Abstract
Purpose
Liposarcoma (LPS) is a one of the most commonly diagnosed soft tissue sarcomas. Little is known about the epidemiology and prognosis of each subtype. We present an analysis of epidemiology and survival of the subtypes of LPS using a national database.
Methods
We queried the Survival Epidemiology, and End Results (SEER) and the Canadian Institute for Clinical Evaluative Sciences (ICES) databases for data on 7 types of LPS. Pearson’s chi square was used to determine associations between variables and subtypes. Kaplan-Meier and Cox Regression analyses were performed for two tests: one using SEER data and the other using variables common to both SEER and ICES.
Results
The well-differentiated subtype was the most common subtype identified. Metastatic disease was associated with decreased survival across all subtypes and age >35 was associated with decreased survival in well-differentiated and myxoid subtypes. Tumor grade was associated with decreased survival in the well-differentiated, myxoid, mixed, and round cell subtypes. In the secondary analysis, age >35 was associated with decreased survival in the myxoid subtype.
Conclusions
The prognosis of liposarcoma differs greatly by subtype. Clinicians should account for patient factors at the time of diagnosis to best navigate treatment of their patients.
Keywords: Liposarcoma, Subtypes, SEER, ICES
1. Introduction
Liposarcoma (LPS) is the most commonly diagnosed adult sarcoma, comprising up to 12.8% of all soft tissue malignancies.1, 2, 3 LPS often presents clinically as a painless, growing mass, and Magnetic Resonance Imaging (MRI) imaging is utilized to visualize the extent of growth and assist in subtype differentiation.4 Complete surgical resection paired with metastasis-opposed radiation therapy has been the principle method of treatment for liposarcoma over the last several decades.5,6 Adjuvant chemotherapy may also be of use but is highly dependent on histological subtype.5,7 While the World Health Organization (WHO) has classified LPS into four subtypes based on distinct histological profiles and the SEER database into seven subtypes,8 there are three broad recognized group classifications of liposarcoma based on histological relation.2 The first group includes the well-differentiated and de-differentiated subtypes, the second the myxoid/round subtypes, and the third the pleomorphic subtype.9 The mixed subtype presents as a histological combination of more than one of the other subtypes, while very little is reported on the fibroblastic variant.10
Present understanding of the prognosis and survival of LPS has primarily been limited to single institution investigations, small scale-meta analyses, or studies that do not investigate the specific subtypes due to the rarity of this tumor.11, 12, 13, 14, 15, 16, 17, 18, 19 There is currently a paucity of data analyzing the demographics, prognosis, clinical characteristics, and survival of LPS’s different subtypes. The purpose of this study is to describe these data for all LPS subtypes using the SEER database. We then externally validate this data with the Canadian Institute for Clinical Evaluative Sciences (ICES)’s database. Our goal is to increase our understanding of liposarcoma and allow surgeons to better navigate treatment based on its different variants to improve outcomes.
2. Materials and Methods
2.1. Patient population
Two datasets were analyzed in this study: the November 2015 release of the SEER database and the Institute for Clinical Evaluative Sciences (ICES) database. The SEER database was created following the National Cancer Act of 1971 and includes over 9.1 million patient cases from 1973 to 2013 maintained by the National Cancer Institute. The database includes data from 18 local cancer registries, which are included based on completeness and representation of the total US population. The SEER database includes a nearly 99% follow up rate and records demographic variables, tumor characteristics, and survival. Additionally, the ICES reports longitudinal data of every patient in Ontario, Canada since 1982. ICES data for this study includes patients from 1993 to 2015. The data that support the findings of this study are available at20 and,21 respectively.
Data were extracted from both the SEER and ICES databases using International Classification of Diseases for Oncology, Third Edition (ICD-O-3) morphology codes. These included codes for well-differentiated (8851/3), dedifferentiated (8858/3), myxoid (8852/3), round (8853/3), mixed (8855/3), pleomorphic (8854/3), and fibroblastic (8857/3) subtypes. Cases with liposarcoma of a “not otherwise specified” subtype (n = 2,986, 20.4% of total) were not included in the study (8850/3).
Patients were stratified by gender, race, and age. For privacy reasons, ICES does not release the exact age of young patients, but instead lists ‘<35’ for any patient 34 years of age or younger. To allow for better comparison between the ICES and SEER datasets, this age grouping was used for SEER data as well. Therefore, age is reported in two categorical variables (less than 35 years and greater or equal to 35 years). Tumors are classified by histological grade and presence of metastatic disease at presentation. As this study does not involve human subjects, Institutional Review Board (IRB) approval was not needed for analysis of SEER data, as per our institution’s policy. Local Research Ethics Board (REB) approval for the use of ICES data was obtained (#3745-C).
2.2. Statistical analysis
For our primary analysis of SEER data, Pearson’s chi-square was used to assess the relationship between age, race, sex, grade and metastatic involvement for each of the liposarcoma subtypes. Kaplan-Meier and Cox proportional hazards analysis were used to determine survival of the subtypes as well as to determine risk factors for decreased survival. Survival was defined as the difference between date of initial diagnosis and date of death. A separate Cox regression was conducted based on treatment with surgery and/or radiation. Incidence was calculated using SEER∗Stat software (version 8.2.1, National Cancer Institute, Bethesda, Maryland) and normalized to US population as per the 2000 Census.
In our secondary analysis, Kaplan-Meier and Cox proportional hazards analysis were similarly conducted with the ICES data. Due to the difference in available variables between the SEER and ICES databases, namely tumor grade and patient race, Cox regression was limited to include only age and gender in this part of the analysis. These variables were common to both datasets and were thus considered for comparison. Incidence was calculated using SEER∗Stat software (version 8.2.1, National Cancer Institute, Bethesda, Maryland) and normalized to US population per the 2000 Census and the 2011 Ontario census. The threshold for significance in all tests was defined as p < 0.05.
3. Results
3.1. Primary analysis of SEER data
A total of 11,680 cases were extracted from the SEER database. The majority of cases were male patients in all except the fibroblastic subtype (p < 0.05). The majority of patients were Caucasian in each subgroup, with an overall mean of 83.1%. The round cell subtype had the greatest proportion of African American patients and the highest rate of reported metastatic disease (Table 1). The lowest median survival was found in the de-differentiated subtype, followed by the pleomorphic, round cell, mixed, well-differentiated, and myxoid subtypes (Table 2). As survival of the fibroblastic subtype did not fall below 50% by 10 years post-diagnosis, the median survival was not calculated.
Table 1.
SEERa demographic and clinical data.
| Variable | Well Differentiated | Myxoid | Round Cell | Pleomorphic | Mixed | Fibroblastic | De-Differentiated | P value |
|---|---|---|---|---|---|---|---|---|
| Total Number | 4298 (36.8%) | 3241 (27.8%) | 304 (2.6%) | 1203 (10.3%) | 453 (3.9%) | 17 (0.2%) | 2164 (18.5%) | |
| Median Age (years) | 63 | 50 | 50 | 66 | 56 | 36 | 66 | <0.01 |
| Race | <0.01 | |||||||
| Caucasian | 3525 (82.0%) | 2694 (83.1%) | 246 (80.9%) | 1004 (83.5%) | 374 (82.6%) | 15 (88.2%) | 1826 (84.4%) | |
| African American | 346 (8.1%) | 309 (9.5%) | 32 (10.5%) | 111 (9.2%) | 43 (9.5%) | 1 (5.9%) | 134 (6.2%) | |
| Other | 381 (8.9%) | 210 (6.5%) | 22 (7.2%) | 85 (7.1%) | 31 (6.8%) | 1 (5.9%) | 195 (9.0%) | |
| GENDER | <0.01 | |||||||
| Males | 2551 | 1895 | 168 | 714 | 279 | 6 | 1448 | |
| Females | 1747 | 1346 | 136 | 489 | 174 | 11 | 716 | |
| Male to Female Ratio | 1.5 | 1.4 | 1.2 | 1.5 | 1.6 | 0.5 | 2.0 | |
| % Female | 40.6 | 41.5 | 44.7 | 40.6 | 38.4 | 64.7 | 33.1 | |
| Metastases | 131 (3.0%) | 127 (3.9%) | 26 (8.5%) | 67 (5.6%) | 25 (5.5%) | 0 (0%) | 201 (0.9%) | <0.01 |
| Grade | <0.01 | |||||||
| 1 | 4095 | 828 | 15 | 44 | 79 | 4 | 168 | |
| 2 | 60 | 873 | 42 | 71 | 70 | 6 | 188 | |
| 3 | 31 | 242 | 89 | 316 | 101 | 1 | 673 | |
| 4 | 20 | 171 | 84 | 416 | 94 | 0 | 843 | |
| Not reported | 92 | 1127 | 74 | 356 | 109 | 6 | 292 | 1061 |
Surveillance, Epidemiology, and End Results Program Database.
Table 2.
Side-to-side comparison of subtypes of liposarcoma, by database.
| Sample Size | Median Age | % Female | Median Survival (IQR) in Years | 5-Year Survival | 10-Year Survival | ||
|---|---|---|---|---|---|---|---|
| Well-Differentiated | SEERc | 4298 | 63 | 40.6 | 15.8 (7.4–26.3) | 0.823 | 0.669 |
| ICESb | 548 | 57 | 39.6 | NAa (22.1 - NAa) | 0.904 | 0.809 | |
| Myxoid | SEER | 3241 | 50 | 41.5 | 17.8 (5.5-NAa) | 0.764 | 0.636 |
| ICES | 319 | 47 | 39.8 | NAa (NAa - NAa) | 0.882 | 0.787 | |
| Round Cell | SEER | 304 | 50 | 44.7 | 7.5 (1.7–25.3) | 0.549 | 0.467 |
| ICES | 60 | 45 | 43.3 | 18.5 (13.1 - NAa) | 0.854 | 0.676 | |
| Pleomorphic | SEER | 1203 | 66 | 40.6 | 5.3 (1.7–16.2) | 0.512 | 0.348 |
| ICES | 132 | 63 | 40.9 | 4.4 (3.1–6.8) | 0.477 | 0.352 | |
| Mixed | SEER | 453 | 56 | 38.4 | 9.7 (2.3–23.2) | 0.628 | 0.498 |
| ICES | 22 | 55 | 50.0 | 6.4 (2.7 - NAa) | 0.707 | 0.530 | |
| Fibroblastic | SEER | 17 | 36 | 64.7 | NAa | 0.941 | 0.753 |
| ICES | 3 | 42 | 66.7 | NAa (NAa - NAa) | 1.000 | NAa | |
| De-Differentiated | SEER | 2164 | 66 | 33.1 | 4.9 (1.6–14.0) | 0.494 | 0.321 |
| ICES | 182 | 67 | 37.4 | 11.4 (7.3 - NAa) | 0.754 | 0.672 | |
Surveillance, Epidemiology, and End Results Program Database.
Canadian Institute for Clinical Evaluative Sciences.
Not applicable due to insufficient sample size or failure of mortality to fall below 0.500.
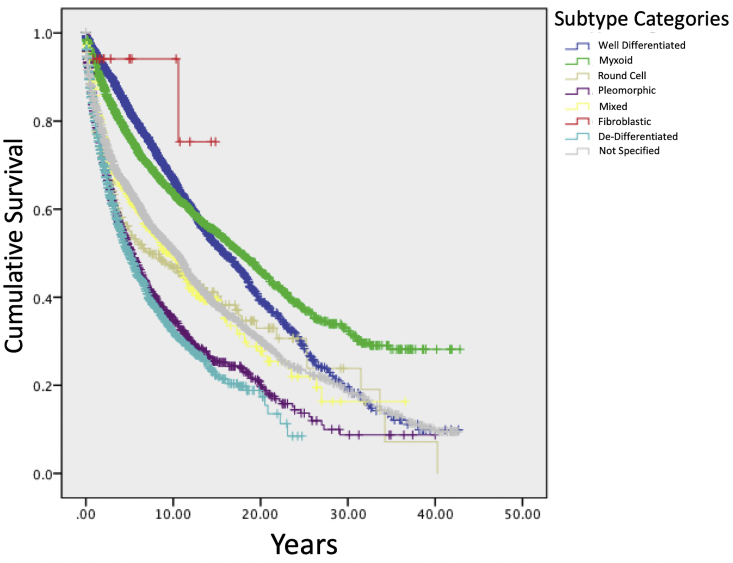
Kaplan Meier analysis demonstrated that fibroblastic liposarcoma had the highest 5-year survival rate of 94.1% (Fig. 1). Rate of 5-year survival was 82.3% for well-differentiated, 76.4% for myxoid, 62.8% for mixed, 54.9% for round cell, 51.2% for pleomorphic, and 49.4% for de-differentiated subtypes (Table 2). Regression analysis revealed that male sex was associated with decreased survival in the well-differentiated subtype and age ≥35 was associated with decreased survival in the myxoid subtype (p < 0.05). Metastatic disease was associated with decreased survival in all subgroups of liposarcoma, with the greatest effect in round cell (HR = 9.03, p < 0.05). There were no significant associations between race and survival in any subtype (Table 3).
Fig. 1.
SEER† survival function, by subtype.
† Surveillance, Epidemiology, and End Results Program Database.
Table 3.
| Risk Factor | Well-Differentiated | Myxoid | Pleomorphic | Mixed | De-Differentiated | Round Cell |
|---|---|---|---|---|---|---|
| Age ≥ 35 (vs. <35) | 3.42 (1.28–9.17)∗ | 3.34 (2.24–4.97)∗ | 0.84 (0.47–1.53) | 1.48 (0.70–3.13) | 1.68 (0.87–3.24) | 2.12 (0.62–7.26) |
| Male (vs. Female) | 1.34 (1.11–1.62)∗ | 1.12 (0.91–1.39) | 1.12 (0.86–1.45) | 0.87 (0.55–1.37) | 0.99 (0.85–1.15) | 1.01 (0.59–1.73) |
| Race | ||||||
| African American | 0.92 (0.64–1.32) | 1.18 (0.87–1.6) | 1.42 (0.92–2.19) | 0.96 (0.53–1.75) | 0.73 (0.53–1.02) | 1.62 (0.71–3.7) |
| Other | 1.12 (0.82–1.54) | 0.75 (0.47–1.19) | 0.71 (0.43–1.18) | 0.43 (0.10–1.79) | 0.78 (0.6–1.01) | 2.91 (1.27–6.67) |
| Caucasian | Reference | Reference | Reference | Reference | Reference | Reference |
| Metastatic Disease | 3.16 (2.35–4.26)∗ | 4.41 (3.42–5.70)∗ | 2.79 (1.95–3.98)∗ | 2.53 (1.38–4.66)∗ | 2.87 (2.39–3.46)∗ | 9.03 (4.85–16.84)∗ |
| Grade | ||||||
| II | 2.22 (1.36–3.60)∗ | 0.98 (0.73–1.33) | 0.58 (0.22–1.57) | 0.33 (0.12–0.89)∗ | 1.26 (0.82–1.93) | 3800.79 (0–3.535E+51) |
| III | 5.50 (3.08–9.82)∗ | 1.95 (1.34–2.83)∗ | 0.92 (0.44–1.93) | 1.42 (0.71–2.83) | 1.82 (1.28–2.58)∗ | 6529.82 (0–6.061E+51) |
| IV | 5.36 (2.65–10.84)∗ | 2.3 (1.62–3.28)∗ | 1.19 (0.58–2.47) | 1.45 (0.73–2.86) | 2.04 (1.44–2.88)∗ | 4963.68 (0–4.608E+51) |
| Unknown | 1.08 (0.62–1.89) | 1.28 (0.95–1.71) | 0.97 (0.45–2.08) | 1.30 (0.63–2.69) | 1.82 (1.25–2.65)∗ | 4498.23 (0–4.178E+51) |
| I | Reference | Reference | Reference | Reference | Reference | Reference |
∗ Significance defined as p < 0.01.
Surveillance, Epidemiology, and End Results Program Database.
Hazard ratio.
Confidence interval.
3.2. Secondary analysis of ICES data
After query of the ICES database, a total of 1266 Ontario patients with biopsy confirmed liposarcoma subtype were included. The most common subtype in this dataset was the well-differentiated subtype (n = 548, 43.3%). As all 3 patients with the fibroblastic survived beyond 5 years, the median survival time was not calculated. Of the remaining subtypes, the greatest 5-year survival was found in the well-differentiated subtype, followed by myxoid, round cell, de-differentiated, mixed, and pleomorphic (Table 2).
In the comparison of the two treatment modalities in the SEER database across all subtypes, treatment with radiation alone was associated with an increased risk of mortality, compared to no surgery or radiation use. On subgroup analysis, treatment with radiation alone was associated with increased risk of mortality in the de-differentiated and myxoid subtypes (Table 4).
Table 4.
SEERb COX regression hazard ratios, by treatment.
| Treatment Modality | Hazard Ratio | (95% CI)a | P value |
|---|---|---|---|
| No Surgery, No Radiation | Reference | ||
| Surgery alone | 1.07 | 0.90–1.27 | 0.09 |
| Radiation alone | 4.39∗ | 1.94–9.94 | <0.01 |
| Surgery, Radiation | 1.09 | 0.91–1.30 | 0.12 |
| SEERbCOX Regression Hazard Ratios, by Treatment and Subtype | |||
| No Surgery, No Radiation | Reference | ||
| Surgery alone | |||
| Well-differentiated | 0.94 | 0.63–1.42 | 0.90 |
| De-differentiated | 0.99 | 0.74–1.34 | 0.97 |
| Mixed | 1.20 | 0.59–2.51 | 0.63 |
| Myxoid | 1.18 | 0.84–1.68 | 0.34 |
| Round cell | 0.84 | 0.34–2.12 | 0.89 |
| Pleomorphic | 1.20 | 0.77–1.87 | 0.43 |
| Radiation alone | |||
| Well-differentiated | 3.11 | 0.84–10.15 | 0.99 |
| De-differentiated | 3.95∗ | 1.23–12.66 | 0.02 |
| Mixed | – | – | – |
| Myxoid | 11.12∗ | 3.45–36.40 | <0.01 |
| Round cell | – | – | – |
| Pleomorphic | 2.73 | 0.67–4.15 | 0.94 |
| Surgery, Radiation | |||
| Well-differentiated | 0.96 | 0.62–1.47 | 0.84 |
| De-differentiated | 0.89 | 0.65–1.21 | 0.45 |
| Mixed | 1.46 | 0.69–3.10 | 0.32 |
| Myxoid | 1.19 | 0.83–1.71 | 0.34 |
| Round cell | 1.06 | 0.42–2.68 | 0.90 |
| Pleomorphic | 1.19 | 0.75–1.88 | 0.46 |
∗indicates significance, blank value indicates incomplete/missing data.
Surveillance, Epidemiology, and End Results Program Database.
Confidence Interval.
When only age and gender were taken into account, age ≥35 was the only significant predictor of decreased survival in the well-differentiated, myxoid, round cell, pleomorphic, and mixed subtypes, while gender was associated with decreased survival in the myxoid subtype (p < 0.01) in the SEER database. In the ICES database, age ≥35 was only associated with decreased survival in the myxoid subtype. There were no associations between gender and survival with any subtype in the ICES database (Table 5).
Table 5.
Side-to-side comparison of cox regression of liposarcoma, by database.
| Database | HRa (95% CIb) for Males | P Value for Males | HRa (95% CIb) for Age ≥35 | P Value for Age >35 | |
|---|---|---|---|---|---|
| Well-Differentiated | SEER | 1.21 (1.08–1.35) | <0.01∗ | 5.21 (3.06–8.87) | <0.01∗ |
| ICES | 1.15 (0.76–1.74) | 0.50 | 2.61 (0.36–18.7) | 0.34 | |
| Myxoid | SEER | 1.14 (1.01–1.27) | 0.03 | 3.94 (3.15–4.93) | <0.01∗ |
| ICES | 0.99 (0.64–1.54) | 0.97 | 3.23 (1.31–7.01) | <0.01∗ | |
| Round Cell | SEER | 1.27 (0.93–1.74) | 0.13 | 1.86 (1.08–3.18) | 0.02∗ |
| ICES | 0.58 (0.22–1.56) | 0.28 | 2.68 (0.73–9.89) | 0.14 | |
| Pleomorphic | SEER | 1.02 (0.88–1.18) | 0.82 | 1.58 (1.08–2.33) | 0.02∗ |
| ICES | 0.88 (0.58–1.35) | 0.56 | 1.63 (0.23–11.7) | 0.63 | |
| Mixed | SEER | 1.09 (0.84–1.43) | 0.51 | 1.99 (1.16–3.42) | 0.01∗ |
| ICES | 0.88 (0.58–1.35) | 0.56 | 1.63 (0.23–11.7) | 0.63 | |
| Fibroblastic | SEER | 1.06 (0.06–17.68) | 0.97 | 0.00 (0.00-Infc) | 0.97 |
| ICES | 4.98 (1.23–19.20) | 0.02 | – | – | |
| De-Differentiated | SEER | 1.49 (0.95–2.35) | 0.09 | 1.07 (0.95–1.22) | 0.27 |
| ICES | 0.97 (0.58–1.61) | 0.90 | 2.7 (0.00 – Infc) | 0.99 |
∗ significance defined as p < 0.05, - Not included in regression as all patient with mixed liposarcoma were >35 years old in the ICES Database.
Hazard ratio.
confidence interval.
Infinity.
4. Discussion
Liposarcoma is the most common histology of soft tissue sarcomas despite its overall rarity.5 Though its growth is often described to be indolent and painless, it generally has a poor prognosis. The most recently published large-scale studies indicate approximate 5-year LPS survival rate averages of 58–95%, depending on tumor grade.22 LPS’s various subtypes present with different natural histories, and post-surgical outcomes vary among them.11 To date, the majority of other studies investigating liposarcoma have been largely focused on specific anatomic sites, specific subtypes, or treatment modalities rather than on LPS’s subtypes.22, 23, 24 Due to the paucity of large, national studies investigating liposarcoma and its subtypes, we present an analysis of two national databases to determine relevant demographic and clinicopathologic factors.
The well-differentiated subtype represents the most common liposarcoma in both the SEER and ICES databases. Arvinius et al.25 retrospectively reviewed 11 cases of well-differentiated and showed that while it may be locally aggressive and recur after surgical resection, well-differentiated LPS rarely metastasizes or dedifferentiates. Mavrogenis et al.26 mirrored these findings in their review of 67 cases of well-differentiated, showing that while there was a 52% local recurrence rate, no patients had evidence of metastasis at 140 months follow-up. We show well-differentiated to have higher median survival rates in comparison to nearly all other subtypes, with the exception of myxoid. Interestingly, a greater 10-year prognosis is seen in the ICES database as with all other subtypes than in SEER, however this may be explained in part by the wider range of dates included in the SEER database (1973–2013) compared to ICES (1993–2015). Advances in the diagnosis and treatment of cancer from the 1970s to the 1990s may introduce a lead-time bias, but this was not investigated in our study.
In contrast to the well-differentiated variant, the de-differentiated subtype may arise de novo, as a recurrence of well-differentiated subtype, or an intermingled transition.27 The diversity in this subtype’s histological patterns may lead to misdiagnosis as it may resemble other malignancies that includes the well-differentiated variant, undifferentiated pleomorphic sarcoma, and spindle cell sarcoma.28 In a 2017 report by Dantey et al.29 the histological grade of de-differentiated was reported in relation to clinical outcomes for 55 patients. While high grade de-differentiated had a significantly higher rate of mortality compared to low grade, both low grade de-differentiated and high grade de-differentiated had poor outcomes, with median survivals of 113 months and 48 months respectively. Our data show that de-differentiated has the lowest median survival among all subtypes, as well as the second highest proportion of high-grade cases.
Round cell and myxoid liposarcomas appear histologically similar, but they differ greatly on clinical assessment.12,30,31 In a retrospective review of 29 patients with either myxoid or round cell liposarcoma, myxoid liposarcoma was found to be typically low-grade and responded well to chemotherapy, resulting in better prognoses. Conversely, round cell had higher rates of metastasis, behaved more aggressively, and did not respond well to chemotherapy.32 Our data show that round cell liposarcoma has higher rates of metastasis and a poorer prognosis than myxoid type. Due to the similarity in histological presentation between myxoid and round cell, these subtypes have proven to be a challenge to clinicians as there is the potential to exist as a combination.10
Unlike the other subtypes of liposarcoma, the pleomorphic variant is unique in that it lacks a molecular signature, thus making it difficult to diagnose.33 It also is often reported to be high grade and may even resemble de-differentiated morphologically. The pleomorphic subtype had the highest proportion of high-grade cases in the SEER database (Grades III & IV = 86.4%), which is similar to other studies.34,35
For each subtype, metastatic involvement as well as higher grading are significantly associated with worse outcomes, which corroborates with existing literature.1,7 Treatment of LPS for the last several decades has revolved around surgery,6 but our analysis showed that radiation alone was associated with an increased risk of mortality compared to no surgical or radiation treatment in the overall study population as well as in the de-differentiated and myxoid subtypes. Despite the well-established efficacy of surgical treatment, our analyses showed that surgery was not associated with a difference in outcomes in both an overall and subtype-stratified populations compared to no radiation or surgery. The only significant finding regarding treatment was an increased risk in some populations treated radiation alone. This may be due to the palliative nature of such a treatment approach, suggesting more severe disease presentation and symptomatology rather than failed treatment.36 Notably, however, detailed treatment modalities and adjunctive therapies are not defined in SEER and thus could not be accounted for in our analysis.
Our study has several limitations. First, neither database offers detailed, granular information about each patient and their clinical progression. Certain elements, such as margin of resection, comorbid diseases, and treatment parameters are not available, which may have provided valuable information in predicting outcomes. Specifically, the SEER database does not include data on chemotherapy or other potentially relevant aspects of adjunctive treatments. These would be important considerations in the final determination of a patient’s prognosis, but could not be evaluated in this study. Therefore, extrapolation of these data, particularly the secondary analysis of treatment modality in Table 4, must be done with caution. The databases also limited the authors’ ability to verify data with a quality review of the results used, and reporting errors may be present. As the database covers several decades, the level of detail of reported information has varied, which can appear as lack of relevant data in years prior to the updated data requirements. Despite these limitations, the statistical power offered by SEER with external validation by ICES provides much insight on the factors and survival of the subtypes of LPS that we hope to use to further our knowledge of this soft tissue sarcoma and assist future clinicians. Future prospective cohort studies are needed to confirm these data and account for other relevant variables, such as margin of resection after surgery, type of radiation used, chemotherapy, location of tumor, and patient comorbidities.
5. Conclusions
Though slow-growing and painless, liposarcoma has the potential to be deadly, and each subtype should be evaluated as a distinct disease process with variable prognosis. The worst outcomes can be expected among the de-differentiated, pleomorphic, and mixed subtypes. These data may better inform orthopaedic and surgical oncologists about disease outcomes to optimize patient care.
Grants and funding
None.
CRediT authorship contribution statement
Kamil M. Amer: Conceptualization. Dominick V. Congiusta: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Resources, Software, Writing - original draft, Writing - review & editing. Jennifer E. Thomson: Investigation, Methodology, Project administration, Resources, Writing - original draft, Writing - review & editing. Samer Elsamna: Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Iftikhar Chaudhry: Conceptualization. Anthony Bozzo: Conceptualization. Rami Amer: Conceptualization. Brianna Siracuse: Conceptualization. Michelle Ghert: Conceptualization. Kathleen S. Beebe: Conceptualization.
Declaration of competing interest
All authors have declared that they have no conflicts of interest for this project. This manuscript has not been submitted to, nor is under review at, another journal or other publishing venue. The authors have no affiliation with any organization with a direct or indirect financial interest in the subject matter discussed in the manuscript.
Acknowledgements
None.
Contributor Information
Kamil M. Amer, Email: kamil.amer@rutgers.edu.
Dominick V. Congiusta, Email: dvc33@njms.rutgers.edu.
Jennifer E. Thomson, Email: jt751@njms.rutgers.edu.
Samer Elsamna, Email: s.samna@rutgers.edu.
Iftikhar Chaudhry, Email: ifmc@comcast.net.
Anthony Bozzo, Email: anthony.bozzo@medportal.ca.
Rami Amer, Email: ramiamer930@gmail.com.
Brianna Siracuse, Email: bls150@njms.rutgers.edu.
Michelle Ghert, Email: ghertm@mcmaster.ca.
Kathleen S. Beebe, Email: beebeka@njms.rutgers.edu.
References
- 1.Gadgeel S.M., Harlan L.C., Zeruto C.A., Osswald M., Schwartz A.G. Patterns of care in a population-based sample of soft tissue sarcoma patients in the United States. Cancer. 2009;115:2744–2754. doi: 10.1002/cncr.24307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henze J., Bauer S. Liposarcomas. Hematol Oncol Clin North Am. 2013;27:939–955. doi: 10.1016/j.hoc.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Weiss S.W. Lipomatous tumors. Monogr Pathol. 1996;38:207–239. [PubMed] [Google Scholar]
- 4.El Ouni F., Jemni H., Trabelsi A. Liposarcoma of the extremities: MR imaging features and their correlation with pathologic data. Orthop Traumatol Surg Res. 2010;96:876–883. doi: 10.1016/j.otsr.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Crago A.M., Liposarcoma M. A. Dickson. Multimodality management and future targeted therapies. Surg Oncol Clin N Am. 2016;25:761–773. doi: 10.1016/j.soc.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manji G.A., Schwartz G.K., Liposarcomas Managing. Cutting through the fat. J Oncol Pract. 2016;12:221–227. doi: 10.1200/jop.2015.009860. [DOI] [PubMed] [Google Scholar]
- 7.Kollar A., Benson C. Current management options for liposarcoma and challenges for the future. Expert Rev Anticancer Ther. 2014;14:297–306. doi: 10.1586/14737140.2014.869173. [DOI] [PubMed] [Google Scholar]
- 8.Jo V.Y., Fletcher C.D. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014;46:95–104. doi: 10.1097/pat.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 9.Liposarcoma A. P. Dei Tos. New entities and evolving concepts. Ann Diagn Pathol. 2000;4:252–266. doi: 10.1053/adpa.2000.8133. [DOI] [PubMed] [Google Scholar]
- 10.de Vreeze R.S., de Jong D., Koops W. Oncogenesis and classification of mixed-type liposarcoma: a radiological, histopathological and molecular biological analysis. Int J Canc. 2011;128:778–786. doi: 10.1002/ijc.25390. [DOI] [PubMed] [Google Scholar]
- 11.Engstrom K., Bergh P., Gustafson P. Liposarcoma: outcome based on the scandinavian sarcoma group register. Cancer. 2008;113:1649–1656. doi: 10.1002/cncr.23784. [DOI] [PubMed] [Google Scholar]
- 12.Fiore M., Grosso F., Lo Vullo S. Myxoid/round cell and pleomorphic liposarcomas: prognostic factors and survival in a series of patients treated at a single institution. Cancer. 2007;109:2522–2531. doi: 10.1002/cncr.22720. [DOI] [PubMed] [Google Scholar]
- 13.Henricks W.H., Chu Y.C., Goldblum J.R., Weiss S.W. Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am J Surg Pathol. 1997;21:271–281. doi: 10.1097/00000478-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Hornick J.L., Bosenberg M.W., Mentzel T., McMenamin M.E., Oliveira A.M., Fletcher C.D. Pleomorphic liposarcoma: clinicopathologic analysis of 57 cases. Am J Surg Pathol. 2004;28:1257–1267. doi: 10.1097/01.pas.0000135524.73447.4a. [DOI] [PubMed] [Google Scholar]
- 15.Lucas D.R., Nascimento A.G., Sanjay B.K., Rock M.G. Well-differentiated liposarcoma. The Mayo Clinic experience with 58 cases. Am J Clin Pathol. 1994;102:677–683. doi: 10.1093/ajcp/102.5.677. [DOI] [PubMed] [Google Scholar]
- 16.McCormick D., Mentzel T., Beham A., Fletcher C.D., liposarcoma Dedifferentiated. Clinicopathologic analysis of 32 cases suggesting a better prognostic subgroup among pleomorphic sarcomas. Am J Surg Pathol. 1994;18:1213–1223. doi: 10.1097/00000478-199412000-00004. [DOI] [PubMed] [Google Scholar]
- 17.ten Heuvel S.E., Hoekstra H.J., van Ginkel R.J., Bastiaannet E., Suurmeijer A.J. Clinicopathologic prognostic factors in myxoid liposarcoma: a retrospective study of 49 patients with long-term follow-up. Ann Surg Oncol. 2007;14:222–229. doi: 10.1245/s10434-006-9043-7. [DOI] [PubMed] [Google Scholar]
- 18.Weiss S.W., Rao V.K. Well-differentiated liposarcoma (atypical lipoma) of deep soft tissue of the extremities, retroperitoneum, and miscellaneous sites. A follow-up study of 92 cases with analysis of the incidence of "dedifferentiation". Am J Surg Pathol. 1992;16:1051–1058. doi: 10.1097/00000478-199211000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Zagars G.K., Goswitz M.S., Liposarcoma A. Pollack. Outcome and prognostic factors following conservation surgery and radiation therapy. Int J Radiat Oncol Biol Phys. 1996;36:311–319. doi: 10.1016/s0360-3016(96)00265-9. [DOI] [PubMed] [Google Scholar]
- 20.SEER Incidence Data, 1975-2016. Jan 4 2019. https://seer.cancer.gov/data/ [Google Scholar]
- 21.ICES Data and Analytic Services. Jan 4 2019. www.ices.on.ca/DAS [Google Scholar]
- 22.Ng V.Y., Scharschmidt T.J., Mayerson J.L., Fisher J.L. Incidence and survival in sarcoma in the United States: a focus on musculoskeletal lesions. Anticancer Res. 2013;33:2597–2604. [PubMed] [Google Scholar]
- 23.Alamanda V.K., Song Y., Schwartz H.S., Holt G.E. Racial disparities in extremity soft-tissue sarcoma outcomes: a nationwide analysis. Am J Clin Oncol. 2015;38:595–599. doi: 10.1097/coc.0000000000000004. [DOI] [PubMed] [Google Scholar]
- 24.Tseng W., Martinez S.R., Tamurian R.M., Borys D., Canter R.J. Histologic type predicts survival in patients with retroperitoneal soft tissue sarcoma. J Surg Res. 2012;172:123–130. doi: 10.1016/j.jss.2010.07.056. [DOI] [PubMed] [Google Scholar]
- 25.Arvinius C., Torrecilla E., Beano-Collado J. A clinical review of 11 cases of large-sized well-differentiated liposarcomas. Eur J Orthop Surg Traumatol. 2017;27:837–841. doi: 10.1007/s00590-017-1968-y. [DOI] [PubMed] [Google Scholar]
- 26.Mavrogenis A.F., Lesensky J., Romagnoli C., Alberghini M., Letson G.D., Ruggieri P. Atypical lipomatous tumors/well-differentiated liposarcomas: clinical outcome of 67 patients. Orthopedics. 2011;34:e893–e898. doi: 10.3928/01477447-20111021-11. [DOI] [PubMed] [Google Scholar]
- 27.Liposarcomas A. P. Dei Tos. Diagnostic pitfalls and new insights. Histopathology. 2014;64:38–52. doi: 10.1111/his.12311. [DOI] [PubMed] [Google Scholar]
- 28.Thway K., Jones R.L., Noujaim J., Zaidi S., Miah A.B., Fisher C. Dedifferentiated liposarcoma: updates on morphology, genetics, and therapeutic strategies. Adv Anat Pathol. 2016;23:30–40. doi: 10.1097/pap.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 29.Dantey K., Schoedel K., Yergiyev O., Bartlett D., Rao U.N.M. Correlation of histological grade of dedifferentiation with clinical outcome in 55 patients with dedifferentiated liposarcomas. Hum Pathol. 2017;66:86–92. doi: 10.1016/j.humpath.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Gimber L.H., Montgomery E.A., Morris C.D., Krupinski E.A., Fayad L.M. MRI characteristics associated with high-grade myxoid liposarcoma. Clin Radiol. 2017;72 doi: 10.1016/j.crad.2017.01.016. 613.e1-613.e6. [DOI] [PubMed] [Google Scholar]
- 31.Lemeur M., Mattei J.C., Souteyrand P., Chagnaud C., Curvale G., Rochwerger A. Prognostic factors for the recurrence of myxoid liposarcoma: 20 cases with up to 8 years follow-up. Orthop Traumatol Surg Res. 2015;101:103–107. doi: 10.1016/j.otsr.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 32.Smith T.A., Easley K.A., Goldblum J.R. Myxoid/round cell liposarcoma of the extremities. A clinicopathologic study of 29 cases with particular attention to extent of round cell liposarcoma. Am J Surg Pathol. 1996;20:171–180. doi: 10.1097/00000478-199602000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Dodd L.G., Sara Jiang X., Rao K., Bui M.M. Pleomorphic liposarcoma: a cytologic study of five cases. Diagn Cytopathol. 2015;43:138–143. doi: 10.1002/dc.23148. [DOI] [PubMed] [Google Scholar]
- 34.Agarwal J., Kadakia S., Agaimy A., Ogadzanov A., Khorsandi A., Chai R.L. Pleomorphic liposarcoma of the head and neck: presentation of two cases and literature review. Am J Otolaryngol. 2017;38:505–507. doi: 10.1016/j.amjoto.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Wang L., Luo R., Xiong Z., Xu J., Fang D. Pleomorphic liposarcoma: an analysis of 6 case reports and literature review. Medicine (Baltim) 2018;97 doi: 10.1097/MD.0000000000009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soyfer V., Corn B.W., Kollender Y., Tempelhoff H., Meller I., Merimsky O. Radiation therapy for palliation of sarcoma metastases: a unique and uniform hypofractionation experience. Sarcoma. 2010;2010:927972. doi: 10.1155/2010/927972. [DOI] [PMC free article] [PubMed] [Google Scholar]