for Model 1 variables and infant age in days at neonatal body composition measurement.
Model 3: Adjusted for Model 1 variables except gestational age at birth, and for infant age in days at follow-up and exclusive breastfeeding.
For traffic density within 150m of the residence, quartiles 1 and 2 were combined due to the large number of zero values.
The examination of potential measurement error in the ambient air pollution exposure assessment revealed a high degree of spatial homogeneity across the study area. The ICCs for trimester-long average concentrations at the 3 nearest monitors ranged from 0.60–0.72 for PM2.5, and 0.74–0.80 for O3.
4. Discussion
In this diverse sample of mother-infant pairs living in Denver, Colorado, we observed limited evidence for an association between prenatal exposure to traffic, PM2.5 and O3 and adiposity among term infants. Third trimester average 8-hour maximum O3 was associated with greater fat mass and adiposity at mid-infancy and a greater rate of change in fat mass and adiposity from birth to ~5 months of age. Additionally, there was limited and inconsistent evidence of effect modification by sex: first trimester average PM2.5 was associated with lower fat mass and adiposity at mid-infancy and a lower rate of growth in adiposity at fat mass in early infancy among female infants only; the association between traffic density within 1000m of the residence and lower adiposity at birth was stronger among male infants.
We hypothesized, based primarily on existing literature on prenatal smoking exposure (36, 37), that weight and adiposity would be lower at birth and higher later in infancy among infants more highly exposed to traffic and ambient air pollution in utero. While we are unaware of other studies specifically examining traffic exposure and infant adiposity at birth, some studies have reported lower continuous birth weight or term low birth weight associated with residential proximity to major roadways (54, 63) or traffic density (14, 29, 63, 64), while others have reported null results (65). Additionally, some studies modeling traffic-related air pollution using models of NO2 or NOx have reported associations with greater odds of term low birth weight (66, 67). A study of infants born in Bradford, England, found that 3rd trimester PM2.5 (mean concentration 12.4 μg/m3) was associated with lower birth weight among infants of white British mothers but greater adiposity (by skinfold thickness) among infants of mothers of Pakistani origin (33). Our findings of an inverse association between adiposity at birth and traffic within 1000m (but not at shorter distances) are difficult to interpret, given that most near roadway pollutants decay to background levels by ~570m from the road edge (55). It is possible that traffic density within 1000m of the residence may indicate other characteristics of the neighborhood built or social environment that are associated with infant adiposity, and these potential spatial confounders merit further study.
Findings at the 5-month follow-up visit were inconsistent across exposures, with greater adiposity and rate of growth in adiposity associated with third trimester 8-hour maximum O3 and no overall association with PM2.5, despite limited evidence of sex-specific results. We observed no evidence of associations between traffic density or distance to roadway measures and 5-month adiposity. A possible explanation for these inconclusive results is that mid-infancy may be too early to observe the increased adiposity associated with prenatal traffic exposure. However, a previous study in a Boston-based cohort (mean third trimester PM2.5: 11.7 ug/m3) found increased growth in weight-for-length between 0 and 6 months of age among infants in the highest vs lowest quartiles of traffic density within 100m (29). There were, however, no associations between 0–6 month growth and distance to roadway or third trimester PM2.5 in that study (29). A later follow-up of the same cohort found greater body mass index and waist circumference at 3.3 years of age and greater fat mass at 7.7 years of age among children of mothers who lived closest to a major roadway during pregnancy, but again PM2.5 was not associated with adiposity outcomes (30).
Our results also provide limited evidence that male and female infants may have different susceptibility and response to prenatal exposure to traffic and ambient air pollution. Some previous studies have reported sex-specific effects. In the French PELAGIE cohort, prenatal NO2 was positively associated with birth weight in boys only, and the authors proposed that males may be more susceptible to the adverse effects of prenatal air pollution (68). This is consistent with the finding that male offspring, but not female offspring, of mice exposed to PM2.5 during pregnancy had lower body weight and reduced food intake compared to the offspring of control mice (69). Another epidemiologic study in a Boston-based cohort found a 3-way interaction between prenatal air pollution exposure, infant sex, and maternal pre-pregnancy obesity, suggesting another dimension of potential heterogeneity (70).
By contrast with a number of previous studies and meta-analyses (16, 21), we did not observe an overall association between PM2.5 exposure during pregnancy and term birth weight. This difference may be explained by the different composition of the ambient air pollution mixture in different regions (71), or by the different prevalence of maternal comorbidities and other factors that may influence susceptibility to the effects of PM2.5 during pregnancy (72, 73). A U.S. national study that stratified by region found an unexpected protective association between Census tract-level PM2.5 and term low birth weight in the Mountain West region (in which Denver is located) (74). Alternatively, the lack of association with term birth weight in this study may be attributed to the low concentrations and low variability of PM2.5 in the study area. The limited exposure variability would also result in relatively wide confidence intervals if presented in common increments of 5–10 μg/m3 of PM2.5.
Several biological mechanisms have been proposed to explain associations between prenatal air pollution exposure and infant weight and adiposity. Many hypotheses center on the role of particulate matter in inducing inflammation and oxidative stress. In the Boston Birth Cohort, ambient concentrations of PM2.5 near the residential address during pregnancy were associated with greater odds of intrauterine inflammation, defined by clinical and pathological features (75). In a large Dutch cohort study, recent exposure to ambient particulate matter during pregnancy was associated with elevated maternal concentrations of C-reactive protein (CRP), and chronic exposure was positively associated with cord blood levels of the same biomarker of inflammation (76). Elevated CRP during pregnancy has been associated with fetal growth restriction (77). In a separate study, maternal plasma concentrations of CRP during pregnancy were positively associated with offspring adiposity in mid-childhood (78).
Other studies have focused on the role of particulate air pollution in disrupting metabolic and endocrine activity. Two epidemiologic studies have reported associations between ambient PM2.5 concentrations during pregnancy and newborn thyroid hormone levels (79, 80), which may contribute to suppression of growth in utero and postnatally. Outdoor (81) and indoor (82) air samples have been shown to contain a wide variety of endocrine-disrupting chemicals, with potential to interfere with estrogens and androgens and produce sex-specific associations. A California-based study found associations between prenatal traffic-related exposures (distance to roadway and non-freeway nitrogen oxides) and cord blood leptin, which in turn predicted greater weight gain in the first 6 months in female but not male infants (83).
Our study has a number of strengths and some limitations. Exposure to air pollution in the Mountain West region of the U.S. has so far been understudied relative to the coastal regions. We used a large, ongoing pre-birth cohort study with detailed information on maternal and infant characteristics from direct measures, questionnaires and medical records. In addition to individual characteristics, we considered socioeconomic confounding at the neighborhood level by adjusting for characteristics of the Census tract of residence. In models of PM2.5 and O3 in different periods of pregnancy, we adjusted for season and year of birth, as well as mean temperature during the specified period of pregnancy.
This analysis was conducted in a relatively low exposure setting with limited exposure variability in ambient air pollutants. While we found few associations with spatially-defined traffic exposure measures, it may be more fruitful to apply models of air pollutants that are expected to have greater intra-urban spatial heterogeneity, such as NO2. Our exposure assessment was limited to the first reported maternal residential address during pregnancy (collected at study enrollment), therefore the potential for misclassification of exposure is likely to be somewhat lower for early compared to late pregnancy. We did not have data available on the timing of changes in residence during pregnancy. A study in New York found that women’s residential moves during pregnancy tended to be of short distance and did not greatly affect the ambient air pollution exposure assessment (84); however, we do not know if this is the case in the Healthy Start cohort.
The methods used here to estimate exposures (proximity-based traffic measures and inverse-distance weighting of ambient air pollution from fixed monitoring sites), while commonly employed in this field, are subject to known limitations (85) that may contribute to misclassification. Additionally, there may be some misclassification of ambient air pollution exposure due to the relatively sparse monitoring network in the Denver metropolitan area. We explored the potential for exposure misclassification due to spatial heterogeneity of ambient air pollutants across the study area. Our findings of moderate to high agreement among the 3 closest monitors indicates that most of the variability in exposure to PM2.5 and O3 in this population is due to differences in the timing of pregnancy relative to temporal (seasonal) changes in air pollution, and suggests that exposure misclassification due to the interpolation procedure is likely to be a relatively minor source of error. We estimated air pollutant concentrations outside the home, but we do not know the concentrations within the home which may be influenced by both indoor and outdoor sources (86). We also did not have available time-activity data to calculate the percentage of time pregnant women spent at work, commuting, or other locations, and we do not have information about exposures received elsewhere.
We cannot rule out the possibility that the associations may be biased by residual confounding. Air pollution is a complex mixture that is frequently associated with other urban features, including traffic noise and lack of proximity to green space, and these may have independent associations with offspring size and adiposity (87, 88). We did not include measurements of noise or green space in this study. Additionally, there may be residual confounding by socioeconomic characteristics and other factors that were not accounted for by our individual-level and neighborhood-level adjustments. Finally, due to the number of statistical tests conducted in this analysis, some findings may be due to chance.
In conclusion, we found limited evidence of associations between prenatal exposure to traffic, PM2.5 and O3 and infant adiposity in this urban population with relatively low ambient PM2.5 concentrations. Continued follow-up of the Healthy Start cohort and similar cohorts such as those participating in the NIH Environmental influences on Child Health Outcomes (ECHO) consortium will allow us to understand the weight and adiposity trajectories of infants exposed prenatally to higher levels of traffic and related air pollutants, and the relative contributions of prenatal and postnatal exposures.
Supplementary Material
Acknowledgements
This work was supported by grants from the National Institute of Environmental Health Sciences (R00ES025817), the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK076648), and the National Institutes of Health Office of the Director (UH3OD023248). Funders had no involvement in the data collection, analysis, or interpretation of results, and were not involved in the writing of the article or the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest Statement
The authors state that they have no competing financial interests related to this work.
Abbreviations: AQS, Air Quality System; CI, confidence interval; CRP, C-reactive protein; ICC, intra-class correlation coefficient; IQR, interquartile range; PM2.5, particulate matter with diameter ≤2.5 microns; O3, ozone; US EPA, United States Environmental Protection Agency.
References
- 1.Vrijheid M, Casas M, Gascon M, Valvi D, Nieuwenhuijsen M. Environmental pollutants and child health-A review of recent concerns. Int J Hyg Environ Health. 2016;219(4–5):331–42. [DOI] [PubMed] [Google Scholar]
- 2.Russ K, Howard S. Developmental Exposure to Environmental Chemicals and Metabolic Changes in Children. Current Problems in Pediatric and Adolescent Health Care. 2016;46(8):255–85. [DOI] [PubMed] [Google Scholar]
- 3.Cortessis VK, Thomas DC, Levine AJ, Breton CV, Mack TM, Siegmund KD, et al. Environmental epigenetics: prospects for studying epigenetic mediation of exposure-response relationships. Hum Genet. 2012;131(10):1565–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11(4):496–506. [DOI] [PubMed] [Google Scholar]
- 5.Laurent O, Wu J, Li L, Chung J, Bartell S. Investigating the association between birth weight and complementary air pollution metrics: a cohort study. Environ Health. 2013;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell ML, Ebisu K, Belanger K. Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ Health Perspect. 2007;115(7):1118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brauer M, Lencar C, Tamburic L, Koehoorn M, Demers P, Karr C. A cohort study of traffic-related air pollution impacts on birth outcomes. Environ Health Perspect. 2008;116(5):680–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dadvand P, Parker J, Bell ML, Bonzini M, Brauer M, Darrow LA, et al. Maternal exposure to particulate air pollution and term birth weight: a multi-country evaluation of effect and heterogeneity. Environ Health Perspect. 2013;121(3):267–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darrow LA, Klein M, Strickland MJ, Mulholland JA, Tolbert PE. Ambient air pollution and birth weight in full-term infants in Atlanta, 1994–2004. Environ Health Perspect. 2011;119(5):731–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleischer NL, Merialdi M, van Donkelaar A, Vadillo-Ortega F, Martin RV, Betran AP, et al. Outdoor air pollution, preterm birth, and low birth weight: analysis of the world health organization global survey on maternal and perinatal health. Environ Health Perspect. 2014;122(4):425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ha S, Hu H, Roussos-Ross D, Haidong K, Roth J, Xu X. The effects of air pollution on adverse birth outcomes. Environ Res. 2014;134C:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laurent O, Hu J, Li L, Cockburn M, Escobedo L, Kleeman MJ, et al. Sources and contents of air pollution affecting term low birth weight in Los Angeles County, California, 2001–2008. Environ Res. 2014;134:488–95. [DOI] [PubMed] [Google Scholar]
- 13.Morello-Frosch R, Jesdale BM, Sadd JL, Pastor M. Ambient air pollution exposure and full-term birth weight in California. Environ Health. 2010;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedersen M, Giorgis-Allemand L, Bernard C, Aguilera I, Andersen AM, Ballester F, et al. Ambient air pollution and low birthweight: a European cohort study (ESCAPE). Lancet Respir Med. 2013;1(9):695–704. [DOI] [PubMed] [Google Scholar]
- 15.Stieb DM, Chen L, Beckerman BS, Jerrett M, Crouse DL, Omariba DW, et al. Associations of Pregnancy Outcomes and PM2.5 in a National Canadian Study. Environ Health Perspect. 2016;124(2):243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu X, Liu Y, Chen Y, Yao C, Che Z, Cao J. Maternal exposure to fine particulate matter (PM2.5) and pregnancy outcomes: a meta-analysis. Environ Sci Pollut Res Int. 2015;22(5):3383–96. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Huang S, Jiao A, Yang X, Yun J, Wang Y, et al. Association between ambient fine particulate matter and preterm birth or term low birth weight: An updated systematic review and meta-analysis. Environ Pollut. 2017;227:596–605. [DOI] [PubMed] [Google Scholar]
- 18.Kingsley SL, Eliot MN, Glazer K, Abu Awad Y, Schwartz JD, Savitz DA, et al. Maternal ambient air pollution, preterm birth and markers of fetal growth in Rhode Island: results of a hospital-based linkage study. Journal of Epidemiology and Community Health. 2017;71(12):1131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kloog I, Melly SJ, Ridgway WL, Coull BA, Schwartz J. Using new satellite based exposure methods to study the association between pregnancy PM₂.₅ exposure, premature birth and birth weight in Massachusetts. Environ Health. 2012;11:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savitz DA, Bobb JF, Carr JL, Clougherty JE, Dominici F, Elston B, et al. Ambient fine particulate matter, nitrogen dioxide, and term birth weight in New York, New York. Am J Epidemiol. 2014;179(4):457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun X, Luo X, Zhao C, Zhang B, Tao J, Yang Z, et al. The associations between birth weight and exposure to fine particulate matter (PM2.5) and its chemical constituents during pregnancy: A meta-analysis. Environ Pollut. 2016;211:38–47. [DOI] [PubMed] [Google Scholar]
- 22.Liang ZJ, Yang Y, Qian ZM, Ruan ZL, Chang JJ, Vaughn MG, et al. Ambient PM2.5 and birth outcomes: Estimating the association and attributable risk using a birth cohort study in nine Chinese cities. Environment International. 2019;126:329–35. [DOI] [PubMed] [Google Scholar]
- 23.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331(7522):929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelishadi R, Poursafa P. A Review on the Genetic, Environmental, and Lifestyle Aspects of the Early-Life Origins of Cardiovascular Disease. Current Problems in Pediatric and Adolescent Health Care. 2014;44(3):54–72. [DOI] [PubMed] [Google Scholar]
- 25.Rogers I, Grp E-BS. The influence of birthweight and intrauterine environment on adiposity and fat distribution in later life. International Journal of Obesity. 2003;27(7):755–77. [DOI] [PubMed] [Google Scholar]
- 26.Perng W, Hajj H, Belfort MB, Rifas-Shiman SL, Kramer MS, Gillman MW, et al. Birth Size, Early Life Weight Gain, and Midchildhood Cardiometabolic Health. J Pediatr. 2016;173:122–30 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollution HPotHEo T-RA. Traffic-Related Air Pollution: A Critical Review of the Literature on Emissions, Exposure, and Health Effects HEI Special Report 17. Boston, MA: Health Effects Institute; 2010. [Google Scholar]
- 28.Alderete TL, Song AY, Bastain T, Habre R, Toledo-Corral CM, Salam MT, et al. Prenatal traffic-related air pollution exposures, cord blood adipokines and infant weight. Pediatr Obes. 2018;13(6):348–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleisch AF, Rifas-Shiman SL, Koutrakis P, Schwartz JD, Kloog I, Melly S, et al. Prenatal exposure to traffic pollution: associations with reduced fetal growth and rapid infant weight gain. Epidemiology. 2015;26(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fleisch AF, Luttmann-Gibson H, Perng W, Rifas-Shiman SL, Coull BA, Kloog I, et al. Prenatal and early life exposure to traffic pollution and cardiometabolic health in childhood. Pediatr Obes. 2017;12(1):48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JS, Alderete TL, Chen Z, Lurmann F, Rappaport E, Habre R, et al. Longitudinal associations of in utero and early life near-roadway air pollution with trajectories of childhood body mass index. Environ Health. 2018;17(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rundle A, Hoepner L, Hassoun A, Oberfield S, Freyer G, Holmes D, et al. Association of childhood obesity with maternal exposure to ambient air polycyclic aromatic hydrocarbons during pregnancy. Am J Epidemiol. 2012;175(11):1163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schembari A, de Hoogh K, Pedersen M, Dadvand P, Martinez D, Hoek G, et al. Ambient Air Pollution and Newborn Size and Adiposity at Birth: Differences by Maternal Ethnicity (the Born in Bradford Study Cohort). Environ Health Perspect. 2015;123(11):1208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei Y, Zhang JJ, Li Z, Gow A, Chung KF, Hu M, et al. Chronic exposure to air pollution particles increases the risk of obesity and metabolic syndrome: findings from a natural experiment in Beijing. FASEB J. 2016;30(6):2115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bove H, Bongaerts E, Slenders E, Bijnens EM, Saenen ND, Gyselaers W, et al. Ambient black carbon particles reach the fetal side of human placenta. Nat Commun. 2019;10(1):3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogers JM. Smoking and pregnancy: Epigenetics and developmental origins of the metabolic syndrome. Birth Defects Res 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oken E, Levitan EB, Gillman MW. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int J Obes (Lond). 2008;32(2):201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McConnell R, Shen E, Gilliland FD, Jerrett M, Wolch J, Chang CC, et al. A longitudinal cohort study of body mass index and childhood exposure to secondhand tobacco smoke and air pollution: the Southern California Children’s Health Study. Environ Health Perspect. 2015;123(4):360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jerrett M, McConnell R, Chang CC, Wolch J, Reynolds K, Lurmann F, et al. Automobile traffic around the home and attained body mass index: a longitudinal cohort study of children aged 10–18 years. Prev Med 2010;50 Suppl 1:S50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang Z, Yang Y, Li J, Zhu X, Ruan Z, Chen S, et al. Migrant population is more vulnerable to the effect of air pollution on preterm birth: Results from a birth cohort study in seven Chinese cities. Int J Hyg Environ Health. 2019;222(7):1047–53. [DOI] [PubMed] [Google Scholar]
- 41.Dong GH, Qian Z, Liu MM, Wang D, Ren WH, Flick LH, et al. Ambient Air Pollution and the Prevalence of Obesity in Chinese Children: The Seven Northeastern Cities Study. Obesity. 2014;22(3):795–800. [Google Scholar]
- 42.EPA U AQS Data Mart [Available from: https://aqs.epa.gov/aqsweb/documents/data_mart_welcome.html.
- 43.Watson JG, Fujita E, Chow JC, Zielinska B, Richards LW, Neff W, et al. Northern Front Range Air Quality Study Final Report. Desert Research Institute; 1998. Contract No.: DRI Document No. 6580–685-8750.1F2. [Google Scholar]
- 44.Xie M, Coons TL, Dutton SJ, Milford JB, Miller SL, Peel JL, et al. Intra-urban spatial variability of PM2.5-bound carbonaceous components. Atmos Environ (1994). 2012;60:486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown SG, Penfold B, Mukherjee A, Landsberg K, Eisinger DS. Conditions Leading to Elevated PM2.5 at Near-Road Monitoring Sites: Case Studies in Denver and Indianapolis. Int J Environ Res Public Health. 2019;16(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu YF, Kuhn T, Mayo P, Hinds WC. Comparison of daytime and nighttime concentration profiles and size distributions of ultrafine particles near a major highway. Environmental Science & Technology. 2006;40(8):2531–6. [DOI] [PubMed] [Google Scholar]
- 47.Hu S, Fruin S, Kozawa K, Mara S, Paulson SE, Winer AM. A Wide Area of Air Pollutant Impact Downwind of a Freeway during Pre-Sunrise Hours. Atmos Environ (1994). 2009;43(16):2541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brauer M, Hoek G, van Vliet P, Meliefste K, Fischer P, Gehring U, et al. Estimating long-term average particulate air pollution concentrations: application of traffic indicators and geographic information systems. Epidemiology. 2003;14(2):228–39. [DOI] [PubMed] [Google Scholar]
- 49.Bell ML, Dominici F, Ebisu K, Zeger SL, Samet JM. Spatial and temporal variation in PM(2.5) chemical composition in the United States for health effects studies. Environ Health Perspect. 2007;115(7):989–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi H, Rauh V, Garfinkel R, Tu Y, Perera FP. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and risk of intrauterine growth restriction. Environ Health Perspect. 2008;116(5):658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rogers IS, Ness AR, Steer CD, Wells JC, Emmett PM, Reilly JR, et al. Associations of size at birth and dual-energy X-ray absorptiometry measures of lean and fat mass at 9 to 10 y of age. Am J Clin Nutr. 2006;84(4):739–47. [DOI] [PubMed] [Google Scholar]
- 52.Urlando A, Dempster P, Aitkens S. A new air displacement plethysmograph for the measurement of body composition in infants. Pediatr Res. 2003;53(3):486–92. [DOI] [PubMed] [Google Scholar]
- 53.Fields DA, Demerath EW, Pietrobelli A, Chandler-Laney PC. Body composition at 6 months of life: comparison of air displacement plethysmography and dual-energy X-ray absorptiometry. Obesity (Silver Spring). 2012;20(11):2302–6. [DOI] [PubMed] [Google Scholar]
- 54.Kingsley SL, Eliot MN, Whitsel EA, Huang YT, Kelsey KT, Marsit CJ, et al. Maternal residential proximity to major roadways, birth weight, and placental DNA methylation. Environ Int. 2016;92–93:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karner AA, Eisinger DS, Niemeier DA. Near-roadway air quality: synthesizing the findings from real-world data. Environ Sci Technol. 2010;44(14):5334–44. [DOI] [PubMed] [Google Scholar]
- 56.Zhu Y, Hinds WC, Kim S, Sioutas C. Concentration and size distribution of ultrafine particles near a major highway. J Air Waste Manag Assoc. 2002;52(9):1032–42. [DOI] [PubMed] [Google Scholar]
- 57.Mobasher Z, Salam MT, Goodwin TM, Lurmann F, Ingles SA, Wilson ML. Associations between ambient air pollution and Hypertensive Disorders of Pregnancy. Environ Res. 2013;123:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Padula AM, Mortimer KM, Tager IB, Hammond SK, Lurmann FW, Yang W, et al. Traffic-related air pollution and risk of preterm birth in the San Joaquin Valley of California. Ann Epidemiol 2014;24(12):888–95.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rivera-González LO, Zhang Z, Sánchez BN, Zhang K, Brown DG, Rojas-Bracho L, et al. An assessment of air pollutant exposure methods in Mexico City, Mexico. J Air Waste Manag Assoc. 2015;65(5):581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Starling AP, Brinton JT, Glueck DH, Shapiro AL, Harrod CS, Lynch AM, et al. Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start study. Am J Clin Nutr. 2015;101(2):302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sauder KA, Kaar JL, Starling AP, Ringham BM, Glueck DH, Dabelea D. Predictors of Infant Body Composition at 5 Months of Age: The Healthy Start Study. J Pediatr. 2017;183:94–9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Selvin S Statistical Analysis of Epidemiologic Data. 3rd ed. New York: Oxford University Press; 2004. [Google Scholar]
- 63.Wilhelm M, Ritz B. Residential proximity to traffic and adverse birth outcomes in Los Angeles County, California, 1994–1996. Environmental Health Perspectives. 2003;111(2):207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Padula AM, Mortimer K, Hubbard A, Lurmann F, Jerrett M, Tager IB. Exposure to traffic-related air pollution during pregnancy and term low birth weight: estimation of causal associations in a semiparametric model. Am J Epidemiol. 2012;176(9):815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van den Hooven EH, Jaddoe VWV, de Kluizenaar Y, Hofman A, Mackenbach JP, Steegers EAP, et al. Residential traffic exposure and pregnancy-related outcomes: a prospective birth cohort study. Environmental Health. 2009;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stieb DM, Chen L, Hystad P, Beckerman BS, Jerrett M, Tjepkema M, et al. A national study of the association between traffic-related air pollution and adverse pregnancy outcomes in Canada, 1999–2008. Environ Res. 2016;148:513–26. [DOI] [PubMed] [Google Scholar]
- 67.Wilhelm M, Ghosh JK, Su J, Cockburn M, Jerrett M, Ritz B. Traffic-related air toxics and term low birth weight in Los Angeles County, California. Environ Health Perspect. 2012;120(1):132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bertin M, Chevrier C, Serrano T, Monfort C, Cordier S, Viel JF. Sex-specific differences in fetal growth in newborns exposed prenatally to traffic-related air pollution in the PELAGIE mother-child cohort (Brittany, France). Environ Res. 2015;142:680–7. [DOI] [PubMed] [Google Scholar]
- 69.Xie P, Zhao C, Huang W, Yong T, Chung ACK, He K, et al. Prenatal exposure to ambient fine particulate matter induces dysregulations of lipid metabolism in adipose tissue in male offspring. Sci Total Environ. 2019;657:1389–97. [DOI] [PubMed] [Google Scholar]
- 70.Lakshmanan A, Chiu YH, Coull BA, Just AC, Maxwell SL, Schwartz J, et al. Associations between prenatal traffic-related air pollution exposure and birth weight: Modification by sex and maternal pre-pregnancy body mass index. Environ Res. 2015;137C:268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ng C, Malig B, Hasheminassab S, Sioutas C, Basu R, Ebisu K. Source apportionment of fine particulate matter and risk of term low birth weight in California: Exploring modification by region and maternal characteristics. Science of the Total Environment. 2017;605:647–54. [DOI] [PubMed] [Google Scholar]
- 72.Lavigne E, Yasseen AS, Stieb DM, Hystad P, van Donkelaar A, Martin RV, et al. Ambient air pollution and adverse birth outcomes: Differences by maternal comorbidities. Environ Res. 2016;148:457–66. [DOI] [PubMed] [Google Scholar]
- 73.Xue T, Zhu T, Han Y. Association between birthweight and ambient PM2.5 in the United States: Individually-varied susceptibility and spatial heterogeneity. Environ Int. 2018;119:388–97. [DOI] [PubMed] [Google Scholar]
- 74.Hao YP, Strosnider H, Balluz L, Qualters JR. Geographic Variation in the Association between Ambient Fine Particulate Matter (PM2.5) and Term Low Birth Weight in the United States. Environmental Health Perspectives. 2016;124(2):250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nachman RM, Mao G, Zhang X, Hong X, Chen Z, Soria CS, et al. Intrauterine Inflammation and Maternal Exposure to Ambient PM2.5 during Preconception and Specific Periods of Pregnancy: The Boston Birth Cohort. Environ Health Perspect. 2016;124(10):1608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van den Hooven EH, de Kluizenaar Y, Pierik FH, Hofman A, van Ratingen SW, Zandveld PY, et al. Chronic air pollution exposure during pregnancy and maternal and fetal C-reactive protein levels: the Generation R Study. Environ Health Perspect. 2012;120(5):746–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tjoa ML, van Vugt JM, Go AT, Blankenstein MA, Oudejans CB, van Wijk IJ. Elevated C-reactive protein levels during first trimester of pregnancy are indicative of preeclampsia and intrauterine growth restriction. J Reprod Immunol. 2003;59(1):29–37. [DOI] [PubMed] [Google Scholar]
- 78.Gaillard R, Rifas-Shiman SL, Perng W, Oken E, Gillman MW. Maternal inflammation during pregnancy and childhood adiposity. Obesity. 2016;24(6):1320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Howe CG, Eckel SP, Habre R, Girguis MS, Gao L, Lurmann FW, et al. Association of Prenatal Exposure to Ambient and Traffic-Related Air Pollution With Newborn Thyroid Function: Findings From the Children’s Health Study. JAMA Netw Open. 2018;1(5):e182172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Janssen BG, Saenen ND, Roels HA, Madhloum N, Gyselaers W, Lefebvre W, et al. Fetal Thyroid Function, Birth Weight, and in Utero Exposure to Fine Particle Air Pollution: A Birth Cohort Study. Environ Health Perspect. 2017;125(4):699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Novak J, Jalova V, Giesy JP, Hilscherova K. Pollutants in particulate and gaseous fractions of ambient air interfere with multiple signaling pathways in vitro. Environ Int. 2009;35(1):43–9. [DOI] [PubMed] [Google Scholar]
- 82.Oziol L, Alliot F, Botton J, Bimbot M, Huteau V, Levi Y, et al. First characterization of the endocrine-disrupting potential of indoor gaseous and particulate contamination: comparison with urban outdoor air (France). Environ Sci Pollut Res Int. 2017;24(3):3142–52. [DOI] [PubMed] [Google Scholar]
- 83.Alderete TL, Song AY, Bastain T, Habre R, Toledo-Corral CM, Salam MT, et al. Prenatal traffic-related air pollution exposures, cord blood adipokines and infant weight. Pediatr Obes. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen L, Bell EM, Caton AR, Druschel CM, Lin S. Residential mobility during pregnancy and the potential for ambient air pollution exposure misclassification. Environ Res. 2010;110(2):162–8. [DOI] [PubMed] [Google Scholar]
- 85.Jerrett M, Arain A, Kanaroglou P, Beckerman B, Potoglou D, Sahsuvaroglu T, et al. A review and evaluation of intraurban air pollution exposure models. Journal of Exposure Analysis and Environmental Epidemiology. 2005;15(2):185–204. [DOI] [PubMed] [Google Scholar]
- 86.Shrestha PM, Humphrey JL, Carlton EJ, Adgate JL, Barton KE, Root ED, et al. Impact of outdoor air pollution on indoor air quality in low-income homes during wildfire seasons. International Journal of Environmental Research and Public Health. 2019;16(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fong KC, Kloog I, Coull BA, Koutrakis P, Laden F, Schwartz JD, et al. Residential Greenness and Birthweight in the State of Massachusetts, USA. International Journal of Environmental Research and Public Health. 2018;15(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Christensen JS, Hjortebjerg D, Raaschou-Nielsen O, Ketzel M, Sorensen TIA, Sorensen M. Pregnancy and childhood exposure to residential traffic noise and overweight at 7 years of age. Environment International. 2016;94:170–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.