Human cytomegalovirus (HCMV) infection is endemic throughout the world regardless of socioeconomic conditions and geographic locations with a seroprevalence reaching up to 100% in some developing countries. Although asymptomatic in healthy individuals, HCMV can cause severe multiorgan disease in immunocompromised or immunonaive patients. HCMV disease is a direct consequence of monocyte-mediated systematic spread of the virus following infection. Because monocytes are short-lived cells, HCMV must subvert the natural short life-span of these blood cells by inducing a distinct activation of Akt, a serine/theonine protein kinase. In this work, we demonstrate that HCMV glycoproteins gB and gH work in tandem to reroute classical host cellular receptor signaling to aberrantly activate Akt and drive survival of infected monocytes. Deciphering how HCMV modulates the cellular pathway to induce monocyte survival is important to develop a new class of anti-HCMV drugs that could target and prevent spread of the virus by eliminating infected monocytes.
KEYWORDS: cytomegalovirus, monocytes
ABSTRACT
Human cytomegalovirus (HCMV) is a major cause of morbidity and mortality among immunocompromised and immunonaive individuals. HCMV-induced signaling initiated during viral entry stimulates a rapid noncanonical activation of Akt to drive the differentiation of short-lived monocytes into long-lived macrophages, which is essential for viral dissemination and persistence. We found that HCMV glycoproteins gB and gH directly bind and activate cellular epidermal growth factor receptor (EGFR) and integrin β1, respectively, to reshape canonical Akt signaling within monocytes. The remodeling of the Akt signaling network was due to the recruitment of nontraditional Akt activators to either the gB- or gH-generated receptor signaling complexes. Phosphoinositide 3-kinase (PI3K) comprised of the p110β catalytic subunit was recruited to the gB/EGFR complex despite p110δ being the primary PI3K isoform found within monocytes. Concomitantly, SH2 domain-containing inositol 5-phosphatase 1 (SHIP1) was recruited to the gH/integrin β1 complex, which is critical to aberrant Akt activation, as SHIP1 diverts PI3K signaling toward a noncanonical pathway. Although integrin β1 was required for SHIP1 recruitment, gB-activated EGFR mediated SHIP1 activation, underscoring the importance of the interplay between gB- and gH-mediated signaling to the unique activation of Akt during HCMV infection. Indeed, SHIP1 activation mediated the increased expression of Mcl-1 and HSP27, two Akt-dependent antiapoptotic proteins specifically upregulated during HCMV infection but not during growth factor treatment. Overall, our data indicate that HCMV glycoproteins gB and gH work in concert to initiate an HCMV-specific signalosome responsible for the atypical activation of Akt required for infected monocyte survival and ultimately viral persistence.
IMPORTANCE Human cytomegalovirus (HCMV) infection is endemic throughout the world regardless of socioeconomic conditions and geographic locations with a seroprevalence reaching up to 100% in some developing countries. Although asymptomatic in healthy individuals, HCMV can cause severe multiorgan disease in immunocompromised or immunonaive patients. HCMV disease is a direct consequence of monocyte-mediated systematic spread of the virus following infection. Because monocytes are short-lived cells, HCMV must subvert the natural short life-span of these blood cells by inducing a distinct activation of Akt, a serine/theonine protein kinase. In this work, we demonstrate that HCMV glycoproteins gB and gH work in tandem to reroute classical host cellular receptor signaling to aberrantly activate Akt and drive survival of infected monocytes. Deciphering how HCMV modulates the cellular pathway to induce monocyte survival is important to develop a new class of anti-HCMV drugs that could target and prevent spread of the virus by eliminating infected monocytes.
INTRODUCTION
Human cytomegalovirus (HCMV) is a ubiquitous betaherpesvirus reaching 60 to 90% seroprevalence among adults in the United States (1). In healthy individuals, HCMV infection is generally asymptomatic, although HCMV can cause acute infectious mononucleosis (2, 3) and has also been linked to chronic inflammatory diseases, such as atherosclerosis, and cancers, including glioblastoma and colon cancer (4, 5). In contrast, HCMV infection of immunocompromised individuals, including AIDS patients and transplant recipients, causes severe morbidity and mortality (6–8). HCMV is also the most common congenital infection occurring in approximately 20,000 to 30,000 infants each year in the United States, with approximately 10 to 15% of infected newborns developing permanent neurological abnormalities (9). HCMV-associated diseases are characterized by end-organ dysfunction, which is a direct consequence of the systemic spread of the virus that occurs during an acute infection (10–12).
Monocytes are the predominant infiltrating cell type found in the infected organs of patients with HCMV disease and are thought to be responsible for viral dissemination (13–16). However, the inability of HCMV to replicate within monocytes contradicts the assertion that monocytes function to mediate virus spread within the infected host (15–20). Furthermore, monocytes are naturally short-lived cells programmed to undergo apoptosis approximately 48 h after their release from the bone marrow in the absence of a differentiation signal (21). To circumvent these obstacles, we have previously shown that HCMV reprograms monocytes to bypass the 48-h viability checkpoint allowing infected monocytes to differentiate into macrophages (22–24). The differentiation of monocytes is critical for HCMV spread within the host, as macrophages are permissive for virus replication and have a life-span ranging from months to years (22). Antiapoptotic viral proteins are not expressed in monocytes during the first 48 h of infection (25), and UV-inactivated particles stimulate monocyte survival similar to “live” virus, suggesting that HCMV entry triggers cellular survival pathways (22, 26). Indeed, HCMV glycoproteins initiate antiapoptotic signaling pathways during viral entry (26–28), indicating a critical role for glycoproteins in the survival of HCMV-infected monocytes.
The HCMV entry process begins with binding of viral envelope glycoproteins to cell surface heparan sulfate proteoglycans (29). This low-affinity reversible binding between the virus and the cell is replaced by a high-affinity irreversible binding of HCMV glycoproteins with cellular receptors (30). The key glycoproteins involved during HCMV entry include gB, gH, gL, gO, and HCMV proteins UL128-131 (31–42). HCMV glycoprotein gH can be found in three complexes as follows: a gH/gL dimer, a gH/gL/gO trimer, and a gH/gL/UL128-131 pentamer (27, 43, 44). Following initial tethering of the virion to the cell surface, gH complexes bind integrins to induce cellular signaling needed for viral entry into target cells (34, 42). Specifically, the trimeric gH complex mediates entry into fibroblasts, while the pentameric gH complex is required for entry into epithelial, endothelial, and myeloid cells (37, 41, 45, 46). HCMV strains expressing the pentameric complex bind to integrin β1 via gH during HCMV entry into monocytes (42). Glycoprotein gB is found as a homotrimeric complex and binds to epidermal growth factor receptor (EGFR) and/or platelet-derived growth factor receptor α (PDGFRα) to trigger HCMV entry into cells, although there are conflicting reports about both receptors being bona fide entry receptors for HCMV (31, 47–53). Studies have demonstrated a direct interaction between gB and EGFR or PDGFRα in fibroblast cells (31, 48). EGFR is thought to be the gB engaging receptor required for HCMV entry into monocytes due to the lack of PDGFRα expression (49). In support, HCMV-induced activation of EGFR signaling is needed for viral entry into monocytes (49). However, it is unknown whether gB directly binds to and activates EGFR during monocyte entry or stimulates the release of EGFR activating factors.
During the entry process of HCMV, activation of EGFR initiates the phosphoinositide 3-kinase (PI3K)/Akt pathway (49). PI3K phosphorylates phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] to phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3] that recruits Akt to the plasma membrane leading to its activation (54–57). PI3K consists of a p85 regulatory subunit and a p110 catalytic subunit, which exists as three different isoforms (58). The p110α and p110β are ubiquitously expressed while p110δ is expressed only in immune cells (59, 60). Although p110δ is the primary isoform responsible for the survival of monocytes under homeostatic conditions (61, 62), we have previously shown that HCMV preferentially utilizes PI3K p110β to stimulate the survival of infected monocytes (63). The activity of PI3K can be reversed by the phosphatase and tensin homolog (PTEN), which dephosphorylates PI(3,4,5)P3 back into PI(4,5)P2 (64, 65). However, HCMV rapidly inactivates PTEN activity during entry into monocytes (63). Alternatively, PI(3,4,5)P3 can also be dephosphorylated to PI(3,4)P2 by SH2 domain-containing inositol 5-phosphatase 1 (SHIP1). Although a negative regulator of the PI3K/Akt pathway under normal circumstances, SHIP1 positively regulates Akt activity within tumor microenvironments (66–68). We have also demonstrated that SHIP1 activity is required for Akt activation during HCMV infection of monocytes and that the SHIP1 product PI(3,4)P2 can rescue the loss of SHIP1 activity (63). The HCMV-induced noncanonical activation of Akt leads to a phosphorylation profile distinct from that of myeloid growth factor-activated Akt (63), which results in the upregulation of select antiapoptotic proteins only within infected monocytes (69). Aberrant Akt activity is necessary to ensure viral dissemination and persistence by promoting monocyte survival and differentiation into macrophages (63).
In this study, we examined how HCMV glycoproteins mediate signaling to induce the nonclassical activation of Akt required for the survival of HCMV-infected monocytes. Specifically, we showed that glycoproteins gB and gH stimulated signaling from EGFR and integrin β1, respectively. We show for the first time that gB directly binds and activates EGFR during HCMV entry into monocytes to promote the recruitment of PI3K to the gB/EGFR complex. Consistent with our previous findings, PI3K p110β was recruited to the gB/EGFR signaling complex. Simultaneously, SHIP1 was recruited to the gH/integrin β1 complex, although EGFR was responsible for the activation of SHIP1. SHIP1 activity was sustained for up to 48 h and needed for the increased expression of antiapoptotic proteins specifically required for infected monocyte survival. Taken together, our data indicate that HCMV glycoproteins gB and gH work in concert to activate SHIP1, leading to the unique activation of Akt and the subsequent survival of infected monocytes.
RESULTS
HCMV activates Akt through gB- and gH-initiated signaling to promote the survival of infected monocytes.
HCMV entry rapidly activates Akt through a preferential phosphorylation at serine 473 (S473), which is essential to the survival of HCMV-infected monocytes past the 48-h viability “gate” (63). HCMV virions neutralized with an anti-gB antibody failed to induce Akt phosphorylation to the level of infected monocytes (63), suggesting the involvement of HCMV glycoproteins in the rapid noncanonical activation of Akt during viral entry. In support, increased expression of Akt-dependent antiapoptotic proteins following HCMV infection requires both gB- and gH-initiated signaling (69). To test whether simultaneous signaling from both gB and gH glycoproteins is required for Akt activation, HCMV was pretreated with neutralizing antibody against either gB or gH. Loss of gB- or gH-initiated signaling reduced the levels of Akt phosphorylation induced during viral entry (Fig. 1A), indicating that gB and gH work together to activate Akt and promote the survival of short-lived monocytes. Moreover, we did not see an enhanced reduction of p-Akt below gB treatment alone when both gB and gH were neutralized, supporting our model that gB and gH signal down a single pathway rather than parallel pathways to activate Akt and stimulate monocyte survival. Accordingly, we found that primary peripheral blood monocytes infected with either gB- or gH-neutralized HCMV exhibited lower viability when compared to that of monocytes infected with untreated virus (Fig. 1B). Consistent across 3 donors, neutralization of gB had a greater effect on lowering monocyte survival following infection, suggesting gB-mediated signaling may have a more prominent role in inducing monocyte survival (Fig. 1C). Additionally, HCMV incubated with an isotype-matched antibody did not have any effect on the Akt activation (Fig. 1A) and survival of infected monocytes (Fig. 1D), indicating that the observed effect of either gB or gH neutralization on monocyte was specific. To directly assess the individual contributions of gB and gH during HCMV-induced Akt-dependent monocyte survival, we generated stable human cell lines expressing soluble versions of gB (sgB) and gH (sgH). The transmembrane and cytoplasmic domains of the soluble glycoproteins were replaced with His tags for convenient purification of the glycoproteins using a nickel column (Fig. 2A). Retroviral vectors containing the sgB or sgH gene were used to transduce human Expi293F cells, which are adapted for high-density, serum-free suspension culture to allow for large-scale production of recombinant proteins. Nickel column-purified secreted recombinant His-tagged sgB and sgH had the expected smaller molecular weights compared to the weights of their full-length counterparts (Fig. 2B). Consistent with neutralizing studies demonstrating that gB and gH were essential components of the virion required to induce monocyte survival, we found that individual treatment with either sgB or sgH resulted in the activation of Akt (Fig. 2C). Accordingly, sgB or sgH induced monocyte survival, albeit not to the levels with whole virus, suggesting that cosignaling from gB and gH may be necessary to induce maximum survival. Although we did not find a statistical difference between sgB and sgB/sgH treatments, we did observe that with each individual donor sgB/sgH treatment always induced slightly greater monocyte survival than sgB (Fig. 2D). The lack of statistical difference is likely due to the donor variability in response levels to experimental treatments that is inherent when using primary monocytes isolated from different blood donors. Nonetheless, these data suggest that there is likely a cooperative effect between gB and gH within a single signaling cascade. In addition, the negative control did not induce monocyte survival, indicating a specific effect of the soluble glycoproteins toward the survival of monocytes (Fig. 2E). Our study indicates that HCMV utilizes both gB and gH during monocyte entry to initiate signaling events required for the atypical Akt activation and survival of infected monocytes. In addition, these data confirm that recombinant sgB and sgH retained proper folding to initiate signaling events consistent with whole virus.
FIG 1.
HCMV gB and gH stimulate Akt activity to promote the survival of HCMV-infected monocytes. (A) Peripheral blood monocytes were mock or HCMV infected or infected with the virus neutralized with antibody against glycoprotein gB or gH or isotype antibody for 15 min. The phospho (p)-Akt (Ser473) and total-Akt were detected by immunoblotting from whole-cell lysates. Actin was used as a loading control. Results are representative of 3 to 5 independent experiments using different donors. Densitometry analysis was performed using Image Lab software (Bio-Rad) and quantification of the specific blots shown is marked as “donor 1,” and data points from another donor (donor 2) have additionally been provided to account for donor variability. (B, C, and D) Monocytes were infected with mock or nonneutralized HCMV or HCMV neutralized with antibody against glycoprotein gB or gH or isotype antibody and incubated for 48 h. Viability was measured by annexin V and propidium iodide (PI) staining using flow cytometry. (B) Monocytes negative for both annexin and PI were considered live cells. (C and D) Lines connect data points from the same experiment using the same donor. Results are representative of 3 to 5 independent experiments using different donors. Statistical significance was measured using paired t test; *, P < 0.05; **, P < 0.005; ns, nonsignificant.
FIG 2.
Soluble sgB and sgH induce monocyte survival via activation of Akt. (A) Schematic representation of the full-length HCMV glycoprotein gB or gH along with the soluble sgB and sgH construct. SS, signal sequence; TMD, transmembrane domain; CPD, cytoplasmic domain; His, histidine tag. (B) HCMV virus particles were lysed and immunoblotted for gB and gH. Soluble His-tagged sgB and sgH were purified from the supernatants of stably transduced Expi293F cells by nickel column purification and immunoblotted for gB, gH, or His tag. (C, D, and E) Monocytes were mock or HCMV infected or treated with sgB (1 μg/ml) and/or sgH (1 μg/ml) or Expi293 cell supernatant negative control (NC). (C) After 15 min, p-Akt (Ser473) and total Akt levels were measured by immunoblotting. (D and E) Monocyte viability was measured after 48 h by annexin V and PI staining using flow cytometry. Results are representative of 3 to 5 independent experiments using different donors. *, P < 0.05; ns, nonsignificant.
HCMV glycoproteins gB and gH directly bind to EGFR and integrin β1, respectively.
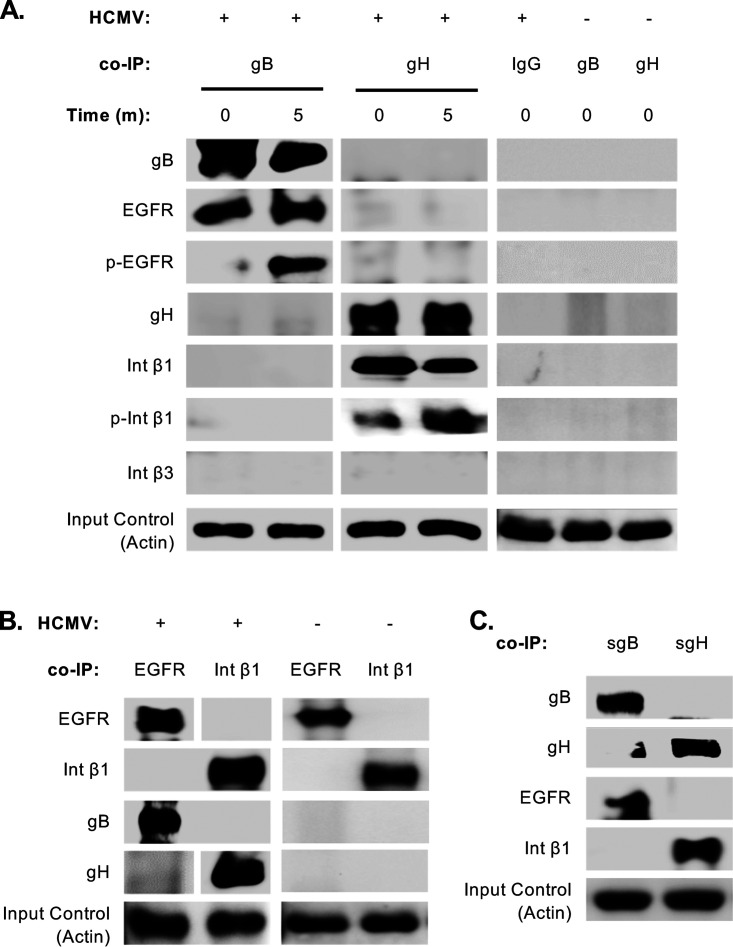
Previous studies have demonstrated that gB directly interacts with EGFR, PDGFRα, and integrins to mediate entry into fibroblasts (31, 48, 70). However, EGFR is thought to be the major gB receptor on monocytes due to the lack of PDGFRα (49). In support, activation of EGFR is required for HCMV entry into monocytes (49). Furthermore, although gH binds and activates integrins during entry into monocytes (42), it remains unclear whether gB also engages integrins as on fibroblasts. To confirm a direct interaction between gB and EGFR, coimmunoprecipitation assays were performed. We found that gB immunoprecipitated with EGFR from monocytes infected with HCMV at 4°C (time, 0 min) (Fig. 3A). Further, activated EGFR was only observed in the immunoprecipitate from infected monocytes briefly temperature shifted to 37°C (time, 5 min), confirming that gB-mediated signaling is required for the activation of EGFR. In agreement with previous reports, we found that gH binds to integrin β1, but not β3, on monocytes (42). Similar to EGFR activation, higher levels of phosphorylated integrin β1 were found in the gH pulldown from temperature-shifted monocytes. Although gB has also been shown to bind integrins to mediate entry into fibroblasts, we did not find gB to interact with either integrin β1 or β3, suggesting that specific glycoprotein and receptor interactors are cell type dependent. Moreover, despite receptor clustering of EGFR and integrin β3 occurring within the first 5 min of HCMV binding onto fibroblast (34), we did not observe the presence of integrin β1 in the immunoprecipitate of gB pulldown or EGFR from gH pulldown from infected monocytes (Fig. 3A). To further validate binding of gB to EGFR and gH to integrin β1, we performed the reverse immunoprecipitation with EGFR and integrin β1. As expected, gB coimmunoprecipitated with EGFR and gH with integrin β1 (Fig. 3B). We also confirmed that sgB and sgH bound to EGFR and integrin β1, respectively (Fig. 3C), indicating that our lab-generated soluble glycoproteins retained proper folding and signaling capacity. These findings demonstrate that gB and gH directly bind to EGFR and integrin β1 during entry into monocytes, respectively, but that the signalosome initiated may be distinct from fibroblasts due to differences in receptor usage and the absence of receptor clustering.
FIG 3.
Glycoproteins gB and gH directly bind to and activate EGFR and integrin β1, respectively. (A, B, and C) Monocytes were mock or HCMV infected or treated with sgB (5 μg/ml) or sgH (5 μg/ml) for 0 min or 5 min (A) or for 0 min (B and C) and immunoprecipitated with antibodies recognizing gB, gH, EGFR, integrin β1, or isotype antibody. Western blot analyses were performed to determine the presence of gB, gH, EGFR, p-EGFR, integrin β1, p-integrin β1, and integrin β3 in the immunoprecipitated samples. Input controls were blotted for actin to confirm homogeneous loading of the samples. (B) Immunoblot is from a single gel from the same experiment. (A, B, and C) Results are representative of at least 3 independent experiments using different donors.
gB and gH initiate monocyte survival signaling through EGFR and integrin β1 activation.
HCMV glycoproteins gB and gH have been shown to directly bind and activate EGFR and integrins to mediate entry into fibroblasts (31, 34). Activation of EGFR and integrins is also required for HCMV entry into monocytes (40, 42, 49, 71). Consistent with these reports, HCMV rapidly phosphorylated EGFR and integrin β1 at 15 min postinfection in monocytes (Fig. 4A). As expected, sgB and sgH activated EGFR and integrin β1, respectively. However, sgB also activated integrin β1 and sgH activated EGFR, suggesting cross talk between gB- and gH-initiated signaling pathways during viral entry into monocytes. Cross talk between EGFR and integrin signaling has been observed with HCMV-infected fibroblasts (34). To test if glycoprotein-mediated activation of EGFR and integrin β1 cross-activates the receptors, monocytes were treated with AG (an EGFR inhibitor) and ATN (an integrin inhibitor) in order to block signaling from the receptor cytoplasmic domain prior to HCMV infection. Pretreatment with either AG or ATN blocked HCMV- or soluble glycoprotein-induced activation of EGFR and integrin β1 (Fig. 4B and C), demonstrating receptor cross talk during HCMV entry into monocytes. Cross talk between EGFR and integrin β1 was required for Akt activation, as loss of signaling from either receptor prevented Akt activation following HCMV infection (Fig. 4D). Our results also suggest that minimal levels of EGFR activity were required to sustain basal Akt activity since EGFR inhibition completely abrogated Akt activity within infected monocytes while suppression of integrin β1 during HCMV infection returned Akt levels to mock-infected levels (Fig. 4D). Accordingly, monocytes treated with either AG or ATN displayed lower survival after HCMV infection with inhibition of EGFR having a more robust effect (Fig. 4E). Mechanistically, we found that EGFR inhibition blocked the upregulation of select Akt-dependent antiapoptotic proteins Mcl-1 and HSP27 following HCMV infection, which was in contrast to integrin β1 inhibition only affecting Mcl-1 expression (Fig. 4F). Overall, these data suggest that EGFR and integrin β1 activation cosignaling mediates survival of infected monocytes.
FIG 4.
Glycoproteins gB and gH activate Akt through EGFR and integrin β1 during monocyte entry. (A) Monocytes were mock or HCMV infected or treated with sgB or sgH. After 15 min, p-EGFR and p-integrin β1 levels were measured by immunoblotting. (B, C, D, E, and F) Monocytes were mock or HCMV infected or treated with sgB, sgH, or negative control (NC). Indicated samples were preincubated with 5 μM AG (an EGFR inhibitor) and 20 μM ATN (an integrin inhibitor) for 1 h at 37°C prior to infection or treatment. (B, C, and D) After 15 min, cells were lysed and levels of p-EGFR, p-integrin β1, p-Akt, and total Akt were measured by immunoblotting. (E) After 48 h, monocyte viability was measured by annexin V and PI staining using flow cytometry. (F) Mcl-1 and HSP27 were detected after 48 h by immunoblotting from whole-cell lysates. Results are representative of at least 3 independent experiments using different donors. *, P < 0.05; **, P < 0.005.
Activation of EGFR and integrin β1 during HCMV entry initiates a unique Akt signaling network.
HCMV induces noncanonical Akt signaling within monocytes during viral entry by recruiting nontraditional Akt activators (63, 69, 72). The role of EGFR and integrins in assembling the HCMV-specific Akt signaling network is unclear. PI3K is directly downstream of EGFR and activated during HCMV entry into monocytes (49). Indeed, we found HCMV binding to the monocyte cell surface stimulated the recruitment of the p85 regulatory subunit of PI3K to EGFR (Fig. 5A). In addition to the regulatory subunit, class 1A PI3K has three different catalytic subunits, p110α, p110β, and p110δ, with p110δ as the primary isoform responsible for the survival of monocytes under homeostatic condition (59, 60). However, HCMV infection utilizes p110β isoform to mediate survival of infected monocytes (63). Accordingly, the p85 regulatory subunit was found to bind to the 110β catalytic subunit (Fig. 5B). We have also shown HCMV to use SHIP1 in concert with PI3K to drive the nonclassical activation of Akt within infected monocytes (63). To determine if SHIP1 is recruited to either the gB/EGFR or gH/integrin β1 complex, we immunoprecipitated EGFR and integrin β1 from infected monocytes and found SHIP1 to interact with integrin β1 but not with EGFR (Fig. 5A). We also found that inhibition of EGFR had a robust effect on suppressing SHIP1 phosphorylation, indicating integrin β1 is responsible for the localization of SHIP1 while EGFR is required for its activation (Fig. 5C). These data argue that HCMV glycoproteins stimulate the synchronous activation of EGFR and integrin β1 in order to selectively recruit and activate PI3K-p110β and SHIP1, which are responsible for the noncanonical activation of Akt.
FIG 5.
Phosphorylation of EGFR and integrin β1 results in the activation of the EGFR/PI3K/SHIP1 signaling pathway. (A and B) EGFR, integrin β1, p85, and SHIP1 were immunoprecipitated after monocytes were infected with or without HCMV for 0 min or 5 min (A) or 5 min (B). (C) Cells were treated with 5 μM AG (an EGFR inhibitor) and 20 μM ATN (an integrin inhibitor) for 1 h at 37°C and then infected with HCMV for 15 min. Cells were then lysed followed by Western blot analyses to determine the presence of different proteins using specific antibodies. Results are representative of at least 3 independent experiments using different donors.
SHIP1 activation is required for the upregulation of prosurvival proteins in HCMV-infected monocytes.
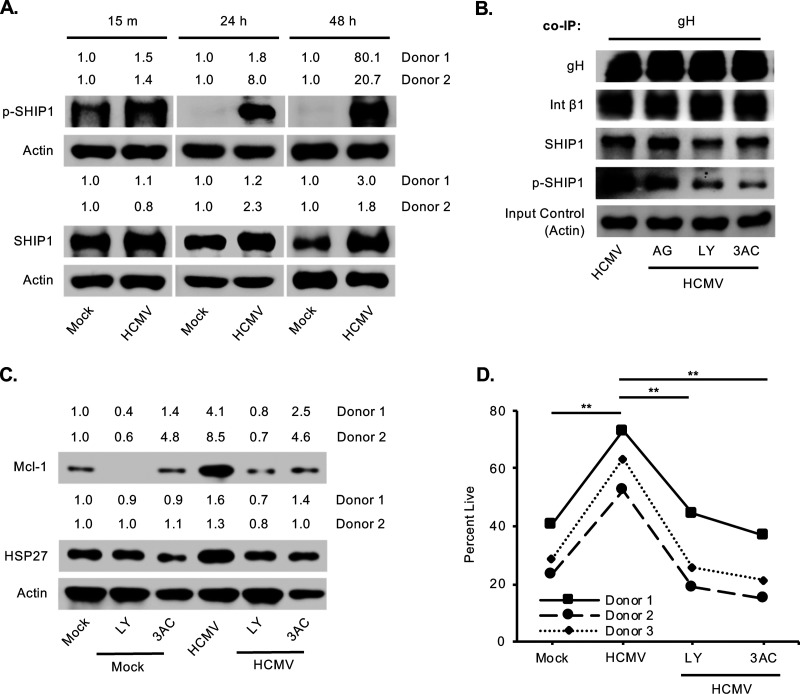
Despite the normal negative regulatory effect on the PI3K/Akt pathway, SHIP1 positively regulates Akt activity in HCMV-infected monocytes similar to leukemic cells (66–68). In cancer cells, the PH domain of Akt binds with greater affinity to the SHIP1 product PI(3,4)P2, leading to a more robust activation of Akt than that of the direct product of PI3K, PI(3,4,5)P3 (73). However, the kinases recruited to the cell membrane to mediate the noncanonical activation of Akt remain unknown. In agreement with our previous studies, total SHIP1 level was elevated by 24 h in HCMV-infected monocytes and maintained for 48 h (Fig. 6A). Here, we further confirmed phosphorylation of SHIP1 and hence activity, which was sustained up to 48 h postinfection. SHIP1 activity was regulated by the EGFR/PI3K signaling pathway, as treatment with EGFR and PI3K inhibitors blocked phosphorylation of integrin-bound SHIP1 similar to 3AC (a SHIP1 inhibitor) (Fig. 6B). Because atypical Akt activation is required for the upregulation of select antiapoptotic proteins, such as Mcl-1 and HSP27, we examined whether blocking SHIP1 and PI3K also prevented the HCMV induction of these prosurvival proteins. Indeed, loss of the PI3K/SHIP1 signaling cascade reduced Mcl-1 and HSP27 expression in HCMV-infected monocytes (Fig. 6C). Subsequently, inhibition of either SHIP1 or PI3K also completely repressed HCMV-induced monocyte survival (Fig. 6D). These data indicate that HCMV glycoprotein-mediated signaling activates SHIP1 to drive the HCMV-specific activation of Akt, leading to monocyte survival through the 48-h viability checkpoint.
FIG 6.
HCMV induces a chronic SHIP1 activation required for the survival of infected monocytes. (A) Monocytes were mock or HCMV infected for 15 min, 24 h, or 48 h, and total or p-SHIP1 was detected from whole-cell lysates. (B, C, and D) Monocytes were treated with 5 μM AG (an EGFR inhibitor), 50 μM LY (a PI3K inhibitor), or 5 μM 3AC (a SHIP1 inhibitor) for 1 h and then mock or HCMV infected. (B) After 5 min infection, anti-gH antibody was used to pull down gH glycoprotein (and any other proteins bound to gH) followed by blotting for gH, integrin β1, SHIP1, and p-SHIP1 in the immunoprecipitate using specific antibodies. Input controls were blotted for actin to confirm homogeneous loading of the samples. (C) After 24 h, Western blot analysis was performed to detect Mcl1 and HSP27 from total cell lysates. (D) Survival of monocytes was measured after 48 h of infection with or without the inhibitors by annexin V and PI staining using flow cytometry. Results are representative of at least 3 to 5 independent experiments using different donors. **, P < 0.005.
DISCUSSION
Monocytes play a critical role in HCMV’s life cycle by bridging the initial acute infection with the establishment of a lifelong persistent infection. Following a primary infection, HCMV-infected peripheral blood monocytes function to disseminate the virus to multiple organ sites (14, 20, 74). Infected monocytes that travel to the bone marrow transmit the virus to CD34+ progenitor cells, which serve as the primary reservoir for latency (75). In order for systemic spread to occur, differentiation of monocytes is required, as macrophages are long-lived cells permissive for HCMV replication (15–20, 22). The first step toward differentiation is the survival of monocytes through a 48-h viability gate, which functions as a critical checkpoint where monocytes either undergo apoptosis or differentiate into macrophages in the presence of appropriate stimuli (21). Despite the plethora of viral antiapoptotic proteins encoded by the HCMV genome, none are expressed during the first 48 h of monocyte infection (25). Consequently, HCMV modulates cellular survival pathways during viral entry to bypass the 48-h viability checkpoint (24, 25, 76). We have previously shown that HCMV stimulates a noncanonical activation of Akt to induce the survival of infected monocytes (63). However, the precise role of HCMV glycoproteins in propagating cellular signaling cascades that guide the aberrant activation of Akt within infected monocytes is not well understood. In this study, we demonstrate that HCMV gB and gH stimulate monocyte survival by spatially regulating EGFR and integrin β1 at site of entry leading to the recruitment of nontraditional Akt activators.
The binding and activation of cellular receptors by viral glycoproteins during entry disrupt normal signaling to promote changes in the host cell microenvironment required for almost every aspect of the viral life cycle (77–79). HCMV has a broad cellular tropism largely due to the wide range of host receptors engaged during viral entry, including EGFR, PDGFRα, integrins, TLR2, Nrp1, CD147, CD90, and BST2 (31, 34, 48, 50–52, 80–84). Despite the plethora of cellular receptors involved in mediating HCMV entry, many ultimately signal to Akt (31, 48, 49, 51, 63, 82). This biological redundancy of HCMV to activate Akt via several mechanisms suggests a critical role of Akt to the viral life cycle. Akt acts as a signaling hub interpreting outside signals to elicit the appropriate response to the microenvironment (85). In other words, activation of different Akt activating receptors stimulates unique biological outcomes by Akt. Thus, Akt function during HCMV infection will be dependent on cell type and receptor repertoire expressed on the cellular surface. The receptors utilized by HCMV glycoproteins to activate Akt during infection of monocytes has been somewhat unclear due to the controversy that surrounds EGFR as a bona fide HCMV entry receptor. EGFR has been shown to facilitate HCMV entry into fibroblasts, trophoblasts, and endothelial cells (31, 49), while others did not observe EGFR activation during infection and found EGFR to be dispensable for virus entry (47). Instead, gB has been shown to bind PDGFRα to activate Akt and mediate HCMV entry (48). Although PDGFRα likely functions as an HCMV entry receptor into certain cell types, monocytes do not express PDGFRα (49). We have previously shown monocytes to express EGFR, which was required for HCMV entry into monocytes (49). However, the lack of direct evidence of gB binding directly to EGFR left open the possibility that EGFR was activated during infection by an ancillary mechanism. Thus, we confirm for the first time that gB directly binds and activates EGFR during HCMV entry into monocytes. These data further suggest the possibility of a hierarchy of Akt activation receptors engaged by HCMV whereby the low-affinity ligand binding of gB allows for substitution of receptors ensuring the initiation of Akt signaling.
HCMV glycoproteins other than gB have also been reported to engage Akt regulatory receptors (51, 82). Consistent with a previous report (42), we demonstrate that gH directly binds to integrin β1 but not to β3 during entry into monocytes, which is in contrast to gH binding to both integrin β1 and β3 on fibroblasts (34). Interestingly, we found sgB and sgH activates both EGFR and integrin β1, suggesting cross talk between the receptors that may be responsible for the unique Akt signaling network following infection of monocytes. In support, sgB immunoprecipitated only with EGFR and sgH only with integrin β1, yet small molecular inhibitors blocking the intracellular signaling domains of either receptors reduce activation of the other. Cross talk between EGFR and integrins has been shown to be important in cell survival, proliferation, and migration (86–88) as well as tumor cell invasion and metastasis (89–91). It appears that HCMV has usurped the synergistic interplay between EGFR and integrin β1 to enhance/modify signaling pathways. Wang et al. reported an early transient receptor clustering between EGFR and integrin β3, which was required to initiate appropriate signaling for HCMV entry into fibroblasts (34). Contrarily, we did not observe a direct interaction between EGFR and integrin β1 in monocytes during the early stages of HCMV entry, indicating that the HCMV-induced signalosome generated in monocytes is different than that of fibroblasts despite activating similar receptors.
HCMV stimulates a noncanonical activation of Akt by inducing a shift away from the PI3K p110δ to the p110β isoform as well as utilizing SHIP1 (63). The role of glycoproteins in coordinating the recruitment and activation of these Akt regulators that are not traditionally associated with Akt activation within monocytes is unclear. Our study demonstrates that gB binding of EGFR leads to the recruitment of PI3K p110β despite p110δ being the predominant isoform found in monocytes (61, 62) and being responsible for Akt regulation under homeostatic conditions (63). Why HCMV-induced activation of EGFR leads to the recruitment of the p110β isoform is unknown. While EGFR may have specificity toward p110β, generally receptor tyrosine kinases (RTKs) are able to bind the different isoforms of PI3K (92). Alternatively, we speculate HCMV induces virus-specific changes to EGFR signaling to promote the recruitment of p110β. Indeed, HCMV stimulates a distinct phosphorylation pattern on the cytoplasmic signaling domain compared to that of EGF activation (93). Regardless, gB activation of EGFR promotes the recruitment of p110β to initiate phosphatidylinositol signaling. Once initiated, SHIP1 then diverts PI(3,4,5)P3 signaling toward PI(3,4)P2 signaling, which preferentially phosphorylates Akt at S473 leading to the upregulation of a select subset of Akt-dependent antiapoptotic proteins (63). The regulation of SHIP1 appears to be cooperatively controlled by both EGFR and integrin β1. SHIP1 is recruited to the monocyte plasma membrane via integrin β1. However, inhibition of integrin β1 did not affect SHIP1 activity, but rather the loss of EGFR signaling prevented HCMV-induced SHIP1 activation. These data indicate that EGFR and integrin β1 work collectively to activate SHIP1, leading predominantly to the phosphorylation of Akt at S473. Because Akt substrate specificity is determined by the phosphorylation ratio between S473 and T308 residues (94, 95), we speculate that HCMV directs the upregulation of a subset of Akt-dependent proteins not induced during growth factor treatment. Indeed, SHIP1 is required for the upregulation of Mcl-1 and HSP27, which are known to be selectively upregulated in HCMV-infected monocytes (69).
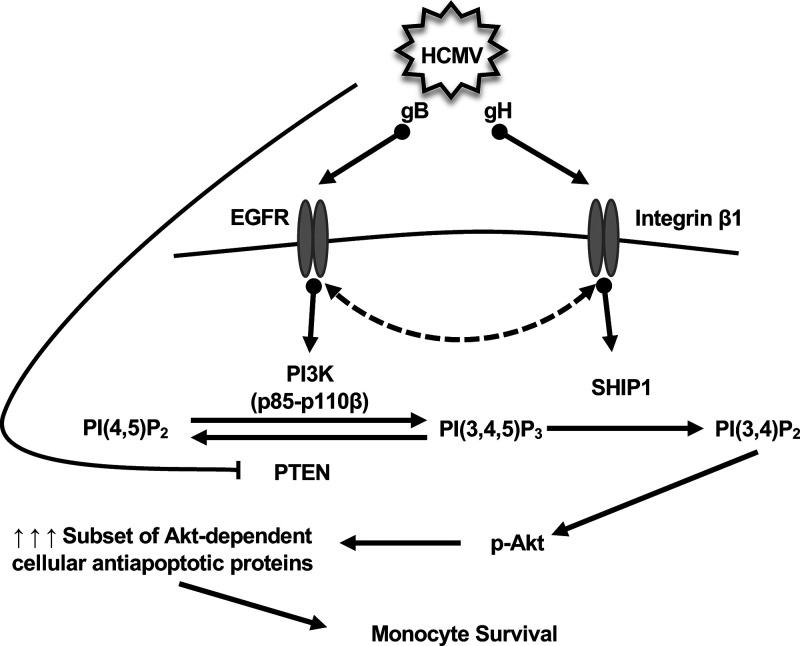
Monocytes are nonpermissive for HCMV replication; therefore, monocyte-to-macrophage differentiation is essential for the dissemination of the virus and establishment of HCMV latency. Here, we demonstrate that during monocyte entry, HCMV glycoproteins are responsible for triggering a noncanonical activation of Akt in order to drive the survival of short-lived monocytes (Fig. 7). Specifically, the spatial regulation of EGFR and integrin β1 by gB and gH leads to the concomitant recruitment and/or activation of PI3K p110β and SHIP1 to the plasma membrane. As observed in cancer cells, SHIP1 diverts signaling from PI(3,4,5)P3 to PI(3,4)P2, which aberrantly activates Akt leading to the upregulation of specific antiapoptotic proteins necessary for survival of infected monocytes. Currently, antiviral therapies against HCMV block specific steps along the virus replication cycle, rendering these drugs ineffective at preventing viral spread in transplant patients at high risk for exposure often resulting in rebound infection upon termination of the antiviral regimen. Thus, deciphering the mechanism of how HCMV modulates cellular signaling to induce monocyte survival may provide insight into new molecular targets aimed at eliminating infected monocytes and inhibiting viral dissemination.
FIG 7.
Proposed model for activation of monocyte receptors by HCMV glycoproteins required for Akt phosphorylation and subsequent survival of infected monocytes. During HCMV entry into monocytes, gB and gH directly bind EGFR and integrin β1, respectively. Activation of either receptor can also lead to the cross-activation of the other receptor. Ultimately, activation of EGFR by HCMV results in the recruitment of the p85 and p110β subunits of PI3K to initiate signaling from PI(3,4,5)P3. However, the concomitant recruitment of SHIP1 to integrin β1 diverts signaling away from canonical PI(3,4,5)P3 signaling to a noncanonical PI(3,4)P2-mediated activation of Akt. The nonclassical activation of Akt leads to the upregulation of a select subset of Akt-dependent antiapoptotic proteins required for the survival of HCMV-infected monocytes.
MATERIALS AND METHODS
Human peripheral blood monocyte isolation.
Isolation of human peripheral blood monocytes was performed as previously described (22, 25). Briefly, blood was drawn from random donors by venipuncture, diluted in RPMI 1640 medium, and centrifuged through Histopaque-1077 (Sigma-Aldrich, St. Louis, MO) to remove red blood cells and neutrophils. Mononuclear cells were collected and washed with saline to remove the platelets and then separated by centrifugation through a Percoll (GE Healthcare, Wilkes-Barre, PA) gradient (40.48% and 47.7%). More than 90% of isolated peripheral blood mononuclear cells were monocytes as determined by CD14-positive staining (24). Cells were washed with saline, resuspended in RPMI 1640 (Lonza, Walkersville, MD), and counted. All experiments were performed in 0 to 1% human serum at 37°C in a 5% CO2 incubator. University Institutional Review Board and Health Insurance Portability and Accountability Act guidelines for the use of human subjects were followed for all experimental protocols in our study.
For the inhibitor studies, the following reagents were purchased from the indicated companies: AG-1478 (AG) (an EGFR inhibitor [96]), 3-α-aminocholestane (3AC) (a SHIP1 inhibitor [66]), and LY294002 (a pan-PI3K inhibitor [97]) from Calbiochem (Billerica, MA) and ATN161 (ATN) (an integrin α5β1 inhibitor [98]) from Selleck Chemicals (Houston, TX).
Virus preparation and infection.
Human embryonic lung 299 (HEL 299) fibroblasts (CCL-137; American Type Culture Collection, Manassas, VA) of low passage (P7-15) were subcultured in Dulbecco’s modified Eagle medium (DMEM) (Lonza) with 2.5 μg/ml Plasmocin (InvivoGen, San Diego, CA) and 10% fetal bovine serum (FBS) (Sigma). When culture reached confluence, cells were infected with HCMV (strain TB40E) in DMEM + 4% FBS. Virus was purified from supernatant on a 20% sorbitol cushion to remove cellular contaminants and resuspended in RPMI 1640 medium. A multiplicity of infection (MOI) of 5 was used for each experiment, as >99% of monocytes were infected with TB40E (49) unless otherwise stated. Mock infection was performed by adding an equivalent volume of RPMI 1640 medium to monocytes. In some experiments, HCMV was pretreated for 1 h with blocking antibodies to glycoprotein B (gB; clone 10B2664; United States Biological, Salem, MA), glycoprotein H (gH; clone 51C1; Thermo Fisher, Rockford, IL), or an isotype control (Abcam, Cambridge, MA) and was used at 5 μg/ml.
Western blotting analysis.
Monocytes were harvested in modified radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl [pH 7.5], 5 mM EDTA, 100 mM NaCl, 1% Triton X-100, 0.1% SDS, 10% glycerol) supplemented with protease inhibitor cocktail (Sigma) and phosphatase inhibitor cocktails 2 and 3 (Sigma) for 30 min on ice. The lysates were cleared from the cell debris by centrifugation at 4°C (5 min, 21,000 × g) and stored at −20°C until further analysis. Protein samples were solubilized in Laemmli SDS sample nonreducing (6×) buffer (Boston Bioproducts, Boston, MA) supplemented with β-mercaptoethanol (Amresco, Solon, OH) by incubation at 95°C for 10 min unless otherwise stated. For detecting EGFR and phospho-EGFR, protein samples were incubated at 60°C for 10 min after solubilizing in Laemmli SDS sample nonreducing (6×) buffer. Equal amounts of total protein from each sample were loaded in each well, separated by SDS-polyacrylamide gel electrophoresis, and transferred to polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA). Blots were blocked in 5% bovine serum albumin (BSA) (Fisher Scientific, Waltham, MA) for 1 h at room temperature (RT) and then incubated with primary antibodies overnight at 4°C. The following antibodies were purchased from the indicated companies: anti-Akt, anti-phospho (p)-Akt (Ser473), anti-SHIP1, anti-p-SHIP1 (Tyr1020), anti-PI3K p110β, anti-EGFR, and anti-p-EGFR (Tyr1068) were from Cell Signaling Technology (Danvers, MA); anti-integrin β1 and anti-p-integrin β1 (Tyr783) were from Abcam (Cambridge, MA); anti-integrin β3 was from Novus Biologicals (Centennial, CO); anti-Mcl1 and anti-HSP27 were from Santa Cruz Biotechnology (Santa Cruz, CA); anti-glycoprotein B was from United States Biological (Salem, MA); anti-glycoprotein gH was from Thermo Fisher Scientific (Rockford, IL); and rhodamine anti-actin antibody was from Bio-Rad (Hercules, CA) and was used as loading control. The blots were then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (Cell Signaling Technology) for 30 min at room temperature, and chemiluminescence was detected using the Clarity Western ECL substrate (Bio-Rad). Densitometry analysis was performed using Image Lab software (Bio-Rad).
Purification of soluble sgB and sgH from stably expressing Expi293F cells.
HCMV (TB40E strain) gB and gH glycoprotein genomic regions were amplified and cloned into pQCXIN retroviral plasmids. During amplification, the transmembrane domains were replaced by His tags using the following primers: sgB-AgeI forward primer, 5′-CCCACCGGTGACGAACATGGAATCCAGGAT-3′; sgB-BamHI reverse primer, 5′-GCGGGATCCCTAATGGTGATGGTGATGATGCTGCTTGTACGAGTTGAATTC-3′; sgH-AgeI forward primer, 5′-CCCACCGGTCCGCGCTATGCGGCCCGGCCT-3′; sgH-BamHI reverse primer, GCGGGATCCCTAATGGTGATGGTGATGATGGTCGGTGGCGTCCACGACGAC-3′. Retroviral vectors containing the gB and gH genes were then generated according to manufacturer’s recommendations (TaKaRa, Mountain View, CA). Briefly, GP2-293 cells were transfected with 15 μg of retroviral plasmid and 15 μg of envelop plasmid (vesicular stomatitis virus G [VSV G]). The retroviruses were harvested after 48 h and used to infect Expi293F cells (Thermo Fisher Scientific, Rockford, IL) at an MOI of 10 to generate stable cell lines expressing sgB and sgH. After 24 h, Geneticin (G418; Thermo Fisher Scientific, Rockford, IL) was used at a 400-μg/ml concentration to select Expi293F cells containing the insert sequences. Once viability dropped to ∼20%, cells were maintained at 200 μg/ml Geneticin concentration until the viability recovered to 90 to 100%. The cells were then harvested and stored in the vapor phase of liquid nitrogen at a concentration of 1 × 107/ml in Expi293 expression medium according to manufacturer’s recommendations. For isolation of sgB/sgH, the stable cells lines were grown in Expi293 expression medium (Thermo Fisher Scientific, Rockford, IL) with 200 μg/ml Geneticin at 8% CO2 on an orbital shaker (125 rpm). Following lysis, recombinant sgB/sgH was then purified using Ni-charged resin (Bio-Rad, Hercules, CA) and dialyzed with PBS at the final stage of purification. Monocytes were treated with the soluble glycoproteins at a 1-μg/ml concentration for each experiment unless otherwise stated. Ni-charged resin-purified lysate of untransfected Expi293 cells was dialyzed with PBS, and the same amount of total volume of soluble glycoproteins was used as a negative control to treat monocytes.
Immunoprecipitation.
Monocytes were infected with HCMV (MOI of 15) or treated with sgB or sgH (5 μg/ml) at 4°C for 1 h. Subsequently, cells were either kept at 4°C or incubated at 37°C for 5 min. Afterward, cells were spun down at 1,000 × g for 5 min at 4°C. The pellet was washed twice with ice-cold PBS and lysed with NP40 cell lysis buffer (Thermo Fisher Scientific, Rockford, IL). The lysates were cleared from the cell debris by centrifugation at 4°C (5 min at 21,000 × g). Antibodies (5 μg) recognizing gB (United States Biological, Salem, MA), gH, EGFR (Thermo Fisher Scientific, Rockford, IL), integrin β1, p85 (Abcam, Cambridge, MA), SHIP1, or IgG isotype controls (Cell Signaling Technology, Danvers, MA) were added to the cleared lysate and incubated overnight at 4°C. Dynabeads protein A/G were added afterward and incubated at 4°C for an additional 4 h. Protein A/G beads with bound protein complexes were magnetically separated and washed with lysis buffer followed by elution of the protein complexes. Samples were then prepared for SDS-polyacrylamide gel electrophoresis and analyzed by Western blot assay.
Flow cytometry.
Monocytes were washed in PBS and incubated in blocking solution consisting of fluorescence-activated cell-sorting buffer, 5% BSA, and human FcR binding inhibitor (eBioscience, San Diego, CA). Cells were stained with an allophycocyanin (APC)-anti-CD14 or APC-anti-mouse IgG1 isotype control antibody (BioLegend, San Diego, CA) on ice and then washed and stained with fluorescein isothiocyanate (FITC)-annexin V and propidium iodide (PI) (ThermoFisher Scientific, Rockford, IL) to detect dead and dying cells. After staining, cells were analyzed by flow cytometry using an LSRFortessa cell analyzer and BD FACSDiva software (BD Biosciences, Franklin Lakes, NJ).
Statistical analysis.
All experiments were performed independently a minimum of 3 times using primary monocytes isolated from different blood donors. Data sets obtained from primary monocytes inherently have substantial variation due to donor variability. Consequently, data is displayed as matched experimental data points from individual donors in a side-by-side comparison. Displaying a side-by-side comparison allows for consistent trends between the different donors to be identified that may otherwise be missed when presenting only the mean and that may not be statistically significant given the high number of donors needed to achieve significance on smaller changes. Nonetheless, data were analyzed using Student's t test comparison with GraphPad Prism software, and P values less than 0.05 were considered statistically significant.
ACKNOWLEDGMENTS
We thank Christine Burrer in the Department of Microbiology and Immunology at SUNY Upstate Medical University for technical support, maintenance of lab operations, and assistance with virus growth and isolation.
This work was supported by grants from the Carol M. Baldwin Breast Cancer Research Fund to G. C. Chan, National Institute of Allergy and Infectious Diseases (R01AI141460) to G. C. Chan, and National Heart, Lung, and Blood Institute (R01HL139824) to G. C. Chan.
REFERENCES
- 1.Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. 2006. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis 43:1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 2.Nerheim PL, Meier JL, Vasef MA, Li WG, Hu L, Rice JB, Gavrila D, Richenbacher WE, Weintraub NL. 2004. Enhanced cytomegalovirus infection in atherosclerotic human blood vessels. Am J Pathol 164:589–600. doi: 10.1016/S0002-9440(10)63148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawlor G, Moss AC. 2010. Cytomegalovirus in inflammatory bowel disease: pathogen or innocent bystander? Inflamm Bowel Dis 16:1620–1627. doi: 10.1002/ibd.21275. [DOI] [PubMed] [Google Scholar]
- 4.Cobbs CS, Harkins L, Samanta M, Gillespie GY, Bharara S, King PH, Nabors LB, Cobbs CG, Britt WJ. 2002. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res 62:3347–3350. [PubMed] [Google Scholar]
- 5.Harkins L, Volk AL, Samanta M, Mikolaenko I, Britt WJ, Bland KI, Cobbs CS. 2002. Specific localisation of human cytomegalovirus nucleic acids and proteins in human colorectal cancer. Lancet 360:1557–1563. doi: 10.1016/S0140-6736(02)11524-8. [DOI] [PubMed] [Google Scholar]
- 6.Crough T, Khanna R. 2009. Immunobiology of human cytomegalovirus: from bench to bedside. Clin Microbiol Rev 22:76–98. doi: 10.1128/CMR.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang XJ, Zhang J, Xiong Y, Jahn G, Xiong HR, Yang ZQ, Liu YY. 2017. Human cytomegalovirus glycoprotein polymorphisms and increasing viral load in AIDS patients. PLoS One 12:e0176160. doi: 10.1371/journal.pone.0176160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koldehoff M, Lindemann M, Ross SR, Elmaagacli AH. 2018. Cytomegalovirus induces HLA-class-II-restricted alloreactivity in an acute myeloid leukemia cell line. PLoS One 13:e0191482. doi: 10.1371/journal.pone.0191482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Britt WJ. 2017. Congenital human cytomegalovirus infection and the enigma of maternal immunity. J Virol 91:e02392-16. doi: 10.1128/JVI.02392-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bissinger AL, Sinzger C, Kaiserling E, Jahn G. 2002. Human cytomegalovirus as a direct pathogen: correlation of multiorgan involvement and cell distribution with clinical and pathological findings in a case of congenital inclusion disease. J Med Virol 67:200–206. doi: 10.1002/jmv.2208. [DOI] [PubMed] [Google Scholar]
- 11.Yurochko AD. 2008. Human cytomegalovirus modulation of signal transduction. Curr Top Microbiol Immunol 325:205–220. doi: 10.1007/978-3-540-77349-8_12. [DOI] [PubMed] [Google Scholar]
- 12.Anderholm KM, Bierle CJ, Schleiss MR. 2016. Cytomegalovirus vaccines: current status and future prospects. Drugs 76:1625–1645. doi: 10.1007/s40265-016-0653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Booss J, Dann PR, Griffith BP, Kim JH. 1989. Host defense response to cytomegalovirus in the central nervous system. Predominance of the monocyte. Am J Pathol 134:71–78. [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor-Wiedeman J, Sissons JG, Borysiewicz LK, Sinclair JH. 1991. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol 72:2059–2064. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- 15.Sinzger C, Jahn G. 1996. Human cytomegalovirus cell tropism and pathogenesis. Intervirology 39:302–319. doi: 10.1159/000150502. [DOI] [PubMed] [Google Scholar]
- 16.Sinclair J, Sissons P. 1996. Latent and persistent infections of monocytes and macrophages. Intervirology 39:293–301. doi: 10.1159/000150501. [DOI] [PubMed] [Google Scholar]
- 17.Ibanez CE, Schrier R, Ghazal P, Wiley C, Nelson JA. 1991. Human cytomegalovirus productively infects primary differentiated macrophages. J Virol 65:6581–6588. doi: 10.1128/JVI.65.12.6581-6588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grefte JM, van der Gun BT, Schmolke S, van der Giessen M, van Son WJ, Plachter B, Jahn G, The TH. 1992. The lower matrix protein pp65 is the principal viral antigen present in peripheral blood leukocytes during an active cytomegalovirus infection. J Gen Virol 73:2923–2932. doi: 10.1099/0022-1317-73-11-2923. [DOI] [PubMed] [Google Scholar]
- 19.Grefte A, Harmsen MC, van der Giessen M, Knollema S, van Son WJ, The TH. 1994. Presence of human cytomegalovirus (HCMV) immediate early mRNA but not ppUL83 (lower matrix protein pp65) mRNA in polymorphonuclear and mononuclear leukocytes during active HCMV infection. J Gen Virol 75:1989–1998. doi: 10.1099/0022-1317-75-8-1989. [DOI] [PubMed] [Google Scholar]
- 20.Taylor-Wiedeman J, Sissons P, Sinclair J. 1994. Induction of endogenous human cytomegalovirus gene expression after differentiation of monocytes from healthy carriers. J Virol 68:1597–1604. doi: 10.1128/JVI.68.3.1597-1604.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitelaw DM. 1972. Observations on human monocyte kinetics after pulse labeling. Cell Tissue Kinet 5:311–317. doi: 10.1111/j.1365-2184.1972.tb00369.x. [DOI] [PubMed] [Google Scholar]
- 22.Smith MS, Bentz GL, Alexander JS, Yurochko AD. 2004. Human cytomegalovirus induces monocyte differentiation and migration as a strategy for dissemination and persistence. J Virol 78:4444–4453. doi: 10.1128/jvi.78.9.4444-4453.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan G, Bivins-Smith ER, Smith MS, Yurochko AD. 2009. NF-κB and phosphatidylinositol 3-kinase activity mediates the HCMV-induced atypical M1/M2 polarization of monocytes. Virus Res 144:329–333. doi: 10.1016/j.virusres.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan G, Nogalski MT, Yurochko AD. 2012. Human cytomegalovirus stimulates monocyte-to-macrophage differentiation via the temporal regulation of caspase 3. J Virol 86:10714–10723. doi: 10.1128/JVI.07129-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan G, Nogalski MT, Bentz GL, Smith MS, Parmater A, Yurochko AD. 2010. PI3K-dependent upregulation of Mcl-1 by human cytomegalovirus is mediated by epidermal growth factor receptor and inhibits apoptosis in short-lived monocytes. J Immunol 184:3213–3222. doi: 10.4049/jimmunol.0903025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yurochko AD, Huang ES. 1999. Human cytomegalovirus binding to human monocytes induces immunoregulatory gene expression. J Immunol 162:4806–4816. [PubMed] [Google Scholar]
- 27.Yurochko AD, Hwang ES, Rasmussen L, Keay S, Pereira L, Huang ES. 1997. The human cytomegalovirus UL55 (gB) and UL75 (gH) glycoprotein ligands initiate the rapid activation of Sp1 and NF-kappaB during infection. J Virol 71:5051–5059. doi: 10.1128/JVI.71.7.5051-5059.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyle KA, Pietropaolo RL, Compton T. 1999. Engagement of the cellular receptor for glycoprotein B of human cytomegalovirus activates the interferon-responsive pathway. Mol Cell Biol 19:3607–3613. doi: 10.1128/mcb.19.5.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Compton T, Nowlin DM, Cooper NR. 1993. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology 193:834–841. doi: 10.1006/viro.1993.1192. [DOI] [PubMed] [Google Scholar]
- 30.Compton T. 2004. Receptors and immune sensors: the complex entry path of human cytomegalovirus. Trends Cell Biol 14:5–8. doi: 10.1016/j.tcb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Huong SM, Chiu ML, Raab-Traub N, Huang ES. 2003. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 424:456–461. doi: 10.1038/nature01818. [DOI] [PubMed] [Google Scholar]
- 32.Hahn G, Revello MG, Patrone M, Percivalle E, Campanini G, Sarasini A, Wagner M, Gallina A, Milanesi G, Koszinowski U, Baldanti F, Gerna G. 2004. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J Virol 78:10023–10033. doi: 10.1128/JVI.78.18.10023-10033.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang D, Shenk T. 2005. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci U S A 102:18153–18158. doi: 10.1073/pnas.0509201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X, Huang DY, Huong SM, Huang ES. 2005. Integrin alphavbeta3 is a coreceptor for human cytomegalovirus. Nat Med 11:515–521. doi: 10.1038/nm1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerna G, Percivalle E, Lilleri D, Lozza L, Fornara C, Hahn G, Baldanti F, Revello MG. 2005. Dendritic-cell infection by human cytomegalovirus is restricted to strains carrying functional UL131-128 genes and mediates efficient viral antigen presentation to CD8+ T cells. J Gen Virol 86:275–284. doi: 10.1099/vir.0.80474-0. [DOI] [PubMed] [Google Scholar]
- 36.Ryckman BJ, Jarvis MA, Drummond DD, Nelson JA, Johnson DC. 2006. Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion. J Virol 80:710–722. doi: 10.1128/JVI.80.2.710-722.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adler B, Scrivano L, Ruzcics Z, Rupp B, Sinzger C, Koszinowski U. 2006. Role of human cytomegalovirus UL131A in cell type-specific virus entry and release. J Gen Virol 87:2451–2460. doi: 10.1099/vir.0.81921-0. [DOI] [PubMed] [Google Scholar]
- 38.Ryckman BJ, Rainish BL, Chase MC, Borton JA, Nelson JA, Jarvis MA, Johnson DC. 2008. Characterization of the human cytomegalovirus gH/gL/UL128-131 complex that mediates entry into epithelial and endothelial cells. J Virol 82:60–70. doi: 10.1128/JVI.01910-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Isaacson MK, Compton T. 2009. Human cytomegalovirus glycoprotein B is required for virus entry and cell-to-cell spread but not for virion attachment, assembly, or egress. J Virol 83:3891–3903. doi: 10.1128/JVI.01251-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nogalski MT, Chan G, Stevenson EV, Gray S, Yurochko AD. 2011. Human cytomegalovirus-regulated paxillin in monocytes links cellular pathogenic motility to the process of viral entry. J Virol 85:1360–1369. doi: 10.1128/JVI.02090-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Straschewski S, Patrone M, Walther P, Gallina A, Mertens T, Frascaroli G. 2011. Protein pUL128 of human cytomegalovirus is necessary for monocyte infection and blocking of migration. J Virol 85:5150–5158. doi: 10.1128/JVI.02100-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nogalski MT, Chan GC, Stevenson EV, Collins-McMillen DK, Yurochko AD. 2013. The HCMV gH/gL/UL128-131 complex triggers the specific cellular activation required for efficient viral internalization into target monocytes. PLoS Pathog 9:e1003463. doi: 10.1371/journal.ppat.1003463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huber MT, Compton T. 1998. The human cytomegalovirus UL74 gene encodes the third component of the glycoprotein H-glycoprotein L-containing envelope complex. J Virol 72:8191–8197. doi: 10.1128/JVI.72.10.8191-8197.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huber MT, Compton T. 1999. Intracellular formation and processing of the heterotrimeric gH-gL-gO (gCIII) glycoprotein envelope complex of human cytomegalovirus. J Virol 73:3886–3892. doi: 10.1128/JVI.73.5.3886-3892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J, Jardetzky TS, Chin AL, Johnson DC, Vanarsdall AL. 2018. The human cytomegalovirus trimer and pentamer promote sequential steps in entry into epithelial and endothelial cells at cell surfaces and endosomes. J Virol 92:e01336-18. doi: 10.1128/JVI.01336-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang D, Shenk T. 2005. Human cytomegalovirus UL131 open reading frame is required for epithelial cell tropism. J Virol 79:10330–10338. doi: 10.1128/JVI.79.16.10330-10338.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Isaacson MK, Feire AL, Compton T. 2007. Epidermal growth factor receptor is not required for human cytomegalovirus entry or signaling. J Virol 81:6241–6247. doi: 10.1128/JVI.00169-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soroceanu L, Akhavan A, Cobbs CS. 2008. Platelet-derived growth factor-alpha receptor activation is required for human cytomegalovirus infection. Nature 455:391–395. doi: 10.1038/nature07209. [DOI] [PubMed] [Google Scholar]
- 49.Chan G, Nogalski MT, Yurochko AD. 2009. Activation of EGFR on monocytes is required for human cytomegalovirus entry and mediates cellular motility. Proc Natl Acad Sci U S A 106:22369–22374. doi: 10.1073/pnas.0908787106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kabanova A, Marcandalli J, Zhou T, Bianchi S, Baxa U, Tsybovsky Y, Lilleri D, Silacci-Fregni C, Foglierini M, Fernandez-Rodriguez BM, Druz A, Zhang B, Geiger R, Pagani M, Sallusto F, Kwong PD, Corti D, Lanzavecchia A, Perez L. 2016. Platelet-derived growth factor-alpha receptor is the cellular receptor for human cytomegalovirus gHgLgO trimer. Nat Microbiol 1:16082. doi: 10.1038/nmicrobiol.2016.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu Y, Prager A, Boos S, Resch M, Brizic I, Mach M, Wildner S, Scrivano L, Adler B. 2017. Human cytomegalovirus glycoprotein complex gH/gL/gO uses PDGFR-alpha as a key for entry. PLoS Pathog 13:e1006281. doi: 10.1371/journal.ppat.1006281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vanarsdall AL, Pritchard SR, Wisner TW, Liu J, Jardetzky TS, Johnson DC. 2018. CD147 promotes entry of pentamer-expressing human cytomegalovirus into epithelial and endothelial cells. mBio 9:e00781-18. doi: 10.1128/mBio.00781-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Altman AM, Mahmud J, Nikolovska-Coleska Z, Chan G. 2019. HCMV modulation of cellular PI3K/AKT/mTOR signaling: new opportunities for therapeutic intervention? Antiviral Res 163:82–90. doi: 10.1016/j.antiviral.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E. 2014. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med 46:372–383. doi: 10.3109/07853890.2014.912836. [DOI] [PubMed] [Google Scholar]
- 55.Kelley TW, Graham MM, Doseff AI, Pomerantz RW, Lau SM, Ostrowski MC, Franke TF, Marsh CB. 1999. Macrophage colony-stimulating factor promotes cell survival through Akt/protein kinase B. J Biol Chem 274:26393–26398. doi: 10.1074/jbc.274.37.26393. [DOI] [PubMed] [Google Scholar]
- 56.Liu H, Perlman H, Pagliari LJ, Pope RM. 2001. Constitutively activated Akt-1 is vital for the survival of human monocyte-differentiated macrophages: role of Mcl-1, independent of nuclear factor (NF)-κB, bad, or caspase activation. J Exp Med 194:113–126. doi: 10.1084/jem.194.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goyal A, Wang Y, Graham MM, Doseff AI, Bhatt NY, Marsh CB. 2002. Monocyte survival factors induce Akt activation and suppress caspase-3. Am J Respir Cell Mol Biol 26:224–230. doi: 10.1165/ajrcmb.26.2.4640. [DOI] [PubMed] [Google Scholar]
- 58.Leverrier Y, Okkenhaug K, Sawyer C, Bilancio A, Vanhaesebroeck B, Ridley AJ. 2003. Class I phosphoinositide 3-kinase p110β is required for apoptotic cell and Fcγ receptor-mediated phagocytosis by macrophages. J Biol Chem 278:38437–38442. doi: 10.1074/jbc.M306649200. [DOI] [PubMed] [Google Scholar]
- 59.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. 2010. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol 11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 60.Engelman JA, Luo J, Cantley LC. 2006. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet 7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 61.Bilancio A, Okkenhaug K, Camps M, Emery JL, Ruckle T, Rommel C, Vanhaesebroeck B. 2006. Key role of the p110δ isoform of PI3K in B-cell antigen and IL-4 receptor signaling: comparative analysis of genetic and pharmacologic interference with p110δ function in B cells. Blood 107:642–650. doi: 10.1182/blood-2005-07-3041. [DOI] [PubMed] [Google Scholar]
- 62.Papakonstanti EA, Zwaenepoel O, Bilancio A, Burns E, Nock GE, Houseman B, Shokat K, Ridley AJ, Vanhaesebroeck B. 2008. Distinct roles of class IA PI3K isoforms in primary and immortalised macrophages. J Cell Sci 121:4124–4133. doi: 10.1242/jcs.032763. [DOI] [PubMed] [Google Scholar]
- 63.Cojohari O, Peppenelli MA, Chan GC. 2016. Human cytomegalovirus induces an atypical activation of Akt to stimulate the survival of short-lived monocytes. J Virol 90:6443–6452. doi: 10.1128/JVI.00214-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gericke A, Leslie NR, Losche M, Ross AH. 2013. PtdIns(4,5)P2-mediated cell signaling: emerging principles and PTEN as a paradigm for regulatory mechanism. Adv Exp Med Biol 991:85–104. doi: 10.1007/978-94-007-6331-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malek M, Kielkowska A, Chessa T, Anderson KE, Barneda D, Pir P, Nakanishi H, Eguchi S, Koizumi A, Sasaki J, Juvin V, Kiselev VY, Niewczas I, Gray A, Valayer A, Spensberger D, Imbert M, Felisbino S, Habuchi T, Beinke S, Cosulich S, Le Novere N, Sasaki T, Clark J, Hawkins PT, Stephens LR. 2017. PTEN regulates PI(3,4)P2 signaling downstream of class I PI3K. Mol Cell 68:566–580. doi: 10.1016/j.molcel.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brooks R, Fuhler GM, Iyer S, Smith MJ, Park MY, Paraiso KH, Engelman RW, Kerr WG. 2010. SHIP1 inhibition increases immunoregulatory capacity and triggers apoptosis of hematopoietic cancer cells. J Immunol 184:3582–3589. doi: 10.4049/jimmunol.0902844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kerr WG. 2011. Inhibitor and activator: dual functions for SHIP in immunity and cancer. Ann N Y Acad Sci 1217:1–17. doi: 10.1111/j.1749-6632.2010.05869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fernandes S, Iyer S, Kerr WG. 2013. Role of SHIP1 in cancer and mucosal inflammation. Ann N Y Acad Sci 1280:6–10. doi: 10.1111/nyas.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peppenelli MA, Arend KC, Cojohari O, Moorman NJ, Chan GC. 2016. Human cytomegalovirus stimulates the synthesis of select Akt-dependent antiapoptotic proteins during viral entry to promote survival of infected monocytes. J Virol 90:3138–3147. doi: 10.1128/JVI.02879-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feire AL, Roy RM, Manley K, Compton T. 2010. The glycoprotein B disintegrin-like domain binds beta 1 integrin to mediate cytomegalovirus entry. J Virol 84:10026–10037. doi: 10.1128/JVI.00710-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim JH, Collins-McMillen D, Caposio P, Yurochko AD. 2016. Viral binding-induced signaling drives a unique and extended intracellular trafficking pattern during infection of primary monocytes. Proc Natl Acad Sci U S A 113:8819–8824. doi: 10.1073/pnas.1604317113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peppenelli MA, Miller MJ, Altman AM, Cojohari O, Chan GC. 2018. Aberrant regulation of the Akt signaling network by human cytomegalovirus allows for targeting of infected monocytes. Antiviral Res 158:13–24. doi: 10.1016/j.antiviral.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Franke TF, Kaplan DR, Cantley LC, Toker A. 1997. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science 275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 74.Gnann JW Jr, Ahlmen J, Svalander C, Olding L, Oldstone MB, Nelson JA. 1988. Inflammatory cells in transplanted kidneys are infected by human cytomegalovirus. Am J Pathol 132:239–248. [PMC free article] [PubMed] [Google Scholar]
- 75.Goodrum F, Caviness K, Zagallo P. 2012. Human cytomegalovirus persistence. Cell Microbiol 14:644–655. doi: 10.1111/j.1462-5822.2012.01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DeMeritt IB, Podduturi JP, Tilley AM, Nogalski MT, Yurochko AD. 2006. Prolonged activation of NF-κB by human cytomegalovirus promotes efficient viral replication and late gene expression. Virology 346:15–31. doi: 10.1016/j.virol.2005.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nemerow GR. 2000. Cell receptors involved in adenovirus entry. Virology 274:1–4. doi: 10.1006/viro.2000.0468. [DOI] [PubMed] [Google Scholar]
- 78.Greber UF. 2002. Signalling in viral entry. Cell Mol Life Sci 59:608–626. doi: 10.1007/s00018-002-8453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mercer J, Schelhaas M, Helenius A. 2010. Virus entry by endocytosis. Annu Rev Biochem 79:803–833. doi: 10.1146/annurev-biochem-060208-104626. [DOI] [PubMed] [Google Scholar]
- 80.Feire AL, Koss H, Compton T. 2004. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc Natl Acad Sci U S A 101:15470–15475. doi: 10.1073/pnas.0406821101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Viswanathan K, Smith MS, Malouli D, Mansouri M, Nelson JA, Fruh K. 2011. BST2/tetherin enhances entry of human cytomegalovirus. PLoS Pathog 7:e1002332. doi: 10.1371/journal.ppat.1002332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Q, Wilkie AR, Weller M, Liu X, Cohen JI. 2015. THY-1 cell surface antigen (CD90) has an important role in the initial stage of human cytomegalovirus infection. PLoS Pathog 11:e1004999. doi: 10.1371/journal.ppat.1004999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li Q, Fischer E, Cohen JI. 2016. Cell Surface THY-1 contributes to human cytomegalovirus entry via a macropinocytosis-like process. J Virol 90:9766–9781. doi: 10.1128/JVI.01092-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martinez-Martin N, Marcandalli J, Huang CS, Arthur CP, Perotti M, Foglierini M, Ho H, Dosey AM, Shriver S, Payandeh J, Leitner A, Lanzavecchia A, Perez L, Ciferri C. 2018. An unbiased screen for human cytomegalovirus identifies neuropilin-2 as a central viral receptor. Cell 174:1158–1171. doi: 10.1016/j.cell.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 85.Manning BD, Cantley LC. 2007. AKT/PKB signaling: navigating downstream. Cell 129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moro L, Dolce L, Cabodi S, Bergatto E, Boeri Erba E, Smeriglio M, Turco E, Retta SF, Giuffrida MG, Venturino M, Godovac-Zimmermann J, Conti A, Schaefer E, Beguinot L, Tacchetti C, Gaggini P, Silengo L, Tarone G, Defilippi P. 2002. Integrin-induced epidermal growth factor (EGF) receptor activation requires c-Src and p130Cas and leads to phosphorylation of specific EGF receptor tyrosines. J Biol Chem 277:9405–9414. doi: 10.1074/jbc.M109101200. [DOI] [PubMed] [Google Scholar]
- 87.Bill HM, Knudsen B, Moores SL, Muthuswamy SK, Rao VR, Brugge JS, Miranti CK. 2004. Epidermal growth factor receptor-dependent regulation of integrin-mediated signaling and cell cycle entry in epithelial cells. Mol Cell Biol 24:8586–8599. doi: 10.1128/MCB.24.19.8586-8599.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Edick MJ, Tesfay L, Lamb LE, Knudsen BS, Miranti CK. 2007. Inhibition of integrin-mediated crosstalk with epidermal growth factor receptor/Erk or Src signaling pathways in autophagic prostate epithelial cells induces caspase-independent death. Mol Biol Cell 18:2481–2490. doi: 10.1091/mbc.e06-04-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ricono JM, Huang M, Barnes LA, Lau SK, Weis SM, Schlaepfer DD, Hanks SK, Cheresh DA. 2009. Specific cross-talk between epidermal growth factor receptor and integrin alphavbeta5 promotes carcinoma cell invasion and metastasis. Cancer Res 69:1383–1391. doi: 10.1158/0008-5472.CAN-08-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Williams KC, Coppolino MG. 2014. SNARE-dependent interaction of Src, EGFR and beta1 integrin regulates invadopodia formation and tumor cell invasion. J Cell Sci 127:1712–1725. doi: 10.1242/jcs.134734. [DOI] [PubMed] [Google Scholar]
- 91.Tai YL, Chu PY, Lai IR, Wang MY, Tseng HY, Guan JL, Liou JY, Shen TL. 2015. An EGFR/Src-dependent β4 integrin/FAK complex contributes to malignancy of breast cancer. Sci Rep 5:16408. doi: 10.1038/srep16408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matheny RW Jr, Adamo ML. 2010. PI3K p110α and p110β have differential effects on Akt activation and protection against oxidative stress-induced apoptosis in myoblasts. Cell Death Differ 17:677–688. doi: 10.1038/cdd.2009.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.LaMarca HL, Nelson AB, Scandurro AB, Whitley GS, Morris CA. 2006. Human cytomegalovirus-induced inhibition of cytotrophoblast invasion in a first trimester extravillous cytotrophoblast cell line. Placenta 27:137–147. doi: 10.1016/j.placenta.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 94.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. 2006. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 95.Yung HW, Charnock-Jones DS, Burton GJ. 2011. Regulation of AKT phosphorylation at Ser473 and Thr308 by endoplasmic reticulum stress modulates substrate specificity in a severity dependent manner. PLoS One 6:e17894. doi: 10.1371/journal.pone.0017894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johns TG, Luwor RB, Murone C, Walker F, Weinstock J, Vitali AA, Perera RM, Jungbluth AA, Stockert E, Old LJ, Nice EC, Burgess AW, Scott AM. 2003. Antitumor efficacy of cytotoxic drugs and the monoclonal antibody 806 is enhanced by the EGF receptor inhibitor AG1478. Proc Natl Acad Sci U S A 100:15871–15876. doi: 10.1073/pnas.2036503100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vlahos CJ, Matter WF, Hui KY, Brown RF. 1994. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem 269:5241–5248. [PubMed] [Google Scholar]
- 98.Stoeltzing O, Liu W, Reinmuth N, Fan F, Parry GC, Parikh AA, McCarty MF, Bucana CD, Mazar AP, Ellis LM. 2003. Inhibition of integrin α5β1 function with a small peptide (ATN-161) plus continuous 5-FU infusion reduces colorectal liver metastases and improves survival in mice. Int J Cancer 104:496–503. doi: 10.1002/ijc.10958. [DOI] [PubMed] [Google Scholar]