Abstract
Background
Bone grafts have been used to enhance bone fracture healing in orthopedic surgery. Bone grafts enhance bone healing either by mechanical support or acting as a scaffold for bone formation. Fresh autograft is the most effective biomaterial because it is histocompatible with less complication about transmissible disease. Hydroxyapatite is a well-established material for bone repair and very comparable to natural apatite providing a strong biomechanical interlock with host tissue. Royal jelly is the principal food for the honeybee queen. This biomaterial has been demonstrated to have several pharmacological activities, such as antiallergic, antitumor and anti-inflammatory effects.
Objectives
This study was design to evaluate the effect of concurrent using of Royal jelly with hydroxyapatite on bone healing in rabbit model.
Methods
15 adult rabbits weighting approximately 2 kg had been used. They were divided into three groups randomly. In first group (N = 5) mid radius bone defect created and left empty. The second group (N = 5) filled with hydroxy apatite alone and the last group (N = 5) filled with royal jelly and hydroxy apatite combination. Radiological evaluation performed on days14th, 28th and 42nd after operation. Histopathological evaluation was done on 56th postoperative day.
Results
Radiological evaluation showed significant superior bone healing in hydroxyapatite and hydroxyapatite-Royal jelly groups in comparison to control group. Control group was the inferior group between three groups. There were not any significant differences between three groups in histopathological group.
Conclusion
In conclusion our study showed the best results with using the hydroxyl apatite and Royal-jelly group because they provide not only scaffold for bone healing but also do, they provide some osteoinduction materials for bone healing.
Keywords: Biomedical engineering, Surgery, Hydroxyapatite, Royal jelly, Rabbit, Bone repair
Biomedical engineering; Surgery; Hydroxyapatite; Royal jelly, Rabbit; Bone repair.
1. Introduction
Nowadays, both veterinary and human orthopaedics apply bone graft to stimulate healing of fractures, speed up formation of joint connection and repair bone defects. New bone autografts are still considered as the gold standard for comparing other stimulating osteogenic factors.
In addition to the healing stimulators, the bone autograft contains cells that do not stimulate immune responses and cannot transmit contagious diseases [1]. In small livestock, bone autografts are obtained from the iliac crest, the inner surface of the upper Tibia and the upper end of humerus bones. Moreover, bone autografts, in human, are also collected from the iliac crest. However, this bone autograft collection results in some complications, such as pain, infections, fractures, blood loss, and increased number of surgical procedures; moreover, a limited amount of bone can be isolated [2]. Currently, due to problems associated with using bone autografts, the tendency to apply non-autografts, such as allografts and xenografts, has been increased. Some of the most important advantages of using non-autografts include the unlimited source for obtaining the grafts and the presence of bone healing stimulating cells and protein substances. Moreover, these types of grafts provide a mechanically supportive scaffold in large bone defects, such as tumor excision and bone loss [3]. However, application of allograft may result in a high risk for transmission of infectious diseases.
Synthetic hydroxyapatite, tricalcium phosphate and the combination of both are commonly used for bone grafting [4]. Hydroxyapatite presents an osteoconduction property and serves as a scaffold for the growth of osteogenic cells, however, it does not have an osteoinduction property [5].
Royal jelly is a white gelatinous substance with a sharp smell that is produced by the glands in the hypopharynx of the worker bees and is the main nutrient source for the queen bee and larvae. Royal jelly has a high nutritional value and has been traditionally used as a food supplement. It consists of water (50%–70%), protein (9%–18%), carbohydrate (7%–18%) lipid and fatty acid (3%–8%), mineral salts (1. 5%) and very small quantities of vitamins and polyphenols [6]. Royal jelly has antibacterial, antioxidant, anti-tumor, and anti-inflammatory effects [7, 8, 9]. Moreover, royal jelly is thought to improve age-related diseases, such as postmenopausal syndrome. Results of various studies performed in animal models have indicated that royal jelly causes vascular distension and can increase blood flow. Furthermore, although royal jelly has an anti-tumor effect, it enhances cell proliferation and differentiation rates. Few studies have investigated the effect of royal Jelly on bone metabolism and its related cellular activities. For instance, it has been reported that oral administration of royal jelly relatively resolved osteoporosis, which was experimentally induced by ovariectomy, in rats [10, 11]. Moreover, it has been shown to induce an estrogen-like effect in osteoblast culture medium [12, 13, 14]. It has been demonstrated that pure apisin which is one of the major royal jelly glycoproteins (MRJPs), enhances cell proliferation and collagen production of healthy neonatal dermal fibroblasts (NB1RGB). Furthermore, apisin improves the differentiation rate of MC3T3-E1 (a mouse osteoblastic cell line) According to these findings, royal Jelly possibly induces inductive effects (partially due to the presence of apisin on cell proliferation and differentiation. The aim of this study was to evaluate the osteogenesis properties of combined application of hydroxyapatite (as an osteoconductor) and royal jelly in bone defect healing process in rabbits.
2. Materials and methods
The experiment was performed under the approval of the State Committee on Animal Ethics, Shahrekord University, Shahrekord, Iran. Also, the recommendations of European Council Directive (86/609/EC) of November 24, 1986, regarding the protection of animals’ rights used for experimental purposes, were considered.
A total of 15 rabbits, which were initially subcutaneously injected with antiparasitic drugs, were used in this study. The rabbits were kept in new environment for 15 days and fed with standard plated food in order to adapt to the new condition.
The 15 rabbits were randomly divided into three groups of five. The bone defect of first group was not filled with any substances. The bone defect of the second group was treated by filling the defect with hydroxyapatite granules, while the third experimental group was treated by filling the defect with hydroxyapatite and 1 mg of royal jelly.
Fresh royal jelly was purchased from approved local bee fields. The natural products have different composition and quality between species and also in different seasons. The Apis mellifera royal jelly was collected in spring and was then characterized. The fresh royal jelly was freeze-dried immediately after purchase, to result in a white and soft powder that was kept away from light and humidity until use.
The rabbits were first anesthetized by intramuscular injection of ketamine 30 mg/kg and acepromazine 0.2 mg/kg followed by shaving their right hand and preparation for surgery. An incision was created on the anterior-internal surface of the radial bone, which was exposed by removing the soft tissues and muscles. A piece of the bone, twice its width (approximately 10 mm), was removed and the defect was filled with the respective materials. After completion of transplantation, first the muscles were sutured and the skin was then subcutaneously closed using 2-0 vicryl sutures. After their complete recovery from anesthesia, the rabbits were released in a cage without external stabilization. All the rabbits received daily doses of intramuscular penicillin (40,000 IU/day) and streptomycin (12 mg/kg) for 3 days post-operation. They were examined every day and the modality of using the hand were evaluated and recorded on a daily basis. Any local wound, inflammation, or lack of healing were noted and addressed.
Lateral view radiographs were obtained from the rabbits immediately after surgery and on days 14, 28, 42 post-operation. Radiographs were taken by Philips Xray machine with exposure parameters of 50 KVP, 8 mA and 0.14 s and the distance between the radiographic film and the X-ray source was about 70 cm and the radiographic device was set at 45 kV (KV) and 20 mA/s. Grid cassette (Fujifilm, USA) and standard radiography film were used. To evaluate and grade the radiographs, the Lane and Sandhu modified radiation scoring system was applied (Table 1). After 8 weeks of treatment, the rabbits were euthanized followedby creating an incision on the forearm and removing the muscles and soft tissues in order to reach the radius and ulna, which were then placed in 10% formalin. The bones were placed in fresh formalin solution after 24 h in order to increase formalin penetration into the tissue. Demineralization of the specimens were performed by immersing them in 15% formaldehyde acid for 2 weeks. The bones were then cut into 4 cm sized fragments, which included both the healthy and defected regions. Each specimen was stained with Hematoxylin and Eosin and evaluated by Emery's bone healing scoring system [15].
Table 1.
Radiograph scoring based on Lane, Sandhu system.
| Items | Score |
|---|---|
| Bone Formation | |
| No evidence of bone formation | 0 |
| Bone formation occupying %25 of defect | 1 |
| Bone formation occupying %50 of defect | 2 |
| Bone formation occupying %75 of defect | 3 |
| Bone formation occupying %100 of defect | 4 |
| Union (Proximal, Superior, Distal/Inferior) | |
| Non union | 0 |
| Possible union | 1 |
| Radiographic/full union | 2 |
| Remodelling | |
| No evidence of remodeling | 0 |
| Poor/weak remodeling | 1 |
| Full remodelling | 2 |
Statistical analyses were performed using the Kruskal-Wallis non parametric ANOVA. Since the obtained P values were less than 0.05, further statistical analyses were conducted by the Mann-Whitney U test.
In the present study, P values less than 0.05 (P < 0.05) were considered statistically significant. Statistical evaluations were performed using SPSS software (SPSS version 17 for Windows, SPSS Inc, Chicago, USA).
3. Results
No post-operative infections were observed in this study. None of the rabbits Limped after the surgery, and they returned to their normal state two days post-operation.
Radiographic evaluation of the recovery process was performed in all the groups on days 14, 28 and 42 post-operation. Scoring of the radiographs was performed in terms of bone formation, rate of upper and lower union, and bone remodeling (Table 2, Figures 1, 2, and 3).
Table 2.
Radiographical findings for healing of the bone defect (sum of the radiological scores) at various post-operative intervals.
| Postoperative weeks | Group 1 | Group 2 | Group 3 | pa |
|---|---|---|---|---|
| 2nd week | 2 (1–4)b,c | 8 (4–10) | 6 (5–8) | 0.024 |
| 4th week | 4 (2–5)d | 6 (4–6) | 8 (4–10) | 0.033 |
| 6th week | 3 (2–7)e | 6 (3–8) | 9 (4–10) | 0.1 |
Kruskal-Wallis non parametric ANOVA.
p = 0. 01 (compared with group 2 by Mann-Whitney U test).
p = 0. 02 (compared with group 3 by Mann-Whitney U test).
p = 0. 04 (compared with group 3 by Mann-Whitney U test).
p = 0. 04 (compared with group 3 by Mann-Whitney U test).
Figure 1.
Hydroxyapatite group Radiographs: A1 2nd week, A2 4th week, A3 6th week.
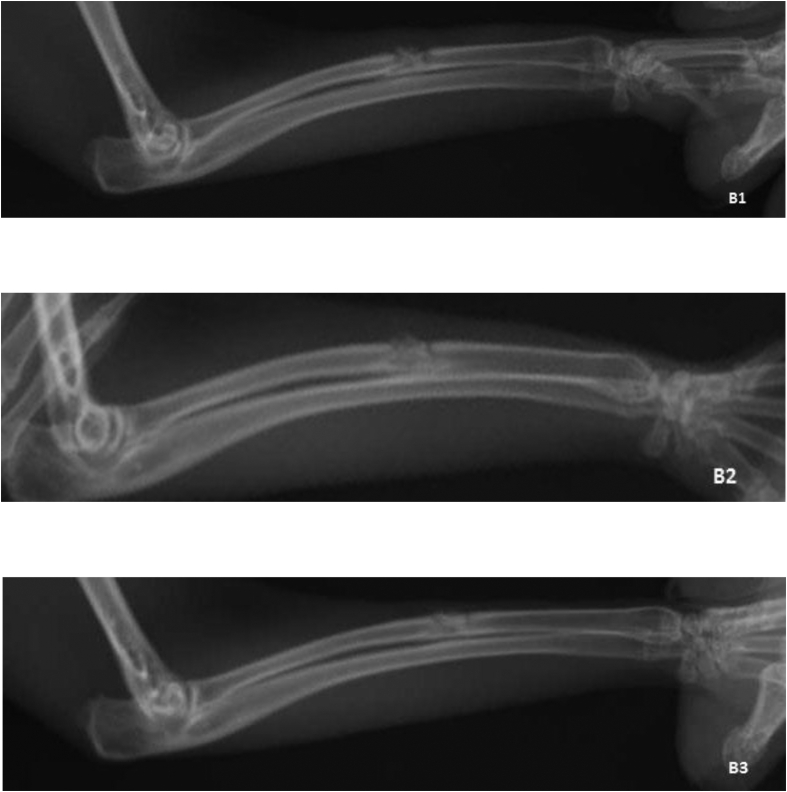
Figure 2.
Hydroxyapatite and Royal Jelly group Radiographs: B1 2nd week, B2 4th week, B3 6th week.
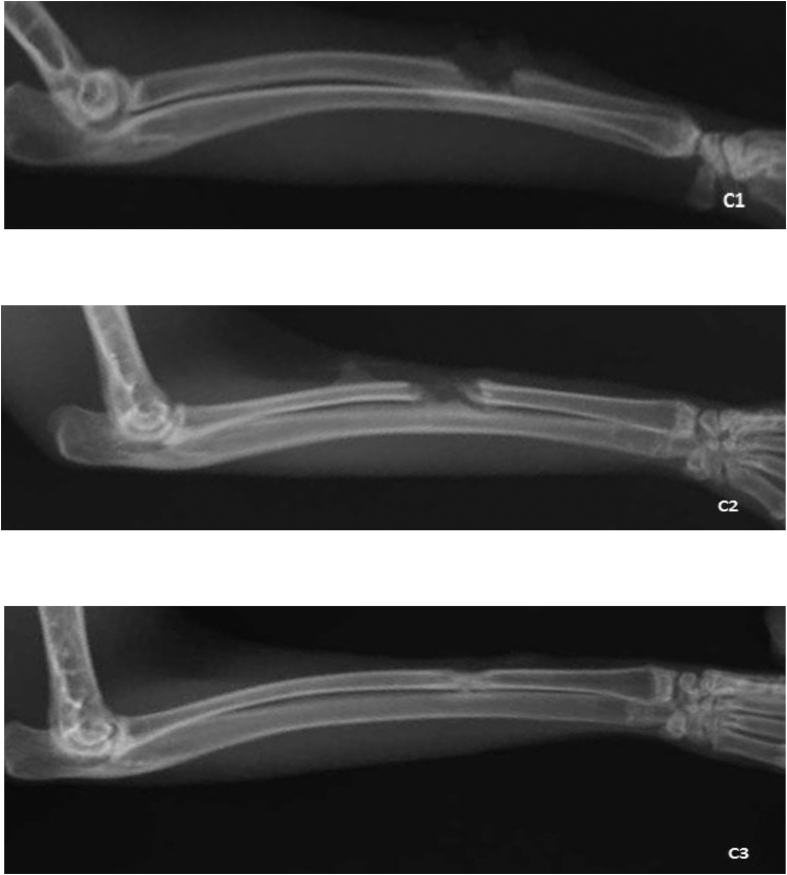
Figure 3.
Control group Radiographs: C1 2nd week, C2 4th week, C3 6th week.
The results indicated significant differences in bone formation, resorption and remodeling between the experimental groups 1 and 2 in the second post-operative week (p = 0.01), demonstrating that the rate of bone healing process was higher in group 2 compared to group 1. A significant difference in bone formation was detected between the groups 1 and 3 in the second post-operative week (p = 0.02). A significant difference in bone formation (p = 0.04) between the groups 1 and 3 in the fourth post-operative week. Moreover, a significant difference was detected between the groups 1 and 3 in the sixth post-operative week (p = 0.04). However, no significant difference was detected between the groups 2 and 3 during post-operative weeks (Table 2, Figures 1, 2, and 3).
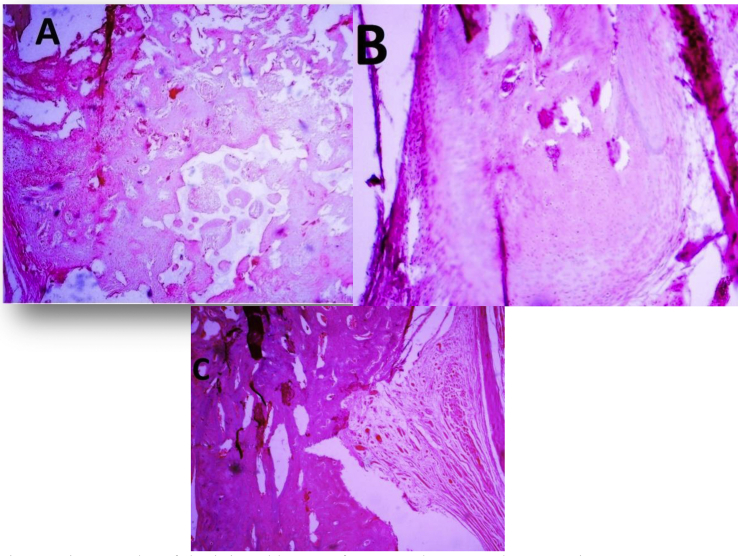
The transplanted samples were extracted 56 days post-operation and referred to the laboratory for histopathological examination. The obtained results were evaluated based on the Emery's bone healing scoring system. Histopathological evaluation indicated no sign of inflammation and infection in the specimens. No significant differences were detected in bone formation, resorption and remodeling between the three groups (Table 3, Figure 4).
Table 3.
Bone measurements at macroscopic and microscopic level.
| Bone type Evaluation based on Emery's scoring system |
Group 1 | Group 2 | Group 3 | pa |
|---|---|---|---|---|
| 3 (2–4) | 6 (5–6) | 4 (4–6) | 0.1 |
Kruskal-Wallis non parametric ANOVA.
Figure 4.
Micrographs of the injured bones after 8 weeks: A Hydroxyapatite group, B Hydroxyapatite and Royal Jelly group, C Control group. Regenerated bone with typical structure of trabecular bone is seen in the defect in the region. (hematoxylin and eosin staining).
4. Discussion
Since the haversian systems in long bones in rabbits are similar to those in the human, it is more appropriate to use rabbit as a model for human in order to investigate the effects of combined application of royal jelly and hydroxyapatite on bone healing process. The selected bone, for the purpose of this study in rabbit, was radius because removing a part of radius does not require applying a stabilizer due to the connection between radius and ulna; therefore, bone healing process could be directly investigated. The defect was created in the middle part of the radius and the length of the extracted part was twice the width of the bone to achieve a discrete fracture model [16].
In this study, the combination of royal jelly and hydroxyapatite was used in order to increase bone activity. The results of this study indicated that osteogenesis was faster in the royal jelly and hydroxyapatite-treated group compared to the hydroxyapatite-treated and the control groups.
Hydroxyapatite is calcium phosphate crystallized phase which creates a stable mechanical scaffold enhances the healing and angiogenesis in the fractured bone. It seems that hydroxyapatite has osteoconductive properties [17]. Moreover, royal jelly plays estrogen-like roles and induces chondriogenesis activity [18]. Therefore, the royal jelly and hydroxyapatite-treated group presented a faster bone healing rate than the hydroxyapatite-treated group. The radiographic findings of this study confirmed these results.
The results of this study demonstrated that combined application of royal jelly and a hydroxyapatite strongly and effectively affected bone healing process. These results indicated that the hydroxyapatite placed in the defected site induced reconstruction, acted as a scaffold in the bone space, enhanced cell differentiation into osteoblasts and scaffold attachment to the bone. Hence, the combined application of hydroxyapatite and royal jelly improved bone healing process. Histopathological findings showed no significant difference between the three groups [17, 18].
Bone formation in the hydroxyapatite-treated group was weaker than that of the royal jelly and hydroxyapatite-treated group, however, it was greater than that in the control group. Hydroxyapatite organized collagen fibers in the defected site, moreover, it controlled and led the bone healing process like a scaffold [17, 18]. Furthermore, the results of radiological studies demonstrated that hydroxyapatite improved bone repair compared to the control group.
According to the radiological and histopathological findings of this study, osteogenesis was weaker in the control group compared to both the experimental groups. In the control group, the defect was filled with fibrous tissue, and the bone and cartilage tissues were seldomly formed. This unorganized and undesirable bone repair in the control group was due to the inadequate chondrification at the defected site and the replacement with fibrous tissue. Discrete cartilaginous structure in the defected region resulted in the fibrocartilage formation between fractured fragments, which disturbed the bone healing process [19].
The present study applied radiological and histopathological evaluations in order to prove the osteogenic properties of hydroxyapatite and royal jelly.
Declarations
Author contribution statement
Amin Bigham-Sadegh: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Haleh Sadat Torkestani, Siavash Sharifi, Sadegh Shirian: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by Shahrekord University Research Council (96GRD1M1880).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
This paper was the result of a DVM thesis submitted to Shahrekord University in the field of Veterinary Medicine.
References
- 1.Fitch R., Kerwin S., Sinibaldi K., Newman-Gage H. Bone autografts and allografts in dogs. Compend. Continuing Educ. Pract. Vet. 1997;19(5):558–578. [Google Scholar]
- 2.Ferguson J. Fracture of the humerus after cancellous hone graft harvesting in a dog. J. Small Anim. Pract. 1996;37(5):232–234. doi: 10.1111/j.1748-5827.1996.tb01776.x. [DOI] [PubMed] [Google Scholar]
- 3.Dorea H., McLaughlin R., Cantwell H., Read R., Armbrust L., Pool R. Evaluation of healing in feline femoral defects filled with cancellous autograft, cancellous allograft or Bioglass. Vet. Comp. Orthop. Traumatol. 2005;18(3):157–168. [PubMed] [Google Scholar]
- 4.Behairy Y., Jasty M. Bone grafts and bone substitutes in hip and knee surgery. Orthopedic Clin. 1999;30(4):661–671. doi: 10.1016/s0030-5898(05)70118-8. [DOI] [PubMed] [Google Scholar]
- 5.Inoue K., Ohgushi H., Yoshikawa T., Okumura M., Sempuku T., Tamai S. The effect of aging on bone formation in porous hydroxyapatite: biochemical and histological analysis. J. Bone Miner. Res. 1997;12(6):989–994. doi: 10.1359/jbmr.1997.12.6.989. [DOI] [PubMed] [Google Scholar]
- 6.Hattori N., Nomoto H., Fukumitsu H., Mishima S., Furukawa S. Royal jelly and its unique fatty acid, 10-hydroxy-trans-2-decenoic acid, promote neurogenesis by neural stem/progenitor cells in vitro. Biomed. Res. 2007;28(5):261–266. doi: 10.2220/biomedres.28.261. [DOI] [PubMed] [Google Scholar]
- 7.Hidaka S., Okamoto Y., Uchiyama S., Nakatsuma A., Hashimoto K., Ohnishi T. Royal jelly prevents osteoporosis in rats: beneficial effects in ovariectomy model and in bone tissue culture model. Evid. base Compl. Alternative Med. 2006;3(3):339–348. doi: 10.1093/ecam/nel019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hosseini S., Delerezh N., Afzal Ahangaran N. The effects of royal jelly on in-vitro cytotoxicity of K562 cells and peripheral blood mononuclear cells. Armaghane danesh. 2014;18(11):869–878. [Google Scholar]
- 9.Kafadar İ.H., Güney A., Türk C.Y., Oner M., Silici S. Royal jelly and bee pollen decrease bone loss due to osteoporosis in an oophorectomized rat model. Eklem Hastalik Cerrahisi. 2012;23(2):100–105. [PubMed] [Google Scholar]
- 10.Mishima S., Suzuki K.-M., Isohama Y., Kuratsu N., Araki Y., Inoue M. Royal jelly has estrogenic effects in vitro and in vivo. J. Ethnopharmacol. 2005;101(1-3):215–220. doi: 10.1016/j.jep.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Moutsatsou P., Papoutsi Z., Kassi E., Heldring N., Zhao C., Tsiapara A. Fatty acids derived from royal jelly are modulators of estrogen receptor functions. PloS One. 2010;5(12) doi: 10.1371/journal.pone.0015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Münstedt K., Bargello M., Hauenschild A. Royal jelly reduces the serum glucose levels in healthy subjects. J. Med. Food. 2009;12(5):1170–1172. doi: 10.1089/jmf.2008.0289. [DOI] [PubMed] [Google Scholar]
- 13.Ramadan M.F., Al-Ghamdi A. Bioactive compounds and health-promoting properties of royal jelly: a review. J. Funct. Foods. 2012;4(1):39–52. [Google Scholar]
- 14.Sabatini A.G., Marcazzan G.L., Caboni M.F., Bogdanov S., Almeida-Muradian L. Quality and standardisation of royal jelly. J. ApiProduct ApiMed. Sci. 2009;1(1):1–6. [Google Scholar]
- 15.Emery S.E., Brazinski M.S., Koka A., Bensusan J.S., Stevenson S. The biological and biomechanical effects of irradiation on anterior spinal bone grafts in a canine model. J. Bone Jt. Surg. Am. Vol. 1994;76(4):540–548. doi: 10.2106/00004623-199404000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Oryan A., Bigham-Sadegh A., Abbasi-Teshnizi F. Effects of osteogenic medium on healing of the experimental critical bone defect in a rabbit model. Bone. 2014;63:53–60. doi: 10.1016/j.bone.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Appleford M.R., Oh S., Oh N., Ong J.L. In vivo study on hydroxyapatite scaffolds with trabecular architecture for bone repair. J. Biomed. Mater. Res. Part A: An Official Journal of The Society for Biomaterials. 2009;89(4):1019–1027. doi: 10.1002/jbm.a.32049. The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu S., Matsushita H., Minami A., Kanazawa H., Suzuki T., Watanabe K. Royal jelly does not prevent bone loss but improves bone strength in ovariectomized rats. Climacteric. 2018:1–6. doi: 10.1080/13697137.2018.1517739. [DOI] [PubMed] [Google Scholar]
- 19.Sadegh A.B., Basiri E., Oryan A., Mirshokraei P. Wrapped omentum with periosteum concurrent with adipose derived adult stem cells for bone tissue engineering in dog model. Cell Tissue Bank. 2014;15(1):127–137. doi: 10.1007/s10561-013-9383-z. [DOI] [PubMed] [Google Scholar]