Abstract
Methanol intoxication can cause irreversible neurologic sequelae if unrecognized and untreated. Ingestion is the most common form of toxicity; however, dermal and inhalational exposures likewise occur but are documented rarely. While acute intoxication is commonly encountered, chronic exposure to methanol should also be highlighted. We report a case of a 57-year old female presenting in the emergency room with progressive dyspnea, metabolic acidosis with high anion gap, and metabolic encephalopathy. After emergency hemodialysis, the patient complained of vision loss on both eyes. Initial non-contrast cranial magnetic resonance imaging (MRI) revealed restricted diffusion of the intraorbital segment of both optic nerves. A thorough history revealed that she was applying a clear colorless liquid bought online all over her body for alleged pruritus for more than a year. The syndrome of metabolic acidosis with high anion gap, metabolic encephalopathy, vision loss, and laboratory findings led us to suspect a diagnosis of chronic methanol poisoning with an acute component. The liquid in question was sent for chemical analysis and result showed that it consisted of 95.5% Methanol. This case highlights the need for high index of clinical suspicion for methanol toxicity in the absence of oral consumption, the complications of chronic form of methanol intoxication, and the uncommon radiologic finding seen in diffusion-weighted imaging (DWI).
Keywords: Methanol, Chronic intoxication, Vision loss, Bilateral optic nerve restricted diffusion
Highlights
-
•
Topical and inhalational exposures are less common but equally significant routes of methanol toxicity.
-
•
Chronic methanol intoxication can present with acute vision loss.
-
•
Restricted diffusion of the optic nerves on MRI is an uncommon finding in methanol intoxication.
1. Introduction
Methanol is a clear and colorless chemical that can usually be seen in household cleaning products, antifreeze, varnishes, and fuel [1,2]. Methanol intoxication is a significant cause of neurologic morbidity and mortality if it is unrecognized and left untreated. Acute methanol toxicity may present with mild symptoms such as headache, altered mentation, blurring of vision, abdominal pain, and vomiting. However, in more severe cases, patients may develop blindness, severe metabolic acidosis, and coma [1]. Intake of adulterated alcoholic beverages is the primary cause of poisoning outbreaks of this substance [2]. Although ingestion is the most commonly implicated route of toxicity, methanol can be absorbed by inhalation and dermal exposure and these serve as uncommon routes of acute and chronic intoxication. Studies on chronic exposure to methanol particularly through dermal and inhalational routes are all the more rare and seen only on limited case reports. Chronic methanol toxicity can have neurologic sequelae such as vision loss that can potentially be irreversible. Vision loss is brought about by the affinity of the toxic metabolite of methanol, formic acid, to the optic pathway [1]. As such, methanol toxicity is an important differential diagnosis in unexplained vision loss. On cranial MR imaging, the most characteristic imaging feature of methanol poisoning is bilateral symmetric basal ganglia necrosis [[3], [4], [5], [6], [7]].
We present a case of chronic exposure to methanol through dermal and inhalational routes manifesting as shortness of breath, vision loss, and altered sensorium. This patient did not present with the typical supratentorial findings of methanol toxicity on initial cranial imaging.
2. Case report
The patient is a 57-year old female, right-handed, who presented with one-week history of cough and progressive shortness of breath few hours before arrival to the emergency room (ER). In the ER, her blood pressure was 130/100 mmHg. She was tachycardic at 140 beats per minute with new-onset atrial fibrillation. She was tachypneic with a respiratory rate of 28 cycles per minute and with an oxygen saturation of only 82% at room air. She was afebrile at 36.5 degrees Celsius. She was agitated and inconsistently followed commands. Arterial blood gases revealed severe metabolic acidosis with a pH of 6.898, carbon dioxide of 20.5, oxygen of 261.6, bicarbonate of 4, and oxygen saturation 99.4% with a high anion gap of 20. The patient was intubated, started on amiodarone and bicarbonate drip, and was sedated with dexmedetomidine. The initial assessment at the ER level was thyroid storm. However, the laboratory findings were consistent with only subclinical hyperthyroidism. A red flag noted in the patient's history was the chronic intake of multiple supplements mostly consisting of herbal extracts. Intoxication with any of these drugs was then considered.
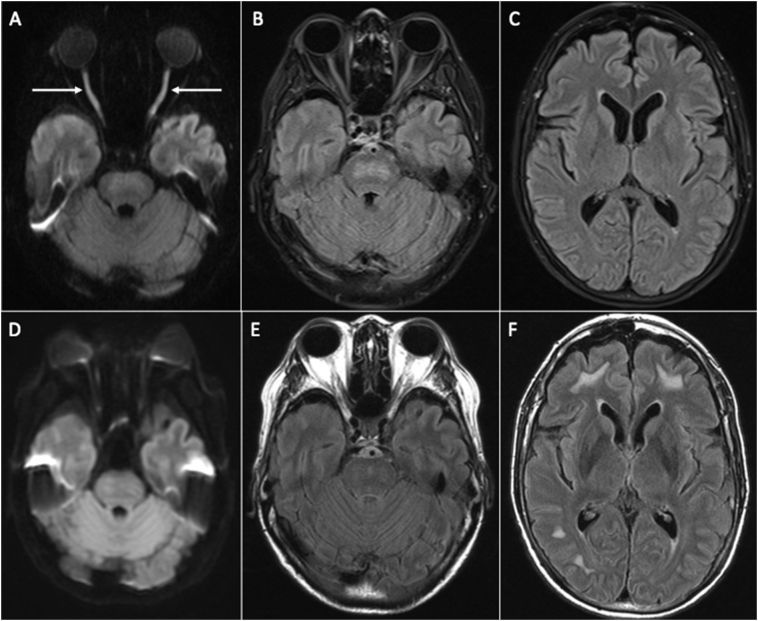
The patient was admitted in the intensive care unit and underwent emergency hemodialysis for the metabolic acidosis with high anion gap. The patient was seen with spontaneous eye opening despite the sedation, with no regard, and without following commands. Her pupils were 4 mm non-reactive to light. She had normal fundoscopic examination with roving eyes movements. There was no focal sensorimotor deficit or meningeal sign. When the sedation was titrated down, she communicated through writing “Darkness” and “Why can't I see?”. At this time, test for visual acuity revealed hand movement at 6 in. on both eyes. Cranial MRI with angiography revealed restricted diffusion involving the intraorbital segment of both optic nerves (Fig. 1A) and confluent white matter hyperintensity in the central pons on fluid attenuation inversion recovery sequence (FLAIR) (Fig. 1B). Considering toxic optic neuropathy, careful pharmacologic review of the herbal supplements taken by the patient and their possible interaction was done but results were not consistent with her signs and symptoms. Unfortunately, no blood sample for any drug or toxic metabolite was collected prior to hemodialysis. Blood and urine heavy metal analysis done after hemodialysis including mercury, lead, and cadmium eventually turned out to be negative. The patient's vision further deteriorated to light perception and ultimately on the 5th hospital day, it progressed to complete vision loss. Visual evoked potential showed abnormal pattern reversal and evidence of failed conduction along both visual pathways consistent with a severe conduction defect seen in lesions anterior to the chiasm. The patient was started on Dexamethasone 5 mg IV every 8 h.
Fig. 1.
Cranial MRI. Axial DWI (A) and FLAIR (B, C) images during admission show restricted diffusion in the intraorbital segment of both optic nerves (arrows) and confluent hyperintense signal in the central pons. Follow-up DWI (D) and FLAIR (E, F) images after two months show resolution of the restricted diffusion in the optic nerves and less confluent signal abnormality in the pons with interval demonstration of hyperintense foci in the bilateral cerebral subcortical white matter.
When the patient's sensorium improved, she was able to report 3-month history of persistent painless darkening of the entire field of vision. On further investigation, the patient disclosed that for the past year, she had been applying clear, odorless liquid on her entire body including the scalp everyday to self-medicate for pruritus. She denied oral intake of this liquid. She acquired this liquid labeled “denatured alcohol” online with claims of it being ethyl alcohol. A sample was sent to the Philippine Institute of Pure and Applied Chemistry (PIPAC) which revealed the liquid to be of 95.5% methanol via gas chromatography.
The patient was discharged after 2 weeks with little improvement in visual acuity: hand movement at 1 ft on the left eye and no light perception on the right eye. Repeat visual evoked potential done 3 weeks after discharge revealed absent response on the right and some potential response on the left which was prominently delayed. Repeat imaging 2 months after the initial cranial MRI showed resolution of the restricted diffusion in the intraorbital segment of both optic nerves (Fig. 1D) and less confluent hyperintense signal in the central pons on FLAIR images (Fig. 1E). However, there was interval appearance of hyperintense signal abnormality in the subcortical white matter (Fig. 1F).
3. Discussion
Our patient presented with restricted diffusion of the intraorbital segment of both optic nerves on initial non-contrast MRI. To our knowledge, this is only the second case report to document such imaging finding in a patient with methanol toxicity. The first case was documented in a 52-year old man who presented with blurring of vision after methanol ingestion. On DWI, restricted diffusion of the bilateral retrobulbar segment of the optic nerves was documented with minimal enhancement on contrast administration [8]. There is a strong affinity of formic acid, a metabolite of methanol, to the retrolaminar optic nerve and optic disc. In these sites, formic acid links with cytochrome oxidase in the mitochondria resulting to decreased ATP production. This ultimately arrests transmission of action potentials along the nerves causing vision loss [1]. Optic nerve pathologies that may present with sudden onset of visual symptoms include demyelination, inflammation, pseudotumor, infection, traumatic, and ischemic optic neuropathy. Methanol toxicity causing toxic optic neuropathy is one of the causes of optic nerve and optic nerve sheath pathologies with gradual onset of symptoms [9]. These diseases may present as restricted diffusion with involvement of the optic nerve.
Additionally in our patient, there was another finding of hyperintense signal involving the central pons. Pathology in the pons was reported in a case where it was demonstrated that foci of T2 hyperintensity in the bilateral pontine tegmentum was one of the imaging findings in severe methanol intoxication [10]. Further, in a case series of 17 patients who died of methanol intoxication, pontine hemorrhage was documented in 2 cases on autopsy [11]. The susceptibility of the pons to methanol is worth investigating, as its vulnerability with the optic nerve may not be a coincidence. In the follow-up imaging of our patient, the less confluent appearance of central pontine hyperintensity coincided with the resolution of diffusion restriction of both optic nerves. The interval appearance of T2 hyperintensity in the bilateral subcortical cerebral hemispheres on repeat MRI is consistent with previously reported imaging findings in the chronic stage of methanol toxicity [5]. These subcortical changes can be consequences of the toxic metabolites of methanol [10]. These are not specific for methanol toxicity but should always be considered in association with other MRI findings [5].
Although ingestion is the most commonly implicated route of toxicity, methanol can be absorbed by inhalation and dermal exposure [2]. These cases, particularly those associated with chronic exposure, are very rare and are confined to case reports. Perhaps one of the earliest reports of methanol toxicity through chronic inhalational route was by Mccormick et al. They documented the case of a young male who sniffed fumes composed of toluene, methanol, and methylene chloride for months. Methanol was isolated on blood examination. He was lethargic and ataxic but had normal ophthalmologic exam and was not in acidosis [12]. His relatively minor symptoms could have been due to low methanol level but with prolonged exposure, visual disturbance and acidosis could be expected as such in our patient.
Some reports of toxicity through dermal route have been published but are of acute exposure. One case was an adult male who presented with lethargy, blurring of vision and severe acidosis after soaking his feet and clothes in methanol while cleaning an industrial tank. Because of the known exposure, ethanol drip was provided readily and he was able to gain back his normal vision [13]. Another case was an adult male with vision changes and metabolic acidosis after using a solvent presumed to be methanol to clean his upper extremities. Despite management, he was unable to recover his vision [14]. One rare case that resulted in mortality was an adult female who presented with the usual signs of symptoms of methanol toxicity after applying a certain spirit to massage her head a few days prior. This report postulated that the high permeability and rich vasculature of the scalp could have readily led to the absorption of methanol [15]. Our patient applied methanol on her scalp and skin of her entire body daily for a year. Interestingly, she did not present with severe symptoms early on. Cutaneous absorption of methanol is affected by many factors. It has been reported that the condition, hydration, temperature, and other characteristics of the skin can influence the rate at which methanol is absorbed [16].
Recently, methanol was highlighted as an occupational hazard through chronic inhalational and dermal exposure in factories in Asia. In South Korea, two factory workers developed blurring of vision after 4 months of being exposed to machines that used methanol to cool down cellular phone parts. No protective devices were used at work exposing the workers to vapor, skin contact, and even eye contact with the fluid [17]. Eight Chinese patients were also reported to have acute vision loss from chronic inhalation of methanol in the workplace. Some patients were exposed for as long as 5 years before developing acute symptoms. It was postulated that the toxic metabolites of methanol could have been accumulating over the years and that acute symptom onset was the result of these metabolites finally reaching the toxic dose [18]. In the same way, our patient presented with vision loss in a background of chronic exposure to methanol through dermal and inhalational routes. The patient applied this solvent throughout her skin and scalp for a year and was exposed to the vapor of this substance upon its application. There was no prior oral intake of methanol or any alcohol-based product. Our patient also had a 3-month history of visual disturbance prior to acute vision loss that was similar to the experience of 3 out of the 8 subjects in China who were exposed to methanol chronically [18]. This underscores that persistent exposure to methanol can present with acute and severely debilitating symptoms on top of probable slowly progressive findings. Such cases warrant further documentation and investigation.
4. Conclusion
We present an uncommon case of vision loss in the background of chronic exposure to methanol through dermal and inhalational routes. Methanol was isolated late in the course of this patient. As such, this case highlights the need for high index of suspicion for methanol in the presence of vision loss and severe metabolic acidosis with high anion gap despite lacking history of toxic ingestion. This case adds to the increasing number of reports of methanol intoxication from vapor and skin contact. The symptoms and course of the patient were similar to those seen in toxic ingestion of methanol. The DWI findings particularly in the optic nerves can be seen in the background of chronic exposure to this substance. History of methanol exposure should therefore be elicited when faced with characteristic clinical findings of vision loss and metabolic acidosis with high anion gap and radiologic finding of restricted diffusion of the optic nerves and confluent hyperintense signal in the central pons. These imaging findings may be hypothesized as unusual imaging features and further case documentation is warranted.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
None declared.
Acknowledgements
We like to thank Dr. Wohldorf, Dr. Yano-Simbulan, Dr. Samson, and Dr. Hawson, for their significant help in managing the case.
Contributor Information
Christianne V. Mojica, Email: cvmojica@stlukes.com.ph.
Jacqueline C. Dominguez, Email: jcdominguez@stlukes.com.ph.
References
- 1.Barceloux D.G., Bond G.R., Krenzelok E.P., Cooper H., Vale J.A. American academy of clinical toxicology practice guidelines on the treatment of methanol poisoning. Clin. Toxicol. 2002;40:415–446. doi: 10.1081/clt-120006745. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Information Note Methanol Poisoning Outbreaks. 2014. https://www.who.int/environmental_health_emergencies/poisoning/methanol_information.pdf
- 3.Blanco M., Casado R., Vásquez F., Pumar J.M. CT and MR imaging findings in methanol intoxication. Am. J. Neuroradiol. 2006;27:452–454. [PMC free article] [PubMed] [Google Scholar]
- 4.Azeemuddin M., Naqi R. MRI findings in methanol intoxication: a report of three cases. J. Pak. Med. Assoc. 2012;62:1099–1101. [PubMed] [Google Scholar]
- 5.Elkhamary S.M., Fahmy D.M., Galvez-Ruiz A., Asghar N., Bosley T.M. Spectrum of MRI findings in 58 patients with methanol intoxication: long-term visual and neurological correlation. Egypt. J. Radiol. Nuclear Med. 2016;47:1049–1055. [Google Scholar]
- 6.Yedavalli V.S., Chowdhry P.S., Bachchav V., Patil A. Potent Potables: examining ccute and chronic CT and MR imaging patterns of ethanol and methanol poisoning. Neurographics. 2018;8:244–253. [Google Scholar]
- 7.De Oliveira A.M., Paulino M.V., Vieira A.P.F., McKinney A.M., da Rocha A.F., dos Santos G.T. Imaging patterns of toxic and metabolic brain disorders. Radiographics. 2019;39:1672–1695. doi: 10.1148/rg.2019190016. [DOI] [PubMed] [Google Scholar]
- 8.Tanrivermis Sayit A., Aslan K., Elmali M., Gungor I. Methanol-induced toxic optic neuropathy with diffusion weighted MRI findings. Cutan. Ocul. Toxicol. 2016;35:337–340. doi: 10.3109/15569527.2015.1122031. [DOI] [PubMed] [Google Scholar]
- 9.Gala F. Magnetic resonance imaging of optic nerve. Indian J. Radiol. Imaging. 2015;25:421–438. doi: 10.4103/0971-3026.169462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaul H.P., Wallace C.J., Auer R.N., Fong T.C. MR findings in methanol intoxication. Am J Neuroradiol. 1995;16:1783–1786. [PMC free article] [PubMed] [Google Scholar]
- 11.Karayel F., Turan A.A., Sav A., Pakis I., Akyildiz E.U., Ersoy G. Methanol intoxication: pathological changes of central nervous system (17 cases) Am. J. Forensic Med. Pathol. 2010;31:34–36. doi: 10.1097/PAF.0b013e3181c160d9. [DOI] [PubMed] [Google Scholar]
- 12.McCormick M.J., Mogabgab E., Adams S.L. Methanol poisoning as a result of inhalational solvent abuse. Ann. Emerg. Med. 1990;19:639–642. doi: 10.1016/s0196-0644(05)82467-9. [DOI] [PubMed] [Google Scholar]
- 13.Downie A., Khattab T.M., Malik M.I., Samara I.N. A case of percutaneous industrial methanol toxicity. Occup. Med. 1992;42:47–49. doi: 10.1093/occmed/42.1.47. [DOI] [PubMed] [Google Scholar]
- 14.Beaton C.R., Meyer C. Accidental transdermal methanol poisoning presenting to a regional emergency department. Can. J. Emerg. Med. 2019;21:435–437. doi: 10.1017/cem.2018.499. [DOI] [PubMed] [Google Scholar]
- 15.Soysal D., Yersal Kabayegit O., Yilmaz S., Tatar E., Ozatli T., Yildiz B. Transdermal methanol intoxication: a case report. Ata Anaesthesiol. Scand. 2007;51:779–780. doi: 10.1111/j.1399-6576.2007.01332.x. [DOI] [PubMed] [Google Scholar]
- 16.Batterman S.A., Franzblau A. Time-resolved cutaneous absorption and permeation rates of methanol in human volunteers. Int. Arch. Occup. Environ. Health. 1997;70:341–351. doi: 10.1007/s004200050228. [DOI] [PubMed] [Google Scholar]
- 17.Ryu J., Lim K.H., Ryu D.R., Lee H.W., Yun J.Y., Kim S.W. Two cases of methyl alcohol intoxication by sub-chronic inhalation and dermal exposure during aluminum CNC cutting in a small-sized subcontracted factory. Ann. Occup. Environ. Med. 2016;28:65. doi: 10.1186/s40557-016-0153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Z., Jiang H., Wang J. Clinical analysis of severe visual loss caused by inhalational methanol poisoning in a chronic process with acute onset: a retrospective clinical analysis. BMC Ophthalmol. 2019;19:124. doi: 10.1186/s12886-019-1127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]