Abstract
Purpose
This study characterizes the clinical and genetic features of nine unrelated patients with de novo variants in the NR4A2 gene.
Methods
Variants were identified and de novo origins were confirmed through trio exome sequencing in all but one patient. Targeted RNA sequencing was performed for one variant to confirm its splicing effect. Independent discoveries were shared through GeneMatcher.
Results
Missense and loss-of-function variants in NR4A2 were identified in patients from eight unrelated families. One patient carried a larger deletion including adjacent genes. The cases presented with developmental delay, hypotonia (six cases), and epilepsy (six cases). De novo status was confirmed for eight patients. One variant was demonstrated to affect splicing and result in expression of abnormal transcripts likely subject to nonsense-mediated decay.
Conclusion
Our study underscores the importance of NR4A2 as a disease gene for neurodevelopmental disorders and epilepsy. The identified variants are likely causative of the seizures and additional developmental phenotypes in these patients.
Keywords: NR4A2, epilepsy, seizures, neurodevelopmental disorder, developmental disorder
INTRODUCTION
The NR4A2 gene encodes a steroid–thyroid hormone–retinoid receptor that acts as a nuclear receptor (NR) transcription factor. The NR transcription factors play a regulatory role in various aspects of mammalian physiology such as neuronal development, inflammation, carcinogenesis, and memory formation. NR4A2 is required for development, function, and neurotransmission of dopaminergic neurons.1
The NR4A2 protein consists of two main domains: a DNA binding domain (DBD) and a ligand binding domain (LBD). The DBD is a highly conserved domain containing two C4 type zinc fingers that bind to specific motifs in DNA hormone response elements, and is connected to the C-terminal LBD via a linker region. The NR4A2 protein functions by binding small molecule ligands within conserved ligand binding patches located in the hydrophobic core of the LBD.2 Ligand binding induces a conformational change in the LBD leading to changes in interaction of nuclear receptor coregulators and other proteins. This alters chromatin structure and gene expression and, therefore, up and down regulation of target genes. A mutated gene may encode for a misfolded protein, dysfunctional ligand binding pocket, or dysfunctional DNA binding.
Haploinsufficiency of the NR4A2 gene caused by heterozygous chromosomal deletions was previously associated with a neurodevelopmental disorder with high penetrance, suggesting that heterozygous loss of NR4A2 is autosomal dominant.3 NR4A2 knockout in midbrain dopaminergic neurons of adult mice has shown to result in neuronal degeneration and impaired motor function.1 Various polymorphisms in NR4A2 have been associated with disorders related to dopaminergic dysfunction such as Parkinson disease, schizophrenia, manic depression, and autism spectrum disorder.4–6 To our knowledge, there is only one previous report of a variant of NR4A2 (NM_006186.3:c.327dup, p.S110Vfs*2) associated with epilepsy.7
Here we report nine patients with variants in NR4A2 and developmental delay/intellectual disability with or without epilepsy.
MATERIALS AND METHODS
Informed consent for genetic testing was obtained from all patients and parents included in the present study. The consent and protocols were approved by the respective institutional ethical review boards (University Medical Centre Utrecht, Mayo Clinic, Children’s Mercy Kansas City, Technical University of Munich, University of Arkansas for Medical Sciences, University of Virginia, Washington University School of Medicine, Boston Children’s Hospital, Ann & Robert H. Lurie Children’s Hospital). All patients underwent exome sequencing; however, the variant identified in patient 2 was detected using a targeted exome analysis for neurodevelopmental genes. The deletion observed in patient 9 was detected by comparative genome hybridization using an Agilent 180K oligoarray. The microdeletion was verified by fluorescence in situ hybridization (FISH). All other cases were analyzed using the complete exome (Supplementary information). By sharing through GeneMatcher,8 we discovered other patients harboring variants in the NR4A2 gene (patients 2, 4, 5, 7). For these patients, exome sequencing was performed at GeneDx (Gaithersburg, MD, USA) using the Clinical Research Exome kit (Agilent Technologies, Santa Clara, CA) or the IDT xGen Exome Research Panel v1.0. The general assertion criteria for variant classification are publicly available on the GeneDx ClinVar submission page (http://www.ncbi.nlm.nih.gov/clinvar/submitters/26957/). All variants identified in patients tested at GeneDx were reported as variants of uncertain significance in accordance with American College of Medical Genetics and Genomics (ACMG) criteria.9
Variants were annotated based on NR4A2 transcript NM_006186.3. De novo status of the variant was confirmed by exome sequencing or Sanger sequencing of parental samples. In silico tools MaxEntScan, NNSPLICE, and Human Splicing Factor predicted altered splicing and potential loss of function (LoF). Targeted RNA sequencing was done using blood-derived RNA of patient 2 to examine the functional consequences of the variant on splicing. Altered splicing was confirmed by reverse transcription polymerase chain reaction (RT-PCR) (details are in Supplementary Information Figures S1 and S2). Plot Protein and RStudio v1.1.463 were used to visualize the variants in Fig. 1. Predicted domain architecture information for the corresponding amino acids in the protein was retrieved from databases SMART, Prosite, InterPro, and Pfam.
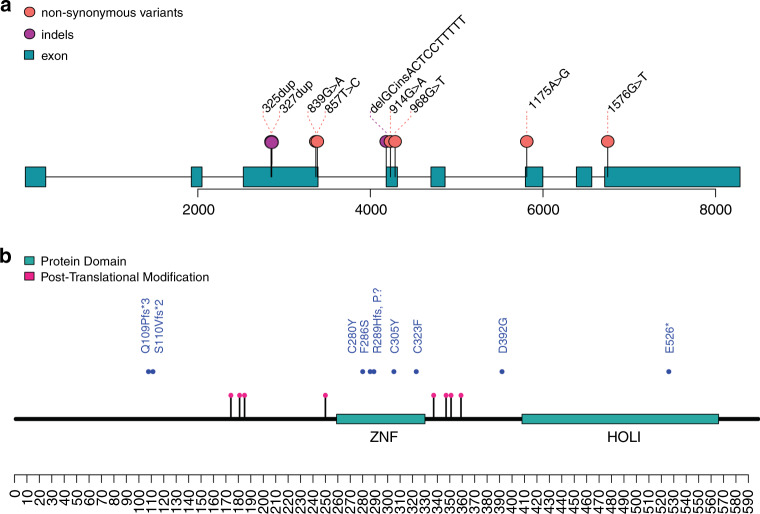
Fig. 1. Schematic view of the distribution of pathogenic variants in NR4A2.
(a) Transcript description and locations (marked by circles) of the variants found in NR4A2 gene. (b) Predicted effects (blue dots) of the pathogenic variants on NR4A2 protein sequence. The c.327dup,p.S110Vfs*2 variant was published previously.7.
RESULTS
We report a case series of nine patients with novel variants in NR4A2, including eight patients with a confirmed de novo variant, and one patient with a larger deletion encompassing NR4A2. The patients (five females, four males; mean age 12.4 years, age range 2–43 years, at the time of inclusion in current study) show heterogeneous phenotypes (detailed phenotypes are given in online Supplementary information). Their neurodevelopmental phenotypes are characterized by delayed psychomotor development (9/9), which was initially normal in two patients. Individuals presented with varying levels (mild to severe) of intellectual disability (ID)/developmental delay (DD). Other features include epilepsy (6/9), speech/language impairment (5/9), behavioral problems (5/9), and movement disorder/hypotonia (8/9). Patients presented with variable epilepsy phenotype, including rolandic epilepsy, generalized encephalopathy, West syndrome, and infantile spasms. One patient showed epileptoform activity and photosensitivity on electroencephalogram (EEG). Seizure type included tonic clonic, generalized, absence, and focal seizures. Seizures remained refractory in two patients and the remaining four became seizure-free on appropriate antiepileptic drugs. Behavioral problems included autism, attention deficit–hyperactivity disorder, hyperactivity, anxiety, and hyposensitivity. Two patients had ataxia. There was no apparent genotype–phenotype correlation.
Genetic results
We identified eight patients with intragenic NR4A2 variants and one patients carrying a larger deletion including NR4A2. Variants were not present in ExAC and gnomAD. De novo occurrence of the variants was confirmed for eight of these patients. The origin of the variant in patient 5 could not be confirmed due to unavailability of the father, but it was not maternal. Five patients had missense variants, one had a microdeletion, and three had nonsense or frameshift variants leading to a premature stop codon (Table 1, Fig. 1). Four missense variants and one splice-acceptor site variant (c.839G>A, p.C280Y; c.914G>A, p.C305Y; c.857T>C, p.F286S; c.968G>T, p.C323F; c.865-1_865delGCinsAAAAAGGAGT) were located in the DBD of the protein that may affect DNA binding of the transcription factor. Patient 4 carried a missense variant (c.1175A>G, p.D392G) affecting a hinge region without any secondary structure in the LBD. Patient 5 had a nonsense variant (c.1576G>T, p.E526*) in the LBD, introducing premature termination. All missense variants were located in a gene region that is enriched for pathogenic variants across the NR4A2 gene family (see Supplementary Figure S4).
Table 1.
Clinical phenotypes of patients with heterozygous de novo and putative de novo NR4A2 variants.
| Patient | Variant (NM_006186.3) | Inheritance | Protein domain/region | Seizures | Age, years/sex/age at seizure onset | Developmental delay | Speech and language impairment | Motor delay | Intellectual disability | Behavioral problems | MRI findings | Neurologic examination findings | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | c.839G>A, p.C280Y | De novo | ZnF_C4 domain, DNA binding domain | Yes | 15/F/6.5 years | Global | NA | NA | Severe | Autism | Normal | Normal | Sleeping difficulties |
| 2 | c.865-1_865delGCinsAAAAAGGAGT, p.? | De novo | ZnF_C4 domain, DNA binding domain | Yes | 12/M/10 years | Global | Yes | Yes | Mild | Hyperactivity, anxiety | Normal | Mild hypotonia | EDS hypermobility |
| 3 | c.914G>A, p.C305Y | De novo | ZnF_C4 domain, DNA binding domain | Yes | 9/F/NA | Moderate | NA | NA | Mild to moderate | NA | Gliosis | Choreoathetoid movements, dystonia, ataxic gait | NA |
| 4 | c.1175A>G, p.D392G | De novo | Hinge region | Yes | 3/F/5 months | Global | NA | NA | Severe | No | Moderate cerebellar atrophy | Severe hypotonia, feeding difficulties, dystonia | None |
| 5 | c.1576G>T, p.E526* | NA | HOLI, ligand binding domain | No | 5/M/Never | Global | Yes | Yes | Mild | Attachment disorder, hyposensitivity | NA | Mild hypotonia, no movement disorder | No |
| 6 | c.325dupC, p.Q109Pfs*3 | De novo | N-terminal regulatory domain | Yes | 2/M/6 months | Global | Yes | Yes | NA | Sensory sensitivity | Pontine hypoplasia, ventriculomegaly | Severe hypotonia, feeding difficulties | Facial dysmorphism, sleep disordered breathing |
| 7 | c.857T>C, p.F286S | De novo | ZnF_C4 domain, DNA binding domain | No | 4/F/Never | Global | Yes | Yes | Moderate | No | Normal | Hypotonia | Mild joint hypermobility, shagreen spot and hypopigmented spot |
| 8 | c.968G>T, p.C323F | De novo | ZnF_C4 domain, DNA binding domain | No | 19/F/Never | Global | Yes | Yes | Moderate to severe | No | Normal | Mild generalized hypotonia | Facial dysmorphism, joint hypermobility |
| 9 | arr[GRCh37]2q23.3q24.1(154790212_158488241)x1 | De novo | NA | yes | 43/M/13 years | Moderate | NA | NA | Moderate to severe | Hyperactivity, aggression | Enlarged cerebrospinal fluid spaces | Progressive ataxia in adulthood | No |
AED antiepileptic drug, EDS Ehlers–Danlos syndrome, EEG electroencephalogram, F female, M male, MRI magnetic resonance image, NA not assessed.
Patient 6 had a frameshift variant (c.325dup) in N-terminal regulatory domain introducing premature termination that is predicted to lead to nonsense-mediated decay (NMD) and LoF, similar to the previously published epilepsy patient (c.327dup).7 Patient 9 carried a chromosomal microdeletion arr[GRCh37]2q23.3q24.1(154790212_158488241)x1 of size >3.6 Mb (3698029 bp) encompassing the NR4A2 gene. The deletion also covered ten flanking genes (KCNJ3, GPD2, GALNT5, ERMN, CYTIP, ACVR1C, ACVR1, UPP2, CCDC148, and PKP4).
Various in silico tools predicted that the variant in patient 2 affects splicing that would lead to LoF. This was confirmed by RT-PCR (see Supplementary Figure S2), which revealed altered splicing leading to aberrant transcripts with an out of frame skipping of exon 4 (130 nucleotides), which will potentially cause truncation and LoF through NMD.
All variants had high predictive scores for a detrimental effect as predicted by SIFT, PolyPhen-2, and CADD scores. The NR4A2 gene appears to be under a high selective strain and extremely intolerant to LoF variation as evidenced from its high probability of being LoF intolerant (pLI) score (1.0) and lower than expected missense variant counts (Z-score = 2.24), as observed in both ExAC and gnomAD. The haploinsufficiency score (HI score = 1.28%) shows this gene to be highly dosage sensitive. Therefore, intolerance to LoF can play an important role in the development of pathogenic phenotypes in patients.3,10,11
DISCUSSION
We report nine patients with early onset epilepsy and/or a developmental disorder, of whom eight carried intragenic variants and on a larger deletion including NR4A2. Six of these patients with de novo NR4A2 variants had epilepsy. The apparent intolerance to LoF and missense variation of NR4A2 suggests that these variants are causing the phenotype in patients.3,10,11 In previous studies, haploinsufficiency of NR4A2 has been implicated in a neurodevelopmental phenotype, including significant language impairment11,12 and ID.3,7 These symptoms overlap with those observed in the patients studied here. We also observed language impairment in five of nine patients, which may be linked to the more prominent expression of NR4A2 in the superior temporal gyrus (STG), a brain region linked to language development.13
Regardless of the similarities among these nine patients, the underlying explanations for the phenotypes of patient 4 and patient 9 may be different. Patient 4 had a deceased sibling with similar phenotype. Therefore, the de novo variant detected in patient 4 may not explain the full phenotype, and another cause, as well as germline mosaicism, should be considered. For patient 9 the microdeletion also affected ten other genes that could have contributed to the clinical features. For example, KCNJ3, also deleted in this patient, encodes for subunit G-protein activated inward rectifier potassium channel 1. Alterations in the function of this potassium channel subunit have been associated with epilepsy.14
It remains unclear how these variants of NR4A2 contribute to the epileptogenesis. However, the physiologic role of NR4A2 provides a clue toward the complex phenotypes of the patients with variants in this gene. NR4A2 is a known transcription factor and it binds to DNA as monomer or homodimer to promote constitutive activation of transcription.15 It exerts concentration-dependent effects on target genes mediating distinct biological processes.16 The product of a mutated gene can be subjected to NMD leading to a misfolded protein, or a dysfunctional ligand binding pocket, or a dysfunctional DNA binding, resulting in impaired or loss of function. It is important to take into consideration that amino acid substitutions can also disrupt the conformation of the protein and such conformational changes might also compromise the function of NR4A2. A large case–control study of patients with neurodevelopmental disorders (such as autism spectrum disorder, DD, ID, and epilepsy) showed significant clustering of the de novo missense variants in cases at the protein level for 200 genes including NR4A2. In many cases, these de novo variants clustered in protein functional domains (such as zinc finger motifs, transmembrane domains, voltage sensors, and channel pores) relevant in the neurodevelopmental pathology. The de novo variants in zinc finger motifs were not present in public control databases. A clustering of variants in specific functional domains emphasized the importance of these de novo variants in characterizing pathogenic genes and functional domains.17 The DBD of NR4A2 contains two highly conserved C4 type zinc fingers that bind to specific motifs in DNA hormone response elements. Two of six patients with epilepsy have a missense variant in the DBD, whereas one patient has a missense variant in a hinge region with no secondary structure in the LBD and in N-terminal regulatory region of the protein. The two missense variants in the highly conserved DBD of NR4A2 may have resulted in impaired DNA binding ability. The other variants observed in patients are truncating variants and deletions, which taken together with the observed clustering of missense variants in a biological relevant protein domain strongly suggest that LoF is the underlying disease mechanism.
It is well established that NR4A2 is important for differentiation, survival and maintenance of dopaminergic neurons.1 Furthermore, the dopamine activity transporter DAT gene has been associated with idiopathic absence epilepsy and seizures.18 Various animal studies have investigated how the DAergic system is linked to epileptogenesis and neurodevelopmental dysfunction.1 Different subtypes of the dopamine receptors can act either as proconvulsant (D1-like receptor) or anticonvulsant (D2-like receptor). Physiological balance of DAergic activity at D1R and D2R can be decisive for complex neuromodulatory response for epileptogenesis.16 The knockout or knockdown of NR4A2 have resulted in poor motor function and lower number of DA neurons, lower levels of protein, and reduced DA at birth in midbrain.19 Studies of NR4A2 heterozygosity in animal models have shown that the affected dopaminergic system is associated with altered locomotor behavior.20 Furthermore, a crucial role of NR4A2 in overall survival was supported by animal studies revealing its involvement in respiratory abnormality and lack of response to hypoxia. NR4A2 is encoded by immediate early genes and has a significant role in development, neuroprotection, learning, and memory formation,19 presenting a reasonable explanation for the associated ID among these patients. These studies indicate that variants in NR4A2 can impair its various functions, and plausibly contribute to the seizure, neurodevelopmental, and global developmental phenotype observed in the described patients.
Supplementary information
Acknowledgements
This study was supported by the "friends of UMC & Willemina Kinder Ziekenhuis" MING funds.
Disclosure
T.B., R.E.S., F.M., and Y.S. are employees of GeneDx. The other authors declare no conflicts of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version of this article (10.1038/s41436-020-0815-4) contains supplementary material, which is available to authorized users.
References
- 1.Kadkhodaei B, Ito T, Joodmardi E, et al. Nurr1 Is required for maintenance of maturing and adult midbrain dopamine neurons. J Neurosci. 2009;29:15923–15932. doi: 10.1523/JNEUROSCI.3910-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Vera IMS, Munoz-Tello P, Zheng J, et al. Defining a canonical ligand-binding pocket in the orphan nuclear receptor Nurr1. Structure. 2019;27:66–77 e65. doi: 10.1016/j.str.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lévy J, Grotto S, Mignot C, et al. NR4A2 haploinsufficiency is associated with intellectual disability and autism spectrum disorder. Clin Genet. 2018;94:264–268. doi: 10.1111/cge.13383. [DOI] [PubMed] [Google Scholar]
- 4.Liu HM, Fu YM, Ren JJ, et al. Association between NR4A2 genetic variation and schizophrenia: a comprehensive systematic review and meta-analysis. Neurosci Lett. 2015;598:85–90. doi: 10.1016/j.neulet.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 5.Smith KM, Bauer L, Fischer M, Barkley R, Navia BA. Identification and characterization of human NR4A2 polymorphisms in attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2005;133B:57–63. doi: 10.1002/ajmg.b.30127. [DOI] [PubMed] [Google Scholar]
- 6.Feliciano P, Zhou X, Astrovskaya I, et al. Exome sequencing of 457 autism families recruited online provides evidence for autism risk genes. NPJ Genom Med. 2019;4:19. doi: 10.1038/s41525-019-0093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramos LLP, Monteiro FP, Sampaio LPB, et al. Heterozygous loss of function of NR4A2 is associated with intellectual deficiency, rolandic epilepsy, and language impairment. Clin Case Rep. 2019;7:1582–1584. doi: 10.1002/ccr3.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sobreira N, Schiettecatte F, Valle D, Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum Mutat. 2015;36:928–930. doi: 10.1002/humu.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reuter MS, Krumbiegel M, Schluter G, Ekici AB, Reis A, Zweier C. Haploinsufficiency of NR4A2 is associated with a neurodevelopmental phenotype with prominent language impairment. Am J Med Genet A. 2017;173:2231–2234. doi: 10.1002/ajmg.a.38288. [DOI] [PubMed] [Google Scholar]
- 12.Leppa VM, Kravitz SN, Martin CL, et al. Rare inherited and de novo CNVs reveal complex contributions to ASD risk in multiplex families. Am J Hum Genet. 2016;99:540–554. doi: 10.1016/j.ajhg.2016.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abrahams BS, Tentler D, Perederiy JV, Oldham MC, Coppola G, Geschwind DH. Genome-wide analyses of human perisylvian cerebral cortical patterning. Proc Natl Acad Sci U S A. 2007;104:17849–17854. doi: 10.1073/pnas.0706128104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y, Zhang YW, Kong SZ, et al. GIRK1-mediated inwardly rectifying potassium current suppresses the epileptiform burst activities and the potential antiepileptic effect of ML297. Biomed Pharmacother. 2018;101:362–370. doi: 10.1016/j.biopha.2018.02.114. [DOI] [PubMed] [Google Scholar]
- 15.Maira M, Martens C, Philips A, Drouin J. Heterodimerization between members of the Nur subfamily of orphan nuclear receptors as a novel mechanism for gene activation. Mol Cell Biol. 1999;19:7549–7557. doi: 10.1128/MCB.19.11.7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson MM, Michelhaugh SK, Bouhamdan M, Schmidt CJ, Bannon MJ. The transcription factor NURR1 exerts concentration-dependent effects on target genes mediating distinct biological processes. Front Neurosci. 2011;5:135.. doi: 10.3389/fnins.2011.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geisheker MR, Heymann G, Wang T, et al. Hotspots of missense mutation identify neurodevelopmental disorder genes and functional domains. Nat Neurosci. 2017;20:1043–1051. doi: 10.1038/nn.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sander T, Berlin W, Ostapowicz A, Samochowiec J, Gscheidel N, Hoehe MR. Variation of the genes encoding the human glutamate EAAT2, serotonin and dopamine transporters and susceptibility to idiopathic generalized epilepsy. Epilepsy Res. 2000;41:75–81. doi: 10.1016/S0920-1211(00)00120-0. [DOI] [PubMed] [Google Scholar]
- 19.Bensinger SJ, Tontonoz P. A Nurr1 pathway for neuroprotection. Cell. 2009;137:26–28. doi: 10.1016/j.cell.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 20.Eells JB, Lipska BK, Yeung SK, Misler JA, Nikodem VM. Nurr1-null heterozygous mice have reduced mesolimbic and mesocortical dopamine levels and increased stress-induced locomotor activity. Behav Brain Res. 2002;136:267–275. doi: 10.1016/S0166-4328(02)00185-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.