Summary
Cell reprogramming has revolutionized cell and regenerative biology field. However, human iPS derivation remains inefficient and variable. A better knowledge of molecular processes and the rationale underlying the importance of somatic cell origin is crucial to uncover reprogramming mechanisms. Here, we analyze the molecular profile of different human somatic cell types. We show menstrual blood-derived stromal cells (MnSCs) have a distinct, reprogramming prone, profile, and we identify SOX15 from their oocyte-related signature as a prominent responsible candidate. SOX15 orchestrates an efficient oocyte-based reprogramming combination when overexpressed with the also oocyte-enriched histone chaperone ASF1A and OCT4 and, through specific mechanism, generates iPSCs with distinguishable pluripotent state that further present higher differentiation capacity than canonical iPSCs. Our work supports the presence of different pluripotency states in reprogramming and the importance of using metaphase-II oocyte and MnSCs information to provide alternative reprogramming combinations and, importantly, to improve and understand pluripotency acquisition.
Subject Areas: Molecular Biology, Stem Cells Research, Transcriptomics
Graphical Abstract

Highlights
-
•
MnSC expression signature reveals SOX15 as a crucial oocyte-enriched reprogramming factor
-
•
SOX15 orchestrates an efficient oocyte-based reprogramming combination in MnSC
-
•
Oocyte-based reprogrammed iPSCs (AOX15) show distinct pluripotent state
-
•
AOX15 iPSCs present higher differentiation capacity than OSKM-iPSCs
Molecular Biology; Stem Cells Research; Transcriptomics
Introduction
Generation of induced pluripotent stem cells (iPSCs) is a complex process, and molecular mechanisms are not fully understood.
The great majority of iPSC lines have been generated from dermal fibroblasts. Nonetheless, a multitude of other cell types have been used (Patel and Yang, 2010) as bone marrow mesenchymal stromal cells (BM-MSCs) (Ohnishi et al., 2011; Park et al., 2008; Shao et al., 2012; Streckfuss-Bomeke et al., 2012). Of note, another mesenchymal cell type, menstrual blood stromal cells (MnSCs), has appeared recently in the reprogramming field with clear advantages in accessibility, and their efficiency potential has not been deeply studied (de Carvalho Rodrigues et al., 2012; Li et al., 2012).
Mammalian metaphase-II (MII) oocyte has widely been shown to have an unpaired reprogramming capacity (Gonzalez-Munoz and Cibelli, 2018; Jullien et al., 2014). By analyzing its specific factors (Assou et al., 2006, 2009; Kocabas et al., 2006) we have previously identified the histone-chaperone ASF1A as crucial for human pluripotency and have shown that overexpression of just ASF1A and OCT4 in human adult dermal fibroblast (hADFs) exposed to GDF9 can reprogram AO9-iPSCs, although noteworthy less efficiently than canonical OSKM (OCT4, SOX2, KLF4, and c-MYC) combination (Gonzalez-Munoz et al., 2014).
Sex-determining region Y-box 15 (SOX15) is a member of the SOX family of transcription factors that is highly expressed in mouse blastocyst ICM and mESCs (Maruyama et al., 2005; Yoshikawa et al., 2006), as well as in human ESCs (Pacini et al., 2010), although its expression is significantly higher in MII oocyte (Assou et al., 2006, 2009; Awe and Byrne, 2013; Kocabas et al., 2006).
Although in vitro analysis modeling indicates SOX15 cooperates with OCT4 on the canonical DNA element (Ng et al., 2012), mouse reprogramming assays (Nakagawa et al., 2008) and SOX15 knockout data (Lee et al., 2004; Meeson et al., 2007) suggested SOX15 has a secondary role during mouse development, and whether it participates in human reprogramming regulation and pluripotency remains elusive.
Here we deeply characterize somatic cells to analyze factors involved in the higher reprogramming efficiency we found in MnSC. We have identified SOX15 as a crucial factor that assembles a reprogramming detonator, together with ASF1A and OCT4 (AOX15), and through specific pathways generates a distinctive pluripotent state, with superior differentiation potential. Our study provides evidence of the importance of using oocyte information to make progress of significance into pluripotency and reprogramming understanding.
Results
MnSCs Show Distinct Expression and Epigenetic Profile and Higher iPSC Reprogramming Efficiency when Compared with hADFs and BM-MSCs
We first characterized the above-mentioned three accessible adult cell types. MnSCs have the highest proliferation rate (Figure 1A) and different morphological appearance, smaller and less spindly (Figure 1B) than hADFs and BM-MSCs. Flow cytometry analysis confirmed the expression of MSC markers, although with different mean fluorescence intensity depending on the cell type (Figure 1C).
Figure 1.
Somatic Cell Lines Characterization
Adult human primary BM-MSC, hADF, and MnSC cell lines derived from three different donors were used.
(A) Average population doubling time (PDT) of the different cell lines (days). Each counting was done by triplicate during four subculture rounds.
(B) Representative bright-field images of adult human primary cell lines (scale bar, 100 μm).
(C) Histograms of flow cytometry analysis using mesenchymal markers.
(D) qRT-PCR for genes characteristic of pluripotent cells was performed as indicated on mRNA collected from hADFs, BM-MSCs, MnSCs, and H9-hESCs. Values indicate average relative expression of the specific gene normalized to GAPDH/Actin in a logarithmic scale. Data correspond to the average of three independent experiments done in triplicate with cells from three different donors (n = 9, mean values ±SEM). Student’s t test was applied for statistical significance: ∗∗p < 0.01.
(E) Histograms of flow cytometry analysis in all somatic cell types compared with pluripotent H9-hESCs, using pluripotent cell markers fluorescent labeling. Percentage of SSEA4-positive cells was calculated from three independent experiments (three donors/cell type).
(F) Immunochemistry analysis of pluripotent markers on the different human somatic cell lines, and H9-hESCs as positive expression control (scale bar, 30 μm).
(G) Immunocytochemistry analysis of specific markers of adipogenic (FBAP4 and oil-red staining), osteogenic (Osteocacin), chondrogenic (Aggrecan), and neural (Nestin, Tuj1) differentiation 21 days after specific differentiation (scale bar, 30 μm).
(H) Quantification of the number of positive cells for each differentiated cell type from 30 images as in (G). Data correspond to the average of three independent immunochemistry experiments done in duplicate with cells from three different donors/cell type (mean values ± SEM).
(I) Correlation heatmap showing the clustering of somatic cell lines (four cell lines obtained from four different donors, except for BM-MSCs where three of the donors were used) using the RNA expression (array-based) data. Euclidean distance and complete agglomeration method were used to compute the heatmap's dendrogram. Correlation was computed with Pearson's method.
(J) Correlation heatmap showing the clustering of genome methylation analysis of somatic cell lines (as in I). Euclidean distance and complete agglomeration method were used to compute the heatmap's dendrogram.
(K) PCA analysis using methylation data as in (J).
(L) Violin plots showing the distribution of the DNA methylation (beta values) in each of the somatic samples and pluripotent hESCs. Data correspond to the average from same four different donors/cell type. Differential global methylation significance is shown (p values: ∗∗∗p < 0.001, ∗<0.05). Median beta-values of each cell population are indicated at the top.
(M) Differentially methylated regions (DMRs) among somatic groups were used to generate Venn diagrams to identify specific and common methylated genomic regions in each pluripotent group. We have highlighted in blue the number of specific MnSC DMRs when compared with both hADF and BM-MSC. See also Figure S1.
We next evaluated the expression of pluripotency markers. Quantitative RT-PCR results confirmed expected lower expression in all somatic cell lines versus pluripotent hESCs (Figure 1D). Flow cytometry and immunofluorescence analysis revealed negligible OCT4 and SOX2 staining compared with hESCs and mild NANOG protein expression. Among somatic cell lines, hADFs showed the highest SSEA4 labeling and MnSCs the lowest (Figures 1E and 1F).
We then evaluated mesenchymal multipotency performing in vitro adipogenic, osteogenic, chondrogenic, and neural differentiation. MnSCs were unable to differentiate, whereas hADF and specially BM-MSC efficiently differentiate to all four lineages (Figures 1G and 1H).
Hierarchical dendrogram based on global transcription profile show MnSCs cluster together and separately to BM-MSCs and hADFs (Figure 1I). Whole-genome CpG DNA methylation analysis with unsupervised hierarchical clustering and principal component analysis (PCA) confirmed the same segregation pattern where MnSCs differ the most (Figures 1J and 1K). Global analysis of methylation profiles (GAMP) indicates a significant difference in MnSCs versus hADFs (p value < 0.05) (Figure 1L). Venn diagrams showing differential methylation regions (DMRs) among somatic groups also identify their unique epigenetic profile (Figure 1M, Table S1).
These results indicate that, although all three cell types show general mesenchymal characteristics, they have different expression and epigenetic signatures, and MnSCs have the most distinct surface marker expression, methylation profile, low pluripotency genes expression, including SSEA4, and less differentiation potential.
We then analyzed iPSC generation potential. MnSCs showed the highest reprogramming efficiency using both canonical—OSKM or OSK (without c-MYC)—(∼20-fold higher) and AO9 combinations (∼10-fold higher). BM-MSCs present similar AO9- and higher OSKM- and OSK-reprogramming capacity than hADFs (Figure 2A).
Figure 2.
MnSCs Expression Signature Reveals SOX15 as Crucial Oocyte-Enriched Reprogramming Factor
(A) Average number of TRA-1-60+ reprogrammed iPSC lines derived from 107 transduced hADFs, BM-MSCs, or MnSCs with the different factor combinations. Mean values (n = 9, triplicates with cells from three different donors/cell type) ± SEM are plotted. TRA-1-60+ reprogrammed iPSC lines were considered those that showed TRA-160+ labeling after at least five expansion passages from initial colony appearance. Student’s t test was applied for statistical significance: ∗∗∗p < 0.001, ∗<0.05 between compared groups.
(B) High-throughput array-based expression analysis of MnSC, BM-MSCs, and hADFs. Differentially regulated genes among groups were used to generate Venn diagrams to identify specific and common regulated genes in each group. We have highlighted in blue the number of specific MnSC differentially regulated genes when compared with both hADF and BM-MSC.
(C) Significant enriched gene ontologies (GO)-key PANTHER pathways (30)-(FDR p < 0.05) associated with MnSCs-specific regulated genes are shown. No GO-key PANTHER pathway with statistically significant results was found for hADF specific regulated genes and only glycolysis pathway (TPI1, PGK1, ALDOA, ENO2, and GPI) for BM-MSCs (fold enrichment 23.43, FDR 9.19 × 10−4).
(D) Venn diagrams showing common genes among MnSCs specific upregulated genes and published lists of oocyte-enriched genes. Representative oocyte-enriched genes are highlighted. When we compared either hADFs or BM-MSCs specific overexpressed genes with available lists of highly specific oocyte factors we found no common genes among lists.
(E) Average number of TRA-1-60+ reprogrammed iPSC lines derived from 107 transduced MnSCs with the different factor combinations. Mean values (n = 9 triplicates with cells from three different donors) ± SEM are plotted. TRA-1-60+ reprogrammed iPSC lines where considered as in Figure 1A. No colonies were found when ASF1A and OCT4 (AO) combination was used. Student’s t test was applied for statistical significance: ∗∗∗p < 0.001 between compared groups.
MnSC Expression Signature Reveals SOX15 as a Crucial Oocyte-Enriched Reprogramming Factor
To define the MnSCs gene expression signature, we identified their specific regulated genes (Figure 2B and Table S1). Gene ontology (GO) analysis revealed a significant enrichment of PANTHER pathways related to the oocyte biology (Figure 2C).
We compared MnSCs-specific overexpressed genes with available lists of highly specific oocyte factors (Assou et al., 2006, 2009; Kocabas et al., 2006) and identified SOX15 as an outlined common gene (Figure 2D). We confirmed SOX15 (Figures S1A–S1C) and ASF1A (Figures S1D and S1E) higher protein overexpression in MnSC over hADFs.
We hypothesized that the oocyte-related expression profile of MnSCs contributes to their high reprogramming efficiency and that overexpression of specific master genes of such signature could contribute to reprogramming initiation. ASF1A fulfills this concurrence, being part of the MnSC signature (Figure 2D) and oocyte-based reprogramming factor (Gonzalez-Munoz et al., 2014); we therefore investigated SOX15.
SOX15 overexpression increased more than 7-fold AO9 reprogramming (Figure 2E) in MnSCs. When we used SOX15, GDF9 is no longer needed for reprogramming, and it does not enhance SOX15-ASF1A-OCT4 (AOX15) efficiency (Figure 2E), suggesting SOX15 overexpression improves GDF9 signaling effect in reprogramming efficiency.
We have therefore identified SOX15 from the oocyte-related MnSCs signature as a reprogramming factor responsible for increased oocyte-based reprogramming combination efficiency.
Reprogramming Occurs through Different Mechanisms and Pathways Depending on Triggering Combination
To gain insight into the mechanism underlying SOX15 role in pluripotency acquisition, we analyzed gene and protein expression showing that SOX15, but not ASF1A, is upregulated early during OSK reprogramming (Figures 3A–3D), whereas its expression decreases during spontaneous differentiation of iPSCs (Figure S4A).
Figure 3.
Reprogramming Occurs Through Different Mechanisms and Pathways Depending on Triggering Combination
(A–D) SOX15 is upregulated early during reprogramming. MnSCs lysates 5 days after retroviral driven overexpression of GFP (MnSC CT), OSK (MnSC-OSK), or AOX15 (MnSC-AOX15) and OSK-iPSCs were used for western blot against SOX15 (A) and ASF1A (B) and loading control GAPDH. (C and D) Quantification of band pixel intensity relative to GAPDH was done. Data correspond to the average of three independent experiments (three different MnSCs donors) done in duplicate (mean values ± SEM). Student’s t test was applied for statistical significance: ∗∗∗p < 0.001.
(E) Proportional Venn diagram showing differentially regulated genes 5 days after OSK, AOX15, or AO9 reprogramming factors overexpression using array-based global gene expression analysis. MnSCs overexpressing GFP were used as control (CT).
(F) SOX15 protein interaction during reprogramming and pluripotency. MnSCs lysates 5 days after retroviral driven overexpression of GFP, OSK, AOX15, or AX15 and fully reprogrammed OSK-iPSCs were used for SOX15 immunoprecipitation (IP) and western blot (Wb) against ASF1A and OCT4. Cell lysate supernatants (SN) were recovered after IP and use for western blot (right panels).
See also Figure S2.
SOX15 overexpression along with OCT4, SOX2, and KLF4 did not affect reprogramming efficiency; however, SOX15 combination with OCT4 and KLF4 (OKX15) strongly reduced it (over 95% reduction). Similarly, SOX2 overexpression with OCT4 and ASF1A (AOS) dramatically decreased iPSC formation (over 90% decrease) (Figure 2E).
Our results indicate SOX15 cannot replace SOX2 during OSK human reprogramming, and vice versa, SOX2 cannot replace SOX15 during AOX15 reprogramming, suggesting described factor combinations function differently and SOX15 exerts a reprogramming triggering effect only when ASF1A is also overexpressed.
To further study the signaling involved in the initiation of reprogramming we analyzed global gene expression profiles of MnSCs 5 days after factors overexpression—OSK, AOX15, and AO9—(Figure 3E and Table S2). Venn diagrams show regulation of different sets of genes. AOX15 includes AKT and ERK phosphorylation regulators BST2 and BST1, TGF-beta signaling regulators SMAD6 or SMAD1, or key mediators of interferon signaling STAT1 and STAT2. Our results show OSK and AOX15 reprogramming onset operate through different initiating pathways.
Both OSK and AOX15 combinations lead to decreased proliferation rate as a consequence of reprogramming initiation, as SOX15 and ASF1A plus SOX15 overexpression did not affect proliferation rate (Figure S2A).
We then questioned the next steps during the reprogramming process. We analyzed the expression of mesenchymal-epithelial transition (MET)/cell adhesion markers before iPSC colonies appear (days 10 and 18) (Figures S2B and S2C) and confirmed both OSK and AOX15 upregulate MET genes, supporting previous studies showing this transition as crucial for reprogramming (Buganim et al., 2013; Li et al., 2010; Subramanyam et al., 2011).
We further investigated whether SOX15 participates in ASF1A-OCT4 reprogramming through interaction with this protein complex (Gonzalez-Munoz et al., 2014).
We found that, although SOX15 protein interacts with both ASF1A and OCT4 in pluripotent iPS cells, during reprogramming initiation, SOX15 only interacts with OCT4 when we use AOX15, but not OSK, combination (Figure 3F).
Similarly, protein interaction between ASF1A and SOX15 is observed not only during AOX15 and not during OSK reprogramming but also when both proteins, ASF1A and SOX15, are overexpressed indicating this interaction is OCT4 independent.
Interestingly, even if at lower degree, we saw ASF1A-SOX15 interaction also in control MnSCs, suggesting a possible hypothesis that MnSCs higher reprogramming capacity may be associated with a basal oocyte-related switched-on state.
Our results show that direct interaction of SOX15 with both ASF1A and OCT4 is part of the specific mechanism of the AOX15 combination and leads to distinct initiation pathways for reprogramming that include later MET activation.
AOX15 Reprogrammed iPSCs Show Distinguishable Pluripotent State
We next characterized the pluripotent cells generated. AOX15-derived colonies were fully reprogrammed, expressing standard stem cell markers after culturing for 25–28 passages (Figures 4A, 4B, and S3A) and showed normal karyotype (Figure S3B). We found no detectable expression of exogenous factors from the retroviral vectors (Figure S3C). When induced to differentiate in vitro, OSK-iPSCs and AOX15-iPSCs can form ecto-, endo-, and mesoderm cell lineages (Figures S4A and S4B) and formed mature teratomas when injected into immunodeficient mice (Figure S4C).
Figure 4.
AOX15 Reprogrammed iPSCs Show Distinguishable Pluripotent State
(A) qRT-PCR for genes characteristic of pluripotent cells was performed as indicated on mRNA collected from source MnSCs and reprogrammed iPSC using OSK or AOX15 combination and H9-hESCs. Values indicate relative expression of the specific gene normalized to GAPDH/Actin relative to MnSC-GFP expression, which was arbitrarily assigned a value of 0, in a logarithmic scale. Data correspond to the average of three independent experiments done in triplicate (three different iPSC clones/MnSC cell line) (n = 9, mean values ± SEM) with cells from three different MnSC donors.
(B) Representative immunofluorescence analysis image of pluripotent markers on OSK and AOX15 iPSC colonies shows similar staining pattern (scale bar, 100 μm).
(C) High-throughput array-based expression analysis of MnSC derived OSK- and AOX15-iPSCs, and H9-hESCs. Differentially regulated genes after either reprogramming combination were used to generate Venn diagrams to identify specific and common regulated genes in each pluripotent group. Representative pluripotency-associated genes are shown.
(D) Violin plots showing the distribution of the DNA methylation (beta values) in each of the pluripotent group and MnSCs. Significant global methylation compared with MnSC is shown (p values: ∗∗∗p < 0.001, ∗∗<0.01).
(E) Heatmap showing the clustering of MnSC derived OSK and AOX15 lines (five lines from four different MnSC donors each) and hESCs (H9 p33 and H9 p67, and H1) using the DNA methylation (array-based) data. Euclidean distance and complete agglomeration method were used to compute the heatmap's dendrogram. Correlation was computed with Pearson's method.
(F) PCA analysis using methylation data as in (E).
(G) High-throughput array-based DNA methylation analysis of MnSC derived OSK- and AOX15-iPSCs, and H9-hESCs. Differentially methylated regions (DMRs) after either reprogramming combination were used to generate Venn diagrams to identify specific and common methylated genomic regions in each pluripotent group.
See also Figures S3 and S4.
We performed whole-transcriptome profiling on our cohort of genetically matched OSK and AOX15 hiPSCs. hESCs were used as control to confirm the pluripotent expression profile of hiPSCs generated (Figure 4C). Although, as expected, there are strong overlaps among all three pluripotent groups, supporting achieved full reprogramming of iPSCs, AOX15-iPSCs specifically regulate the expression of ∼13% of the genes compared with OSK-iPSCs (Figure 4C) (Table S3). Their GO analysis revealed a significant enrichment for biological processes where we found a number of genes that are also either highly expressed or have an essential functional role in human MII oocytes (Table S4).
Whole-genome CpG DNA methylation analysis confirmed expected hypermethylation (Nishino et al., 2011) of all pluripotent groups compared with MnSC cells (Figure 4D). Unsupervised hierarchical clustering and PCA of whole-genome CpG DNA methylation data revealed separated clustering of OSK from AOX15 hiPSCs (from same MnSCs donors) as well as from hESCs (Figures 4E and 4F), and we can identify specific differentially methylated regions (DMRs) for each of the reprogramming combinations (Figure 4G and Table S3).
Together, these results further support that differential pathways to reach pluripotency, depending on triggering combination factors, leads to a distinguishable pluripotent state.
Superior Differentiation Potential of AOX15-iPSCs
We hypothesized differences found in OSK and AOX15-iPSCs have a functional effect on their differentiation capacity.
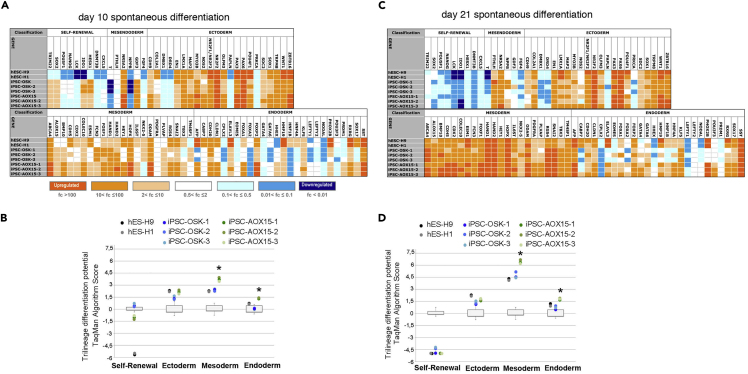
We assessed the potential of pluripotent lines to spontaneously differentiate in vitro and quantified trilineage potential using ScoreCard assays (Bock et al., 2011) at two time points (10 and 21 days). We analyzed the expression plots of 94 genes, relative to the undifferentiated reference set (Figures 5A and 5C). Although, as expected, the Scorecard analysis algorithm predicts trilineage potential for all cell lines, we found a significant higher trend for mesodermal and endodermal differentiation in AOX15-iPSCs compared with OSK-iPSCs, both at early and late stages, suggesting their highest differentiation potential (Figures 5B and 5D).
Figure 5.
AOX15 and OSK iPSCs Show Differential Spontaneous Differentiation Potential
Embryo bodies were derived from hESCs (H9 and H1) or MnSC derived OSK and AOX15 iPSCs (three iPSC lines from three different donors/combination) at two time points of spontaneous in vitro differentiation. Heatmap expression (A and C) and dot blot scores (B and D) generated by the ScoreCard algorithm showing expression of pluripotency and differentiation markers at day 10 of floating EBs culture or after 11 more days (21 days) of attached EBs culture. ∗p < 0.05 one-way ANOVA with TUKEY post hoc test.
To prove the functional consequences of differential activation of lineage-specific transcription, we evaluated specific mesoderm and endoderm cell types in vitro for differentiation potential toward cardiomyocytes and pancreatic progenitor cells, respectively.
We examined the relative amount of cardiac troponin T-positive cells by immunofluorescence, flow cytometry, and relative gene expression of cardiac-specific markers (Figures 6A–6C). Our results confirmed the highest cardiac differentiation potential of AOX15-iPSCs.
Figure 6.
AOX15-iPSCs Show Increased Cardiomyocyte, Pancreatic Progenitors, and Primordial Germ Cell (PGC) Differentiation Efficiency
(A–C) Efficiency of in vitro differentiation toward cardiomyocytes of OSK- and AOX15-iPSCs 14 days after induction. (A) Immunocytochemistry analysis of cardiomyocyte cell marker Cardiac troponin T (cTnT). DAPI was used for nucleus labeling (scale bar, 80 μm). (B) Flow cytometric analysis at 14 days post induction of cardiac differentiation using OSK- or AOX15-iPSCs. Data are expressed as mean ± SEM (n = 9) with cells from three different donor-iPSCs. ∗p < 0.05 t test. (C) qRT-PCR data for cardiomyocyte differentiation markers NKX2.5, αMHC, MEF2C, MLC2C at day 14 of in vitro differentiation. Average expression values ±SEM are represented relative to undifferentiated iPSCs (normalized to GAPDH/Actin). Data correspond to the average of three independent experiments done in triplicate with cells from three different donor-iPSCs (n = 9). Student’s t test was applied for statistical significance: ∗p < 0.05 between OSK and AOX15 groups.
(D–F) Efficiency of in vitro differentiation toward pancreatic progenitors of OSK- and AOX15-iPSCs generated after 15 days of differentiation using the STEMdiff directed differentiation kit. (D) Immunocytochemistry analysis of pancreatic progenitors marker PDX1 with cells from three different donor-iPSCs (scale bar, 80 μm). (E) Flow cytometric analysis at 15 days post induction of pancreatic progenitor differentiation using OSK- or AOX15-iPSCs. Data are expressed as mean ± SEM (n = 9) with cells from three different donor-iPSCs. ∗p < 0.05 t test. (F) qRT-PCR data for pancreatic progenitor differentiation markers PDX1, NKX6.1, SOX9, NGN3, HNF6, FOXA2. Average expression values ±SEM are represented relative to undifferentiated iPSCs (normalized to GAPDH/Actin). Data correspond to the average of three independent experiments done in triplicate with cells from three different donor-iPSCs (n = 9). Student’s t test was applied for statistical significance: p∗<0.05 between OSK and AOX15 groups.
(G and H) Efficiency of in vitro differentiation toward primordial germ cell (PGC) fate. (G) Immunocytochemistry images of PGC differentiation markers VASA and PRMT5 (scale bar, 60 μm). (H) qRT-PCR data for PGC differentiation markers. Average expression values ±SEM are represented relative to undifferentiated iPSCs (normalized to GAPDH/Actin). Data correspond to the average of three independent experiments done in triplicate with cells from three different donor-iPSCs (n = 9). Student’s t test was applied for statistical significance: p∗<0.05 between OSK and AOX15 groups.
Similarly, AOX15-iPSCs efficiently differentiate to PDX1-positive cells (Figures 6D and 6E) and show the highest pancreatic progenitor markers expression (Figure 6F).
We further assessed their capacity for primordial germ cells (PGC-like) specification as a number of reports have shown the low efficiency of this process using hiPSCs and the importance of the highly orchestrated combination of transcriptional and epigenetic state of the original cells (Canovas et al., 2017; Irie et al., 2015; Sasaki et al., 2015). We found iPSCs differentiate to VASA and PRMT5-positive cells (Figure 6G), and qPCR showed AOX15-iPSCs present significantly higher relative expression of PGC markers than OSK-iPSCs (Figure 6H). Together, our data support that AOX15-iPSCs have superior differentiation capacity.
Discussion
We provide compelling evidence of the importance of deep characterization of original somatic cells to provide crucial information involved in the reprogramming phenomenon.
We found MnSCs have unique expression and epigenetic and potency profiles and show higher reprogramming efficiency than hADFs and BM-MSCs. Although high reprogramming capacity has been shown previously for these cells (de Carvalho Rodrigues et al., 2012; Li et al., 2012), no rigorous comparative analysis has been made and, contrary to our data, high efficiency has been attributed to theoretical basal multipotent state, although marker analysis has been controversial and discordant among publications (de Carvalho Rodrigues et al., 2012; Khanjani et al., 2014; Li et al., 2012), probably due to an absence of appropriate controls.
Our analysis, focused on the oocyte-related signature of MnSCs, has uncovered SOX15 as a crucial human reprogramming factor. We found SOX15 expression is upregulated in human pluripotent cells, matching mouse ES data (Maruyama et al., 2005), and also during early reprogramming.
We show SOX15 overexpression, together with ASF1A and OCT4, creates an efficient “oocyte-based” reprogramming combination (AOX15) that follows a reprogramming mechanism different from the canonical OSK combinations.
Our co-immunoprecipitation assays show AOX15, but not OSK, reprogramming initiation functions by SOX15 interaction with both ASF1A and OCT4, generating specific early transcriptional activation program, and finally reaches a unique pluripotent state that has relevant functional consequences with increased differentiation potential, including challenging PGC-like generation.
Our work supports the hypothesis that pluripotency is not a single state—previous revolutionary publications in this field have shown the existence of naive and ground human iPSC and ESC, and the following research confirms the complexity of such definitions—and more progress is needed to explore the possibility of additional pluripotent states or, probably more accurately, sequences of pluripotency states, that can resemble the natural progression after fertilization.
Exogenous factor reprogramming through iPSCs constitutes an exceptional tool for such study allowing the analysis of specific triggering combinations as the one described here that will help uncover other crucial factors for pluripotency, reprogramming, and development. We believe the use of oocyte significant factors together with somatic cell nuclear transfer information will comprise a valuable source for this goal.
Limitations of the Study
We have focused on analysis and characterization of AOX15 reprogramming with MnSCs; however, full characterization of the role of SOX15 in oocyte-based reprogramming in cell types of diverse origin would also bring valuable information of the reprogramming process. Our results indicate SOX15 oocyte-based reprogramming operates also in cell types of mesenchymal origin as hADFs and BM-MSCs, although at lower efficiency than MnSCs. Our hypothesis considers endogenous basal-activated pathways associated with oocyte-factors in somatic cells influence their reprogramming efficiency depending on the combination we choose; further studies are needed to decipher such basal-activated pathways and their relation with the ease for reprogramming.
Also, it would be interesting to provide deeper mechanistic insight into this process. A possible strategy would be to disrupt SOX15 interaction with ASF1A, OCT4, or both. Information of SOX15 and ASF1A interaction domains at protein level would be needed, and it is part of our future projects to perform biochemical and molecular characterization of ASF1A and SOX15 complex to uncover factors involved in reprogramming initiation.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact Elena Gonzalez-Munoz (egonmu@uma.es).
Materials Availability
All material used are listed in Transparent Methods section and Key Resources Table in Supplemental Information, and any further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact.
Data and Code Availability
The accession number for the expression and methylation arrays data reported in this paper is NCBI’s Gene Expression Omnibus (GEO) repository: GSE139085 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE139085).
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We acknowledge the assistance and support of Laboratory for Cell Reprogramming and BIONAND students, colleagues, and collaborators. We thank members of the LARCEL laboratory and Prof. J.B. Cibelli (Michigan State University) for comments, discussion, and support; Dr. Ariane Wittgreen for intellectual input and discussion; and Biobanco del Sistema Sanitario Público de Andalucía for karyotyping and teratoma assay service.
The authors thankfully acknowledge the computer resources (IPA software) provided by the PAB (Andalusian Bioinformatics Platform) center located at the University of Malaga (www.scbi.uma.es).
This work was funded by Ministerio de Economía y Competitividad Gobierno de España (MINECO-SAF2015-66105-R and RYC-2014-15410) and Fundación Progreso y Salud.
Author Contributions
Funding acquisition: E.G.-M. Conceived and designed experiments: E.G.-M. Performed experiments: E.G.-M., L.L.-C. Analyzed the data: E.G.-M. Bioinformatic analysis: J.M.-M., P.C.-S. Wrote the manuscript: E.G.-M.
Declaration of Interests
The authors declare no competing interests.
Published: August 21, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101376.
Supplemental Information
For differential methylation analysis of MSC, BM-MSCs, and hADFs, table contains sheets with significant DMRs exclusive of each comparison as long as sheets with significant DMRs shared in different comparisons. For differential Gene expression analysis of MSC, BM-MSCs, and hADFs, table contains sheets with significant genes exclusive of each comparison as long as sheets with significant genes shared in different comparisons.
Table contains sheets with significantly differently regulated genes of each comparison.
For differential gene expression analysis of hES, OSK-iPSC, and AOX15-iPSCs, table contains sheets with significant genes exclusive of each comparison as long as sheets with significant genes shared in different comparisons. For differential methylation analysis of hES, P1-iPSC, and P2-iPSCs, table contains sheets with significant DMRs exclusive of each comparison as long as sheets with significant DMRs shared in different comparisons.
(Mi et al., 2019) for identification of biological processes associated with AOX15-iPSC expression signature.
References
- Assou S., Anahory T., Pantesco V., Le Carrour T., Pellestor F., Klein B., Reyftmann L., Dechaud H., De Vos J., Hamamah S. The human cumulus--oocyte complex gene-expression profile. Hum. Reprod. 2006;21:1705–1719. doi: 10.1093/humrep/del065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assou S., Cerecedo D., Tondeur S., Pantesco V., Hovatta O., Klein B., Hamamah S., De Vos J. A gene expression signature shared by human mature oocytes and embryonic stem cells. BMC Genomics. 2009;10:10. doi: 10.1186/1471-2164-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awe J.P., Byrne J.A. Identifying candidate oocyte reprogramming factors using cross-species global transcriptional analysis. Cell Reprogram. 2013;15:126–133. doi: 10.1089/cell.2012.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock C., Kiskinis E., Verstappen G., Gu H., Boulting G., Smith Z.D., Ziller M., Croft G.F., Amoroso M.W., Oakley D.H. Reference Maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell. 2011;144:439–452. doi: 10.1016/j.cell.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buganim Y., Faddah D.A., Jaenisch R. Mechanisms and models of somatic cell reprogramming. Nat. Rev. Genet. 2013;14:427–439. doi: 10.1038/nrg3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canovas S., Campos R., Aguilar E., Cibelli J.B. Progress towards human primordial germ cell specification in vitro. Mol. Hum. Reprod. 2017;23:4–15. doi: 10.1093/molehr/gaw069. [DOI] [PubMed] [Google Scholar]
- de Carvalho Rodrigues D., Asensi K.D., Vairo L., Azevedo-Pereira R.L., Silva R., Rondinelli E., Goldenberg R.C., Campos de Carvalho A.C., Urmenyi T.P. Human menstrual blood-derived mesenchymal cells as a cell source of rapid and efficient nuclear reprogramming. Cell Transplant. 2012;21:2215–2224. doi: 10.3727/096368912X653048. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Munoz E., Arboleda-Estudillo Y., Otu H.H., Cibelli J.B. Cell reprogramming. Histone chaperone ASF1A is required for maintenance of pluripotency and cellular reprogramming. Science. 2014;345:822–825. doi: 10.1126/science.1254745. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Munoz E., Cibelli J.B. Somatic cell reprogramming informed by the oocyte. Stem Cells Dev. 2018;27:871–887. doi: 10.1089/scd.2018.0066. [DOI] [PubMed] [Google Scholar]
- Irie N., Weinberger L., Tang W.W., Kobayashi T., Viukov S., Manor Y.S., Dietmann S., Hanna J.H., Surani M.A. SOX17 is a critical specifier of human primordial germ cell fate. Cell. 2015;160:253–268. doi: 10.1016/j.cell.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullien J., Miyamoto K., Pasque V., Allen G.E., Bradshaw C.R., Garrett N.J., Halley-Stott R.P., Kimura H., Ohsumi K., Gurdon J.B. Hierarchical molecular events driven by oocyte-specific factors lead to rapid and extensive reprogramming. Mol. Cell. 2014;55:524–536. doi: 10.1016/j.molcel.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanjani S., Khanmohammadi M., Zarnani A.H., Akhondi M.M., Ahani A., Ghaempanah Z., Naderi M.M., Eghtesad S., Kazemnejad S. Comparative evaluation of differentiation potential of menstrual blood- versus bone marrow-derived stem cells into hepatocyte-like cells. PLoS One. 2014;9:e86075. doi: 10.1371/journal.pone.0086075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocabas A.M., Crosby J., Ross P.J., Otu H.H., Beyhan Z., Can H., Tam W.L., Rosa G.J., Halgren R.G., Lim B. The transcriptome of human oocytes. Proc. Natl. Acad. Sci. U S A. 2006;103:14027–14032. doi: 10.1073/pnas.0603227103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.J., Goring W., Ochs M., Muhlfeld C., Steding G., Paprotta I., Engel W., Adham I.M. Sox15 is required for skeletal muscle regeneration. Mol. Cell. Biol. 2004;24:8428–8436. doi: 10.1128/MCB.24.19.8428-8436.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Liang J., Ni S., Zhou T., Qing X., Li H., He W., Chen J., Li F., Zhuang Q. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell. 2010;7:51–63. doi: 10.1016/j.stem.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Li Y., Li X., Zhao H., Feng R., Zhang X., Tai D., An G., Wen J., Tan J. Efficient induction of pluripotent stem cells from menstrual blood. Stem Cells Dev. 2012;22:1147–1158. doi: 10.1089/scd.2012.0428. [DOI] [PubMed] [Google Scholar]
- Maruyama M., Ichisaka T., Nakagawa M., Yamanaka S. Differential roles for Sox15 and Sox2 in transcriptional control in mouse embryonic stem cells. J. Biol. Chem. 2005;280:24371–24379. doi: 10.1074/jbc.M501423200. [DOI] [PubMed] [Google Scholar]
- Meeson A.P., Shi X., Alexander M.S., Williams R.S., Allen R.E., Jiang N., Adham I.M., Goetsch S.C., Hammer R.E., Garry D.J. Sox15 and Fhl3 transcriptionally coactivate Foxk1 and regulate myogenic progenitor cells. EMBO J. 2007;26:1902–1912. doi: 10.1038/sj.emboj.7601635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H., Muruganujan A., Ebert D., Huang X., Thomas P.D. PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic. Acids Res. 2019;47:D419–D426. doi: 10.1093/nar/gky1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M., Koyanagi M., Tanabe K., Takahashi K., Ichisaka T., Aoi T., Okita K., Mochiduki Y., Takizawa N., Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- Ng C.K., Li N.X., Chee S., Prabhakar S., Kolatkar P.R., Jauch R. Deciphering the Sox-Oct partner code by quantitative cooperativity measurements. Nucleic Acids Res. 2012;40:4933–4941. doi: 10.1093/nar/gks153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino T., Kanata S., Umezawa Y. Selective visualization of point defects in carbon nanotubes at the atomic scale by an electron-donating molecular tip. Chem. Commun. (Camb.) 2011;47:7467–7469. doi: 10.1039/c1cc12045j. [DOI] [PubMed] [Google Scholar]
- Ohnishi H., Oda Y., Aoki T., Tadokoro M., Katsube Y., Ohgushi H., Hattori K., Yuba S. A comparative study of induced pluripotent stem cells generated from frozen, stocked bone marrow- and adipose tissue-derived mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2011;6:261–271. doi: 10.1002/term.428. [DOI] [PubMed] [Google Scholar]
- Pacini S., Carnicelli V., Trombi L., Montali M., Fazzi R., Lazzarini E., Giannotti S., Petrini M. Constitutive expression of pluripotency-associated genes in mesodermal progenitor cells (MPCs) PLoS One. 2010;5:e9861. doi: 10.1371/journal.pone.0009861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I.H., Arora N., Huo H., Maherali N., Ahfeldt T., Shimamura A., Lensch M.W., Cowan C., Hochedlinger K., Daley G.Q. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M., Yang S. Advances in reprogramming somatic cells to induced pluripotent stem cells. Stem Cell Rev. 2010;6:367–380. doi: 10.1007/s12015-010-9123-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K., Yokobayashi S., Nakamura T., Okamoto I., Yabuta Y., Kurimoto K., Ohta H., Moritoki Y., Iwatani C., Tsuchiya H. Robust in vitro induction of human germ cell fate from pluripotent stem cells. Cell Stem Cell. 2015;17:178–194. doi: 10.1016/j.stem.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Shao K., Koch C., Gupta M.K., Lin Q., Lenz M., Laufs S., Denecke B., Schmidt M., Linke M., Hennies H.C. Induced pluripotent mesenchymal stromal cell clones retain donor-derived differences in DNA methylation profiles. Mol. Ther. 2012;21:240–250. doi: 10.1038/mt.2012.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streckfuss-Bomeke K., Wolf F., Azizian A., Stauske M., Tiburcy M., Wagner S., Hubscher D., Dressel R., Chen S., Jende J. Comparative study of human-induced pluripotent stem cells derived from bone marrow cells, hair keratinocytes, and skin fibroblasts. Eur. Heart J. 2012;34:2618–2629. doi: 10.1093/eurheartj/ehs203. [DOI] [PubMed] [Google Scholar]
- Subramanyam D., Lamouille S., Judson R.L., Liu J.Y., Bucay N., Derynck R., Blelloch R. Multiple targets of miR-302 and miR-372 promote reprogramming of human fibroblasts to induced pluripotent stem cells. Nat. Biotechnol. 2011;29:443–448. doi: 10.1038/nbt.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T., Piao Y., Zhong J., Matoba R., Carter M.G., Wang Y., Goldberg I., Ko M.S. High-throughput screen for genes predominantly expressed in the ICM of mouse blastocysts by whole mount in situ hybridization. Gene Expr. Patterns. 2006;6:213–224. doi: 10.1016/j.modgep.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For differential methylation analysis of MSC, BM-MSCs, and hADFs, table contains sheets with significant DMRs exclusive of each comparison as long as sheets with significant DMRs shared in different comparisons. For differential Gene expression analysis of MSC, BM-MSCs, and hADFs, table contains sheets with significant genes exclusive of each comparison as long as sheets with significant genes shared in different comparisons.
Table contains sheets with significantly differently regulated genes of each comparison.
For differential gene expression analysis of hES, OSK-iPSC, and AOX15-iPSCs, table contains sheets with significant genes exclusive of each comparison as long as sheets with significant genes shared in different comparisons. For differential methylation analysis of hES, P1-iPSC, and P2-iPSCs, table contains sheets with significant DMRs exclusive of each comparison as long as sheets with significant DMRs shared in different comparisons.
(Mi et al., 2019) for identification of biological processes associated with AOX15-iPSC expression signature.
Data Availability Statement
The accession number for the expression and methylation arrays data reported in this paper is NCBI’s Gene Expression Omnibus (GEO) repository: GSE139085 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE139085).