Abstract
Background
Total knee arthroplasty (TKA) is one of the most effective ways to treat end-stage painful conditions of the knee. However, non-standardized reporting patterns can make quantitative analysis of patient outcomes difficult.
Methods
A systematic review of the literature was performed using keywords “total knee arthroplasty” and “total knee replacement.” Randomized controlled trials (RCTs) meeting the inclusion criteria were sorted and reviewed. Type of study, outcome measures used to report their results, and the actual results were recorded. Quantitative analysis was performed.
Results
A total of 233 RCTs were included. There was significant variability in the reporting of short term and long term outcomes in total knee arthroplasty. The most common treatment domains in order of decreasing frequency were objective knee function, subjective knee function, perioperative complications, and pain. Range of motion was the most common outcome metric reported in all the RCTs and also was the most common metric used to assess objective knee function. The most common patient reported outcome measure used to assess postoperative function was the Knee Society Score followed by Knee Injury and Osteoarthritis Outcome Score. The Visual Analog Scale was the most common measurement tool used to assess postoperative pain. Most studies assessed patient outcomes in three treatment domains. None reported outcomes in all seven domains.
Conclusion
There is significant variability in outcome reporting patterns in TKA literature. Most studies do not track outcomes comprehensively, with a significant minority of the RCTs tracking outcomes in only one treatment domain.
Keywords: Total knee arthroplasty, Joint replacement, Outcome measures, Knee society score, Patient reported outcomes, Oxford knee score
1. Introduction
Total knee arthroplasty (TKA) is an effective treatment for end-stage osteoarthritis, which is one of the most common causes of disability in the United States.1,2 The number of TKAs performed in the United States in 2012 alone was more than 700,000.3 With increasing human lifespan and rising prevalence of obesity leading to more arthritis-related disability, the demand for TKA is projected to increase by over 143% by 2050.4,5 These statistics underscore the importance of clinical research on TKA, whether the focus of the research is cost effectiveness, new innovations, or clinical outcomes. A standardized approach to this research would enhance collaborative efforts, and the ability to pool data from multiple studies in order to increase the power of the conclusions with larger cohorts of patients.
Clinical outcomes in TKA are measured using a variety of tools including physical exam findings, such as range of motion (ROM), stability, and ability to perform a straight leg raise, complication rate, radiographic parameters, implant survivorship, and subjective metrics such as patient experience and satisfaction. With recent focus on capturing quality of life (QOL) and functional improvement as experienced by patients, patient reported outcome measures (PROMs) have gained popularity and acceptance as tools to assess outcomes after many orthopaedic procedures.6,7
With so many different options available for reporting outcomes after TKA, it is important to assess the reporting variability and trends in clinical research pertaining to TKA. High variability in reporting outcomes can hinder comparison of results across studies and prevent pooled analysis.8 Furthermore, the comprehensiveness of different outcome measurement tool utilized is highly variable. While the primary goal of TKA is to relieve pain, secondary goals include restoring function, improving quality of life, and reducing dependence on medical aids, among others.9 Thus, it is important for researchers to use outcome measurement tools that address all of the treatment domains of TKA. The goal of this review was to determine if there is variability in types of outcomes reported in the literature and to assess the comprehensiveness of the outcome reporting after TKA.
2. Materials and methods
2.1. Search strategy
The following combination of keywords was used for a comprehensive literature search of the electronic database PUBMED: “total knee arthroplasty” and “total knee replacement.” The following date range was used: June 1, 2014 to June 24, 2019. Only randomized controlled trials written in English and published in ten high-impact orthopaedic journals were considered for inclusion (Table 1). The ten orthopaedic journals were chosen based on their impact factor rating and their track record of publishing studies on TKA.10
Table 1.
Ten high-impact orthopaedic journals included in the review.
| Journal | Country of Origin |
|---|---|
| Acta Orthopaedica | United Kingdom (UK) |
| Clinical Orthopaedics and Related Research (CORR) | United States of America (USA) |
| Journal of American Academy of Orthopaedic Surgeons (JAAOS) | USA |
| Journal of Arthroplasty (JOA) | USA |
| Journal of Bone and Joint Surgery (JBJS) | USA |
| Journal of Bone and Mineral Research (JBMR) | USA |
| Journal of Orthopaedic Research (JOR) | USA |
| Knee | Netherlands |
| Knee Surgery, Sports Traumatology, and Arthroscopy (KSSTA) | Germany |
| Osteoarthritis and Cartilage | UK |
2.2. Study selection
Titles relevant to TKA were retained for further scrutiny. Any duplicates were merged in a citation manager. The following inclusion criteria were used when screening studies: (a) randomized controlled trial, (b) pertaining to total knee arthroplasty, (c) reporting on clinical outcomes, and (d) published in the last 5 years.
The following exclusion criteria were used: (a) case series, case control studies, cohort studies, case reports, expert opinions, or other studies that are not randomized controlled trials, (b) reviews, (c) studies published in languages other than English, (d) any studies involving non-human subjects or cadaveric studies, (e) basic science studies, (f) biomechanical studies, (g) radiographic studies, (h) studies on unicompartmental knee arthroplasty, and (i) published in journals other than the ten chosen ones. The study selection algorithm and results are displayed in Fig. 1.
Fig. 1.
Study selection algorithm and results.
2.3. Data extraction
Data were independently extracted by two independent reviewers for detailed analysis. Information recorded included the sample size in each study, the study design (level of evidence), average duration of follow up, the research question the study was trying to answer, outcome measurement tools used to report results, and actual outcomes reported in the study. The collected data were reviewed and analyzed independently by two authors. They also independently performed a risk of bias assessment for each study. Any discrepancies in data analysis were resolved by means of discussion.
2.4. Quality appraisal
Both reviewers independently analyzed the methodological quality of each study meeting inclusion criteria in regard to each of the following domains: comprehensiveness of outcomes reported and pertinence of outcomes reported to the question the study was trying to answer. Comprehensiveness of each study was assessed based on whether or not the study reported outcomes in each of the seven treatment domains of TKA: (1) perioperative complications, (2) pain, (3) patient satisfaction, (4) subjective knee function (measured by PROMs), (5) objective knee function (as measured by physician), (6) implant survivorship, and (7) radiographic findings.7,8 Internal and external validity of each study was assessed using ten quality criteria described in prior published research.11,12 Only trials meeting at least 7 out of the 10 quality criteria were included for analysis.
3. Results
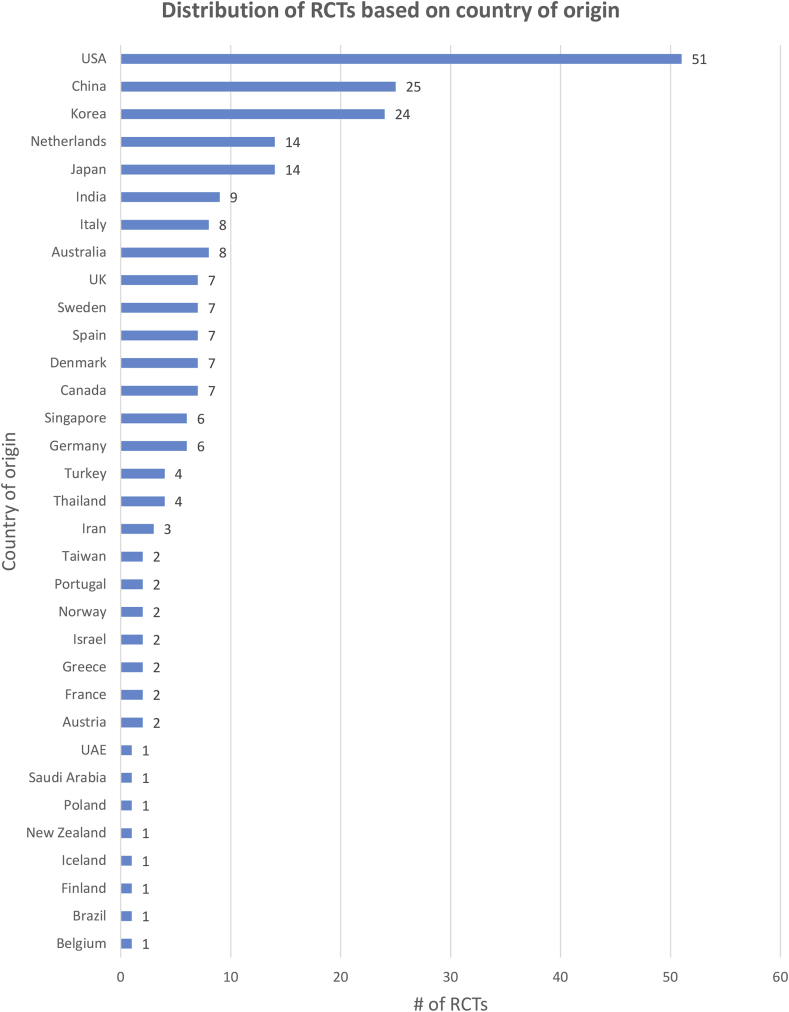
A total of 233 studies (Appendix 1) met the inclusion and quality criteria. The majority of RCTs included for analysis originated in USA. The country that was next largest source of research was China. The breakdown of RCTs based on country of origin is displayed in Fig. 2.
Fig. 2.
Country of origin of RCTs included for analysis.
The most productive year for research was 2017, with 57 of the 233 included RCTs having been published that year. There were 40 RCTs published in the last six months of 2014 while only 7 RCTs were published in the first six months of 2019. The breakdown of RCTs by year of publication is displayed in Fig. 3.
Fig. 3.
Number of RCTs published on TKA outcomes per year.
The most common topics of interest in RCTs were perioperative anesthesia, tranexamic acid (TXA) administration, implant design, and the role of computer navigation in TKA. Some of the less common topics of study included deep vein thrombosis (DVT) prophylaxis, non-TXA topical and injectable agents used to reduce blood loss, and role of drains in TKA. These results are displayed in Fig. 4. TKA topics on which there was no more than one RCT published were grouped under “other.” Some of these topics included use of melatonin in the perioperative period, different techniques for medial release in TKA, role of Botox in improving range of motion after TKA, the effect of albumin transfusion in reducing postoperative complications, use of bone wax in TKA, efficacy of various anti-nausea medications in the perioperative period, the effect of sealing femoral canal on reducing blood loss during TKA, role of denosumab in TKA, use of fentanyl patch for pain control, the effect of leg position in immediate perioperative period on blood loss, and use of cryotherapy for perioperative analgesia.
Fig. 4.
Most common TKA topics studied in RCTs.
Follow-up period was defined as “short-term” if the average duration of follow-up was less than 2 years, “mid-term” if it was 2–10 years, and “long-term” if it was greater than 10 years in an RCT. These definitions were based on prior published parameters in knee arthroplasty research.13, 14, 15, 16 Most of the RCTs included for analysis reported short-term outcomes in TKA (69.5%) while a small minority of the RCTs reported on long-term outcomes (3.9%). These results are displayed in Fig. 5.
Fig. 5.
Duration of follow-up in RCTs included in systematic review.
The sample size of RCTs was defined as “small” if it included less than 50 patients, “medium” if it included 50–200 patients, and “large” if it included more than 200 patients. Most of the RCTs included for analysis had medium sample size (75%). Twelve percent of the RCTs consisted of large sample size and 13% consisted of small sample size.
Of the seven treatment domains of TKA described above, the two domains most commonly assessed to report outcomes after TKA were complications and objective knee function. Complications reported included length of stay, blood loss, and transfusion rate. For objective assessment of knee function, range of motion (ROM) was the most commonly used metric, followed by strength as measured by dynamometer and ability to do a straight leg raise (SLR).
For subjective assessment of knee function, the Knee Society Score (KSS) was the most commonly used PROM. Other commonly used PROMs include The Knee Injury and Osteoarthritis Outcome Score (KOOS), The Western Ontario and McMaster Universities Arthritis Index (WOMAC), 36-Item Short Form Health Survey (SF-36), Oxford Knee Score (OKS), 12-Item Short Form Health Survey (SF-12), Hospital for Special Surgery Knee Score (HSS), University of California Los Angeles (UCLA) Activity Score, and Forgotten Joint Score (FJS) in descending order of popularity.
Pain was most commonly assessed by the Visual Analog Scale (VAS) followed by the Numerical Rating Scale (NRS). The distribution of various metrics used to report outcomes in order of frequency is displayed in Fig. 6. The distribution of most common outcome domains assessed is provided in Fig. 7.
Fig. 6.
Most common parameters used to report outcomes in RCTs.
Fig. 7.
Frequency of the seven outcome domains assessed in RCTs.
Comprehensiveness of outcome reporting in each RCT was determined based on how many treatment domains were assessed in the outcomes reported out of the seven total domains: perioperative complications, knee subjective function, knee objective function, pain, implant survivorship, radiographic findings, and patient satisfaction. Thirty-two percent of the RCTs included in this review reported outcomes in three treatment domains while only 1.7% of the RCTs reported outcomes in six treatment domains. None of the RCTs reported outcomes in all seven domains and an astounding 17.2% of the RCTs reported outcomes in only one treatment domain. These results are displayed in Fig. 8.
Fig. 8.
Comprehensiveness of outcome reporting in RCTs.
4. Discussion
The demand for TKA is expected to increase in the next two decades due to an aging population and rising obesity rates.4 TKA is one of the few high cost procedures performed on Medicare patients, with total joint replacement (TJR) costing more than $7 billion to the healthcare system in 2014.17 These rising healthcare costs have increased the scrutiny of patient outcomes after TJR.18
Patient outcomes have traditionally been assessed by objective findings such as physical exam findings, implant survivorship, and radiographic evidence of loosening in older studies.19,20 However, with the advent of bundled payment plans such as Comprehensive Care for Joint Replacement (CJR) and Bundled Payments for Care Improvement (BPCI), surgeons and institutions offering TJR are incentivized to report and track patient-reported outcome measures (PROMs) in order to monitor improvement in QOL after surgery.21 While the growing popularity of PROMs does underscore the importance of considering patient perspectives in improving treatment outcomes after TKA, PROMs are not without their limitations and some studies have raised concerns that these subjective measures of knee function by themselves may not be the most accurate way to assess outcomes after TKA.22 Thus, it is important to take a holistic approach consisting of subjective measures, objective exam findings, radiographic analysis, complication rate, revision-free survival, and other parameters affecting patient satisfaction when assessing outcomes after TKA.
Prior research in other specialties of orthopaedics has shown that there is significant variability in outcome reporting.23, 24, 25 Stone et al. showed that more than 20 PROMs are currently used to track outcomes in hip arthroscopy research, making comparison across studies difficult.23 Makhni et al. found that there is significant variation in the type and method of laxity reporting in high impact ACL literature.24 Similarly, our review revealed that there is significant variability in outcome reporting in TKA literature. We found that the most common metric used to track outcomes after TKA was ROM and this objective measure of knee function was reported in only 133 of the 233 RCTs included for analysis. The rest of the RCTs used a variety of other metrics such as strength, ability to do a straight leg raise, Timed Up and Go (TUG) test, stair climb test, etc., for assessing knee function objectively. There was even more variability in PROMs used to assess patient perspectives of knee function. While KSS was the most commonly used PROM to assess improvement in knee function from the patient’s standpoint, this was used in only 120 of the 233 RCTs included for analysis. The amount of variability was even higher when it came to reporting outcomes in other treatment domains such as pain, perioperative complications, patient satisfaction, etc. This variability in outcome reporting is challenging because lack of standardization can prevent comparison of results across different studies. This lack of standardization in outcome reporting is not unique to TKA literature and is recognized as one of the areas of methodological deficiencies found to be pervasive in orthopaedic literature across many specialties.26
Furthermore, our review results show that not all outcome domains are assessed adequately by clinical studies. The median number of outcome domains assessed was three out of a total of seven and a significant portion of the RCTs (17.2%) only assessed outcomes in one domain. None of the RCTs included for analysis reported outcomes in all seven domains. This incomplete reporting may compromise the accuracy of reported results and prevent comparison of new techniques, implant types, and other innovations with existing technology in the field of arthroplasty.
Our review was subject to several important limitations. First, only studies published in ten high-impact journals were included for analysis. While this was done to exclude low quality studies, the authors recognize that this inclusion criterion may have also excluded several high quality studies published in other journals. Second, limiting our review to the past 5 years may have allowed us to identify the current outcome reporting trends but may have excluded other metrics that were used in the past to measure outcomes after total knee arthroplasty. Despite these limitations, one of the strengths of our review was that it only included randomized controlled trials, which are studies consisting of the highest level of evidence in scientific research. Even at this level of impact, our review revealed that outcome reporting in knee arthroplasty literature is variable and not very comprehensive. This lack of standardization in outcome reporting is likely to be even more profound and widespread in studies with lower levels of evidence.
5. Conclusion
The results of the study show that there is significant variability in outcome reporting patterns in total knee arthroplasty literature. Most studies do not track outcomes comprehensively, with a significant minority of the RCTs tracking outcomes in only one treatment domain. We suggest that standardization of reported outcomes and domains in TKA studies should be mandated by the appropriate societies and journals in order to more readily enable meaningful comparisons between and pooling of data from multiple studies.
Ethical committee statement
This systematic review of the literature did not require approval by the Biomedical Institutional Review Board of The Ohio State University.
Declaration of competing interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcot.2020.05.014.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Oliveria S.A., Felson D.T., Reed J.I., Cirillo P.A., Walker A.M. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995;38(8):1134–1141. doi: 10.1002/art.1780380817. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Prevalence and most common causes of disability among adults--United States, 2005. MMWR Morb Mortal Wkly Rep. 2009;58(16):421–426. [PubMed] [Google Scholar]
- 3.Healthcare Cost and Utilization Project (HCUP). Nationwide Inpatient Sample (NIS). Statistical Brief #186. 2004. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb186-Operating-Room-Procedures-United-States-2012.pdf Accessed October 3, 2019. [Google Scholar]
- 4.Inacio M.C.S., Paxton E.W., Graves S.E., Namba R.S., Nemes S. Projected increase in total knee arthroplasty in the United States - an alternative projection model. Osteoarthritis Cartilage. 2017;25(11):1797–1803. doi: 10.1016/j.joca.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 5.Sloan M., Premkumar A., Sheth N.P. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018;100(17):1455–1460. doi: 10.2106/JBJS.17.01617. [DOI] [PubMed] [Google Scholar]
- 6.Makhni E.C., Hamamoto J.T., Higgins J.D. How comprehensive and efficient are patient-reported outcomes for rotator cuff tears? Orthop J Sports Med. 2017;5(3) doi: 10.1177/2325967117693223. 2325967117693223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deshpande P.R., Rajan S., Sudeepthi B.L., Abdul Nazir C.P. Patient-reported outcomes: a new era in clinical research. Perspect Clin Res. 2011;2(4):137–144. doi: 10.4103/2229-3485.86879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makhni E.C., Meyer M.A., Saltzman B.M., Cole B.J. Comprehensiveness of outcome reporting in studies of articular cartilage defects of the knee. Arthroscopy. 2016;32(10):2133–2139. doi: 10.1016/j.arthro.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Lange T., Schmitt J., Kopkow C., Rataj E., Gunther K.P., Lutzner J. What do patients expect from total knee arthroplasty? A delphi consensus study on patient treatment goals. J Arthroplasty. 2017;32(7):2093–2099. doi: 10.1016/j.arth.2017.01.053. e2091. [DOI] [PubMed] [Google Scholar]
- 10.Scimago S.J.R. Journal & Country Rank. 2019 https://www.scimagojr.com/journalrank.php?category=2732 Accessed October 3, 2019. [Google Scholar]
- 11.Akobeng A.K. Assessing the validity of clinical trials. J Pediatr Gastroenterol Nutr. 2008;47(3):277–282. doi: 10.1097/MPG.0b013e31816c749f. [DOI] [PubMed] [Google Scholar]
- 12.Vajapey S.P., Blackwell R.E., Maki A.J., Miller T.L. Treatment of extensor tendon disruption after total knee arthroplasty: a systematic review. J Arthroplasty. 2019;34(6):1279–1286. doi: 10.1016/j.arth.2019.02.046. [DOI] [PubMed] [Google Scholar]
- 13.Ishii Y., Noguchi H., Sato J., Sakurai T., Toyabe S.I. Quadriceps strength impairment in the mid- to long-term follow-up period after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2017;25(11):3372–3377. doi: 10.1007/s00167-016-4333-5. [DOI] [PubMed] [Google Scholar]
- 14.Scior W., Hilber F., Hofstetter M., Graichen H. Short-term and mid-term results of lateral condyle sliding osteotomy in the treatment of valgus total knee arthroplasty: a successful therapy option in Grade 2 valgus total knee arthroplasty. Knee. 2018;25(3):466–472. doi: 10.1016/j.knee.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Glover A.W., Santini A.J.A., Davidson J.S., Pope J.A. Mid- to long-term survivorship of oxidised zirconium total knee replacements performed in patients under 50years of age. Knee. 2018;25(4):617–622. doi: 10.1016/j.knee.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Mahawar K.K. Defining short-term, medium-term, long-term, and very long-term follow-up after bariatric Surgery. Obes Surg. 2018;28(5):1425–1426. doi: 10.1007/s11695-018-3183-2. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Medicare and Medicaid Services Comprehensive care for joint replacement model. https://innovation.cms.gov/initiatives/cjr
- 18.Gutacker N., Bojke C., Daidone S., Devlin N.J., Parkin D., Street A. Truly inefficient or providing better quality of care? Analysing the relationship between risk-adjusted hospital costs and patients’ health outcomes. Health Econ. 2013;22(8):931–947. doi: 10.1002/hec.2871. [DOI] [PubMed] [Google Scholar]
- 19.Sledge C.B., Walker P.S. Total knee arthroplasty in rheumatoid arthritis. Clin Orthop Relat Res. 1984;182(182):127–136. [PubMed] [Google Scholar]
- 20.Barck A.L. 10-year evaluation of compartmental knee arthroplasty. J Arthroplasty. 1989;4:S49–S54. doi: 10.1016/s0883-5403(89)80007-5. Suppl(54) [DOI] [PubMed] [Google Scholar]
- 21.Lyman S., Yin K.L. Patient-reported outcome measurement for patients with total knee arthroplasty. J Am Acad Orthop Surg. 2017;25:S44–S47. doi: 10.5435/JAAOS-D-16-00637. Suppl 1(1) [DOI] [PubMed] [Google Scholar]
- 22.Hossain F.S., Konan S., Patel S., Rodriguez-Merchan E.C., Haddad F.S. The assessment of outcome after total knee arthroplasty: are we there yet? Bone Joint Lett J. 2015;97-B(1):3–9. doi: 10.1302/0301-620X.97B1.34434. [DOI] [PubMed] [Google Scholar]
- 23.Stone A.V., Jacobs C.A., Luo T.D. High degree of variability in reporting of clinical and patient-reported outcomes after hip arthroscopy. Am J Sports Med. 2018;46(12):3040–3046. doi: 10.1177/0363546517724743. [DOI] [PubMed] [Google Scholar]
- 24.Makhni E.C., Padaki A.S., Petridis P.D. High variability in outcome reporting patterns in high-impact ACL literature. J Bone Joint Surg Am. 2015;97(18):1529–1542. doi: 10.2106/JBJS.O.00155. [DOI] [PubMed] [Google Scholar]
- 25.Vajapey S.P., Morris J., Li D., Greco N., Spitzer A. Outcome reporting patterns in total hip arthroplasty: a systematic review of randomized clinical trials. JBJS Rev. 2020;8(4) doi: 10.2106/JBJS.RVW.19.00197. [DOI] [PubMed] [Google Scholar]
- 26.Coleman B.D., Khan K.M., Maffulli N., Cook J.L., Wark J.D. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports. 2000;10(1):2–11. doi: 10.1034/j.1600-0838.2000.010001002.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.