Foot-and-mouth disease virus (FMDV) nonstructural protein 3A plays important roles in virus replication, host range, and virulence. To further understand the role of 3A during FMDV infection, identification of host cell factors that interact with FMDV 3A is needed. Here, we found that vimentin is a direct binding partner of FMDV 3A, and manipulation of vimentin has a negative effect on virus replication. We also demonstrated that amino acid residues 15 to 21 at the N-terminal region of the FMDV 3A are responsible for the interaction between 3A and vimentin and that the 3A-vimentin interaction is critical for viral replication since the full-length cDNA clone harboring mutations in 3A, which were disrupt 3A-vimentin reactivity, could not produce viable virus progeny. This study provides information that not only provides us a better understanding of the mechanism of FMDV replication but also helps in the development of novel antiviral strategies in the future.
KEYWORDS: 3A, foot-and-mouth disease virus, vimentin
ABSTRACT
Nonstructural protein 3A of foot-and-mouth disease virus (FMDV) is a partially conserved protein of 153 amino acids that is in most FMDVs examined to date, and it plays important roles in virus replication, virulence, and host range. To better understand the role of 3A during FMDV infection, we used coimmunoprecipitation followed by mass spectrometry to identify host proteins that interact with 3A in FMDV-infected cells. Here, we report that cellular vimentin is a host binding partner for 3A. The 3A-vimentin interaction was further confirmed by coimmunoprecipitation, glutathione S-transferase (GST) pull down, and immunofluorescence assays. Alanine-scanning mutagenesis indicated that amino acid residues 15 to 21 at the N-terminal region of the FMDV 3A are responsible for the interaction between 3A and vimentin. Using reverse genetics, we demonstrate that mutations in 3A that disrupt the interaction between 3A and vimentin are also critical for virus growth. Overexpression of vimentin significantly suppressed the replication of FMDV, whereas knockdown of vimentin significantly enhanced FMDV replication. However, chemical disruption of the vimentin network by acrylamide resulted in a significant decrease in viral yield, suggesting that an intact vimentin network is needed for FMDV replication. These results indicate that vimentin interacts with FMDV 3A and negatively regulates FMDV replication and that the vimentin-3A interaction is essential for FMDV replication. This study provides information that should be helpful for understanding the molecular mechanism of FMDV replication.
IMPORTANCE Foot-and-mouth disease virus (FMDV) nonstructural protein 3A plays important roles in virus replication, host range, and virulence. To further understand the role of 3A during FMDV infection, identification of host cell factors that interact with FMDV 3A is needed. Here, we found that vimentin is a direct binding partner of FMDV 3A, and manipulation of vimentin has a negative effect on virus replication. We also demonstrated that amino acid residues 15 to 21 at the N-terminal region of the FMDV 3A are responsible for the interaction between 3A and vimentin and that the 3A-vimentin interaction is critical for viral replication since the full-length cDNA clone harboring mutations in 3A, which were disrupt 3A-vimentin reactivity, could not produce viable virus progeny. This study provides information that not only provides us a better understanding of the mechanism of FMDV replication but also helps in the development of novel antiviral strategies in the future.
INTRODUCTION
Foot-and-mouth disease virus (FMDV), a member of the Aphthovirus genus within the Picornaviridae family, is the causative agent of foot-and-mouth disease (FMD), a highly contagious and economically important disease of cloven-hoofed animals, both domestic and wild (1, 2). FMDV is a nonenveloped, single-stranded positive-sense RNA virus with a genome of approximately 8,400 nucleotides and exists as 7 immunologically distinct serotypes: O, A, C, Asia 1, South African Territories type 1 (SAT1), SAT2, and SAT3 (3, 4). The viral RNA genome contains a single open reading frame encoding a polyprotein, which is processed co- and posttranslationally by virus-encoded proteases to yield a set of intermediate precursors and the mature viral proteins, including four structural proteins (VP1, VP2, VP3, and VP4) and eight nonstructural proteins (L, 2A, 2B, 2C, 3A, 3B, 3C, and 3D) (5, 6). Both the protein intermediates and the mature viral proteins are critical for viral replication (7, 8).
FMDV infection causes a major rearrangement of intracellular membranes into vesicle structures to form sites of viral replication (9, 10), where viral nonstructural proteins and some host proteins compose the replication complex. FMDV nonstructural protein 3A, as an essential part of the replication complex, contains a membrane-binding hydrophobic domain which is thought to anchor the 3A protein to intracellular membranes, thereby mediating the location of the viral RNA replication complex within a membrane context (11). FMDV 3A is unique among the picornaviruses by extending its carboxy terminus in at least 60 amino acids and is a partially conserved protein of 153-amino acids long in most FMDVs examined to date. The amino-terminal (N-terminal) half of the FMDV 3A protein, which contains an N-terminal hydrophilic domain, a hydrophobic transmembrane domain (residues 59 to 76) (12, 13), and a dimerization domain with a hydrophobic interface (residues 25 to 44) (14), is highly conserved among all FMDVs and has been thought to be important for FMDV replication. In contrast, the carboxy-terminal (C-terminal) half of 3A is variable and tolerates large deletions, which have been involved in alterations in virulence, host tropism, and replication ability (15–17). Therefore, the structure and the membrane-binding properties of 3A suggest that FMDV 3A plays multiple roles and may interact with several host cellular factors in the process of virus replication. Although some host cellular factors have been identified to interact with FMDV 3A and to modulate the replication of FMDV (18, 19), the role of 3A and the interactions of 3A with cellular proteins during FMDV replication have not yet been fully elucidated.
Vimentin, a major component of type III intermediate filaments, is detected in cells of mesenchymal origin and is also present in cells adapted to tissue culture and many transformed cell lines (20). The main function of vimentin is to maintain the shape and mechanical integrity of cells (21). Vimentin has also been shown to participate in a variety of other cell functions, such as organelle positioning, vesicular and organelle transport, cell signaling, cell adhesion, and migration (22, 23). In addition, recent studies demonstrate that vimentin plays important roles during viral infection. Vimentin is involved in the process of viral entry of Japanese encephalitis virus (24, 25), enterovirus 71 (26), porcine reproductive and respiratory syndrome virus (27), severe acute respiratory syndrome coronavirus (SARS-CoV) (28), cowpea mosaic virus (29), and human cytomegalovirus (30). Vimentin also plays a critical role in the process of viral replication of dengue virus (31), transmissible gastroenteritis virus (32), and vaccinia virus (33) and in the viral egress of bluetongue virus via interactions with the outer capsid protein VP2 (34). Moreover, virus infection, such as frog virus 3 (35), vaccinia virus (36), and African swine fever virus (37), can induce rearrangement of vimentin to form vimentin cages around the viral factories, which is important for virus survival. On the other hand, infection with human immunodeficiency virus (38) or adenovirus type 2 (39) results in the cleavage of host vimentin, although the function of this cleavage remains elusive. Similarly, a recent study has demonstrated that FMDV 2C can interact with cellular vimentin to modulate virus replication (40). However, the role of vimentin during FMDV infection remains to be characterized.
In this study, we used coimmunoprecipitation followed by mass spectrometric analyses to identify host cell proteins that interact with FMDV 3A during FMDV infection. We discovered that vimentin is an interacting partner of FMDV 3A, and the interaction was confirmed by coimmunoprecipitation and GST pull down. Using overexpression and knockdown strategies, we found that modulations of the expression of vimentin have a negative effect on FMDV replication in cell culture. In addition, acrylamide treatment, which causes disruption of vimentin intermediate filaments, resulted in a significant decrease in viral yield, suggesting that an intact vimentin network is required for FMDV replication. The regions within 3A that mediate the interaction with vimentin were mapped by alanine-scanning mutagenesis. Using reverse genetics, we demonstrated that changes to amino acid residues in 3A that are responsible for interactions with vimentin are clearly detrimental to virus replication. Overall, these results indicate that the vimentin and its interaction with 3A may play a significant role in FMDV replication.
RESULTS
FMDV 3A interacts with cellular vimentin.
FMDV 3A has been documented to be involved in the replication of FMDV; however, the exact role of 3A during FMDV replication has not yet been well defined. Additionally, the interactions of FMDV 3A with host cellular proteins are not well known. To search for host cellular proteins that interact with FMDV 3A, we performed a combination of coimmunoprecipitate assays with mass spectrometry. Proteins derived from the whole-cell lysate of FMDV-infected FBK cells were coimmunoprecipitated with antibodies specific for the FMDV 3A protein. The immunoprecipitated proteins were subjected to SDS-PAGE, visualized using Coomassie brilliant blue staining, and identified by liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis. Several host proteins were shown to interact with FMDV 3A (Table 1). Among those proteins, host cellular vimentin, which was identified with high confidence by MS with 12 unique peptides to the proteins and a 29.61% sequence coverage, was selected for further study due to its biological function in organelle and vesicular transport and organelle positioning (22). To verify the interaction of vimentin with FMDV 3A, FLAG-tagged vimentin was coexpressed with Myc-tagged FMDV 3A in HEK293T cells, and then a coimmunoprecipitation assay was performed using anti-c-Myc agarose beads and blotted with an anti-FLAG antibody. As shown in Fig. 1A, FLAG-vimentin was coimmunoprecipitation with Myc-3A but not with empty vector control, indicating that vimentin interacted with FMDV 3A and confirming the mass spectrometry results. Furthermore, to further confirm the interaction between vimentin and FMDV 3A, a GST pulldown assay was performed using GST-fused 3A (GST-3A) and His-fused vimentin (His-vimentin) expressed in Escherichia coli BL21. His-vimentin was pulled down by GST-3A but not by GST alone (Fig. 1B). Moreover, to further confirm that the interaction between vimentin and 3A occurs during FMDV infection, the locations of vimentin and 3A were detected in FMDV-infected PK-15 cells by using a double-label immunofluorescence assay, and the result indicated that vimentin colocalized with FMDV 3A during FMDV infection (Fig. 1C). Taken together, these results indicate that FMDV 3A directly interacts with cellular vimentin.
TABLE 1.
A list of the top 10 proteins interacting with the FMDV 3A protein
| UniProt accession no. | Protein_qscore | No. of unique peptidesa | %Covb | MWc | Identified name |
|---|---|---|---|---|---|
| P48616 | 41.86 | 12 | 29.61 | 53,695.08 | Vimentin |
| F6S1Q0 | 39.51 | 10 | 37.06 | 47,935.48 | Uncharacterized protein |
| P08728 | 33.84 | 7 | 28.07 | 43,858.06 | Keratin, type I cytoskeletal 19 |
| A6QNZ7 | 26.87 | 7 | 15.4 | 54,816.18 | Keratin 10 |
| O02717 | 20.93 | 6 | 11.20 | 72,326.97 | Nonmuscle myosin heavy chain |
| A4IF97 | 17.44 | 5 | 29.82 | 19,679.48 | Myosin regulatory light chain 12B |
| Q3T149 | 14.17 | 4 | 23.88 | 22,379.33 | Heat shock protein beta-1 |
| Q861U5 | 13.95 | 4 | 31.3 | 15,388.51 | Similar to 40S ribosomal protein S18 |
| B5B3R8 | 10.46 | 3 | 17.29 | 24,427.39 | Alpha S1 casein |
| Q56JX8 | 10.46 | 3 | 24.5 | 17,211.67 | 40S ribosomal protein S13 |
The number of peptide sequences unique to a protein group.
The percentage of the protein sequence covered by identified peptides [(number of the matched residues/total number of residues in the entire sequence) × 100%].
Molecular weight.
FIG 1.
Interaction of FMDV 3A with vimentin. (A) Coimmunoprecipitation analysis of Myc-3A and FLAG-vimentin. Plasmids encoding Myc-3A and FLAG-vimentin were transfected into HEK293T cells together or alone. At 24 h after transfection, cell lysates were immunoprecipitated with anti-c-Myc agarose beads. The bound proteins were eluted and detected by Western blotting with anti-Myc and anti-FLAG monoclonal antibody (MAb). (B) GST pulldown assay. The soluble proteins GST-3A or GST expressed in E. coli BL21 were conjugated to glutathione beads and incubated with the soluble His-vimentin protein expressed in E. coli BL21. After being washed, the bound proteins were eluted from the beads and detected by immunoblotting with anti-GST antibody and anti-Myc antibody. (C) Colocalization analysis of vimentin with FMDV 3A in PK-15 cells. PK-15 cells were infected (MOI, 10) or mock infected with FMDV O/GZSB/2011. At 3.5 hpi, the cells were fixed and stained with antibodies against vimentin (red) and FMDV 3A (green).
Identification of the vimentin binding site on FMDV 3A.
To identify the critical amino acid residues for the interaction between FMDV 3A and vimentin, alanine-scanning mutagenesis of FMDV 3A was performed. A total of 22 mutant 3A proteins which contain 7 consecutive alanine residues to substitute the native amino acid residues were constructed (Fig. 2A). The ability of these mutated 3A proteins to bind vimentin was assessed using GST pulldown assay. GST pulldown experiments showed that the 3A protein containing mutations in area 3 was unable to bind vimentin (Fig. 2B), demonstrating that amino acid residues in area 3 of FMDV 3A were critical for the interaction between 3A and vimentin.
FIG 2.
Alanine-scanning mutagenesis of FMDV 3A identifies residues required for the interaction with vimentin. (A) Schematic diagram of FMDV 3A alanine mutants used in the present study. Each alanine 3A mutant name is followed by the amino acid residues mutated for that mutant. All of the indicated native residues were mutated to an alanine. (B) E. coli-expressed GST-fusion proteins encoding FMDV 3A mutants were conjugated to glutathione beads and then incubated with the soluble His-vimentin protein expressed in E. coli BL21. Having been washed, the bound proteins were detected by Western blotting using the anti-GST antibody and the anti-Myc antibody.
Binding between FMDV 3A and host vimentin appears critical for virus replication.
To further investigate the significance of the interaction between host vimentin and FMDV 3A, 7 alanine substitutions at positions 15 to 21 of 3A, which were shown to alter 3A-vimentin reactivity in the GST pull down, were introduced into the full-length cDNA clone prGZSB of FMDV O/GZSB/2011 to produce pSB3A-3m. These constructs were transfected into BSR-T7/5 cells, and supernatants from transfected cells were then serially passaged in BHK-21 cells up to 6 times to amplify the clone-derived viruses. Transfection with prGZSB produced viable virus progeny; however, transfection with pSB3A-3m was not able to produce viable progeny, suggesting that amino acid residues determining the FMDV 3A-vimentin interaction are also critical for virus replication. These results indicate that the interaction between 3A and vimentin is essential for virus replication. However, we cannot rule out the possibility that these mutations in 3A have other effects by changing the structure of 3A or by inhibiting 3A to interact with other proteins.
Overexpression of vimentin impairs FMDV replication.
To explore the role of vimentin in FMDV replication, PK-15 cells were transfected with a plasmid encoding a triple-peptide FLAG (3×FLAG) alone (pCMV-3FLAG-1) or fused to full-length vimentin (pFLAG-vim). The overexpression of vimentin in transfected cells was checked at 24 h posttransfection by Western blotting with an anti-FLAG antibody (Fig. 3A). Furthermore, at 24 h posttransfection, the cells were infected (multiplicity of infection [MOI], 1) with FMDV O/GZSB/2011, and the virus replication was assessed at 12 hours postinfection (hpi) by real-time reverse transcriptase PCR (RT-PCR) and Western blotting. The results revealed that the viral RNA and viral protein levels in vimentin-overexpressing cells decreased 93% and 55%, respectively, compared with that in pCMV-3FLAG-1-transfected cells (Fig. 3B to D). Additionally, the virus titer in the cell culture supernatant was also measured, and the result demonstrated that the virus titer in vimentin-overexpressing cell supernatants also presented a reduction (∼0.8 logs) compared with that in pCMV-3FLAG-1-transfected cell supernatants (Fig. 3E). Together, these results indicate that the overexpression of vimentin inhibits FMDV replication in PK-15 cells.
FIG 3.
Effects of vimentin overexpression on FMDV replication. (A) PK-15 cells were transfected with a plasmid encoding a triple-peptide FLAG (3×FLAG) alone (pCMV-3FLAG-1) or fused to full-length vimentin (pFLAG-vim) for 24 h. Western blot analysis for detecting protein expression levels of FLAG-vimentin with a FLAG-specific antibody. As a loading control, the detection of the levels of intracellular β-actin was performed. (B to D) At 24 h after transfection, PK-15 cells were infected with FMDV O/GZSB/2011 at an MOI of 1. The lysates of the infected cells were collected at 12 hpi. The expression of vimentin and viral protein were detected by Western blotting (B). Viral protein abundance in the Western blot shown in B was measured and normalized to β-actin (C). Viral RNA levels were detected by real-time RT-PCR (D). (E) Virus yield in the supernatants of FMDV-infected negative-control or vimentin-overexpressing cells at 12 hpi were determined by viral plaque assay on BHK-21 cells. Data are presented as the mean ± SD from three independent experiments. *, P < 0.05; ***, P < 0.001.
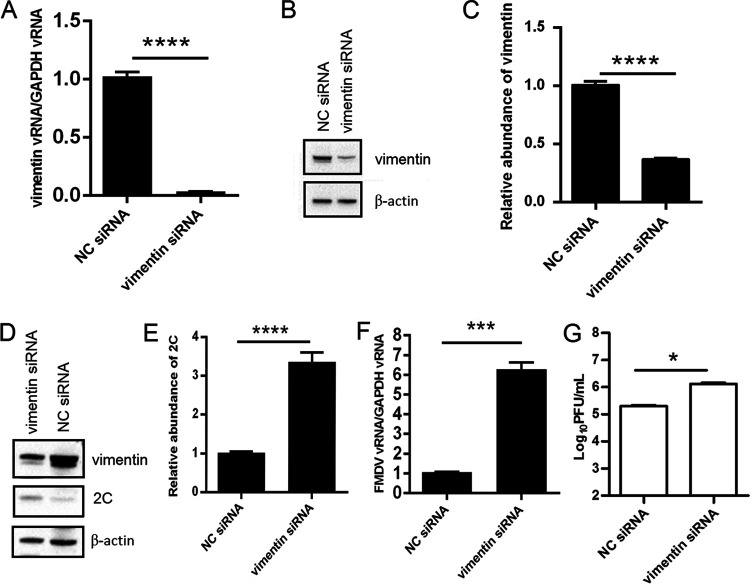
Knockdown of vimentin promotes FMDV replication.
To further investigate the effect of vimentin on FMDV replication, small interfering RNA (siRNA) knockdown assays were performed. PK-15 cells were transfected with siRNA against vimentin or a nontargeting negative-control siRNA for 48 h. The knockdown efficiency of the siRNAs was tested by real-time RT-PCR and Western blotting. The results showed that vimentin expression was significantly lower in cells transfected with vimentin siRNA than in cells transfected with nontargeting (NC) siRNA (Fig. 4A to C), thus indicating that the knockdown was effective. At 48 h after transfection, PK-15 cells were infected with the FMDV O/GZSB/2011 at an MOI of 1 for 12 h. Viral replication was analyzed by real-time RT-PCR, Western blotting, and virus titration. Real-time RT-PCR analysis showed that the viral RNA in the vimentin knockdown cells was 6-fold higher than that in negative-control cells (Fig. 4F). Similarly, an approximate 3-fold increase in the expression level of the FMDV 2C protein was observed in the vimentin knockdown cells compared with negative-control cells (Fig. 4D and F). Moreover, the virus titer in the supernatants of vimentin-knockdown cells was higher (∼1 logs) than that in negative-control cells (Fig. 4G). These results indicate that downregulation of vimentin significantly enhances FMDV replication.
FIG 4.
Effects of vimentin-specific siRNA treatment on FMDV replication. (A, B) PK-15 cells were transfected with an siRNA-targeted vimentin or with a nontargeting negative-control siRNA for 48 h, as described in the Materials and Methods. The expression of vimentin was detected by real-time RT-PCR (A) and Western blotting (B). (C) Quantification of vimentin bands in the Western blot shown in B using ImageJ analysis software and normalized to β-actin. (D to F) At 48 h after transfection, PK-15 cells were infected with FMDV O/GZSB/2011 at an MOI of 1. The lysates of the infected cells were collected at 12 hpi. Expression of vimentin and viral proteins was detected by Western blotting (D). Viral protein abundance in the Western blot shown in D was quantitated and made reference to β-actin control bands (E). Viral RNA levels were measured by real-time RT-PCR (F). (G) Virus yield in the supernatants of FMDV-infected NC siRNA or vimentin siRNA cells at 12 hpi were determined by viral plaque assay on BHK-21 cells. Data are presented as the mean ± SD from three independent experiments. *, P < 0.05; ***, P < 0.001; ****, P < 0.0001.
Disruption of vimentin networks inhibits FMDV replication.
Considering that downregulation of vimentin significantly enhances FMDV replication, it is necessary to investigate whether the disruption of the endogenous vimentin networks affect FMDV replication. Thus, acrylamide was used since it can specifically and reversibly disrupt the vimentin intermediate filament networks. PK-15 cells were treated with acrylamide at the indicated concentrations for 0.5 h, and the treated cells were then observed under an inverted microscope. As shown in Fig. 5A, the mock-treated PK-15 cells maintained normal cell morphology, while cells treated with acrylamide present a change in cell morphology to a more rounded shape in a dose-dependent manner. Additionally, the cytotoxic activity of acrylamide against PK-15 cells was also measured using cell counting kit-8. The result showed that cell viability was not affected by treatment with 300 μM acrylamide (Fig. 5B). Therefore, 300 μM acrylamide was used for subsequent experiments.
FIG 5.

Effects of acrylamide on FMDV replication in PK-15 cells. (A) PK-15 cells were treated with acrylamide at the indicated concentrations for 30 min and then observed under the microscope. (B) PK-15 cells were treated with the indicated concentrations of acrylamide for 30 min, and the cell viability was determined by cell counting kit-8, as described in the Materials and Methods. (C) PK-15 cells were either mock treated or treated with 300 μM acrylamide for 30 min, and the cells were then fixed, immunostained with an antibody against vimentin, and visualized by immunofluorescence microscopy. (D) PK-15 cells were treated with acrylamide at a concentration of 300 μM or mock treated for 30 min and then infected with FMDV O/GZSB/2011 (MOI, 1) for 12 h at 37°C. Virus titers were determined by plaque assay on BHK-21 cells. Data are presented as the mean ± SD from three independent experiments. *, P < 0.05; ns, P > 0.05.
To determine whether the vimentin networks of PK-15 cells were disrupted by treatment with 300 μM acrylamide for 0.5 h, immunofluorescence staining was performed. PK-15 cells were treated with acrylamide at a concentration of 300 μM for 0.5 h, and the treated cells were fixed, immunostained with an antibody against vimentin, and visualized by immunofluorescence microscopy. The results indicated that the mock-treated PK-15 cells displayed an intact vimentin structure dispersed throughout the cytoplasm, while cells treated with acrylamide present a collapse of the vimentin networks (Fig. 5C).
To examine the effect of these changes in vimentin distribution on FMDV replication, PK-15 cells were pretreated with 300 μM acrylamide for 0.5 h and then infected with FMDV O/GZSB/2011 at an MOI of 1 for 12 h. As documented in Fig. 5D, treatment with 300 μM acrylamide for 0.5 h resulted in a reduction (0.5 logs) in virus titers compared with the mock-treated control. These results suggest that disruption of vimentin inhibits FMDV replication.
Vimentin is not cleaved in FMDV-infected PK-15 cells.
Previous studies have reported that vimentin was specifically cleaved during infection with adenovirus (39), human immunodeficiency viruses (41), and bovine leukemia virus (42). Similarly, a recent study also showed that vimentin is degraded in FMDV-infected MCF-10A cells (40). To determine whether vimentin is cleaved during FMDV infection of PK-15 cells, PK-15 cells were infected with FMDV O/GZSB/2011 at an MOI of 1, and cell lysates collected from different times postinfection were tested by Western blotting. As illustrated in Fig. 6, no cleavage of vimentin was detected during the course of FMDV infection, indicating that FMDV infection does not lead to the degradation of vimentin in PK-15 cells.
FIG 6.

Vimentin is not cleaved in FMDV-infected PK-15 cells. PK-15 cells were mock infected or infected with FMDV O/GZSB/2011 at an MOI of 1. At the indicated times postinfection, cells were collected and detected by Western blotting using an antibody against vimentin.
DISCUSSION
A virus extensively interacts with host cellular factors to create a more favorable environment for viral replication during viral infection, and understanding the mechanisms of viral replication is important for the development of novel preventive and therapeutic strategies. However, the molecular mechanism of FMDV replication is still unclear. FMDV 3A, which is an important component of the viral replication complex (RC), plays a critical role in FMDV replication. Hence, studies on the interaction of cellular protein with FMDV 3A will provide new information for understanding the mechanism of FMDV replication. However, few interactions between FMDV 3A and cellular proteins have been reported thus far. Here, we report that vimentin is a binding partner of FMDV nonstructural protein 3A and that both vimentin and its interaction with FMDV 3A play essential roles during FMDV replication.
Vimentin, an important component of the cytoskeleton, belongs to class III intermediate filament and plays critical roles in many cellular processes, such as cell adhesion, cell migration, and signal transduction (22, 23). Furthermore, an increasing number of studies have suggested that vimentin is also involved in the life cycle of a variety of viruses, starting from entry until egress. For example, surface-expressed vimentin is critical for virus entry, such as human cytomegalovirus (30), Japanese encephalitis virus (24, 25), porcine reproductive and respiratory syndrome virus (27), and cowpea mosaic virus (29). Vimentin is required for virus replication, such as dengue virus (31, 43) and Junin virus (44). Vimentin interacts with the outer capsid protein VP2 of bluetongue virus to facilitate viral egress (34). In the present study, we used coimmunoprecipitation followed by mass spectrometric analyses to identify cellular proteins that interact with FMDV 3A during FMDV infection. Our results revealed that vimentin is an interacting partner of FMDV 3A. To further verify this interaction, in vitro coimmunoprecipitation and GST pull down were carried out, and our results suggested that there was a direct interaction between FMDV 3A and vimentin (Fig. 1). Interestingly, a recent report demonstrated that cellular vimentin is also a specific binding partner for FMDV 2C and this 2C-vimentin interaction is essential for FMDV replication (40). Therefore, we speculated that vimentin may interact with a great variety of viral proteins during FMDV replication. So, further work is necessary to clarify whether the other viral proteins of FMDV can interact with vimentin.
To investigate the effect of vimentin on FMDV replication, we attempted to manipulate the levels of vimentin in cells infected with FMDV. Moreover, given that PK-15 cells are derived from a naturally susceptible species of FMDV, we used PK-15 cells to evaluate the role of vimentin on FMDV replication. The results showed that overexpression of vimentin in PK-15 cells resulted in a significant decrease in viral yield (Fig. 3) and that downregulation of vimentin levels with siRNA in PK-15 cells led to a significant increase in viral yield (Fig. 4), suggesting that vimentin has a negative effect on FMDV replication in PK-15 cells. However, these results are different from a recent report showing that the overexpression of vimentin has no effect on FMDV replication in MCF-10A cells (40). The reason for this discrepancy maybe that the cell types used in these two experiments were different, since PK-15 cells were derived from a naturally susceptible animal, while the MCF-10A cell line which was derived from human fibrocystic mammary tissue is an artificial system for FMDV infection. So, we speculated that FMDV may utilize different mechanisms to complete its replication in different cell types. In addition, these results are in contrast to previous studies suggesting that knockdown of vimentin significantly impairs the replication of transmissible gastroenteritis virus (32), human immunodeficiency virus (45), and enterovirus 71 (46). The basis for this discrepancy may be that vimentin plays different roles in different viral infections. However, it remains obscure why vimentin is differentially involved in viral replication. On the other hand, acrylamide has been widely used to disrupt the vimentin network without altering the structure of the microtubule (47, 48, 56) and has been used in studies illuminating the role of vimentin in Junin, dengue, and bluetongue virus replication (34, 44, 49). Thus, to further understand the role of vimentin during FMDV replication, we tested the effect of acrylamide on FMDV replication. Our result showed that chemical disruption of vimentin with acrylamide resulted in reduced viral yield (Fig. 5), indicating that an intact vimentin network is required for FMDV replication. This result is consistent with the findings in a recent study, which reported that disruption of the vimentin intermediate filament decreased FMDV replication in MCF-10A cells (40), and is also in agreement with previous reports showing that disruption of the vimentin intermediate filament reduced the replication of hepatitis C virus (50), dengue virus (31, 49), and parvovirus minute virus of mice (51). Furthermore, overexpression of a truncated form of vimentin, which can disrupt vimentin function, also resulted in a significant decrease in viral yield (data not shown). Hence, our results suggested that vimentin has a negative effect on FMDV replication in PK-15 cells and that an intact vimentin network is required for efficient FMDV replication in PK-15 cells. The mechanism of the negative effect of vimentin on the FMDV replication and the exact role of vimentin during FMDV infection in PK-15 cells remain elusive and require further study.
Previous studies have shown that many viral infections, such as human immunodeficiency viruses (41), bovine leukemia virus (42), and adenovirus (39), can induce the cleavage of vimentin. For human immunodeficiency viruses (38, 41, 52) and bovine leukemia virus (42), it has been suggested that vimentin is cleaved by a viral protease. In the case of adenovirus, however, it has been demonstrated that a cellular protease, rather than a viral protease, may result in the degradation of vimentin. Moreover, a recent study also reported that vimentin is degraded in FMDV-infected MCF-10A cells (40). However, our results showed that no cleaved bands were observed during the course of FMDV infection in PK-15 cells (Fig. 6), indicating that vimentin is not cleaved in FMDV-infected PK-15 cells. The reason for this discrepancy maybe due to the difference in cell types in these two experiments, since PK-15 cells were derived from pig which is a naturally susceptible animal of FMDV, while MCF-10A cells were derived from human (nonhost animal of FMDV) as described above. Further studies are needed to ascertain the precise role of vimentin during FMDV infection in PK-15 cells since PK-15 cells are derived from a natural host of FMDV.
Given that FMDV 3A is a partially conserved protein with a highly conserved N-terminal region and a hypervariable C-terminal region and that the hypervariable C-terminal region is associated with host range, it is necessary to determine the specific region in 3A responsible for vimentin binding. Using alanine-scanning mutagenesis, we found that area 3 (residues 15 to 21) of 3A is essential for binding to vimentin since mutations of these residues (residues 15 to 21) to alanine in 3A of FMDV disrupted the 3A-vimentin interaction (Fig. 2). Sequence alignment analysis showed that residues 15 to 21 of 3A are highly conserved among FMDVs of different serotypes (Fig. 7), suggesting that these residues or its interaction with vimentin may be critical for viral survival. This is confirmed by reverse genetics since mutated FMDV genomes containing the 3A mutations that disrupted the interaction between vimentin and 3A produced nonviable virus. Additionally, a recent study has shown that vimentin is also an interacting partner of FMDV 2C and that this 2C-vimentin interaction is critical for FMDV replication (40). Considering that 2C and 3A are the important components of the viral replication complex of FMDV and that vimentin was rearranged during FMDV infection (9), we hypothesize that vimentin may also interact with other viral proteins encoded by FMDV and serves as a scaffold for the generation of the viral replication complex. On the other hand, although the regions in 2C and 3A responsible for vimentin binding were identified, additional studies mapping the 2C- or 3A-binding region in vimentin would be needed to further understand how vimentin functions during FMDV infection.
FIG 7.
Alignment of the amino acid sequences of the 3A protein from different serotypes of FMDV, namely, O1Campos/Bra/58 (GenBank accession no. AJ320488), O/HKN/21/70 (AJ294984), c-s8c1 (AJ133357), Asia1/MAY/9/99 (HQ632774), SAT2/ZIM/14/90 (KJ144910), A12/U/119/32 (M10975), A22/UssR/65 (X74812), Asia1/LEB/83 (AJ295004), O/CAM/12/94 (AJ294980), SAT1/NIG/1/15 (MF678823), and SAT3-4bech/1/65 (AY593853).
In conclusion, our results show for the first time that cellular vimentin interacts with FMDV 3A and negatively modulates the FMDV replication in PK-15 cells. The FMDV 3A-vimentin interaction appears crucial for virus survival since FMDV genomes containing 3A mutations that disrupted the interaction between 3A and vimentin led to viruses unable to replicate in cell cultures. Additionally, the results also indicate that an intact vimentin network is required for FMDV replication. Thus, the present study not only provides us a better understanding of the mechanism of FMDV replication in PK-15 cells but also will help in the development of novel antiviral strategies in the future. However, further work will be needed to fully understand the role of vimentin on FMDV infection and to identify whether other viral proteins also interact with vimentin.
MATERIALS AND METHODS
Cell lines, viruses, and reagents.
Baby hamster kidney (BHK-21) cells and pig kidney epithelial (PK-15) cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, USA) containing 10% heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin sulfate at 37°C in a humidified 5% CO2 incubator. Primary fetal bovine kidney (FBK) cells and human embryonic kidney (HEK293T) cells were maintained in DMEM (Gibco) supplemented with 10% FBS and 4.5 g/ml d-glucose. BSR-T7/5 cells, which constitutively express the T7 RNA polymerase, were grown as previously described (53).
FMDV strain O/GZSB/2011 which belongs to the O/SEA/Mya-98 lineage was isolated from cattle in Guizhou Province, China, during the 2011 outbreak. The virus was propagated in BHK-21 cells, and the titer was determined by the plaque-forming assays on BHK-21 cells using standard techniques.
Acrylamide and cell counting kit-8 were purchased from Sigma-Aldrich and Beyotime, respectively.
Antibodies and plasmids.
Mouse monoclonal antibodies against FMDV 3A and 2C were generated in our laboratory. A mouse anti-vimentin monoclonal antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Horseradish peroxidase (HRP)-conjugated mouse anti-Myc monoclonal antibody, HRP-conjugated mouse anti-FLAG monoclonal antibody, rabbit anti-vimentin polyclonal antibody, Alexa Fluor 647-conjugated goat anti-rabbit IgG H&L antibody, and mouse anti-β-actin monoclonal antibody were purchased from Abcam (Cambridge, MA, USA). Fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse secondary antibody was purchased from Sigma (St. Louis, MO). HRP-conjugated goat anti-mouse secondary antibody was purchased from CWBio (Beijing, China). Mouse anti-GST monoclonal antibody was purchased from Novagen.
The coding sequence for 3A was amplified by PCR from the infectious clone prGZSB that encodes the genomic RNA of the FMDV O/GZSB/2011 strain (54). The PCR product was cloned into pGEX-6P-1 and pcDNA3.1 to generate pGEX-6p-1-3A and pMyc-3A, respectively. The coding region of vimentin was amplified by RT-PCR using total RNA prepared from PK-15 cells and cloned into pET-28a and pCMV-3FLAG-1 to yield pET-28a-vim and pFLAG-vim, respectively. The sequences of the primers used for plasmid construction are available upon request, and all plasmids were confirmed by restriction digestion and sequencing. To generate mutant 3A proteins in which amino acids were substituted with alanine, the 3A coding region containing mutations were synthesized by GeneWiz, Inc., and cloned into pGEX-6p-1 to produce plasmids (p3A-1 to p3A-22).
Coimmunoprecipitation and mass spectrometry assay.
FBK cells grown to 90% confluence were infected with O/GZSB/2011 at a multiplicity of infection (MOI) of 10 or mock infected. At 5 h postinfection (hpi), the cells were rinsed twice with phosphate-buffered saline (PBS) and lysed using immunoprecipitation (IP) lysis/wash buffer (25 mM Tris [pH 7.4] 150 mM NaCl, 1 mM EDTA, 1% NP-40, and 5% glycerol) containing a protease inhibitor cocktail (Thermo Fisher Scientific) on ice for 30 min. The cell lysates were cleared by centrifugation at 14,000 × g for 10 min at 4°C, and the supernatants were incubated with anti-3A antibody overnight at 4°C and then incubated with Protein G-coated beads (Thermo Fisher Scientific) at 4°C for 1 h. The beads were washed two times using IP lysis/wash buffer and one time with ultrapure water, and the bound proteins were eluted by boiling with SDS-PAGE loading buffer for 5 min, separated using SDS-PAGE, and then visualized by Coomassie brilliant blue stain (Pierce). The lysates of mock-infected FBK cells was used as a control.
Protein bands of interest were manually excised from the 12% SDS-PAGE gel, minced into 1-mm3 pieces, and destained with washing solution (25 mM NH4HCO3, 50% [vol/vol] acetonitrile) until the gel slice became colorless. The protein samples were reduced and alkylated with dithiothreitol (DTT) and iodoacetamide, respectively, and then subjected to in-gel digestion with trypsin (10 ng/μl) at 37°C overnight. The tryptic peptides were injected onto a desalting column and subsequently chromatographically separated on a Biobasic C18 capillary column. The resultant peptide masses were analyzed using the TripleTOF 5600 system (Sciex, Framingham, MA, USA).
The mass spectrum produced from each sample was searched against the protein databases (Uniprot and NCBI nonredundant [nr]) using the Mascot v2.3 search engine, and proteins were identified. Mascot was set up to search the bovine database, with a fragment mass tolerance of 0.1 Da, a peptide mass tolerance of 0.05 Da, and strict trypsin specificity, allowing for up to 1 missed cleavages. The oxidation of methionine, N-terminal pyroglutamate of glutamine, and deamidation of asparagine/glutamine were set as variable modifications, whereas carbamidomethylation of cysteine was set as a fixed modification.
Coimmunoprecipitation of FMDV 3A and vimentin.
To identify the interaction of FMDV 3A and vimentin, coimmunoprecipitation was performed. Briefly, HEK293T cells were cotransfected with pMyc-3A and pFLAG-vim using Lipofectamine 3000 (Invitrogen) as per the manufacturer’s instructions. Empty vectors were used as controls. At 24 h posttransfection, the cells were rinsed with PBS and lysed in IP lysis/wash buffer (25 mM Tris [pH 7.4] 150 mM NaCl, 1 mM EDTA, 1% NP-40, and 5% glycerol) containing a protease inhibitor cocktail (Thermo Fisher Scientific) on ice for 30 min. The cell lysates were clarified by centrifugation at 12,000 × g for 20 min at 4°C and utilized for coimmunoprecipitation assays using anti-c-Myc agarose beads (Thermo Fisher Scientific) according to the manufacturer’s instructions. The precipitates were subjected to Western blotting with HRP-conjugated mouse anti-FLAG monoclonal antibody and HRP-conjugated mouse anti-Myc monoclonal antibody.
GST pulldown assay.
GST pulldown assays were performed according to the manufacturer’s protocols. Briefly, GST-3A, GST-3A mutants, GST, and His-vimentin proteins were expressed in Escherichia coli BL21(DE3) cells under induction of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). The induced E. coli BL21 cells were harvested and disrupted by sonication. The supernatants of the cell lysates containing GST or GST-fusion proteins were mixed with 50% glutathione-Sepharose slurry (GE Healthcare) and incubated for 1 h at 4°C with gentle rocking motion on a rotating platform. After being washed five times with a 1:1 wash solution of Tris-buffered saline (TBS; 25 mM Tris-HCl and 0.15 M NaCl [pH 7.2]):pulldown lysis buffer, the resins were incubated with the supernatants of the cell lysates containing the His-vimentin proteins expressed by E. coli BL21 cells for 1 h at 4°C. Having been washed five times with wash buffer, proteins bound to the resin were eluted with elution buffer and analyzed by SDS-PAGE, followed by immunoblotting with mouse anti-vimentin monoclonal antibody or mouse anti-GST monoclonal antibody. Independent pulldown assays were conducted at least three times.
Immunofluorescence and confocal microscopy.
PK-15 cells grown onto 12-mm glass coverslips were infected with FMDV O/GZSB/2011 at an MOI of 10 or mock infected. At 3.5 hpi, the cells were fixed with 4% paraformaldehyde (EMS, Hatfield, PA) for 30 min, permeabilized with 0.5% Triton X-100 in phosphate-buffered saline (PBS) for 10 min, and blocked in blocking buffer (PBS and 5% bovine serum albumin) for 1 h. Then, cells were incubated with monoclonal antibody to 3A and polyclonal antibody to vimentin and stained with FITC-conjugated goat anti-mouse antibody and Alexa Fluor 647-conjugated rabbit anti-goat IgG H&L antibody. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen) for 5 min. Having been washed five times, the cells were observed using a laser scanning confocal microscope (TCS SP8; Leica, Germany).
Construction of mutant FMDV.
Plasmid prGZSB containing the full-length cDNA of the highly virulent FMDV strain O/GZSB/2011 was constructed as previously described (54). pSB3A-3m is a derivative of prGZSB that contains 7 alanine substitutions at positions 15 to 21 of 3A. prGZSB and pSB3A-3m were linearized at the unique NotI site located downstream of the poly(A) tract, and the linearized plasmid DNAs were then transfected into BSR-T7/5 cells using Lipofectamine 3000 (Invitrogen, USA) according to the manufacturer’s protocol. The supernatants from transfected cells were harvested at 60 h posttransfection and serially passaged in BHK-21 cells until a cytopathic effect (CPE) appeared or were blind passaged in BHK-21 cells 6 times. The virus stocks were prepared, and the viral genomes were completely sequenced.
Overexpression of vimentin.
The pFLAG-vim plasmid was transfected into PK-15 cells in 6-well plates as described above, and the empty vector pCMV-3FLAG-1 was used as a control. At 24 h after transfection, the cells were washed three times with PBS. The cells were then infected with FMDV O/GZSB/2011 at an MOI of 1 at 37°C. After adsorption for 1 h, the cells were washed three times with PBS and cultured in 2 ml of fresh medium at 37°C. At 12 hpi, the supernatants and lysates of the infected cells were then collected, and the titers of the infectious viruses in the supernatant were determined by plaque assay on BHK-21 cells, as described previously (55).
RNA interference.
Small interfering RNA (siRNA) targeting vimentin (GenBank accession number XM_005668106) were synthesized by GenePharma (China). The target sequence for vimentin was 5′-GGAAGCUGCUAACUACCAATT-3′. Nontargeting siRNA (NC siRNA) was used as a negative control. PK-15 cells grown on a 12-well plate were transfected with 50 nM siRNA using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer’s protocol. At 48 h after transfection, the siRNA-transfected cells were infected with FMDV O/GZSB/2011 at an MOI of 1 and cultured for 12 h. The supernatants and lysates of the infected cells were then collected, and the virus titers in the supernatant were determined by plaque assay on BHK-21 cells.
RNA extraction and real-time RT-PCR.
Total RNA was extracted from FMDV-infected cells using the RNeasy minikit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. The cDNA was synthesized from 2 μg of total RNA using a reverse transcription kit (TaKaRa, Dalian, China) as per the manufacturer’s instruction. Real-time RT-PCR was performed using the SYBR green PCR master mix (TaKaRa) on a 7500 real-time PCR system (Applied Biosystems, Foster City, CA). The relative expression levels of the target gene mRNA were normalized to GAPDH mRNA using the threshold cycle (2−ΔΔCT) method. Three replicates were performed. The primers used in this study are shown in Table 2.
TABLE 2.
Oligonucleotide primers used in this study
| Primera | Sequence (5′-3′) | Usage |
|---|---|---|
| qVimF | CCGCACCAACGAGAAGGT | RT-qPCR vimentin |
| qVimR | CGGCTAGCAGAATCTTGTTC | |
| q3DF | CAAACCTGTGATGGCTTCGA | RT-qPCR FMDV |
| q3DR | CCGGTACTCGTCAGGTCCA | |
| qGAPDHF | CAAGAAGGTGGTGAAGCA | RT-qPCR GAPDH |
| qGAPDHR | AAGTGGAAGAGTGAGTGTC |
Forward and reverse primers are indicated by F and R, respectively.
Cell viability assay.
Cell viability was determined by using the cell counting kit-8. Briefly, 1 day after PK-15 cells were seeded in a 96-well plate, cells were treated with acrylamide at desired concentrations for 0.5 h. Alternatively, cells were transfected with siRNAs for 48 h or with plasmids for 24 h. Then, 10 μl of a CCK-8 solution was added into each well of the plates, and the plates were incubated for another 5 h at 37°C. The optical density (OD) was measured at 450 nm using a microplate reader (Gene Company Limited, China).
Drug treatment assay.
PK-15 cells were seeded in 12-well plates at 37°C overnight and then treated with 300 μM acrylamide for 0.5 h. The treated cells were washed twice with minimal essential medium (MEM) and infected with FMDV O/GZSB/2011 at an MOI of 1 for another 12 h. Then, the cultures were harvested and subjected to three freeze-thaw cycles, and the total (intracellular and extracellular) virus yield was determined by plaque assay in BHK-21 cells.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grant number 31702266) and Open Foundation of State Key Laboratory of Veterinary Etiological Biology (grant number SKLVEB2018KFKT003).
We thank Karl-Klaus Conzelmann (Max von Pettenkofer Institute and Gene Center, Germany) for generously supplying the BSR-T7/5 cells used in the present study.
REFERENCES
- 1.Alexandersen S, Zhang Z, Donaldson AI, Garland AJM. 2003. The pathogenesis and diagnosis of foot-and-mouth disease. J Comp Pathol 129:1–36. doi: 10.1016/S0021-9975(03)00041-0. [DOI] [PubMed] [Google Scholar]
- 2.Brown F. 2003. The history of research in foot-and-mouth disease. Virus Res 91:3–7. doi: 10.1016/S0168-1702(02)00268-X. [DOI] [PubMed] [Google Scholar]
- 3.Domingo E, Escarmís C, Baranowski E, Ruiz-Jarabo CM, Carrillo E, Núñez JI, Sobrino F. 2003. Evolution of foot-and-mouth disease virus. Virus Res 91:47–63. doi: 10.1016/S0168-1702(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 4.Knowles NJ, Samuel AR. 2003. Molecular epidemiology of foot-and-mouth disease virus. Virus Res 91:65–80. doi: 10.1016/S0168-1702(02)00260-5. [DOI] [PubMed] [Google Scholar]
- 5.Belsham GJ. 1993. Distinctive features of foot-and-mouth disease virus, a member of the picornavirus family; aspects of virus protein synthesis, protein processing and structure. Prog Biophys Mol Biol 60:241–260. doi: 10.1016/0079-6107(93)90016-D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sobrino F, Saiz M, Jimenez-Clavero MA, Nunez JI, Rosas MF, Baranowski E, Ley V. 2001. Foot-and-mouth disease virus: a long known virus, but a current threat. Vet Res 32:1–30. doi: 10.1051/vetres:2001106. [DOI] [PubMed] [Google Scholar]
- 7.Grubman MJ, Baxt B. 2004. Foot-and-mouth disease. Clin Microbiol Rev 17:465–493. doi: 10.1128/cmr.17.2.465-493.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grubman MJ, Baxt B. 1982. Translation of foot-and-mouth disease virion RNA and processing of the primary cleavage products in a rabbit reticulocyte lysate. Virology 116:19–30. doi: 10.1016/0042-6822(82)90399-3. [DOI] [PubMed] [Google Scholar]
- 9.Armer H, Moffat K, Wileman T, Belsham GJ, Jackson T, Duprex WP, Ryan M, Monaghan P. 2008. Foot-and-mouth disease virus, but not bovine enterovirus, targets the host cell cytoskeleton via the nonstructural protein 3C(pro). J Virol 82:10556–10566. doi: 10.1128/JVI.00907-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monaghan P, Cook H, Jackson T, Ryan M, Wileman T. 2004. The ultrastructure of the developing replication site in foot-and-mouth disease virus-infected BHK-38 cells. J Gen Virol 85:933–946. doi: 10.1099/vir.0.19408-0. [DOI] [PubMed] [Google Scholar]
- 11.Xiang WK, Cuconati A, Hope D, Kirkegaard K, Wimmer E. 1998. Complete protein linkage map of poliovirus P3 proteins: interaction of polymerase 3D(pol) with VPg and with genetic variants of 3AB. J Virol 72:6732–6741. doi: 10.1128/JVI.72.8.6732-6741.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forss S, Strebel K, Beck E, Schaller H. 1984. Nucleotide sequence and genome organization of foot-and-mouth disease virus. Nucleic Acids Res 12:6587–6601. doi: 10.1093/nar/12.16.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.González-Magaldi M, Martín-Acebes MA, Kremer L, Sobrino F. 2014. Membrane topology and cellular dynamics of foot-and-mouth disease virus 3A protein. PLoS One 9:e106685. doi: 10.1371/journal.pone.0106685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.González-Magaldi M, Postigo R, de la Torre BG, Vieira YA, Rodríguez-Pulido M, López-Viñas E, Gómez-Puertas P, Andreu D, Kremer L, Rosas MF, Sobrino F. 2012. Mutations that hamper dimerization of foot-and-mouth disease virus 3A protein are detrimental for infectivity. J Virol 86:11013–11023. doi: 10.1128/JVI.00580-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beard CW, Mason PW. 2000. Genetic determinants of altered virulence of Taiwanese foot-and-mouth disease virus. J Virol 74:987–991. doi: 10.1128/jvi.74.2.987-991.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knowles NJ, Davies PR, Henry T, O'Donnell V, Pacheco JM, Mason PW. 2001. Emergence in Asia of foot-and-mouth disease viruses with altered host range: characterization of alterations in the 3A protein. J Virol 75:1551–1556. doi: 10.1128/JVI.75.3.1551-1556.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pacheco JM, Gladue DP, Holinka LG, Arzt J, Bishop E, Smoliga G, Pauszek SJ, Bracht AJ, O'Donnell V, Fernandez-Sainz I, Fletcher P, Piccone ME, Rodriguez LL, Borca MV. 2013. A partial deletion in non-structural protein 3A can attenuate foot-and-mouth disease virus in cattle. Virology 446:260–267. doi: 10.1016/j.virol.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Gladue DP, O'Donnell V, Baker-Bransetter R, Pacheco JM, Holinka LG, Arzt J, Pauszek S, Fernandez-Sainz I, Fletcher P, Brocchi E, Lu Z, Rodriguez LL, Borca MV. 2014. Interaction of foot-and-mouth disease virus nonstructural protein 3A with host protein DCTN3 is important for viral virulence in cattle. J Virol 88:2737–2747. doi: 10.1128/JVI.03059-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li D, Lei C, Xu Z, Yang F, Liu H, Zhu Z, Li S, Liu X, Shu H, Zheng H. 2016. Foot-and-mouth disease virus non-structural protein 3A inhibits the interferon-beta signaling pathway. Sci Rep 6:21888. doi: 10.1038/srep21888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azumi N, Battifora H. 1987. The distribution of vimentin and keratin in epithelial and nonepithelial neoplasms. A comprehensive immunohistochemical study on formalin- and alcohol-fixed tumors. Am J Clin Pathol 88:286–296. doi: 10.1093/ajcp/88.3.286. [DOI] [PubMed] [Google Scholar]
- 21.Janmey PA, Euteneuer U, Traub P, Schliwa M. 1991. Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J Cell Biol 113:155–160. doi: 10.1083/jcb.113.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eriksson JE, Dechat T, Grin B, Helfand B, Mendez M, Pallari HM, Goldman RD. 2009. Introducing intermediate filaments: from discovery to disease. J Clin Invest 119:1763–1771. doi: 10.1172/JCI38339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ivaska J, Pallari HM, Nevo J, Eriksson JE. 2007. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp Cell Res 313:2050–2062. doi: 10.1016/j.yexcr.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 24.Das S, Ravi V, Desai A. 2011. Japanese encephalitis virus interacts with vimentin to facilitate its entry into porcine kidney cell line. Virus Res 160:404–408. doi: 10.1016/j.virusres.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Liang J-J, Yu C-Y, Liao C-L, Lin Y-L. 2011. Vimentin binding is critical for infection by the virulent strain of Japanese encephalitis virus. Cell Microbiol 13:1358–1370. doi: 10.1111/j.1462-5822.2011.01624.x. [DOI] [PubMed] [Google Scholar]
- 26.Du N, Cong H, Tian H, Zhang H, Zhang W, Song L, Tien P. 2014. Cell surface vimentin is an attachment receptor for enterovirus 71. J Virol 88:5816–5833. doi: 10.1128/JVI.03826-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J-K, Fahad A-M, Shanmukhappa K, Kapil S. 2006. Defining the cellular target(s) of porcine reproductive and respiratory syndrome virus blocking monoclonal antibody 7G10. J Virol 80:689–696. doi: 10.1128/JVI.80.2.689-696.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu YT-C, Chien S-C, Chen I-Y, Lai C-T, Tsay Y-G, Chang SC, Chang M-F. 2016. Surface vimentin is critical for the cell entry of SARS-CoV. J Biomed Sci 23:14. doi: 10.1186/s12929-016-0234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koudelka KJ, Destito G, Plummer EM, Trauger SA, Siuzdak G, Manchester M. 2009. Endothelial targeting of cowpea mosaic virus (CPMV) via surface vimentin. PLoS Pathog 5:e1000417. doi: 10.1371/journal.ppat.1000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller MS, Hertel L. 2009. Onset of human cytomegalovirus replication in fibroblasts requires the presence of an intact vimentin cytoskeleton. J Virol 83:7015–7028. doi: 10.1128/JVI.00398-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanlaya R, Pattanakitsakul SN, Sinchaikul S, Chen ST, Thongboonkerd V. 2010. Vimentin interacts with heterogeneous nuclear ribonucleoproteins and dengue nonstructural protein 1 and is important for viral replication and release. Mol Biosyst 6:795–806. doi: 10.1039/b923864f. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Shi H, Chen J, Shi D, Dong H, Feng L. 2015. Identification of the interaction between vimentin and nucleocapsid protein of transmissible gastroenteritis virus. Virus Res 200:56–63. doi: 10.1016/j.virusres.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Risco C, Rodríguez JR, López-Iglesias C, Carrascosa JL, Esteban M, Rodríguez D. 2002. Endoplasmic reticulum-Golgi intermediate compartment membranes and vimentin filaments participate in vaccinia virus assembly. J Virol 76:1839–1855. doi: 10.1128/jvi.76.4.1839-1855.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhattacharya B, Noad RJ, Roy P. 2007. Interaction between Bluetongue virus outer capsid protein VP2 and vimentin is necessary for virus egress. Virol J 4:7. doi: 10.1186/1743-422X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murti KG, Chen M, Goorha R. 1985. Interaction of frog virus 3 with the cytomatrix. III. Role of microfilaments in virus release. Virology 142:317–325. doi: 10.1016/0042-6822(85)90340-X. [DOI] [PubMed] [Google Scholar]
- 36.Ferreira LR, Moussatché N, Neto VM. 1994. Rearrangement of intermediate filament network of BHK-21 cells infected with vaccinia virus. Arch Virol 138:273–285. doi: 10.1007/BF01379131. [DOI] [PubMed] [Google Scholar]
- 37.Stefanovic S, Windsor M, Nagata K, Inagaki M, Wileman T. 2005. Vimentin rearrangement during African swine fever virus infection involves retrograde transport along microtubules and phosphorylation of vimentin by calcium calmodulin kinase II. J Virol 79:11766–11775. doi: 10.1128/JVI.79.18.11766-11775.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shoeman RL, Mothes E, Honer B, Kesselmeier C, Traub P. 1991. Effect of human immunodeficiency virus type 1 protease on the intermediate filament subunit protein vimentin: cleavage, in vitro assembly and altered distribution of filaments in vivo following microinjection of protease. Acta Histochem Suppl 41:129–141. [PubMed] [Google Scholar]
- 39.Belin MT, Boulanger P. 1987. Processing of vimentin occurs during the early stages of adenovirus infection. J Virol 61:2559–2566. doi: 10.1128/JVI.61.8.2559-2566.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gladue DP, O'Donnell V, Baker-Branstetter R, Holinka LG, Pacheco JM, Fernández Sainz I, Lu Z, Ambroggio X, Rodriguez L, Borca MV. 2013. Foot-and-mouth disease virus modulates cellular vimentin for virus survival. J Virol 87:6794–6803. doi: 10.1128/JVI.00448-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shoeman RL, Höner B, Stoller TJ, Kesselmeier C, Miedel MC, Traub P, Graves MC. 1990. Human immunodeficiency virus type 1 protease cleaves the intermediate filament proteins vimentin, desmin, and glial fibrillary acidic protein. Proc Natl Acad Sci U S A 87:6336–6340. doi: 10.1073/pnas.87.16.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snásel J, Shoeman R, Horejsí M, Hrusková-Heidingsfeldová O, Sedlácek J, Ruml T, Pichová I. 2000. Cleavage of vimentin by different retroviral proteases. Arch Biochem Biophys 377:241–245. doi: 10.1006/abbi.2000.1776. [DOI] [PubMed] [Google Scholar]
- 43.Teo CSH, Chu JJH. 2014. Cellular vimentin regulates construction of dengue virus replication complexes through interaction with NS4A protein. J Virol 88:1897–1913. doi: 10.1128/JVI.01249-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cordo SM, Candurra NA. 2003. Intermediate filament integrity is required for Junin virus replication. Virus Res 97:47–55. doi: 10.1016/S0168-1702(03)00221-1. [DOI] [PubMed] [Google Scholar]
- 45.Fernandez-Ortega C, Ramirez A, Casillas D, Paneque T, Ubieta R, Dubed M, Navea L, Castellanos-Serra L, Duarte C, Falcon V, Reyes O, Garay H, Silva E, Noa E, Ramos Y, Besada V, Betancourt L. 2016. Identification of vimentin as a potential therapeutic target against HIV infection. Viruses-Basel 8:98. doi: 10.3390/v8060098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cong HL, Ning D, Tian HC, Yang Y, Zhang W, Zhang H, Zhang WL, Song L, Tien P. 2013. Enterovirus 71 VP1 activates calmodulin-dependent protein kinase II and results in the rearrangement of vimentin in human astrocyte cells. PLoS One 8:e73900. doi: 10.1371/journal.pone.0073900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aggeler J, Seely K. 1990. Cytoskeletal dynamics in rabbit synovial fibroblasts: I. Effects of acrylamide on intermediate filaments and microfilaments. Cell Motil Cytoskeleton 16:110–120. doi: 10.1002/cm.970160205. [DOI] [PubMed] [Google Scholar]
- 48.Olink-Coux M, Huesca M, Scherrer K. 1992. Specific types of prosomes are associated to subnetworks of the intermediate filaments in PtK1 cells. Eur J Cell Biol 59:148–159. [PubMed] [Google Scholar]
- 49.Chen W, Gao N, Wang JL, Tian YP, Chen ZT, An J. 2008. Vimentin is required for dengue virus serotype 2 infection but microtubules are not necessary for this process. Arch Virol 153:1777–1781. doi: 10.1007/s00705-008-0183-x. [DOI] [PubMed] [Google Scholar]
- 50.Nitahara-Kasahara Y, Fukasawa M, Shinkai-Ouchi F, Sato S, Suzuki T, Murakami K, Wakita T, Hanada K, Miyamura T, Nishijima M. 2009. Cellular vimentin content regulates the protein level of hepatitis C virus core protein and the hepatitis C virus production in cultured cells. Virology 383:319–327. doi: 10.1016/j.virol.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 51.Fay N, Panté N. 2013. The intermediate filament network protein, vimentin, is required for parvoviral infection. Virology 444:181–190. doi: 10.1016/j.virol.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 52.Honer B, Shoeman RL, Traub P. 1991. Human immunodeficiency virus type 1 protease microinjected into cultured human skin fibroblasts cleaves vimentin and affects cytoskeletal and nuclear architecture. J Cell Sci 100:799–807. [DOI] [PubMed] [Google Scholar]
- 53.Li P, Bai XW, Cao YM, Han CH, Lu ZJ, Sun P, Yin H, Liu ZX. 2012. Expression and stability of foreign epitopes introduced into 3a nonstructural protein of foot-and-mouth disease virus. PLoS One 7:e41486. doi: 10.1371/journal.pone.0041486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma X, Li P, Bai X, Sun P, Bao H, Lu Z, Cao Y, Li D, Chen Y, Qiao Z, Liu Z. 2014. Sequences outside that of residues 93–102 of 3A protein can contribute to the ability of foot-and-mouth disease virus (FMDV) to replicate in bovine-derived cells. Virus Res 191:161–171. doi: 10.1016/j.virusres.2014.07.037. [DOI] [PubMed] [Google Scholar]
- 55.Ma X, Li P, Sun P, Bai X, Bao H, Lu Z, Fu Y, Cao Y, Li D, Chen Y, Qiao Z, Liu Z. 2015. Construction and characterization of 3A-epitope-tagged foot-and-mouth disease virus. Infect Genet Evol 31:17–24. doi: 10.1016/j.meegid.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 56.Sager PR. 1989. Cytoskeletal effects of acrylamide and 2,5-hexanedione: selective aggregation of vimentin filaments. Toxicol Appl Pharmacol 97:141–155. doi: 10.1016/0041-008X(89)90063-X. [DOI] [PubMed] [Google Scholar]