Abstract
Background
We sought to investigate the possible associations of rest-activity patterns with cortical amyloid burden, medial temporal lobe (MTL) neurodegeneration, and cognitive function in patients in the early stage of cognitive impairment.
Methods
Rest-activity patterns were assessed in 100 participants (70 with mild cognitive impairment and 30 with mild dementia) using wrist actigraphy. All participants underwent 18F-flutemetamol positron emission tomography (PET) imaging to quantify cortical amyloid burden, structural brain magnetic resonance imaging (MRI) to quantify MTL grey matter volume, neuropsychological testing, and clinical diagnosis. We used multiple linear regression models adjusted for covariates, including demographics, diabetes, hypertension, depressive symptom, psychotropic medication, sleep medication, weekend effect, and apolipoprotein-ε allele status.
Findings
After adjusting for possible confounders, we found that the midline estimation of statistic of rhythm (MESOR) associated positively with frontal/executive function (estimate = 1.17, standard error [SE] = 0.37, p = 0.002). The least active 5-h (L5) onset time associated positively with MTL grey matter volume and memory function (estimate = 1.24, SE = 0.33, p = 0.001, and estimate = 3.77, SE = 1.22, p = 0.003, respectively), particularly in amyloid-negative participants. Additional path analysis revealed that MTL grey matter volume partially mediated the association between L5 onset time and memory function in amyloid-negative participants.
Interpretation
Decreased MESOR and advanced L5 onset time may be useful as early signs of cognitive decline or MTL neurodegeneration. Furthermore, amyloid pathology may act as a moderator of the relationships between rest-activity patterns, neurodegeneration, and cognitive function.
Funding
Korea Centres for Disease Control and Prevention (#4845–303); National Research Foundation of Korea (2019M3C7A1031905, 2019R1A5A2026045).
Keywords: Rest-activity patterns; Mild cognitive impairment; Dementia; Cognition; Medial temporal lobe; Amyloid burden; Mesor, L5 onset time
Research in context.
Evidence before this study
Recent animal studies indicate that a circadian rhythm deficit in the central nervous system accelerates neurodegenerative changes by increasing oxidative stress, inflammation, astrogliosis, blood-brain barrier permeability, and amyloid plaque formation. Nonetheless, relatively little is known about the impact of circadian rhythm disturbance and rest-activity pattern changes on neurodegeneration in humans. Especially, much more research needs to be done in patients with early stage of cognitive impairment to elucidate the relationships between rest-activity pattern, neurodegeneration, and cognitive function. A few case-control studies have been reported; however, the data are inconsistent regarding changes in rest-activity pattern in this population. Active phase advance, rest or sleep phase advance, decreased rhythm amplitude, and increased daytime nap with dampened melatonin profile have been reported, but these studies were limited by small sample sizes as well as a lack of neuroimaging biomarkers and detailed neuropsychological tests.
Added value of this study
In the present study, we investigated associations of rest-activity patterns with cortical amyloid burden, medial temporal lobe (MTL) neurodegeneration, and cognitive function in patients in the early stage of cognitive impairment. We found that MESOR, which corresponds to the rhythm-adjusted mean activity, associated positively with frontal/executive function. Furthermore, L5 onset time, which corresponds to the time of rest period onset, associated positively with MTL grey matter volume and memory function, particularly in amyloid-negative participants. Additional path analysis revealed that MTL grey matter volume partially mediates the relationship between L5 onset time and memory function in amyloid-negative participants.
Implications of all the available evidence
The findings of our study suggest rest-activity pattern changes in early stage of cognitive impairment may be useful as early signs of cognitive decline or neurodegeneration. In addition, future research efforts should consider the potential moderating effects of cortical amyloid burden on relationships between rest-activity patterns, neurodegeneration, and cognitive function.
Alt-text: Unlabelled box
1. Introduction
Circadian rhythms are near 24-h oscillations in behavioural, physiological, cellular, and biochemical processes [1]. In mammals, the principal circadian oscillator is comprised of cell-autonomous transcriptional and post-translational feedback loops in the suprachiasmatic nucleus (SCN) of the hypothalamus [2]. The SCN carries circadian rhythm output signals via projection neurons to multiple brain regions that contain the local circuits responsible for various biological processes. For example, the spontaneous firing rate in SCN neurons affects daily rest-activity patterns in mammals [3].
Circadian rhythm output from the SCN decreases with age [4, 5]. Whether the reduced circadian output is a physiological change due to ageing or a pathological process remains controversial; however, increasing evidence suggests that circadian rhythm disturbances occur early in the course of neurodegenerative disorders and may potentially contribute to the pathogenesis and progression of disease [6]. Recent animal studies indicate that a circadian rhythm deficit in the central nervous system accelerates neurodegenerative changes by increasing oxidative stress, inflammation, astrogliosis, blood-brain barrier permeability, and amyloid plaque formation [7], [8], [9], [10]. Despite these reports, relatively little is known about the impact of circadian rhythm disturbance and rest-activity pattern changes on neurodegeneration in humans.
Dementia, a common health problem in older adults, is defined by the deterioration of cognitive function and ability to perform everyday activities. Alzheimer's disease (AD) with cortical amyloid burden as a prominent neuropathological biomarker is the most common neurodegenerative disease causing dementia. Non-Alzheimer's dementia that is free of prominent cortical amyloid burden, includes frontotemporal lobe dementia (FTLD), Lewy body dementia (LBD), vascular dementia, or a mixture of these pathologies [11], [12], [13]. Importantly, vascular pathologies can contribute to AD aetiology as well. Evaluation of dementia patients requires not only cognitive and neurological examination, but also assessment of available disease biomarkers such as cortical amyloid burden. However, it is still unclear whether or not rest-activity pattern changes depend on the aetiology of neurodegeneration [14].
Rest-activity pattern changes have been reported in numerous studies of patients with moderate to severe dementia, as reviewed in [14]. Decreased total activity, fragmented 24-h activity rhythm, blunted rhythm amplitude, and phase delay have been documented in these patients [15], [16], [17], [18], [19]. Nonetheless, much more research needs to be conducted in patients in the early stage of cognitive impairment to elucidate the relationships between rest-activity pattern, neurodegeneration, and cognition [14, 20]. A few case-control studies have been reported; however, the data are inconsistent regarding changes in rest-activity pattern in this population [21]. Active-phase advance [22], rest or sleep phase advance [22, 23], decreased rhythm amplitude [24], and increased daytime nap with a dampened melatonin profile [25] have been reported. However, most of these studies were limited by small sample sizes as well as a lack of neuroimaging biomarkers and detailed neuropsychological tests.
In the present study, we examined the rest-activity patterns of patients with mild cognitive impairment (MCI) and mild dementia using actigraphy. In addition, we performed detailed neuropsychological tests and assessed neuroimaging biomarkers such as cortical amyloid burden, and medial temporal lobe (MTL) neurodegeneration. We investigated the possible associations of rest-activity patterns with cortical amyloid burden, MTL neurodegeneration, and cognitive function, thereby evaluating the possibility of actigraphy-based rest-activity pattern variables as biomarkers in patients with MCI and mild dementia.
2. Materials and methods
2.1. Participants
Study participants were from the Chronic Cerebrovascular Disease Consortium at Ajou University Hospital (Suwon, Korea), which is a disease-orientated biobank supported and funded by the Korea Centres for Disease Control and Prevention. The Chronic Cerebrovascular Disease Consortium recruited participants who complained of changes in cognition by an affected individual or observers from six hospital-based outpatient clinics at tertiary referral centres and two community-based mental health centres. Rest-activity patterns were measured in participants recruited at Ajou University Hospital only. Of the 170 participants who registered for the Chronic Cerebrovascular Disease Consortium at Ajou University Hospital, our final analysis included 70 patients who presented with MCI and 30 patients who displayed mild dementia from November 2016 to December 2018. Given that MCI and mild dementia lie on a continuum of the early stages of cognitive impairment, those individuals were combined in our analysis. A flow diagram illustrating how study participants were selected is shown in Fig. S1.
Patients with MCI were evaluated using the expanded Mayo Clinic criteria [26]. Patients with mild dementia were evaluated using the National Institute on ageing-Alzheimer's Association core clinical criteria for all-cause dementia [27] with two additional conditions: a Clinical Dementia Rating (CDR) global score of 1 and a Mini-Mental State Examination (MMSE) score of at least 15. We excluded patients who met at least one of the following criteria: (1) possible behavioural variant frontotemporal lobar degeneration, (2) possible Lewy body dementia, and (3) history of a neurological or medical condition, such as territorial cerebral infarction, intracranial haemorrhage, idiopathic parkinsonism, heart failure, or renal failure, or other conditions that could interfere with the study.
2.2. Standard protocol approval, registration, and patient consent
The Chronic Cerebrovascular Disease Consortium is registered at the Korean National Clinical Trial Registry CRIS (identifier: KCT0003391). The study was approved by the Institutional Review Board of Ajou University Hospital (AJIRB-BMR-SUR-16–362). Written informed consent was obtained from all participants and caregivers.
2.3. Measurement of rest-activity patterns
All participants were invited to wear a research-grade triaxial accelerometer (Fitmeter; Fit.Life Inc, Suwon, Korea) [28, 29] on their non-dominant wrist for at least 7 days while performing their usual daily activities in a home setting. Activity counts in 1-min epochs from the first 4 consecutive days of data, starting at midnight, were processed to calculate rest-activity pattern variables. We adjusted for the number of weekend days to control for a potential weekend effect. In fact, the majority of participants (85%) reported that they did not have a regular job during the assessment period. Of the 100 participants, 17 participants had irregular missing data periods, indicating device removal during their 4-day assessment period. To control for this, we applied the recently developed imputation method ‘accelmissing’, which was developed for accelerometer data and validated using 2003−2004 National Health and Nutrition Examination Survey data [30]. In our study, the mean imputation time was 77 min/day from the 17 participants, representing less than 1% of the total data time of all participants.
We calculated rest-activity pattern variables using two different methods, namely cosinor analysis and nonparametric analysis. Cosinor analysis fits the raw activity counts to cosine curves using the least-square method [31]. From the cosinor analysis, we calculated the (1) robustness (goodness-of-fit for cosine curve, generally represents the rhythmicity of the circadian activity pattern), (2) midline estimation of statistic of rhythm (MESOR, generally represents the mean of the activity fitted curve), (3) amplitude (peak to nadir difference in activity), and (4) acrophase (timing of peak activity). We also performed nonparametric analysis, which does not have a priori assumptions about the waveform of daily activity but instead calculates variables based on raw activity counts [32]. We calculated the (1) inter-daily stability (IS, generally represents the strength of coupling of a rhythm to environmental zeitgebers), (2) intra-daily variability (IV, generally represents activity fragmentation in a day), (3) least active 5-h (L5) onset time, and (4) most active 10-h (M10) onset time from the nonparametric analysis. Analyses were performed using in-house-developed Python code, which was validated using ‘Cosinor’ software (developed by Refinetti) and the ‘nparACT’ package in the R Statistical software [33, 34]. The distribution of eight rest-activity pattern variables is shown in Fig. S2.
2.4. Cognitive function assessment
Cognitive function was evaluated using a standardised neuropsychological test called the Seoul Neuropsychological Screening Battery (SNSB) [35]. The SNSB includes tests of language, visuospatial, memory, frontal/executive function, and depressive symptoms. The language function was based on the Boston Naming Test (range, 0–60). The visuospatial function was based on the Rey Complex Figure Test (range, 0–36). The memory function was calculated by summing scores from verbal memory (Seoul Verbal Learning Test immediate recall, delayed recall, and recognition score) and visual memory tests (Rey Complex Figure Test immediate recall, delayed recall, and recognition score; range, 0–144). The frontal/executive function was calculated by summing scores from the Controlled Oral Word Association Test and Stroop Colour Reading Test (range, 0–55). The depressive symptoms were evaluated by the Korean version of the short-form geriatric depression scale (SGDS; range, 0–15).
2.5. Neuroimaging biomarkers
All participants underwent an 18F-flutemetamol positron emission tomography (PET) scan to quantify cortical amyloid burden using a Discovery STe PET/CT scanner (GE, Milwaukee, WI, USA) with identical settings. The 18F-flutemetamol was injected into an antecubital vein as a bolus with a mean dose of 185 MBq. After 90 min, a 20-min PET scan was performed. The 18F-flutemetamol PET scans were co-registered with individual magnetic resonance imaging (MRI) scans, which were normalised to a T1-weighted MRI template. Using transformation parameters, MRI-co-registered 18F-flutemetamol PET images were normalised to the MRI template. To quantify 18F-flutemetamol retention, a standard uptake value ratio (SUVR) was obtained by using the pons as a reference region. Global cortical 18F-flutemetamol retention was calculated from the volume-weighted average SUVR of bilateral 28 cortical volumes of interest from frontal, posterior cingulate, lateral temporal, parietal, and occipital lobes using the Annotated Anatomical Labelling atlas [36]. Based on a previous report on the elderly Korean population and our observed data distribution, participants were considered to be positive for amyloid if their global cortical SUVR was greater than 0.65 [37].
All participants underwent structural brain MRI scanning to quantify MTL grey matter (GM) volume. We performed voxel-based morphometry with the diffeomorphic anatomical registration through an exponentiated lie algebra (DARTEL) procedure on T1-weighted images [38, 39]. All MRI data acquisition was performed using a 3T GE Healthcare Discovery 750 M scanner. High-resolution axial three-dimensional fast spoiled gradient recalled echo structural images (3D-T1 FSPGR: 200 × 200 matrix, TR = 8 ms, TE = 3 ms, flip angle = 12°, FOV = 20 cm, slices = 176, slice thickness = 1.0 mm) were collected using a magnetisation-prepared rapid gradient echo sequence. Pre-processing of the structural data was performed using the Statistical Parametric Mapping software (SPM12; Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB (Mathworks Inc., Natick, MA, USA). At first, using the segmentation algorithm implemented in SPM12, three-dimensional T1-weighted structural images of each individual were segmented into six tissue sections. Default parameters were used in the segmentation process with the exception that affine regularisation was performed using the international consortium for brain mapping template for east Asian brains. We then progressed to the DARTEL process implemented in SPM12. First, we created grey matter and white matter templates from our entire data set using the DARTEL technique. We then normalised each participant's image into the Montreal Neurological Institute space with the normalised images modulated to ensure that relative grey and white matter volumes were preserved following spatial normalisation. Subsequently, all images were smoothed by convolving them with an isotropic Gaussian kernel of 6 mm full width at half maximum.
To assess subcortical vascular pathology, we evaluated the severity of white matter hyperintensities (WMHs) according to the modified criteria of Fazekas et al. using the fluid-attenuated inversion recovery images [40]. The severity of periventricular white matter hyperintensities (PVWMHs) were categorised into the following groups, namely mild (cap or band < 5 mm), moderate (5 mm ≤ cap or band < 10 mm), and severe (cap or band ≥ 10 mm), based on the size of the cap or band that was perpendicular or horizontal to the ventricle. The severity of deep white matter hyperintensities (DWMHs) were divided into the following groups: mild (maximum diameter of deep white matter lesion < 10 mm), moderate (10 mm ≤ lesion < 25 mm), and severe (lesion ≥ 25 mm). The severity of overall WMHs was assigned to one of three groups by combining PVWMHs (P1: mild, P2: moderate, P3: severe) and DWMHs (D1: mild, D2: moderate, D3: severe), such as the mild (D1P1, D1P2), moderate (neither mild nor severe), and severe (D3P3) groups.
2.6. Other measures
The covariates assessed in this study were age, sex, education, living alone, diabetes, hypertension, depressive symptom, acetylcholine esterase inhibitor use, antidepressant use, antipsychotics use, benzodiazepine use, sleep medication use, number of weekend days, and apolipoprotein-ε allele status.
2.7. Statistical analysis
Continuous variable data are reported as the mean with the standard deviation (SD) or median with the interquartile range (IQR) after verifying a normal distribution using the Shapiro–Wilk test. For group comparisons, we used the Student's t-test and the Mann–Whitney U test for variables exhibiting a normal or non-normal distribution, respectively. For categorical variables, we examined group differences using the chi-squared test. For exploratory analyses, we performed Pearson's correlation tests. Rest-activity pattern variables that had at least one significant correlation (p < 0.01) with a neuroimaging biomarker or cognitive function were selected for subsequent regression analyses. We performed multiple linear regression analyses to examine the significance of observed associations. To adjust possible covariates with parsimonious models, we used forward stepwise methods. The criterion for covariate selection involved entering variables p < 0.05 and exit variables p > 0.10. The local effect size of the rest-activity pattern variable within the regression model was assessed by a variation of Cohen's f square [41]. We evaluated model assumptions of normality and homoscedasticity by examining the distribution of standardised residuals. To correct for multiple independent variables, dependant variables, and subgroup analyses, false discovery rate (FDR)-corrected p-values less than 0.05 were considered as statistically significant. Finally, we performed path analysis using the PROCESS macro in SPSS [42], which uses a bootstrap approach that can avoid the power problem by non-normality and is less restricted by sample size [43]. All analyses were performed using SPSS, version 22 (IBM, Chicago, IL, USA) or the R Statistical Software, version 3.5.3, (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Demographic characteristics of study participants
The demographic characteristics of the study participants are listed in Table 1. The median (IQR) age was 73 (67–77) years, number of years of education was 12 (6–12), SGDS score was 4.5 (2–9), and MMSE score was 25 (22–27). Of the 100 participants, 68% were female, 23% presented with diabetes, and 52% were diagnosed with hypertension. Approximately 57% were taking antidepressants and 35% were taking sleep medication, consistent with recent studies suggesting a high prevalence of depression or insomnia in patients with cognitive impairment [44, 45]. Finally, 46% were identified as amyloid-positive by 18F-flutemetamol PET imaging.
Table 1.
Demographic characteristics of study participants.
|
Categories based on cortical amyloid burden |
||||
|---|---|---|---|---|
| Characteristics |
All participants (n = 100) |
Amyloid-negative (n = 54) |
Amyloid-positive (n = 46) |
pa |
| Age, median (IQR), yr | 73 (67–77) | 71 (64–75) | 74 (72–78) | <0.001 |
| Female, No. (%) | 68 (68.0) | 37 (68.5) | 31 (67.4) | 0.90 |
| Education, median (IQR), yr | 12 (6–12) | 9 (6–12) | 12 (6–14) | 0.10 |
| Living alone, No. (%) | 14 (14.0) | 10 (18.5) | 4 (8.7) | 0.16 |
| Comorbidity, No. (%) | ||||
| Diabetes | 23 (23.0) | 13 (24.1) | 10 (21.7) | 0.78 |
| Hypertension | 52 (52.0) | 28 (51.9) | 24 (52.2) | 0.97 |
| SGDS, median (IQR) | 4.5 (2.0–9.0) | 5.5 (2.0–11.0) | 3.5 (2.0–8.0) | 0.08 |
| Psychotropic medication use, No. (%) | ||||
| Acetylcholinesterase inhibitor | 30 (30.0) | 9 (16.7) | 21 (45.7) | 0.002 |
| Antidepressant | 57 (57.0) | 27 (50.0) | 30 (65.2) | 0.13 |
| Antipsychotics | 11 (11.0) | 4 (7.4) | 7 (15.2) | 0.21 |
| Benzodiazepine (daytime) | 14 (14.0) | 7 (13.0) | 7 (15.2) | 0.75 |
| Sleep medicationb | 35 (35.0) | 20 (37.0) | 15 (32.6) | 0.64 |
| APOE genotype, No. (%) | ||||
| ε2 allele carrier | 16 (16.0) | 13 (24.1) | 3 (6.5) | 0.02 |
| ε4 allele carrier | 30 (30.0) | 5 (9.3) | 25 (54.3) | <0.001 |
| MMSE, median (IQR) | 25 (22–27) | 26 (23–28) | 23 (20–26) | 0.003 |
| CDR-SB, median (IQR) | 2.0 (1.1–4.5) | 2.0 (1.0–3.0) | 4.0 (1.5–5.1) | <0.001 |
APOE = apolipoprotein E; CDR-SB = Clinical Dementia Rating Sum of Boxes; IQR = interquartile range; MMSE = Mini-Mental Status Examination; SGDS = Short-form Geriatric Depression Scale.
Student's t-test was performed for normally distributed continuous variables, and the Mann-Whitney U test was conducted for continuous variables that did not exhibit a normal distribution. Chi-square tests were performed for categorical variables.
Benzodiazepine, zolpidem, and trazodone at bedtime.
Next, we categorised the participants into two groups based on cortical amyloid burden. These demographic characteristics were also compared between subgroups (Table 1). In brief, amyloid-positive participants were slightly older, more likely to be treated with acetylcholinesterase inhibitor (ACEi) and less likely to be an APOE ε2 carrier. Moreover, these participants were more likely to be an APOE ε4 allele carrier and exhibited lower MMSE scores with higher CDR-Sum of Boxes (CDR-SB).
3.2. Analysis of rest-activity patterns, neuroimaging biomarkers, and cognitive function
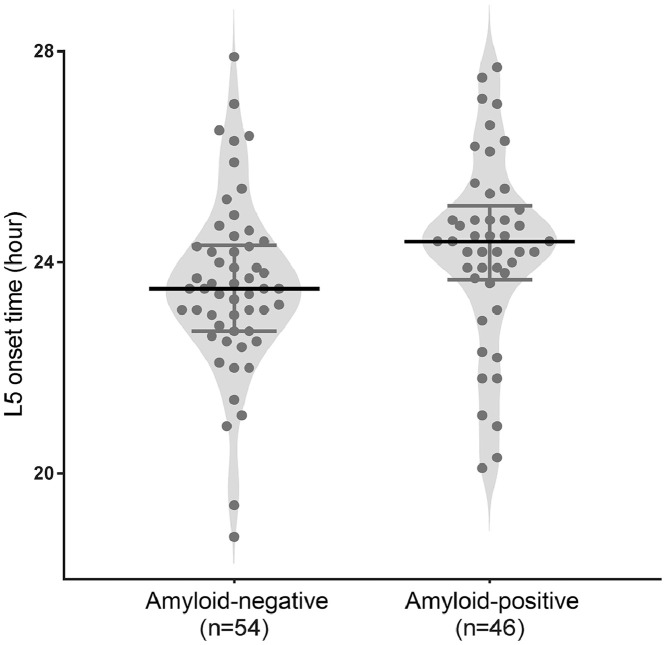
Descriptive statistics for rest-activity patterns, neuroimaging biomarkers, and cognitive function are reported in Table 2. We first examined whether rest-activity pattern variables were different between the subgroups. Although other variables did not exhibit differences, L5 onset time, which is akin to the rest period onset time, differed with the presence of cortical amyloid burden. Amyloid-positive participants displayed a later L5 onset time than amyloid-negative participants (mean [SD], 23.6 [1.7] vs. 24.2 [1.8]; p = 0.04). This difference remained significant after accounting for six covariates that varied between the demographic characteristics, including age, ACEi use, APOE -ε2, -ε4 allele carrier, MMSE, and CDR-SB (estimated marginal means [standard error], 23.4 [0.3] vs. 24.5 [0.3]; p = 0.02; Fig. 1). Furthermore, amyloid-positive participants exhibited a higher cortical amyloid burden, more MTL neurodegeneration, and lower language and memory functions. However, visuospatial and frontal/executive functions did not differ between the two groups.
Table 2.
Rest-activity pattern variables, neuroimaging biomarkers, and cognitive function of study participants.
| Characteristics |
Categories based on cortical amyloid burden |
||||
|---|---|---|---|---|---|
| Mean (SD) or Median (IQR)a | Range |
All participants (n = 100) |
Amyloid-negative (n = 54) |
Amyloid-positive (n = 46) |
pb |
| Rest-activity pattern variables | |||||
| Cosinor analysis | |||||
| Robustness | 0.02–0.48 | 0.14 (0.08–0.18) | 0.13 (0.09–0.17) | 0.14 (0.07–0.19) | 0.75 |
| MESOR, rhythm-adjusted mean activity | 1.06–10.34 | 4.93 (2.03) | 4.97 (1.86) | 4.87 (2.22) | 0.69 |
| Amplitude | 0.66–8.12 | 3.36 (2.32–4.82) | 3.46 (2.50–5.07) | 3.25 (2.26–4.74) | 0.56 |
| Acrophase, timing of peak activity, time | 11.5–18.1 | 13.7 (12.7–14.6) | 13.6 (12.6–14.4) | 13.9 (12.7–14.6) | 0.57 |
| Nonparametric analysis | |||||
| IS, day to day consistency | 0.27–0.85 | 0.60 (0.49–0.69) | 0.59 (0.51–0.68) | 0.64 (0.45–0.71) | 0.86 |
| IV, fragmentation | 0.38–1.66 | 0.93 (0.28) | 0.93 (0.30) | 0.92 (0.28) | 0.81 |
| L5 onset time, rest phase, time | 18.8–27.9 | 23.9 (1.7) | 23.6 (1.7) | 24.2 (1.8) | 0.04 |
| M10 onset time, active phase, time | 3.5–12.4 | 8.1 (6.8–9.8) | 8.2 (7.0–9.5) | 7.8 (6.6–10.0) | 0.86 |
| Neuroimaging biomarkers | |||||
| Cortical amyloid burden, SUVR | 0.49–1.28 | 0.62 (0.57–0.87) | 0.57 (0.55–0.60) | 0.88 (0.79–1.00) | <0.001 |
| Medial temporal lobe GM volume | 0.26–0.53 | 0.39 (0.06) | 0.41 (0.05) | 0.36 (0.05) | <0.001 |
| Cognitive function | |||||
| Language function | 8.0–57.0 | 42.0 (32.0–48.0) | 45.5 (36.0–50.3) | 40.0 (29.5–48.0) | 0.03 |
| Visuospatial function | 2.5–36.0 | 28.5 (24.1–32.0) | 29.0 (24.9–32.0) | 27.8 (21.6–30.3) | 0.11 |
| Memory function | 6.0–105.0 | 49.3 (19.7) | 56.7 (19.2) | 40.6 (16.8) | <0.001 |
| Frontal/executive function | 0.0–41.0 | 18.7 (10.1) | 19.7 (10.1) | 17.5 (10.0) | 0.28 |
GM = grey matter; IQR = interquartile range; IS = inter-daily stability; IV = intra-daily variability; L5 onset time = least active 5-h onset time; M10 onset time = most active 10-h onset time; MESOR = midline estimation statistic of rhythm; SD = standard deviation; SUVR = standardised uptake value ratio.
Values represented as the mean ± standard deviation (SD) for normally distributed variables and median (IQR) for variables that did not exhibit a normal distribution.
Student's t-test was performed for normally distributed continuous variables, and the Mann-Whitney U test was conducted for continuous variables that did not exhibit a normal distribution.
Fig. 1.
Comparison of least active 5-h onset time between amyloid-negative and -positive participants. L5 onset time, which is defined as the least active 5-h onset time, was compared between the two groups by applying analysis of covariance (ANCOVA) adjusted for age, acetylcholine esterase inhibitor use, Apolipoprotein -ε2, -ε4 allele carrier, Mini-Mental State Examination (MMSE) score, and Clinical Dementia Rating Sum of Boxes. These six covariates exhibited statistically significant differences between the two groups for the demographic characteristics listed in Table 1 (estimated marginal means [standard error], 23.4 [0.3] vs 24.5 [0.3]; p = 0.02). Circles represent patients; bars in the middle, median; error bars, interquartile range.
3.3. Association of MESOR with frontal/executive function and L5 onset time with memory function
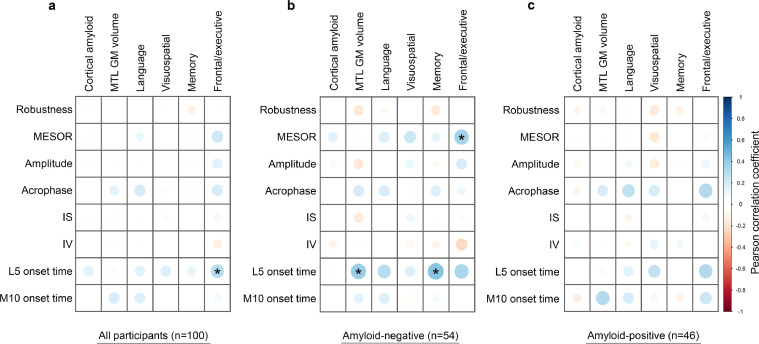
To enable further analysis, we first performed a Pearson correlation test to narrow down possible associations of the rest-activity pattern variables with neuroimaging biomarkers and cognitive domains (Fig. 2). MESOR and L5 onset time correlated significantly (p < 0.01) with at least one neuroimaging biomarker, or cognitive domain.
Fig. 2.
Exploratory correlation analysis between rest-activity pattern variables, neuroimaging biomarkers, and cognitive domain measures. We performed Pearson correlation tests to narrow down the possible associations between the eight rest-activity pattern variables (vertical axis) with two neuroimaging biomarkers and four cognitive domain measures (horizontal axis) in all participants (a), in amyloid-negative participants (b), and in amyloid-positive participants (c). The colour and diameter of each circle represent a Pearson correlation coefficient. Note that statistically significant associations were observed between L5 onset time and frontal/executive function (r = 0.29, p = 0.003) in all participants. In addition, significant associations were observed between MESOR and frontal/executive function (r = 0.36, p = 0.008), as well as between L5 onset time and MTL GM volume (R = 0.38, p = 0.004) and memory function (r = 0.43, p = 0.001) in amyloid-negative participants. Asterisk indicates p < 0.01. IS = inter-daily stability; IV = intra-daily variability; L5 onset time = least active 5-h onset time; M10 onset time = most active 10-h onset time; MESOR = midline estimation statistic of rhythm, MTL GM = medial temporal lobe grey matter.
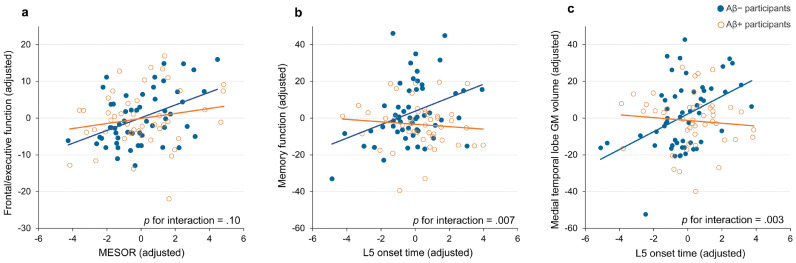
To investigate the robustness and effect size of these associations after controlling for possible covariates, we next conducted a multiple linear regression analysis. MESOR associated positively with frontal/executive function in all participants, as well as in amyloid-negative participants (estimate = 1.17, standard error [SE] = 0.37, p = 0.002, estimate = 2.12, SE = 0.52, p < 0.001; Table 3). The local effect sizes for MESOR on frontal/executive function in all participants and amyloid-negative participants were low (Cohen's f2 = 0.08) and medium (Cohen's f2 = 0.17), respectively (Table S1). Next, to assess the moderating effect of cortical amyloid burden, we performed interaction analyses. No significant moderating effect on the association between MESOR and frontal/executive function was observed with cortical amyloid burden (p for interaction = 0.1; Fig. 3a).
Table 3.
Multiple linear regression analysis for associations of MESOR and L5 onset time with neuroimaging biomarkers and cognitive function.
|
Independent variable: MESOR |
||||||
|---|---|---|---|---|---|---|
| All participants (n = 100) |
Amyloid-negative (n = 54) |
Amyloid-positive (n = 46) |
||||
| Dependant variables | estimate (se) | pa | estimate (se) | p | estimate (se) | p |
| Neuroimaging biomarkers | ||||||
| Cortical amyloid burden, SUVRb | 0.46 (0.83) | 0.58 | 0.26 (0.23) | 0.26 | −0.25 (1.00) | 0.80 |
| Medial temporal lobe GM volumeb | −0.39 (0.21) | 0.08 | −0.22 (0.33) | 0.52 | −0.15 (0.25) | 0.54 |
| Cognitive function | ||||||
| Language function | 0.76 (0.46) | 0.10 | 0.78 (0.62) | 0.21 | 0.63 (0.70) | 0.37 |
| Visuospatial function | 0.17 (0.32) | 0.59 | 0.64 (0.36) | 0.08 | −0.31 (0.46) | 0.51 |
| Memory function | 0.39 (0.78) | 0.62 | 0.91 (0.12) | 0.45 | −0.06 (0.90) | 0.95 |
| Frontal/executive function | 1.17 (0.37) | 0.002c | 2.12 (0.52) | <0.001c | 0.54 (0.52) | 0.30 |
|
Independent variable: L5 onset time |
||||||
|---|---|---|---|---|---|---|
| All participants (n = 100) |
Amyloid-negative (n = 54) |
Amyloid-positive (n = 46) |
||||
| Dependant variables | estimate (se) | pa | estimate (se) | p | estimate (se) | p |
| Neuroimaging biomarkers | ||||||
| Cortical amyloid burden, SUVRb | 1.39 (0.96) | 0.15 | −0.12 (0.25) | 0.64 | −0.08 (1.26) | 0.95 |
| Medial temporal lobe GM volumeb | 0.47 (0.25) | 0.06 | 1.24 (0.33) | 0.001c | −0.13 (0.35) | 0.72 |
| Cognitive function | ||||||
| Language function | 0.95 (0.55) | 0.09 | 1.17 (0.69) | 0.10 | 0.59 (0.91) | 0.52 |
| Visuospatial function | 0.53 (0.36) | 0.14 | 0.50 (0.41) | 0.23 | 0.87 (0.57) | 0.13 |
| Memory function | 1.21 (0.91) | 0.19 | 3.77 (1.22) | 0.003c | −0.22 (1.13) | 0.85 |
| Frontal/executive function | 1.13 (0.45) | 0.01 | 1.36 (0.65) | 0.04 | 1.48 (0.61) | 0.02 |
GM, grey matter; L5 onset time, least active 5-h onset time; MESOR, midline estimation statistic of rhythm; SUVR, standardised uptake value ratio.
All p-values were adjusted for age, sex, education, living alone, diabetes, hypertension, depressive symptom, acetylcholine esterase inhibitor use, antidepressant use, antipsychotics use, benzodiazepine use, sleep medication use, number of weekend days, and apolipoprotein-ε2 and -ε4 allele carrier status using a forward stepwise method. The criterion for covariate selection involved entering variables p < 0.05 and exit variables p > 0.10. Independent variables were directly entered into the regression model.
Cortical amyloid burden and medial temporal lobe GM volume were multiplied by 100 before regression analysis for ease of interpreting estimates.
p < 0.05 after false discovery rate correction for two independent variables, six dependant variables, and subgroup analysis (correction for 36 tests).
Fig. 3.
Association of MESOR with frontal/executive function and of L5 onset time with memory function and medial temporal lobe GM volume. Multiple linear regression analyses were performed after controlling for the covariates using a forward stepwise method. Associations of MESOR and frontal/executive function (a), L5 onset time and memory function (b), and L5 onset time and medial temporal lobe GM volume (c) are shown. The values on the graph represent residuals from regression of the rest-activity pattern variable and the cognitive function or medial temporal lobe GM. The selected covariates in each graph are: education, living alone, acetylcholine esterase inhibitor use, antipsychotics use, and APOE ε2 allele carrier status (a); education, acetylcholine esterase inhibitor use, APOE ε2 allele carrier status, and APOE ε4 allele carrier status (b); and age, sex, acetylcholine esterase inhibitor use, and APOE ε4 allele carrier status (c). Amyloid-negative participants are indicated by blue circles with blue linear trendlines. Amyloid-positive participants are indicated by orange circles with orange linear trendlines. APOE = apolipoprotein E; GM = grey matter; L5 onset time = least active 5-h onset time; MESOR = midline estimation statistic of rhythm.
Our analysis also revealed that L5 onset time associated positively with memory function in amyloid-negative participants (estimate = 3.77, SE = 1.22, p = 0.003; Table 3). Furthermore, L5 onset time associated positively with MTL GM volume in amyloid-negative participants (estimate = 1.24, SE = 0.33, p = 0.001). The local effect sizes of L5 onset time for memory function and MTL GM volume in amyloid-negative participants were medium (Cohen's f2 = 0.19 for memory function, Cohen's f2 = 0.28 for MTL GM volume; Table S2). We also found a significant moderating effect by cortical amyloid burden on associations of L5 onset time with memory function and MTL GM volume (p for interaction = 0.007 and p = 0.003, respectively; Fig. 3b and c).
3.4. Model of relationships between L5 onset time, MTL GM volume, memory function, and cortical amyloid burden
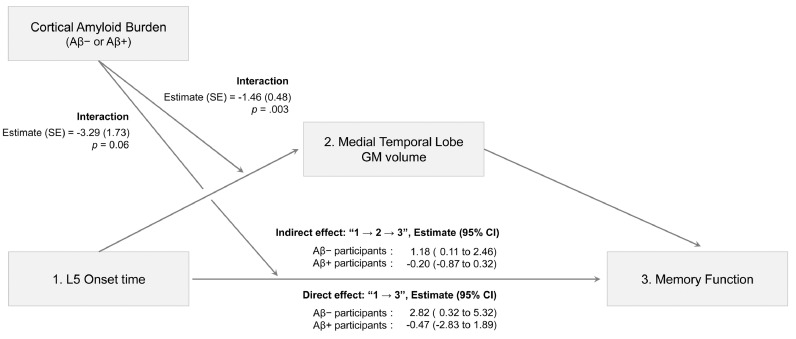
To further understand the complex relationships between L5 onset time, MTL GM volume, memory function, and cortical amyloid burden, we performed path analysis, which enabled us to consider different variables together in one model. Based on our regression results and previous evidence suggesting a pathophysiological role for disrupted circadian rhythm on neurodegeneration [21, 23], we examined a model that involves MTL GM volume mediation in the association between L5 onset time and memory function. Furthermore, we added cortical amyloid burden as a possible moderator in the association of L5 onset time with MTL GM volume and memory function. Six variables selected as significant covariates in our multiple linear regression analyses were adjusted in the final path model (Fig. 4).
Fig. 4.
Path analysis of L5 onset time for memory function. In this path model, we adjusted for six covariates (age, sex, education, APOE ε2 allele carrier status, APOE ε4 allele carrier status, and acetylcholine esterase use). These variables were significant covariates in either or both of the two relationships examined for associations of L5 onset time with memory function and L5 onset time with medial temporal lobe GM volume (as shown in Fig. 3b and 3c). 95% confidence intervals were calculated using the bootstrap method (5000 resampling). APOE = apolipoprotein E; Aβ = amyloid beta; CI = confidence interval; GM = grey matter; L5 onset time = least active 5-h onset time; SE = standard error.
This analysis revealed that both the direct (L5 onset time → memory function) and indirect paths (L5 onset time → MTL GM volume → memory function) were significant in amyloid-negative participants. On the other hand, neither path was significant in amyloid-positive participants. Collectively, our results indicate that MTL GM volume partially mediates the association between L5 onset time and memory function in amyloid-negative participants.
4. Discussion
In this study, we explored the relationships between rest-activity pattern, neuroimaging biomarkers, and cognitive function in patients with MCI and mild dementia. We found that MESOR, which corresponds to the rhythm-adjusted mean activity, associated positively with frontal/executive function. Furthermore, L5 onset time, which corresponds to the time of rest period onset, associated positively with MTL GM volume and memory function, particularly in amyloid-negative participants. Additional path analysis revealed that MTL GM volume partially mediates the relationship between L5 onset time and memory function in amyloid-negative participants.
Our finding that MESOR is associated with frontal/executive function is consistent with a previous study that showed a positive association between MESOR and frontal/executive function in elderly individuals. Indeed, Walsh et al. reported that the lowest quartile for MESOR at baseline was associated with lower frontal/executive function 5 years later in 1287 older women without dementia (mean age, 82.8 years; mean MMSE score, 28.4) [46]. Their study included only women with advanced age, MESOR was treated as a quartile variable, and neither baseline frontal/executive function nor any neuroimaging biomarkers were assessed. Thus, our analyses expand on these findings on several important considerations. Our study is first to report a positive association between MESOR and frontal/executive function in patients with cognitive impairment. In addition, we treated MESOR as a continuous variable, enabling us to estimate a more accurate association between variables. Finally, we assessed cortical amyloid burden using 18F-flutemetamol PET imaging, revealing a more prominent association between MESOR and frontal/executive function specifically in amyloid-negative participants. Recently, several studies have suggested unidirectional or bidirectional associations between low physical activity and frontal/executive dysfunction in elderly individuals with normal cognition and in MCI patients [47, 48]. Thus, one possible mechanistic explanation for the association of MESOR with frontal/executive function could involve the positive relationship between MESOR and physical activity. In fact, several previous reports have emphasised intensity and quality of exercise as important factors involved in the protective effects against cognitive impairment [49, 50]. However, it should be noted that, although MESOR and physical activity are conceptually similar, they measure different characteristics. In other words, MESOR does not reflect the quality of exercise. Future studies that examine both MESOR and physical activity will confirm our hypothesis more thoroughly.
Another interesting finding of our study is the positive association between L5 onset time and memory function in amyloid-negative participants (Table 3 and Fig. 4). We found that individuals with earlier rest-phase are more likely to have poor memory function, suggesting that rest-phase advance is a potential biomarker for memory impairment. Two previous cross-sectional studies also suggested circadian phase advance as a possible state marker for MCI. Naismith, et al. reported phase advance of dim-light melatonin onset (DLMO) in 26 MCI patients relative to 26 matched controls [23]. They also found a significant association between early DLMO with poorer memory function. Other cognitive domain functions, however, were not assessed in their study, hampering the interpretation of a specific effect of phase advance on memory. Another study assessed the circadian phases of participants using a combined analysis of wrist temperature, motor activity, and body position [22]. Compared to 19 healthy controls, 21 MCI patients exhibited more phase advance in both L5 and M10 onset times, however, cognitive function was not analysed specifically. Another recent longitudinal study of 2754 older men without dementia showed that an advance in acrophase, which indicates the timing of peak activity, was associated with greater cognitive impairment 3.4 years later [51]. A significant effect of acrophase on general cognitive function, as assessed by a modified MMSE, was noted. Taken together with our findings, these results imply that phase advance is linked to the clinical and cognitive trajectories in patients with cognitive impairment. The underlying mechanisms of this association, however, remain uncertain. Thus, the need for a proper assessment of neural correlates of cognitive impairment is paramount in resolving uncertainty [20].
In line with this perspective, we performed brain amyloid PET imaging with structural MRI and found a positive association between L5 onset time and MTL GM volume in amyloid-negative participants (Fig. 3c). MTL is an anatomical component of the memory system, and its degeneration is associated with memory impairment and increased risk for dementia [52], [53], [54]. Several reports suggested a pathophysiological role of circadian disruption and rest-activity pattern alteration on neurodegeneration [21, 23]. We thus performed path analysis and found a partial mediating effect of MTL neurodegeneration on the association of L5 onset time and memory function. Taken together, these findings suggest the possibility that an advance of L5 onset time might precede and affect memory impairment via MTL neurodegeneration.
Such an advance in the L5 onset time and its association with MTL neurodegeneration suggests two possible hypotheses. First, L5 onset time advance may reflect the circadian clock system disruption in affected individuals. However, in our study, active-phase (acrophase and M10 onset time) did not significantly associate with MTL neurodegeneration. In addition, no associations were observed between other circadian rest-activity pattern variables and neurodegeneration in our study. However, a previous report demonstrated the contribution of fragmented rhythm as represented by high IV values to preclinical amyloid pathophysiology [55]. Therefore, we cannot conclude that L5 onset time advance and its association with neurodegeneration were attributed to circadian clock system disruption. Second, L5 onset time advance may reflect changes in sleep phase or architecture in affected individuals. Sleep phase advance, fragmented sleep, and decreased slow-wave sleep, which are common in patients with cognitive impairment, could result in L5 onset time advance in the affected individuals of our study [56]. Future studies to examine both actigraphy measurement and sleep monitoring will be required to examine this hypothesis.
Intriguingly, significant associations of MESOR and L5 onset time with cognition were more robust in amyloid-negative participants. To delineate the moderating effect of cortical amyloid burden on this association, we performed an interaction test. Cortical amyloid burden did not significantly moderate the relationship between MESOR and frontal/executive function. Thus, we do not support the idea that the association of MESOR with frontal/executive function is specific to cognitive impairment with non-Alzheimer's pathologic changes [57]. Different baseline characteristics, such as the presence of slightly advanced disease, small sample size, and unknown confounding factors, may contribute to the lack of significance in the relationship between MESOR and L5 onset time with cognition in amyloid-positive participants. On the other hand, we found that cortical amyloid burden exerted a significant moderating effect on the association of L5 onset time with MTL neurodegeneration. Thus, we reasoned that this association may be specific to cognitive impairment with non-Alzheimer's pathologic changes [57].
Nevertheless, the question remains of why this association was not significant in amyloid-positive participants. These participants exhibited somewhat later L5 onset time than amyloid-negative participants, suggesting that the presence of cortical amyloid influenced L5 onset time and restricted the association between L5 onset time and other variables (Fig. 1). Interestingly, a recent study showed that physiologically relevant concentrations of Aβ1–42 lengthened the circadian rhythm period in human fibroblasts and mouse primary neurons [58]. In addition, the lower MTL GM volume and memory function observed in amyloid-positive participants may also be attributed to nonsignificant associations. Based on these results, future research efforts should consider the potential moderating effect of cortical amyloid burden on the relationship between rest-activity patterns, neuroimaging biomarkers, and cognitive function.
There are several possibilities for pathophysiology of cognitive impairment in amyloid-negative participants in our study. FTLD and LBD are common possible causes of cognitive impairment in non-Alzheimer's pathologic changes. In our study, we excluded ‘possible behavioural variant FTLD’ or ‘possible LBD’ patients using validated diagnostic criteria for each disease [59, 60]. However, considering the lack of early stage FTLD or LBD biomarkers, the possibility exists that we inadvertently included cognitively impaired FTLD or LBD patients as amyloid-negative participants, especially those who had mild or atypical clinical features due to early stage disease or mixed pathology. Vascular cognitive impairment is another common cause of cognitive impairment in non-Alzheimer's pathologic changes. We excluded participants who had a history of large vessel disease, such as territorial cerebral infarction and intracranial haemorrhage, as they usually possess lesion-specific motor or cognitive defects. However, we did not exclude participants who had small vessel disease with subcortical vascular pathology, which is very common in Alzheimer's and non-Alzheimer's patients and contributes to increased risk of dementia [61]. About 64.8%, 25.9%, and 9.3% of amyloid-negative participants in our study displayed mild, moderate, and severe WMHs, respectively (Table S3). These were similar in proportion to the number of amyloid-positive participants in our study and that of a previous one examining 4253 older adults with a similar stage of cognitive impairment [62].
Our study had some limitations. Given that all participants displayed cognitive impairment, associations between rest-activity patterns and participant characteristics pertaining to disease onset and preclinical stage of disease may have been missed in our study. Future studies which include cognitively normal older adults combined with numerous neurodegenerative disease-associated biomarkers (e.g., amyloid, tau, vascular, MTL neurodegeneration, whole-brain functional, and structural connectivity, etc.) are warranted. Lack of subjective and objective sleep assessments was another limitation. Physical activity should be assessed in future study. Additionally, this study was cross-sectional, making causal relationships of our findings difficult to demonstrate. Finally, the small sample size potentially limits the ability to generalise our results. Thus, future studies of longitudinal design with a greater number of participants are necessary to confirm our results.
In conclusion, we investigated the associations between rest-activity patterns, neuroimaging biomarkers, and cognitive function in patients with MCI and mild dementia. We found that decreased MESOR and advanced L5 onset may be useful as early indicators of cognitive decline or MTL neurodegeneration. In addition, our findings suggest for the first time that cortical amyloid pathology may function to moderate the relationship between rest-activity patterns, neurodegeneration, and cognitive function. Our study will undoubtedly inform future animal and human research to probe the neurobiology of circadian rhythm disturbances and rest-activity pattern alterations in neurodegenerative disorders.
Funding sources
This study was provided with biospecimens and data from the biobank of Chronic Cerebrovascular Disease Consortium and biobank of Ajou University Hospital, a member of Korea Biobank Network. The consortium was supported and funded by the Korea Centres for Disease Control and Prevention (#4845-303). This research was supported by the National Research Foundation of Korea (NRF) grants funded by the Korean government (Ministry of Science and ICT) (Grant Nos. 2019M3C7A1031905, 2019R1A5A2026045). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declaration of Competing Interest
All authors declare no competing interests.
Data statement
Because there is a possibility that participants could be identified based on their clinical and biological sampling data, the datasets generated and/or analysed in this study cannot be made publicly available. In the event that an ethical approval is obtained, anonymised data and the analytical methods used in this study can be provided from the corresponding author upon reasonable request.
Acknowledgements
The authors thank the Chronic Cerebrovascular Disease Consortium participants, staff, and investigators for their contributions to this study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2020.102881.
Contributor Information
Sang Joon Son, Email: sjsonpsy@ajou.ac.kr.
Eun Young Kim, Email: ekim@ajou.ac.kr.
Appendix. Supplementary materials
References
- 1.Reppert S.M., Weaver D.R. Coordination of circadian timing in mammals. Nature. 2002;418(6901):935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 2.Weaver D.R. The suprachiasmatic nucleus: a 25-year retrospective. J Biol Rhythms. 1998;13(2):100–112. doi: 10.1177/074873098128999952. [DOI] [PubMed] [Google Scholar]
- 3.Hastings M.H., Maywood E.S., Brancaccio M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat Rev Neurosci. 2018;19(8):453–469. doi: 10.1038/s41583-018-0026-z. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura T.J., Takasu N.N., Nakamura W. The suprachiasmatic nucleus: age-related decline in biological rhythms. J Physiol Sci. 2016;66(5):367–374. doi: 10.1007/s12576-016-0439-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Logan R.W., McClung C.A. Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat Rev Neurosci. 2019;20(1):49–65. doi: 10.1038/s41583-018-0088-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musiek E.S., Holtzman D.M. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science. 2016;354(6315):1004–1008. doi: 10.1126/science.aah4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kress G.J., Liao F., Dimitry J., Cedeno M.R., FitzGerald G.A., Holtzman D.M. Regulation of amyloid-beta dynamics and pathology by the circadian clock. J Exp Med. 2018;215(4):1059–1068. doi: 10.1084/jem.20172347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musiek E.S., Lim M.M., Yang G., Bauer A.Q., Qi L., Lee Y. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest. 2013;123(12):5389–5400. doi: 10.1172/JCI70317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakazato R., Kawabe K., Yamada D., Ikeno S., Mieda M., Shimba S. Disruption of Bmal1 impairs blood-brain barrier integrity via pericyte dysfunction. J Neurosci. 2017;37(42):10052–10062. doi: 10.1523/JNEUROSCI.3639-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lananna B.V., Nadarajah C.J., Izumo M., Cedeno M.R., Xiong D.D., Dimitry J. Cell-autonomous regulation of astrocyte activation by the circadian clock protein BMAL1. Cell Rep. 2018;25(1):1-9 e5. doi: 10.1016/j.celrep.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bang J., Spina S., Miller B.L. Frontotemporal dementia. Lancet. 2015;386(10004):1672–1682. doi: 10.1016/S0140-6736(15)00461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker Z., Possin K.L., Boeve B.F., Aarsland D. Lewy body dementias. Lancet. 2015;386(10004):1683–1697. doi: 10.1016/S0140-6736(15)00462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Brien J.T., Thomas A. Vascular dementia. Lancet. 2015;386(10004):1698–1706. doi: 10.1016/S0140-6736(15)00463-8. [DOI] [PubMed] [Google Scholar]
- 14.Smagula S.F., Gujral S., Capps C.S., Krafty R.T. A systematic review of evidence for a role of rest-activity rhythms in dementia. Front Psychiatry. 2019;10:778. doi: 10.3389/fpsyt.2019.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartman Y.A.W., Karssemeijer E.G.A., van Diepen L.A.M., Olde Rikkert M.G.M., Thijssen D.H.J. Dementia patients are more sedentary and less physically active than age- and sex-matched cognitively healthy older adults. Dement Geriatr Cogn Disord. 2018;46(1–2):81–89. doi: 10.1159/000491995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ancoli-Israel S., Klauber M.R., Jones D.W., Kripke D.F., Martin J., Mason W. Variations in circadian rhythms of activity, sleep, and light exposure related to dementia in nursing-home patients. Sleep. 1997;20(1):18–23. [PubMed] [Google Scholar]
- 17.Hatfield C.F., Herbert J., van Someren E.J., Hodges J.R., Hastings M.H. Disrupted daily activity/rest cycles in relation to daily cortisol rhythms of home-dwelling patients with early Alzheimer's dementia. Brain. 2004;127(Pt 5):1061–1074. doi: 10.1093/brain/awh129. [DOI] [PubMed] [Google Scholar]
- 18.Harper D.G., Stopa E.G., Kuo-Leblanc V., McKee A.C., Asayama K., Volicer L. Dorsomedial SCN neuronal subpopulations subserve different functions in human dementia. Brain. 2008;131(Pt 6):1609–1617. doi: 10.1093/brain/awn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satlin A., Volicer L., Stopa E.G., Harper D. Circadian locomotor activity and core-body temperature rhythms in Alzheimer's disease. Neurobiol Aging. 1995;16(5):765–771. doi: 10.1016/0197-4580(95)00059-n. [DOI] [PubMed] [Google Scholar]
- 20.Musiek E.S. Circadian rhythms in AD pathogenesis: a critical appraisal. Curr Sleep Med Rep. 2017;3(2):85–92. doi: 10.1007/s40675-017-0072-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leng Y., Musiek E.S., Hu K., Cappuccio F.P., Yaffe K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 2019;18(3):307–318. doi: 10.1016/S1474-4422(18)30461-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortiz-Tudela E., Martinez-Nicolas A., Diaz-Mardomingo C., Garcia-Herranz S., Pereda-Perez I., Valencia A. The characterization of biological rhythms in mild cognitive impairment. Biomed Res Int. 2014;2014 doi: 10.1155/2014/524971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naismith S.L., Hickie I.B., Terpening Z., Rajaratnam S.M., Hodges J.R., Bolitho S. Circadian misalignment and sleep disruption in mild cognitive impairment. J Alzheimers Dis. 2014;38(4):857–866. doi: 10.3233/JAD-131217. [DOI] [PubMed] [Google Scholar]
- 24.La Morgia C., Ross-Cisneros F.N., Koronyo Y., Hannibal J., Gallassi R., Cantalupo G. Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann Neurol. 2016;79(1):90–109. doi: 10.1002/ana.24548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weissova K., Bartos A., Sladek M., Novakova M., Sumova A. Moderate changes in the circadian system of Alzheimer's disease patients detected in their home environment. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0146200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winblad B., Palmer K., Kivipelto M., Jelic V., Fratiglioni L., Wahlund L.O. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 27.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim D.Y., Jung Y.S., Park R.W., Joo N.S. Different location of triaxial accelerometer and different energy expenditures. Yonsei Med J. 2014;55(4):1145–1151. doi: 10.3349/ymj.2014.55.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin D.W., Yun J.M., Shin J.H., Kwon H., Min H.Y., Joh H.K. Enhancing physical activity and reducing obesity through smartcare and financial incentives: a pilot randomized trial. Obesity. 2017;25(2):302–310. doi: 10.1002/oby.21731. [DOI] [PubMed] [Google Scholar]
- 30.Ae Lee J., Gill J. Missing value imputation for physical activity data measured by accelerometer. Stat Methods Med Res. 2018;27(2):490–506. doi: 10.1177/0962280216633248. [DOI] [PubMed] [Google Scholar]
- 31.Ancoli-Israel S., Cole R., Alessi C., Chambers M., Moorcroft W., Pollak C.P. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 32.Witting W., Kwa I.H., Eikelenboom P., Mirmiran M., Swaab D.F. Alterations in the circadian rest-activity rhythm in aging and Alzheimer's disease. Biol Psychiatry. 1990;27(6):563–572. doi: 10.1016/0006-3223(90)90523-5. [DOI] [PubMed] [Google Scholar]
- 33.Blume C., Santhi N., Schabus M. 'nparACT' package for R: a free software tool for the non-parametric analysis of actigraphy data. MethodsX. 2016;3:430–435. doi: 10.1016/j.mex.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Refinetti R., Lissen G.C., Halberg F. Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res. 2007;38(4):275–325. doi: 10.1080/09291010600903692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahn H.J., Chin J., Park A., Lee B.H., Suh M.K., Seo S.W. Seoul Neuropsychological Screening Battery-dementia version (SNSB-D): a useful tool for assessing and monitoring cognitive impairments in dementia patients. J Korean Med Sci. 2010;25(7):1071–1076. doi: 10.3346/jkms.2010.25.7.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 37.Hwang J., Jeong J.H., Yoon S.J., Park K.W., Kim E.J., Yoon B. Clinical and biomarker characteristics according to clinical spectrum of Alzheimer's disease (ad) in the validation cohort of Korean brain aging study for the early diagnosis and prediction of AD. J Clin Med. 2019;8(3) doi: 10.3390/jcm8030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Ashburner J., Friston K.J. Voxel-based morphometry–the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 40.Noh Y., Lee Y., Seo S.W., Jeong J.H., Choi S.H., Back J.H. A new classification system for ischemia using a combination of deep and periventricular white matter hyperintensities. J Stroke Cerebrovasc Dis. 2014;23(4):636–642. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Selya A.S., Rose J.S., Dierker L.C., Hedeker D., Mermelstein R.J. A practical guide to calculating Cohen's f(2), a measure of local effect size, from PROC MIXED. Front Psychol. 2012;3:111. doi: 10.3389/fpsyg.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayes A.F. Guilford Press; New York, US: 2013. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. [Google Scholar]
- 43.Hayes A.F. An index and test of linear moderated mediation. Multivariate Behav Res. 2015;50(1):1–22. doi: 10.1080/00273171.2014.962683. [DOI] [PubMed] [Google Scholar]
- 44.Ismail Z., Elbayoumi H., Fischer C.E., Hogan D.B., Millikin C.P., Schweizer T. Prevalence of depression in patients with mild cognitive impairment: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(1):58–67. doi: 10.1001/jamapsychiatry.2016.3162. [DOI] [PubMed] [Google Scholar]
- 45.da Silva R.A. Sleep disturbances and mild cognitive impairment: a review. Sleep Sci. 2015;8(1):36–41. doi: 10.1016/j.slsci.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walsh C.M., Blackwell T., Tranah G.J., Stone K.L., Ancoli-Israel S., Redline S. Weaker circadian activity rhythms are associated with poorer executive function in older women. Sleep. 2014;37(12):2009–2016. doi: 10.5665/sleep.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daly M., McMinn D., Allan J.L. A bidirectional relationship between physical activity and executive function in older adults. Front Hum Neurosci. 2014;8:1044. doi: 10.3389/fnhum.2014.01044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frederiksen K.S., Verdelho A., Madureira S., Bazner H., O'Brien J.T., Fazekas F. Physical activity in the elderly is associated with improved executive function and processing speed: the LADIS study. Int J Geriatr Psychiatry. 2015;30(7):744–750. doi: 10.1002/gps.4220. [DOI] [PubMed] [Google Scholar]
- 49.Groot C., Hooghiemstra A.M., Raijmakers P.G., van Berckel B.N., Scheltens P., Scherder E.J. The effect of physical activity on cognitive function in patients with dementia: a meta-analysis of randomized control trials. Ageing Res Rev. 2016;25:13–23. doi: 10.1016/j.arr.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 50.Ohman H., Savikko N., Strandberg T.E., Pitkala K.H. Effect of physical exercise on cognitive performance in older adults with mild cognitive impairment or dementia: a systematic review. Dement Geriatr Cogn Disord. 2014;38(5–6):347–365. doi: 10.1159/000365388. [DOI] [PubMed] [Google Scholar]
- 51.Rogers-Soeder T.S., Blackwell T., Yaffe K., Ancoli-Israel S., Redline S., Cauley J.A. Rest-activity rhythms and cognitive decline in older men: the osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2018;66(11):2136–2143. doi: 10.1111/jgs.15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Korf E.S., Wahlund L.O., Visser P.J., Scheltens P. Medial temporal lobe atrophy on MRI predicts dementia in patients with mild cognitive impairment. Neurology. 2004;63(1):94–100. doi: 10.1212/01.wnl.0000133114.92694.93. [DOI] [PubMed] [Google Scholar]
- 53.Barkhof F., Polvikoski T.M., van Straaten E.C., Kalaria R.N., Sulkava R., Aronen H.J. The significance of medial temporal lobe atrophy: a postmortem MRI study in the very old. Neurology. 2007;69(15):1521–1527. doi: 10.1212/01.wnl.0000277459.83543.99. [DOI] [PubMed] [Google Scholar]
- 54.Squire L.R., Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253(5026):1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 55.Musiek E.S., Bhimasani M., Zangrilli M.A., Morris J.C., Holtzman D.M., Ju Y.S. Circadian rest-activity pattern changes in aging and preclinical Alzheimer disease. JAMA Neurol. 2018;75(5):582–590. doi: 10.1001/jamaneurol.2017.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang C., Holtzman D.M. Bidirectional relationship between sleep and Alzheimer's disease: role of amyloid, tau, and other factors. Neuropsychopharmacology. 2020;45(1):104–120. doi: 10.1038/s41386-019-0478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmitt K., Grimm A., Eckert A. Amyloid-beta-induced changes in molecular clock properties and cellular bioenergetics. Front Neurosci. 2017;11:124. doi: 10.3389/fnins.2017.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McKeith I.G., Dickson D.W., Lowe J., Emre M., O'Brien J.T., Feldman H. Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 61.Dichgans M., Leys D. Vascular Cognitive Impairment. Circ Res. 2017;120(3):573–591. doi: 10.1161/CIRCRESAHA.116.308426. [DOI] [PubMed] [Google Scholar]
- 62.Chang K.J., Lee S., Lee Y., Lee K.S., Back J.H., Jung Y.K. Severity of white matter hyperintensities and length of hospital stay in patients with cognitive impairment: a CREDOS (Clinical Research Center for Dementia of South Korea) study. J Alzheimers Dis. 2015;46(3):719–726. doi: 10.3233/JAD-142823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.