Abstract
Plants have been used in cosmetic products since ancient times and are the subject of scientific investigation even nowadays. During the years, a deeper understanding of both the behavior of skin and of plants have become available drawing increasingly complex pictures. Plants are complex organisms that produce different metabolites responding to the environment they live in. Applied to the skin, phytomolecules interact with skin cells and affect the skin well-being and appearance. Ethnobotanical studies on the one hand and physico-chemical analyses on the other have pictured a rich inventory of plants with potential to enrich modern cosmetic products.
Subject Areas: Biological Sciences, Plant Biotechnology, Plants
Graphical Abstract

Biological Sciences; Plant Biotechnology; Plants
Introduction
We might not be fully aware of the extent to which plants lend us a hand during the day. Other than providing construction material and food, plants supply active molecules of high value for pharmaceutical and cosmetic use through their metabolic machinery.
Preparations aimed at the amelioration of human skin conditions and appearance have accompanied humanity for millennia and have evolved into modern cosmetics. Skin is our interface to the environment and it actively protects us from aggressions. Plants have played a crucial role by providing ingredients able to soothe and protect the skin. Modern cosmetics can now not only improve hydration and relieve redness but also tune skin elastic properties (Ahshawat et al., 2008), and protect from ambient pollutants (Juliano and Magrini, 2018a; Pawar et al., 2017). Their relevant role in our life is reflected in the worth of the cosmetic market that is forecasted to reach $429.8 billion by 2022 (Cosmetics Market by Category, n.d. [skin and sun care products, hair care products, deodorants, makeup and color cosmetics, fragrances] and by distribution channel [general departmental store, supermarkets, drug stores, brand outlets]). The role of these formulations often goes beyond their intended use and tackle into the emotional sphere (Segot-Chicq et al., 2007). A personal cosmetic routine can feed feelings of attractivity and well-being (Graham and Kligman, 1985).
An official definition of natural cosmetics has not yet been produced by either the US Food and Drug Administration or the European Union, and different consumers might thus identify these products differently and have different expectations, e.g., as not containing chemically synthesized molecules, assuring a gentle action on the skin, coming from an ecologically friendly production process, or produced by an animal-testing-free process. We define here natural cosmetics as products whose efficacy is ascribed to their plant-derived ingredients. Countries leading in the development of natural cosmetics are China, South Korea, and Japan, with France being the most active at the European level, at least according to their fervent patent activity on anticellulite, tanning, or whitening cosmetics (Caesar et al., 2017).
Focusing on natural cosmetics, this work highlights how rich the world of plants is and how it can provide opportunities for innovation at different levels. As the knowledge on how the skin behaves is deepened and, at the same time, more scientific evidence is collected on plant physiology, the potential of plants in helping the skin soars, and yet, is still to be fully explored.
Skin and Plants Are Complex Responsive Systems
Skin is both a protective layer and a sensing structure for our body. Skin faces environmental and internal stressors that impact its integrity and has evolved molecular coping mechanisms (Suárez et al., 2012; França et al., 2017). Similarly, plants suffer and respond to external changes tuning their metabolism and communicating with their community (Leopold, 2014; Mancuso and Viola, 2015; Ninkovic, 2003). With the years, the understanding of both skin physiology and of plant behavior has grown and we are now more aware of their complexity.
As skin is the largest organ in the body, it is not surprising that our quality of life is affected when it is in a compromised state (Taieb et al., 2012). We know today that the skin is far from being a static homogeneous layer of cells as it might appear, especially to consumers, and the degree of its complexity has been uncovered in numerous studies. In addition to the interpersonal differences occurring in the population (Robinson 1999, 2002), the skin is inherently a complex organ. Different populations of cells have been identified, and the communication among them is crucial for skin homeostasis and healing. Cells undergo maturation and rapid migrations toward the surface of the epidermis or wounds. This is important in the case of wounds when only certain types of cells must undergo intense proliferation (Rognoni and Watt, 2018). Skin has also a tightly regulated circadian rhythm and its activities change day and night (Wu et al., 2018). Moreover, each cell of the skin can respond differently to stressors over time and behave differently from its surrounding resulting in a very heterogeneous tissue. For example, of all the fibroblasts that are responsible for the generation of the connective tissue in our skin, only some of them produce inflammatory molecules affecting the surrounding cells and promoting skin aging (Mahmoudi et al., 2019). The concept of skin has also expanded to include the resident bacteria on the surface. An additional degree of complexity has been added in the last decade. The skin is far from a passive surface. To ensure a balanced status, the skin produces bioactive molecules targeted at controlling the resident bacterial population such as peptides, and even some lipids with antimicrobial activity (Chen et al., 2018). The presence of bacteria on the skin has long been known and kept under control with cleaning products. Current efforts are targeted instead toward tuning the resident skin bacteria composition to achieve a healthy skin status with the use of either prebiotic molecules, to support the growth of reduced bacterial populations, or even living bacteria to directly supplement the skin flora. Interestingly, the composition of the bacterial population on skin does not change significantly during a person’s life as much, or depending on the environment as when in the case of a skin disease (Byrd et al., 2018).
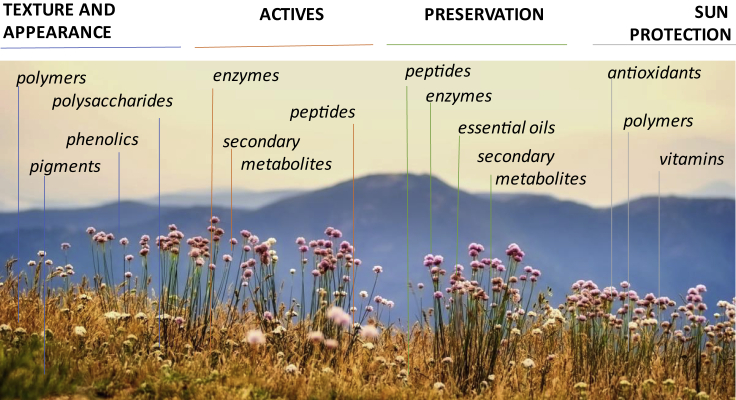
A deeper understanding of the complexity of plant metabolism and behaviors is also on the way. Plant complexity can be seen at different levels such as in their organization, their response to the environment, and in the preparations they are used in. Preparations such as extracts contain indeed a wide range of phytomolecules and thus offer multiple benefits to the skin (Figure 1).
Figure 1.
Schematic View of the Types of Phytomolecules that Plants Provide and the Activity They Can Deliver to Cosmetic Products
For a Figure360 author presentation of Figure 1, see https://doi.org/10.1016/j.isci.2020.101358.
Plants have grown to face the challenges posed by biotic and abiotic environmental stressors that come in different sizes, from the nanoscale of chemicals and nanomaterials to the milli-scale of insect and even herbivores. In addition to the internal accumulation of deterrent molecules for defense from herbivores, for example, a complex communication system based on the release in the air of molecules has also evolved (Gális et al., 2009; Leopold, 2014). Different plants have responded in unique ways but by mainly producing chemicals, i.e., secondary metabolites, that are also valuable for medical and cosmetic application. Within the secondary metabolism, plants have developed enzymatic and non-enzymatic mechanisms to cope with oxidative stress, such as the one due to the interaction with the nanomaterials encountered in the soil (Siddiqi and Husen, 2017; Czarnocka and Karpiński, 2018). Another example of abiotic stressors is the exposure to light (photoperiod) and how it affects plant metabolism; phenolic compounds, i.e., chlorogenic acid and catechins, are synthesized in a higher amount when the photoperiod is longer (Yang et al., 2018). Similarly, temperature promotes the production of different secondary metabolites such as alkaloids (Yang et al., 2018). Alkaloids have also been reported in response to drought stress, together with salinity that causes oxidative stress and leads to an increase in phenolic molecules. By identifying the optimal cultivation conditions (Papaioanou et al., 2018), it is possible to tune the composition in secondary metabolites and antioxidant activity as shown for raspberry Rubus idaeus L. and the effects on quantitative and qualitative fruit characteristics. Altitude with its higher solar UV radiation and lower temperatures also plays an important role, as winemakers challenged by climate change well know (Falcao et al., 2010), and is reflected especially in the composition of phenolic compounds, i.e., total amount of UV-absorbing caffeic acid derivatives (Spitaler et al., 2008). A comprehensive review on the response of plants to the environment by using secondary metabolites is available (McClintock, 1984). On accepting the Nobel Prize in Physiology or Medicine in 1983, Barbara McClintock already hinted at plant cells as “thoughtful” as they must interpret the environmental situation to adjust their metabolism (McClintock, 1984). Interestingly, the “intelligence” of plants is currently under investigation using plants such as Mimosa pudica that has been shown to remember experiences and is able to distinguish the stimuli received; mimosa leaves can differentiate whether they are touched by waterdrops or fingertips (Abramson and Chicas-Mosier, 2016). The complexity observed in the composition of plants is just a hint, and their behavior is now an active subject of research.
Plants in Cosmetics
Years of traditional use have led to the appreciation of plants for their effects on the human skin. Traditional treatment of skin with plants include teas, infusions, decoctions, ointments, and creams to target discolorations, changes in elasticity, and even medical conditions (Tabassum and Hamdani, 2014). Scientific investigation has later identified the active molecules that plants provide and are responsible for these benefits; chemical or biochemical industrial processes have then made their production at a higher scale feasible.
More than five thousand years ago, Egyptians were already using plants we use today as natural ingredients for cosmetics, such as thyme, chamomile, lavender, lily, peppermint, rosemary, and aloe, and the practice was later adopted by Roman and Greek civilizations (Gonzalez-Minero and Bravo-Diaz, 2018). The knowledge on the use of plants to treat the skin has been preserved through the centuries not only in populations living in rural areas but even in highly industrialized countries of Europe; through interviews of local people, tens of species of local plants are often identified as ailments in each study and the knowledge resides with the women of the community (Quave et al., 2008; Ambu et al., 2020; Miraldi et al., 2017; Pieroni and Cattero, 2019; Voeks, 2007; Zobolo and Mkabela, 2006). These studies often, however, denounce a loss of knowledge with the generations.
The use of natural ingredients such as plant extracts, offers a raw material with a complex composition of different active molecules. This complexity can explain the use of similar plants for different skin conditions and their ability to provide different benefits. As an example, extracts from plants belonging to the Salvia genus have proven beneficial in topical preparations with whitening, anti-aging, soothing, and brightening properties (for more examples see Caesar et al., 2017). At the same time, different parts of the same plant specialize and accumulate a complex range of metabolites that can benefit human skin (Table 1).
Table 1.
Different Parts of Plants Can Provide Cosmetic Ingredients (Examples)
| Location | Plant | Compound | Cosmetic Application | Reference |
|---|---|---|---|---|
| Bark | Prunus padus | Polyphenol and flavonoid | Skin firming and whitening | (Hwang et al., 2014) |
| Betula | Betulin | Anti-inflammatory, anti-microbial | (Krasutsky, 2007) | |
| Flowers | Dendrobium | Pelargonidin, sinapic, ferulic acids | Skin firming and whitening | (Kanlayavattanakul et al., 2018) |
| Clitoria ternatea | Phenolics | Antioxidant in eye gel | (Kamkaen and Wilkinson, 2009) | |
| Fruit | Carica papaya | Papain | Scar treatment | (Manosroi et al., 2013) |
| Rosa canina | Beta carotene, lycopene, β-chryptoxanthin, rubixanthin, zeaxanthin, lutein | Anti-inflammatory | (Hodisan et al., 1997; Deliorman Orhan et al., 2007) | |
| Leaves | Taraxacum officinale | Chologenic acid | Antioxidant | (Xie et al., 2018) |
| Fragaria vesca | Ellagitannin | Antioxidant, skin whitening | (Couto et al., 2020) | |
| Nuts | Sapindus mukorossi | Saponins | Detergent | (Almutairi and Ali, 2014; Góral and Wojciechowski, 2020) |
| Corylus avellana | Phenols | Antioxidant | (Delgado et al., 2010) | |
| Petals | Rosa gallica | Anthocyanins, polyphenols, flavonoids | Anti-inflammatory | (Lee et al., 2018) |
| Crocus sativus | Flavonol kaempferol | Skin whitening | (Kubo and Kinst-Hori, 1999) | |
| Pollen | Echium plantagineum | Anthocyanin petunidin-3-O-rutinoside | Varia | (Di Paola-Naranjo et al., 2004; Xi et al., 2018) |
| Helianthus annuus | Safflospermidines | Skin whitening | (Khongkarat et al., 2020) | |
| Roots | Morus nigra | Phenolics 2,4,2′,4′-tetrahydroxychalcone and morachalcone A | Skin whitening | (Zheng et al., 2010) |
| Paeonia lactiflora | Penta-O-galloyl-β-D-glucose | Skin barrier support | (Kim et al., 2020) | |
| Seeds | Aesculus hippocastanum | Saponin escin, flavonoids quercetin and kaempferol | Reduce capillary fragility, cell protective | (Wilkinson and Brown, 1999) |
| Moringa pterygosperma | Tocopherols | Carrier oil | (Kleiman et al., 2008) | |
| Sprouts | Polygonum hydropiper | (2R,3R)-(+)-taxifolin | Skin whitening | (Miyazawa and Tamura, 2007) |
| Glycine max | Proteins and polysaccharides | Anti-aging, whitening | (Lai et al., 2012) | |
| Stems | Chromolaena odorata | α- and β-Pinenes | Aromatic | (Owolabi et al., 2010) |
| Opuntia ficus-indica | ND | Soothing | (Schmid et al., 2020) | |
| Volatiles | varia | Methyl jasmonate | Scent | (Scognamiglio et al., 2012) |
| Eugenia uniflora and others | Terpenoids | Scent, anti-nociceptive, hypothermic | (Kappers et al., 2008; Yazaki et al., 2017; Amorim et al., 2009) |
What is an advantage can, however, turn into a drawback. One concern on the use of plant ingredients is their variability in composition due to environmental changes and cultivation conditions. These natural ingredients offer sometimes a composition hard to control and low stability in terms of color and activity, and can possibly contain undesired contaminants such as heavy metals (Abou-Arab and Abou Donia, 2000). Variations can be seen not only in the composition of secondary metabolites, e.g., flavonoids and anthocyanins (Akula and Ravishankar, 2011), but also in the high molecular weight constituents such as starch and lignin (Waring et al., 1985). Standing on strong analytics, standardization can provide an answer to ensure a constant content of an active molecule of interest (Ong, 2004; Kunle et al., 2012).
It is important to notice that plants can, however, provide not only efficacy through their bioactive molecules (Figure 1, Table 1) but also a means to improve the product texture by providing gums and polysaccharides, and alternative preservation strategies while enriching the product with a plethora of aromatic compounds and molecules, i.e., oils and pigments to alter the appearance and feeling of the product. Although cosmetics account as very minor contributors to the microplastic emergency, plants are offering alternatives to the microbeads found in scrubbing products such as cellulose-based, alginate-based, and lignin-based beads (Lee et al., 2020; Coombs Obrien et al., 2017; Nagaoka et al., 2007).
Discovery of Plants for Cosmetics: Between Tradition and Science
Long gone are the days of small gardens growing herbs used in domestic preparations based on recipes passed in the family through the years. Although many plant ingredients in cosmetics are well known from tradition, discoveries of species and activities are continuously reported, and the action of many plants has received confirmation in laboratory studies. Commercial cosmetic products rely not only on the long use of the plants but also on the scientific knowledge acquired through the years.
Plants and Tradition
One way to discover plant ingredient is by enquiring oral traditions and non-conventional medicine. Study of the ethnobotanical features of a region offers a high chance of innovation (Saikia et al., 2006). Although they often concentrate on small geographical areas, these can fully unlock the knowledge available to the population and this often expands toward a medical application (Schultz et al., 2020; Pieroni et al., 2004). Many ethnobotanical studies of century-long traditional uses offer plants that are well known, although maybe only in small regions. In Togo, for example, different habitats, i.e., from savanna to forest, are present and different ethnical groups are also distributed. Not only has the use of plants for cosmetic purposes thus evolved over time based on the availability of the plants in a specific ecological region but also the knowledge has spread and grown through interaction of the ethnic communities. Ethnobotany combined with genomic tools can luckily help to identify and classify plants that are rare or less known (Newmaster and Ragupathy, 2010). Moreover, the ethnobotanical knowledge can offer information for the optimization of biotechnological processes as it can indicate the optimal harvesting conditions and the richest plant parts (De La Parra and Quave, 2017). Ethnobotanical studies often sadly denounce, however, a loss of traditional use on plants, also due to loss of habitat due to climate change (Caballero-Serrano et al., 2019; Paniagua Zambrana et al., 2017).
Plants and Analytics
One approach to identify interesting plants is the screening of whole environmental collections, or a big number of natural isolates, using biochemical or chemical tests. By screening some 100 tropical plants and testing them, different species can be ranked based on their activity, e.g., antioxidant activity (Kim et al., 1997) or anti-tyrosinase activity (Baurin et al., 2002). The Mediterranean region is a hotspot of biodiversity, and a screening study has identified multiple plants with antibacterial action (March et al., 1991). The rich biodiversity of environments such as the amazons (Burlando and Cornara, 2017) or Australia has also offered plants that are traditionally well known for their valuable activities and that are also fast growing, a characteristic interesting when envisioning cultivation for commercialization (Atanasov et al., 2015). Even in the old continent, plants are still offering interesting cosmetic activities such as Hypericum scruglii, which is endemic to Sardinia and has potential anti-age action with its collagenase and tyrosinase inhibiting activity (Mandrone et al., 2017; Chiocchio et al., 2018). An analytical investigation of even plants that are widely distributed and long known in all continents like stinging nettle can harbor small molecules, i.e., such as ursolic acid together with quercetin and other phenolic compounds, providing strong anti-oxidant and anti-aging action by inhibiting the skin enzymes elastase and collagenase (Bourgeois et al., 2016). The comparison of the properties of different plant species can, however, be often difficult as the tests on their activities have been performed under different conditions.
Selection of plants that proceeds by screening relies on laboratory analyses based on biochemical or cell-based in vitro assays. Plants identification and their traceability are key nowadays to guarantee quality. To assure this, genetic-based techniques such as DNA metabarcoding that use next-generation sequencing (NGS) techniques have been adopted. Extraction processes and maceration can, however, lead to genomic DNA degradation hampering the analysis. Cold press extraction and supercritical carbon dioxide (sCO2) extraction allow a better identification at the species level and excludes the need of organic solvents. When looking at the composition of a plant preparation, mass spectrometry allows the analysis of all chemicals from a sample with very little or no prior preparation. Similarly, extractive electrospray ionization mass spectrometry has been developed to analyze cosmetic products and their ingredients, and it can detect contaminants at levels below 1 ppb (parts per billion), e.g., sun filters, hormones, and antibiotics (Zhang et al., 2013). Additionally, liquid chromatography has been used to distinguish active ingredients such as propolis from its counterfeit alternatives, i.e., poplar tree gum (from buds), which carries a characteristic chemical salicin (Zhang et al., 2011), i.e., salicin is hydrolyzed during propolis maturation and it is thus not detectable in the final product.
Ingredient Alternatives from Plants
Natural cosmetics are often preferred by consumers as their action is not based on molecules produced by chemical synthesis in the laboratory but through plant metabolic pathways that have evolved through the centuries. The use of plant ingredients can offer support or even replace synthetic ingredients such as preservatives, whitening, anti-pollution, and sun-protection agents. As an example, typical skin-whitening agents such as hydroquinone, retinoic acids, and corticoids are currently challenged by natural molecules with a whitening action on skin due to a tyrosinase-inhibiting action. This section focuses on three functions such as anti-pollution, sun protection, and natural preservation approaches. The topic of anti-aging has been recently reviewed (Pratsinis and Kletsas, 2019).
Anti-pollution
Awareness and evidence of the link between atmospheric pollution, premature aging, and an increase cellular oxidative stress and increase in reactive oxygen species (ROS) and matrix-metalloproteases (MMPs) is increasing (Kim et al., 2016; Valacchi et al., 2012). Molecules can act by forming a barrier (film) on the skin, directly interact with the pollutant by chelation, or help the skin fight by providing antioxidant activity. Camellia sinensis extracts (tea) is a plant producing multiple polyphenols that promote collagen synthesis and have an antiaging effect on the skin (Lee et al., 2014). Extract of white horehound Marrubium vulgare is often used in anti-pollution products as it has a strong antioxidant activity due to the presence of flavonoids such as ladanein, verbascoside, and forsythoside B. Similarly, the Chinese magnolia-vine Schisandra chinensis contributes a strong antioxidant action to the skin thanks to the lignans (polyphenols) such as schisandrin and other compounds such as chlorogenic acid, isoquercitrin, and quercetin that it contains (Juliano and Magrini, 2018b).
Sun Protection
Molecules with aromatic rings in their structure such as flavonoids (Cefali et al., 2016) naturally absorb UV radiation between 200 and 400 nm (UVA and UVB), provide protection to the plant from excessive solar radiation, and can act as alternative sunlight filters in cosmetics. Antioxidant molecules from plants can help to support the natural antioxidant mechanism of skin and can be used to stabilize UV filters by preventing the accumulation of radicals (Lorigo and Cairrao, 2019). Not only vitamin A, C, or E can be used in support but also the flavonoids rutin and quercetin have provided an acceptable sun protection (SPF) comparable with homosalate, but only at a 10% concentration when in an oil-in-water preparation. Rich in quercetin, a kaempferol glycoside, and a caffeic acid-derivative, the methanolic extract from Baccharis antioquensis leaves has showed good absorbance of UVA and UVB radiation giving a satisfactory photostability and an SPF of five when included in a formulation (Mejía-Giraldo et al., 2016). The use of these natural alternatives might impact the cost-effectiveness of the final product, whereas their use in combination with existing filters might be more feasible and their action could be exploited as boosters. The addition of rutin at a 0.1% concentration, a concentration much lower than the chemical filters, i.e., at a 2%- to 3.5% range, enhanced two points the SPF value of a formulation containing UVA (benzophenone-3) and UVB (ethylhexyl methoxycinnamate) filters (Velasco et al., 2008). A similar boosting action has been suggested also for lignin, a by-product of the paper industry (Qian et al., 2017). Added to a standard sunscreen formulation, with lignin formula with an SPF of 15 could become SPF of 30 with the addition of 2% lignin solution (Qian et al., 2015). As compared with standard UV filters that are produced by chemical synthesis, plant-derived extracts and materials offer a higher degree of biodegradability to the product and positively impact the sustainability of the process.
Preservation
In response to the critical opinions of the public toward preservatives and the possible sensitization, irritation, and effect on the endocrine system, plants and natural products in general have been investigated as possible alternatives. A broad antibacterial action has been discovered in many plant preparations. The essential oils from thyme and eucalyptus have proven action against both Gram (+) and Gram (−) bacteria and even antibiotic-resistant Staphylococcus aureus (MRSA) (Tohidpour et al., 2010). This activity is due to the presence in essential oils of aromatic terpenes (Mekonnen et al., 2016), e.g., eugenol, β-pinene, and α-pinene (Medeiros Leite et al., 2007). Since the extract from cotton lavender Santolina chamaecyparissus showed activity against P. aeruginosa, S. aureus, and Aspergillus niger in ranges comparable with methylparaben, this was further analyzed to isolate the active molecule spiroketalenol (Kerdudo et al., 2016). A performance better than methylparaben has been reported especially for essential oils, e.g., from true lavender Lavandula officinalis, tea tree Melaleuca alternifolia, and true cinnamon Cinnamomum zeylanicum (Herman et al., 2013).
Essential oils have a preservative action and, if insufficient working alone, their action can be used to support traditional preservative strategies. The essential oil from lesser calamint Calamintha officinalis at a 2% concentration has shown to be an effective preservative satisfying the regulatory requirements; its effect is, however, influenced by the preparation and proved more effective when in a cream than in a shampoo formulation, and when in the presence of EDTA (Nostro et al., 2002, 2004). Essential oils from lavender, tea tree, and lemon used at a 0.1%–0.5% concentration in formulations have proved effective against bacteria and fungi after 7 days when combined with a synthetic preservative and able to meet regulatory requirements (Kunicka-Styczyńska et al., 2009). Found in various plant sources, hydroxytyrosol is a potential bio-preservative able to potentially replace synthetic food and cosmetic additives such as butylated hydroxytoluene (BHT) (Bernini et al., 2013). Hydroxytyrosol is also produced in olive tree plant tissues. Using a green-chemistry-inspired extraction process, olive leaves and olive pulp can be a source of a juice containing hydroxytyrosol and not a high-cost waste, because of their high level of toxicity.
It is noteworthy that the action of compounds on bacteria is often measured with agar-based methods, and their correspondent efficacy in a liquid such as a cosmetic formulation might need some tuning and testing. One other concern is, however, that oils need sometimes to be used in a high non-cost-efficient concentration, that, in addition to the cost of the final product, might also affect the resulting smell and texture of the product (Deans and Ritchie, 1987; Muyima et al., 2002). Issues facing the use of natural preservatives might also be the availability, their stability during the manufacturing process, the optimal required concentration, and especially their selective action on specific strains, i.e., not broad-spectrum agents (Flanagan, 2011). Essential oils can also undergo oxidation and lose their aromatic profile and bioactivity; a technique such as microencapsulation can, however, help to protect the oil in the formulation (Carvalho et al., 2016).
Future Developments
The environment has offered powerful ingredients for natural cosmetics, whereas science and technology helped to uncover and better understand their action. Plants are the source of inspiration for their ability to thrive in changing and challenging environments. However, biodiversity loss and climate change can quickly affect the distribution of plants and their chemicals. Lists of plants threatened by global warming and under the risk of extinction are available and should be considered when looking for ingredients (The IUCN Red List of Threatened Species, n.d.). Interestingly, plant ingredients can offer multiple functions and even the ones that are already included in formulations might play additional roles and benefit the skin, e.g., natural dyes and radical scavenging (Boo et al., 2012).
Plants have offered innovative solutions to medicine, material science, architecture, robotics, and engineering and can surely further cosmetic products (Burris et al., 2017; Li and Wang, 2015; Momeni and Ni, 2018; Rian and Sassone, 2014; Koch et al., 2009; Bar-Cohen, 2012). Plants have, for example, devised climbing strategies based on either particles or adhesive polymers that might find application to promote the interaction of the cosmetic products with the skin surface (Burris et al., 2017). The surface of leaves have evolved specialized structures able to lower the interaction with atmospheric particles and bacteria, absorb or reflect specific light wavelengths, or efficiently absorbing water; these are all functions sought for by cosmetic products and plants can offer inspiration for new devices or concepts (Koch et al., 2009). In addition, extracts of a wide variety of plants offer sustainable synthetic routes for biofunctional materials currently used in cosmetics such as silver particles (one example is Chandran et al., 2006). Considering the microbiome and how important a balance with the skin is, the mechanisms that plants have evolved against pathogens and based on proteins, peptides, and small molecules will offer new strategies, also when looking for alternative preservation strategies (Goyal and Mattoo, 2016; Tiku, 2020; Aljbory and Chen, 2018; Lim et al., 2017). The contribution of plants to cosmetic products can clearly go beyond the isolation of bioactive compounds.
Focusing on the plant material, the concern over the quality of the plant material used can be met by genome-based analytics for identification, metabolite fingerprinting by mass spectroscopic methods, and the transparency offered by the blockchain technology (Heinrich et al., 2019). Moreover, optimized cultivation conditions, traditional breeding techniques, and manipulation at the gene level have already offered the possibility of improving the plant in terms of cultivability and composition, i.e., removal of toxic compounds and enriching for desired active molecule (Alagoz et al., 2016). The development of in vitro cultivation of plant cells has provided a big step toward the reproducible production of plant-based ingredients. The techniques currently developed toward cellular agriculture might offer a reliable and reproducible supply of plant material on a larger scale in the future (Stephens et al., 2018). On the other hand, more complex and representative skin models might be developed on the fashion of mini-organs and organoids (Davies, 2018). If skin models could include a bigger variety of skin cell types, reproduce their interaction in vitro, and ideally include the microbial outer component, i.e., the microbiome, a better understanding and design of the action of cosmetics could be achieved.
Plant ingredients can actively help to improve the skin appearance or support traditional ingredients in their action. Plants play a crucial role in natural cosmetics, products whose sales are rising, but whose future might be harmed by climate change that threatens habitats and leads to loss of species and traditions (European Environment Agency, 2020). As a temporary response, the investment in ethnobotanical studies can minimize the loss of knowledge also in industrialized countries, while specialized analytics and more complex in vitro skin models are developed and help to better understand the activity of plants on human skin.
Acknowledgments
The critical comments of Ramon Weishaupt and the kind support of Rosemary Barnes is acknowledged during the manuscript preparation.
Funding
This research received no external funding.
Conflicts of Interest
The author declare no conflict of interest.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101358.
Supplemental Information
References
- Abou-Arab A., Abou Donia M.A. Heavy metals in Egyptian spices and medicinal plants and the effect of processing on their levels. J. Agric. Food Chem. 2000;48:2300–2304. doi: 10.1021/jf990508p. [DOI] [PubMed] [Google Scholar]
- Abramson C.I., Chicas-Mosier A. Learning in plants: lessons from Mimosa pudica. Front. Psychol. 2016;7:417. doi: 10.3389/fpsyg.2016.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahshawat M.S., Saraf S., Saraf S. Preparation and characterization of herbal creams for improvement of skin viscoelastic properties. Int. J. Cosmet. Sci. 2008;30:183–193. doi: 10.1111/j.1468-2494.2008.00442.x. [DOI] [PubMed] [Google Scholar]
- Akula R., Ravishankar G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signaling Behav. 2011;6:1720–1731. doi: 10.4161/psb.6.11.17613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagoz Y., Gurkok T., Zhang B., Unver T. Manipulating the biosynthesis of bioactive compound alkaloids for next-generation metabolic engineering in opium poppy using CRISPR-Cas 9 genome editing technology. Sci. Rep. 2016;6:30910. doi: 10.1038/srep30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aljbory Z., Chen M. Indirect plant defense against insect herbivores: a review. Insect Sci. 2018;25:2–23. doi: 10.1111/1744-7917.12436. [DOI] [PubMed] [Google Scholar]
- Almutairi M., Ali M. Direct detection of saponins in crude extracts of soapnuts by FTIR. Nat. Prod. Res. 2014;29:1–5. doi: 10.1080/14786419.2014.992345. [DOI] [PubMed] [Google Scholar]
- Ambu G., Chaudhary R.P., Mariotti M., Cornara L. Traditional uses of medicinal plants by ethnic people in the Kavrepalanchok district, Central Nepal. Plants. 2020;9:759. doi: 10.3390/plants9060759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim A.C.L., Lima C.K.F., Hovell A.M.C., Miranda A.L.P., Rezende C.M. Antinociceptive and hypothermic evaluation of the leaf essential oil and isolated terpenoids from Eugenia uniflora L. (Brazilian Pitanga) Phytomedicine. 2009;16:923–928. doi: 10.1016/j.phymed.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Atanasov A.G., Waltenberger B., Pferschy-Wenzig E., Linder T., Wawrosch C., Uhrin P., Temml V., Wang L., Schwaiger S., Heiss E.H., Rollinger J.M. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol. Adv. 2015;33:1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Cohen Y. Nature as a model for mimicking and inspiration of new technologies. Int. J. Aeronaut. Space Sci. 2012;13:1–13. [Google Scholar]
- Baurin N., Arnoult E., Scior T., Do Q.T., Bernard P. Preliminary screening of some tropical plants for anti-tyrosinase activity. J. Ethnopharmacol. 2002;82:155–158. doi: 10.1016/s0378-8741(02)00174-5. [DOI] [PubMed] [Google Scholar]
- Bernini R., Merendino N., Romani A., Velotti F. Naturally occurring hydroxytyrosol: synthesis and anticancer potential. Curr. Med. Chem. 2013;20:655–670. doi: 10.2174/092986713804999367. [DOI] [PubMed] [Google Scholar]
- Boo H., Hwang S., Bae C., Park S., Heo B., Gorinstein S. Extraction and characterization of some natural plant pigments. Ind. Crops Prod. 2012;40:129–135. [Google Scholar]
- Bourgeois C., Leclerc É.A., Corbin C., Doussot J., Serrano V., Vanier J., Seigneuret J., Auguin D., Pichon C., Lainé É., Hano C. Nettle (Urtica dioica L.) as a source of antioxidant and anti-aging phytochemicals for cosmetic applications. Comptes Rendus Chim. 2016;19:1090–1100. [Google Scholar]
- Burlando B., Cornara L. Revisiting amazonian plants for skin care and disease. Cosmetics. 2017;4:25. [Google Scholar]
- Burris J., Lenaghan S., Stewart C. Climbing plants: attachment adaptations and bioinspired innovations. Plant Cell Rep. 2017;37:565–574. doi: 10.1007/s00299-017-2240-y. [DOI] [PubMed] [Google Scholar]
- Byrd A.L., Belkaid Y., Segre J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018;16:143–155. doi: 10.1038/nrmicro.2017.157. [DOI] [PubMed] [Google Scholar]
- Caballero-Serrano V., Mclaren B., Carrasco J.C., Alday J.G., Fiallos L., Amigo J., Onaindia M. Traditional ecological knowledge and medicinal plant diversity in Ecuadorian Amazon home gardens. Glob. Ecol. Conservat. 2019;17:e00524. [Google Scholar]
- Caesar F.C.S., Carnevale Neto F., Porto G.S., Campos P.M.B.G.M. Patent analysis: a look at the innovative nature of plant-based cosmetics. Quim. Nova. 2017;40:840–847. [Google Scholar]
- Carvalho I.T., Estevinho B.N., Santos L. Application of microencapsulated essential oils in cosmetic and personal healthcare products – a review. Int. J. Cosmet. Sci. 2016;38:109–119. doi: 10.1111/ics.12232. [DOI] [PubMed] [Google Scholar]
- Cefali L.C., Ataide J.A., Moriel P., Foglio M.A., Mazzola P.G. Plant-based active photoprotectants for sunscreens. Int. J. Cosmet. Sci. 2016;38:346–353. doi: 10.1111/ics.12316. [DOI] [PubMed] [Google Scholar]
- Chandran S.P., Chaudhary M., Pasricha R., Ahmad A., Sastry M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol. Prog. 2006;22:577–583. doi: 10.1021/bp0501423. [DOI] [PubMed] [Google Scholar]
- Chen Y.E., Fischbach M.A., Belkaid Y. Skin microbiota-host interactions. Nature. 2018;553:427–436. doi: 10.1038/nature25177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiocchio I., Mandrone M., Sanna C., Maxia A., Tacchini M., Poli F. Screening of a hundred plant extracts as tyrosinase and elastase inhibitors, two enzymatic targets of cosmetic interest. Ind. Crops Prod. 2018;122:498–505. [Google Scholar]
- Coombs Obrien J., Torrente-Murciano L., Mattia D., Scott J.L. Continuous production of cellulose microbeads via membrane emulsification. ACS Sustain. Chem. Eng. 2017;5:5931–5939. [Google Scholar]
- Couto J., Figueirinha A., Batista M., Paranhos A., Nunes C., Gonçalves L., Marto J., Fitas M., Pinto P., Ribeiro H., Pina M. Fragaria vesca L. Extract: a promising cosmetic ingredient with antioxidant properties. Antioxidants. 2020;9:154. doi: 10.3390/antiox9020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmetics Market by Category (n.d.), (Skin & sun care products, Hair care products, Deodorants, Makeup & color cosmetics, Fragrances) and by Distribution Channel (general departmental store, Supermarkets, Drug stores, Brand outlets) - global opportunity analysis and industry Forecast, 2014-2022. Available: https://www.alliedmarketresearch.com/cosmetics-market.
- Czarnocka W., Karpiński S. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radic. Biol. Med. 2018;122:4–20. doi: 10.1016/j.freeradbiomed.2018.01.011. [DOI] [PubMed] [Google Scholar]
- Davies J.A. Academic Press; 2018. Chapter 1-Organoids and Mini-Organs: Introduction, History, and Potential; pp. 3–23. [Google Scholar]
- Deans S.G., Ritchie G. Antibacterial properties of plant essential oils. Int. J. Food Microbiol. 1987;5:165–180. [Google Scholar]
- Delgado T., Malheiro R., Pereira J.A., Ramalhosa E. Hazelnut (Corylus avellana L.) kernels as a source of antioxidants and their potential in relation to other nuts. Ind. Crops Prod. 2010;32:621–626. [Google Scholar]
- Deliorman Orhan D., Hartevioğlu A., Küpeli E., Yesilada E. In vivo anti-inflammatory and antinociceptive activity of the crude extract and fractions from Rosa canina L. fruits. J. Ethnopharmacol. 2007;112:394–400. doi: 10.1016/j.jep.2007.03.029. [DOI] [PubMed] [Google Scholar]
- European Environment Agency, (2020), Distribution shifts of plant and animal species. Available: https://www.eea.europa.eu/data-and-maps/indicators/distribution-of-plant-species-2/assessment.
- Falcao L.D., Burin V.M., Eduardo S.C., Hamilton J.V., Brighenti E., Rosier J.P., Bordignon-Luiz M.T. Vineyard altitude and mesoclimate influences on the phenology and maturation of Cabernet-Sauvignon grapes from Santa Catarina State. OENO One. 2010;44:135–150. [Google Scholar]
- 37.Flanagan J. Preserving cosmetics with natural preservatives and preserving natural cosmetics. In: Dayan N., Kromidas L., editors. Formulating, packaging, and marketing of natural cosmetic products, 10. Wiley; 2011. pp. 169–178. [Google Scholar]
- França K., França A.P., De França R. Environmental psychodermatology: stress, environment and skin. In: França K., Jafferany M., editors. Stress and Skin Disorders: Basic and Clinical Aspects. Cham. Springer International Publishing; 2017. pp. 47–53. [Google Scholar]
- Gális I., Gaquerel E., Pandey S.P., Baldwin I.T. Molecular mechanisms underlying plant memory in JA-mediated defence responses. Plant. Cell Environ. 2009;32:617–627. doi: 10.1111/j.1365-3040.2008.01862.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Minero J.F., Bravo-Diaz L. The use of plants in skin-care products. Cosmetics and Fragrances: past and Present. Cosmetics. 2018;5:50. [Google Scholar]
- Góral I., Wojciechowski K. Surface activity and foaming properties of saponin-rich plants extracts. Adv. Colloid Interfaces Sci. 2020;279:102145. doi: 10.1016/j.cis.2020.102145. [DOI] [PubMed] [Google Scholar]
- Goyal R.K., Mattoo A.K. Plant antimicrobial peptides. In: Epand R.M., editor. Host Defense Peptides and Their Potential as Therapeutic Agents. Springer International Publishing; 2016. pp. 111–136. [Google Scholar]
- Graham J.A., Kligman A.M. Physical attractiveness, cosmetic use and self-perception in the elderly. Int. J. Cosmet. Sci. 1985;7:85–97. doi: 10.1111/j.1467-2494.1985.tb00399.x. [DOI] [PubMed] [Google Scholar]
- Heinrich M., Scotti F., Booker A., Fitzgerald M., Kum K.Y., Label K. Unblocking high-value botanical value chains: is there a role for blockchain systems? Front. Pharmacol. 2019;10:396. doi: 10.3389/fphar.2019.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman A., Herman A.P., Domagalska B.W., Maynarczyk A. Essential oils and herbal extracts as antimicrobial agents in cosmetic emulsion. Indian J. Microbiol. 2013;53:232–237. doi: 10.1007/s12088-012-0329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodisan T., Socaciu C., Ropan I., Neamtu G. Carotenoid composition of Rosa canina fruits determined by thin-layer chromatography and high-performance liquid chromatography. J. Pharm. Biomed. Anal. 1997;16:521–528. doi: 10.1016/s0731-7085(97)00099-x. [DOI] [PubMed] [Google Scholar]
- Hwang D., Kim H., Shin H., Jeong H., Kim J., Kim D. Cosmetic effects of Prunus padus bark extract. Korean J. Chem. Eng. 2014;31:2280–2285. [Google Scholar]
- Juliano C., Magrini A.G. Cosmetic functional ingredients from botanical sources for anti-pollution skincare products. Cosmetics. 2018;5:19. [Google Scholar]
- Juliano C., Magrini A.G. Methylglyoxal, the major antibacterial factor in manuka honey: an alternative to preserve natural cosmetics? Cosmetics. 2018;6:1. [Google Scholar]
- Kamkaen N., Wilkinson J.M. The antioxidant activity of Clitoria ternatea flower petal extracts and eye gel. Phytother. Res. 2009;23:1624–1625. doi: 10.1002/ptr.2832. [DOI] [PubMed] [Google Scholar]
- Kanlayavattanakul M., Lourith N., Chaikul P. Biological activity and phytochemical profiles of Dendrobium: a new source for specialty cosmetic materials. Ind. Crops Prod. 2018;120:61–70. [Google Scholar]
- Kappers I.F., Dicke M., Bouwmeester H.J. Terpenoids in plant signaling: chemical ecology. Wiley Encyclopedia Chem. Biol. John Wiley Sons. 2008 doi: 10.1002/9780470048672.wecb652. [DOI] [Google Scholar]
- Kerdudo A., Burger P., Merck F., Dingas A., Rolland Y., Michel T., Fernandez X. Development of a natural ingredient – natural preservative: a case study. Comptes Rendus Chim. 2016;19:1077–1089. [Google Scholar]
- Khongkarat P., Ramadhan R., Phuwapraisirisan P., Chanchao C. Safflospermidines from the bee pollen of Helianthus annuus L. exhibit a higher in vitro antityrosinase activity than kojic acid. Heliyon. 2020;6:e03638. doi: 10.1016/j.heliyon.2020.e03638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.J., Kim J.H., Kim H.P., Heo M.Y. Biological screening of 100 plant extracts for cosmetic use (II): anti-oxidative activity and free radical scavenging activity. Int. J. Cosmet. Sci. 1997;19:299–307. doi: 10.1046/j.1467-2494.1997.171726.x. [DOI] [PubMed] [Google Scholar]
- Kim K.E., Cho D., Park H.J. Air pollution and skin diseases: adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016;152:126–134. doi: 10.1016/j.lfs.2016.03.039. [DOI] [PubMed] [Google Scholar]
- Kim K., Shim J.S., Kim H., Son E.D. Penta-O-galloyl-β-D-glucose from Paeonia lactiflora Pall. root extract enhances the expression of skin barrier genes via EGR3. J. Ethnopharmacol. 2020;248:112337. doi: 10.1016/j.jep.2019.112337. [DOI] [PubMed] [Google Scholar]
- Kleiman R., Ashley D.A., Brown J.H. Comparison of two seed oils used in cosmetics, moringa and marula. Ind. Crops Prod. 2008;28:361–364. [Google Scholar]
- Koch K., Bhushan B., Barthlott W. Multifunctional surface structures of plants: an inspiration for biomimetics. Prog. Mater. Sci. 2009;54:137–178. [Google Scholar]
- Krasutsky P. Birch bark research and development. Nat. Prod. Rep. 2007;23:919–942. doi: 10.1039/b606816b. [DOI] [PubMed] [Google Scholar]
- Kubo I., Kinst-Hori I. Flavonols from saffron flower: tyrosinase inhibitory activity and inhibition mechanism. J. Agric. Food Chem. 1999;47:4121–4125. doi: 10.1021/jf990201q. [DOI] [PubMed] [Google Scholar]
- Kunicka-Styczyńska A., Sikora M., Kalemba D. Antimicrobial activity of lavender, tea tree and lemon oils in cosmetic preservative systems. J. Appl. Microbiol. 2009;107:1903–1911. doi: 10.1111/j.1365-2672.2009.04372.x. [DOI] [PubMed] [Google Scholar]
- Kunle O., Folashade, Egharevba H., Omoregie, Ochogu P. Standardization of herbal medicines -A review. Int. J. Biodivers. Conservat. 2012;4:101–112. [Google Scholar]
- De La Parra J., Quave C.L. Ethnophytotechnology: harnessing the power of ethnobotany with biotechnology. Trends Biotechnol. 2017;35:802–806. doi: 10.1016/j.tibtech.2017.07.003. [DOI] [PubMed] [Google Scholar]
- Lai J., Xin C., Zhao Y., Feng B., He C., Dong Y., Fang Y., Wei S. Study of active ingredients in black soybean sprouts and their safety in cosmetic use. Molecules. 2012;17:11669–11679. doi: 10.3390/molecules171011669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.O., Kim S.N., Kim Y.C. Anti-wrinkle effects of water extracts of teas in hairless mouse. Toxicol. Res. 2014;30:283–289. doi: 10.5487/TR.2014.30.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M., Nam T.G., Lee I., Shin E.J., Han A., Lee P., Lee S., Lim T. Skin anti-inflammatory activity of rose petal extract (Rosa gallica) through reduction of MAPK signaling pathway. Food Sci. Nutr. 2018;6:2560–2567. doi: 10.1002/fsn3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.S., Yoo E., Lee H.S., Won K. Preparation and application of light-colored lignin nanoparticles for broad-spectrum sunscreens. Polymers (Basel) 2020;12:699. doi: 10.3390/polym12030699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold A.C. Smart plants: memory and communication without brains. Plant Signal. Behav. 2014;9:e972268. doi: 10.4161/15592316.2014.972268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Wang K. Fluidic origami with embedded pressure dependent multi-stability: a plant inspired innovation. J. R. Soc. Interfaces. 2015;12:20150639. doi: 10.1098/rsif.2015.0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim G., Singhal R., Kachroo A., Kachroo P. Fatty acid– and lipid-mediated signaling in plant defense. Annu. Rev. Phytopathol. 2017;55:505–536. doi: 10.1146/annurev-phyto-080516-035406. [DOI] [PubMed] [Google Scholar]
- Lorigo M., Cairrao E. Antioxidants as stabilizers of UV filters: an example for the UV-B filter octylmethoxycinnamate. Biomed. Dermatol. 2019;3:11. [Google Scholar]
- Mahmoudi S., Mancini E., Xu L., Moore A., Jahanbani F., Hebestreit K., Srinivasan R., Li X., Devarajan K., Pralot L. Heterogeneity in old fibroblasts is linked to variability in reprogramming and wound healing. Nature. 2019;574:553–558. doi: 10.1038/s41586-019-1658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso S., Viola A. Island Press; 2015. Brilliant Green: The Surprising History and Science of Plant Intelligence. [Google Scholar]
- Mandrone M., Scognamiglio M., Fiorentino A., Sanna C., Cornioli L., Antognoni F., Bonvicini F., Poli F. Phytochemical profile and α-glucosidase inhibitory activity of Sardinian Hypericum scruglii and Hypericum hircinum. Fitoterapia. 2017;120:184–193. doi: 10.1016/j.fitote.2017.06.020. [DOI] [PubMed] [Google Scholar]
- Manosroi A., Chankhampan C., Manosroi W., Manosroi J. Transdermal absorption enhancement of papain loaded in elastic niosomes incorporated in gel for scar treatment. Eur. J. Pharm. Sci. 2013;48:474–483. doi: 10.1016/j.ejps.2012.12.010. [DOI] [PubMed] [Google Scholar]
- March C., Sanz I., Yufera E.P. Antimicrobial activities on mediterranean plants. Zentralbl Mikrobiol. 1991;146:291–295. [PubMed] [Google Scholar]
- McClintock B. The significance of responses of the genome to challenge. Science. 1984;226:792. doi: 10.1126/science.15739260. [DOI] [PubMed] [Google Scholar]
- Medeiros Leite A., De Oliveira Lima E., De Souza E.L., De Fátima Formiga Melo Diniz M., Nogueira Trajanoii V., Almeida De Medeirosi I. Inhibitory effect of b-pinene, a-pinene and eugenol on the growth of potential infectious endocarditis causing Gram-positive bacteria. Rev. Bras. Cienc. Farm. 2007;43:121–126. [Google Scholar]
- Mejía-Giraldo J.C., Winkler R., Gallardo C., Sánchez-Zapata A.M., Puertas-Mejía M.A. Photoprotective potential of Baccharis antioquensis (asteraceae) as natural sunscreen. Photochem. Photobiol. 2016;92:742–752. doi: 10.1111/php.12619. [DOI] [PubMed] [Google Scholar]
- Mekonnen A., Yitayew B., Tesema A., Taddese S. In Vitro antimicrobial activity of essential oil of Thymus schimperi, Matricaria chamomilla, Eucalyptus globulus, and Rosmarinus officinalis. Int. J. Microbiol. 2016;2016:9545693. doi: 10.1155/2016/9545693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miraldi E., Governa P., Biagi M., Giordani P., Cornara L. Skin wound healing: from mediterranean ethnobotany to evidence based phytotherapy. Athens J. Sci. 2017;4:199–211. [Google Scholar]
- Miyazawa M., Tamura N. Inhibitory compound of tyrosinase activity from the sprout of Polygonum hydropiper l. (benitade) Biol. Pharm. Bull. 2007;30:595–597. doi: 10.1248/bpb.30.595. [DOI] [PubMed] [Google Scholar]
- Momeni F., Ni J. Nature-inspired smart solar concentrators by 4D printing. Renew. Energy. 2018;122:35–44. [Google Scholar]
- Muyima N.Y.O., Zulu G., Bhengu T., Popplewell D. The potential application of some novel essential oils as natural cosmetic preservatives in an aqueous cream formulation. Flavour Fragr. J. 2002;17:258–266. [Google Scholar]
- Nagaoka S., Nagata M., Arinaga K., Shigemori K., Takafuji M., Ihara H. Environmentally friendly coloured materials: cellulose/titanium dioxide/inorganic pigment composite spherical microbeads prepared by viscose phase-separation method. Color. Technol. 2007;123:344–350. [Google Scholar]
- Newmaster S.G., Ragupathy S. Ethnobotany genomics - discovery and innovation in a new era of exploratory research. J. Ethnobiol. Ethnomedicine. 2010;6:2. doi: 10.1186/1746-4269-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkovic V. Volatile communication between barley plants affects biomass allocation. J. Exp. Bot. 2003;54:1931–1939. doi: 10.1093/jxb/erg192. [DOI] [PubMed] [Google Scholar]
- Nostro A., Cannatelli M.A., Morelli I., Cioni P.L., Bader A., Marino A., Alonzo V. Preservative properties of Calamintha officinalis essential oil with and without EDTA. Lett. Appl. Microbiol. 2002;35:385–389. doi: 10.1046/j.1472-765x.2002.01216.x. [DOI] [PubMed] [Google Scholar]
- Nostro A., Cannatelli M.A., Morelli I., Musolino A.D., Scuderi F., Pizzimenti F., Alonzo V. Efficiency of Calamintha officinalis essential oil as preservative in two topical product types. J. Appl. Microbiol. 2004;97:395–401. doi: 10.1111/j.1365-2672.2004.02319.x. [DOI] [PubMed] [Google Scholar]
- Ong E.S. Extraction methods and chemical standardization of botanicals and herbal preparations. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004;812:23–33. doi: 10.1016/j.jchromb.2004.07.041. [DOI] [PubMed] [Google Scholar]
- Owolabi M., Ogundajo A., Yusuf K., Lajide L., Villanueva H., Tuten J., Setzer W. Chemical composition and bioactivity of the essential oil of Chromolaena odorata from Nigeria. Rec. Nat. Prod. 2010;4:72–78. [Google Scholar]
- Paniagua Zambrana N.Y., Bussmann R.W., Hart R.E., Moya Huanca A.L., Ortiz Soria G., Ortiz Vaca M., Ortiz Ãlvarez D., Soria Morãn J., Soria Morãn M., Chãvez S. Traditional knowledge hiding in plain sight - twenty-first century ethnobotany of the Chàcobo in Beni, Bolivia. J. Ethnobiol. Ethnomedicine. 2017;13:57. doi: 10.1186/s13002-017-0179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paola-Naranjo R.D., Sánchez-Sánchez J., González-Paramás A.M., Rivas-Gonzalo J.C. Liquid chromatographic–mass spectrometric analysis of anthocyanin composition of dark blue bee pollen from Echium plantagineum. J. Chromatogr. A. 2004;1054:205–210. doi: 10.1016/j.chroma.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Papaioanou M., Chronopoulou G.E., Ciobotari G., Efrose C.R., Sfichi-Duke L., Chatzikonstantinou M., Pappa E., Ganopoulos I., Madesis P., Nianiou-Obeidat I. Cosmeceutical properties of two cultivars of red raspberry grown under different conditions. Cosmetics. 2018;5:20. [Google Scholar]
- Pawar G., Abdallah M.A., De Sãa E.V., Harrad S. Dermal bioaccessibility of flame retardants from indoor dust and the influence of topically applied cosmetics. J. Expo. Sci. Environ. Epidemiol. 2017;27:100–105. doi: 10.1038/jes.2015.84. [DOI] [PubMed] [Google Scholar]
- Pieroni A., Cattero V. Wild vegetables do not lie: comparative gastronomic ethnobotany and ethnolinguistics on the Greek traces of the Mediterranean Diet of southeastern Italy. Acta Botanica Brasilica. 2019;33:198–211. [Google Scholar]
- Pieroni A., Quave C.L., Villanelli M.L., Mangino P., Sabbatini G., Santini L., Boccetti T., Profili M., Ciccioli T., Rampa L.G. Ethnopharmacognostic survey on the natural ingredients used in folk cosmetics, cosmeceuticals and remedies for healing skin diseases in the inland Marches, Central-Eastern Italy. J. Ethnopharmacol. 2004;91:331–344. doi: 10.1016/j.jep.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Pratsinis H., Kletsas D. Special Issue: anti-aging properties of natural compounds. Cosmetics. 2019;6:67. [Google Scholar]
- Qian Y., Qiu X., Zhu S. Lignin: a nature-inspired sun blocker for broad-spectrum sunscreens. Green. Chem. 2015;17:320–324. [Google Scholar]
- Qian Y., Zhong X., Li Y., Qiu X. Fabrication of uniform lignin colloidal spheres for developing natural broad-spectrum sunscreens with high sun protection factor. Ind. Crops Prod. 2017;101:54–60. [Google Scholar]
- Quave C.L., Pieroni A., Bennett B.C. Dermatological remedies in the traditional pharmacopoeia of Vulture-Alto Bradano, inland southern Italy. J. Ethnobiol. Ethnomedicine. 2008;4:5. doi: 10.1186/1746-4269-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rian I.M., Sassone M. Tree-inspired dendriforms and fractal-like branching structures in architecture: a brief historical overview. Front. Arch. Res. 2014;3:298–323. [Google Scholar]
- Robinson M.K. Population differences in skin structure and physiology and the susceptibility to irritant and allergic contact dermatitis: implications for skin safety testing and risk assessment. Contact Dermatitis. 1999;41:65–79. doi: 10.1111/j.1600-0536.1999.tb06229.x. [DOI] [PubMed] [Google Scholar]
- Robinson M.K. Population differences in acute skin irritation responses. Contact Dermatitis. 2002;46:86–93. doi: 10.1034/j.1600-0536.2002.460205.x. [DOI] [PubMed] [Google Scholar]
- Rognoni E., Watt F.M. Skin cell heterogeneity in development, wound healing, and cancer. Trends Cell Biol. 2018;28:709–722. doi: 10.1016/j.tcb.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikia A.P., Ryakala V.K., Sharma P., Goswami P., Bora U. Ethnobotany of medicinal plants used by Assamese people for various skin ailments and cosmetics. J. Ethnopharmacol. 2006;106:149–157. doi: 10.1016/j.jep.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Schmid D., Suter F., Zülli F. An Opuntia cactus extract to treat sensitive and dry skin. Mibelle Biochem. Bolimattstrasse. 2020;1:5033. [Google Scholar]
- Schultz F., Anywar G., Wack B., Quave C.L., Garbe L. Ethnobotanical study of selected medicinal plants traditionally used in the rural Greater Mpigi region of Uganda. J. Ethnopharmacol. 2020;256:112742. doi: 10.1016/j.jep.2020.112742. [DOI] [PubMed] [Google Scholar]
- Scognamiglio J., Jones L., Letizia C.S., Api A.M. Fragrance material review on methyl jasmonate. Food Chem. Toxicol. 2012;50:S572–S576. doi: 10.1016/j.fct.2012.03.035. [DOI] [PubMed] [Google Scholar]
- Segot-Chicq E., Compan-Zaouati D., Wolkenstein P., Consoli S., Rodary C., Delvigne V., Guillou V., Poli F. Development and validation of a questionnaire to evaluate how a cosmetic product for oily skin is able to improve well-being in women. J. Eur. Acad. Dermatol. Venereol. 2007;21:1181–1186. doi: 10.1111/j.1468-3083.2007.02193.x. [DOI] [PubMed] [Google Scholar]
- Siddiqi K.S., Husen A. Plant response to engineered metal oxide nanoparticles. Nanoscale Res. Lett. 2017;12:92. doi: 10.1186/s11671-017-1861-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitaler R., Winkler A., Lins I., Yanar S., Stuppner H., Zidorn C. Altitudinal variation of phenolic contents in flowering heads of Arnica montana cv. ARBO: a 3-Year Comparison. J. Chem. Ecol. 2008;34:369–375. doi: 10.1007/s10886-007-9407-x. [DOI] [PubMed] [Google Scholar]
- Stephens N., Di Silvio L., Dunsford I., Ellis M., Glencross A., Sexton A. Bringing cultured meat to market: technical, socio-political, and regulatory challenges in cellular agriculture. Trends Food Sci. Technol. 2018;78:155–166. doi: 10.1016/j.tifs.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez A.L., Feramisco J.D., Koo J., Steinhoff M. Psychoneuroimmunology of psychological stress and atopic dermatitis: pathophysiologic and therapeutic updates. Acta Derm. Venereol. 2012;92:7–15. doi: 10.2340/00015555-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabassum N., Hamdani M. Plants used to treat skin diseases. Pharmacognosy Rev. 2014;8:52–60. doi: 10.4103/0973-7847.125531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taieb C., Rahhali N., Moingeon V., Perez-Cullell N., Sibaud V. CosmeceutiQoL: a tool for assessing dermo-cosmetic products’ impact on quality of life. J. Cosmet. Laser Ther. 2012;14:18–23. doi: 10.3109/14764172.2011.634420. [DOI] [PubMed] [Google Scholar]
- The IUCN Red List Of Threatened Species (n.d.), More than 30,000 species are threatened with extinction. Available: https://www.iucnredlist.org/.
- Tiku A.R. Antimicrobial compounds (phytoanticipins and phytoalexins) and their role in plant defense. In: Mérillon J., Ramawat K.G., editors. Co-Evolution of Secondary Metabolites. Springer International Publishing; 2020. pp. 845–868. [Google Scholar]
- Tohidpour A., Sattari M., Omidbaigi R., Yadegar A., Nazemi J. Antibacterial effect of essential oils from two medicinal plants against methicillin-resistant Staphylococcus aureus (MRSA) Phytomedicine. 2010;17:142–145. doi: 10.1016/j.phymed.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Valacchi G., Sticozzi C., Pecorelli A., Cervellati F., Cervellati C., Maioli E. Cutaneous responses to environmental stressors. Ann. N. Y. Acad. Sci. 2012;1271:75–81. doi: 10.1111/j.1749-6632.2012.06724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco M.V.R., Balogh T.S., Pedriali C.A., Sarruf F.D., Pinto C.A.S.O., Kaneko T.M., Rolim Baby A. Associação da rutina com p-Metoxicinamato de Octila e Benzofenona-3: avaliação In vitro da eficácia fotoprotetora por espectrofotometria de refletância. Lat. Am. J. Pharm. 2008;27:23–27. [Google Scholar]
- Voeks R.A. Are women reservoirs of traditional plant knowledge? Gender, ethnobotany and globalization in northeast Brazil. Singapore J. Trop. Geogr. 2007;28:7–20. [Google Scholar]
- Waring R.H., Mcdonald A.J.S., Larsson S., Ericsson T., Wiren A., Arwidsson E., Ericsson A., Lohammar T. Differences in chemical composition of plants grown at constant relative growth rates with stable mineral nutrition. Oecologia. 1985;66:157–160. doi: 10.1007/BF00379849. [DOI] [PubMed] [Google Scholar]
- Wilkinson J.A., Brown A.M.G. Horse Chestnut – aesculus Hippocastanum: potential applications in cosmetic skin-care products. Int. J. Cosmet. Sci. 1999;21:437–447. doi: 10.1046/j.1467-2494.1999.234192.x. [DOI] [PubMed] [Google Scholar]
- Wu G., Ruben M.D., Schmidt R.E., Francey L.J., Smith D.F., Anafi R.C., Hughey J.J., Tasseff R., Sherrill J.D., Oblong J.E. Population-level rhythms in human skin with implications for circadian medicine. Proc. Natl. Acad. Sci. U S A. 2018;115:12313. doi: 10.1073/pnas.1809442115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi X., Li J., Guo S., Li Y., Xu F., Zheng M., Cao H., Cui X., Guo H., Han C. The potential of using bee pollen in cosmetics: a review. J. Oleo Sci. 2018;67:1071–1082. doi: 10.5650/jos.ess18048. [DOI] [PubMed] [Google Scholar]
- Xie P., Huang L., Zhang C., Ding S., Deng Y., Wang X. Skin-care effects of dandelion leaf extract and stem extract: antioxidant properties, tyrosinase inhibitory and molecular docking simulations. Ind. Crops Prod. 2018;111:238–246. [Google Scholar]
- Yang L., Wen K., Ruan X., Zhao Y., Wei F., Wang Q. Response of plant secondary metabolites to environmental factors. Molecules. 2018;23:E762. doi: 10.3390/molecules23040762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazaki K., Arimura G., Ohnishi T. ‘Hidden’ terpenoids in plants: their biosynthesis, localization and ecological roles. Plant Cell Physiol. 2017;58:1615–1621. doi: 10.1093/pcp/pcx123. [DOI] [PubMed] [Google Scholar]
- Zhang C., Zheng H., Liu G., Hu F. Development and validation of HPLC method for determination of salicin in poplar buds: application for screening of counterfeit propolis. Food Chem. 2011;127:345–350. [Google Scholar]
- Zhang X., Wang N., Zhou Y., Liu Y., Zhang J., Chen H. Extractive electrospray ionization mass spectrometry for direct characterization of cosmetic products. Anal. Methods. 2013;5:311–315. [Google Scholar]
- Zheng Z., Cheng K., Zhu Q., Wang X., Lin Z., WANG M. Tyrosinase Inhibitory constituents from the roots of Morus nigra: a structure−activity relationship study. J. Agric. Food Chem. 2010;58:5368–5373. doi: 10.1021/jf1003607. [DOI] [PubMed] [Google Scholar]
- Zobolo A., Mkabela Q. Traditional knowledge transfer of activities practised by Zulu women to manage medicinal and food plant gardens. Afr. J. Range Forage Sci. 2006;23:77–80. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.