Abstract
The natural history of cognitive growth in the neuronopathic form of Mucopolysaccharidosis type II (MPS II) is not well defined especially their patterns of development and decline. The ability to predict the developmental course of the neurologically impaired patient is necessary to assess treatment outcomes aimed at the brain. Thirteen intravenous enzyme replacement therapy-treated Japanese patients with neuronopathic MPSII who had mutation analysis were followed on one standard measure of cognitive development over time. Six children in Group MS had missense mutations and 7 children in Group NT had null type mutations such as deletions, recombination with the pseudogene, and nonsense mutations. The patients as a whole demonstrated cognitive growth until about 36–42 months of age, followed by a plateau in development. The mean age equivalent score at age 3 was similar to that at age 6. While the decline was slow for the entire group, the patients in Group NT showed a more rapid decline than those in Group MS. Two patients with deletions showed decline to a very low level by age 5. The long plateau in cognitive development in patents with MPS II was substantiated and was consistent with other studies. This is the first demonstration that different mutation types within the neuronopathic MPS II patients are associated with different rates of decline. We also were able to identify the chronological age before which a trial would need to start in order to maintain cognitive growth and a ceiling beyond which a relatively normal outcome would not be likely.
Keywords: Mucopolysaccharidosis type II, Hunter syndrome, Developmental age, Cognitive natural history, Kyoto Scale of Psychological Development, Genotype
Abbreviations: CA, chronological age; AEq, age equivalent scores; KSPD, Kyoto Scale of Psychological Development; BSID-III, Bayley Scale of Infant Development- Third Edition
1. Introduction
The natural history of cognitive growth in the progressive neuronopathic form of Mucopolysaccharidosis type II (MPS II) is poorly defined due to the lack of data for children under four years of age and associations between mutation analysis and cognitive assessments. This study provides such data for 13 patients, clarifying the early course and the timing of decline. As new treatments designed to treat the central nervous system problems in brain are being developed and tested in clinical trials, the need for cognitive natural history of MPS II as a comparator is crucial [1]. The ability to predict the developmental course of the patient is necessary to assess treatment outcomes. The data presented here augments the data already in the literature about the cognitive developmental course in neuronopathic MPS II and will provide new information about patterns of decline among neuronopathic patients with various mutation types.
MPS II is an X-linked disorder defined by a deficiency of iduronidate-2-sulfatase resulting in accumulation of dermatan sulfate and heparan sulfate [2]. Two forms have been described [3]; both the neuronopathic form and the non-neuronopathic form are characterized by significant somatic disease affecting almost all bodily systems. Manifestations include hepatosplenomegaly, abnormal facies, joint stiffness, skeletal deformity, cardiac disease, lung disease, communicating hydrocephalus, and hearing loss [2]. The neuronopathic form has been characterized by relatively normal development in the first two years, increased slowing of cognitive development until age 5, and then a halt in the acquisition of new skills [4,5]. Variability in the timing of the halt in cognitive development and decline has been described clinically but not documented. Unlike patients with the other neuronopathic MPS disorders such as MPS IH and MPS III, some patients with MPS II appear to have a prolonged plateau of undefined length [6]. Other questions include whether some patients rapidly decline, whether eventual decline in skills is inevitable, and what the rate of decline might be. Both hydrocephalus and almost universal hearing loss also affect cognitive development, occurring in both forms the neuronopathic and non-neuronopathic forms of the disease [2].
No method can definitively predict whether a patient is neuronopathic or not. Biomarkers are not completely predictive, although, in general, GAGs are higher in children with the neuronopathic form [7]. MRI in early development has not been shown to differentiate the two forms until later in development when brain atrophy signals advanced neuronopathic disease [8]. Another specific characteristic of the neuronopathic form is behavioral abnormality which has been noted at the approximate time of cognitive change, although that timing has not been carefully documented [5].
Kosuga et al. [9] and a host of other authors [[10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26]] have found that deletions, recombination, frameshift and in most but not all cases, nonsense mutations are associated with severe forms of the disease. This literature also points to a subset of missense mutations that also lead to severe forms, while the majority of missense mutations result in attenuated disease. To classify patients into those who were more likely to decline at a more rapid rate, for our sample of severe neuronopathic patients, we separated the missense mutations from those with null mutations, deletions, and recombination to examine whether their patterns of cognitive decline would differ.
2. Methods
Subjects: Thirteen Japanese neuronopathic patients with MPSII were enrolled in this study. All were treated with enzyme replacement therapy (ERT). As enzyme administered intravenously does not cross the blood-brain-barrier, the cognitive status of the patient can be assumed not to be affected by this treatment. The diagnosis of MPSII was carried out based on enzyme activity of iduronate-2-sulfatase in leukocytes in addition to concentration of urinary glycosaminoglycans (GAGs). We recruited 13 already ascertained male patients with neuronopathic MPSII for this study. They all had received the Kyoto Scale of Psychological Development [27] administered by several different centers which regularly see children with rare diseases.
Subjects were divided into two groups based on literature findings; those with missense mutations, hypothesized to be somewhat milder, and those with deletions, recombination, and nonsense mutations, thought to be more severe. In group MS, six patients had the following missense mutations: G140V, K234N, R468Q, R88H, K236N, and Q121R. In Group NT, there were three patients with recombination with pseudogene IDS2 [27], one patient with a single base deletion (del163G), two patients with nonsense mutations (W337*, Y242*), and one patient with a gene deletion including all exons of IDS gene, respectively. Group NT was hypothesized to have a more severe clinical course.
Cognitive ability was evaluated by Kyoto Scale of Psychological Development (KSPD). All subtests were used to calculate the developmental age. The KSPD [27,28] is a standardized developmental assessment tool, developed and widely used in Japan for all age groups. To demonstrate its relationship to other more commonly used measures for very young children, it has been compared to the Bayley Scales of Infant Development III (BSID-III) in infants who were seven and 18 months of age. At 18 months the KSPD was shown to be significantly correlated with Bayley Scale cognitive age equivalents and DQ as well as motor skills, but not language and social skills [29]. An additional study suggests it correlates highly with the Japanese version of the Stanford Binet Intelligence Scale (Tanaka-Binet) in children with pervasive developmental disorder [30]. It was suggested to be an example of an alternative test for the BSID-III by the Consensus Conference on Cognitive endpoints [1].
To analyze the relationship between the developmental and chronological age of each group (MS and NT), we used a linear mixed-effects model, where random intercept, group (MS and NT), age-in-month (linear, quadratic, cubic), and interaction of group and age-in-months were included to this model. Least squared means of developmental age by the group for each age-in-months were estimated based on this model. In addition, both age equivalent scores and developmental quotients were plotted by chronological age for both groups.
3. Results
Table 1 provides details regarding individual patient data, chronological age at baseline and last visit, mutation type and group membership (group MS or group NT), age equivalent scores (developmental ages) and developmental quotients at baseline and last follow-up. Also, number of visits and elapsed time between baseline and last visit for each patient are documented.
Table 1.
Age and cognitive ability assessment in Group MS and Group NT patients with means and standard deviations.
| Chrono-logical age at baseline | Chrono-logical age at last visit | Number of visits | Develop-mental age at baseline | Develop-mental age at last visit | Change in develop-mental age | Elapsed time from baseline to last visit | Develop-mental quotient baseline | Develop-mental quotient last visit | |
|---|---|---|---|---|---|---|---|---|---|
| Group Group MS | |||||||||
| 1.G140V | 28 | 47 | 6 | 24 | 26 | 2 | 19 | 86 | 55 |
| 2.K234N | 15⁎ | 78 | 12 | 11 | 39 | 28 | 63 | 73 | 50 |
| 3. R468Q | 45 | 116 | 9 | 30 | 27 | −3 | 71 | 67 | 23 |
| 4. K236N | 46 | 60 | 3 | 36 | 38 | 2 | 14 | 78 | 63 |
| 5. R88H | 37 | 69 | 3 | 18 | 19 | 1 | 32 | 49 | 28 |
| 6. Q121R | 30 | 95 | 8 | 18 | 16 | −2 | 65 | 60 | 17 |
| Mean | 37.2 | 77.5 | 6.8 | 22.8 | 27.5 | 4.7 | 44.0 | 68.8 | 39.4 |
| Standard deviation | 8.3 | 24.9 | 3.5 | 9.1 | 9.5 | 11.6 | 25.3 | 13.3 | 19.2 |
| Group NT | |||||||||
| 1. W337X | 43 | 90 | 7 | 22 | 20 | −2 | 47 | 51 | 22 |
| 2.Y242X | 36 | 64 | 3 | 15 | 19 | 4 | 28 | 42 | 30 |
| 3. 163delG | 22 | 83 | 7 | 18 | 12 | −6 | 61 | 82 | 14 |
| 4. Whole gene deletion | 34 | 78 | 10 | 24 | 12 | −12 | 44 | 71 | 15 |
| 5. Recom-bination with IDS 2 | 35 | 56 | 2 | 23 | 26 | 3 | 21 | 66 | 46 |
| 6. Recom-bination with IDS 2 | 39 | 54 | 2 | 19 | 21 | 2 | 15 | 49 | 39 |
| 7. Recom-bination with IDS 2 | 6⁎ | 58 | 8 | 5 | 34 | 29 | 52 | 83 | 59 |
| Mean | 34.8 | 69.0 | 5.6 | 18.0 | 20.6 | 2.6 | 38.3 | 63.4 | 32.2 |
| Standard deviation | 7.1 | 14.5 | 3.2 | 6.5 | 7.7 | 13.0 | 17.1 | 16.4 | 16.7 |
All timed data is in months with the exception of gain/loss which is calculated by year.
child is under 1.5 years of age
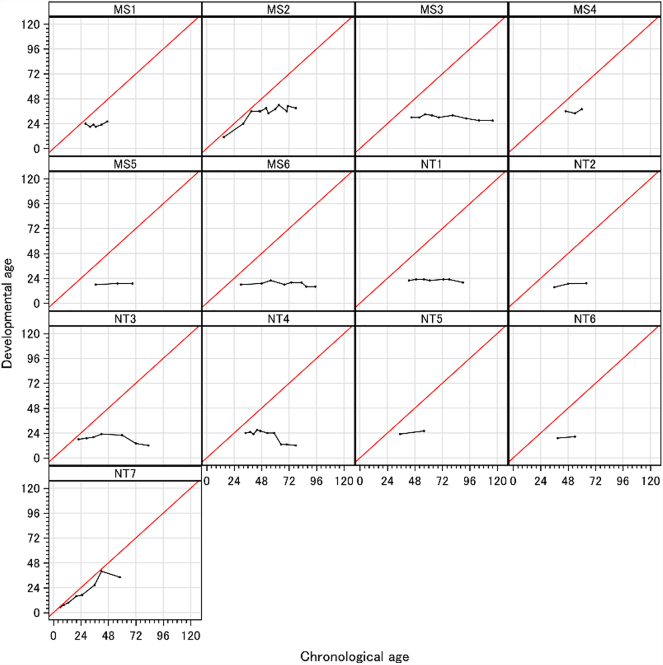
Groups MS and NT show no difference in mean age. All patients in group MS demonstrate little change in developmental age over time, all falling within 3 months gain or loss with the exception of patient MS2 who was the youngest in that group at baseline (15 months) who continued to show cognitive developmental gain over time. In group NT, with the exception of patient NT7 (who was 6 months of age and showed a developmental gain) and patient NT4 (who lost cognitive skills), patients also showed little change over time. The results indicate that the Group MS has a slightly slower cognitive developmental decline than group NT. The calculated overall p value between difference in change of developmental age Group MS and Group NT was calculated to be p = .00033. Age equivalent scores (developmental ages) for the two groups are plotted in Fig. 1.
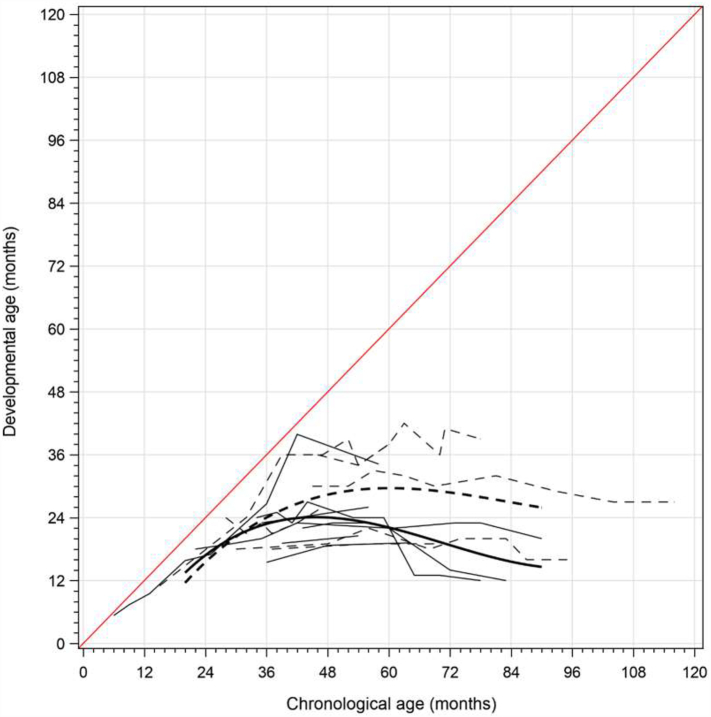
Fig. 1.
(a) Growth curve of the developmental age of each patient. (b) Growth curve of the developmental age by the group. The growth curve was estimated by the least squared means based on the linear mixed-effect model. X and Y axis indicates actual chronological age and the developmental age, respectively. Dashed and solid lines were in group MS and NT, respectively. Linear curves with lighter colors show individual developmental age for each visit.
Case results: Group MS, consisting of patients with missense mutations, had a variable course. Patient MS2 with the K234N mutation, was the highest functioning of all 13 patients. He showed marked growth up to 39 months of age (chronological age, CA) at which time he was functioning at 42 months (age equivalent scores, AEq). But after 39 months (CA), he gained only 6 months in age equivalent score in the next 29 months up until his last visit at 78 months old (CA). Patient MS4 with the K236N mutation, was followed from 46 to 60 months (CA) and neither gained or lost skills. These two patients, while significantly impaired relative to their peers, demonstrate a plateau in skills after 36 months (CA) but no documented decline. The other four missense patients were functioning at a much lower level (at a 30 months AEq level or less) but did not vary more than 3 age equivalent months from their baseline score which is likely to represent the limits of measurement error. Patient MS6 with the Q121R mutation, was followed for a total of 65 months from age 30 months to 95 months (CA); he was functioning at 18 months (AEq) at baseline and 16 months (AEq) at last follow-up. The plateau in development in which the patient did neither loss or gain skills was noted up to 65 months (CA). The remainder of the missense mutation patients also demonstrated this plateau.
In Group NT, patient NT7 with a recombination, was first assessed at 6 months (CA) and was within normal limits. He continued to gain skills until 42 months (CA). He only had one visit afterwards at 58 months (CA), but he lost 6 months of skills. Two other patients NT5 and NT6 who were 35 and 39 months (CA) at baseline were stable, but very low in AEqs over a 15–20 month period. Similarly, the patients with nonsense mutations were quite low functioning, starting from 36 and 43 months AEqs, neither losing skills over the period they were followed (28 to 47 months CA).
Patients NT3 and NT4 with deletions showed a similar pattern, one initially stable from 22 to 60 months (CA) and the other from 34 to 59 months (CA). Both declined after this and by the time they were 83/78 months (CA), they were functioning at a 12 months AEq level. This may be due either to their genotype or as a result of the long period they were followed. The three patients who were followed who were older than 6.5–7.5 years of age functioned at a very low level in group NT (12, 12, and 20 months DA), but slightly higher in group MS (39, 16, 27 DA).
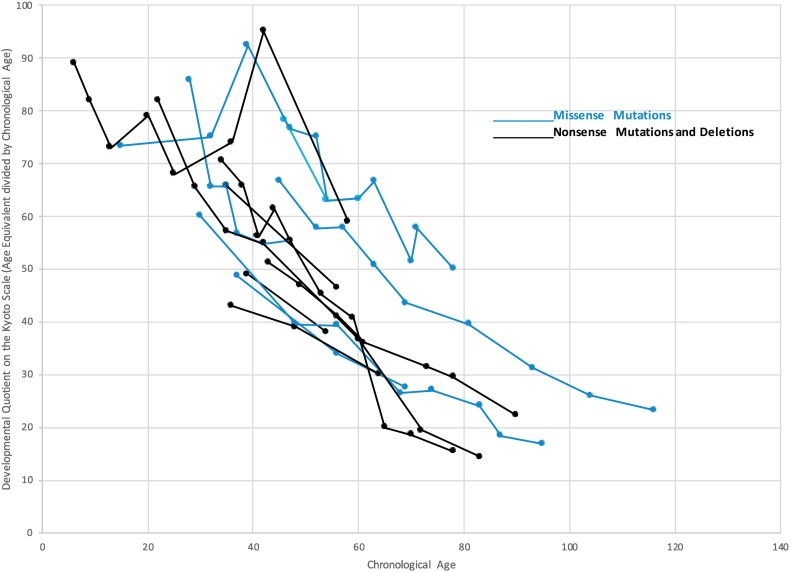
Developmental quotients did not separate out those who were still developing as clearly as age equivalents did, as all patients showed steady decline relative to same age peers. Fig. 2 demonstrates the steady downward trajectory of developmental quotients but does not reveal the early addition of cognitive skills in the youngest patients.
Fig. 2.
Developmental quotients of groups MS and NT by chronological age.
4. Discussion
Studies from many countries of genotype/phenotype correlations have documented that patients with deletions, recombination, frameshifts, and splicing abnormalities usually have a severe phenotype [[9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26]]. Also, severe phenotypes may be associated with most but not all nonsense mutations. Some nonsense mutations such as R443*, R8*, and Y103* are associated with intermediate or attenuated disease [9,10,13,17,18,24,25]. Missense mutations are even more problematic because there are many associated with the neuronopathic phenotype. Kosuga et al. [9] found of children with 33 missense mutations, 21 were non-neuronopathic. In addition, of the 8 nonsense mutations, 6 were neuronopathic. From these studies, severity can be predicted for deletions, recombination, frameshifts and splicing mutations. Because of the many private mutations, heterogeneity, and uncertainty regarding phenotype, prediction of the phenotype from the genotype is uncertain in many cases but it will be of increasing importance in clinical trials with new treatments to determine the patient's developmental trajectory.
Of reported studies of genotype/phenotype correlation, only two [21,26] had objective measurements to document severity. Even in these two cases, criteria varied. Only in the study by Vollebregt et al. [26], did neuropsychological testing contribute to the classification. In all other studies, clinical judgment of developmental delay was used. Using objective criteria is particularly crucial in the case of intermediate severity in which no specific demarcation criteria exists between intermediate and mild or severe cases. In our study, this need for objective measurement was addressed through the use of the KSPD at every visit for these patients.
Importantly, these studies in the literature do not examine the cognitive developmental trajectory of various levels of severity associated with mutation type. While the trajectories can distinguish attenuated from neuronopathic, we wanted to know whether the patterns of decline of the more predictably neuronopathic mutations, as in Group NT, were different from those with the missense mutations in Group MS. While we can say that a difference is supported by our data, especially for the patients with deletions, is the difference sufficient to alter expectations for these two groups in a clinical trial? We clearly need a larger subject group to verify these results.
This may be the first study of differing patterns of cognitive development in neuronopathic patients by mutation type with actual assessments on one instrument. The other longitudinal studies such as Holt et al. [5] and Young et al. [31] have examined developmental trajectories, but both relied on multiple instruments. While they are consistent with the findings in our overall group, the overall levels are higher in the Young et al. study and lower in the Holt et al. study. This may be due to the effects of the measures, differing patient ascertainment or samples, or differing incidences of mutations as our study was in Japan, the Young et al. study in the UK, and the Holt et al. study in the US.
For the entire group, our results demonstrate the cognitive clinical course of genetically defined patients with MPS II. The range of chronological age which our patients received KSPD is from 6 to 116 months as described in Table 1. All patients continued to add skills through age 3. There were two very young patients who continued to show cognitive growth. One patient, recruited at 15 months CA in Group MS, demonstrated a significant increase in AEq scores prior to ceasing development at 39 months CA, varying from 36 to 42 months AEq score until his last visit at a CA of 78 months. Similarly, a patient in Group NT, who was seen initially at 5 months continued to develop until 42 months CA at which time he showed an AEq score of 40 months, but by 58 months CA declined to an AEq of 34 months. AEqs allow examination of such cognitive growth, whereas DQ does not as demonstrated in Fig. 2.
While these very young two patients continued to develop until 36 to 42 months similar to the other patients in the study, a plateau followed that lasted for some children for several years. However, many of the children very gradually lost skills over time. For the group as a whole the mean AEq at age 3 was similar to that at age 6.
We had hypothesized that missense mutations, even when found in the neuronopathic patients, would show a more attenuated decline than in nonsense mutations, deletions, and rearrangements. By classifying our patients into two groups, we found a statistically significant different trajectory of decline. Whether the size of the differences between the patterns of decline in the two groups is clinically relevant remains to be seen.
In this study, we have shown the difference of rate of cognitive decline by genotype of IDS gene. Patients in Group MS shows slower rate of decline than those in Group NT. These differences could be partially explained by cross-reactive immunological material (CRIM) status of the mutant IDS enzyme. Most of the patients in Group MS are considered to be CRIM-positive, and they may have residual IDS activity. On the other hand, most of the patients in Group NT are considered to be CRIM-negative, and they do not have any residual IDS activity. To evaluate CRIM status and enzyme activity accurately, further analysis using skin culture fibroblasts is necessary.[32].
In conclusion, the present study examined the overall cognitive trajectory of neuronopathic MPS II as well as differing patterns by mutation type. The limitation of this study is that the patients were seen at different intervals and frequencies. Overall, despite this limitation, the pattern of long plateaus in development was apparent and consistent with other studies [7]. Even though one might hypothesize that they may be more responsive to treatment, Group MS showed notably long plateaus in development, which will be a significant challenge in to demonstrate treatment effect in clinical trials. However, because no patient showed cognitive development past an AEq of 4 years, for these children, beginning a trial before they plateau (before three years of age) with success defined as cognitive developmental progression past an AEq of 4 years might be an advantageous approach.
In Group NT, two patients with deletions declined to a very low level; and by a CA of 5 to 6 years of age, they declined to under an AEq of 18 months. This suggests a somewhat more severe and earlier decline in patients with deletions. Individualized expectations depending on genotype could shorten the time period if this more rapid decline is verified in a larger number of patients. Clearly, larger samples of children with specific MPS II mutations in a prospective treatment study would be ideal if their rate of decline might be anticipated. However, waiting to treat before decline is anticipated may be too late. With protein and gene replacement imminent, early treatment, initiated before the child reaches a plateau in development, is a better alternative.
Developmental quotients were obtained from Kyoto scale of psychological development,2001. Blue lines show the data of Group MT, and black lines shows the data of Group NT.
Acknowledgements
The authors would like to thank Eisuke Inoue and Masashi Mikami for their support of statistical analysis. This research was sponsored by JCR Pharmaceuticals Co., Ltd.
Refferences
- 1.van der Lee J.H., Morton Jonathan, Adams Heather R., Clarke Lorne, Ebbink Berendine Johanne, Escolar Maria L., Giugliani Roberto, Harmatz Paul, Hogan Melissa, Jones Simon, Kearney Shauna, Muenzer Joseph, Rust Stewart, Semrud-Clikeman Margaret, Wijburg Frits A., Yu Zi-Fan, Janzen Darren, Shapiro Elsa. Cognitive endpoints for therapy development for neuronopathic mucopolysaccharidoses: results of a consensus procedure. Mol. Genet. Metab. 2017;121:70–79. doi: 10.1016/j.ymgme.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Muenzer J., Beck M., Eng C.M., Escolar M.L., Giugliani R., Guffon N.H., Harmatz P., Kamin W., Kampmann C., Koseoglu S.T., Link B., Martin R.A., Molter D.W., Munoz Rojas M.V., Ogilvie J.W., Parini R., Ramaswami U., Scarpa M., Schwartz I.V., Wood R.E., Wraith E. Multidisciplinary management of Hunter syndrome. Pediatrics. 2009;124:e1228–e1239. doi: 10.1542/peds.2008-0999. [DOI] [PubMed] [Google Scholar]
- 3.Tylki-Szymańska A. Mucopolysaccharidosis type II, Hunter’s syndrome. Pediatr. Endocrinol. Rev. 2014;12(Suppl. 1):107–113. [PubMed] [Google Scholar]
- 4.Barone R., Pellico A., Pittalà A., Gasperini S. Neurobehavioral phenotypes of neuronopathic mucopolysaccharidoses. Ital. J. Pediatr. 2018;44:121. doi: 10.1186/s13052-018-0561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holt J.B., Poe M.D., Escolar M.L. Natural progression of neurological disease in mucopolysaccharidosis type II. Pediatrics. 2011;127:e1258–e1265. doi: 10.1542/peds.2010-1274. [DOI] [PubMed] [Google Scholar]
- 6.Muenzer Joseph, Burton Barbara K., Harmatz Paul, Amartino Hernan, Jones Simon A., González Luis, Gutiérrez-Solana Matilde Ruiz-Garcia, Wu Yuna, Alexanderian David. Neurodevelopmental status and adaptive behavior of pediatric patients with Hunter syndrome: a longitudinal observational study. Mol. Genet. Metab. 2019;126:S103. doi: 10.1186/s13023-023-02805-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hendriksz C.J., Muenzer J., Vanderver A., Davis J.M., Burton B.K., Mendelsohn N.J., Wang N., Pan L., Pano A., Barbier A.J. Levels of glycosaminoglycans in the cerebrospinal fluid of healthy young adults, surrogate-normal children, and Hunter syndrome patients with and without cognitive impairment. Mol. Genet. Metab. Rep. 2015;5:103–106. doi: 10.1016/j.ymgmr.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan Z., Styner M., Muenzer J., Poe M., Escolar M. Correlation of automated volumetric analysis of brain MR imaging with cognitive impairment in a natural history study of mucopolysaccharidosis II. Am. J. Neuroradiol. 2010;31:1319–1323. doi: 10.3174/ajnr.A2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kosuga M., Mashima R., Hirakiyama A., Fuji N., Kumagai T., Seo J.H., Nikaido M., Saito S., Ohno K., Sakuraba H., Okuyama T. Molecular diagnosis of 65 families with mucopolysaccharidosis type II (Hunter syndrome) characterized by 16 novel mutations in the IDS gene: genetic, pathological, and structural studies on iduronate-2-sulfatase. Mol. Genet. Metab. 2016;118:190–197. doi: 10.1016/j.ymgme.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Alcántara-Ortigoza M.A., García-de Teresa B., González-del Angel A., Berumen J., Guardado-Estrada M., Fernández-Hernández L., Navarrete-Martínez J.I., Maza-Morales M., Rius-Domínguez R. Wide allelic heterogeneity with predominance of large IDS gene complex rearrangements in a sample of Mexican patients with Hunter syndrome. Clin. Genet. 2016;89:574–583. doi: 10.1111/cge.12738. [DOI] [PubMed] [Google Scholar]
- 11.Alkhzouz C., Lazea C., Bucerzan S., Nascu I., Kiss E., Denes C.L., Grigorescu-Sido P. Clinical and genetic characteristics of romanian patients with mucopolysaccharidosis type II. JIMD Rep. 2016;33:19–25. doi: 10.1007/8904_2016_535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amartino H., Ceci R., Masllorens F., Gal A., Arberas C., Bay L., Ilari R., Dipierri J., Specola N., Cabrera A., Rozenfeld P. Identification of 17 novel mutations in 40 Argentinean unrelated families with mucopolysaccharidosis type II (Hunter syndrome) Mol. Genet. Metab. Rep. 2014;1:401–406. doi: 10.1016/j.ymgmr.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brusius-Facchin A.C., Schwartz I.V., Zimmer C., Ribeiro M.G., Acosta A.X., Horovitz D., Monlleó I.L., Fontes M.I., Fett-Conte A., Sobrinho R.O., Duarte A.R. Mucopolysaccharidosis type II: identification of 30 novel mutations among Latin American patients. Mol. Genet. Metab. 2014;111:133–138. doi: 10.1016/j.ymgme.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Bunge S., Steglich C., Zuther C., Beck M., Morris C.P., Schwinger E., Schinzel A., Hopwood J.J., Gal A. Iduronate-2-sulfatase gene mutations in 16 patients with mucopolysaccharidosis type II (Hunter syndrome) Hum. Mol. Genet. 1993;2:1871–1875. doi: 10.1093/hmg/2.11.1871. [DOI] [PubMed] [Google Scholar]
- 15.Chang J.H., Lin S.P., Lin S.C., Tseng K.L., Li C.L., Chuang C.K., Lee-Chen G.J. Expression studies of mutations underlying Taiwanese Hunter syndrome (mucopolysaccharidosis type II) Hum. Genet. 2005;116:160–166. doi: 10.1007/s00439-004-1234-3. [DOI] [PubMed] [Google Scholar]
- 16.Chiong M.A., Canson D.M., Abacan M.A., Baluyot M.M., Cordero C.P., Silao C.L. Clinical, biochemical and molecular characteristics of Filipino patients with mucopolysaccharidosis type II-Hunter syndrome. Orphanet J. Rare Dis. 2017;12:7. doi: 10.1186/s13023-016-0558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dvorakova L., Vlaskova H., Sarajlija A., Ramadza D.P., Poupetova H., Hruba E., Hlavata A., Bzduch V., Peskova K., Storkanova G., Kecman B. Genotype–phenotype correlation in 44 Czech, Slovak, Croatian and Serbian patients with mucopolysaccharidosis type II. Clin. Genet. 2017;91:787–796. doi: 10.1111/cge.12927. [DOI] [PubMed] [Google Scholar]
- 18.Filocamo M., Bonuccelli G., Corsolini F., Mazzotti R., Cusano R., Gatti R. Molecular analysis of 40 Italian patients with mucopolysaccharidosis type II: new mutations in the iduronate-2-sulfatase (IDS) gene. Hum. Mutat. 2001;18:164–165. doi: 10.1002/humu.1169. [DOI] [PubMed] [Google Scholar]
- 19.Froissart R., Da Silva I.M., Guffon N., Bozon D., Maire I. Mucopolysaccharidosis type II–genotype/phenotype aspects. Acta Paediatr. 2002;91:82–87. doi: 10.1111/j.1651-2227.2002.tb03116.x. [DOI] [PubMed] [Google Scholar]
- 20.Froissart R., Da Silva I.M., Maire I. Mucopolysaccharidosis type II: an update on mutation spectrum. Acta Paediatr. 2007;96:71–77. doi: 10.1111/j.1651-2227.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 21.Galvis J., González J., Uribe A., Velasco H. Deep genotyping of the IDS gene in Colombian patients with Hunter syndrome. JIMD Rep. 2014;192:101–109. doi: 10.1007/8904_2014_376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gort L., Chabas A., Coll M.J. Hunter disease in the Spanish population: molecular analysis in 31 families. J. Inherit. Metab. Dis. 1998;21:655–661. doi: 10.1023/a:1005432600871. [DOI] [PubMed] [Google Scholar]
- 23.Isogai K., Sukegawa K., Tomatsu S., Fukao T., Song X.Q., Yamada Y., Fukuda S., Orii T., Kondo N. Mutation analysis in the iduronate-2-sulphatase gene in 43 Japanese patients with mucopolysaccharidosis type II (Hunter disease) J. Inherit. Metab. Dis. 1998;21:60–70. doi: 10.1023/a:1005363414792. [DOI] [PubMed] [Google Scholar]
- 24.Sohn Y.B., Ki C.S., Kim C.H., Ko A.R., Yook Y.J., Lee S.J., Kim S.J., Park S.W., Yeau S., Kwon E.K., Han S.J. Identification of 11 novel mutations in 49 Korean patients with mucopolysaccharidosis type II. Clin. Genet. 2012;81:185–190. doi: 10.1111/j.1399-0004.2011.01641.x. [DOI] [PubMed] [Google Scholar]
- 25.Vafiadaki E., Cooper A., Heptinstall L.E., Hatton C.E., Thornley M., Wraith J.E. Mutation analysis in 57 unrelated patients with MPS II (Hunter’s disease) Arch. Dis. Child. 1998;79:237–241. doi: 10.1136/adc.79.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vollebregt A.A., Hoogeveen-Westerveld M., Kroos M.A., Oussoren E., Plug I., Ruijter G.J., van der Ploeg A.T., Pijnappel W.P. Genotype–phenotype relationship in mucopolysaccharidosis II: predictive power of IDS variants for the neuronopathic phenotype. Dev. Med. Child Neurol. 2017;59:1063–1070. doi: 10.1111/dmcn.13467. [DOI] [PubMed] [Google Scholar]
- 27.Ikuzawa M., Iwachidou S., Oogami R. In: A guide of Kyoto Scale of psychological development. Ikuzawa M., Matsushita Y., Nagase A., editors. Kyoto Kokusai Shakaifukushi Center; Kyoto: 2001. [Google Scholar]
- 28.Janzen D., Delaney K., Shapiro E. Cognitive and adaptive measurement endpoints for clinical trials in mucopolysaccharidoses types I, II, and III: a review of the literature. Mol. Genet. Metab. 2017;121:57–69. doi: 10.1016/j.ymgme.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Kono Y., Yonemoto N., Kusuda S., Hirano S., Iwata O., Tanaka K., Nakazawa J. Developmental assessment of VLBW infants at 18 months of age: A comparison study between KSPD and Bayley III. Brain Dev. 2016 Apr;38(4):377–385. doi: 10.1016/j.braindev.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Koyama T., Osada H., Tsujii H., Kurita H. Utility of the Kyoto Scale of Psychological Development in cognitive assessment of children with pervasive developmental disorders. Psychiatry Clin. Neurosci. 2009;63:241–243. doi: 10.1111/j.1440-1819.2009.01931.x. [DOI] [PubMed] [Google Scholar]
- 31.Young I.D., Harper P.S., Archer I.M., Newcombe R.G. A clinical and genetic study of Hunter’s syndrome. 1 Heterogeneity. J. Med. Genet. 1982;19:401–407. doi: 10.1136/jmg.19.6.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karageorgos L., Brooks D.A., Pollard A., Melville E.L., Hein L.K., Clements P.R., Ketteridge D., Swiedler S.J., Beck M., Giugliani R., Harmatz P., Wraith J.E., Guffon N., Leão Teles E., Sá Miranda M.C., Hopwood J.J. Mutational analysis of 105 mucopolysaccharidosis type VI patients. Hum. Mutat. 2007 Sep;28(9):897–903. doi: 10.1002/humu.20534. [DOI] [PubMed] [Google Scholar]