Abstract
Lipophilic compounds constitute a majority of therapeutics in the pipeline of drug discovery. Despite possessing enhanced efficacy and permeability, some of these drugs suffer poor solubility necessitating the need of a suitable drug delivery system. Nanoemulsion is a drug delivery system that provides enhanced solubility for poorly soluble drugs in an attempt to improve the oral bioavailability. The purpose of this study is to develop a nanoemulsion system using ibuprofen as a model drug in order to investigate the potential of this colloidal system to enhance the absorption of poorly water-soluble drugs. Ibuprofen loaded-nanoemulsion with different drug concentrations (1.5, 3 and 6% w/w) were formulated from olive oil, sucrose ester L-1695 and glycerol using D-phase emulsification technique. A pseudoternary phase diagram was utilised to identify the optimal excipient composition to formulate the nanoemulsion system. In vitro diffusion chamber studies using rodent intestinal linings highlighted improved absorption profile when ibuprofen was delivered as nanoemulsion in comparison to microemulsions and drug-in-oil systems. This was further corroborated by in vivo studies using rat model that highlighted a two-fold increase in ibuprofen absorption when the drug was administered as a nanoemulsion relative to drug-in-oil system. On the other hand, when ibuprofen was administered as microemulsions, only a 1.5-fold increase in absorption was observed relative to drug-in-oil system. Thus, this study highlights the potential of using nanoemulsion as a drug delivery system to enhance the oral bioavailability of hydrophobic drugs.
Keywords: Ibuprofen, Nanoemulsion, Intestinal transport, Pharmacokinetic profile, Oral delivery, Bioavailability, Transepithelial electrical resistance (TEER), Nanomaterials, Materials characterization, Pharmaceutical science, Dose-response relationship, Nanotechnology
Ibuprofen; Nanoemulsion; Intestinal transport; Pharmacokinetic profile; Oral delivery; Bioavailability, Transepithelial electrical resistance (TEER); Nanomaterials; Materials characterization; Pharmaceutical science; Dose-response relationship; Nanotechnology.
1. Introduction
Advancement in drug discovery has led to the development of a variety of compounds with enhanced efficacy and therapeutic potential. However, such promising drug molecules often suffers poor oral bioavailability due to low aqueous solubility. This serves as a major obstacle to pharmaceutical industries in the attempt to bring such therapeutics to market. Thus, the discovery of such drug molecules ought to be complemented with the development of novel and intelligent drug delivery systems which is able to deliver the therapeutics with enhanced bioavailability in order meet the drug's therapeutic window of efficacy. The solubility and dissolution rate of the drugs are two main factors that govern the absorption rate of the drug products in the gastrointestinal fluids [1, 2].
In order to increase their solubility and bioavailability in the gastrointestinal fluids, several approaches have been used to improve the bioavailability of poorly soluble drugs. These include the use of an ionic liquid (IL) formulation of active pharmaceutical ingredients (APIs) [3], salt formation [4] prodrug synthesis [5], solid dispersion [6] and lipid-based drug delivery systems [7].
In recent years, there has been an impetus on using lipid-based formulations to improve oral bioavailability of lipophilic drugs [8]. Some of these lipid-based formulations include self-dispersing formulations such as self-emulsifying, self-micro/nano-emulsifying drug delivery system (SMEDDS/SNEDDS). These systems offer an elegant approach for the delivery of poorly water-soluble drugs due to their self-emulsifying behavior. In addition, the small droplet sizes that is formed upon dispersion has been shown to improve drug absorption from the intestinal tracts [9, 10].
Besides that, nanotechnology offers various approaches in the area of dissolution enhancement of poorly water-soluble drugs [11]. By combining the concept of nanotechnology along with the principle of lipid-based formulation, nanoemulsion has emerged as a drug delivery strategy to enhance the oral bioavailability of poorly water soluble drugs [12, 13, 14].
Nanoemulsion is a heterogeneous system that consists of an oil phase dispersed in aqueous medium which is stabilised by surfactants [14, 15]. Unlike conventional colloidal system, nanoemulsion displays much smaller droplet diameter of about 20–200 nm. The small droplet size confers larger colloidal surface area that promote solubility and dissolution rate which ultimately translate to enhance mucosal permeability across the intestinal tracts [16, 17, 18]. Besides that, nanoemulsions circumvents issues such flocculation and coalescence during long-term storage because the nanometer-sized droplets are more kinetically stable relative to conventional emulsion systems [19, 20, 21]. Construction of pseudoternary phase diagram is a critical step in the development of such lipid-based formulation. This system will self-disperse itself as a thermodynamically stable nano drug carrier within the gastrointestinal (GI) lumen [12]. In addition, such ternary phase diagrams provide precise and accurate data of the various composition of the components needed to produce a nanoemulsion.
Ibuprofen, a phenyl propionic acid derivative, plays a significant role in the treatment of rheumatoid arthritis, osteoarthritis and related conditions [22, 23]. This molecule has been chosen as model dug in this study. This is because ibuprofen suffers poor solubility and gastrointestinal absorption upon oral administration [24, 25]. Thus, it is postulated that nanoemulsion may serve as a promising drug delivery strategy to improve the oral absorption [26]. Currently, ibuprofen is commercially available in the market as tablets, gel and oral suspensions [27, 28].
In this study, we investigate the ability of nanoemulsion system in improving the solubility and oral bioavailability of ibuprofen. A pseudoternary phase diagram was constructed in order to assess and identify a nanoemulsion region from oil (olive oil), surfactant (glycerol) and different sucrose esters (co-surfactant). Upon identifying such nanoemulsion region, the colloidal system was then developed as a carrier system for ibuprofen. The selected nanoemulsion (NE) region was characterised by measuring droplet size, polydispersity index, zeta potential and morphology. In addition, in vitro and in vivo intestinal study were conducted to evaluate the ability of ibuprofen loaded nanoemulsion in enhancing the oral bioavailability of the drug upon administration.
2. Materials and methods
2.1. Materials
Ibuprofen [2-(4-isobutylphenyl)-propionic acid] was purchased from Sigma-Aldrich (UK). HPLC grade acetonitrile and orthophosphoric acid were obtained from Merck (Darmstadt, Germany). Distilled water was purified before use with ELGA Water Purification System R15 supplied with pump and tank (Elga Water System, UK). Olive oil, glycerol, propyl paraben and methyl paraben were supplied by Zulat Pharmacy (Malaysia). Sucrose Ester (SE) Laurate (L-1695), SE Oleate (O-1570) and SE Palmitate (P-1570) were obtained from Ryoto Mitsubishi-Kagaku (Japan). All other chemicals and solvents were of analytical grade.
2.2. Formulation development
2.2.1. Preparation of nanoemulsion
A series of nanoemulsion formulation was prepared by D-phase emulsification method to produce concentrated emulsion as previously reported [29]. The concentrated nanoemulsion which also known as nanophase gel (NPG) was initially formed during the emulsification process. The composition of NPG formulations (A-E) could be observed in Table 1. SE was dissolved in glycerol with gentle agitation at 65 °C. Meanwhile, olive oil was heated up to 65 °C. This was followed by adding the oil phase gradually into the surfactant phase at a similar temperature by gentle stirring. This process was carried out until the mixture eventually becomes a gel-like consistency. The produced NPG was stored in a glass container at 4 °C. All experiments were carried out at room temperature (25 °C).
Table 1.
Composition of nanophase gel (NPG) formulations (%). The NPG were formulated from olive oil as the oil phase, sucrose ester (SE) laurate (L-1695) as a co-surfactant and glycerol as a surfactant.
| Formulation | Oil to surfactant ratio | Olive oil | SE L-1695 | Glycerol |
|---|---|---|---|---|
| A | 3:1 | 60.0 | 20.0 | 20.0 |
| B | 4:1 | 60.0 | 15.0 | 25.0 |
| C | 5:1 | 60.0 | 12.0 | 28.0 |
| D | 6:1 | 60.0 | 10.0 | 30.0 |
| E | 7:1 | 60.0 | 8.6 | 31.4 |
Prior to the nanoemulsion preparation, NPG was weighed separately to obtain three different percentage; 10%, 20% and 30% (w/w) oil, respectively. All three different compositions of oil percentage were introduced into distilled water and mixed using Eurostar Digital IKA® WERKE at a speed of 200 rpm for 10 min. The altered composition of nanoemulsion formulations is shown in Table 2. The nanoemulsion were analysed using Mastersizer (Malvern Instrument, UK) which separate each nanoemulsion based on their mean droplet size distribution and uniformity.
Table 2.
Composition of nanoemulsion formulations (%) upon diluting nanophase gel (NPG) in distilled water.
| Formulation | NPG | Water |
|---|---|---|
| 10% | 16.7 | 83.3 |
| 20% | 33.3 | 66.7 |
| 30% | 50.0 | 50.0 |
2.2.2. Pseudoternary phase diagram study
A series of formulated nanoemulsion was used to construct the pseudoternary phase diagram consisting of oil (olive oil), surfactant (glycerol) and non-ionic co-surfactant (sucrose esters). SE Laurate (L-1695), SE Oleate (O-1570) and SE Palmitate (P-1570) were used in order to study the effect of co-surfactant on the formulation. The pseudo-ternary phase diagram A comprised of olive oil, SE Laurate (L-1695) and glycerol, while pseudo-ternary phase diagram B consisted of olive oil, SE Oleate (O-1570) and glycerol and finally pseudo-ternary phase diagram C contained olive oil, SE Palmitate (P-1570) and glycerol.
The resulting mixture was evaluated based on the droplet size distribution and uniformity by using Mastersizer 2000 with Hydrosizer 2000MU module (Malvern Instruments, UK). The identification of the emulsion region in the phase region would be classified as nanoemulsion (NE) region (<1 μm), microemulsion (ME) region (>1 μm) and separation of emulsion (SE) region. Then, the optimum nanoemulsion formulations from the experiment were selected to be loaded with ibuprofen.
2.2.3. Ibuprofen loading at different percentages in nanoemulsion
Ibuprofen has been chosen as an active ingredient to be incorporated into selected nanoemulsion formulations at different concentrations (1.5% w/w, 3.0% w/w and 6.0% w/w). Ibuprofen was dissolved in olive oil and heated up to 65 °C before being loaded into nanoemulsion formulations. Ibuprofen nanoemulsion produced were further analysed using Zetasizer (Malvern Instrument, UK).
2.3. Characterisation study
2.3.1. Droplet size, polydispersity index (PDI) and zeta potential analysis
In a beaker, 250 μl of oil/surfactant mixture was introduced to 50 ml of distilled water under gentle agitation using a glass rod. The droplet size of the emulsions was analysed using photon correlation spectroscopy (PCS) (Mastersizer 2000MU, Malvern Instruments, UK) in pseudoternary phase diagram study. Following that, formulations that produce nanometer-range droplet size from the pseudoternary phase diagram (Formulation A, B, C, D, and E) were subsequently analysed for droplet size distribution and zeta potential analysis using Zetasizer Nano ZS (Malvern Instruments, UK).
2.3.2. Microscopic observation
Scanning electron microscopy (SEM) (FEI Quanta 450, Netherlands) was carried out to study the surface morphology and to determine the droplet size and the uniformity of nanoemulsion distribution. Ibuprofen nanoemulsion (1.5% w/w, 3.0% w/w and 6.0% w/w) and control (3% ibuprofen in olive oil) were separately spread on the sample holder and coated with gold under an argon atmosphere to a thickness of 6.5 mm. The samples were observed under SEM to examine the surface morphology and to determine the droplet size of nanoemulsions as well as the uniformity of droplet size distribution.
2.4. In vitro diffusion chamber method
The transport of ibuprofen nanoemulsion across the rat intestinal membrane was conducted using in vitro diffusion chamber method [30]. This study was performed in accordance with the guidelines and approval from the animal ethics committee of Universiti Teknologi MARA (UiTM) (UiTM Care:53/2014). Male Sprague Dawley rats, weighing 230–250 g were anesthesized with a mixture of ketamil and xylazil (3:2), intraperitoneally (i.p.) after being fasted overnight. By doing a midline abdominal incision, the intestine was exposed and removed and then washed with phosphate buffered saline (PBS). The intestinal segment was isolated and cut open. The muscle layer on the external surface of the segment was stripped off and the intestinal sheets were then mounted to the pins of the diffusion chamber (Harvard Navicyte, Warner Instruments, USA).
The drug solution was added to the donor compartment and the same volume of buffer was added to the receiver site. 95% O2 and 5% CO2 gas was aerated in each chamber in order to mix each solution and also to maintain the viability of the membrane. Throughout the experiment, the temperature was maintained at 37 °C. A volume of 0.1 ml aliquot was taken from the receiver site at predetermined time intervals over 120 min. The aliquot was immediately replaced with an equal volume of buffer solution and then drugs were assayed. The apparent permeability coefficient was calculated using the following equation (Eq. (1)):
| Papp = Flux × (1/Area) × (1/60) × (1/C0) | (1) |
Where Papp is referred as apparent permeability coefficient (cm/s), while the flux, F, is the slope of linear portion of cumulative transport amount to time at the steady state (pmol/ml). Area refers to the area of diffusion chamber for transport (fix value), 1.78 cm2, and C0 is the initial drug concentration (pmol/ml). In this instance, the best microemulsions and nanoemulsion identified from pseudoternary phase diagram study and characterisation study were evaluated. In addition, 3.0% w/w of ibuprofen in olive oil, an emulsion formed without the use of glycerol and sucrose ester surfactant is used as a control.
2.5. Transepithelial electrical resistance (TEER) study
In order to elucidate if the ibuprofen nanoemulsion was transported paracellularly or transcellularly, a TEER study was conducted. The transepithelial electrical resistance (TEER) of cell monolayer was measured at room temperature using Multichannel Epithelial Voltage Clamps (Warner Instruments, USA) and the result obtained was corrected with the blank film resistance. Again, the microemulsions and nanoemulsion identified from pseudoternary phase diagram study and characterisation study were evaluated for its effect on transepithelial electrical resistance. In addition, 3.0% w/w of ibuprofen in olive oil, an emulsion formed without the use of glycerol and sucrose ester surfactant was used as a control in this experiment.
2.6. In vivo oral absorption study
Male Sprague Dawley rats (weighing from 230 - 250 g) were obtained from Laboratory Animal Facility and Management (LAFAM), UiTM Puncak Alam. All animal experiments were performed according to guidelines of the Committee on Animal Research & Ethics (CARE) of Faculty of Pharmacy, Universiti Teknologi MARA (UiTM) (UiTM Care:43/2014).
The animals were fasted overnight, 12 h prior to the start of experiment with water ad libitum and prepared according to the method previously reported [31]. The rats were randomized to be administered orally (30 mg/kg) with either nanoemulsion, microemulsion and control formulation, respectively. In this instance, 3.0% w/w of ibuprofen in olive oil, an emulsion formed without the use of glycerol and sucrose ester surfactant was used as a control. Then, rats were anesthetized with ketamine: xylazine; ratio of 2:1. Blood samples (0.25 ml) were collected from the jugular vein using heparinized syringes at predetermined time intervals of 0, 0.25, 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, 5.0 and 6.0 h. Plasma samples were treated with DA prior to UPLC (Waters, USA) analysis.
The ibuprofen concentration in plasma was measured using UPLC method. Prior to the analysis, 100 μl of plasma samples were treated with 200 μl of acetonitrile with propanol (1:1) and vortexed for 1 min. The samples were then centrifuged at 10,000 rpm for 10 min. 50 μl of the supernatant was filtered and injected into the UPLC system.
The chromatographic conditions applied in this study were adapted and modified from previous study by Abdullah et al [32] in order to acquire the specific and sensitive analytical methods. The filtered and degassed mobile phase comprised a mixture of acetonitrile and water adjusted to pH 2.5 with concentrated orthophosphoric acid at ratios of 70:30. The UPLC eluting conditions were performed by isocratic elution at a flow rate of 0.5 ml/min and detected by UV-Vis detector at a wavelength of 223 nm.
3. Results and discussion
3.1. Pseudoternary phase diagram study
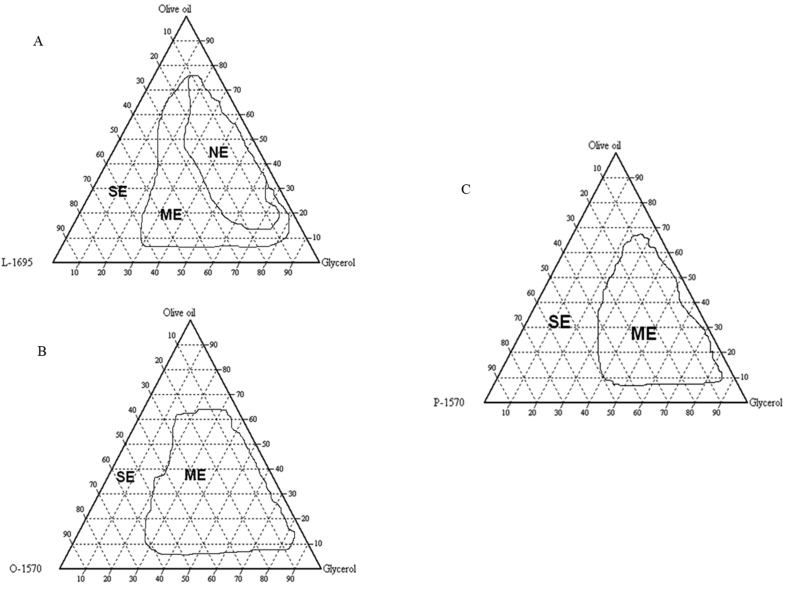
Emulsification involves the mixing of oil and water to form a two-phase system that is stabilised by a surfactant [33] as well as to promote particle size reduction [13]. The oil phase consists of non-polar and hydrophobic molecules that contains hydrocarbons, natural triglycerides and other derivatives [34] while the aqueous phase consists of electrolytes and other solutes dissolved water. In this study, pseudoternary phase diagrams were developed without the addition of ibuprofen in order to obtain precise concentration range of the components that give rise to regions of nanoemulsion. Three phase diagrams were constructed by pairing the three different surfactants as shown in Figure 1(A-C).
Figure 1.
(A–C) The pseudoternary phase diagram of oil, co-surfactant and three different surfactants: System A (olive oil, glycerol and SE laurate, L-1695), System B (olive oil, glycerol and SE oleate, O-1570) and System C (olive oil, glycerol and SE palmitate, P-1570).
These pseudoternary phase diagrams show different areas that correspond to different types of emulsions such as nanoemulsion (NE), microemulsion (ME) and separated emulsion (SE). It could be observed that System A that utilised SE laurate L-1695 has the largest region of NE formation compared to the other two systems which do not have any NE regions. System A formed a stable and broad region of NE due to the emulsification properties of SE Laurate [14]. On the other hand, System B which involved the usage of SE Oleate 1570 shows moderate region of emulsification. This is because most of the series of formulations produced were in the ME region while pseudo ternary phase diagram C which comprised of SE Palmitate 1570 displayed poor emulsification properties evidenced by the presence of large ME and SE areas which were not stable after being left overnight. Based on the results, system A that uses SE Laurate 1695 as emulsifier have the best emulsification properties and formed a stable nanoemulsion system when compared with SE Oleate 1570 and SE Palmitate 1570.
In this study, different types of SEs were used to determine the effects of hydrophilic-lipophilic balance (HLB) values on the development of colloidal drug delivery systems. As the degree of sucrose esterification and fatty acid chain length increased, the HLB value of SE became decreased [35]. SE Laurate 1695 that has a high HLB value of 16 and was able to produce a large region of NE with small droplet size distribution and uniformity. This is because SE Laurate has good droplets entrapment and stabilisation which is explained by the low amounts of di-, tri- and polylaurate content (20%), high amount of monolaurate content (80%) and short chain length of lauric acid. Meanwhile, system B and system C that uses SE Oleate 1570 (HLB:15) and SE Palmitate 1570 (HLB:15) respectively, could only produce ME region instead of NE region. These surfactants contain a lower monoesters composition of approximately 70%. Such finding is further corroborated by the study by Leong et al [36] who discovered that preparation of phytosterol dispersions by using SE Laurate 1695 resulted in smaller particle size below than 100 nm compared to SE Palmitate 1570, SE Stearate 1570 and SE Oleate 1570, respectively. Hence, SE Laurate 1695 that displayed a high HLB value is selected as a suitable excipient to produce nanoemulsion formulations with small droplet size distribution and high stability.
3.2. Characterisation of nanoemulsion
3.2.1. Droplet size, polydispersity index (PDI) and zeta potential analysis
Initially, ibuprofen was added at several concentrations relative to the weight of formulation which was 1.5%, 3.0% and 6.0% (w/w). From our work, formulation containing 3% (w/w) ibuprofen resulted in the smallest mean droplet size with improved stability based on the droplet size, PDI and zeta potentials value relative to other concentration of ibuprofen nanoemulsion. However, when more than 6% (w/w) of ibuprofen was loaded into the nanoemulsion, the formulation undergoes phase separation between the oil and aqueous phase. The optimum composition of nanoemulsion for oral delivery of ibuprofen and the effects of the nanoemulsion formulations on the droplet size, polydispersity index and zeta potential are shown in Table 3.
Table 3.
Compositions, droplet size, polydispersity index and zeta potential of the selected formulations. Results are expressed as the mean ± S.E of n ≥ 3 experiments.
| Vehicle | Formulations % (w/w) |
||||
|---|---|---|---|---|---|
| A | B | C | D | E | |
| Ibuprofen | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| L-1695 | 20.0 | 15.0 | 12.0 | 10.0 | 8.6 |
| Glycerol | 20.0 | 25.0 | 28.0 | 30.0 | 31.4 |
| Olive oil | 57.0 | 57.0 | 57.0 | 57.0 | 57.0 |
| Mean droplet size (nm) | 318.0 ± 1.079 | 232.1 ± 0.451 | 243.0 ± 0.443 | 266.9 ± 1.388 | 372.0 ± 1.518 |
| PDI | 0.112 ± 0.021 | 0.047 ± 0.014 | 0.056 ± 0.006 | 0.058 ± 0.001 | 0.115 ± 0.016 |
| Zeta Potential (mV) | -25.4 ± 0.423 | -31.7 ± 0.361 | -30.2 ± 0.173 | -29.7 ± 0.553 | -27.8 ± 0.890 |
In this investigation, Malvern Zetasizer was used in standard autosizer mode to measure emulsions with the droplets within submicron range. Formulation B, C and D showed small droplet size, low PDI and good zeta potential values which were more than -29 mV among the five formulations, with formulation B displaying the smallest mean droplet size. The PDI value varied depending upon the droplet distribution from the nanoemulsions. PDI is an indicator of broadness of molecular weight distribution [15]. The PDI numerical value ranges from 0.0 (for a perfectly uniform particle size sample) to 1.0 (for a highly polydisperse sample). For drug loaded nanoparticles, values of 0.2 and below are generally deemed acceptable criteria in formulation development [16]. Therefore, in this study, we managed to develop several ibuprofen nanoemulsions with low PDI values. In addition, Formulation B demonstrates the lowest PDI value (0.047 ± 0.014) which is smaller than 0.05 indicating high monodispersity [16].
Zeta potential is also an important physicochemical characteristic of the formulations. Zeta potential measurements were used to determine the stability of colloidal systems. It has been shown that colloidal system displaying a zeta potential more than -30 mV shows good stability over time [37, 38]. As shown in Table 1, Formulation B loaded with 3% (w/w) ibuprofen represents the highest zeta potential value which was -31.7 mV. Hence, Formulation B loaded with 3% (w/w) ibuprofen was the most stable nanoemulsion formulation based on the droplet size, PDI and zeta potential value.
Nanoemulsions are isotropic dispersed systems that are thermodynamically unstable causing the systems to be susceptible to Oswald ripening. This may ultimately lead to unwanted instabilities such as creaming and flocculation. However, such limitation may be circumvented through the introduction of surfactants [39]. In this study, we have identified that the addition of the surfactant, sucrose ester L-1695, resulted in the formation of a nanoemulsion system as shown in Figure 1 and Table 3. The optimised nanoemulsion, formulation B also displayed enhanced stability in terms of particle size for up to 90 days when stored at 4 °C as shown in Figure S1. Such observations are in alignment with the findings from Xu et al [40] reported that the use of surfactants such as soybean protein isolate, β-conglycinin and glycinin enhanced the stability of nanoemulsion systems for up to 45 days. However, in this study, we have demonstrated that the use of sucrose ester L-1695 resulted in the formation of a nanoemulsion system that was stable for up to 90 days when stored at 4 °C. Sucrose ester L-1695 is a non-ionic amphiphilic surfactant that consist of a sucrose moiety acting as the hydrophilic head and a fatty acid lipophilic tail. The surfactant resides at the oil-water interface with the fatty acid tail embedded in the oil phase while the sucrose head faces the aqueous phase providing a steric barrier against droplet coalescence leading to enhanced nanoemulsion stability [41].
3.2.2. Morphological study using scanning electron microscopy (SEM)
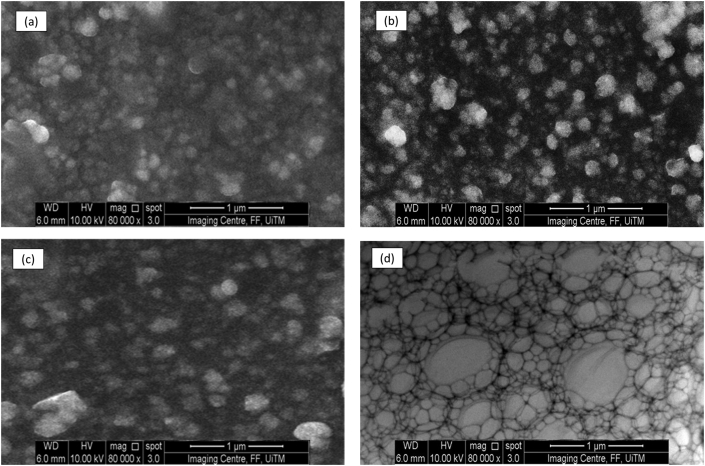
SEM analysis was conducted in order to obtain information pertaining the structure, size and shape of the nanoemulsion droplets in comparison to the control formulation. Information detailing very small droplet size could not be obtain via light microscopy as it is beyond the detection limits of light microscopy (<1 μm) necessitating the use of SEM. (a) 1.5 % w/w (b) 3.0 % w/w (c) 6.0% w/w and (d) control formulation containing 3% w/w ibuprofen in olive oil are shown in Figure 2.
Figure 2.
Scanning electron microscope images of Formulation B which are nanoemulsion loaded with different ibuprofen concentration. 1.5 % w/w (a), 3.0 % w/w (b) and 6.0 % w/w (c). (d) is a control formulation which is an oil in water emulsion containing 3 % (w/w) ibuprofen, the oil phase consists of olive oil. All images were taken at x 80 000 magnification.
The nanoemulsion droplets are spherical with diameters ranging between 200 to 300 nm. Spherical nanoemulsion were formed when ibuprofen was loaded at 1% (w/w) and 3% (w/w) concentrations. In addition, nanoemulsion loaded with 3% (w/w) ibuprofen showed the most uniform droplet distribution among all the formulations with comparable droplets size measurement as shown in Table 3. However, when the drug concentration was increased to 6% (w/w), the size distribution displayed larger and non-uniform droplet size. Vasconcelos et al reported that at low resveratrol drug loading (2% and 5% w/w), the group discovered that there was minimal impact of drug loading on nanoemulsion droplet size [42]. However, such results contradict the more recent findings by Wik et al that reported an increment in size after loading hydrophobic drugs into nanoemulsion. Wik et al discovered that when hydrophobic drugs such as curcumin are loaded into nanoemulsion, they observed fused nanoemulsion globules that such suggest coalescence between nanoemulsion droplets are taking place during drug loading [43].
Sucrose esters have been used in formulation science to produce microemulsions, nanoparticles and nanosuspension due to their excellent emulsification and stabilisation properties [14]. However, the surfactant itself may solubilise poorly soluble drug through micellar solubilisation [44]. It is postulated that when the drug loading was increased from 3% to 6 w/w, the addition of higher drug loading during the emulsification step may have caused some of the surfactant to directly interact with the ibuprofen molecules via micellar solubilisation. This resulted in less sucrose ester L-1695 available to stabilise the interphase of the newly formed nanoemulsion droplets via the Gibbs-Marangoni effect during the homogenisation step in D phase emulsification [45]. The lack of surfactant at the interphase of the newly formed nanoemulsion may lead to some of the droplet to coalesce leading non-uniform droplet size distribution as shown in Figure 2 (c).
However, the most non-uniform droplet distribution is shown in the control formulation in Figure 2 (d) which was a standard oil in water emulsion. The standard oil in water emulsion displayed irregular shape with very distinct non uniform droplet size distribution. Such observation is attributed to the absence of any surfactant such as sucrose ester L-1695 needed to stabilise the newly formed emulsion droplets after the homogenisation step in D phase emulsification.
3.3. In vitro diffusion chamber
An ex vivo model of gut permeation via the use of diffusion chamber lined with intestinal sheets was utilised to predict the degree of oral absorption and to elucidate the absorption mechanisms across rodent intestinal tissues as shown in Table 4. Three different sucrose esters were used as surfactant to determine the best surfactant to be used to formulate ibuprofen nanoemulsion. By using SE laurate as the surfactant, ibuprofen nanoemulsion (NE) was produced with an average particle size that is lower than 300 nm. SE oleate formed ibuprofen microemulsion (ME A) possesses an average particle size of 1μm when it was used in the formulation. Ibuprofen microemulsion (ME B), displayed an average particle size of 3 μm, when SE palmitate was used as the surfactant. In addition, 3.0% w/w of ibuprofen in olive oil, an emulsion formed without the use of glycerol and sucrose ester surfactant was used as a control in this experiment. The comparison of Papp between the emulsions can be observed as shown in Table 4.
Table 4.
Papp of ibuprofen in the ileum and the enhancement ratio of the emulsion formulations when compared with control. Results are expressed as the mean ± S.E of at least 3 experiments. ∗P < 0.01, ∗P < 0.05, N.S. no significance different, compared with the control.
| Formulation | Ibuprofen concentration (w/w) | Papp (x10−6 cm/s) | Enhancement ratio |
|---|---|---|---|
| Control | 3.0% | 1.3 ± 0.2 | - |
| Ibuprofen nanoemulsion (NE) | 1.5% | 8.1 ± 1.9∗∗ | 6.2 |
| 3.0% | 13.8 ± 2.2∗∗ | 10.6 | |
| 6.0% | 8.6 ± 0.5∗∗ | 6.6 | |
| Ibuprofen microemulsion A (ME A) |
1.5% | 3.9 ± 0.2∗∗ | 3.0 |
| 3.0% | 6.9 ± 2.5∗∗ | 5.3 | |
| 6.0% | 5.4 ± 0.6∗∗ | 4.2 | |
| Ibuprofen microemulsion B (ME B) |
1.5% | 1.4 ± 0.1N.S. | 1.1 |
| 3.0% | 1.8 ± 0.2N.S. | 1.4 | |
| 6.0% | 1.6 ± 0.1N.S. | 1.2 |
When SE L-1695 was used as the surfactant, 3.0 % w/w ibuprofen loaded nanoemulsion demonstrates the highest Papp value (13.8 × 10−6 cm/s) across the small intestine tissue. The Papp value of 3.0% w/w ibuprofen loaded microemulsion, ME A formulated SE O-1570 displayed a lower Papp value of 6.9 × 10−6 cm/s across the small intestine. The Papp value of 3.0% w/w ibuprofen ME B formulated using SE P-1570 displayed the lowest Papp value (1.76 × 10−6 cm/s). The values of Papp for nanoemulsion (NE) loaded with 3% (w/w) ibuprofen in the ileum were 10.6-fold higher than control values. Overall, the transport of ibuprofen NE across ileum was shown to be higher than the MEs and control as illustrated by the higher ibuprofen transported over 120 min.
An increase in drug concentration, from 1.5 to 3.0% w/w, was observed to lead to a higher penetration rate across the small intestine membrane. This was proven by the data obtained from laurate, oleate and palmitate, as 3.0% concentration of ibuprofen gave higher rates of Papp values compared to 1.5% concentration. On the other hand, should the drug concentration be too high, the droplet size would become too large leading to a decrease in the rate of ibuprofen transported as seen in all the formulations loaded with 6% w/w of ibuprofen.
It has been shown that particle size has a major influence on the efficiency of particle uptake within the intestinal tissue [46]. The larger particle size in Ibuprofen ME B relative to ibuprofen ME A may provide a probable reason for the lower Papp value of ME B relative to ME A. As the particle size in a colloidal system increases, the rate Fickian diffusion of ibuprofen across the intestinal wall will also decrease leading to reduction in the rate of drug transport. This is because the effective diffusivity of particles in intestinal mucosa decreases with increasing particle size [17]. A study by Yildiz et al reported that the permeation rate of carboxylate-modified particles increased as particle size decreased from 500 nm to 20nm [17]. Histological evaluation of the tissue sections revealed that 100 nm particles were diffused across the submucosal layers while larger size microparticles were primarily localized in the epithelial lining of tissue [46]. In general, smaller particles size would increase intestinal permeation regardless of using nanoemulsion or nanoparticles as drug delivery system [18]. A recent study by Meirelles et al also corroborates our findings where benzopyran HP1, a compound isolated from Hypericum polyanthemum increased approximately 5.3 times after its incorporation in a nanoemulsions system as compared to solubilisation in cyclodextrin alone [47]. It is apparent from the in vitro study that nanoemulsion provides an elegant approach to improve the delivery of drug molecules across the gut epithelium. In addition, it is also worth noting that nanoemulsion has also been reported to display improved shelf-life due to their small particle size conferring protection against flocculation, coalescence, aggregation and Ostwald ripening [48].
3.4. Transepithelial electrical resistance (TEER) analysis
In an attempt to elucidate the mechanism that is involved in enhancing the permeation of ibuprofen across the ileum, further experimental analysis was performed on the systems using TEER. TEER was used to assess the effect of drug presence on the intestinal barrier function as well as indicators of intercellular passage of the intestinal mucosa [19]. Since the major route of ions occur paracellularly, TEER has been used for assessing the permeability of tight junctions [20] and its association with an increase in paracellular permeability [21].
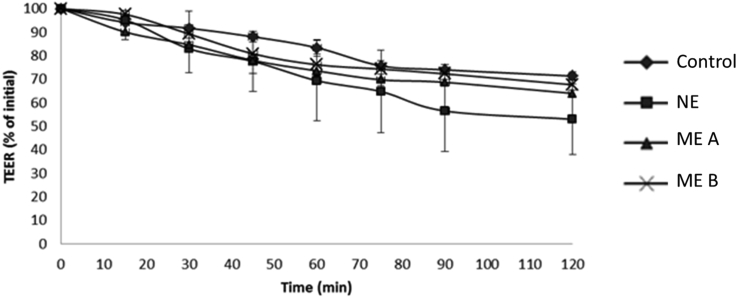
All three formulations displayed lower TEER value than the control. According to Figure 3, ibuprofen NE with a concentration of 3.0% w/w had the lowest time-dependent decrease in TEER values leading to an increase in ibuprofen absorption as compared to microemulsions and oil solution. In a previous work by Asmar et al, a time-dependent decrease TEER is an indicator of tight junction disassembly [49]. This is further supported by the work of Stuettgen et al who showed that the addition of phenylpiperazine (PPZ) and its derivatives induced concentration-dependent decreases in TEER leading to an increase paracellular uptake across the intestine [21]. Therefore, by comparing our results, it is postulated that the enhancement in ibuprofen absorption across small intestine may involve the time-dependent dilation of tight junction between the epithelial cells leading to enhanced drug transport via the paracellular pathway [50].
Figure 3.
Effects of Ibuprofen formulations on the transepithelial electrical resistance (TEER) values of the ileum. Results are expressed as the mean ± S.E. (n = 3). NE, nanoemulsion; ME, microemulsion.
Ibuprofen nanoemulsion may loosen the tight junction by reducing the expression of claudin-4, which is a major tight junction protein that imposes a control over the selectivity and permeability ions and small molecules [50, 51]. The loosening of the tight junction may be attributed to the presence of sucrose ester on the surface of the nanoemulsion droplet that actively interact with the ileum membrane leading to a reduction in TEER over time. Mechanistic study has shown that exposure of intestinal lining to sucrose ester leads to a reduction in claudin-1 and claudin 4 expression that results in loosening of tight junctions which culminates in enhanced paracellular transport [52]. On the other hand, Kiss et al also demonstrated that sucrose esters decreased the resistance and impedance of the Caco-2 epithelial cell layers via enhanced fluidisation of Caco-2 plasma membrane. This enhanced fluidisation of the plasma membrane may lead to enhanced transcellular transport across the intestinal lining in addition to enhanced paracellular transport [53].
In addition, it is also known that the tight junction strands are composed of proteins structures that are also sensitive to rapid changes within the luminal lipidic microenvironment [54]. The resistance of the apical membrane along intestinal lining as measured via TEER, decreased most rapidly in the presence of ibuprofen nanoemulsion in comparison to microemulsion and standard emulsion (control) thereby promoting better absorption of ibuprofen across the intestinal membrane. Such observation is attributed to the smaller droplet size of the nanoemulsion leading to greater surface area of interaction between the nanoemulsion droplets and the intestinal lining relative to the other formulations [55]. This leads to much more rapid changes within the luminal lipidic microenvironment near the membrane of the ileum as well as enhanced membrane fluidisation of the ileum and loosening of the tight junctions which culminate in rapid reduction in TEER in the nanoemulsion treated group.
3.5. In vivo oral absorption study
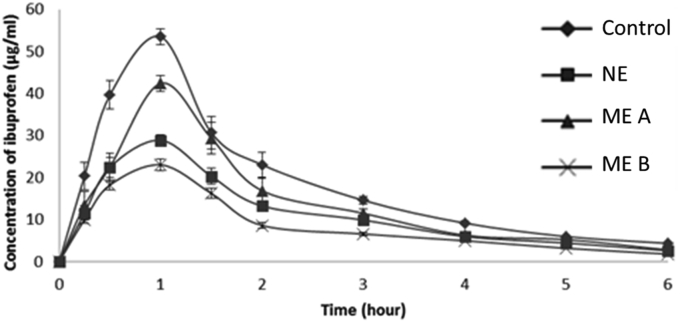
Ibuprofen is mostly absorbed from the small intestine into the lymphatic vessel which then enters the systemic circulation [56]. It has been shown that a smaller emulsion droplet size may lead to a higher ibuprofen drug transport as can be observed from our in vitro diffusion chamber study. Following this, we proceeded with an in vivo study to further evaluate ibuprofen nanoemulsion uptake in a rat model. In this study, emulsion using L-1695 forms a nanoemulsion system with nano droplet size range between 200 – 250 nm. Meanwhile, SE O-1570 and P-1570 form an ibuprofen microemulsion, with a droplet size of 1 μm and 3 μm respectively. Figure 4 displays the pharmacokinetic profiles of ibuprofen after oral administration in rats. The ibuprofen concentration in plasma of the four dosage forms increased rapidly within one hour after oral administration before being eliminated. Table 5 highlights the pharmacokinetic parameters (Cmax, Tmax, T1/2 and AUC) of ibuprofen after oral administration.
Figure 4.
Plasma concentration of ibuprofen versus time following oral administration of ibuprofen (30 mg/kg) in rats. The results are expressed as the mean ± S.D (n = 5). NE, nanoemulsion; ME, microemulsion.
Table 5.
Pharmacokinetic parameters of ibuprofen (30 mg/kg) following oral administration in rats. Results are expressed as the mean ± S.D (n = 5) ∗P < 0.05, compared with the control. NE, nanoemulsion; ME, microemulsion.
| Cmax (μg/ml) | Tmax (h) | T1/2(h) | AUC0–6h (μg/ml∙h) | |
|---|---|---|---|---|
| Control | 23.0 ± 1.4 | 1.0 | 1.87 ± 0.41 | 3060.3 ± 169.9 |
| NE | 53.5 ± 1.9 | 1.0 | 2.02 ± 0.28 | 6670.1 ± 283.8∗ |
| ME A | 42.4 ± 1.3 | 1.0 | 1.89 ± 0.27 | 5084.0 ± 246.3∗ |
| ME B | 28.9 ± 1.0 | 1.0 | 1.87 ± 0.41 | 4044.1 ± 79.0∗ |
According to Figure 4, the ibuprofen concentration in plasma of the four dosage forms increased rapidly within one hour after oral administration before being eliminated. The maximum ibuprofen concentration in plasma (Tmax) of the dosage forms was recorded similarly at 1.0 h. The incorporation of ibuprofen in a nanoemulsion system significantly increased the absorption of ibuprofen through gastrointestinal tract (GIT) as evidenced by the higher area under the curve AUC as compared to the ibuprofen oil solution and microemulsion.
Based on Table 5, the Cmax value of ibuprofen NE was 53.5 ± 1.9 μg/ml and in comparison, ibuprofen ME A was 42.4 ± 1.3 μg/ml. The Cmax value of ibuprofen ME B was 28.9 ± 1.0 μg/ml. The Cmax value of ibuprofen oil solution was 23.0 ± 1.4 μg/ml. The AUC of oral absorption was calculated using trapezoidal method from zero to final sampling time. The AUC0–6h values of ibuprofen NE and ibuprofen oil solution were 6670.1 ± 283.8 μg/ml∙h and 3060.3 ± 169.9 μg/ml∙h respectively. The AUC value of ibuprofen NE was statistically significant (p < 0.05) compared to the ibuprofen oil solution. The AUC of ibuprofen increased 2.2-fold after the oral administration of ibuprofen nanoemulsion. On the other hand, the AUC values of ibuprofen ME A and ME B were 5084.0 ± 246.3 μg/ml∙h and 4044.1 ± 79.0 μg/ml∙h respectively. The difference in rate of absorption between ibuprofen ME A and oil formulation was 1.7-fold, while the rate of absorption of ME B compared with oil formulation increased by 1.3-fold. These results confirmed that the absorption of ibuprofen formulated as nanoemulsion enhanced the oral absorption in rats compared to ibuprofen MEs and ibuprofen oil solution. This might be due to the improved solubility of ibuprofen in nanoemulsion permitting more time for the drug to diffuse across the unstirred water layer in intestine and across the epithelial layer [25], In addition, the nanoemulsion system confers greater surface area of interaction with the intestinal villi relative to microemulsion thus promoting intestinal absorption [57]. Furthermore, the dilation of tight junction as explained earlier may also serve as an explanation for the enhanced permeation ibuprofen across the gut.
From Table 5, the elimination half-life values were calculated from ibuprofen plasma concentration in rats following formulation administration. The results were closely similar and were not statistically significant. On the basis of the results obtained from this analysis, it could be concluded that ibuprofen NE may have a higher rate of drug absorption compared with ME A and ME B while displaying similar elimination half-life. Previously, Kim et al also reported similar results in which doxorubicin (DOX) was formulated in a medium chain glycerides-based colloidal microemulsion to enhance the intestinal paracellular absorption of DOX. The group discovered that DOX microemulsions markedly enhanced the intestinal absorption as compared to DOX solution [58]. A recent study by Hou et al [59] reported that curcumin loaded self-nanomicellizing solid dispersion based on rebaudioside A (RA) displayed improved aqueous solubility and exhibited enhanced pharmacokinetic profiles when compared to free curcumin, highlighting the value of colloidal nanocarriers as a drug delivery vehicle to improve intestinal absorption.
Sucrose esters are of one the excipients which are typically used in formulation science in order to modify the bioavailability of drug molecules. Such enhancement in bioavailability as observed in Figure 4 may be attributed to dissolution enhancement conferred by sucrose ester when the excipient is incorporated into the formulation. Sucrose ester have been reported to increase the solubility and release of various poorly-water soluble drug such as verapamil [60], fenofibrate [61] and chlorin e6 [62]. In addition to improving drug dissolution, sucrose ester also actively interacts with biological barriers which culminate in enhanced penetration and absorption across the gastrointestinal tract. Alama et al explored the use of sucrose ester in enhancing the intestinal absorption of alendronate, a drug used in the management of osteoporosis [52]. The group discovered upon conducting in vivo studies in rodents that co-delivery of alendronate in the presence of sucrose ester resulted in enhanced intestinal absorption in a dose-dependent manner. Upon conducting extensive mechanistic study, they discovered such enhancement in intestinal absorption was attributed to the increased in membrane fluidity as well as transient loosening of the tight junctions of the intestinal lining leading to improved absorption. However, in our current work we identified that incorporation of sucrose ester into an emulsion of decreasing droplet size further enhance intestinal absorption.
By comparing our results with the findings previously reported in the literature, it can be postulated that incorporating sucrose ester into a nanoemulsion systems improves the dissolution and absorption enhancing properties of the surfactant. Sucrose esters are non-ionic surfactants that are located at the oil-water interphase in a colloidal system. In the case of nanoemulsion, the dispersed oil phase possesses greater surface area due to the smaller droplet size relative microemulsion and standard emulsions [55]. Since the sucrose ester is located at the surface of the droplet, the surfactant will have more interfacial area to interact with both the drug in the oil phase as well as the luminal membrane along the intestinal tract. The enhanced surface area of interaction between the surfactant and the drug will improve drug dissolution while the improved interaction between the surfactant with the luminal membrane will increased intestinal membrane fluidity and loosening of the tight junctions. Collectively, these processes will lead to enhanced drug absorption when delivered using a nanoemulsion system leading to enhanced bioavailability.
In general, it is evident that the enhancement in ibuprofen absorption when formulated as a nanoemulsion highlights the utility of the nanocarrier as a drug delivery platform for improved oral bioavailability. Such drug delivery platform may be of great utility in enhancing the absorption of poorly water-soluble drugs that frequently suffer poor oral bioavailability. To the best of our knowledge, this study is the first reported study on the oral delivery of ibuprofen through the enhancement of intestinal permeation via the paracellular pathway. The use of surfactants such as SE laurate (L-1695) alongside olive oil and glycerol in an optimised combination has resulted in the successful development of a stable nanoemulsion system capable of enhancing oral bioavailability via tight junction modulation culminating in enhanced ibuprofen permeation.
4. Conclusions
Nanoemulsion has the potential of enhancing the oral bioavailability of ibuprofen. A pseudoternary phase diagram was successfully constructed to optimise the concentration of oil, surfactant and co-surfactant mixture in order to determine the nanoemulsion region suitable for drug delivery. We have identified that the combination of olive oil, SE L-1695 and glycerol produced a large nanoemulsion region which could be utilised for drug delivery. In vitro study utilising gut epithelium lining showed that the Papp for the optimised nanoemulsion loaded with 3% (w/w) ibuprofen displayed 10.6 times higher drug transport than the control formulations. Furthermore, the oral bioavailability for ibuprofen nanoemulsion was 2.2-folds higher relative to the control formulation when evaluated in vivo. Collectively, this work demonstrates that through judicious selection of excipients, a stable nanoemulsion for ibuprofen was developed that provide enhanced oral bioavailability.
Declarations
Author contribution statement
K.A. Hamid: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
N. Anuar: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
A.H. Sabri: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
T.J.B. Effendi: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
The authors wish to thank Ministry of Higher Education of Malaysia for providing financial assistance in the form of FRGS 600-RMI/ST/FRGS 5/3/Fst (115/2013) grant.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supporting Information_Khuriah
References
- 1.Yousaf A.M., Malik U.R., Shahzad Y., Mahmood T., Hussain T. Silymarin-laden PVP-PEG polymeric composite for enhanced aqueous solubility and dissolution rate: preparation and in vitro characterization. J. Pharm. Anal. 2019;9:34–39. doi: 10.1016/j.jpha.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devadasu V.R., Deb P.K., Maheshwari R., Sharma P., Tekade R.K. Elsevier Inc.; 2018. Physicochemical, Pharmaceutical, and Biological Considerations in GIT Absorption of Drugs. [Google Scholar]
- 3.Groo A.C., De Pascale M., Voisin-Chiret A.S., Corvaisier S., Since M., Malzert-Fréon A. Comparison of 2 strategies to enhance pyridoclax solubility: nanoemulsion delivery system versus salt synthesis. Eur. J. Pharmaceut. Sci. 2017;97:218–226. doi: 10.1016/j.ejps.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 4.Putra O.D., Umeda D., Fujita E., Haraguchi T., Uchida T., Yonemochi E., Uekusa H. Solubility improvement of benexate through salt formation using artificial sweetener. Pharmaceutics. 2018;10 doi: 10.3390/pharmaceutics10020064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma H., Chen G., Wang T., Li Q., Liu Y. Design, synthesis, and biological evaluation of a novel water-soluble prodrug of docetaxel with amino acid as a linker. Chem. Biol. Drug Des. 2016:363–369. doi: 10.1111/cbdd.12762. [DOI] [PubMed] [Google Scholar]
- 6.Wilson V., Lou X., Osterling D.J., Stolarik D.F., Jenkins G., Gao W., Zhang G.G.Z., Taylor L.S. Relationship between amorphous solid dispersion in Vivo absorption and in Vitro dissolution: phase behavior during dissolution, speciation, and membrane mass transport. J. Contr. Release. 2018;292:172–182. doi: 10.1016/j.jconrel.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Schultz H.B., Thomas N., Rao S., Prestidge C.A. Supersaturated silica-lipid hybrids (super-SLH): an improved solid-state lipid-based oral drug delivery system with enhanced drug loading. Eur. J. Pharm. Biopharm. 2018;125:13–20. doi: 10.1016/j.ejpb.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Vithani K., Jannin V., Pouton C.W., Boyd B.J. Colloidal aspects of dispersion and digestion of self-dispersing lipid-based formulations for poorly water-soluble drugs. Adv. Drug Deliv. Rev. 2019;142:16–34. doi: 10.1016/j.addr.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Sun C., Gui Y., Hu R., Chen J., Wang B., Guo Y., Lu W., Nie X., Shen Q., Gao S., Fang W. Preparation and pharmacokinetics evaluation of solid self-microemulsifying drug delivery system (S-SMEDDS) of Osthole. AAPS PharmSciTech. 2018;19:2301–2310. doi: 10.1208/s12249-018-1067-3. [DOI] [PubMed] [Google Scholar]
- 10.Sindi A.M., Hosny K.M. Preparation and evaluation of protective effect of pumpkin seed oil based self nanoemulsifying oral delivery system against ibuprofen-induced peptic ulcer. J. Drug Deliv. Sci. Technol. 2019;52:415–420. [Google Scholar]
- 11.Dizaj S.M., Vazifehasl Z., Salatin S., Adibkia K., Javadzadeh Y. Nanosizing of drugs: effect on dissolution rate. Res. Pharm. Sci. 2015;10:95–108. http://www.ncbi.nlm.nih.gov/pubmed/26487886 [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmad J., Amin S., Kohli K., Mir S.R. Construction of pseudoternary phase diagram and its evaluation: development of self-dispersible oral formulation. Int. J. Drug Dev. Res. 2013;5:84–90. [Google Scholar]
- 13.Cerqueira M.Â., Pinheiro A.C., Ramos O.L., Silva H., Bourbon A.I., Vicente A.A. Elsevier Inc.; 2017. Advances in Food Nanotechnology. [Google Scholar]
- 14.Szuts A., Szabó-Révész P. Sucrose esters as natural surfactants in drug delivery systems - a mini-review. Int. J. Pharm. 2012;433:1–9. doi: 10.1016/j.ijpharm.2012.04.076. [DOI] [PubMed] [Google Scholar]
- 15.Shrivastava A. 2018. Introduction to Plastics Engineering. [Google Scholar]
- 16.Danaei M., Dehghankhold M., Ataei S., Davarani F.H., Javanmard R., Dokhani A., Khorasani S., Id M.R.M. 2018. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems; pp. 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yildiz H.M., McKelvey C.A., Marsac P.J., Carrier R.L. Size selectivity of intestinal mucus to diffusing particulates is dependent on surface chemistry and exposure to lipids. J. Drug Target. 2015;23:768–774. doi: 10.3109/1061186X.2015.1086359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams K.M., Gokulan K., Cerniglia C.E., Khare S. Size and dose dependent effects of silver nanoparticle exposure on intestinal permeability in an in vitro model of the human gut epithelium. J. Nanobiotechnol. 2016;14:8–13. doi: 10.1186/s12951-016-0214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mineo H., Hara H., Shigematsu N., Okuhara Y., Tomita F. Melibiose, difructose anhydride III and difructose anhydride IV enhance net calcium absorption in rat small and large intestinal epithelium by increasing the passage of tight junctions in vitro. J. Nutr. 2002;132:3394–3399. doi: 10.1093/jn/132.11.3394. [DOI] [PubMed] [Google Scholar]
- 20.Chen S., Einspanier R., Schoen J. Transepithelial electrical resistance (TEER): a functional parameter to monitor the quality of oviduct epithelial cells cultured on filter supports. Histochem. Cell Biol. 2015;144:509–515. doi: 10.1007/s00418-015-1351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stuettgen V., Brayden D.J. Investigations of piperazine derivatives as intestinal permeation enhancers in isolated rat intestinal tissue mucosae. AAPS J. 2020;22:33. doi: 10.1208/s12248-020-0416-9. [DOI] [PubMed] [Google Scholar]
- 22.Salim N., Basri M., Rahman M.B., Abdullah D.K., Basri H. Modification of palm kernel oil esters nanoemulsions with hydrocolloid gum for enhanced topical delivery of ibuprofen. Int. J. Nanomed. 2012;7:4739–4747. doi: 10.2147/IJN.S34700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen H., Chang X., Du D., Li J., Xu H., Yang X. Microemulsion-based hydrogel formulation of penciclovir for topical delivery. Int. J. Pharm. 2006;315:52–58. doi: 10.1016/j.ijpharm.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Jiang B., Hu L., Gao C., Shen J. Ibuprofen-loaded nanoparticles prepared by a co-precipitation method and their release properties. Int. J. Pharm. 2005;304:220–230. doi: 10.1016/j.ijpharm.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Hu L., Yang J., Liu W., Li L. Preparation and evaluation of ibuprofen-loaded microemulsion for improvement of oral bioavailability. Drug Deliv. 2011;18:90–95. doi: 10.3109/10717544.2010.522613. [DOI] [PubMed] [Google Scholar]
- 26.Kim B.S., Won M., Lee K.M., Kim C.S. In vitro permeation studies of nanoemulsions containing ketoprofen as a model drug. Drug Deliv. 2008;15:465–469. doi: 10.1080/10717540802328599. [DOI] [PubMed] [Google Scholar]
- 27.Hu L., Tang X., Cui F. Solid lipid nanoparticles (SLNs) to improve oral bioavailability of poorly soluble drugs. J. Pharm. Pharmacol. 2004;56:1527–1535. doi: 10.1211/0022357044959. [DOI] [PubMed] [Google Scholar]
- 28.Dingler A., Gohla S. Production of solid lipid nanoparticles (SLN): scaling up feasibilities. J. Microencapsul. 2002;19:11–16. doi: 10.1080/02652040010018056. [DOI] [PubMed] [Google Scholar]
- 29.Salleh S.H.A., Hamid K.A., Effendi T.J.B., Jamil N. 2012 IEEE Symp. Business, Eng. Ind. Appl. IEEE; 2012. Characterization and stability evaluation of olive oil nanoemulsion-based hydrogel formulation by nanophase emulsification technique; pp. 824–828. [Google Scholar]
- 30.Hamid K.A., Lin Y., Gao Y., Katsumi H., Sakane T., Yamamoto A. The effect of wellsolve, a novel solubilizing agent, on the intestinal barrier function and intestinal absorption of griseofulvin in rats. Biol. Pharm. Bull. 2009;32:1898–1905. doi: 10.1248/bpb.32.1898. http://www.ncbi.nlm.nih.gov/pubmed/19881305 [DOI] [PubMed] [Google Scholar]
- 31.Jaafar M.H.M., Hamid K.A. Chitosan-coated alginate nanoparticles enhanced absorption profile of insulin via oral administration. Curr. Drug Deliv. 2019;16:672–686. doi: 10.2174/1567201816666190620110748. [DOI] [PubMed] [Google Scholar]
- 32.Abdullah G.Z., Abdulkarim M.F., Salman I.M., Ameer O.Z., Chitneni M., Mahdi E.S., Yam M.F., Hameem S., Basri M., Sattar M.A., Noor A.M. Stability studies of nano-scaled emulsions containing Ibuprofen for topical delivery. Int. J. Drug Deliv. 2011;3:74–82. doi: 10.2147/IJN.S14667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishii F., Nii T. Elsevier B.V.; 2014. Lipid Emulsions and Lipid Vesicles Prepared from Various Phospholipids as Drug Carriers. [Google Scholar]
- 34.Chanama R., Horn G., McClements D.J. Influence of oil polarity on droplet growth in oil-in-water emulsions stabilized by a weakly adsorbing biopolymer or a nonionic surfactant. J. Colloid Interface Sci. 2002;247:167–176. doi: 10.1006/jcis.2001.8110. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe T., Kawai T., Nonomura Y. Effects of fatty acid addition to oil-in-water emulsions stabilized with sucrose fatty acid ester. J. Oleo Sci. 2018;67:307–313. doi: 10.5650/jos.ess17097. [DOI] [PubMed] [Google Scholar]
- 36.Leong W.F., Che Man Y.B., Lai O.M., Long K., Nakajima M., Tan C.P. Effect of sucrose fatty acid esters on the particle characteristics and flow properties of phytosterol nanodispersions. J. Food Eng. 2011;104:63–69. [Google Scholar]
- 37.Honary S., Zahir F. Effect of zeta potential on the properties of nano-drug delivery systems - a review (Part 1) Trop. J. Pharmaceut. Res. 2013;12:255–264. [Google Scholar]
- 38.Meor Mohd Affandi M.M.R., Julianto T., Majeed A.B.A. Development and stability evaluation of Astaxanthin nanoemulsion. Asian J. Pharmaceut. Clin. Res. 2011;4:143–148. [Google Scholar]
- 39.Simonazzi A., Cid A.G., Villegas M., Romero A.I., Palma S.D., Bermúdez J.M. 2018. Nanotechnology Applications in Drug Controlled Release. [Google Scholar]
- 40.Xu J., Mukherjee D., Chang S.K.C. Physicochemical properties and storage stability of soybean protein nanoemulsions prepared by ultra-high pressure homogenization. Food Chem. 2018;240:1005–1013. doi: 10.1016/j.foodchem.2017.07.077. [DOI] [PubMed] [Google Scholar]
- 41.Isailović T.M., Todosijević M.N., Dordević S.M., Savić S.D. Physicochemical and Biopharmaceutical Characteristics/Performances; 2017. Natural Surfactants-Based Micro/Nanoemulsion Systems for NSAIDs- Practical Formulation Approach. [Google Scholar]
- 42.Vasconcelos T., Marques S., Sarmento B. Measuring the emulsification dynamics and stability of self-emulsifying drug delivery systems. Eur. J. Pharm. Biopharm. 2018;123:1–8. doi: 10.1016/j.ejpb.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 43.Wik J., Bansal K.K., Assmuth T., Rosling A., Rosenholm J.M. Facile methodology of nanoemulsion preparation using oily polymer for the delivery of poorly soluble drugs. Drug Deliv. Transl. Res. 2019:19–21. doi: 10.1007/s13346-019-00703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seedher N., Kanojia M. Micellar solubilization of some poorly soluble antidiabetic drugs: a technical note. AAPS PharmSciTech. 2008;9:431–436. doi: 10.1208/s12249-008-9057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh Y., Meher J.G., Raval K., Khan F.A., Chaurasia M., Jain N.K., Chourasia M.K. Nanoemulsion: concepts, development and applications in drug delivery. J. Contr. Release. 2017;252:28–49. doi: 10.1016/j.jconrel.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Desai M.P., Labhasetwar V., Amidon G.L., Levy R.J. Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharm. Res. 1996;13:1838–1845. doi: 10.1023/a:1016085108889. [DOI] [PubMed] [Google Scholar]
- 47.Meirelles G.C., Mendes C., Caon T., Teixeira H.F., von Poser G., Ponchel G. Intestinal permeability enhancement of benzopyran HP1-loaded nanoemulsions. Eur. J. Pharmaceut. Sci. 2019;127:115–120. doi: 10.1016/j.ejps.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 48.Rezaee M., Basri M., Raja R.N.Z., Rahman A., Salleh A.B., Chaibakhsh N., Karjiban R.A. Formulation development and optimization of palm kernel oil esters-based nanoemulsions containing sodium diclofenac. Int. J. Nanomed. 2014;9:539–548. doi: 10.2147/IJN.S49616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El Asmar R., Panigrahi P., Bamford P., Berti I., Not T., Coppa G.V., Catassi C., Fasano A. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology. 2002 doi: 10.1053/gast.2002.36578. [DOI] [PubMed] [Google Scholar]
- 50.Bergmann K.R., Liu S.X.L., Tian R., Kushnir A., Turner J.R., Li H.L., Chou P.M., Weber C.R., De Plaen I.G. Bifidobacteria stabilize claudins at tight junctions and prevent intestinal barrier dysfunction in mouse necrotizing enterocolitis. Am. J. Pathol. 2013;182:1595–1606. doi: 10.1016/j.ajpath.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakaya Y., Takaya M., Hinatsu Y., Alama T., Kusamori K., Katsumi H., Sakane T., Yamamoto A. Enhanced oral delivery of bisphosphonate by novel absorption enhancers: improvement of intestinal absorption of alendronate by N-acyl amino acids and N-acyl taurates and their absorption-enhancing mechanisms. J. Pharmacol. Sci. 2016;105:3680–3690. doi: 10.1016/j.xphs.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 52.Alama T., Katayama H., Hirai S., Ono S., Kajiyama A., Kusamori K., Katsumi H., Sakane T., Yamamoto A. Enhanced oral delivery of alendronate by sucrose fatty acids esters in rats and their absorption-enhancing mechanisms. Int. J. Pharm. 2016;515:476–489. doi: 10.1016/j.ijpharm.2016.10.046. [DOI] [PubMed] [Google Scholar]
- 53.Kiss L., Hellinger É., Pilbat A.M., Kittel Á., Tö Rök Z., Furedi, Szakács G., Veszelka S., Sipos P., Ózsvári B.É., Puskás L.G., Vastag M., Szabó -Révész P., Deli M.A. Sucrose esters increase drug penetration, but do not inhibit P-glycoprotein in Caco-2 intestinal epithelial cells. J. Pharmacol. Sci. 2014;103:3107–3119. doi: 10.1002/jps.24085. [DOI] [PubMed] [Google Scholar]
- 54.Trimble W.S., Grinstein S. Barriers to the free diffusion of proteins and lipids in the plasma membrane. 2015;208:259–271. doi: 10.1083/jcb.201410071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Priya L.B., Baskaran R., Padma V.V. Elsevier Inc.; 2017. Chapter 21 - Phytonanoconjugates in Oral Medicine. [Google Scholar]
- 56.Mazaleuskaya L.L., Theken K.N., Gong L., Thorn C.F., Fitzgerald G.A., Altman R.B., Klein T.E. PharmGKB summary: ibuprofen pathways. Pharmacogenetics Genom. 2015;25:96–106. doi: 10.1097/FPC.0000000000000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karamanidou T., Bourganis V., Kammona O., Kiparissides C. Lipid-based nanocarriers for the oral administration of biopharmaceutics. Nanomedicine. 2016;11:3009–3032. doi: 10.2217/nnm-2016-0265. [DOI] [PubMed] [Google Scholar]
- 58.Kim J.E., Yoon I.S., Cho H.J., Kim D.H., Choi Y.H., Kim D.D. Emulsion-based colloidal nanosystems for oral delivery of doxorubicin: improved intestinal paracellular absorption and alleviated cardiotoxicity. Int. J. Pharm. 2014;464:117–126. doi: 10.1016/j.ijpharm.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 59.Hou Y., Wang H., Zhang F., Sun F., Xin M., Li M., Li J., Wu X. Novel self-nanomicellizing solid dispersion based on rebaudioside A: a potential nanoplatform for oral delivery of curcumin. Int. J. Nanomed. 2019;14:557–571. doi: 10.2147/IJN.S191337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Horoz B.B., Kiliçarslan M., Yüksel N., Baykara T. Influence of aluminum tristearate and sucrose stearate as the dispersing agents on physical properties and release characteristics of Eudragit RS microspheres. AAPS Pharm. Sci. Technol. 2006;7:1–7. doi: 10.1208/pt070116. [DOI] [PubMed] [Google Scholar]
- 61.Vithani K., Hawley A., Jannin V., Pouton C., Boyd B.J. Solubilisation behaviour of poorly water-soluble drugs during digestion of solid SMEDDS. Eur. J. Pharm. Biopharm. 2018;130:236–246. doi: 10.1016/j.ejpb.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 62.Paul S., Heng P.W.S., Chan L.W. Improvement in dissolution rate and photodynamic efficacy of chlorin e6 by sucrose esters as drug carrier in nanosuspension formulation: optimisation and in vitro characterisation. J. Pharm. Pharmacol. 2018;70:1152–1163. doi: 10.1111/jphp.12947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information_Khuriah