Abstract
Many of the metals used in orthopaedic surgical implants are immunologically active and can cause hypersensitivity reactions. Most of these metal hypersensitivity (MHS) reactions are type IV/delayed-type hypersensitivity reactions. The most common form of all metal hypersensitivity reactions is allergic contact dermatitis (ACD) caused by nickel. The purpose of this review is to examine the evidence regarding hypersensitivity to orthopaedic implants and provide current recommendations for evaluating these patients. We report on four case examples of patients where it was determined that metal allergy led to complications related to surgery. The most common symptoms for patients with MHS-associated failures are localized soft tissue reaction including delayed wound healing and/or recurrent wound issues. The best way to avoid postoperative issues is to routinely ask patients prior to surgery if they have any known MHS including problems with cosmetic jewelry. If this is known before surgery, titanium or carbon fiber implants should be used for fracture fixation and arthroplasty implantation choice should be modified based on the specific arthroplasty performed. MHS-associated failures are a diagnosis of exclusion and must be contemplated after judicious workup of localized soft tissue reaction including delayed wound healing and/or recurrent wound issues.
Keywords: Metallic implants, Nickel allergy, Metal hypersensitivity, Allergic contact dermatitis, Delayed infection
1. Introduction/review of literature
Surgical metallic implants have a wide array of therapeutic uses. In orthopaedic surgery, these implants are used in arthroplasty, bone augmentation, and bone fixation including fractures, osteotomies, and fusions (e.g., plates, screws, rods). Many of the metals are immunologically active and cause allergic (hypersensitivity) reactions.1 These reactions have been reported in orthopaedics, dentistry, gynecology, cardiology, and less commonly urology.2 The great majority of metal hypersensitivity (MHS) reactions in orthopaedic implants are type IV/delayed-type hypersensitivity reactions.
The most common form of all metal hypersensitivity is allergic contact dermatitis (ACD) caused by nickel.3,4 Interestingly, the prevalence of contact skin sensitivity in patients with a joint replacement device is higher than that in the general population.5 As the number of arthroplasties and the use of orthopaedic implants continues to increase, the number of allergy-related implant complications likewise is expected to increase.6,7 Common symptoms from MHS reactions may include one or more of the following: localized pain, swelling, redness, warmth, itching/burning, drainage, and implant loosening or instability1 that mimic suspected infection.
As such, implant MHS reactions present diagnostic and therapeutic challenges.8 The lack of general treatment guidelines leads to individual management and potential inconsistency that in turn make it difficult to develop guidelines.8 MHS reactions are diagnostically challenging because they are a diagnosis of exclusion.9 Surgeons must first rule out more common differential diagnoses including infection, instability, implant wear, and/or fracture.8 Additionally, many patients presenting with MHS-associated implant failure present with different symptoms than others with the same failure mechanism (see “2. Case Reports” section). Another challenge is the lack of a definitive diagnostic test. However, skin patch testing and lymphocyte transformation tests have shown some value.10 Exacerbating this issue, some patients have reported sensitivity to nickel-cobalt-chromium, yet have no evident MHS reactions and have no symptoms.11 Therefore, the association between metal sensitivity and allergic reactions has not been fully elucidated.12 Because diagnosis is difficult, a summary of general guidelines excluding differential diagnoses and the combination of different allergy diagnostic tools is needed.13
MHS orthopaedic implant reaction is becoming more important to understand. The number of total joint arthroplasty patients testing positive for MHS has increased exponentially over the last 20 years.14 Furthermore, it is estimated that 11 million individuals will be living with total hip or knee arthroplasties (4 million total hip; 7.4 million total knee) by 2030.15 Therefore, a better understanding of the risk, diagnostic, and treatment dilemmas is important. This review article will provide a guide to understand the epidemiology and pathophysiology of hypersensitivities, assess and interpret the clinical history, and provide our clinical recommendations.
1.1. Epidemiology
Between 10% and 15% of people exhibit cutaneous sensitivity to metal.16 The most common form of metal hypersensitivity is ACD caused by nickel.3,4 It is estimated that cutaneous hypersensitivity reactions to nickel, cobalt, and chromium in the general population is between 10% and 15%, with nickel the highest at approximately 14%.4,16 There are characteristics that increase this risk. For example, females are four times more likely than males to have nickel-associated contact dermatitis.17 People with ear-piercings are 3.2 times as likely to have contact dermatitis to nickel.8 The prevalence of metal hypersensitivity is also increased in certain occupations, such as metalwork, bricklaying, and pottery.18 Lastly, the prevalence of contact skin sensitivity in arthroplasty may be as high as 25%.5,16 Therefore, the growing number of arthroplasties, and the increased use of metallic implants used for fracture fixation, will lead to an increasing number of allergy-related orthopaedic implant complications.6,7
The most commonly reported failure mechanism for metallic implants has been reported for total joint arthroplasty (TJA). In the United States it is estimated that 8.4% of total knee arthroplasties (TKAs) are revisions, while 10.7% of total hip arthroplasties (THA) are revisions.19 Other studies have estimated annual revision rates between 10% and 20% of all lower extremity TJAs.1,20 Sharkey et al. described the reason for revision of 781 TKAs that included loosening (40%), infection (27.5%), instability (7.5%), periprosthetic fracture (4.5%), and arthrofibrosis (4.5%).21 Similarly, Sadoghi et al. reported the most common causes for TKA revision were aseptic loosening (29.8%), septic loosening (14.8%), pain (9.5%), and wear (8.2%).22 For THAs, the most common causes for revision were aseptic loosening (55.2%), dislocation (11.8%), septic loosening (7.5%), and periprosthetic fractures (6%).22 Jafari et al. in another study equated the failure mechanisms for THA revisions as aseptic loosening (51%), instability (15%), wear (14%), infection (8%), fracture (5%), and miscellaneous (7%).23 Because routine evaluation for MHS is uncommon, the role of MHS as a cause of failure is poorly understood and underappreciated in mechanism and incidence.
1.2. Pathophysiology
Hypersensitivity reactions are a function of the adaptive immune system. The Gell and Coomb’s classification types of hypersensitivity reactions are Type I, Type II (antibody-mediated), Type III, and Type IV (cell-mediated, delayed).24,25 Type I hypersensitivity reactions are the most common type and occur swiftly, within seconds to minutes. Free antigens cross-link immunoglobulin E (IgE) antibodies on pre-sensitized mast cells. As a result, there is an immediate release of vasoactive amines (e.g., histamine) that act on postcapillary venules. The release of these amines results in vasodilation, inflammation, and subsequent edema. Additionally, there is a delayed release of inflammatory cytokines from mast cells and basophils that induces cellular inflammation. An example of a Type I hypersensitivity reaction is a reaction to a bug sting, drug, food, or seasonal allergy.25 Type I reactions to nickel complexes are extremely rare, but have been reported.26
Type II hypersensitivity reactions occur when immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies bind to antigens present on cell surfaces.24 As a result, this may lead to direct cellular destruction, inflammation, or cellular dysfunction. Examples of Type II hypersensitivity reactions resulting in cellular destruction include hemolytic anemia and blood transfusion reactions. Examples of Type II hypersensitivity reactions that precede inflammation include rheumatic fever and hyper-acute transplant rejection. Examples of Type II hypersensitivity reactions that lead to cellular dysfunction are autoimmune diseases, such as Grave’s disease and Myasthenia Gravis.25
Type III hypersensitivity reactions occur when antibodies bind to antigens present in serum. This leads to the development of an antigen-antibody IgG complex that leads to the activation of the complement system.24 Examples of Type III hypersensitivity reactions include serum sickness, systemic lupus erythematosus, and poststreptococcal glomerulonephritis.25
Type IV hypersensitivity reactions involve T-cells, and unlike the other types do not require antibodies.24 These reactions are delayed, usually peaking at three days, and takes the longest to occur of all the types.8 There are two mechanisms for Type IV hypersensitivity reactions. One involves cluster of differentiated 8 (CD8+) cytotoxic T-cells directly killing targeted cells. The other requires cluster of differentiated 4 (CD4+) helper T-cells to become activated, release inflammatory cytokines, and activate macrophages that kill targeted cells. An example of CD8+ direct cell killing is the autoimmune disease Type I diabetes mellitus, in which the beta-cells of the pancreas are targeted. An example of CD4+ indirect cell killing is a nickel allergy, positive purified protein derivative (PPD) skin test, or graft-versus-host-disease.25 Most hypersensitivities to metallic and nonmetallic orthopaedic implants occur via the CD4+ mechanism.8 Nickel MHS is acquired through intimate and extended contact to nickel metal or nickel salts.27 Once this sensitization has occurred, exposure to sufficient amounts of nickel may result in a MHS reaction.27 All metals in contact with biological systems undergo corrosion, and nickel is no exception.16 Nickel is considered to function as a hapten.28 In this Type IV nickel MHS reactions, CD4+ cytotoxic T-cells release inflammatory cytokines that attack complexes of nickel ion and endogenous proteins. This ultimately promotes osteoclast activity while inhibiting osteoblast activity, leading to localized osteopenia, osteolysis, loosening, and this may lead to ultimate failure.29
1.3. Clinical presentation
Common symptoms of MHS reactions include localized pain, swelling, redness, warmth, loosening, draining, itching/burning, and implant instability.1 Patients with MHS reactions to implants typically have prolonged eczematous dermatitis.8 Although the majority of MHS reactions are localized, more widespread inflammation has been reported.18 Although MHS reactions are a diagnosis of exclusion,6 these patients present similarly to patients with infection, and therefore should not be overlooked. If cutaneous signs of a hypersensitivity reaction are seen after the introduction of a metallic implant, MHS should be considered.16 The first step to correctly recognizing the diagnosis of MHS-associated failure is to consider it.
2. Materials and methods
After institutional review board approval, we reviewed our institution’s medical records to identify cases in which it was determined that metal allergy led to complications related to surgery. Data was compiled by reviewing relevant clinical notes, operative notes, and radiographic findings stored in our institution’s electronic medical record. All the patients represented in the following case presentations were contacted about our intent to publish these findings and they gave verbal permission.
3. Case reports
3.1. Case 1
A 53-year-old female presented with a left Schatzker type II tibial plateau fracture30 after a fall. Her past medical history included hypertension, depression, and dermatomyositis. Past surgical history included open reduction internal fixation (ORIF) for an arm fracture, for which hardware had been removed (no specific reason given), and a cholecystectomy with retained gallbladder clips. The patient denied alcohol, tobacco, and drug use, and her body mass index (BMI) was 34. Two days following initial presentation, the patient underwent ORIF of her left tibial lateral plateau using a stainless steel 6-hole variable angle anterolateral proximal tibial locking plate with 45 cubic centimeters (cc) of allograft bone.
Five weeks post-operatively, there was concern for deep infection. The patient stated she had some purulent drainage the previous day with an increase in redness of the wound site. After wound evaluation, it appeared to be localized epidermolysis without stitch abscess. She was instructed to apply a topical antibiotic ointment. Three days later, there was no significant improvement, so she was started on clindamycin 300 mg (mg) for 14 days with dry dressing changes. Ten weeks post-operatively, she complained of aching in her joint line at night but overall denied any new wound problems. She advanced her activity and was instructed to begin weight bearing as tolerated. The patient also began use of a recumbent bike and outpatient physical therapy. Her wound stopped draining and healed uneventfully.
She was seen 28 weeks postoperatively, and her wound had been draining for a couple weeks. Other than occasional stiffness in the morning, she had minimal pain/discomfort. There were no radiographic signs of failure or osteomyelitis (Fig. 1). Due to the length of time since surgery, the plan was to evaluate her for deep infection. Her C-reactive protein (CRP) was 0.80 mg/dL (dl) (0–1), erythrocyte sedimentation rate (ESR) was 33 mm/h (mm/hr) (0-30), and white blood cells (WBCs) were 7.14 × 109/liter (L) (3.5–10.5). Since she had an elevated ESR, there was concern for deep infection. She underwent deep hardware removal and intra-operative deep cultures were obtained.
Fig. 1.
Anteroposterior (A) and lateral (B) views prior to hardware removal.
Ten weeks after debridement and deep hardware removal, the patient’s knee pain developed to the point where she had difficulty with stairs and had pain with each step. At the time, WBCs were 7.21 × 109/L (3.5–10.5). Due to her increased pain and fracture displacement, revision ORIF of her left lateral tibial plateau fracture was performed with titanium implants. Intra-operative gram stain showed no signs of infection, and WBCs were 5.38 × 109/L (3.5–10.5).
At 32 weeks post-operatively, the patient reported no pain with a score of 0/10 and no wound issues. The patient stated she felt as if her recovery process was easier this time. Radiographs taken demonstrated that her fracture was continuing to heal (Fig. 2).
Fig. 2.
Anteroposterior (A) and lateral (B) views taken showing ORIF with titanium implants.
3.2. Case 2
A 58-year-old male sustained a distal tibia fracture and displaced ipsilateral clavicle fracture after a motorcycle accident. He underwent ORIF of the distal tibia fracture with an anteromedial stainless steel pre-contoured distal tibial locking plate (Fig. 3), and ORIF of his clavicle fracture with a non-locking 2.7 mm non-locking stainless steel dynamic compression plate (DCP). Of note, he had prior orthopaedic surgeries including unspecified left and right shoulder surgeries, and left total knee and right total hip arthroplasties. The patient denied alcohol and drug use and quit smoking within the last year. His BMI was 32 and he had no documented allergies. At 14 weeks post-operatively, his pain improved significantly, and he only had dull and achy pain at night. He was walking without an assistive device and was overall very happy with his progress including no left clavicle complaints.
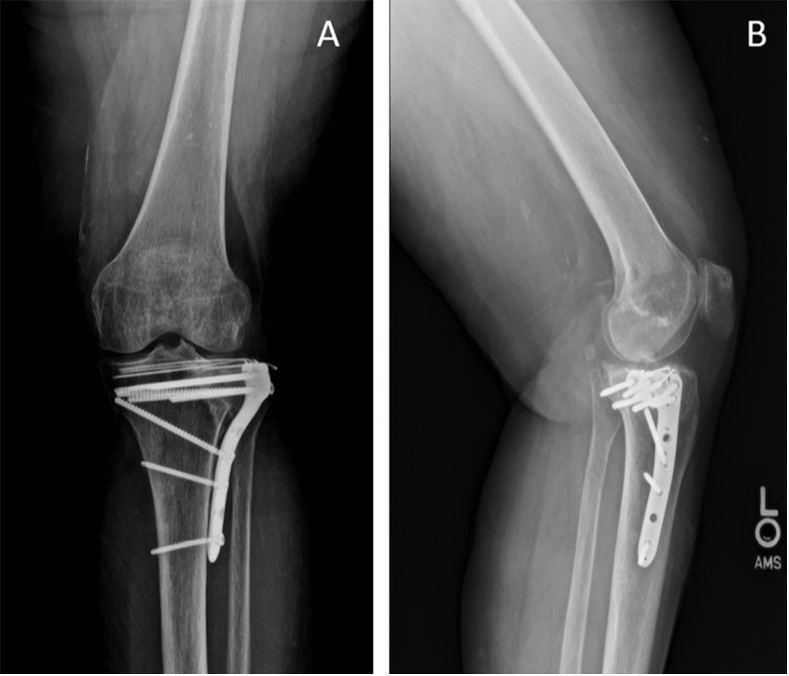
Fig. 3.
Anteroposterior (A) and lateral (B) views of distal ORIF.
At 123 weeks postoperative, he complained of skin changes over his left leg and described them as a nuisance. Coincidently, his dermatologist diagnosed him as having a nickel allergy. He stated he had occasional aching in his left tibia, but no consistent pain. He reported immense itchiness and the skin was described as dry and flaky. Additionally, the area that was involved was directly over his anteromedial tibial plate. He was prescribed a corticosteroid cream that was not effective to his satisfaction. Therefore, he underwent deep hardware removal.
At 6 weeks follow-up after deep hardware removal, he was asymptomatic, including the skin changes. He had no pain and was able to walk without an assistive device. Radiographs of the left leg (Fig. 4) showed fracture healing.
Fig. 4.
Anteroposterior (A) and lateral (B) views after ORIF hardware removal.
3.3. Case 3
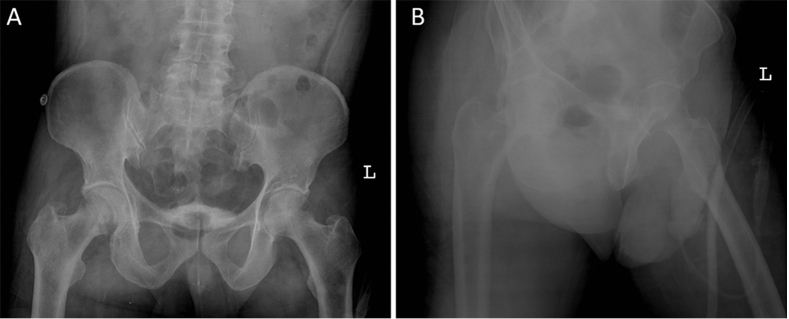
A 55-year-old male who was involved in a motor vehicle accident had a right posterior wall with significant comminution and marginal impaction (Fig. 5). ORIF was performed through a standard Kocher-Langenbeck approach. The patient had no prior surgical history and no documentation of allergies. The patient denied alcohol, drug use, or smoking. His BMI was 27. His initial recovery included wound dehiscence that required a return to the operating room for delayed primary closure, but he had no signs of infection. Otherwise, his recovery was unremarkable and he returned to his normal activities without complaint.
Fig. 5.
Anteroposterior (A) and lateral (B) views of right acetabular fracture.
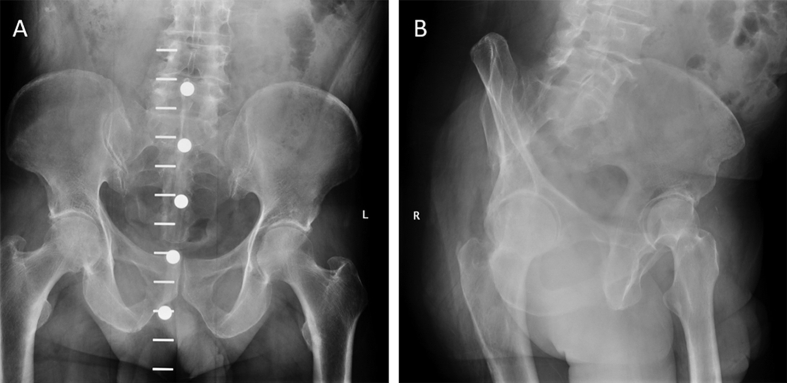
Four years after surgery, he presented with right hip pain, up to 7/10 on the Visual Analog Scale (VAS),31 especially after sitting for a long time. Pain improved with ibuprofen. The patient recently saw a dermatologist for a rash around his surgical incision. The dermatologist felt that the patient had psoriasis and prescribed a corticosteroid cream. Radiographs showed loss of joint space, healed posterior wall fracture, and intact hardware of his right acetabulum with a potential osteophyte forming inferiorly (Fig. 6). Ten weeks later the patient presented with a positive nickel test from another dermatology appointment, complaining mainly of skin lesions. Due to the dermatological changes near his incision and the subsequent pain, deep hardware removal and debridement was performed. Intra-operative cultures were negative. After his hardware was removed, his hip pain improved, skin lesions resolved, and there were mild degenerative changes noticed on the radiograph (Fig. 7).
Fig. 6.
Anteroposterior (A) and lateral (B) views of healed acetabular wall fracture prior to removal of hardware.
Fig. 7.
Anteroposterior (A) and lateral (B) views of healed acetabular wall fracture following removal of hardware.
3.4. Case 4
A 24-year-old female presented with a history of left groin pain for 7 months with no precipitating event. Previously, the patient had undergone conservative treatment (8 weeks of physical therapy, which did help with strengthening but did not help with pain and a steroid injection that helped with pain for a few weeks). The patient had a documented allergy to nickel (however, this was discovered after surgery) and no history of smoking, alcohol, or drug use. The patient had increased femoral anteversion and a cam lesion with retroversion of her acetabulum. One week later, left hip surgical dislocation with greater trochanteric osteotomy and fixation with a 22-modifier was performed. In addition, a left proximal femoral rotational osteotomy, left open femoral head and neck osteoplasty, left labral repair, and acetabuloplasty for the retroversion of the acetabulum and left hip wound vacuum-assisted closure (VAC) application measuring 30 cm2 was performed.
Five weeks later the patient was doing physical therapy and using crutches. She had some drainage from her incision that began the previous week. Radiographs of her left hip showed good callus formation at her rotational osteotomy site with no change in her hardware (Fig. 8). There was no change in her femoral head or joint space. She was directed to continue to progress with her therapy protocol and was also recommended to use a stationary bike.
Fig. 8.
Anteroposterior (A) and lateral (B) views of left hip with hardware.
Four weeks later, the patient presented with 2/10 pain on the VAS scale and was ambulating with a single crutch. On exam, she had two areas of her incision that were draining serous fluid. There was no cellulitis or erythema. Eight weeks later there was still draining of the postoperative wound, and she had fatigue. It was discussed with her that a CT scan was needed prior to plate removal to see if she needed a titanium plate placed. A nonunion workup and rheumatoid panel were performed for evaluation of her fatigue. A nickel tape was strapped to her right forearm and she was to report back as to what happened after wearing for three days. Three weeks later, the patient underwent deep hardware removal of the left proximal femur with wound VAC application of the left thigh measuring 36 cm2. She was to be weightbearing as tolerated with crutches and to use two crutches for two weeks.
Six weeks later the patient’s radiographs showed that she was healing well (Fig. 9), and her incisions were no longer draining. She was told to work on strengthening and stamina. Eight weeks later, she finished physical therapy.
Fig. 9.
Anteroposterior view of left hip following hardware removal.
4. Discussion
The most common symptoms for patients with MHS-associated failures are those exhibited by the presented cases—localized soft tissue reaction including delayed wound healing and/or recurrent wound issues. They may also have significantly delayed dermatological reactions similar to Case 3. These patients present similar to those with an indolent deep infection. A standard infection work-up should be performed. Furthermore, it should be verified with the patient if there is any history of metal sensitivity. Infection should be meticulously excluded prior to deducing MHS-associated failure. If it becomes necessary for the patient to have surgery for infection or consideration of MHS, deep cultures should be obtained to avoid missing a concomitant infection. We also recommend dermatological testing in patients with a history of autoimmune disorders, or multiple medical allergies due to the presence of a reactive immune system that may increase the risk of MHS. Finally, if a patient that has metallic implants in place with a history of multiple wound issues—or recurrent problems after operative debridement and appropriate infection management—dermatological testing for nickel allergy should be considered. In the setting of fractures, surgeons should be prepared to revise the implants with titanium or carbon fiber implants if bone stabilization is still required.
The best way to avoid postoperative issues is to routinely ask patients prior to surgery if they have any known MHS including problems with cosmetic jewelry. If this is known prior to surgery, titanium or carbon fiber implants should be used for fracture fixation and arthroplasty implantation choice should be modified based on the specific arthroplasty performed. It is important to remember that titanium implants still have nickel in them, but it is a minimal amount, and is a more readily available material from implant manufacturers compared to carbon fiber for fracture fixation. For instance, if a THA is being performed, titanium femoral and acetabular components should be used with a ceramic femoral head and polyethylene acetabular liner. Alternatively, one approach could be to use these implant materials for every fracture or arthroplasty patient to minimize the risk of MHS reaction.
5. Conclusion
Hypersensitivity reactions to nickel-containing orthopaedic implants may pose many therapeutic and diagnostic challenges. Hypersensitivity symptoms closely mimic infection and implant failure. As a result, MHS-associated failures are a diagnosis of exclusion and must be contemplated after judicious workup of localized soft tissue reaction including delayed wound healing and/or recurrent wound issues.6 In this review we attempt to help orthopaedic surgeons understand the epidemiology and pathophysiology of MHS, and to assess and interpret the clinical history that may direct one towards considering MHS as a reason for complication. However, due to the lack of consensus on this subject, better diagnostic tools and formal guidelines are needed.
Author contributions
C. Baumann: Collected the review materials and case study data, interpreted the data and designed the review, wrote and edited the manuscript.
B. Crist: Concept idea and supervision of the medical student, analysis of case series patient data, evaluated and edited the manuscript.
Funding statement
C. Baumann: Reports no conflicts either related to this study or to a commercial interest.
B. Crist: Reports being a paid consultant for Globus Medical, is a paid presenter and speaker for Kinetic Concepts, Inc., has stock or stock options in the Orthopaedic Implant Company, is a paid consultant for Pacira, is an unpaid consultant for SMV, and receives research support from Depuy Synthes.
No external funding was used in the execution of this study.
Declaration of competing interest
The authors report no conflicts of interest.
References
- 1.Pacheco K.A. Allergy to surgical implants. J Allergy Clin Immunol Pract. 2015;3:683–695. doi: 10.1016/j.jaip.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Stamm A., Kozlowski P., Brandenberger J. Surgical solution to an intracorporeal nickel allergy. Rev Urol. 2017;19:195–197. doi: 10.3909/riu0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thyssen J.P., Menne T. Metal allergy--a review on exposures, penetration, genetics, prevalence, and clinical implications. Chem Res Toxicol. 2010;23:309–318. doi: 10.1021/tx9002726. [DOI] [PubMed] [Google Scholar]
- 4.Schäfer T., Böhler E., Ruhdorfer S. Epidemiology of contact allergy in adults. Allergy. 2001;56:1192–1196. doi: 10.1034/j.1398-9995.2001.00086.x. [DOI] [PubMed] [Google Scholar]
- 5.Delimar D., Bohacek I., Pastar Z. Orthopedic and cutaneous reactions to nickel after total hip replacement. Acta Dermatovenerol Croat. 2018;26:39–43. [PubMed] [Google Scholar]
- 6.Thomas P. Clinical and diagnostic challenges of metal implant allergy using the example of orthopaedic surgical implants: Part 15 of the Series Molecular Allergology. Allergo J Int. 2014;23:179–185. doi: 10.1007/s40629-014-0023-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goon A.T., Goh C.L. Metal allergy in Singapore. Contact Dermatitis. 2005;52:130–132. doi: 10.1111/j.0105-1873.2005.00518.x. [DOI] [PubMed] [Google Scholar]
- 8.Roberts T.T., Haines C.M., Uhl R.L. Allergic or hypersensitivity reactions to orthopaedic implants. J Am Acad Orthop Surg. 2017;25:693–702. doi: 10.5435/JAAOS-D-16-00007. [DOI] [PubMed] [Google Scholar]
- 9.Lachiewicz P.F., Watters T.S., Jacobs J.J. Metal hypersensitivity and total knee arthroplasty. J Am Acad Orthop Surg. 2016;24:106–112. doi: 10.5435/JAAOS-D-14-00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akil S., Newman J.M., Shah N.V. Metal hypersensitivity in total hip and knee arthroplasty: current concepts. J Clin Orthop Trauma. 2018;9:3–6. doi: 10.1016/j.jcot.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thienpont E., Berger Y. No allergic reaction after TKA in a chrome-cobalt-nickel-sensitive patient: case report and review of the literature. Knee Surg Sports Traumatol Arthrosc. 2013;21:636–640. doi: 10.1007/s00167-012-2000-z. [DOI] [PubMed] [Google Scholar]
- 12.Basko-Plluska J.L., Thyssen J.P., Schalock P.C. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65–79. [PubMed] [Google Scholar]
- 13.Thomas P., Summer B. Diagnosis and management of patients with allergy to metal implants. Expet Rev Clin Immunol. 2015;11:501–509. doi: 10.1586/1744666X.2015.1016501. [DOI] [PubMed] [Google Scholar]
- 14.Granchi D., Cenni E., Giunti A. Metal hypersensitivity testing in patients undergoing joint replacement: a systematic review. J Bone Joint Surg Br. 2012;94:1126–1134. doi: 10.1302/0301-620X.94B8.28135. [DOI] [PubMed] [Google Scholar]
- 15.Maradit Kremers H., Larson D.R., Crowson C.S. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97:1386–1397. doi: 10.2106/JBJS.N.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hallab N., Merritt K., Jacobs J.J. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001;83-A:428–436. doi: 10.2106/00004623-200103000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Lu L.K., Warshaw E.M., Dunnick C.A. Prevention of nickel allergy: the case for regulation? Dermatol Clin. 2009;27:155–161. doi: 10.1016/j.det.2008.11.003. vi-vii. [DOI] [PubMed] [Google Scholar]
- 18.Yoshihisa Y., Shimizu T. Metal allergy and systemic contact dermatitis: an overview. Dermatol Res Pract. 2012;2012:749561. doi: 10.1155/2012/749561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wengler A., Nimptsch U., Mansky T. Hip and knee replacement in Germany and the USA: analysis of individual inpatient data from German and US hospitals for the years 2005 to 2011. Dtsch Arztebl Int. 2014;111:407–416. doi: 10.3238/arztebl.2014.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong K.L., Mowat F.S., Chan N. Economic burden of revision hip and knee arthroplasty in Medicare enrollees. Clin Orthop Relat Res. 2006;446:22–28. doi: 10.1097/01.blo.0000214439.95268.59. [DOI] [PubMed] [Google Scholar]
- 21.Sharkey P.F., Lichstein P.M., Shen C. Why are total knee arthroplasties failing today--has anything changed after 10 years? J Arthroplasty. 2014;29:1774–1778. doi: 10.1016/j.arth.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Sadoghi P., Liebensteiner M., Agreiter M. Revision surgery after total joint arthroplasty: a complication-based analysis using worldwide arthroplasty registers. J Arthroplasty. 2013;28:1329–1332. doi: 10.1016/j.arth.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Jafari S.M., Coyle C., Mortazavi S.M. Revision hip arthroplasty: infection is the most common cause of failure. Clin Orthop Relat Res. 2010;468:2046–2051. doi: 10.1007/s11999-010-1251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajan T.V. The Gell-Coombs classification of hypersensitivity reactions: a re-interpretation. Trends Immunol. 2003;24:376–379. doi: 10.1016/s1471-4906(03)00142-x. [DOI] [PubMed] [Google Scholar]
- 25.Uzzaman A., Cho S.H. Chapter 28: classification of hypersensitivity reactions. Allergy Asthma Proc. 2012;33(Suppl 1):96–99. doi: 10.2500/aap.2012.33.3561. [DOI] [PubMed] [Google Scholar]
- 26.Walsh M.L., Smith V.H., King C.M. Type 1 and type IV hypersensitivity to nickel. Australas J Dermatol. 2010;51:285–286. doi: 10.1111/j.1440-0960.2010.00664.x. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y., Dai S. Structural basis of metal hypersensitivity. Immunol Res. 2013;55:83–90. doi: 10.1007/s12026-012-8351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuroishi T., Bando K., Tanaka Y. CXCL4 is a novel nickel-binding protein and augments nickel allergy. Clin Exp Allergy. 2017;47:1069–1078. doi: 10.1111/cea.12926. [DOI] [PubMed] [Google Scholar]
- 29.Hallab N.J., Vermes C., Messina C. Concentration- and composition-dependent effects of metal ions on human MG-63 osteoblasts. J Biomed Mater Res. 2002;60:420–433. doi: 10.1002/jbm.10106. [DOI] [PubMed] [Google Scholar]
- 30.Schatzker J., McBroom R., Bruce D. The tibial plateau fracture. The Toronto experience 1968--1975. Clin Orthop Relat Res. 1979:94–104. [PubMed] [Google Scholar]
- 31.Wewers M.E., Lowe N.K. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health. 1990;13:227–236. doi: 10.1002/nur.4770130405. [DOI] [PubMed] [Google Scholar]