Previous studies have identified sex-specific differences during chronic HIV-1 infection, but little is known about sex differences in the acute phase, or how disparities in the initial response to the virus may affect disease. We demonstrate that restriction of viral load in women begins during acute infection and is maintained into chronic infection. Despite this, women exhibit more rapid CD4+ T cell loss than men. These profound differences are influenced by 17β-estradiol, which contributes both to T cell activation and to reduced viral replication. Thus, we conclude that estradiol plays a key role in shaping responses to early HIV-1 infection that influence the chronic phase of disease.
KEYWORDS: estrogen, human immunodeficiency virus, sex differences, T cell activation, viral load, viral pathogenesis, viral replication
ABSTRACT
The influence of biological sex on disease progression in HIV-1-infected individuals has been focused on the chronic stage of infection, but little is known about how sex differences influence acute HIV-1 infection. We observed profound differences in viral load and CD4+ T cell activation from the earliest time points in men and women in a Zambian heterosexual acute infection cohort. Women exhibited a >2-fold higher rate of CD4+ T cell loss despite significantly lower viral loads (VL) than men. The importance of studying acute infection was highlighted by the observation that very early in infection, women exhibited significantly higher levels of CD4+ T cell activation, a difference that was lost over the first 3 years of infection as activation in men increased. In women, activation of CD4+ T cells in the acute phase was significantly correlated with plasma levels of 17β-estradiol (E2). However, unlike in men, higher CD4+ T cell activation in women was not associated with higher VL. In contrast, a higher E2 level in early infection was associated with lower early and set-point VL in women. We attribute this to an inhibitory effect of estradiol on virus replication, which we were able to observe with relevant transmitted/founder viruses in vitro. Thus, estradiol plays a key role in defining major differences between men and women during early HIV-1 infection by contributing to both viral control and CD4+ T cell loss, an effect that extends into the chronic phase of the disease.
IMPORTANCE Previous studies have identified sex-specific differences during chronic HIV-1 infection, but little is known about sex differences in the acute phase, or how disparities in the initial response to the virus may affect disease. We demonstrate that restriction of viral load in women begins during acute infection and is maintained into chronic infection. Despite this, women exhibit more rapid CD4+ T cell loss than men. These profound differences are influenced by 17β-estradiol, which contributes both to T cell activation and to reduced viral replication. Thus, we conclude that estradiol plays a key role in shaping responses to early HIV-1 infection that influence the chronic phase of disease.
INTRODUCTION
Although sex differences in response to pathogenic infections have been well documented in the literature (1–3), many studies of HIV-1 transmission and pathogenesis to date utilize single cohorts (commonly men who have sex with men [MSM] or female sex workers) or cohorts that are significantly biased toward one sex and are thus unable to directly compare disease course and outcomes between men and women. As a result, robust studies comparing how the immune response to HIV-1 differs between men and women, particularly during the early phase of infection, have been difficult to perform. Current estimates suggest that 17.8 million women are infected with HIV-1 and that it is the leading cause of death in women of reproductive age (15 to 49 years) (4). Thus, a detailed understanding of the biology of HIV-1 infection in women is essential to combating the global HIV-1 epidemic in general.
A growing body of data has demonstrated that women exhibit more robust immune responses to both primary viral infections and vaccination compared to men (2, 3). In HIV-1 specifically, several studies have reported that chronically infected women have significantly lower plasma viremia than men, although the reason for this discrepancy has yet to be identified (5–10). In addition, a study comparing men and women chronically infected with HIV-1 found that infected women had a higher proportion of activated (CD38+ HLA-DR+) CD8+ T cells, but not CD4+ T cells, prior to antiretroviral therapy (ART) initiation, suggesting higher levels of detrimental immune activation (11). Furthermore, several interferon-stimulated genes (ISGs) were found to be expressed at significantly higher levels in women than men during the chronic stage of HIV-1 infection, after correcting for plasma viral load, implying a more activated innate phenotype in females given similar exposure to viral antigen (12). However, existing studies are limited by their utilization of patient samples from individuals of different ethnic backgrounds, in different geographical locations, or with unknown dates and routes of infection. The present study has focused on a cohort of Zambian, heterosexual, acutely infected individuals, allowing for comparison of epidemiological and immunological parameters in a group that share a similar environment and genetic background. To our knowledge, these data represent the first longitudinal comparison of the immune response to natural HIV-1 infection that encompasses both acute and chronic infections of both men and women. The studies presented here demonstrate that the discrepancy in viral load observed in women is established early in infection and that CD4+ T cell loss is double that of men in the first few years of infection. These differences are linked in women to estradiol levels, which correlate with CD4+ T cell activation and lower viral loads.
RESULTS
Women exhibit a higher rate of CD4+ T cell decline and lower plasma viral loads early in HIV-1 infection.
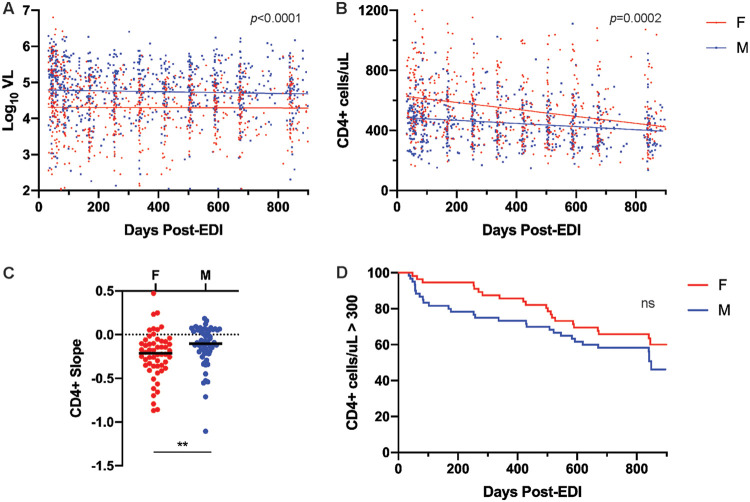
In order to understand the impact of biological sex on HIV-1 disease course, viral loads and CD4+ T cell counts were compared in acutely individuals enrolled in the Zambia Emory HIV Research Project (ZEHRP) cohort at sites in Lusaka and Ndola, Zambia. Postpeak viral loads of male and female participants (n = 158; 72 female [F], 86 male [M]) were plotted between 30 and 900 days after the estimated date of infection (EDI). A total of 97% of these individuals were infected with a subtype C variant. The estimated date of infection was calculated as previously described based on the date of the last seronegative test, the date of first seropositive test, and the presence or absence of p24 antigen (13). Subjects were selected on the basis of ART-naive status and the availability of at least two viral load measurements within the time interval, including measurements within 90 days of the beginning and end of the interval. The median age of these subjects was 31 at the time of study enrollment. During this period, women had consistently lower plasma viral loads (VL) than men, as indicated by the difference in the y intercepts but not the slopes of the linear regression line for each sex (Fig. 1A).
FIG 1.
Higher rate of CD4+ T cell decline despite lower plasma viral load in women. (A) Linear regression of log10VL values of Zambian male (n = 86) and female (n = 72) subjects between 30 and 900 days post-EDI (analysis of covariance [ANCOVA] for intercepts, P < 0.0001). (B) Linear regression lines of CD4+ T cells counts from male (n = 60) and female (n = 56) subjects between 30 and 900 days post-EDI (ANCOVA for slopes, P = 0.0002). (C) Comparison of slopes of individual linear regression lines of CD4+ cell counts between 30 and 900 days post-EDI for individuals in panel B (Mann-Whitney U test, P = 0.005). (D) Survival curves indicating time to a CD4+ cell count of <300 cells/μl by sex (log rank test, P = 0.2).
CD4+ T cell counts of a subset of 116 individuals (56 F, 60 M) were then plotted for individuals who met the aforementioned criteria and for whom CD4+ cell counts were available. When the linear regression lines for CD4+ T cell counts of each sex are compared, the slopes differ significantly (Fig. 1B). Comparison of slopes of individual linear regression lines of CD4+ cell counts over this interval indicates that women exhibit a >2-fold higher rate of loss (median slope of −0.21 versus −0.10 for men, Fig. 1C). Importantly, CD4+ T cell counts are significantly higher in HIV-1 uninfected women than uninfected men, as previously reported in individuals in Lusaka, Zambia (14). Thus, CD4+ T cell counts initially remain higher in women than men after infection, although the rate of CD4+ decline is higher in women, such that by 900 days men and women have similar numbers of CD4+ T cells. Indeed, a Kaplan-Meier survival analysis of the time to a CD4+ T cell count of <300/μl over the first 900 days of infection yielded curves that are not significantly different for men and women (Fig. 1D), despite women starting out with higher CD4+ T cells counts.
CD4+ T cells are more highly activated in women during acute infection.
Chronic immune activation is a hallmark of HIV-1 infection and a reliable predictor of CD4+ T cell loss (15). In order to determine whether differential T cell activation contributes to higher rates of CD4+ cell loss in women despite consistently lower levels of plasma viremia, markers of cellular activation on peripheral blood mononuclear cells (PBMCs) were analyzed by flow cytometry in 47 (22 F, 25 M) HIV-1-infected individuals at 1, 9, and 30 months postinfection. Subjects were randomly selected based on sample availability and ART-naive status. All subjects were infected through heterosexual contact with HIV-1 group M subtype C viruses.
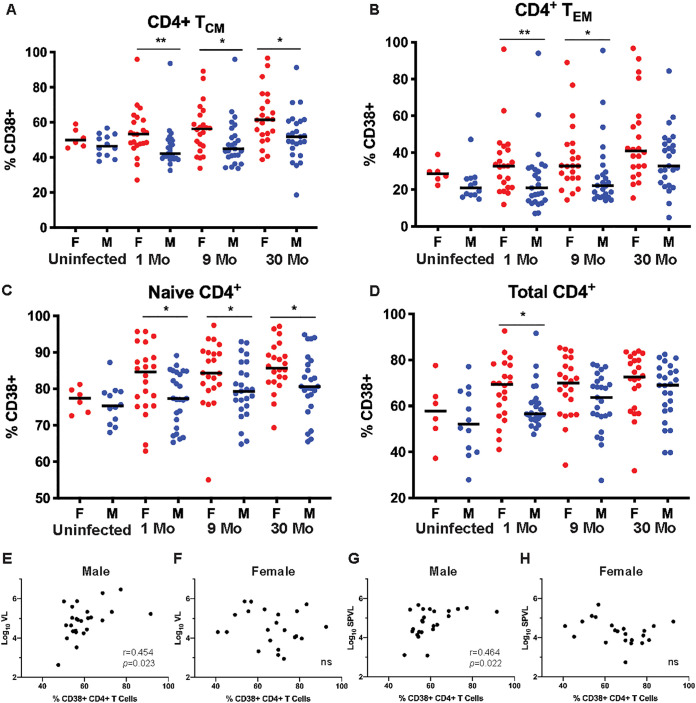
At 1 month post-EDI, a greater percentage of CD4+ T cells from women than men expressed the activation marker CD38 (Fig. 2D). This difference was apparent in central (CCR7+ CD27+ CD45RO+, Fig. 2A) and effector (CCR7– CD27− CD45RO+, Fig. 2B) memory T cell subsets, and as well as in naive (CCR7+ CD27+ CD45RO–, Fig. 2C) CD4+ T cell subsets. These sex differences are still significant in the memory CD4+ T cell subsets at 9 months postinfection. By 30 months post-EDI, there were no significant sex differences in the levels of CD4+ T cell activation in the memory subsets, although there was still a significantly greater proportion of CD38+ naive CD4+ T cells in women than men. Interestingly, the reduced difference during follow-up was primarily because the percentage of CD38+ T cells in men increased to a level similar to that of women over time; while the total CD38+ CD4+ T cells increased between uninfected women and women at 1 months post-EDI, it was not until the chronic stage of infection that there was a significant increase in men.
FIG 2.
CD4+ T cells in women are more highly activated than in men but do not correlate with VL. Proportion of CD4+ T cells expressing CD38 in uninfected women and men, and individuals at 1, 9, and 30 months post-EDI was assessed by flow cytometry. (A) TCM cells (CCR7+ CD27+ CD45RO+) (Mann-Whitney U test, P = 0.004, 0.037, and 0.010 at 1, 9, and 30 months post-EDI, respectively). (B) TEM cells (CCR7− CD27− CD45RO+) (Mann Whitney U test, P = 0.009 and 0.013, at 1 and 9 months post-EDI respectively). (C) TNaive cells (CCR7+ CD27+ CD45RO−) (Mann-Whitney U test, P = 0.032, 0.025, and 0.023 at 1, 9, and 30 months post-EDI, respectively). (D) Total CD4+ T cells (Mann Whitney U test, P = 0.024 at 1 month post-EDI). (E and F) Correlations between the proportions of CD4+ T cells expressing CD38 at 1 month post-EDI and log10VL values at the time of sampling in men (Spearman’s correlation, P = 0.023 and r = 0.454) (E) and women (Spearman’s correlation, P = 0.471 and r = −0.162) (F). (G and H) Correlations between the proportions of CD4+ T cells expressing CD38 at 1 month post-EDI and SPVL in men (Spearman’s correlation, P = 0.022 and r = 0.464) (G) and women (Spearman’s correlation, P = 0.101 and r = −0.359) (H).
In order to understand how CD4+ T cell activation affected the extent of viral replication and VL, we compared CD4+ T cell activation at 1 month post-EDI with VL at the time of PBMC collection (Fig. 2E and F) and with set-point VL (log10SPVL calculated as the geometric mean of VL from 1 to 12 months) (Fig. 2G and H). In men, there was a significant positive association between total CD4+ T cell activation at 1 month post-EDI and both VL at sampling (Fig. 2E) and log10SPVL (Fig. 2G). However, there was no relationship between these parameters in women (Fig. 2F and H), demonstrating that despite heightened CD4+ T cell activation, there was no commensurate increase in VL. These data are consistent with the observation that women consistently maintain lower plasma viremia than men despite higher CD4+ T cell activation.
Estradiol is associated with viral load and CD4+ T cell activation.
In order to address the cause of heightened T cell activation in acutely infected women, we measured estradiol (E2) in time point-matched plasma samples. While E2 and its metabolites estrone and estriol act in concert with other sex steroid hormones, E2 is the major form of estrogen found in nonpregnant premenopausal women in terms of absolute serum levels and affinity for estrogen receptor α (ERα) (16, 17). It plays a role in immune signaling in CD4+ T cells primarily via binding to ERα triggering nuclear translocation and transcription from estrogen response elements in target genes. This impacts the expression of several genes involved in the immune response, including the genes for AP-1 and gamma interferon (18, 19).
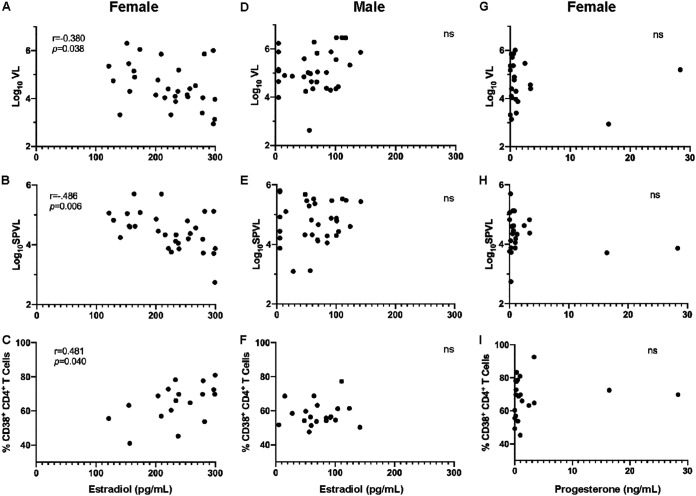
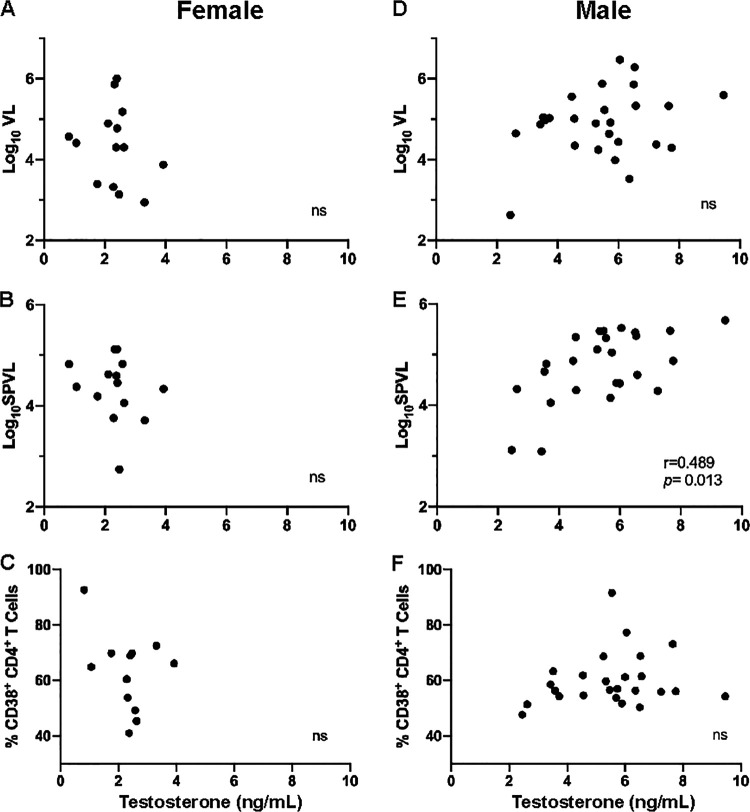
We measured plasma levels of E2 by conventional ELISA at a median of 44 days after the estimated date of HIV-1 infection in women and men who were included in our flow cytometry studies and in additional subjects for whom VL data were available. The plasma levels of E2 were inversely correlated with time point-matched log10VL in women, but not men, who had predictably lower E2 levels (Fig. 3A and D). Further, E2 levels at infection predicted lower log10SPVL values in women but not in men (Fig. 3B and E). Despite its association with lower levels of plasma viremia in women, we found that in women the plasma E2 was positively correlated with total CD4+ T cell activation at 1 month post-EDI (Fig. 3C). We also measured the plasma levels of progesterone in women and, based on these results, only two female subjects appeared to be in the midluteal phase of the menstrual cycle at the time of sampling. We did not see a significant effect of progesterone on the viral load or cellular activation (Fig. 3G to I). Finally, we measured the plasma levels of testosterone in order to understand whether this sex steroid hormone also played a role in HIV-1 pathogenesis. We found that there was no significant relationship between plasma levels of testosterone and the log10VL value at the time of sample collection (Fig. 4A and D) or CD4+ T cell activation in men or women (Fig. 4C and F). However, we did find a positive association between plasma levels of testosterone in men and the log10SPVL value (Fig. 4E). Interestingly, this was in contrast to the negative association observed in women between E2 and log10SPVL values.
FIG 3.
Plasma levels of 17β-estradiol but not progesterone at 1 month postinfection correlate with VL, SPVL, and CD4+ T cell activation in women. 17β-estradiol (E2) was measured in the plasma at 1 month postinfection. (A and D) Correlations between plasma levels of E2 and log10VL at 1 month post-EDI in women (Spearman’s correlation, P = 0.038 and r = −0.380) (A) and men (Spearman’s correlation, P = 0.241 and r = −0.184) (D). (B and E) Correlations between plasma levels of E2 and log10SPVL in women (Spearman’s correlation, P = 0.006 and r = −0.486) (B) and men (Spearman’s correlation, P = 0.782 and r = −0.051) (E). (C and F) Correlations between plasma levels of E2 and CD4+ T cell activation at 1 month post-EDI in women (Spearman’s correlation, P = 0.0404 and r = 0.487) (C) and men (Spearman’s correlation, P = 0.582 and r = 0.131) (F). (G to I) Correlations between plasma levels of progesterone and log10VL (G), log10SPVL (H), and CD4+ T cell activation (I) at 1 month post-EDI in women (Spearman’s correlation, P = 0.640 and r = −0.12, P = 0.527 and r = −0.14, and P = 0.329 and r = 0.224, respectively).
FIG 4.
Relationship between plasma levels testosterone at 1 month postinfection and VL, SPVL, and CD4+ T cell activation. Testosterone was measured in the plasma at 1 month postinfection. (A and D) Correlations between plasma levels of testosterone and log10VL at 1 month post-EDI in women (Spearman’s correlation, P = 0.445 and r = −0.22) (A) and men (Spearman’s correlation, P = 0.304 and r = 0.210) (D). (B and E) Correlations between plasma levels of testosterone and log10SPVL in women (Spearman’s correlation, P = 0.292 and r = −0.30) (B) and men (Spearman’s correlation, P = 0.013 and r = 0.489) (E). (C and F) Correlations between plasma levels of testosterone and CD4+ T cell activation at 1 month post-EDI in women (Spearman’s correlation, P = 0.592 and r = −0.17) (C) and men (Spearman’s correlation, P = 0.495 and r = 0.143) (F).
Estradiol restricts viral replication in vitro.
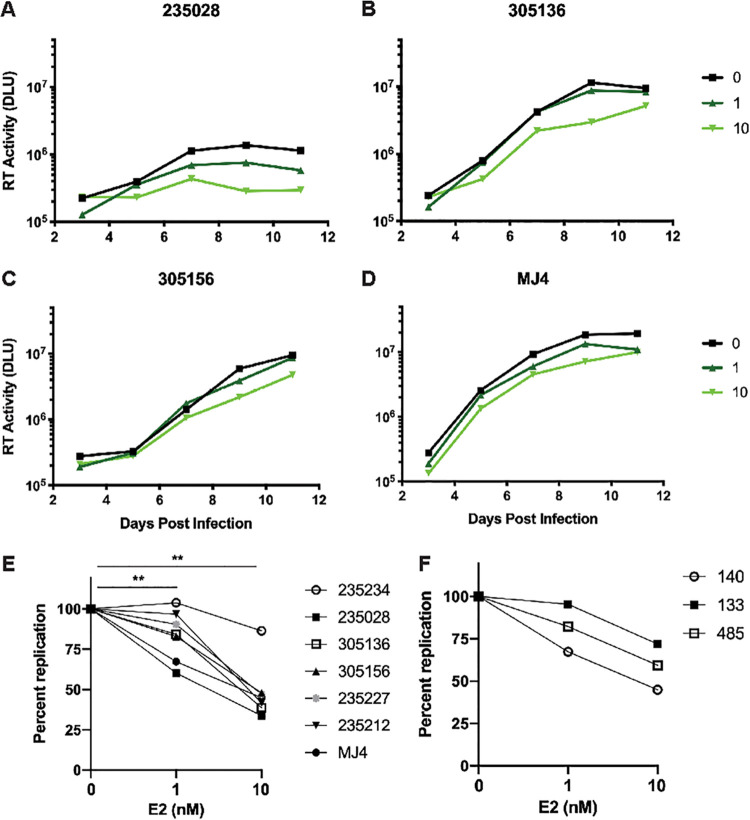
After observing that plasma E2 was inversely correlated with VL and SPVL in women, we sought to understand whether E2 could directly impact HIV-1 replication. Previous studies have attempted to identify the effect of sex hormones on viral replication, particularly of hepatitis C virus (HCV) and HIV-1. These studies vary in their conclusions, likely as a result of variable methodology with respect to the dosage of E2, the presence or absence of additional sex hormones, the cell type, and the virus strain used (20–23). We assessed the in vitro replication of six patient-derived transmitted/founder (T/F) viruses, as well as a subtype C laboratory isolate (MJ4), in the presence or absence of physiological levels of E2. Infectious molecular clones of T/F viruses were generated as previously described and used to infect PBMCs from healthy female donors in the presence of 0, 1, or 10 nM E2 (24). PBMCs were not activated prior to infection. Virus growth was measured between 3 and 11 days postinfection, and the area under the curve (AUC) was calculated for each virus and compared between E2 treatment groups. Overall, we found that E2 treatment resulted in a modest but consistent restriction of viral replication. Representative replication curves for four of the T/F viruses are shown in Fig. 5A to D. Collectively, E2 treatment significantly restricted viral replication in the viruses assayed, as measured by the percent AUC compared to untreated samples. The median replication was 84.29% of untreated at 1 nM E2 and 44.95% of untreated at 10 nM E2 (Fig. 4E). A similar pattern of viral restriction of MJ4 replication by E2 was observed in three donors (Fig. 4F). This suggests that while E2 appears to be involved in cellular activation pathways, it can simultaneously induce a restriction on viral replication.
FIG 5.
Estradiol inhibits replication of transmitted/founder viruses and MJ4. T/F viruses, as well as MJ4, were used to infect PBMCs from a healthy female donor (i.e., donor 140) in media containing 0, 1, or 10 nM E2. (A to D) Representative in vitro viral replication curves over an 11-day period for T/F viruses from 235028, 305136, 305156, and MJ4. The median values of three technical replicates are shown for each treatment at each time point. (E) Percent replication in treated compared to untreated control cells as determined from the AUC in six T/F viruses and MJ4 between days 3 and 11 for each of the treatment groups (Freidman test with Dunn’s multiple-comparison tests, P = 0.003 [between 0 and 10 nM E2] and P = 0.008 [between 0 and 1 nM E2]). (F) Percent replication in treated compared to untreated control cells as determined from the AUC of MJ4 between days 3 and 11 in donors 140, 133, and 485.
DISCUSSION
A growing body of research indicates that primary infection of women by viruses such as influenza and HCV is characterized by an enhanced inflammatory response that can lead to viral clearance (1, 3). The present study indicates that, consistent with what has been reported in acute infection by other viruses and in vaccination studies, women exhibit a heightened cellular immune activation upon initial infection with HIV-1. The data presented shed light on how inherent sex differences in the immune response manifest in the context of HIV-1 infection, where virus is not cleared and inflammation contributes to disease progression.
While numerous studies have previously reported lower HIV-1 viral loads in chronically infected women compared to men (5–10), the present study constitutes the first report wherein viral loads are compared in the absence of treatment in men and women in the same cohort, beginning in acute infection through to chronic stages. We were therefore able to demonstrate that, in this study population, women consistently have lower viral loads than men regardless of disease stage. We have further compared longitudinal CD4+ T cell counts in the same population. Uninfected women in the Zambian cohort had significantly higher CD4 counts than men, as has been reported in other populations (14). As might be predicted, therefore, we observed that during very early HIV-1 infection women also have higher CD4+ T cell counts than men. While this could be interpreted as reduced CD4+ T cell loss in women, we demonstrate here that the rate of CD4+ T cell decline in these women is actually >2-fold greater than that in men, such that by 30 months post-EDI, there is no significant difference in the CD4+ T cell counts between the two sexes. This result is consistent with a study of women enrolled in Women’s Interagency HIV Study and men in the Multicenter AIDS Cohort Study, which also reported a higher rate of CD4 decline in women (25). However, the present study allows for a more direct comparison of men and women by utilizing data from a single cohort where individuals have all been infected via heterosexual contact. Moreover, we show that this accelerated loss of CD4 T cells starts to occur during the first months of HIV-1 infection.
The apparent discrepancy that we and others have identified, wherein women maintain lower viral loads yet reach CD4+ T cell counts indicative of extreme immunodeficiency at a similar time postinfection to men, appears to be the result of the increased cellular activation we observe in women during acute infection. Indeed, we found that total CD4+ T cells in women were significantly more highly activated during acute infection, but that the difference between sexes waned over time as CD4+ T cell activation increased in men. Interestingly, while the percentage of total CD38+ CD4+ T cells was increased in women at 1 month post-EDI versus uninfected women, it was not until the chronic stage of infection that there was a significant increase in this percentage in men. Meier et al. (11) did not observe sex differences in total CD4+ T cell activation in HIV-1-infected individuals prior to ART initiation in AIDS Clinical Trials Group (ACTG) study 384; however, this is consistent with our observations, since the ACTG 384 subjects were mostly chronically infected individuals with CD4+ T cell counts <500 cells/mm3. This highlights the significance of our observation that sex differences in immune activation are most prominent during acute infection, where women exhibit a more activated CD4+ T cell phenotype than men.
Moreover, we found that E2 is likely a factor influencing enhanced CD4+ T cell activation due to their direct association. However, this is likely via a mechanism that involves other cells of the immune system. A significant body of research indicates that many effects of estradiol on CD4+ T cells are anti-inflammatory, with evidence to suggest that estradiol inhibits expression of the interleukin-2 (IL-2) receptor and promotes the suppressive function of regulatory CD4+ T cells (26–31). It is possible that estradiol and the estrogen receptor are involved in a positive-feedback loop that contributes to the increased production of type I interferons and ISGs in women, which in turn could contribute to a more generalized immune activation (11, 12, 32–35).
We also observed a striking difference in the role CD4+ T cell activation plays in terms of virus replication between men and women. In men, the level of early CD4+ T cell activation was significantly correlated with both contemporaneous VL and early set-point VL (determined as the geometric mean of VL between 1 and 12 months postinfection). This is consistent with a previous study of both untreated and treated MSM (15). In contrast in women no correlation between CD4+ T cell activation and VL was observed. This difference between the sexes also appears to be linked to the action of estrogen, since we observed a significant negative association between E2 levels and VL at the time of sampling, an effect that extended over the first year of infection and did not appear to be influenced by plasma levels of progesterone. Furthermore, we found that plasma levels of testosterone were in fact positively correlated with SPVL in men, although there was not a significant relationship between contemporaneous testosterone levels and viral load, as was observed with E2. The strong negative correlation with E2 could provide an explanation, at least in part, for the consistently lower early and SPVL observed in women versus men.
This conclusion is supported by the observation that E2 could reduce viral replication in PBMCs in vitro. Since cells involved in the immune response, including CD4+ T cells, are known to express the estrogen receptor, we sought to determine whether estrogen could impact the replication of authentic transmitted founder viruses in PBMCs (36). While previous studies have observed various effects of sex hormones on HIV-1 replication, we show here that patient-derived T/F virus replication can be inhibited after treatment of PBMCs with E2 (20–23). This may involve some of the same interferon-based pathways influenced by E2 that govern CD4+ T cell activation, since the transcriptional levels of several ISGs have been shown to be elevated in immune cells of women and some of these may contribute to more effective control of virus replication. For example, Chang et al. (12) identified ISG15, which interferes with HIV-1 virion release from infected cells (37), as being more highly expressed in the CD4+ T cells of women than men. On the other hand, Das et al. recently reported that estradiol treatment of CD4+ T cells in an in vitro latency model reduced reactivation of latent virus, by inducing higher levels of its receptor which bound to the HIV-1 LTR and suppressed viral transcription directly (38). Additional studies will be important to differentiate between these different possibilities. A limitation of the present study is that the data shown here regarding the relationship between E2, CD4+ T activation, and viral replication are correlative and will require further studies to identify the direct and indirect mechanisms by which these factors are related.
In this first study of sex differences in a cohort of heterosexually infected men and women from Zambia, we have observed distinct differences in immune activation and CD4 loss during acute HIV-1 infection. We identified E2 as a significant effector molecule that can inhibit viral replication, while simultaneously exacerbating CD4+ T cell activation and a more rapid loss of CD4+ cells in HIV-1 infected women. Collectively, these observations suggest a complex interplay between sex hormones and cells of the immune system early in HIV infection in women, which may lead to limited control of viral load and yet exacerbate CD4+ T cell death.
MATERIALS AND METHODS
Study subjects.
All participants in the Zambia Emory HIV Research Project discordant couples cohort in Zambia and International AIDS Vaccine Initiative (IAVI) Protocol C were enrolled in human subjects protocols approved by both the University of Zambia Research Ethics Committee and the Emory University Institutional Review Board. Before enrollment, individuals received counseling and signed a written informed consent form agreeing to participate. All subjects selected for this study were ART naive at the time of sample collection or recording of viral load or CD4+ T cell count in accordance with standard of care and ART availability at the time (2006 to 2010). None of the female participants included in the assays described here were pregnant at the time of sample collection. The ages (medians and interquartile ranges [IQR]) of male and female subjects are shown in Table 1.
TABLE 1.
Ages of male and female subjects
The algorithm used to determine the EDI has been previously described (13). Viral load measurements below the limit of detection (LOD) were imputed using the formula LOD/sqrt(2) (39). Set-point viral load (SPVL) was calculated as the mean of the log10VL values determined between 30 and 365 days post-EDI.
Flow cytometry.
Cryopreserved PBMCs isolated from 47 HIV-1-infected Zambians at 1, 9, and 30 months postinfection were analyzed by flow cytometry. Frozen PBMCs were thawed, washed once with R20 (RPMI 1640 [Sigma-Aldrich, St. Louis, MO] containing 20% fetal bovine serum [FBS; Sigma-Aldrich], 1% 1 M HEPES buffer [Sigma-Aldrich], 1% l-glutamine [Sigma-Aldrich], 1% penicillin-streptomycin [Sigma-Aldrich], and 1% sodium pyruvate [Sigma-Aldrich]). Cells were counted using an automated cell counter (Beckman Coulter, Brea, CA), and 1 million cells were held in R10 (10% FBS) medium for staining. At that time, the cells were washed with 3 ml of Dulbecco phosphate-buffered saline (PBS) without Ca/Mg (Sigma-Aldrich) and stained for 20 min at room temperature with Aqua Live/Dead amine dye-AmCyan (Invitrogen, Carlsbad, CA). The cells were then washed once with R10, and anti-CCR7-APCR700 was added to the cells, followed by incubation at 37°C for 15 min. The rest of the monoclonal antibodies, except anti-Ki67, were then added, followed by incubation at room temperature for 20 min. After a washing step with PBS, Cytofix/Cytoperm (BD, Franklin Lakes, NJ) was added to cells, followed by incubation at 4°C for 20 min. After a washing step with Perm wash buffer, anti-Ki67-FITC was added to the cells. The 12-parameter panel is shown in Table 2. All flow cytometry data were collected on an LSRFortessa (BD). Analyses of these data were performed using FlowJo version 10 software (Tree Star, Ashland OR).
TABLE 2.
Flow cytometry panel used for immunophenotyping
| Antibody target | Clone | Fluorochrome | Manufacturer |
|---|---|---|---|
| Viability | NA | AmCyan | Invitrogen |
| CD3 | SK7 | APC H7 | BD Pharmingen |
| CD4 | RPA-T4 | BV 605 | BioLegend |
| CD8 | RPA-T8 | BV655 | BD Horizon |
| CD45RO | UCLH1 | PECF594 | BD Horizon |
| CCR7 | 3D12 | APCR700 | BD Horizon |
| CD27 | M-T271 | PeCy7 | BD Pharmingen |
| HLADR | L243 | PE | EBioscience |
| CD38 | HB7 | APC | BD Horizon |
| PD1 | EH12.1 | BV421 | BD Horizon |
| Ki67 | 35/Ki67 | FITC | BD Horizon |
| CD57 | HNK-1 | PerCP Cy5.5 | BioLegend |
Sex steroid hormone ELISAs.
Plasma levels of 17β-estradiol, testosterone, and progesterone were measured in duplicate using commercially available enzyme-linked immunosorbent assays (ELISAs; Thermo Fisher, Waltham, MA, for E2; Abcam, Cambridge, UK, for testosterone and progesterone) according to the manufacturer’s instructions.
Viral replication.
T/F virus sequences for six acutely infected Zambian individuals (235234, 235028, 305136, 305156, 235227, and 235212) were identified after near full-length genomic PCR amplification and sequenced using Pacific Biosciences single-molecule long-read technology. The sequences were corrected for base-call errors and assembled using the MFPseq platform (40). Infectious molecular clones were generated from second-round amplicons as previously described (24) (GenBank accession numbers KR820325, KR820393, and MT347678 to MT347681). Virus stocks were recovered after transfection of 293T cells, as described previously (41). E2 was resuspended in 100% ethanol and stored at −20°C in 100 mM aliquots. Frozen PBMCs from buffy coats were thawed and incubated for 72 h in R10 (Phenol Red-free RPMI 1640 supplemented with 10% charcoal-stripped fetal bovine serum [Thermo Fisher] and penicillin-streptomycin) containing 20 U/ml of IL-2 and 1 or 10 nM gamma-irradiated 17β-estradiol or vehicle control (Sigma-Aldrich). The cells were then infected in 24-well plates by 2 h of spinoculation at 2,200 rpm at a multiplicity of infection of 0.02. After spinoculation, the medium in the infection plate was diluted to a 2-ml total volume with the same estradiol-containing medium. On days 2 and 3, the medium was replaced with hormone-containing medium. Then, 50 μl of supernatant was sampled every 48 h starting after the replacement of cell culture medium on day 3, and the reverse transcriptase activity was measured at each time point using a radiolabeled reverse transcriptase assay, as previously described (42).
Statistical analyses.
All statistical analysis was performed using Prism 8 (GraphPad Software, Inc., San Diego, CA) except for generalized linear models, which were generated using JMP Pro 13 (SAS Institute, Inc., Cary, NC). Sequence analysis to determine T/F virus consensus genomes were performed with Geneious (version 9; Biomatters, Auckland, New Zealand) using MUSCLE, followed by hand aligning. The statistical tests used are indicated in each figure legend.
Data availability.
Sequences are available in GenBank under accession numbers MT347678 to MT347681.
ACKNOWLEDGMENTS
We thank all the volunteers in Zambia who participated in this study and all the staff in the Rwanda Zambia HIV Research Group who made this study possible. We also thank Jon Allen and Paul Farmer for technical assistance, sample management, and database management and Zachary Ende, who provided extensive technical advice regarding T/F virus replication.
This study was funded by grants R01 MH095503-05 (S.A.), R01 AI51231, and R01 AI64060 (E.H.). This study was also supported, in part, by the Virology Core at the Emory Center for AIDS Research by performing viral load determinations (grant P30 AI050409) and a Yerkes National Primate Research Center base grant through the Office of Research Infrastructure Programs/OD P51OD11132. This study was supported in part by IAVI, whose work is made possible by the generous support from many donors, including the Bill & Melinda Gates Foundation, the Ministry of Foreign Affairs of Denmark, Irish Aid, the Ministry of Finance of Japan, the Ministry of Foreign Affairs of the Netherlands, the Norwegian Agency for Development Cooperation, the United Kingdom Department for International Development (DFID), and the U.S. Agency for International Development (USAID). A full list of IAVI donors is available (www.iavi.org).
The contents of this study are the responsibility of the study authors and do not necessarily reflect the views of USAID, the U.S. Department of Defense, the National Institutes of Health (NIH), or the U.S. Government. E.E.-B., K.B., and D.C. received training support through an Action Cycling Atlanta AIDS Vaccine 200 fellowship. E.H. is a Georgia Eminent Scholar. We declare there are no competing financial interests.
REFERENCES
- 1.Markle JG, Fish EN. 2014. SeXX matters in immunity. Trends Immunol 35:97–104. doi: 10.1016/j.it.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Klein SL, Marriott I, Fish EN. 2015. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg 109:9–15. doi: 10.1093/trstmh/tru167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischer J, Jung N, Robinson N, Lehmann C. 2015. Sex differences in immune responses to infectious diseases. Infection 43:399–403. doi: 10.1007/s15010-015-0791-9. [DOI] [PubMed] [Google Scholar]
- 4.UNAIDS. 2017. Ending AIDS: progress towards the 90-90-90 targets. UNAIDS, Geneva, Switzerland: http://www.unaids.org/sites/default/files/media_asset/Global_AIDS_update_2017_en.pdf. [Google Scholar]
- 5.Evans JS, Nims T, Cooley J, Bradley W, Jagodzinski L, Zhou S, Melcher GP, Burke DS, Vahey M. 1997. Serum levels of virus burden in early-stage human immunodeficiency virus type 1 disease in women. J Infect Dis 175:795–800. doi: 10.1086/513973. [DOI] [PubMed] [Google Scholar]
- 6.Farzadegan H, Hoover DR, Astemborski J, Lyles CM, Margolick JB, Markham RB, Quinn TC, Vlahov D. 1998. Sex differences in HIV-1 viral load and progression to AIDS. Lancet 352:1510–1514. doi: 10.1016/S0140-6736(98)02372-1. [DOI] [PubMed] [Google Scholar]
- 7.Lyles CM, Dorrucci M, Vlahov D, Pezzotti P, Angarano G, Sinicco A, Alberici F, Alcorn TM, Vella S, Rezza G. 1999. Longitudinal human immunodeficiency virus type 1 load in the Italian seroconversion study: correlates and temporal trends of virus load. J Infect Dis 180:1018–1024. doi: 10.1086/314980. [DOI] [PubMed] [Google Scholar]
- 8.Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, Quinn TC. 2001. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med 344:720–725. doi: 10.1056/NEJM200103083441003. [DOI] [PubMed] [Google Scholar]
- 9.Gandhi M, Bacchetti P, Miotti P, Quinn TC, Veronese F, Greenblatt RM. 2002. Does patient sex affect human immunodeficiency virus levels? Clin Infect Dis 35:313–322. doi: 10.1086/341249. [DOI] [PubMed] [Google Scholar]
- 10.Govender S, Otwombe K, Essien T, Panchia R, de Bruyn G, Mohapi L, Gray G, Martinson N. 2014. CD4 counts and viral loads of newly diagnosed HIV-infected individuals: implications for treatment as prevention. PLoS One 9:e90754. doi: 10.1371/journal.pone.0090754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, Kulkarni S, Wen TF, Lindsay RJ, Orellana L, Mildvan D, Bazner S, Streeck H, Alter G, Lifson JD, Carrington M, Bosch RJ, Robbins GK, Altfeld M. 2009. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med 15:955–959. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang JJ, Woods M, Lindsay RJ, Doyle EH, Griesbeck M, Chan ES, Robbins GK, Bosch RJ, Altfeld M. 2013. Higher expression of several interferon-stimulated genes in HIV-1-infected females after adjusting for the level of viral replication. J Infect Dis 208:830–838. doi: 10.1093/infdis/jit262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haaland RE, Hawkins PA, Salazar-Gonzalez J, Johnson A, Tichacek A, Karita E, Manigart O, Mulenga J, Keele BF, Shaw GM, Hahn BH, Allen SA, Derdeyn CA, Hunter E. 2009. Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1. PLoS Pathog 5:e1000274. doi: 10.1371/journal.ppat.1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karita E, Ketter N, Price MA, Kayitenkore K, Kaleebu P, Nanvubya A, Anzala O, Jaoko W, Mutua G, Ruzagira E, Mulenga J, Sanders EJ, Mwangome M, Allen S, Bwanika A, Bahemuka U, Awuondo K, Omosa G, Farah B, Amornkul P, Birungi J, Yates S, Stoll-Johnson L, Gilmour J, Stevens G, Shutes E, Manigart O, Hughes P, Dally L, Scott J, Stevens W, Fast P, Kamali A. 2009. CLSI-derived hematology and biochemistry reference intervals for healthy adults in eastern and southern Africa. PLoS One 4:e4401. doi: 10.1371/journal.pone.0004401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deeks SG, Kitchen CM, Liu L, Guo H, Gascon R, Narvaez AB, Hunt P, Martin JN, Kahn JO, Levy J, McGrath MS, Hecht FM. 2004. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood 104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh S, Klein RS. 2017. Sex drives dimorphic immune responses to viral infections. J Immunol 198:1782–1790. doi: 10.4049/jimmunol.1601166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker ME. 2013. What are the physiological estrogens? Steroids 78:337–340. doi: 10.1016/j.steroids.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Pernis AB. 2007. Estrogen and CD4+ T cells. Curr Opin Rheumatol 19:414–420. doi: 10.1097/BOR.0b013e328277ef2a. [DOI] [PubMed] [Google Scholar]
- 19.Pennell LM, Galligan CL, Fish EN. 2012. Sex affects immunity. J Autoimmun 38:J282–J291. doi: 10.1016/j.jaut.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Garcia M, Biswas N, Patel MV, Barr FD, Crist SG, Ochsenbauer C, Fahey JV, Wira CR. 2013. Estradiol reduces susceptibility of CD4+ T cells and macrophages to HIV infection. PLoS One 8:e62069. doi: 10.1371/journal.pone.0062069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asin SN, Heimberg AM, Eszterhas SK, Rollenhagen C, Howell AL. 2008. Estradiol and progesterone regulate HIV type 1 replication in peripheral blood cells. AIDS Res Hum Retroviruses 24:701–716. doi: 10.1089/aid.2007.0108. [DOI] [PubMed] [Google Scholar]
- 22.Devadas K, Biswas S, Ragupathy V, Lee S, Dayton A, Hewlett I. 2018. Modulation of HIV replication in monocyte derived macrophages (MDM) by steroid hormones. PLoS One 13:e0191916. doi: 10.1371/journal.pone.0191916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tasker C, Ding J, Schmolke M, Rivera-Medina A, García-Sastre A, Chang TL. 2014. 17β-Estradiol protects primary macrophages against HIV infection through induction of interferon-alpha. Viral Immunol 27:140–150. doi: 10.1089/vim.2013.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deymier MJ, Ende Z, Fenton-May AE, Dilernia DA, Kilembe W, Allen SA, Borrow P, Hunter E. 2015. Heterosexual transmission of subtype C HIV-1 selects consensus-like variants without increased replicative capacity or interferon-alpha resistance. PLoS Pathog 11:e1005154. doi: 10.1371/journal.ppat.1005154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anastos K, Gange SJ, Lau B, Weiser B, Detels R, Giorgi JV, Margolick JB, Cohen M, Phair J, Melnick S, Rinaldo CR, Kovacs A, Levine A, Landesman S, Young M, Munoz A, Greenblatt RM. 2000. Association of race and gender with HIV-1 RNA levels and immunologic progression. J Acquir Immune Defic Syndr 24:218–226. doi: 10.1097/00126334-200007010-00004. [DOI] [PubMed] [Google Scholar]
- 26.Prieto GA, Rosenstein Y. 2006. Oestradiol potentiates the suppressive function of human CD4 CD25 regulatory T cells by promoting their proliferation. Immunology 118:58–65. doi: 10.1111/j.1365-2567.2006.02339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tai P, Wang J, Jin H, Song X, Yan J, Kang Y, Zhao L, An X, Du X, Chen X, Wang S, Xia G, Wang B. 2008. Induction of regulatory T cells by physiological level estrogen. J Cell Physiol 214:456–464. doi: 10.1002/jcp.21221. [DOI] [PubMed] [Google Scholar]
- 28.Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, Ziegler SF, Offner H. 2004. Cutting edge: estrogen drives expansion of the CD4+ CD25+ regulatory T cell compartment. J Immunol 173:2227–2230. doi: 10.4049/jimmunol.173.4.2227. [DOI] [PubMed] [Google Scholar]
- 29.Moulton VR, Holcomb DR, Zajdel MC, Tsokos GC. 2012. Estrogen upregulates cyclic AMP response element modulator alpha expression and downregulates interleukin-2 production by human T lymphocytes. Mol Med 18:370–378. doi: 10.2119/molmed.2011.00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMurray RW, Ndebele K, Hardy KJ, Jenkins JK. 2001. 17β-Estradiol suppresses IL-2 and IL-2 receptor. Cytokine 14:324–333. doi: 10.1006/cyto.2001.0900. [DOI] [PubMed] [Google Scholar]
- 31.Azenabor AA, Hoffman-Goetz L. 2001. 17β-Estradiol increases Ca2+ influx and down regulates interleukin-2 receptor in mouse thymocytes. Biochem Biophys Res Commun 281:277–281. doi: 10.1006/bbrc.2001.4341. [DOI] [PubMed] [Google Scholar]
- 32.Choubey D, Moudgil KD. 2011. Interferons in autoimmune and inflammatory diseases: regulation and roles. J Interferon Cytokine Res 31:857–865. doi: 10.1089/jir.2011.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seillet C, Laffont S, Trémollières F, Rouquié N, Ribot C, Arnal J-F, Douin-Echinard V, Gourdy P, Guéry J-C. 2012. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor α signaling. Blood 119:454–464. doi: 10.1182/blood-2011-08-371831. [DOI] [PubMed] [Google Scholar]
- 34.Griesbeck M, Ziegler S, Laffont S, Smith N, Chauveau L, Tomezsko P, Sharei A, Kourjian G, Porichis F, Hart M, Palmer CD, Sirignano M, Beisel C, Hildebrandt H, Cenac C, Villani AC, Diefenbach TJ, Le Gall S, Schwartz O, Herbeuval JP, Autran B, Guery JC, Chang JJ, Altfeld M. 2015. Sex differences in plasmacytoid dendritic cell levels of IRF5 drive higher IFN-α production in women. J Immunol 195:5327–5336. doi: 10.4049/jimmunol.1501684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seillet C, Rouquié N, Foulon E, Douin-Echinard V, Krust A, Chambon P, Arnal J-F, Guéry J-C, Laffont S. 2013. Estradiol promotes functional responses in inflammatory and steady-state dendritic cells through differential requirement for activation function-1 of estrogen receptor alpha. J Immunol 190:5459–5470. doi: 10.4049/jimmunol.1203312. [DOI] [PubMed] [Google Scholar]
- 36.Phiel KL, Henderson RA, Adelman SJ, Elloso MM. 2005. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett 97:107–113. doi: 10.1016/j.imlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Okumura A, Lu G, Pitha-Rowe I, Pitha PM. 2006. Innate antiviral response targets HIV-1 release by the induction of ubiquitin-like protein ISG15. Proc Natl Acad Sci U S A 103:1440–1445. doi: 10.1073/pnas.0510518103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Das B, Dobrowolski C, Luttge B, Valadkhan S, Chomont N, Johnston R, Bacchetti P, Hoh R, Gandhi M, Deeks SG, Scully E, Karn J. 2018. Estrogen receptor-1 is a key regulator of HIV-1 latency that imparts gender-specific restrictions on the latent reservoir. Proc Natl Acad Sci U S A 115:E7795–E7804. doi: 10.1073/pnas.1803468115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Succop PA, Clark S, Chen M, Galke W. 2004. Imputation of data values that are less than a detection limit. J Occup Environ Hyg 1:436–441. doi: 10.1080/15459620490462797. [DOI] [PubMed] [Google Scholar]
- 40.Dilernia DA, Chien JT, Monaco DC, Brown MP, Ende Z, Deymier MJ, Yue L, Paxinos EE, Allen S, Tirado-Ramos A, Hunter E. 2015. Multiplexed highly-accurate DNA sequencing of closely-related HIV-1 variants using continuous long reads from single molecule, real-time sequencing. Nucleic Acids Res 43:e129. doi: 10.1093/nar/gkv630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deymier MJ, Claiborne DT, Ende Z, Ratner HK, Kilembe W, Allen S, Hunter E. 2014. Particle infectivity of HIV-1 full-length genome infectious molecular clones in a subtype C heterosexual transmission pair following high fidelity amplification and unbiased cloning. Virology 468–470:454–461. doi: 10.1016/j.virol.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Claiborne DT, Prince JL, Hunter E. 2014. A restriction enzyme based cloning method to assess the in vitro replication capacity of HIV-1 subtype C Gag-MJ4 chimeric viruses. J Vis Exp 2104:51506. doi: 10.3791/51506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequences are available in GenBank under accession numbers MT347678 to MT347681.