Abstract
Inhalation of aerosolized products generated by different electronic devices is called vaping. E-cigarettes or Vaping product use Associated Lung Injury (EVALI) outbreak peaked in August–September 2019 and gradually declined. EVALI remains a diagnosis of exclusion which presents as an acute lung injury in the vaping population. Vitamin E acetate and its products are implicated as one of the cytotoxic agents causing airway centered pneumonitis. Lipid laden macrophages are found in samples of BAL fluid but their role in cytopathology of the disease remains unclear. We present a 57 years old man who came to the emergency department at Monmouth Medical Center, New Jersey in fall, 2019. Reportedly he has been vaping THC about 100g every day for past three days. At initial presentation, he had fever, shortness of breath and hypoxia requiring supplemental oxygen. He was empirically treated with levofloxacin 500 mg for five days without a significant improvement in his symptoms. Non-contrast chest CT scan showed bilateral ground-glass opacities, indicative of diffuse alveolar damage. He underwent flexible bronchoscopy to rule out infective pneumonia followed by auto-immune work-up that was non-conclusive. He was given 1 mg/kg methylprednisolone with a quick taper of oral steroids leading to the resolution of symptoms. At six months follow-up, imaging showed near resolution of ground-glass opacities.
Keywords: Vaping, Acute lung injury, Pneumonia, Tetrahydrocannabinol
Highlights
-
•
EVALI remains a diagnosis of exclusion in the population who actively vapes.
-
•
Vitamin E acetate and its products are implicated as one of the cytotoxic agent causing airway centered pneumonitis.
-
•
Patients with severe and persistent symptoms should have flexible bronchoscopy with BAL to rule out infective etiologies.
-
•
Treatment with supplemental oxygen, antibiotics, and systemic steroids can help resolve the symptoms with a favorable prognosis.
-
•
Follow-up visits should be scheduled in order to monitor clinical and radiological improvement and to monitor long term outcomes.
1. Background
Inhalation of aerosolized products created by using different electronic devices is called vaping. The process involves heating the liquid or wax containing different products including cannabis, tetrahydrocannabinol (THC), nicotine, flavors, vitamin E acetate, and glycerol. Fourth Generation e-cigarettes have been increasingly popular among adolescents as they are smaller and cartridges can be replaced. Though cigarette smoking has been discouraged for decades, e-cigarettes or vaping have been increasingly popular, and is a multibillion industry [1]. EVALI is considered as an acute or subacute respiratory illness in people who had used e-cigarettes (vaping) or dabbing in the last ninety days. Vitamin E acetate and THC is implicated for the lung injury. Blount BC et al. observed vitamin E acetate, and THC or its metabolite in bronchoalveolar lavage (BAL) of 29 patients. Likely, it is an exaggerated inflammatory response to vitamin E acetate, THC or other additives in the product leading to acute lung injury [2,3]. In many states, EVALI is a reportable disease. A study suggested that emergency department visits secondary to EVALI outbreak started in July 2019 and peaked till September 2019 [4]. As of Feb18, 2020, a total of 2807 hospitalized cases of e-cigarette, or vaping, product use-associated lung injury (EVALI) have been reported to the Centers for Disease Control (CDC). Among these patients, 66% were male with a median age of 24 years [5,6]. A case series from Illinois reported that patients presented with constitutional (100%), respiratory (97%), gastrointestinal symptoms (77%), and similar trends have been seen in other case series [7,8]. Approximately 25% of patients have a pulse oxygen saturation below 88% at the time of presentation. While evaluating these patients, diagnostic labs are obtained to rule out other possible differentials like infectious pneumonia, autoimmune and rheumatological etiologies. Most of these patients have radiological evidence of ground-glass opacities [9]. There is a paucity of literature in terms of agreement on diagnostic criteria, leaving it as a diagnosis of exclusion. This report presents a patient who has been vaping THC containing products for more than three years without any change in quantity. We will discuss the disease presentation, diagnostic work-up, the clinical course, cytological and radiological pictures and the appropriate treatment with close follow up.
2. Case presentation
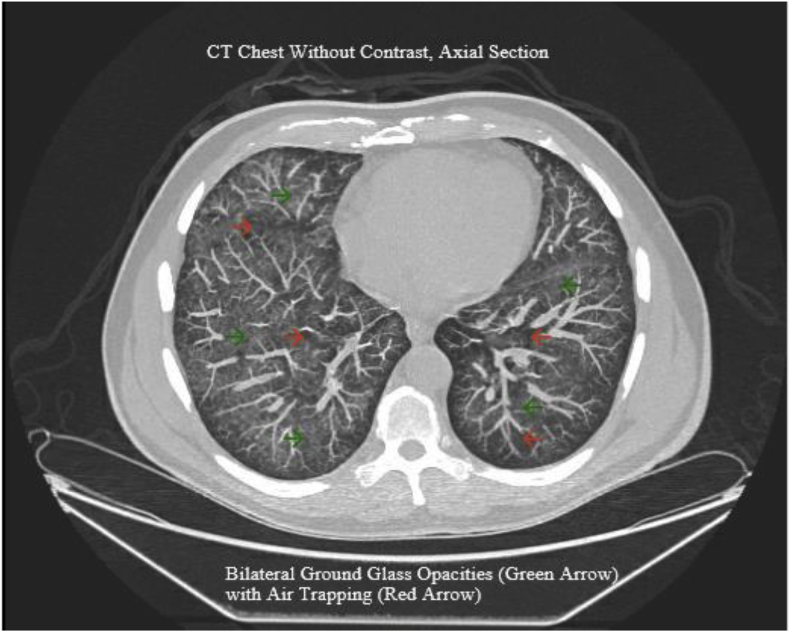
In December 2019, a 57-year-old male with the complaint of left lower abdominal pain, dry cough, and dyspnea on exertion came to the emergency department of a community hospital in New Jersey. His past medical history was insignificant, and he was not taking any medication. The patient was a former smoker with 30 packs per year smoking history for more than 10 years. He started vaping as a smoking cessation tool 3 years ago. Reportedly he has been vaping THC about 100g every day for past three days. On the day of his admission, he had fever, increasing dyspnea and shortness of breath on exertion. In the emergency department (ED) his vital signs were as follows: blood pressure 110/78 mmHg, temperature Tmax 103.3 F, respiratory rate 31 breaths/minute, heart rate 129 beats/minute and his pulse oxygen saturation was 92% at room air. On physical examination, he was found to be in mild respiratory distress. His lung exam had bilateral sporadic crackles. He was placed on supplemental oxygen via nasal cannula. Hematological laboratory findings included white cell count (WBC) of 14.8 K/CMM, elevated ESR of 95 mm/hour, C-reactive protein (CRP) 414.8 mg/L, total bilirubin 3.6 mg/dl, procalcitonin 1.39 ng/ml, alanine aminotransferase (ALT) 83 units/L, aspartate aminotransferase (AST) 90 units/L, and alkaline phosphatase 150 units/L. A chest computed tomography scan was performed, which showed multifocal ground-glass opacities throughout the lungs with air trapping/sparing (Fig. 1, Fig. 3).
Fig. 1.
A non-contrast computed tomography scan was performed, which showed bi-lateral ground-glass opacities throughout the lungs with air trapping/sparing.
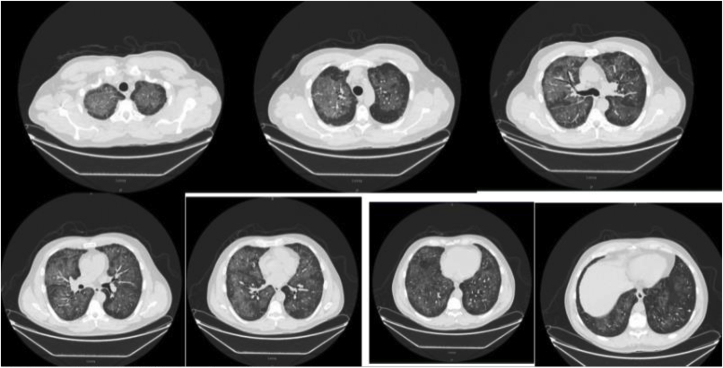
Fig. 3.
Shows bilateral GGO's at the time of diagnosis.
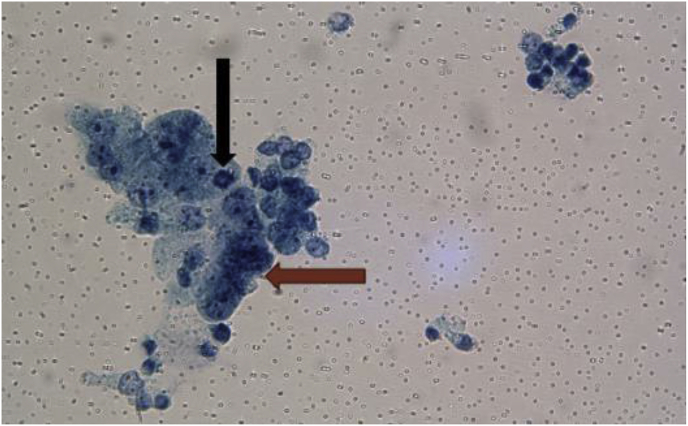
The patient was admitted as a possible case of infective pneumonia (PNA). Diagnostic work-up for bacterial, viral and fungal pneumonia including sputum samples, blood cultures, nasal swabs for respiratory viral polymerase chain reaction and Fungitell turned out to be negative. His WBC's, transaminases, and bilirubin remained elevated. Autoimmune workup including but not limited to anti-nuclear antibody (ANA), anti-double-stranded DNA antibody (anti-dsDNA), smooth muscle antibody (SMA) was non-conclusive. The patient remained febrile, tachypneic and had increasing oxygen requirements up to 5 L/minute even after completing a 5-day course of Levofloxacin. So, decision was made to do a flexible bronchoscopy with bronchoalveolar lavage (BAL). Bronchoscopy revealed bronchitic and bronchiectatic changes without endobronchial lesions suggesting a picture of pneumonitis [10]. Cytology of the bronchial washings showed acute inflammation with reactive pneumocytes. BAL did not reveal evidence of alveolar hemorrhage, and showed a differential count of macrophages 78%, lymphocytes 5%, and neutrophils 17%. These findings are very non-specific and can be seen in a host of other lung diseases (Fig. 2).
Fig. 2.
Cytology from Bronchoalveolar lavage, PAP stain demonstrating reactive pneumonocytes (brown arrow) and mitosis (black arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
A diagnosis of EVALI was made based on a negative infectious and autoimmune workup in a patient who actively vapes. The patient was given a dose of 1 mg/kg methylprednisolone intravenously and started on prednisone 40 mg daily for seven days.
2.1. Treatment
After five days of empiric Levaquin 500 mg, the patient was given a dose of methylprednisolone 40 mg intravenously and subsequently, started on prednisone 40 mg daily the following day, which was tapered quickly.
2.2. Outcome and follow-up
Patient's symptoms including fever, dyspnea, and shortness of breath improved with decreased oxygen requirement following a short course of steroids. He was saturating above 95% on room air on the day of discharge. He was instructed to stop vaping and discharged home. A close follow up was established. On the third day of discharge, he did not report any symptom, and complete resolution of symptoms at 4 week outpatient follow-up. Six months after the discharge from the hospital a follow-up CT scan of the chest was obtained that showed near resolution of ground glass opacities (Fig. 3, Fig. 4).
Fig. 4.
Shows near resolution of GGO's at 6 months follow up.
3. Discussion
EVALI outbreak peaked in August–September 2019 and gradually declined [11]. As of December 2019, CDC is reporting hospitalized EVALI cases and EVALI related deaths regardless of their hospital admission status. Public awareness during this national outbreak and the legislation has played a key role in a precipitous drop in new cases. The most common ingredients in vaping that have been implicated for acute lung injury are THC, vitamin E acetate, and their products. An experimental study done by Wu, D., & O'Shea, D. has shown that vaping of vitamin E acetate produces carcinogen benzene, alkenes, and an exceptionally toxic ketene gas which is likely responsible for lung injury in vaping [12]. There is limited data available to diagnose, treat, and screen these patients. Patients commonly present with cough, fever, shortness of breath associated with mild to severe hypoxic respiratory failure. A case series reported 100% of patients had constitutional symptoms alongside respiratory and gastrointestinal symptoms. The diagnostic workup includes ruling out other possible infective, autoimmune, and rheumatological etiologies. The role of bronchoscopy remains controversial as a diagnostic tool. Patients who have persistent or severe respiratory symptoms need flexible bronchoscopy with BAL to rule out alveolar hemorrhage and infectious causes of pneumonitis. Lipid laden macrophages are seen in the samples of BAL fluids, consistently with oil-red O staining [13]. The cytopathological role and clinical impact of lipid-laden macrophages are not clear. Yasmeen M. Butt et al. studied 17 biopsies and mentioned that none of them showed evidence of exogenous lipoid pneumonia. Though most of the cases had lipid-laden macrophages in BAL fluid, the authors recommended interpreting lipid-laden macrophages as a possible marker of exposure instead of toxicity [10]. The case definition of EVALI includes abnormal chest imaging (chest x-ray and, or chest CT scan) showing a pattern of acute lung injury. A chest x-ray should be the first imaging modality in all suspected EVALI patients, and a chest CT scan is warranted if the chest x-ray is non-conclusive. A case series showed that 100% of patients had bi-lateral opacities. Typically, these opacities are ground glass (GGO's) in density and show underlying diffuse alveolar damage (DAD) or acute eosinophilic pneumonia [7,9]. GGO's in EVALI are more likely to have a uniform distribution, basilar predominance, and subpleural or lobular sparing as seen in different imaging patterns reported by Henry et al. [14]. Recognition of this pattern in the right clinical context of active vaping can help clinicians to narrow down the differential diagnosis. Inflammatory dysregulation, especially elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) as seen in our patient can lead to coagulopathy explaining elevated INR in EVALI patients. Khanijo, S. suggested that procalcitonin can be falsely positive in these patients, limiting the utility of procalcitonin in suspected cases of EVALI [15]. The decision regarding outpatient management of suspected EVALI can be considered on a case to case basis, provided that the patient does not have hypoxemia at presentation (oxygen saturation<95%) and a close follow up (within 24–48 hours) can be arranged. While patients who have hypoxemia (oxygen saturation <95%) at presentation should be hospitalized. Patients who do not have worsening signs or symptoms of hypoxemia and respiratory distress can be managed on the medical floor. Supportive treatment includes supplemental oxygen via nasal cannula, face mask, or high flow nasal cannula to keep oxygen saturation between 88 and 92%. Consulting pulmonary and critical care physician earlier in the course of the disease can help determine the optimal management of respiratory failure in these patients. David A. Siegel et al. reported 47% of the patients received intensive care unit (ICU) level care. In terms of intubation, one of the largest case series (n = 98) reported a total of 26% received invasive mechanical ventilation [7]. Patients with older age (>50 years) had a higher percentage of intubation and the total length of hospital stay [16]. Data is suggestive of resolution of symptoms when treated with antibiotics and systemic steroids with a favorable prognosis even in severe cases [17]. However, the efficacy of steroids is yet to be established. There is a paucity of literature in terms of long term effects or recurrence of EVALI in those patients who continue to vape. Vaping has measurable acute adverse effects on cellular and organ health. Long term side effects of smoking may not be obvious until decades of chronic smoking, we can speculate to see long term outcomes of EVALI till mid of the 21st century [18]. Nevertheless, CDC recommends complete cessation of tetrahydrocannabinol (THC) containing E-cigarettes, and vitamin E acetate should not be added to any e-cigarette. As most of the deaths occurred within 48 hours after discharge from the hospital, a follow-up no later than two weeks should be arranged. On the follow-up visit imaging should be considered alongside pulse oximetry [19]. In addition, if a patient requires home oxygen therapy ongoing care with a pulmonologist must be established at the time of discharge.
Funding
None.
Credit authorship contribution statement
Mohsin Sheraz Mughal: Conceptualization, Writing the manuscript, Review & editing. Denise Lauren V. Dalmacio: Writing original draft and Data curation. Hasan Mahmood Mirza, Ikwinder Preet Kaur, and Maria Amanda Dela Cruz: Writing - review & editing. Violet E Kramer: Manuscript revision & Supervision.
Declaration of competing interest
I, Mohsin S. Mughal declare no conflict of interest on the behalf of all co-authors. I have read the terms and conditions of respiratory Medicine Case Reports and agreed to them.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2020.101174.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Barrington-Trimis J.L., Leventhal A.M. Adolescents' use of “Pod mod” e-cigarettes — urgent concerns. N. Engl. J. Med. 2018;379(12):1099–1102. doi: 10.1056/nejmp1805758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blount B.C., Karwowski M.P., Shields P.G., Morel-Espinosa M., Valentin-Blasini L., Gardner M., Braselton M., Brosius C.R., Caron K.T., Chambers D., Corstvet J., Cowan E., De Jesús V.R., Espinosa P., Fernandez C., Holder C., Kuklenyik Z., Kusovschi J.D., Newman C.…Pirkle J.L. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N. Engl. J. Med. 2020;382(8):697–705. doi: 10.1056/nejmoa1916433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blount B.C., Karwowski M.P., Morel-Espinosa M., Rees J., Sosnoff C., Cowan E., Gardner M., Wang L., Valentin-Blasini L., Silva L., De Jesús V.R., Kuklenyik Z., Watson C., Seyler T., Xia B., Chambers D., Briss P., King B.A., Delaney L.…Pirkle J.L. Evaluation of Bronchoalveolar lavage fluid from patients in an outbreak of E-cigarette, or vaping, product use–associated lung injury — 10 states, August–October 2019. MMWR. Morbid. Mortal. Weekly Rep. 2019;68(45):1040–1041. doi: 10.15585/mmwr.mm6845e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartnett K.P., Kite-Powell A., Patel M.T., Haag B.L., Sheppard M.J., Dias T.P., King B.A., Melstrom P.C., Ritchey M.D., Stein Z., Idaikkadar N., Vivolo-Kantor A.M., Rose D.A., Briss P.A., Layden J.E., Rodgers L., Adjemian J. Syndromic surveillance for E-cigarette, or vaping, product use–associated lung injury. N. Engl. J. Med. 2020;382(8):766–772. doi: 10.1056/nejmsr1915313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products. Centers for Disease Control and Prevention. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html#overview. Accessed on 2020, February 25.
- 6.Lozier M.J., Wallace B., Anderson K. Update: demographic, product, and substance-use characteristics of hospitalized patients in a nationwide outbreak of E-cigarette, or vaping, product use–associated lung injuries — United States. MMWR. Morbid. Mortal. Weekly Rep. December 2019;68(49):1142–1148. doi: 10.15585/mmwr.mm6849e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Layden J.E., Ghinai I., Pray I., Kimball A., Layer M., Tenforde M.W., Navon L., Hoots B., Salvatore P.P., Elderbrook M., Haupt T., Kanne J., Patel M.T., Saathoff-Huber L., King B.A., Schier J.G., Mikosz C.A., Meiman J. Pulmonary illness related to E-cigarette use in Illinois and Wisconsin — final report. N. Engl. J. Med. 2020;382(10):903–916. doi: 10.1056/nejmoa1911614. [DOI] [PubMed] [Google Scholar]
- 8.Davidson K., Brancato A., Heetderks P. Outbreak of electronic-cigarette–associated acute lipoid pneumonia — North Carolina, July–August 2019. MMWR. Morbid. Mortal. Weekly Rep. 2019;68(36):784–786. doi: 10.15585/mmwr.mm6836e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kligerman S., Raptis C., Larsen B., Henry T.S., Caporale A., Tazelaar H., Schiebler M.L., Wehrli F.W., Klein J.S., Kanne J. Radiologic, pathologic, clinical, and physiologic findings of electronic cigarette or vaping product use–associated lung injury (EVALI): evolving knowledge and remaining questions. Radiology. 2020;294(3):491–505. doi: 10.1148/radiol.2020192585. [DOI] [PubMed] [Google Scholar]
- 10.Butt Y.M., Smith M.L., Tazelaar H.D., Vaszar L.T., Swanson K.L., Cecchini M.J., Boland J.M., Bois M.C., Boyum J.H., Froemming A.T., Khoor A., Mira-Avendano I., Patel A., Larsen B.T. Pathology of vaping-associated lung injury. N. Engl. J. Med. 2019;381(18):1780–1781. doi: 10.1056/nejmc1913069. [DOI] [PubMed] [Google Scholar]
- 11.Krishnasamy V.P., Hallowell B.D., Ko J.Y., Board A., Hartnett K.P., Salvatore P.P., Danielson M., Kite-Powell A., Twentyman E., Kim L., Cyrus A., Wallace M., Melstrom P., Haag B., King B.A., Briss P., Jones C.M., Pollack L.A., Ellington S. Update: characteristics of a nationwide outbreak of E-cigarette, or vaping, product use–associated lung injury — United States, August 2019–January 2020. MMWR. Morbid. Mortal. Weekly Rep. 2020;69(3):90–94. doi: 10.15585/mmwr.mm6903e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu D., O'Shea D. 2019. Potential for Release of Pulmonary Toxic Ketene from Vaping Pyrolysis of Vitamin E Acetate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choe J., Chen P., Falk J.A., Nguyen L., Ng D., Parimon T., Ghandehari S. A case series of vaping-associated lung injury requiring mechanical ventilation. Crit. Care Explor. 2020;2(1):e0079. doi: 10.1097/cce.0000000000000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henry T.S., Kanne J.P., Kligerman S.J. Imaging of vaping-associated lung disease. N. Engl. J. Med. 2019;381(15):1486–1487. doi: 10.1056/nejmc1911995. [DOI] [PubMed] [Google Scholar]
- 15.Khanijo S., Lou B.X., Makaryus M., Weber A., Fryman C., Iakovou A. Coagulopathy and inflammatory dysregulation with E-cigarette use. Am. J. Med. 2019 doi: 10.1016/j.amjmed.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Siegel D.A., Jatlaoui T.C., Koumans E.H., Kiernan E.A., Layer M., Cates J.E., Kimball A., Weissman D.N., Petersen E.E., Reagan‐Steiner S., Godfred‐Cato S., Moulia D., Moritz E., Lehnert J.D., Mitchko J., London J., Zaki S.R., King B.A., Jones C.M. Update: interim guidance for health care providers evaluating and caring for patients with suspected E‐cigarEttE, or vaping, product use associated lung injury — United States, October 2019. Am. J. Transplant. 2019;19(12):3420–3428. doi: 10.1111/ajt.15690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gotts J.E., Jordt S., McConnell R., Tarran R. What are the respiratory effects of e-cigarettes? BMJ. 2019;l5275 doi: 10.1136/bmj.l5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maddock S.D., Cirulis M.M., Callahan S.J., Keenan L.M., Pirozzi C.S., Raman S.M., Aberegg S.K. Pulmonary Lipid-Laden macrophages and vaping. N. Engl. J. Med. 2019;381(15):1488–1489. doi: 10.1056/nejmc1912038. [DOI] [PubMed] [Google Scholar]
- 19.Mikosz C.A., Danielson M., Anderson K.N. Characteristics of patients experiencing rehospitalization or death after hospital discharge in a nationwide outbreak of E-cigarette, or vaping, product use-associated lung injury - United States, 2019. MMWR Morb. Mortal. Wkly. Rep. 2020;68:1183. doi: 10.15585/mmwr.mm685152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.