Abstract
The evergreen forests of the Ecuadorian Amazon Region are altered by anthropogenic and natural factors. This provokes the need to look for tools that facilitate decision-making in support of restoration. We characterized the ecological structure and distribution of an evergreen Amazonian-Andean forest in the upper Puyo River micro-basin as a base for establishing an ecological quality index. The inventory was assessed using transects 0.1 ha (10 m × 100 m), registering the species with diameters at 1.30 m height ≥10 cm. We encountered a high degree of richness (30 families, 65 species and 322 individuals). The structural parameters indicated the predominance of a heterogeneous forest, with characteristics belonging to disturbed forests. A non-continuous vertical distribution pattern was found, determined by 78% of the species in a single stratum. The mixing ratio resulted in a higher proportion (1:5) for the lower class. The species Wettinia maynensis, Alchornea glandulosa, Miconia splendens, Piptocoma discolor and Inga velutina had the highest ecological value. The principal components analysis distinguished the abundance of species that had a high correlation with the sites and a distinct pattern was found in transect three. We developed the Structural Ecological Quality Index, which considers the social position of various species as well as their ecological importance, to reflect alterations in structural parameters. Abundance was concentrated in the 10–20 m strata (10–20 cm diameter class), which showed the presence of disturbance indicator species and a low ecological value. This indicated the real value of the historical succession of the forest, thus we recommend carrying out a restoration of this important reservoir.
Keywords: Amazonian forests, Micro-basin, Structural ecological quality index, Species of ecological importance, Heterogeneity patterns, Agroforestry, Applied ecology, Ecological restoration, Flora, Forestry, Environmental science, Ecology
Amazonian forests; Micro-basin; Structural ecological quality index; Species of ecological importance; Heterogeneity patterns, Agroforestry; Applied ecology; Ecological restoration; Flora; Forestry, Environmental science, Ecology
1. Introduction
Tropical forests are important in Ecuador due to the expanse of land they occupy, the ecological values they encompass, the multiple benefits and goods they produce and the fact that they are recognized as some of the richest in the world in terms of tree species (Yaduv et al., 2018). Amazon forests are part of the natural capital that serves as the subsistence of many local communities (Turner and Daily, 2008) and in turn provide ecosystem services such as the connectivity of fragmented landscapes, the regulation of water cycles and biodiversity conservation. These ecosystems are characterized by being rich in tree species, with multiple direct and indirect benefits as a source of timber and non-wood resources and differ in their characteristics in terms of composition and structure (Colín, 2002; Murthy et al., 2016).
In this context, studies on forest composition, structure and dynamics represent an initial step towards gaining knowledge of these forests (Aguirre and Pollito, 2011; de Castilho et al., 2006; Torres et al., 2019). Associated with this initial step, a theoretical basis can be built to support the practices of management and conservation of forest resources as a starting point for the determination of criteria, conservation and recovery methods (Garavito et al., 2012). Vegetation structure patterns and ecosystem processes have been identified as essential components for the long-term survival of natural systems (Ruiz-Jaén and Aide, 2005). Knowledge of the vegetation's structure and composition provides important information on species that are more susceptible to disturbances in a given region and helps to predict succession patterns (Alonso-Torrens et al., 2016; Templeton et al., 2019) and make decisions about the application of management techniques (Nascimento et al., 2001; Nordström et al., 2019).
Amazonian evergreen forests are characterized by a complex and poorly understood structure (Dueñas et al., 2007), thus studies are needed to understand the behavior of patterns of ecological and spatial heterogeneity structure. These patterns suffer alterations, mainly due to deforestation practices, changes in land use, the expanding agricultural frontier and cattle ranching (Bravo-Medina et al., 2017; Caballero et al., 2018; Qin et al., 2017; Rasmussen et al., 2017). Human exploitation across a gradient of increasing intensities alters composition, landscape structure, habitat quality and ecosystem services (DeFries et al., 2002; Malhi et al., 2008; Vera-Vélez et al., 2019). The forest ecosystems that make up the upper Puyo River micro-basin have not been heavily studied. Moreover, these ecosystems are vulnerable, since human activities related to agriculture and cattle ranching bring about the progressive degradation of this reservoir that forms part of the important functions and services derived from the micro-basin (MAE, 2012). This constitutes a threat to the integrity of the ecosystem, its services, biodiversity, structure and hydrological functions. Hence, the objective of this work was to characterize the patterns of ecological structure and species distribution as a criterium for defining a structural ecological quality index in an evergreen Amazonian-Andean forest in the upper Puyo River micro-basin. This new index has a wide application in forest ecology as it provides real value as a measure one can take to evaluate the current state of a forest in succession. It provides relevant information for decision-making regarding the restoration of degraded ecosystems, which aids the conservation of Amazonian biodiversity.
2. Materials and methods
2.1. Location
The study was conducted of an evergreen Amazonian-Andean peri-urban forest in the upper Puyo River micro-basin. This is found within the hydrographic demarcation of the Pastaza River where the water catchment of the Pindo Mirador Biological Station is located, in the foothills of the community named 24 de May and within the buffer zone of the Llanganates National Park, Mera canton, Pastaza province, Ecuador (Figure 1).
Figure 1.
Geographical location of the study area comprising an evergreen foothills forest in the upper Puyo River micro-basin, Pastaza, Ecuador, South America.
2.2. Study area characteristics
The predominant bioclimatic conditions vary between humid and hyper-humid rainforests (MAE, 2012), with an average annual temperature of 23 °C and an annual rainfall of 411.9 cm. The lowest precipitation occurs from January to April, while the highest occurs from May to July and the temperature varies throughout the year. The average altitude where the sampling areas were established was 1228.72 m above sea level (masl). The geographic and physiographic characteristics of the study areas are presented in Table 1. The coordinates, altitude and gradient were taken with a GPS RTK of 1 cm precision.
Table 1.
Geographical and physiographical characteristics and degree of disturbance of five transects in an evergreen foothills forest.
| Transect | Geographic coordinates |
Altitude |
Gradient |
Level of disturbance | |
|---|---|---|---|---|---|
| X | Y | (masl) | (%) | ||
| T1 | 825462 | 9839971 | 1122.4 | 20 | High |
| T2 | 825488 | 9840077 | 1221.3 | 15 | High |
| T3 | 825581 | 9840022 | 1234.8 | 10 | Low |
| T4 | 825822 | 9830132 | 1272.6 | 45 | Medium |
| T5 |
825749 |
9840209 |
1292.5 |
30 |
Medium |
| Total | |||||
2.3. Floristic list
The Gentry transect model (Gentry, 1982) was adapted and used to perform a floristic inventory. Thus, five permanent transects of 0.1 ha (10 m × 100 m), oriented in the direction of the maximum slope separated by intervals of 100 linear meters were implemented, considering logistical, economic and precision factors (Schreader et al., 1983; Tomppo et al., 2011) for this type of periurban forest; where all species ≥10 cm in diameter at 1.30 m height (DBH) were registered. Botanical identification was made possible with the collaboration of local botanists, the accuracy of species names was assessed by cross-referencing the original dataset with current determinations of collections stored at the Herbarium ECUAMZ from Universidad Estatal Amazónica and confirmed by specialized platforms accessible through the Tropicos database (http://www.tropicos.org); The Plan List (TPL) (http://www.theplantlist.org/) and the classification system proposed by the Angiosperm Phylogeny Group (APG, 2003).
In addition, systematic sampling was used to install the transects by considering the accessibility, topography and lack of floristic information about the study area, based on satellite images, orthophotos from SIGTIERRAS and topographic maps at a scale of 1:50,000 from the Military Geographic Institute (IGM for its Spanish initials) and also considering the experiences of similar studies carried out by Torres et al. (2019) and Patiño et al. (2015) along the altitudinal gradient of an evergreen foothills forest in the Amazon Region.
The disturbance was evaluated by direct observation in the transects (Table 1), whereby a value of 1 represented no disturbance, 2 meant a slight disturbance, 3 meant moderate disturbance and 4 was high disturbance. The types of disturbance were: selective logging, extraction of firewood, presence of early successional species, extraction of non-timber forest products, and forest clearings by landslides or the effects of the wind. With this information, three levels of disturbance were established (high, medium and low), based on those established by González et al. (2016). The level was considered high when the disturbances were severe (at least 4 disturbances with scores of 4), medium when they are moderate (3 disturbances that scored less than 2) and low when disturbances were scarce or absent.
2.4. Structural parameters
From the inventory information parameters associated with the vertical and horizontal structure, the vertical structure was analyzed according to height range, for which we employed the relative social position index (RSPI) (Hernández and Giménez, 2016). The horizontal structure was described through the distribution of diameter classes where the mixing ratio (MR) and ecological importance value index (IVI) were calculated (Melo and Vargas, 2003). To define height range, the following three strata, were established according to the criteria of Ibarra and López (2002): lower stratum (h ≤ 10 m); middle stratum, in which h was found in a range of 10–20 m; and upper stratum (h > 20 m). The diameter classes were established with 10 cm width ranges, where the number of individuals belonging to each class was grouped according to outer limits. This was achieved using the methodology described by (López-Pérez et al., 2014).
The RSPI of each tree species was determined using Finol's methodology (Finol, 1976) by considering the total number of individuals for each substratum. For this calculation, the phytosociological value of the substratum was needed, which was obtained using VF = n/N, where VF: phytosociological value of the substratum; n: number of individuals from the substratum and N: total number of individuals from all species. With this value, the absolute social position of the species was calculated through PSa = VF(l)∗n(l)+VF(m)∗n(m)+VF(u)∗n(u), where PSa: absolute social position; VF(l), VF(m), VF(u): phytosociological value of lower, middle and upper substratum, respectively; n(l), n(m), n(u): number of individuals in the lower, middle and upper substratum, respectively. The RSPI was obtained from the percentage of each species as a function of the total sum of the absolute values. This index reports the decline of community species driven by disturbances (Hernández and Giménez, 2016), low reproductive potential and the predominant vertical distribution pattern (Melo and Vargas, 2003). The MR was calculated by: MR = Number of species/Number of trees (Kees and Michela, 2019). This expressed the degree of homogeneity or heterogeneity in the floristic composition (Bascopé and Jorgensen, 2005).
The IVI was obtained using the sum of the phytosociological parameters based on the criteria of (Melo and Vargas, 2003). This was calculated as follows: IVI = RA + RD + RF, where IVI: importance value index; RA: relative abundance; RD: relative dominance and RF: relative frequency. Relative abundance was calculated thus: RA= (n/N)∗100, where n: Number of individuals of each species; N: Total number of individuals. Relative dominance was found by RD=(Ga/GT)∗100, where Ga: basal area of each species and GT: total basal area. The basal area was determined as Ga=(π/4)∗(d1.30)2. The relative frequency was obtained by considering that: RF=(aF/TF)∗100, where aF: absolute frequency and TF: total frequency. This index allowed us to identify the species with the highest ecological value in the forest and the existence of disturbance indicator species (Catalá, 2011; Gordo, 2009). The aforementioned indices provide relevant elements of forest structure, however, it was important to develop a new index that provides complete information on ecological succession.
2.5. The new structural quality index
The new index was made possible by evaluating on a scale of 1–3 the following parameters: relative ecological position, ecological importance value index, mixing ratio and abundance of species in disturbed places (ASDP). As regards REP, a score of 1 was given when more than 60% of the species were present in a single stratum, 2 was given if 40–60% of the species were found in a single stratum and 3 meant there was a proportional relationship of the number of species present in the three strata. When the value assigned was lower, this reported the species alteration in the vertical structure of the community (Camacho and Plonczak, 2012; González et al., 2016) as a result of the disturbances. In relation to the IVI, a score of 1 was given when more than two species of secondary forest occupied high ecological positions, in which this position was considered from the registration of the ten first species with greater ecological, value 2 was scored if at least one species of secondary forest occupied a high ecological position and 3 if no species of secondary forest were found within the high positions. The category of secondary forest species was granted for those species that had little variation in diameter distribution, a low basal area, a regular canopy height and a high abundance in cleared areas. The lowest value of this component indicated the low ecological value of the most important species represented in the stands (Melo and Vargas, 2003). For MR, the score of 3 was given when more than 50% of the diameter classes resulted in a ratio greater than 1:3, 2 if 40–50% of the diameter classes resulted in a mixing ratio between 1:3 and 1:5, and 1 if less than 40% of the diameter classes were a ratio of 1:3 and 1:5. Low values were assigned to measure the result of a heterogeneous environment that has suffered alterations due to the historical succession of the stands (Aguirre-Mendoza et al., 2018; Melo and Vargas, 2003). Lastly, the ASDP was rated 3 when no disturbance indicator species were reported in the evergreen forest area, 2 if one to three were present and 1 if there were more than three disturbance indicator species. The lowest value of ASDP was assigned to estimate the scarce impact that ecological succession has had on species richness in the community (Benítez-Malvido and Martínez-Ramos, 2003; Patiño et al., 2015; Silva et al., 2010; Villa et al., 2019). The maximum score for the four structural parameters (3) corresponded to the desired state for the ecological structure of the evergreen Amazonian-Andean forest of the Puyo River micro-basin and the lowest score (1) when alterations and disruption in the ecological structure occurred.
The new structural quality index was expressed in relative terms and ranged from 1 to 3, in which 3 signified a forest with high structural ecological quality, because it maintains its structure and is well preserved, so does not require restoration activities. A score of 2 denoted a forest with medium structural ecological quality, due to the progressive alteration of its structural parameters and, therefore, restoration activities are necessary. A score of 1 indicated low structural ecological quality due to the total or partial rupture of its structural parameters, so it requires restoration activities. A new ecological index called Structural Ecological Quality Index (SEQI) was calculated by means of the following equation: SEQI = (REP + IVI + MR + ASDP)/4. This index provides a real value as a measure one can take to evaluate the successional state of a forest, which facilitated making decisions regarding restoration needs.
2.6. Information processing
With the data on the abundance of species for each transect, a hierarchical dendrogram was carried out, based on the Bray-Curtis measures, which allowed for the classification of ecological groups. This was made possible by the statistical program SPSS ver. 22.0, which allows one to calculate diversity measures for a sample data set. The information regarding species matrix and study transects was processed by analyzing the main components using the CANOCO version 5.0 ecological program. The purpose of this was to determine which species contributed most to the abundance of each transect and to measure correlations between study areas.
3. Results
The results of the inventory reported a total of 30 botanical families, 65 species and 322 individuals in the area of 0.5 ha (For more details, see Supplementary Material 1). The floristic list included the presence of many families with low species representations. 15 families, which made up 50% of the total inventory, were represented by a single species (Araliaceae, Asteraceae, Boraginaceae, Celastraceae, Cyatheaceae, Elaeocarpaceae, Lamiaceae, Lecythidaceae, Phyllanthaceae, Proteaceae, Rosaceae, Rutaceae, Sapotaceae, Sapindaceae and Siparunaceae). The families with the largest number of species were: Fabaceae (8), Lauraceae (5) and Urticeaceae (5), which represented 27.69% of the total species. The families with the highest number of individuals were Arecaceae (101), Euphorbiaceae (58) and Melastomataceae (33), together accounting for 59.63% of the total. The three species with the highest number of individuals in all five transects were: Wettinia maynensis (98), Alchornea glandulosa (50) y Miconia splendens (25).
The average distribution of individuals with diameter at 1.30 m height d1.30 ≥ 10 cm by height and diameter classes was irregular (Figure 2, a and b). The height behavior of the trees resembled a bell-shaped curve with values between 4 and 30 m. The height range indicated that the intermediate stratum (10–20 m) was the most represented (45 average of individuals/0.5 ha), followed by the lower stratum (≤10 m) (18 average of individuals/0.5 ha), and finally the upper stratum (>20m) (1.4 average of individuals/0.5 ha). In the lower stratum, there were more individuals in the 8–10 m class and fewer in the 4–6 m class. In the middle stratum, the largest number of individuals was in the 10–12 m range and the smallest in the 18–20 m class. In the upper stratum, the maximum number of trees was found in the >24 cm class and the smallest in the 22–24 m class.
Figure 2.
Distribution of the average number of individuals by height class (a) and diameter class (b) in five 0.5 ha transects of an evergreen foothills forest.
The distribution of individuals per diameter class showed a behavior similar to a curve in the form of an inverted J. It is notable that the largest number of individuals occurred in the lower diameter class (10–20 cm). It was found that the trees showed a tendency to decrease the number of individuals as diameter at 1.30 m height (d1.30) increased. In the first diametric class (10–20 cm), the distribution of average number of individuals was 42.4; in the second (20–30 cm), it was 12.2; in the third (30–40 cm), it was 5.4; in the fourth (40–50 cm), it was 2.8; in the fifth (50–60 cm) it was 1; in the sixth (60–70 cm), it was 0.4; in the seventh (70–80 cm), it was nil; and in the eighth (>80 cm), it was 0.2.
Using the dispersion diagram, we verified the point distribution trend in a concentrated manner, which denotes the absence of defined clusters. The trees' behavior displayed a continuous stratum without demarcation of substrata (Figure 3). The dominant trees in this forest, with values above the average tree mass, reached heights of more than 22 m. Only three species (Alchornea glandulosa, Dacroydes olivifera and Inga velutina) were dominant trees with few individuals (7) (highlighted in red). This forest was distinguished by the predominance of the social class of co-dominant trees (trees forming the general level of the tree canopy), although the presence of suppressed trees (trees with crowns below the general level of the tree canopy) is notable.
Figure 3.
Scatter plot showing the distribution trend of trees in relation to total height and crown insertion height of an evergreen foothills forest wit dominant trees highlighted in red.
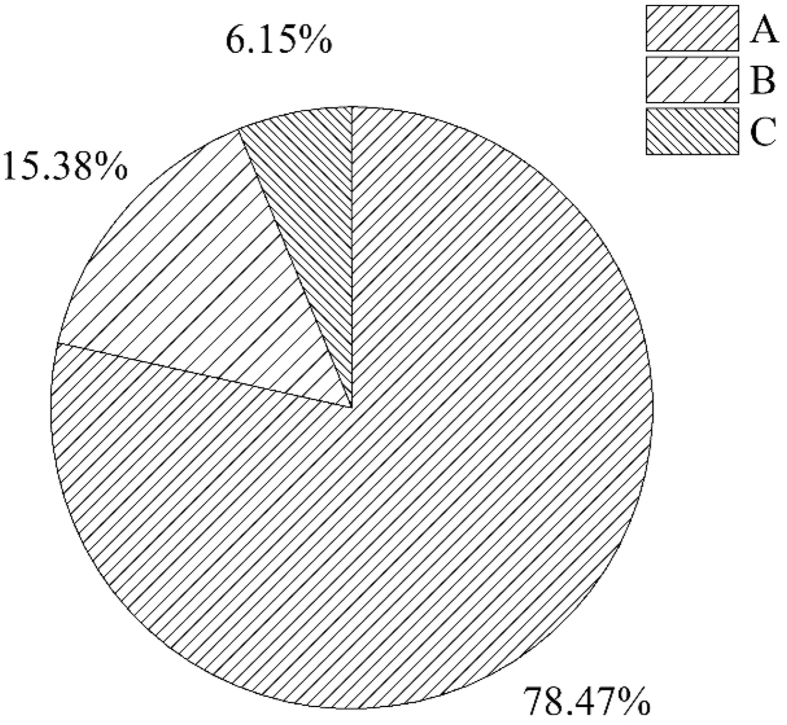
According to the relative social position index (RSPI), the species Wettinia maynensis, Alchornea glandulosa, Inga velutina, Miconia spendens, Piptocoma discolor y Dacroydes olivifera, had higher values than the other species in the floristic list (68.75; 15.41; 3.82; 3.72; 3.10 and 2.45%, respectively, for more details, see Supplementary Material 2). The species Wettinia maynensis, Alchornea glandulosa, Inga velutina y Dacroydes olivifera presented continuous vertical distribution (presence in the three substrata). In contrast, we also found a large amount of unrepresentative species, which were present in only one substratum and had low RSPI (Cecropia marginalis, Cecropia sciadophylla, Siparuna poeppigii, Casearia arborea, Zanthoxylum riedelianum, Chimarrhis glabriflora, Hieronyma alchorneoides, Roupala montana, Sorocea pubivena, Ficus paraensis, Guarea purusana, Guarea kunthiana, Calyptranthes bipennis, Aniba hostmanniana, Aegiphila cordata, Nectandra cissiflora, Inga nobilis, Stryphnodendron porcatum, Lonchocarpus seorsus, Maytenus macrocarpa, Protium sagotianum, Unonopsis veneficiorum, Rollinia chrysocarpa and Duguetia hadrantha). The highest percentage of species (78.46%) was present in a single stratum (51 species), 15.38% were shared between two strata (10 species) and only 6.15% of the species were present in all three strata (4 species) (Figure 4).
Figure 4.
Percentage of species represented in each forest substratum as a measurement for defining the vertical distribution pattern. Species present in one stratum (A), species present in two strata (B), and species present in three strata (C).
The mixture ratio reported that as diameter at 1.30 m height (d1.30) decreases, the value tends to increase, reaching a maximum of 4.71. The mixing ratio resulted in a higher proportion (1:5) for the diameter class (10–20 cm), meaning that for every five individuals, it is possible to find a different species. In the upper classes (>60 cm), this proportion was lower and indicated a greater heterogeneity of species (Table 2).
Table 2.
Mixing ratio (relationship between species and individuals) by diameter classes in a 0.5 ha area of an evergreen foothills forest.
| Diameter Class (cm) | Number of Species | Number of Individuals | Mixing Ratio |
|---|---|---|---|
| 10–20 | 45 | 212 | 4.71 (1:5) |
| 20–30 | 26 | 61 | 2.34 (1:2) |
| 30–40 | 9 | 27 | 3.0 (1:3) |
| 40–50 | 8 | 14 | 1.75 (1:2) |
| 50–60 | 3 | 5 | 1.66 (1:2) |
| 60–70 | 2 | 2 | 1.0 (1:1) |
| 70–80 | 0 | 0 | 0.0 (0:0) |
| >80 | 1 | 1 | 1.0 (1:1) |
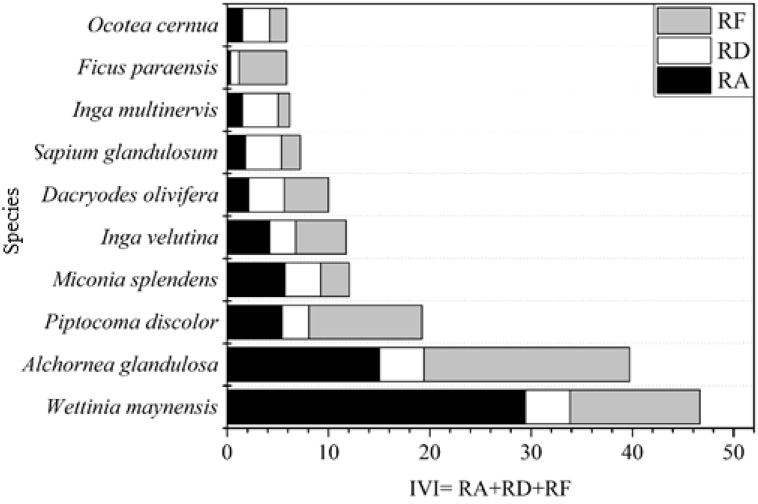
Among the ten species of greater ecological value, which represented 15.38% of the flora present, Wettinia maynensis, Alchornea glandulosa and Piptocoma discolor (Figure 5) occupied the first three positions with IVI values of 46.65, 39.73 and 19.22, respectively. A variation in abundance, dominance and frequency was discovered. The three species stood out due to their high frequency and, fundamentally, for their high abundance, together making up 28% of the importance value. The high ecological position occupied by floristic elements that typify events of ecological succession, determined by their high abundance (Piptocoma discolor, Miconia splendens, Inga velutina and Inga multinervis) is significant. Similarly, the species Dacroydes olivifera, Sapium glandulosum and Inga multinervis were distinguished by their dominance (trees with large dimensions). The rest of the species (55), which represented 84.62% of the flora found, presented an IVI of less than four, reflecting their scarce representation in the physiognomy of the forest.
Figure 5.
Representation of the ten species of greatest ecological importance based on abundance, dominance and relative frequency in the upper area of the evergreen foothills forest. IVI (ecological importance value index), RA (relative abundance), RD (relative dominance), RF (relative frequency).
The principal component analysis (PCA) resulted in eigenvalues of 0.36 and 0.27 for the first two axes, and a high inertia value (51.85), which indicated a high correlation between sampling units and abundance of species. Between the first two axes, 64% of the total variance was explained and only the first component 36% of the total variability of the data (Table 3). The spatial arrangement diagram (Figure 6 a) described a pattern of spatial distribution determined by abundance of species, showing a low correlation between species grouped in T5, T1 and T4 (they formed an approximate angle of 90 °). In these sites, the predominance of rare species was reported due to their scarce presence (Cordia panamensis, Duguetia hadrantha, Guarea kunthian, Guarea purusana, Endlicheria serícea, Eschweilera caudiculata, Hieronyma alchorneoides, Isertia laevis, Matisia longiflora and Maytenus macrocarpaen), and these also contributed to the species richness. Through the hierarchical dendrogram, with 50% similarity in abundance of species (Figure 6 b), two ecological groups were identified, one consisting of T1, T2, T4 and T5 and the other of T3. The two groups were differentiated for their physiognomy, ecological structure, floristic composition, conservation status, topography and location in the study area.
Table 3.
Results of the explained variance of the Principal Component Analysis showing the axis correlation between sampling units and species abundance.
| Axes | 1 | 2 | 3 | 4 | Total Variance (Inertia) |
|---|---|---|---|---|---|
| Values | 0.3658 | 0.2749 | 0.2049 | 0.1544 | 51.85559 |
| Explained variance (accumulative) | 36.58 | 64.07 | 84.56 | 100.00 |
Figure 6.
Spatial arrangement diagram based on the Principal Component Analysis showing the distribution of species in the study transects (a), and hierarchical dendrogram with the classification of vegetation groups according to species abundance (b). Laetia procera (LaetProc); Inga ilta (IngaIlta); Sapium glandulosum (SapiGlan); Cecropia membranácea (CecrMemb); Aegiphila cordata (AegyCord); Cyathea lasiosora (CyatLasi); Chimarrhis glabriflora (ChimGlab); Aniba hostmanniana (AnibHost); Cecropia sciadophylla (CecrScia); Allophylus floribundus (AlloFlor); Piptocoma discolor (PiptDisc); Miconia dielsii (MicoDiel); Ficus paraensis (FicuPara); Cecropia ficifolia (CecrFici); Nectandra membranacea (NectMemb); Ocotea cernua (OcotCern); Bactris setulosa (BactSetu); Tovomitopsis membranacea (TovoMemb); Calyptranthes bipennis (CalyBipe); Inga nobilis (IngaNobi); Inga venusta (IngaVenu); Ficus trigona (FicuTrig); Guatteria guianensis (GuatGuia); Roupala montana (RoupMont); Alchornea glandulosa (AlchGlan); Miconia splendens (MicoSple); Rollinia chrysocarpa (RollChry); Prunus debilis (PrunDebi); Siparuna poeppigii (SipaPoep); Sloanea meianthera (SloaMeia); Protium sagotianum (ProtSago); Sapium marmieri (SapiMarm); Cordia panamensis (CordPana); Trichilia pallida (TricPall); Guarea purusana (GuarPuru); Stryphnodendron porcatum (StryPorc); Unonopsis veneficiorum (UnonVene); Lonchocarpus seorsus (LoncSeor); Guarea kunthiana (GuarKunt); Duguetia hadrantha (DuguHadr); Wettinia maynensis (WettMayn); Sterculia colombiana (SterColo); Oreopanax palamophyllus (OreoPala); Calliandra trinervia (CallTrin); Hieronyma alchorneoides (HierAlch); Isertia laevis (IserLaev); Nectandra cissiflora (NectCiss); Sorocea pubivena (SoroPubi); Cecropia marginalis (CerMa); Tovomita weddelliana (TovoWeda); Casearia arborea (CaseArbo); Pouteria torta (PoutTort); Matisia longiflora (MatiLong); Endlicheria sericea (EndlSer); Psychotria cuspidulata (PsyCusp); Maytenus macrocarpa (MaytMarc); Inga multinervis (IngaMult); Eschweilera caudiculata (EschCaud); Miconia rivalis (MicoRiva); Dacryodes olivifera (DacrOliv); Inga velutina (IngaVelu); Pourouma tomentosa (PourTome); Quararibea cordata (QuarCord); Duguetia spixiana (DuguSpix).
It was shown that T5 had the greatest abundance of species (size of circles, Figure 7), followed by T4 and T1, then T2 and finally T3. The transects T1 y T2 had greater disturbance and at the same time greater abundance of species but were, for the most part, dominated by intermediate pioneer species, typical of forest clearings or open areas. Notable species there included those from the genera Cecropia, Pourouma, Piptocoma, Inga and Miconia. On the other hand, T3 was the area with the highest conservation status. Few species were evidenced and they were mainly species of primary forest, such as Wittinia maynensis, Bactris setulosa and Sterculia colombiana. The species that showed the greatest abundance were Wittinia maynensis, Alchornea glandulosa, Miconia splendens, Piptocoma discolor, Inga velutina and Dacroydes olivifera.
Figure 7.
Distribution of species abundance in study transects by spatial management analysis (An increase in the size of the circles indicates a greater number of species).
The structural ecological quality index proposed by the authors of this research project resulted in a value of 1 on a scale of 1–4. This result is due to the fact that more than 60% of the species reported in the inventory were present in a single stratum (78% of species in a single stratum according to Figure 4). More than two species of secondary forest occupied a high ecological position, taking into account the first ten ecological positions (four species reported with high IVI in Figure 5). The mixing ratio reported that less than 50% of the diameter classes presented a proportion greater than 1:3 (only two diameter classes of the eight reported in Table 1 presented a ratio greater than 1:3, which reflected 25% of the total diameter classes). Furthermore, the abundance of species in areas with disturbances reflected that at least three disturbance-indicating species abound in disturbed areas (the following genera were found: Cecropia, Pourouma, Piptocoma, Inga and Miconia, Figure 7).
4. Discussion
The floristic composition of the upper zone of the evergreen foothills forest of the Puyo river micro-basin, represented in the floristic list table, indicated a characteristic pattern of tropical humid forests (da Luz et al., 2019; Louman et al., 2001), where there is a high richness of species and low abundance. The disproportionate distribution in terms of number of families, individuals and species in the evergreen foothills forest in the upper Puyo River micro-basin is due to the levels of disturbance and the state of ecological succession (Cascante, 2001; Condit et al., 1996; Gentry, 1988; González et al., 2016; Yepes and Villa, 2010), that was evidenced in the study areas. The pattern of richness found in this study confirmed what has been reported in general for the tree communities of the Amazon rainforest (Cintra et al., 2005), where few species are abundant.
The results obtained in terms of floristic composition and ecological structure in the evergreen foothills forest in the upper Puyo River micro-basin are encouraging, because there are no up-to-date floristic reports in this important forest area. It is recognized for its significant protective and hydrological functions, subject to high rates of wood extraction and sensitive to endogenous factors such as landslides and heavy rains that cause the tallest trees to fall. These factors are responsible for the forest's state of ecological succession and its complex floral composition. The floristic composition at the level of botanical families determined that it is a forest in which many species dominant. These species are represented by few individuals, which is in line with Gordo (2009) and Ter Steege et al. (2013), who reported that in this type of ecosystem, one fundamental characteristic is the large number of species represented by few individuals, with complex spatial patterns. It is also consistent with the data obtained by various authors in Amazonian evergreen forests where the Arecaceae and Euphorbiaceae families are presented as the most abundant, indicating a good correspondence between genera and abundant families. The high representativeness of the Arecaceae family was due to the abundance of palm species. This is consistent with the richness reported in Amazonian ecosystems by various authors (Patiño et al., 2015; Pintaud et al., 2008; Svenning, 2001; Torres et al., 2019; Villacis et al., 2017), where the presence of Wettinia maynensis was found to be highly distributed in all the sites of our study. In this study, the Melastomataceae family appears as one of the most represented, distinguished by a group of perennial shrubs and arboreal species (Palacios, 2016) that appear to be opportunistic in places where there is a high disturbance, as in this study.
The irregular distribution reported for tree height and diameter, which is an indicator of the heterogeneity present in this Amazonian forest ecosystem (Cintra et al., 2005; de Castilho et al., 2006; Freitas et al., 2019; Lutz et al., 2013). The high density of individuals in the middle and lower strata are consistent with those reported by various authors (Aguirre-Mendoza et al., 2018; Dueñas et al., 2007; López-Hernández et al., 2017), who mention a greater number of individuals in the lower strata, with a characteristic pattern determined by a progressive decrease in the number of individuals as height increases. The differences observed in the height classes reflect the characteristic physiognomy of the evergreen Amazonian foothills forest in the Puyo River micro-basin, characterized by trees between 10 and 20 m tall, which means a variable vertical structure that can respond to the dynamics of clearing. This coincides with Cortés et al. (2009) who stated that variation in height of trees is due to the dynamics of clearing in mountainous ecosystems with high-altitude slopes and marked floristic differences between the forest's strata. These results indicated the state of succession of the vegetation patches, due to the presence of natural and anthropic factors. The trends observed are similar to those reported by Jadán et al. (2016) and Aguirre-Mendoza et al. (2018).
The distribution presented in the diameter classes, determined by the largest number of individuals in the lower classes, and the absence of trees in some upper classes is due to the characteristic of a contemporary forest that has suffered due to anthropic and natural factors, which is representative of a natural forest that is heterogeneous or has a high tendency to be heterogeneous (Caballero et al., 2018). The results obtained in our investigation coincide with what has been described by various authors (Bascopé and Jorgensen, 2005; Cascante, 2001; Cortés et al., 2009). According to numerous researchers (García et al., 2016; Louman et al., 2001; Melo and Vargas, 2003) the pattern of diameter structure found could be associated with the presence of mixed populations of different growth rates, ages and successional states, as well as competition between individuals for the resources, land use changes and, fundamentally, selective logging of timber-yielding trees. On the other hand, García et al. (2016), González et al. (2016), Aguirre-Mendoza et al. (2018) and da Luz et al. (2019) state that the diameter distribution of individuals is presented in the form of an inverted J when one deals with native forests or in recovery processes after having suffered from the felling of trees of commercial interest. The extraction of wood due to selective logging is considered one of the aspects that is most responsible for this forest's diameter structure. This has not only diminished the presence of adult trees, but has also affected natural regeneration. It is therefore clear that the pattern observed indicates a low reproductive potential of the species recorded. These characteristics are typical of disturbed forest communities in a state of succession (Aguirre-Mendoza et al., 2018; Leal et al., 2017; Vieira et al., 2003; Yepes and Villa, 2010), where the largest number of individuals present were in the lower class. The total absence of trees in certain larger-sized classes (70–80 cm), is noteworthy and implies that trees of commercial interest had been pruned. Meanwhile (Palacios et al., 2003), reported that one aspect that greatly influences the presence of small or medium diameters is the occurrence of many natural clearings, caused by several trees falling due to strong winds, landslides or the natural dynamics of the forest, such as high rainfall and density. The pattern found in the diameter classes is due to the natural and anthropogenic changes that frequently occur in the study area. In the context of large conservation and restoration initiatives for forests in a state of succession, the identification of aspects related to structure can serve as a key element for such activities.
The predominance of species in the social class of co-dominant trees indicated a characteristic pattern of heterogeneous forests (Markgraf et al., 2020), where the highest abundance of trees is to be found forming the general level of the tree canopy. However, the presence of few species in the social class of dominant trees is given by the nature of the species and the presence of natural phenomena, such as the opening of clearings (Melo and Vargas, 2003). Taller individuals take advantage of this by filling the open spaces from above and expanding their canopies. The presence of trees in different social classes (dominant, co-dominant and suppressed) indicated that the forest is growing in patches, such that these differently-sized patches are at various phases of the forest's growth cycle (Aguirre-Mendoza et al., 2018; Dueñas et al., 2007; Silva et al., 2010). This is even more the case if one considers the strong pressure to which they have been subjected.
These results obtained in terms of relative social position index allowed us to identify the predominance of a pattern of vertical distribution not continued in the study area. This makes the species vulnerable to future scenarios of environmental changes, which are very propitious in the upper Puyo River micro-basin and include: landslides, strong winds, land use changes and deforestation. Therefore, these species were considered to be in decline in the vertical structure of the community, driven by the disturbances, with low reproductive potential. This suggests the need to look for strategies to guarantee natural regeneration according to which ecological group they belong to (pioneers or tolerant of shade).
The factors that may have influenced species predominance in a single forest stratum (78%) are: availability of nutrients, high competition for the presence of pioneer species, growth rates, mortality and the dynamics of the forest itself. This affects the state of development of the species, so that their growth in some study areas could be limited and prevent them from forming part of the higher forest strata. These results are in line with the criteria of Acebey et al. (2017); Brzeziecki et al. (2018); González et al. (2016); and Jadán et al. (2016). Meanwhile, Aguirre-Mendoza et al. (2018) states that these characteristics correspond to different ecological requirements of the species and their ability to compete with others for the availability of resources. It is worth mentioning that a species has its place strengthened in the vertical structure of the forest when it is represented in the three substrata, however, its presence in the climatic stage will be uncertain (de Oliveira Fragoso et al., 2016). The results obtained in relation to the relative social position provided pertinent information about the floristic composition and permanence of the species in the different substrata, as well as the role that the species play in each of them, indicating the existence of a low reproductive potential of the species. This suggests that one must act in order to assist the permanence of the species in the three strata of the forest, through special forestry techniques aimed at natural regeneration, collection of fruits and seeds for the promotion and subsequent enrichment of the forest in most of the species reported in this inventory. The exceptions to this are Wettinia maynensis, Alchornea glandulosa, Inga velutina and Dacryodes olivifera, which apparently reproduce without difficulty. Given there is no risk to their permanence in the climatic stages, it is not necessary to channel silvicultural actions towards helping this small group.
The reported variation in the mixing ratio by diameter classes (1:1/1:5) indicated an average of approximately 1–5 individuals per species. This situation showed the high degree of mixing as a reflection of the tree species' heterogeneity (Melo and Vargas, 2003; Mostacedo et al., 2006). However, it is remarkable that there was a certain degree of alteration in some diameter classes with a lower proportion than what was previously reported for Amazon forests (Aguirre-Mendoza et al., 2018). This parameter describes the horizontal structure of the forest and allowed us to gain insight into the mixture's intensity, or in other words, the way in which the individuals of the different species are distributed within the forest according to diameter class. From this, one can infer that the study areas are characterized by a heterogeneous environment, with a certain degree of alteration in the diameter classes, represented by a high species richness as a result of the historical succession that the study stands of this tree community have undergone.
The high percentage of species (84.62%) with low ecological importance value indicate that though there is high species richness that actually makes up a minor component of the forest ecosystem and accordingly has a relatively low ecological importance index. The variation found in frequency and relative abundance was explained by the presence of rare species in the study areas that generally register low abundance (Aguirre-Mendoza et al., 2018; Silva et al., 2010). In contrast, dominance was determined by the basal area occupied by some species (de Castilho et al., 2006). The IVI was strongly influenced by the presence of large individuals. Such is the case of Ficus paraensis, which presented one of the highest IVI values and had only four individuals, two of which had diameters greater than 45 cm. The above coincides with data reported in tropical forests where a few tall trees with large diameters are those with the highest IVI within the forest (Dueñas et al., 2007). The high ecological positions achieved by some species such as Miconia splendens, Piptocoma discolor, Inga velutina and Inga multinervis are due to a predominant pattern of disturbances in the area causing these species to colonize open spaces. These species can be considered heliophytes of secondary forests. They were characterized by their high abundance in areas of small clearings with low basal area (Melo and Vargas, 2003). The pioneer species of secondary forests that are typical of ecological succession can be considered as indicative of the changes that have occurred in the forest areas of the upper Puyo River micro-basin. This corresponds to what was stated by Valdés-Sáenz et al. (2014), namely that the IVI values may be a reflection of the anthropization that some species of commercial interest are subject to. Benítez-Malvido and Martínez-Ramos (2003) indicated that forest degradation affects structural parameters and depends on the characteristics of the disturbed site (Pickett et al., 1987). The ecological value presented by many pioneer species in this research is a reflection of the structural attributes (abundance, dominance and frequency). The structural behavior determined by the greater value of importance for the Wettinia genus was similar to that observed by Cortés et al. (2009) and Gómez (2005), who reported high ecological importance for this floristic group within the ecosystem. This contrasts with Cerón and Montalvo (1997), who found that the IVI in a Secoya community in Sucumbíos province, Ecuador, was represented more by Iriartea deltoidea. Whilst it is true that this appeared in our study area, it had very low values due to its abundance. In areas of lower altitude, Jadán et al. (2016), Patiño et al. (2015) and Torres et al. (2019) also reported Irialtea deltoidea as a species of greater ecological importance. These contrasts could be due to the distribution pattern of palm species determined by an altitudinal gradient where apparently the genus Wittinia occupies the higher parts and Irialtea the lower. This provides key information for decision-making in relation to forest management and conservation.
The relationship found between the abundance of species and the study areas, through the analysis of the main components, agrees with that expressed by Jiménez et al. (2017), who stated that the distribution of the species allows one to evaluate the relationship established between the areas, the species and some variables of the environment. One is able to establish which species reached the highest abundance values in environments with certain levels of disturbance.
Variation in species abundance indicated that this is a forest that comprises different stages of plant succession, ranging from early and intermediate to more advanced stages. The factors that explain the wide distribution of species by transects were: location, accessibility, state of succession, level of disturbance and the heterogeneity (fragmentation) of the environment. The T5 site, recognized by its highest abundance of species, was less accessible, located in the highest part beside the river bank (the other sites were on the other side of the river), with medium disturbance, a more advanced state of ecological succession and more isolated fragments or patches of vegetation with some discontinuity. DeWalt et al. (2003) indicated that species composition tends to be different between successional stages. Sheil (1999) and Villa et al. (2019) showed that the abundance of species is greater in areas where succession is more advanced until the moment in which it begins to decline. The average degree of disturbance corroborates the hypothesis of intermediate disturbance outlined by Connell (1978), where the opening of clearings in the forest and the ability of certain species to thrive favor a much higher level of diversity than the absence of disturbance does. In these places, there will always be newly disturbed sites (clearings) typically occupied by pioneer species, and others of intermediate age and closed canopy (occupied by non-pioneer species). Benítez-Malvido and Martínez-Ramos (2003) indicated that vegetation fragmentation allows for greater species richness, because it creates more distinct habitats to occupy. These results correspond to those obtained by Valdés-Sáenz et al. (2014) in forest areas with different levels of disturbance, and Patiño et al. (2015) in an evergreen foothills forest in the Piatúa River basin, Napo, Ecuador. In our study, it is remarkable how in the transects that showed a higher level of disturbance due to natural or anthropic factors, there was a greater abundance of species, although they were generally pioneer species typical of secondary forests that have the characteristic of colonizing disturbed habitat. The presence of pioneer species such as those mentioned in this research project are similar to those reported by Patiño et al. (2015). It is worth noting the results obtained from the ecological structure of the evergreen foothills forest, as they reflect the practices of land use and changes that have occurred in the ecosystem.
The low values for structural ecological quality index proposed by the authors of this work reflect the constant disturbances and the historical succession that the stands in this area have undergone. The index resulted in a low value of 1 for this evergreen Amazonian-Andean forest presented. This is a result of the anthropic and natural disturbances that have led to the rupture of the ecological parameters that make up the forest's horizontal and vertical structure. The rupture is due to the low species representativeness in the forest substrata, a predominance of trees of low diametric structure, alterations in the mixing ratio and the low impact of the ecosystem's species richness. Hence, it is essential to understand the role played by successional processes and to determine the limits of anthropic disturbance (Hobbs and Harris, 2001). In this sense, Benítez-Malvido and Martínez-Ramos (2003) pointed out that the loss of structural and functional attributes requires actions that allow for the recovery of some desirable properties of the original ecosystem. Consequently, restoration is necessary in order to allow this important forest reservoir's functions and ecological structure to recover. This index is widely applicable to forest ecology, and could be important to for decision-making in forestry programs, since it provides valuable information to guide management and restoration actions in areas with high potential for Amazonian biodiversity that have been altered mainly by the intensity of selective logging.
5. Conclusion
The micro-basin of the Puyo River is a relatively diverse ecosystem, with a low structural and ecological quality index, due to the fact that a large part of the species richness was represented by a minority and the diversity of the ecosystem is mainly grouped in the 10–20 m height classes. This made it possible to evaluate the degree of forest succession and therefore, to suggest actions for its restoration.
We identified the predominance of a discontinuous vertical distribution pattern that estimates a high amount of species whose presence has diminished in the community's substrata and had low reproductive potential. This suggests that it is necessary to search for strategies to guarantee natural regeneration in line with the ecological group to which they belong. The species Wettinia maynensis, Alchornea glandulosa, Inga velutina and Dacryodes olivifera had the best relative social position indices.
The ecological importance of the species, represented by IVI values, suggest that the species with the highest ecological weight are Wettinia maynensis, Alchornea glandulosa and Miconia splendens. It was shown that many of the species that obtained high ecological positions (Miconia splendens, Piptocoma discolor, Inga velutina and Inga multinervis) are characteristic of secondary forests, so they could be considered indicators of environmental changes. The results allowed us to identify key species (those with the greatest abundance) as contributors to biodiversity in this Amazonian-Andean forest in the upper Puyo River micro-basin.
It was verified by analyzing the main components that the most disturbed sites (T1, T2, T4 and T5) are generally associated with a group of pioneer species typical of late secondary forests that have the characteristic of colonizing disturbed habitat. It was also found that in the areas with a more conserved pattern (T3), typical species of the evergreen foothills forest predominated.
Declarations
Author contribution statement
Yudel García-Quintana: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Yasiel Arteaga-Crespo: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Bolier Torres-Navarrete, Marco Robles-Morillo, Carlos Bravo-Medina, Alexandra Sarmiento-Rosero: Performed the experiments; Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors wish to thank the Universidad Estatal Amazónica, the Provincial Decentralized Autonomous Government of Pastaza and the NGO known as The Nature Conservancy, Ecuador, for all financial support for field work, and Gabriel Grefa for his hard work in supporting the identification of botanical material. We also extend our gratitude to all those who in one way or another collaborated in the development of this research as a contribution to the inventory of the evergreen foothills forest in the upper Puyo River micro-basin.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Acebey A.R., Krömer T., Kessler M. Species richness and vertical distribution of ferns and lycophytes along an elevational gradient in Los Tuxtlas, Veracruz, Mexico. Flora. 2017;235:83–91. [Google Scholar]
- Aguirre G.A., Pollito P.A.Z. Estructura y composición florística de un bosque de terraza baja en Tambopata, Madre de Dios. Biodiversidad Amazónica. 2011;3:44–53. [Google Scholar]
- Aguirre-Mendoza Z., Celi Delgado H., Herrera Herrera C. Estructura y composición florística del bosque siempreverde montano bajo de la parroquia San Andrés, cantón Chinchipe, provincia de Zamora Chinchipe, Ecuador. Arnaldoa. 2018;25:923–938. [Google Scholar]
- Alonso-Torrens Y., Hernández Martínez F.R., Barrero-Medel H., López-Ibarra G., Madanes N., Prieto-Méndez J. Estructura y composición de la vegetación de pinares de Alturas de Pizarras en la Empresa Agroforestal Minas, Cuba. Madera Bosques. 2016;22:75–86. [Google Scholar]
- APG An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Bot. J. Linn. Soc. 2003;141:399–436. [Google Scholar]
- Bascopé S.F., Jorgensen P. Caracterización de un bosque montano húmedo: Yungas, La Paz. Ecología en Bolivia. 2005;40:365–379. [Google Scholar]
- Benítez-Malvido J., Martínez-Ramos M. Impact of forest fragmentation on understory plant species richness in Amazonia. Conserv. Biol. 2003;17:389–400. [Google Scholar]
- Bravo-Medina C., Marín H., Marrero-Labrador P., Ruiz M.E., Torres-Navarrete B., Navarrete-Alvarado H., Durazno-Alvarado G., Changoluisa-Vargas D. Evaluación de la sustentabilidad mediante indicadores en unidades de producción de la provincia de Napo, Amazonia Ecuatoriana. Bioagro. 2017;29:23–36. [Google Scholar]
- Brzeziecki B., Bielak K., Bolibok L., Drozdowski S., Zajączkowski J., Żybura H. Structural and compositional dynamics of strictly protected woodland communities with silvicultural implications, using Białowieża Forest as an example. Ann. For. Sci. 2018;75:89. [Google Scholar]
- Caballero E.J., Messinger M., Román-Dañobeytia F., Ascorra C., Fernandez L.E., Silman M. Deforestation and forest degradation due to gold mining in the Peruvian Amazon: a 34-year perspective. Rem. Sens. 2018;10:1903. [Google Scholar]
- Camacho Y.J., Plonczak M. Estudio fitosociológico de dos lotes en el piso de bosque premontano en la parroquia Carayaca, estado Vargas, Venezuela. La Revista Forestal Venezolana. 2012;56:63–73. [Google Scholar]
- Cascante M. Composición florística y estructura de un bosque húmedo premontano en el Valle Central de Costa Rica. Rev. Biol. Trop. 2001;49:213–225. [PubMed] [Google Scholar]
- Catalá E.I. Los conceptos de especies indicadoras, paraguas, banderas y claves: su uso y abuso en ecología de la conservación. Interciencia. 2011;36:31–38. [Google Scholar]
- Cerón C., Montalvo C. EcoCiencia; Quito, Ecuador: 1997. Composición de una hectárea de bosque en la comunidad Huaorani de Quehueiri-Ono, zona de amortiguamiento del Parque Nacional Yasunı, Napo, Ecuador. Estudios biológicos para la conservación; pp. 279–298. [Google Scholar]
- Cintra R., Ximenes A.d.C., Gondim F.R., Kropf M.S. Forest spatial heterogeneity and palm richness, abundance and community composition in Terra Firme forest, Central Amazon. Braz. J. Bot. 2005;28:75–84. [Google Scholar]
- Colín J.S. La importancia de rescatar, preservar, mantener y cuidar la micro cuenca del Río Magdalena, Distrito Federal. Revista del Centro de Investigación de la Universidad la Salle. 2002;5:5. 5. [Google Scholar]
- Condit R., Hubbell S.P., Lafrankie J.V., Sukumar R., Manokaran N., Foster R.B., Ashton P.S. Species-area and species-individual relationships for tropical trees: a comparison of three 50-ha plots. J. Ecol. 1996:549–562. [Google Scholar]
- Connell J.H. Diversity in tropical rain forests and coral Reefs. Science. 1978:1302–1310. doi: 10.1126/science.199.4335.1302. [DOI] [PubMed] [Google Scholar]
- Cortés W.A., Murillo J.L.T., Medina A.L. Análisis florístico y estructural de los bosques premontanos en el municipio de Amalfi (Antioquia, Colombia) Colombia Forestal. 2009;12:81–102. [Google Scholar]
- da Luz F.J., dos Santos E.S., Junior F.d.O.C., Borges C.H.A., dos Santos A.C. Structural analysis and distribution patterns in lowland tropical forest, eastern Amazon. J. Agric. Stud. 2019;7:240–255. [Google Scholar]
- de Castilho C.V., Magnusson W.E., de Araújo R.N.O., Luizao R.C., Luizao F.J., Lima A.P., Higuchi N. Variation in aboveground tree live biomass in a central Amazonian Forest: effects of soil and topography. For. Ecol. Manag. 2006;234:85–96. [Google Scholar]
- de Oliveira Fragoso R., Temponi L.G., Pereira D.C., Guimaraes A.T.B. Rehabilitation of a degraded area in the field of semideciduous seasonal forest under different treatments/Recuperacao de area degradada no dominio floresta estacional semidecidual sob diferentes tratamentos. Ciência Florest. 2016;26:699–712. [Google Scholar]
- DeFries R.S., Houghton R.A., Hansen M.C., Field C.B., Skole D., Townshend J. Carbon emissions from tropical deforestation and regrowth based on satellite observations for the 1980s and 1990s. Proc. Natl. Acad. Sci. Unit. States Am. 2002;99:14256–14261. doi: 10.1073/pnas.182560099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWalt S.J., Maliakal S.K., Denslow J.S. Changes in vegetation structure and composition along a tropical forest chronosequence: implications for wildlife. For. Ecol. Manag. 2003;182:139–151. [Google Scholar]
- Dueñas A., Betancur J., Galindo R. Estructura y composición florística de un bosque húmedo tropical del parque nacional natural Catatumbo Barí, Colombia. Colombia Forestal. 2007;10:26–39. [Google Scholar]
- Finol H. Estudio fitosociológico de las unidades 2 y 3 de la Reserva Forestal de Caparo, Estado Barinas. Acta Bot. Venez. 1976;11:15–103. [Google Scholar]
- Freitas W.K., Ferreira J., Noronha G.D., Ramos M.C.G., Esper P.M.O. Diametric structure of a deciduous forest fragment in the agribusiness region of western Santa Catarina State, Brazil. Biosci. J. 2019;35:267–276. [Google Scholar]
- Garavito N.T., Álvarez E., Caro S.A., Murakami A.A., Blundo C., Espinoza T.B., Cuadros M.L.T., Gaviria J., Gutiérrez N., Jørgensen P. Evaluación del estado de conservación de los bosques montanos en los Andes tropicales. Revista Ecosistemas. 2012;21:148–166. [Google Scholar]
- García Y., Torres Y.A., Guerrero J., González O., Gaibor C.S., Jara M. Efecto de la extracción de arena sílice en la estructura y composición de especies de dos sitios de bosques de pinares de la región Occidental de Pinar del Río. Revista Amazónica Ciencia y Tecnología. 2016;5:218–232. [Google Scholar]
- Gentry A.H. Neotropical floristic diversity: phytogeographical connections between Central and South America, Pleistocene climatic fluctuations, or an accident of the Andean orogeny? Ann. Mo. Bot. Gard. 1982;69:557–593. [Google Scholar]
- Gentry A.H. Changes in plant community diversity and floristic composition on environmental and geographical gradients. Ann. Mo. Bot. Gard. 1988:1–34. [Google Scholar]
- Gómez D. Análisis florístico de los bosques premontanos en el municipio de Anorí (Antioquia). Informe Final. Corantioquia. Medellín. Pg. 2005;150 [Google Scholar]
- González A.J., Andrade G.A.P., Sospedra R.S., Rodríguez M.P.R. SATHIRI; 2016. Perturbaciones humanas sobre la composición y estructura del bosque semideciduo mesófilo, reserva de la biósfera Sierra del Rosario, Cuba; pp. 196–206. [Google Scholar]
- Gordo J.F.A. Análisis estructural de un bosque natural localizado en zona rural del municipio de Popayán. Biotecnología en el Sector Agropecuario y Agroindustrial. 2009;7:115–122. [Google Scholar]
- Hernández P., Giménez A.M. Diversidad, composición florística y estructura en el Chaco Serrano, Argentina. Madera Bosques. 2016;22:37–48. [Google Scholar]
- Hobbs R.J., Harris J.A. Restoration ecology: repairing the Earth's ecosystems in the new millennium. Restor. Ecol. 2001;9:239–246. [Google Scholar]
- Ibarra O.G., López L. Estructura, composición, riqueza y diversidad de árboles en tres muestras de selva mediana subperennifolia. An. Inst. Biol. - Ser. Bot. 2002;73:283–314. [Google Scholar]
- Jadán O., Torres B., Selesi D., Peña D., Rosales C., Günter S. Diversidad florística y estructura en cacaotales tradicionales y bosque natural (Sumaco, Ecuador) Colombia Forestal. 2016;19:5–18. [Google Scholar]
- Jiménez L., Gusmán J., Capa-Mora D., Quichimbo P., Mezquida E.T., Benito M., Rubio A. Riqueza y diversidad vegetal en un bosque siempreverde piemontano en los Andes del sur del Ecuador. Bosques Latitud Cero. 2017;7 [Google Scholar]
- Kees S.M., Michela J.F. Estructura y composición florística de tres tipos de bosques de la provincia del Chaco. Avances. 2019;22:21–33. [Google Scholar]
- Leal V., Costa T., Silva A. A forest growth dynamic indicator. J. Environ. Sci. Eng. 2017:68–84. [Google Scholar]
- López-Hernández J.A., Aguirre-Calderón Ó.A., Alanís-Rodríguez E., Monarrez-Gonzalez J.C., González-Tagle M.A., Jiménez-Pérez J. Composición y diversidad de especies forestales en bosques templados de Puebla, México. Madera Bosques. 2017;23:39–51. [Google Scholar]
- López-Pérez D., Castillo-Acosta O., Zavala-Cruz J., Hernández-Trejo H. Estructura y composición florística de la vegetación secundaria en tres regiones de la sierra norte de Chiapas, México. Polibotánica. 2014:1–23. [Google Scholar]
- Louman B., David Q., Margarita N. Centro Agronómico de Investigación y Enseñanza (CATIE); 2001. Silvicultura de bosques latifoliados húmedos con énfasis en América Central. [Google Scholar]
- Lutz J.A., Larson A.J., Freund J.A., Swanson M.E., Bible K.J. The importance of large-diameter trees to forest structural heterogeneity. PloS One. 2013;8 doi: 10.1371/journal.pone.0082784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAE . Ministerio del Ambiente del Ecuador. Manejo forestal sustentable. Norma técnica Bosque Tropical. In: Forestal O.T., editor. Napo. Ecuador. 2012. p. 30. [Google Scholar]
- Malhi Y., Roberts J.T., Betts R.A., Killeen T.J., Li W., Nobre C.A. Climate change, deforestation, and the fate of the Amazon. Science. 2008;319:169–172. doi: 10.1126/science.1146961. [DOI] [PubMed] [Google Scholar]
- Markgraf R., Doyon F., Kneeshaw D. Forest landscape heterogeneity increases shrub diversity at the expense of tree seedling diversity in temperate mixedwood forests. Forests. 2020;11:160. [Google Scholar]
- Melo O., Vargas R. Universidad del Tolima; Ibagué, Colombia: 2003. Evaluación Ecológica y Silvicultural de Ecosistemas Boscosos. [Google Scholar]
- Mostacedo B., Balcazar J., Montero J.C. Tipos de bosque, diversidad y composición florística en la Amazonia sudoeste de Bolivia. Ecología en Bolivia. 2006;41:99–116. [Google Scholar]
- Murthy I.K., Bhat S., Sathyanarayan V., Patgar S., Beerappa M., Bhat P., Bhat D., Ravindranath N., Khalid M., Prashant M. Vegetation structure and composition of tropical evergreen and deciduous forests in Uttara Kannada District, Western Ghats under different disturbance regimes. Trop. Ecol. 2016;57:77–88. [Google Scholar]
- Nascimento A.R.T., Longhi S.J., Brena D.A. Estrutura e padrões de distribuição espacial de espécies arbóreas em uma amostra de Floresta Ombrófila Mista em Nova Prata, RS. Ciência Florest. 2001;11:105–119. [Google Scholar]
- Nordström E.-M., Nieuwenhuis M., Başkent E.Z., Biber P., Black K., Borges J.G., Bugalho M.N., Corradini G., Corrigan E., Eriksson L.O. Forest decision support systems for the analysis of ecosystem services provisioning at the landscape scale under global climate and market change scenarios. Eur. J. For. Res. 2019;138:561–581. [Google Scholar]
- Palacios J., Ramos Y., Mosquera A., Castro F., García F., Arroyo J., Cogollo A. Estructura de un bosque pluvial tropical (Bp-T) en Salero, Unión Panamericana. In: García Chocó., Ramos F., Palacios Y., Arroyo J., JE Mena A., González M., editors. Salero: diversidad biológica de un bosque pluvial tropical (bp-T) Editorial Guadalupe Ltda; Bogotá, DC: 2003. [Google Scholar]
- Palacios W. Universidad Técnica del Norte Ibarra-Ecuador; 2016. Árboles del Ecuador: Familias y géneros; especies representativas, Ciudadela el Olivo. [Google Scholar]
- Patiño J., Lozano P., Tipán C., Navarrete H., López R., Asanza M., Torres B. Composición florística y estructura de un bosque siempreverde piemontano de 600 a 700 m snm en la cuenca del río Piatúa, Napo, Ecuador. Revista Amazónica Ciencia y Tecnología. 2015;4:166–214. [Google Scholar]
- Pickett S., Collins S., Armesto J. A hierarchical consideration of causes and mechanisms of succession. Vegetatio. 1987;69:109–114. [Google Scholar]
- Pintaud J.-C., Galeano G., Balslev H., Bernal R., Borchsenius F., Ferreira E., de Granville J.-J., Mejía K., Millán B., Moraes M. Las palmeras de América del Sur: diversidad, distribución e historia evolutiva. Rev. Peru. Biol. 2008;15:7–30. [Google Scholar]
- Qin Y., Xiao X., Dong J., Zhou Y., Wang J., Doughty R.B., Chen Y., Zou Z., Moore B., III Annual dynamics of forest areas in South America during 2007–2010 at 50-m spatial resolution. Rem. Sens. Environ. 2017;201:73–87. [Google Scholar]
- Rasmussen L.V., Watkins C., Agrawal A. Forest contributions to livelihoods in changing agriculture-forest landscapes. For. Pol. Econ. 2017;84:1–8. [Google Scholar]
- Ruiz-Jaén M.C., Aide T.M. Vegetation structure, species diversity, and ecosystem processes as measures of restoration success. For. Ecol. Manag. 2005;218:159–173. [Google Scholar]
- Schreader H., Gregorie T., Wood G. J. Willey and Sons; New York: 1983. Sampling Methods for Multiresource forest Inventory. [Google Scholar]
- Sheil D. Tropical forest diversity, environmental change and species augmentation: after the intermediate disturbance hypothesis. J. Veg. Sci. 1999;10:851–860. [Google Scholar]
- Silva J.S.B., Montoya Á.J.D., López D.C., Hurtado F.H.M. Variación florística de especies arbóreas a escala local en un bosque de tierra firme en la Amazonia colombiana. Acta Amazonica. 2010;40:179–188. [Google Scholar]
- Svenning J.-C. Environmental heterogeneity, recruitment limitation and the mesoscale distribution of palms in a tropical montane rain forest (Maquipucuna, Ecuador) J. Trop. Ecol. 2001;17:97–113. [Google Scholar]
- Templeton L.K., Neel M.C., Groffman P.M., Cadenasso M.L., Sullivan J.H. Changes in vegetation structure and composition of urban and rural forest patches in Baltimore from 1998 to 2015. For. Ecol. Manag. 2019;454:117665. [Google Scholar]
- Ter Steege H., Pitman N.C., Sabatier D., Baraloto C., Salomão R.P., Guevara J.E., Phillips O.L., Castilho C.V., Magnusson W.E., Molino J.-F. Hyperdominance in the Amazonian tree flora. Science. 2013;342:1243092. doi: 10.1126/science.1243092. [DOI] [PubMed] [Google Scholar]
- Tomppo E., Heikkinen J., Henttonen H.M., Ihalainen A., Katila M., Mäkelä H., Tuomainen T., Vainikainen N. Springer Science & Business Media; 2011. Designing and Conducting a forest Inventory-Case: 9th National Forest Inventory of Finland. [Google Scholar]
- Torres B., Vasseur L., López R., Lozano P., García Y., Arteaga Y., Bravo C., Barba C., García A. Structure and above ground biomass along an elevation small-scale gradient: case study in an Evergreen Andean Amazon forest, Ecuador. Agrofor. Syst. 2019:1–11. [Google Scholar]
- Turner R., Daily G. The ecosystem services framework and natural capital conservation. Environ. Resour. Econ. 2008;39:25–35. [Google Scholar]
- Valdés-Sáenz M.A., García-Quintana Y., Escarré-Esteve A., Flores J., Geada-López G., Arteaga-Crespo Y., Valdés-Sáenz C.R. Estructura de un bosque natural perturbado de Pinus tropicalis Morelet en Galalón, Cuba. Bot. Sci. 2014;92:417–423. [Google Scholar]
- Vera-Vélez R., Grijalva J., Cota-Sánchez J.H. New Forests; 2019. Cocoa Agroforestry and Tree Diversity in Relation to Past Land Use in the Northern Ecuadorian Amazon; pp. 1–20. [Google Scholar]
- Vieira I.C.G., de Almeida A.S., Davidson E.A., Stone T.A., de Carvalho C.J.R., Guerrero J.B. Classifying successional forests using Landsat spectral properties and ecological characteristics in eastern Amazonia. Rem. Sens. Environ. 2003;87:470–481. [Google Scholar]
- Villa P.M., Martins S.V., Rodrigues A.C., Safar N.V.H., Bonilla M.A.C., Ali A. Testing species abundance distribution models in tropical forest successions: implications for fine-scale passive restoration. Ecol. Eng. 2019;135:28–35. [Google Scholar]
- Villacis H.G.S., Quintana Y.G., López G.G., Crespo Y.A., Obregon J.R., Rubio J.G. Efecto del grado de antropización en la estructura, en tres sitios fragmentados bosque siempreverde piemontano. Revista Cubana de Ciencias Forestales. 2017;5:172–180. [Google Scholar]
- Yaduv V., Srivastava A., Khare P. Tropical forest and ecosystems services in Indian context. Curr. World Environ. 2018;13:151. [Google Scholar]
- Yepes A.P., Villa J.A. Sucesión vegetal luego de un proceso de restauración ecológica en un fragmento de bosque seco tropical (La Pintada, Antioquia) Rev. Lasallista Invest. 2010;7:24–34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.