Highlights
-
•
Even slow growing mammary lesions in a male child may be malignant.
-
•
Secretory breast cancer positive for estrogen and progesterone receptors is very rare in a male child.
-
•
Secretory breast cancer after surgical excision usually does not require adjuvant therapy.
Keywords: Breast neoplasms, Male, Mastectomy, Excision margins, Case report
Abstract
Introduction
The secretory breast carcinoma is very rare in children. It represents less than 1% of malignancy cases in childhood and is even less common in males, with 15 cases reported until 2004. Therefore, the aim of this study is to report a male child with breast carcinoma and review literature.
Presentation of case
A 14-year-old male patient with a history of a painless slow-growing lump in the left breast and, five years later, onset of yellow discharge from the papilla. Ultrasound scanning demonstrated a well- defined, regular, homogeneous and hypoechoic mass.
Nodule excision was initially performed, followed by mastectomy, due to compromised radial margin. Immunohistochemistry revealed weakly positive estrogen and progesterone receptors. Adjuvant therapy was not required. Sixteen months after resection, the patient is well with no complaints or recurrence.
Discussion
Due to its rarity, there is no therapeutic guideline. Although the recommended treatment is still surgical excision, there is no consensus as to its extent. Prognosis is usually favorable. Our patient was submitted to mastectomy with sentinel lymph node biopsy due to compromised radial margin.
Conclusion
Secretory breast carcinoma is a rare form of breast cancer, especially in male children; which hampers standardization of diagnosis, treatment and prognosis establishment.
1. Introduction
Secretory breast cancer is the most common cause of breast cancer in children, nevertheless it is still considered rare [[1], [2], [3], [4]], since it does not even comprise 1% of malignancy cases in childhood [[3], [4], [5]]. It is even rarer in males, with a total of 15 reported cases until 2004 [6].
Most breast masses in children are benign, which is why surgeons are reluctant to perform biopsies, fearing post-operative deformities during breast development [[4], [5], [6], [7]].
Due to its rarity, there are no therapeutic guidelines for secretory breast cancer, nonetheless, the consensus is that the first step is surgical removal [3,4]. Even with its auspicious prognosis, there are cases of late recurrence [[1], [2], [3], [4], [5], [6], [7]].
This report was made in line with the SCARE criteria [8].
2. Presentation of case
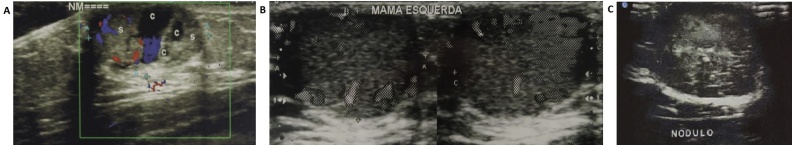
A 9-year-old male patient was referred to another pediatric surgery service because of a lump in his left breast. At the time, an ultrasound showed a vascularized and miscellaneous nodule in the mammary region, compatible with cavernous hemangioma (Fig. 1A). A year later, despite no clinical changes, a new ultrasound scan, requested by the same service, showed a solid, 16 × 14 × 16 mm, hypervascularized, retroareolar mass (Fig. 1B).
Fig. 1.
(A) Ultrasound findings at first presentation - vascularized and heterogenous nodule in left breast, compatible with cavernous hemangioma. (B) Ultrasound image one year after first appointment: solid, hypervascularized, retroareolar, 16 × 14 × 16 mm mass. (C) Ultrasound image four years after second ultrasound: solid, regular, homogeneous, well delimitated and hypoechoic 27 × 24 × 19 mm mass in the retroareolar region.
The patient quit follow-up consultations and was referred to our hospital four years later, with a significant growth of the mass in the left breast. It was a 3 cm solid, well delimitated, regular, mobile, painless and retroareolar mass with passive yellowish discharge from the nipple. Ultrasound imaging revealed a solid, regular, homogeneous, well delimitated and hypoechoic mass, measuring, in the retroareolar region (Fig. 1C).
Five months later, the mass was still well delimited and painless with approximately four centimeters. He was submitted to node excision, which during the surgery revealed itself to be solid and adhered to the adjoining tissues. Consequently, removal of the areola was necessary.
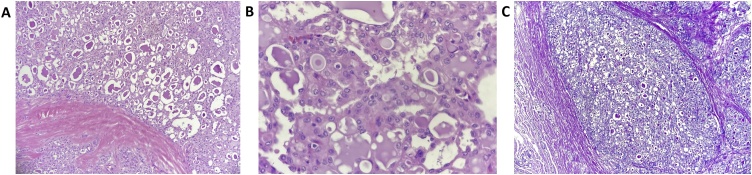
Gross dissection revealed a 3.5 cm well circumscribed, soft-to-firm nodule. Histological sections showed a cystic tumor with microcystic, tubular and papillary architecture, and intraluminal eosinophilic secretions, positive for periodic acid-Schiff (PAS) stain (Fig. 2). Radial margin was positive. Mild atypia, uniform nuclei, evident nucleoli and a low mitotic rate were observed. There was perineural and lymphovascular invasion. Immunohistochemical analysis demonstrated tumor cells with positive expression for S-100, GCDFP-15 and weak and patchy expression for estrogen and progesterone receptors (Fig. 3A–D). EMA (epithelial membrane antigen) was strongly positive (Fig. 3C). Immunohistochemistry for HER2/neu (c-erbB2) was negative. Ki-67 (MIB1) labeling index was low, with less than 15% positive neoplastic cells (Fig. 3E and F). (epithelial Molecular tests were not available. Due to its infiltration of skin and areola, the clinical classification was T4.
Fig. 2.
(A) Hematoxylin and eosin stain, 10X – tumor exhibiting microcyst architecture with intraluminal eosinophilic secretions. (B) Hematoxylin and eosin stain, 40X – The neoplastic cells are large and polygonal and show eosinophilic and foamy cytoplasm, vesicular chromatin nuclei and evident nucleoli. (C) Periodic acid-Schiff stain (PAS), 10X – Secretory materials are positive for PAS.
Fig. 3.
(A) On immunohistochemistry, the neoplasm was positive for GCFDP-15, (B) S-100 and (C) EMA (epithelial membrane antigen); weak and patchy for (D) estrogen and (E) progesterone receptors (nuclear stain). (F) HER2/neu (c-erbB2) was completely negative (score IHC = 0) and (G) Ki67 index was low (<15%).
Abdominal ultrasound and a chest x-ray were performed and no metastasis was found. Thus, the patient underwent a left mastectomy (Fig. 4) with sentinel lymph node biopsy (Fig. 5A and B), which was free from malignancy. The mammary tissue revealed microscopic atypical glandular proliferation (residual carcinoma) with foreign-body reaction and free margins.
Fig. 4.
Mammary gland excision.
Fig. 5.
(A) Sentinel lymph node identification by blue dye. (B) Lymphatic network flow into the sentinel lymph node.
The patient had a good recovery and no adjuvant therapy was necessary. Sixteen months post-op, he shows no signs of recurrence.
3. Discussion
Breast cancer is the most common type of neoplasm in females. Yearly, it is responsible for 28% of all new cancer cases, and most have a good prognosis [9]. It can also occur in males, however, it corresponds to only 1% of all breast cancer cases [9]. Also, it’s relatively rare in patients under 35 years old, with risks increasing progressively with age, especially after 50 years of age [9].
Breast cancer of epithelial origin have various subtypes, which differ on biology, treatment strategy, metastasis pattern and prognosis [10]. They can be divided in non-invasive, which are the intraductal and in situ lobular carcinomas, and invasive, which include various types of breast cancer, including the secretory carcinomas [11], which are responsible for less than 1% of all malignant breast neoplasms [12]. Thus, the current case has very rare elements: a secretory carcinoma in a male child.
Clinically, secretory breast cancer has a favorable prognosis. Its characteristics are: slow growing, painless, well circumscribed, movable mass. The ultrasound shows a single, microlobular and hypoechoic mass appearing to be benign, similar to a fibroadenoma and other well circumscribed carcinomas. The malignant cells are characteristically PAS positive, have a granular eosinophilic cytoplasm, and secretory abundance (intra and extracellular) of sulfated mucopolysaccharides and mucin containing material [[1], [2], [3], [4],8]. The secretory breast carcinoma is usually negative for estrogen and progesterone receptors, which suggests that this tumor is not influenced by sexual hormones [1,13]. Usually, it does not present HER2 gene amplification [1]. In our case, the tumor was weakly positive for estrogen and progesterone receptors, and negative for HER2.
Genetically, it is characterized by the translocation t(12,15)(p13,q15) in the ETV6-NTRK3 gene, which despite being present in other neoplasms like acute myeloid leukemia, in the breast seems specific of the secretory carcinoma [14,15]. In the pediatric population, a reliable image of the mammary mass is hard to obtain, because the diagnostic methods used routinely in adults are not as effective in children. While some methods have not yet been proven effective in children, others may expose them to radiation or have bad quality images. According to literature, ultrasonography is the main exam in pediatric evaluation [16]. Biopsy is usually postponed and rarely performed, due to the improbability of a malignant tumor, since those are exceedingly rare in children, and because of the fear of breast deformities during development [16].
In this case, the follow up of the breast nodule was performed using ultrasound, and the biopsy was not indicated previously for the reasons mentioned above. However, the patient’s missing follow-up consultations caused the carcinoma diagnosis to be delayed. As already mentioned, the secretory carcinoma has a slow growth, thus, even though the resection was performed 6 years after the first evaluation, there was no dissemination and the breast excision was potentially curative.
The most adequate treatment for secretory breast carcinomas is surgical removal [17]. There is no consensus yet as to the extension of the procedure. The main complications are local recurrence and axillary lymph node metastasis [17]. Ideally, removal of the most adjacent mammary tissue to the tumor as possible should be attempted, for the analysis and investigation of intraductal invasion, since it can indicate a higher risk of local recurrence. When the primary tumor is big or when there is a high risk of relapse, mastectomy is indicated [18]. The size of the tumor can vary from 0.5–16 cm, but most tend to stay around 1.5 to 3 cm [1]. Tumors below 3 cm have lower risk of relapse [19]. Since metastasis are rare, there is no evidence of the benefit of adjuvant therapy [17,18].
In the presented case, mastectomy was performed due the compromised radial margin. A traditional axillary lymph node dissection was not necessary, since the sentinel lymph node revealed no neoplastic disease. The present case has a potentially favorable prognosis due to the young age of the patient, regardless of the tumor size (3.5 cm), which requires a long-term follow-up and indicates a higher rate of local recurrence. Furthermore, in this case, adjuvant therapy was not recommended because of the low risk of metastasis, as reported in the literature.
The patient is still undergoing follow-up, with no signs of metastasis or recurrence.
4. Conclusion
Secretory breast carcinomas are a rare form of breast cancer, and even rarer in male children. This case report takes on importance due to its infrequency, and the fact that there are few papers published about this subject, which hampers the standardization of diagnosis, treatment and establishing a prognosis. Therefore, the presented case can contribute to the literature with information about this rare type of breast tumor in children.
Declaration of Competing Interest
There is no conflict of interest.
Funding
There is no source of funding.
Ethical approval
This study was submitted and approved by the Ethic Committee of the Hospital of Clinics of Federal University of Paraná.
CAAE: 21477719.6.0000.0096.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Elis Novochadlo Klüppel: Validation, Investigation, Writing - Original Draft, Writing - Review & Editing, Visualization, Project administration Luiza Rodrigues da Costa: Validation, Investigation, Writing - Original Draft, Writing - Review & Editing, Visualization, Project administration Carolina Marquetto Tognolo: Conceptualization, Methodology, Validation, Investigation, Resources, Data Curation, Writing - Review & Editing, Visualization, Project administration Alexandre do Nascimento: Validation, Investigation, Resources, Visualization Melyssa Grignet Ribeiro: Conceptualization, Methodology, Validation, Investigation, Resources, Data Curation, Writing - Original Draft, Writing - Review & Editing, Visualization Camila Girardi Fachin: Conceptualization, Methodology, Validation, Investigation, Resources, Data Curation, Writing - Original Draft, Writing - Review & Editing, Visualization, Supervision, Project administration.
Registration of research studies
-
1.
Name of the registry: SECRETORY BREAST CARCINOMA IN A MALE CHILD: CASE REPORT AND LITERATURE REVIEW.
-
2.
Unique identifying number or registration ID: not a “first in man” case report.
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked): not a “first in man” case report.
Guarantor
Camila Girardi Fachin.
Provenance and peer review
Not commissioned, externally peer-reviewed.
References
- 1.Lombardi A., Maggi S., Bersigotti L., Lazzarin G., Nuccetelli E., Amanti C. Secretory breast cancer. Case report. G Chir. 2013;34:125–127. [PMC free article] [PubMed] [Google Scholar]
- 2.Osako T., Takeuchi K., Horii R., Iwase T., Akiyama F. Secretory carcinoma of the breast and its histopathological mimics: value of markers for differential diagnosis. Histopathology. 2013;63 doi: 10.1111/his.12172. [DOI] [PubMed] [Google Scholar]
- 3.Cabello C., Alvarenga M., Alvarenga C.A., Duarte G.M., Pereira P.N., Marshall P.S. Case report and review of the literature: secretory breast cancer in a 13-year-old boy—10 years of follow up. Breast Cancer Res. Treat. 2012;133:813–820. doi: 10.1007/s10549-011-1869-4. [DOI] [PubMed] [Google Scholar]
- 4.Szántó J., András C., Tsakiris J., Gomba S., Szentirmay Z., Bánlaki S., Szilágyi I., Kiss C., Antall P., Horváth Á., Lengyel L., Castiglione-Gertsch M. Secretory breast cancer in a 7.5-year old boy. Breast. 2004;13:439–442. doi: 10.1016/j.breast.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Brandt S.M., Swistel A.J., Rosen P.P. Secretory carcinoma in the axilla. Am. J. Surg. Pathol. 2009;33:950–953. doi: 10.1097/PAS.0b013e31819c2628. [DOI] [PubMed] [Google Scholar]
- 6.Kavalakat A.J., Covilakam R.K., Culas T.B. Secretory carcinoma of breast in a 17-year-old male. World J. Surg. Oncol. 2004;2:1–6. doi: 10.1186/1477-7819-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alenda C., Aranda F.I., Seguí F.J., Laforga J. Secretory carcinoma of the male breast: correlation of aspiration cytology and pathology. Diagn. Cytopathol. 2005;32:47–50. doi: 10.1002/dc.20157. [DOI] [PubMed] [Google Scholar]
- 8.Agha R.A., Borrelli M.R., Farwana R., Koshy K., Fowler A., Dennis P. The SCARE 2018 statement: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2018;(60):132–136. doi: 10.1016/j.ijsu.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 9.Instituto Nacional de Câncer José Alencar Gomes da Silva (INCA), (2018). http://www.inca.gov.br/estimativa/2016/sintese-de-resultados-comentarios.asp.
- 10.Lambertini M., Santoro L., Del Mastro L., Nguyen B., Livraghi L., Ugolini D., Peccatori F.A., Azim H.A. Reproductive behaviors and risk of developing breast cancer according to tumor subtype: a systematic review and meta-analysis of epidemiological studies. Cancer Treat. Rev. 2016;49:65–76. doi: 10.1016/j.ctrv.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 11.The World Health Organization Histological Typing of Breast Tumors—Second Edition. The World Organization. Am. J. Clin. Pathol. 1982;78:806–816. doi: 10.1093/ajcp/78.6.806. http://www.ncbi.nlm.nih.gov/pubmed/7148748 [DOI] [PubMed] [Google Scholar]
- 12.Page D.L., Anderson T.J. Diagnostic Histopathol. Breast. Churchill Livingstone; 1987. Uncommon types of invasive carcinoma; pp. 236–239. [DOI] [Google Scholar]
- 13.Yorozuya K., Takahashi E., Kousaka J., Mouri Y., Yoshida M., Fujii K., Akizuki M., Nakano S., Fukutomi T., Umemoto Y., Yokoi T., Imai H. A case of estrogen receptor positive secretory carcinoma in a 9-year-old girl with ETV6-NTRK3 fusion gene. Jpn. J. Clin. Oncol. 2012;42:208–211. doi: 10.1093/jjco/hyr187. [DOI] [PubMed] [Google Scholar]
- 14.Weigelt B., Reis-Filho J.S. Histological and molecular types of breast cancer: is there a unifying taxonomy? Nat. Rev. Clin. Oncol. 2009;6:718–730. doi: 10.1038/nrclinonc.2009.166. [DOI] [PubMed] [Google Scholar]
- 15.Tognon C., Knezevich S.R., Huntsman D., Roskelley C.D., Melnyk N., Mathers J.A., Becker L., Carneiro F., MacPherson N., Horsman D., Poremba C., Sorensen P.H.B. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell. 2002;2:367–376. doi: 10.1016/S1535-6108(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 16.Murphy J.J., Morzaria S., Gow K.W., Magee J.F. Breast cancer in a 6-year-old child. J. Pediatr. Surg. 2000;35:765–767. doi: 10.1053/jpsu.2000.6064. [DOI] [PubMed] [Google Scholar]
- 17.Herz H., Cooke B., Goldstein D. Metastatic secretory breast cancer. Non-responsiveness to chemotherapy: case report and review of the literature. Ann. Oncol. 2000;11:1343–1347. doi: 10.1023/A:1008387800525. [DOI] [PubMed] [Google Scholar]
- 18.Rosen P.P., Cranor M.L. Secretory carcinoma of the breast. Arch. Pathol. Lab. Med. 1991;115:141–144. http://europepmc.org/abstract/MED/1992979 [PubMed] [Google Scholar]
- 19.D’Amore E.S.G., Maisto L., Gatteschi M.B., Toma S., Canavese G. Secretory carcinoma of the breast. Report of a case with fine needle aspiration biopsy. Acta Cytol. 1986;30:309–312. [PubMed] [Google Scholar]