Summary
This study shows that multiple modes of mitochondrial stress generated by partial mtDNA depletion or cytochrome c oxidase disruption cause ryanodine receptor channel (RyR) dysregulation, which instigates the release of Ca2+ in the cytoplasm of C2C12 myoblasts and HCT116 carcinoma cells. We also observed a reciprocal downregulation of IP3R channel activity and reduced mitochondrial uptake of Ca2+. Ryanodine, an RyR antagonist, abrogated the mitochondrial stress-mediated increase in [Ca2+]c and the entire downstream signaling cascades of mitochondrial retrograde signaling. Interestingly, ryanodine also inhibited mitochondrial stress-induced invasive behavior in mtDNA-depleted C2C12 cells and HCT116 carcinoma cells. In addition, co-immunoprecipitation shows reduced FKBP12 protein binding to RyR channel proteins, suggesting the altered function of the Ca2+ channel. These results document how the endoplasmic reticulum-associated RyR channels, in combination with inhibition of the mitochondrial uniporter system, modulate cellular Ca2+ homeostasis and signaling under mitochondrial stress conditions.
Subject Areas: Biological Sciences, Molecular Biology, Cell Biology
Graphical Abstract

Highlights
-
•
Multiple types of mitochondrial stress induce the expression of RyR channel genes
-
•
Stress-induced RyR channels are leaky, causing the release of Ca2+ in the cytosol
-
•
Mitochondrial stress also impairs Ca2+ uptake through mitochondrial uniporter
-
•
RyR antagonists blocked Ca2+ leak, downstream signaling, and target gene expression
Biological Sciences; Molecular Biology ; Cell Biology
Introduction
Mitochondria (mt) are the powerhouse of cells as they produce most cellular ATP through respiration-linked oxidative phosphorylation. In addition to harnessing energy from food material, they participate in several essential cellular functions including the integration of metabolism; synthesis of intermediates for lipid, sterol, nucleotide, and amino acid synthesis; co-ordination of apoptotic signals; and production of signaling molecules such as reactive oxygen species (Friedman and Nunnari, 2014; Tait and Green, 2012). Another important function of mitochondria, in association with the endoplasmic reticulum (ER), is to modulate intracellular Ca2+ homeostasis (Giorgi et al., 2009). The ER is the major reservoir of Ca2+ in resting cells. Shuttling Ca2+ from the ER to the mitochondria through the IP3 receptor calcium ion channel regulates ATP production to meet the cellular energy needs (Cardenas et al., 2010).
The mitochondrial function requires constant communication with the nucleus, which is achieved by tightly orchestrated signaling networks that control both the biogenesis and function of mitochondria (Guha and Avadhani, 2013; Yong and Tang, 2018). Mitochondrial biogenesis is maintained by anterograde signals from the nucleus to the mitochondria that control the import of proteins, mtDNA maintenance, and gene expression as demanded by the energy and growth requirements of the cell. Metabolic stress due to impaired mitochondrial respiration, partial or complete mtDNA depletion, disruption of cytochrome c oxidase (CcO) complex, suppression of mitochondrial transcription, and hypoxia can induce mitochondria-to-nucleus stress signaling pathway, called mitochondrial retrograde signaling (MtRS) (Butow and Avadhani, 2004; Guha and Avadhani, 2013). Recent research has focused on signaling initiated by mitochondria under stress to play a key role in cellular function and homeostasis (Yang and Kim, 2019). This could involve either physical or chemical stress, which ranges from acute to chronic. Mitochondrial stress signaling affecting nuclear gene expression brings about phenotypic changes in cell morphology, cell migration, and growth characteristics (Amuthan et al., 2001), which could make a change in stochastic cellular behaviors. Different MtRS signaling mechanisms have been reported in a variety of metazoan organisms and experimental contexts. The occurrence of MtRS has been reported in mtDNA mutations, deletions, recombinations, and mitochondrial unfolded protein response (mtUPR). The importance of MtRS has been implicated in multiple diseases including cancer progression, myopathies, neurodegeneration, and other disorders (Amuthan et al., 2001, 2002; Arnould et al., 2002; Desideri et al., 2015; Fang et al., 2010; He et al., 2010; Ishikawa et al., 2008).
Intracellular Ca2+ acts as a second messenger to regulate a wide range of cellular functions, including muscle contraction, neurotransmission, and regulation of transcription through activation of specific transcription factors (Clapham, 2007; Demaurex and Nunes, 2016). We and others have shown that increased [Ca2+]c and activation of calcineurin (Cn) are integral components of the signaling cascade involved in MtRS (Biswas et al., 1999; Goffart and Wiesner, 2003). We also showed that partial mtDNA depletion, hypoxia, environmental toxins, and other factors that affect mitochondrial function and disrupt mitochondrial membrane potential (ΔΨm) initiate Ca2+/Cn-dependent retrograde signaling (Srinivasan and Avadhani, 2007). One hallmark of this signaling is the elevation of [Ca2+]c, which is maintained by the ER and mitochondrial Ca2+ stores (Rizzuto et al., 2012). Indeed, this intracellular organelle communication is functionally important for cellular metabolism and cell survival (Duchen, 1999; Franzini-Armstrong, 2007). Even though mitochondrial affinity for Ca2+ is relatively low, they play a vital role in taking up Ca2+ and releasing it back to the cytosol to regulate signaling (Giorgi et al., 2009; Rizzuto et al., 2012). Under conditions of impaired mitochondrial function and disruption of ΔΨm, we showed increased steady-state [Ca2+]c and activation of Cn, which in turn activates NFAT, CREB, CEBPδ, and a unique IkBβ-dependent nuclear factor-κB pathway (Biswas et al., 2005b). In addition, elevated [Ca2+]c also activates many kinases such as PKC, CaMKIV (Arnould et al., 2002), JNK, and MAPK (Amuthan et al., 2002; Friis et al., 2014). Furthermore, the Ca2+/Cn pathway activates heterogeneous nuclear ribonuclear protein hnRNPA2, which acts as a transcriptional co-activator for mitochondrial stress-induced transcription factors cRel:p50, CREB, CEBPδ, and NFAT (Guha et al., 2009). HnRNPA2 plays a critical role in the assembly or stability of enhanceosome complexes at promoter sequences leading to synergistic activation of >120 stress response genes (Biswas et al., 2005b; Guha and Avadhani, 2013) and telomere maintenance (Guha et al., 2018).
We have previously shown that partial depletion of mtDNA in C2C12 cells causes increased [Ca2+]c and initiates MtRS (Biswas et al., 1999). However, the precise mechanism of altered Ca2+ homeostasis in cells subjected to mitochondrial stress remains unresolved. In this study, we show that partial depletion of mtDNA or disruption of the CcO complex induces RyR1 and 3 Ca2+ channel mRNA and protein expression. This is accompanied by a decrease in steady-state levels of FKBP12, a critical regulator of RyR Ca2+ channel gating. Altered FKBP12-RyR binding is known to cause intracellular Ca2+ leak, causing increased [Ca2+]c and initiating MtRS (Dirksen and Avila, 2002; Marx et al., 2000). A steady transfer of Ca2+ from the ER to mitochondria is vital for maintaining cellular bioenergetics (Green and Wang, 2010). We present evidence that mitochondrial dysfunction impairs Ca2+ uniporter function and uptake of Ca2+, leading to increased Ca2+ pool in the cytosol. Furthermore, short hairpin RNA (shRNA)-mediated knockdown (KD) of RyR1 mRNAs in cells with dysfunctional mitochondria reversed Cn activity and abrogated the signaling-associated transcription factor activation and gene expression, suggesting that overexpression of RyR Ca2+ channel is a critical factor in the induction and maintenance of MtRS. This study provides a unified mechanism of altered Ca2+ homeostasis in cells subjected to two different types of mitochondrial dysfunctional stress: mtDNA depletion and CcO4KD.
Results
A Distinctive Agonist-Induced Ca2+ Release Pattern in Cells with Dysfunctional Mitochondria
Previously, we showed that activation of Cn is an early step of MtRS in partial mtDNA-depleted C2C12 skeletal myoblasts and A549 lung carcinoma cells (Amuthan et al., 2001; Biswas et al., 1999). In the present study, we determined the role of mitochondrial dysfunction-induced changes in Ca2+ homeostasis by using CcO subunit IVi1-silenced (CcO4KD) C2C12 cells and mtDNA depletion in HCT116 and C2C12 cells. The mtDNA contents of both C2C12 cells and HCT116 cells were reported before and also presented in Figures S1A and S1B. Figure S1 shows that 2′,3′-Dideoxycytidine (ddC; Millipore Sigma, D5782) or ethidium bromide treatment caused about 70%–80% reduction in mtDNA content in HCT116 and C2C12 cells by about 5–10 growth cycles. All primer information is provided in Table S1. The CcO4KD cells show reduced CcO activity, a marked metabolic shift toward glycolysis, and morphological changes similar to the mtDNA-depleted myoblasts described before (Srinivasan et al., 2016).
Acetylcholine is an agonist for IP3 receptor-induced Ca2+ release, whereas caffeine sensitizes the RyR channel opening to induce Ca2+ release (Pessah et al., 1987). These two agonists were used to trace the Ip3R- and RyR-mediated Ca2+ release in live-cell imaging. Finally, an irreversible ER Ca2+-ATPase inhibitor thapsigargin was used for the depletion of the ER Ca2+ store. Intracellular free Ca2+ concentration ([Ca2+]i) and Ca2+ release properties were monitored and recorded using the fluorescent dye Fura-2-AM in individual cells. Acetylcholine (10 μM), caffeine (5 mM), and thapsigargin (2 μM) were added sequentially to control and CcO4KD C2C12 cells as shown in Figures 1A and 1B (mean values of 30 cells in triplicate for each group). Control C2C12 cells showed a robust response to acetylcholine with a higher peak at 360 ± 25 nM ([Ca2+]c) (mean of 3 replicates and 30 cells per replicate, p < 0.05), compared with CcO4KD cells, which gave a peak at 166 ± 10 nM ([Ca2+]c). However, caffeine-stimulated sarcoplasmic reticulum (SR)-Ca2+ release was detected only in CcO4KD cells where the ([Ca2+]c) was raised up to 249 nM ± 14 (p < 0.001) (Figures 1A and 1B). Figure 1C represents the area under the curve quantifications of Ca2+ response with the aforementioned agonists in control and CcO4KD C2C12 cells (Figures 1A and 1B). The acetylcholine-mediated ([Ca2+]c) rise and ensuing recovery were faster in control cells compared with CcO4KD cells (Figure S2A). The response to caffeine was maximal with 5 mM as 20 mM caffeine (Figure S2B) did not induce a significantly higher [Ca2+]c rise (Figure 1B).
Figure 1.
CcO Disruption in C2C12 Cells Alters RyR and IP3R Agonist-Induced Ca2+ Release
(A–E) [Ca2+]c measurements were carried out with control and CcO4KD cells as described in the Transparent Methods. Traces show the average response of three identical runs (average ≤ 30 cells/run) to different agonists in all cell lines. Recorded data were further analyzed by SpectralyzerLSMDeluxe version 2009092525 software to subtract the background fluorescence before calculating the F380/340 ratio and finally represented as nanomolars. (A) Ca2+ release in control cells. (B, D, and E) Ca2+ release for CcO4KD cells. (C) The area under the curve of increases in [Ca2+]c release in response to different agonists and inhibitors. The values (μM Ca2+) are mean of tracings with >90 cells presented in (B, D, and E). Caff, caffeine; Ach, acetylcholine; Tg, thapsigargin.
ATP, another IP3-mobilizing agonist, duplicated the Ca2+ mobilization pattern observed for acetylcholine in both CcO4KD and control cells (Figures S2C and S2D). The addition of caffeine after ATP (0.1 mM) induced similar [Ca2+]c patterns to that for caffeine after acetylcholine. The role of the ryanodine receptor in caffeine-mediated calcium release was confirmed in experiments showing that ryanodine (50 μM), an antagonist of RyR channels, caused a decrease in the caffeine-mediated Ca2+ release (Figure 1E). Similarly, depletion of the ER store by pretreatment with thapsigargin essentially eliminated the caffeine-mediated Ca2+ release (Figure 1D). These results show that disruption of the CcO complex alters ER Ca2+ release by downregulating the IP3R-dependent mechanism and upregulating the RyR-mediated pathway.
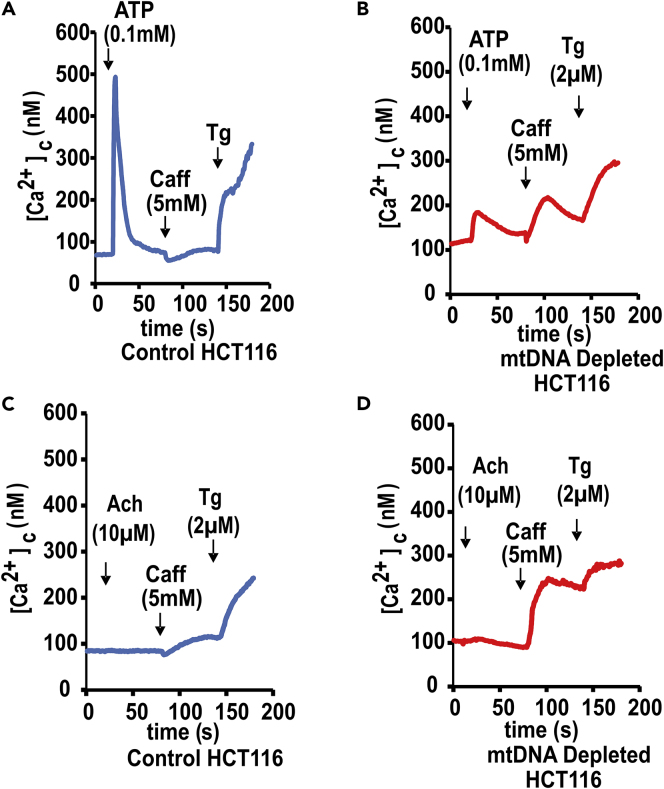
To test the similarity in response to multiple forms of mitochondrial stress, we also tested the agonist-induced Ca2+ release in control and mtDNA-depleted colon carcinoma HCT116 cells. Figures 2A and 2B show that the peak area of ATP-induced Ca2+ release is smaller in mtDNA-depleted cells compared with control cells. However, treatment with caffeine following ATP stimulation evoked a [Ca2+]c response only in mtDNA-depleted HCT116 cells but not in the control cells. Pretreatment with ryanodine completely abrogated caffeine response in mtDNA-depleted cells (not shown), suggesting the role of RyR channels in the altered pattern of Ca2+ release. The addition of acetylcholine (10 μM) did not show Ca2+ release in either control or mtDNA-depleted cells, suggesting the lack of muscarinic receptors. After treatment with acetylcholine, caffeine (5 mM) induced a markedly larger Ca2+ release in mtDNA-depleted cells than in control cells (Figures 2C and 2D). Furthermore, the addition of thapsigargin (2 μM) caused activation of store-operated [Ca2+]c rises in control and mtDNA-depleted cells (Figures 2A–2D). These results confirm that mitochondrial stress initiated by both mtDNA depletion and disruption of CcO complex sensitizes cells to RyR agonist-induced Ca2+ release.
Figure 2.
Ca2+ Release in Response to RyR Channel and IP3R Channel Agonists in Partial mtDNA-Depleted HCT116 Cells
(A–D) [Ca2+]c measurements were carried out with control and mtDNA-depleted HCT116 cells as in Figure 1. Traces show the average response of three different runs (average ≤ 30 cells/run) following the addition of different agonists in all cell lines. Recorded data were further analyzed by SpectralyzerLSMDeluxe version 2009092525 software to subtract the background fluorescence before calculating the F380/340 ratio and finally represented as nanomolars. (A and C) Ca2+ release in control HCT116 cells in response to different agonists as indicated in figures. (B and D) Ca2+ release in response to different agonists in mtDNA-depleted HCT116 cells. Caff, caffeine; Ach, acetylcholine; Tg, thapsigargin.
Impaired Calcium Clearance by Dysfunctional Mitochondria
To test more directly the mechanism of the mitochondrial dysfunction-induced increase in the cytoplasmic [Ca2+], we measured the rate of mitochondrial Ca2+ uptake in suspensions of saponin-permeabilized cells with Fura2-FF/FA dye. The influence of ER on Ca2+ clearance was negated by pretreatment with thapsigargin. Dissipation of the [Ca2+]c rise caused by a Ca2+ bolus corroborates the mtCa2+ uptake. Figures 3A and 3C show the time course of a 30 μM CaCl2 pulse-induced mitochondrial Ca2+ uptake and subsequent FCCP (carbonyl cyanide 4-trifluoromethoxy phenylhydrazone) addition, which fully releases the stored mitochondrial Ca2+. The initial rate of Ca2+ clearance following the addition of CaCl2 in C2C12 and HCT116 cells is presented in Figures 3E and 3F. The [Ca2+]c rise was cleared faster in control cells than in cells with dysfunctional mitochondria (Figures 3A, 3C, 3E, and 3F). Control C2C12 cell mitochondria removed Ca2+ at 0.125 nM/s, whereas the clearance was nearly 30% slower in CcO4KD cells (Figure 3E). A similar decrease in Ca2+ clearance rate was also seen in HCT116 cells following mtDNA depletion (Figures 3C and 3F). The ΔΨm in both CcO4KD cells and mtDNA-depleted HCT116 cells was significantly lower than in the respective control cells with normal mtDNA contents or intact CcO complex (Figures 3B and 3D). Previously, we reported respiratory complex defects and reduced ΔΨm in both CcO4KD cells (Srinivasan et al., 2016) and mtDNA-depleted HCT116 cells (Chowdhury et al., 2017). The mitochondrial respiratory chain defects in mitochondrial-associated deceased patients show reduced ΔΨm along with reduced ATP synthesis, which in turn causes altered [Ca2+]mt uptake under physiological stimulations (Visch et al., 2006). In conjunction with previous findings (Chowdhury et al., 2017; Srinivasan et al., 2016; Visch et al., 2006), these results show that cells with mitochondrial dysfunction indeed have disrupted ΔΨm and a lower rate of mitochondrial Ca2+ uptake that may represent the immediate upstream event for MtRS.
Figure 3.
Impaired Mitochondrial Ca2+ Clearance in CcO4KD and mtDNA-Depleted Cells
Representative scans of [Ca2+]c clearance by saponin-permeabilized cells following a 30 μM CaCl2 spike in the presence of thapsigargin (2 μM). Recorded data were analyzed in PTI Felix and Felix GX software.
(A) Ca2+ clearance in control and CcO4KD C2C12 cells.
(B) Tetramethylrhodamine (TMRE) fluorescence in control and CcO4KD C2C12 cells.
(C) Ca2+ clearance in control and mtDNA-depleted HCT116 cells.
(D) TMRE fluorescence in control and mtDNA-depleted HCT116 cells.
(E) Rates of Ca2+ uptake in control and CcO4KD C2C12 cells.
(F) Rates of Ca2+ uptake by control and mtDNA-depleted HCT116 cells. Significance was calculated by one-way ANOVA with Tukey's multiple-comparison test, and data are presented as a mitochondrial dysfunctional group versus control. In all experiments, error bars represent standard deviations (∗p < 0.05, ∗∗p ≤ 0.01). Caff, caffeine; Ach, acetylcholine; FCCP, carbonyl cyanide 4-trifluoromethoxy phenylhydrazone; Tg, thapsigargin.
Activation of Cn in Response to Mitochondrial Dysfunction
In addition to altered agonist-induced Ca2+ release by cells with dysfunctional mitochondria (Figure 1), both CcO4KD C2C12 cells and mtDNA-depleted HCT116 cells also showed elevated steady-state [Ca2+]c (Figures 4A and 4C). It is seen that the basal Ca2+ level in CcO4KD cells increased to about 80 nM as opposed to 60 nM in control C2C12 cells (Figure 4A). Similarly, the steady-state Ca2+ level increased in mtDNA-depleted HCT116 cells to about 130 nM from about 60 nM in control cells (Figure 4C). Consistent with the basal Ca2+ increase, the Cn activity was increased nearly 3-fold in CcO4KD C2C12 cells and about 2.7-fold in mtDNA-depleted HCT116 cells (Figures 4B and 4D). This is consistent with our published reports (Srinivasan et al., 2016) that mRNA for the catalytic subunit of Cn (CnA) was increased by 2.5-fold in CcO4KD cells. These results show that in addition to altered agonist-induced Ca2+ release, cells with dysfunctional mitochondria also show markedly increased Cn activity.
Figure 4.
Elevated Cytosolic Ca2+ and Cn Activity in Response to Mitochondrial Dysfunction
(A and B) (A) Basal [Ca2+]c levels in control and CcO4KD C2C12 cells and (B) Cn activity in control and CcO4KD C2C12 cells.
(C and D) (C) Basal [Ca2+]c levels in control and mtDNA-depleted HCT116 cells, and (D) Cn activity in control and mtDNA-depleted HCT116 cells. The bar diagrams represent the mean ± SEM from three independent experiments. Significance was calculated by one-way ANOVA with Tukey's multiple-comparison test, and data are presented as a mitochondrial dysfunctional group versus control. In all experiments error bars represent standard deviations (∗p < 0.05, ∗∗p ≤ 0.01).
Altered Ca2+ Uniporter System Is Insufficient to Induce Retrograde Signaling
The mitochondrial uniporter complex (MCUC) is the major mitochondrial inner membrane system for the import of Ca2+ from the cytoplasm (Baughman et al., 2011; Bick et al., 2012; Mammucari et al., 2017; Mishra et al., 2017). To understand the reason for inefficient Ca2+ clearance associated with dysfunctional mitochondria in Figure 3, we assessed the levels of the three key components of the MCUC: MCU, MICU1, and EMRE (C22orf32). MCU is the main channel-forming protein for the transport of Ca2+; EMRE is a mandatory scaffold that is required for MCU channel conductance, whereas MICU1 is a regulatory protein that mediates Ca2+ gating (Kamer and Mootha, 2015) of MCU. As seen from Figures 5A and 5B, MCU mRNA levels were significantly reduced in mtDNA-depleted HCT116 cells, whereas there was no change in the MICU1 mRNA level. Surprisingly, the EMRE mRNA level was increased in mtDNA-depleted cells (Figure 5C). A somewhat similar pattern of expression was seen in CcO4KD C2C12 myoblasts (Figure 5D), although the level of MICU1 was also reduced significantly. Immunoblots in Figures 5E and 5F show that MCU protein level was decreased in mtDNA-depleted HCT116 cells, whereas the EMRE level was increased. The level of MICU1 was reduced marginally (Figure 5F). In CcO4KD C2C12 cells, both the MCU and MICU1 levels were significantly lower than in control cells in terms of both protein (Figure 5G) and mRNA levels (Figure 5D). However, as in HCT116 cells, the EMRE levels were higher than in control cells. These results suggest that altered uniporter abundance might contribute to the slower rate of Ca2+ clearing. The levels of succinate dehydrogenase (SDHA) used as a mitochondrial loading control did not change significantly.
Figure 5.
Altered Mitochondrial Uniporter System in mtDNA-Depleted HCT116 Cells and CcO IVi1 KD C2C12 Cells
(A–C) Levels of (A) MCU, (B) MICU1, and (C) EMRE mRNAs in control and mtDNA-depleted HCT116 cells.
(D) Quantitative mRNA analysis of MCU, MICU1 and EMRE in control and CcO4KD C2C12 cells.
(E and F) Immunoblot analysis of MCU, MICU1, and EMRE proteins from control and mtDNA-depleted HCT116. 30 μg proteins from whole-cell extracts were used in each case. Immunoblots were developed using respective primary (1:1,000 dilution) and 1:10,000 dilutions of secondary antibodies. The blots were imaged as described in the Transparent Methods. GAPDH was used as a loading control and SDHA as mitochondrial loading control.
(G) Relative protein levels of MCU, MICU1, and EMRE in control, CcO4KD, and mtDNA-depleted cells. GAPDH was used as a loading control and SDHA as mitochondrial loading control.
(H) Levels of MCU mRNA in control and stable MCU-specific shRNA-expressing (MCU-KD) cells.
(I, J, and K) The levels of (I) IGF1R, (J) Glut4, and (K) RyR (1,2, 3) mRNAs, respectively, in control and MCU-KD cells. Results represent the mean ± SEM from three separate experiments. Significance was calculated by one-way ANOVA with Tukey's multiple-comparison test, and data are presented as a mitochondrial dysfunctional group versus control. In all experiments error bars represent standard deviations (∗p < 0.05, ∗∗p ≤ 0.01).
To test if the reduced MCU mRNA played a direct role in inducing MtRs, we generated MCU KD cells by stably expressing shRNA for MCU mRNA in C2C12 cells (Figure 5H). We performed bioenergetic profile experiments with MCU KD and control cells to obtain respiratory parameters such as oxygen consumption rate (OCR), the percentage of oxygen consumption devoted to ATP production as well as the amount devoted to maintain the proton (H+) leak (ECAR), and the maximal respiratory rate under conditions of uncoupled respiration. All the OCR and ECAR measurements were performed at 37°C under air and were recorded simultaneously in an XF-24 analyzer platform. Figure S3A shows reduced basal as well as maximal respiration in MCU KD cells. Data were corrected by normalizing with total protein concentrations. We also calculated the ratio of ECAR/OCR as a measure of the relative magnitude of glycolysis versus oxidative phosphorylation. MCU KD cells showed elevated ECAR indicative of increased glycolysis and lactate production (Figure S3B). We tested transcriptional patterns of MtRS-specific marker genes such as insulin like growth factor 1 receptor (IGF1R), glucose transporter protein 4 (Glut4), and ryanodine receptor 1 (RyR1), which respond to mitochondrial respiratory stress signaling in different cells (Biswas et al., 1999, 2008b; Guha et al., 2007). Results in Figures 5H-5K show that all three markers of MtRS including mRNAs for IGF1R, Glut4 and RyR1 were downregulated in MCU KD cells. Thus, MCU mRNA KD and an associated reduction in Ca2+ clearance alone are not sufficient to induce MtRS.
The Role of RyR Ca2+ Channels in Increased [Ca2+]c and Activation of MtRS Marker Gene Expression
In compliance with increased caffeine-induced Ca2+ release in cells with mitochondrial dysfunction in Figures 1 and 2, RyR3 mRNA and proteins were increased in CcO4KD C2C12 cells (Figures 6A and 6B) and both RyR1 and RyR3 proteins were induced in mtDNA-depleted HCT116 cells (Figures 6C and 6D). Notably, IP3R mRNA was not induced in CcO4KD cells. This is consistent with our previous report showing no increase in IP3R protein in mtDNA-depleted C2C12 cells (Biswas et al., 1999). As the RyR channels are known to be leaky (Dirksen and Avila, 2002; Marx et al., 2000), we reasoned that increased RyR expression may be the reason for increased cytosolic Ca2+ levels. To test this possibility, we silenced all three RyR mRNAs using shRNA targeted to the common conserved region among all three genes. Figure 6E shows the levels of shRNA KD of RyR genes in control and mtDNA-depleted C2C12 cells. Immunoblots in Figure 6E show that the RyR protein levels were markedly lower in mtDNA-depleted cells stably transduced with shRNA expression vectors than in control cells and cells transduced with a scrambled shRNA expression vector (top panel). Levels of IGF1R (second panel from the top) and matured cathepsin L protein (second panel from the bottom) were also reduced in cells expressing RyR shRNA. Both these proteins are important markers of MtRS (Amuthan et al., 2001, 2002; Biswas et al., 1999; Guha et al., 2007). Control cells with normal mtDNA levels with or without expression of shRNA for RyR mRNAs do not express these genes (Figure 6E). These results confirm that increased RyR expression is essential for the initiation and/or propagation of MtRS.
Figure 6.
Role of RyR Ca2+ Channel Activation in Increased [Ca2+]c in Response to Mitochondrial Stress
(A) Real-Time PCR data on RyR and IP3R channel mRNA induction in CcO4KD C2C12 cells.
(B) Immunoblot data showing the induction of RyR protein level in control, mtDNA-depleted, and CcO4KD C2C12 cells. 50 μg protein was loaded in each lane, and GAPDH was used as the loading control.
(C) Immunoblot showing RyR1 and RyR3 induction in mtDNA-depleted HCT116 cells.
(D) The qPCR data showing induction of RyR mRNA in mtDNA-depleted HCT116 cells.
(E) Immunoblot analysis of proteins from control and mtDNA-depleted C2C12 cells expressing scrambled shRNA and two different RyR-specific shRNAs (named shRNA 1 or shRNA 2). Different molecular weight range areas of the same blot (∼500 kDa, ∼110 kDa, and about 40 kDa) were excised and probed with antibodies against RyR1, IGF1R, and cathepsin L proteins. GAPDH was used as a loading control.
(F and G) The agonist induced Ca2+ release in (F) mtDNA-depleted and (G) mtDNA-depleted/RyR KD cells. Note that caffeine failed to induce Ca2+ release in mtDNA-depleted/RyR KD cells. Traces show the average response of three identical runs (average ≤ 30 cells/run) following the addition of different agonists in each cell line. Recorded data were further analyzed by SpectralyzerLSMDeluxe version 2009092525 software to subtract the background fluorescence before calculate the F380/340 ratio and finally represented as nM.
(H) The steady-state [Ca2+]c levels in mtDNA-depleted and mtDNA-depleted/RyR KD cells. Caff, caffeine; Ach, acetylcholine; Tg, thapsigargin.
(I) Relative Cn activity in control, mtDNA-depleted C2C12 cells, and depleted cells expressing scrambled and specific shRNAs against all three RyR genes. Note that shRNA expression against RyR genes downregulated the activity. Mean ± SD was calculated from three independent experiments. Significance was calculated by one-way ANOVA with Tukey's multiple-comparison test, and data are presented as mitochondrial dysfunctional group versus control. In all experiments error bars represent standard deviations (∗p < 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001).
To ascertain the role of RyR channel activity in the induced expression of MtRS target genes, we measured agonist-induced Ca2+ release in RyR KD C2C12 cells. Figures 6F and 6G show the agonist-induced Ca2+ release patterns in mtDNA-depleted (Figure 6F) and mtDNA-depleted/RyR KD (Figure 6G) C2C12 cells. RyR KD eliminated caffeine-mediated Ca2+ release in mtDNA-depleted cells. Notably, the basal [Ca2+]c level was reduced to ∼38 nM in RyR KD cells from ∼90 nM in mtDNA-depleted cells (Figure 6H). Consistent with the reduced [Ca2+]c, Cn activity was also reduced to control cell levels in mtDNA-depleted/RyR KD cells (Figure 6I). These results together show that induced activation of the RyR channel is a critical factor in the activation of Cn and downstream signaling cascade.
To further ascertain the role of induced or activated RyR Ca2+ channel in inducing Cn activation and MtRS, which in turn induces cell proliferation and invasive behavior (Chowdhury et al., 2017; Srinivasan et al., 2016), we tested the effects of RyR channel KD in C2C12 cells in a scratch test (Figure S4) and the effects of RyR channel blocking with 50 μM ryanodine in C2C12 cells by Matrigel invasion (Figure 7A). Results show that KD of all three RyR mRNAs markedly reduced the migration of mtDNA-depleted C2C12 cells in a scratch test (Figure S4). This assay revealed that the original scratch area was reduced by about 20% in control cells, whereas in mtDNA-depleted cells it was reduced by about 83% of the area in 24 h. There was no change in scrambled shRNA-expressing cells when compared with mtDNA-depleted cells, whereas RyR mRNA KD cells showed a remarkable recovery close to the control cell level (Figures S4A and S4B). Similarly, mtDNA-depleted C2C12 cells showed nearly 2-fold higher Matrigel invasion, which was effectively reduced by treatment with 50 μM ryanodine, an RyR antagonist that showed a marked reduction in Matrigel invasion as shown in Figures 7A and 7B. We also used different concentrations of (5 and 10 μM) cell-permeable derivative of S107, which marginally reduced the invasion of cells through Matrigel. S107 is known to block the leaky intracellular calcium release by stabilizing FKBP12 and RyR associations. This treatment (Figure 7A, last two panels) significantly reduced the number of invasive cells compared with the mtDNA-depleted C2C12 cells. We further assessed the levels of FKBP12 association with RyR proteins by immunoprecipitation (Figure 7C). We found a marked increase in RyR protein in the immunoprecipitate and a considerably lower level of FKBP12 protein compared with that found in control cells. It has been reported that 1:1 stoichiometry of RyR and FKBP protein subunit, i.e., 4:4 is necessary for efficient regulation of tetrameric RyR channels (Brillantes et al., 1994; Meissner, 2010). In light of this, our results confirm that stress-induced RyR channels are leaky and responsible for increased [Ca2+]c, which is a critical factor in MtRS.
Figure 7.
Effects of RyR Channel Antagonists and Stabilizers on the Invasive Property of mtDNA-Depleted C2C12 Cells
Ryanodine, an antagonist of RyR channels, and S107, an effective stabilizer of RyR1 and FKBP association, were used.
(A) Top panel: Matrigel invasion of control C2C12 cells treated with or without 50 μM ryanodine for 16 h before cells were loaded in the chamber. Lower panel: In vitro Matrigel invasion of mtDNA-depleted C2C12 cells treated with or without 50 μM ryanodine or different concentrations of RyR-FKBP stabilizer S107. Scale bars, 100 μM.
(B) Quantitation of invading cells from three separate experiments. The significance was calculated by one-way ANOVA with Tukey's multiple-comparison test, and data are presented as the treatment group versus no-treatment control. In all experiments error bars represent standard deviations (∗p < 0.05, ∗∗∗∗p ≤ 0.001).
(C) Immunoblot analysis (n = 3) of FKBP proteins coimmunoprecipitated with RyR from control and mtDNA-depleted C2C12 cells. The total lysate (Input) and immunoprecipitated proteins were probed with antibody to RyR and FKBP 12. IB, immunoblotting; IP, immunoprecipitation.
Discussion
Multiple mechanisms of mitochondria to nucleus cross talk have been described to explain changes in nuclear gene expression in response to mitochondrial genetic or metabolic defects (Butow and Avadhani, 2004; Yang and Kim, 2019). These include Ca2+/Cn activation, mitochondrial unfolded protein response, mitochondrial reactive oxygen species-induced HIF1α or MAP kinase activation, and activation of microRNA (Butow and Avadhani, 2004; Carden et al., 2017; Guha and Avadhani, 2013). In previous studies, we showed that partial mtDNA depletion by chemical treatment of ethidium bromide or ddC caused a steady and sustained increase in the cytoplasmic Ca2+, which in turn caused activation of protein phosphatase, Cn (Amuthan et al., 2001; Biswas et al., 1999, 2005b). In some cells, Cn activation occurred at the transcription level by inducing the expression of Cn Aα mRNA, whereas in other cells, the activation was mostly post-translational through Ca2+/calmodulin binding without a significant increase in transcription (Klee et al., 1979). This signaling cascade induced the expression of >120 nuclear genes involved in an array of cellular processes including plasma membrane receptors, cell cycle regulators, cytoskeletal proteins, and proteins associated with mitochondrial oxidative metabolism (Biswas et al., 2005b). We also showed that in immortalized cells, MtRS induced changes in cell morphology and invasive behavior, typical of tumor cells (Biswas et al., 2005a; Srinivasan et al., 2016). In this study, we elucidated the mechanism of altered Ca2+ homeostasis in cells subjected to mitochondrial stress using partial mtDNA depletion and also CcO subunit IVi1 mRNA silencing (CcO4KD) as stress inducers in two different cell lines. Our results show that a combination of altered uniporter function and markedly increased RyR Ca2+ channel activity in response to mitochondrial stress together contribute to increased cytoplasmic Ca2+ levels.
Mitochondria play a critical role in the regulation of cellular Ca2+ homeostasis, mainly by acting as an important storage compartment (Bagur and Hajnoczky, 2017). The MCUC has been shown to be the main system for the transport of Ca2+ released from IP3 and other channels in response to agonist-induced activation (Taylor and Tovey, 2010). We examined the MCUC components, and MCU was consistently downregulated in both cell types in response to mitochondrial stress. This is consistent with impaired Ca2+ clearance by mitochondria from stressed cells. However, KD of MCU alone did not induce MtRS markers, suggesting the need for other factors in inducing MtRS.
In extension of our previous observations in mtDNA-depleted C2C12 cells and A549 lung carcinoma cells (Amuthan et al., 2001; Biswas et al., 1999), we observed marked variations in the activity of RyR channels versus IP3R channels in cells with dysfunctional mitochondria. In control cells, there were high levels of Ca2+ release in response to IP3 channel agonist acetylcholine, but no detectable Ca2+ release in response to RyR channel agonist caffeine. In CcO4KD cells and mtDNA-depleted HCT116 cells, there was a marked reduction in acetylcholine-invoked Ca2+ release, whereas caffeine invoked a 5- to 10-fold higher Ca2+ release, suggesting a dramatic change in the inducibility or activity of these two channels. The overall Ca2+ pool that is released from the ER in the control and CcO4KD cells appears to be similar as seen from the total Ca2+ release invoked by ATP. Although the precise reason for this change in response to mitochondrial dysfunction remains unclear, it is most likely related to changes in the regulation of channels and also in the steady-state protein levels. We observed a large increase in RyR channel protein level and a corresponding increase in mRNA levels in response to the mitochondrial membrane or DNA damage. Similar patterns of RyR mRNA and protein increase, as well as a large increase in caffeine-evoked Ca2+ release, are observed in response to mitochondrial respiratory and metabolic inhibitors, including FCCP, antimycin A, oligomycin, and hypoxia (Biswas et al., 1999, 2008a; Srinivasan and Avadhani, 2007). Our results show that increased expression and altered activation of RyR Ca2+ channels is a hallmark of mitochondrial dysfunction. Notably, IP3R-evoked Ca2+ release is markedly reduced in cells with mitochondrial stress.
The three isoforms of RyR receptor Ca2+ channels RyR1, RyR2, and RyR3 show nearly 70% homology in terms of amino acid sequences and are expressed in a cell- and tissue-specific manner. In C2C12 myocytes mostly RyR1 and RyR2 are expressed, whereas RyR3 is predominantly expressed in HCT116 colon cancer cells. RyR channel dysfunction or dysregulation has been implicated in many musculoskeletal disorders, and myocardial diseases associated with many mutations in RYR genes have been reported (Bellinger et al., 2009). RyR channels are tightly regulated by binding of tetrameric FK binding proteins 12.0 to the partially opened tetrameric receptor complex (Ahern et al., 1994). In the striated muscle cells, RyR channels are tightly regulated by binding to FKBP proteins in a stoichiometric ratio. The complex is destabilized by FKBP dissociation, implicating Ca2+ mishandling, which is associated with cardiac and skeletal myopathies (Bellinger et al., 2008; Chelu et al., 2004).
Protein kinase A (PKA)-mediated phosphorylation of channel proteins reduces the affinity of the tetrameric complex for binding to FKBP tetramers thus causing a dysregulated or “leaky” channel (Ahern et al., 1994; Dirksen and Avila, 2002; Ivarsson et al., 2019; Marx et al., 2000). Our results show that the steady-state levels of both FKBP 12.0 proteins are markedly reduced (Figure S5) in cells with mitochondrial dysfunction. Based on this, we propose that a combination of increased RyR channel proteins and reduction of regulatory FKBP subunits results in the assembly of leaky channels, which result in increased [Ca2+]c. The possible involvement of RyR Ca2+ channel function was further tested in two ways: first, RyR channel mRNA KD in mtDNA-depleted cells aborted the expression of MtRS markers including Cn, IGF1R, and cathepsin L. Furthermore, RyR mRNA KD also reduced the invasive potential of mtDNA-depleted C2C12 cells as tested by wound healing assay (Figure S4). In the second approach, the use of ryanodine, a specific antagonist of the RyR channel, essentially abrogated the increase in [Ca2+]c (data not shown), activation of Cn, and the invasive property of mtDNA-depleted C2C12 cells. We, therefore, provide direct and rigorous proof for the involvement of activated RyR Ca2+ channels in inducing MtRS.
Results presented here also show that mitochondrial Ca2+ clearance is severely curtailed in cells with mitochondrial dysfunction (mtDNA depletion or CcO subunit KD). It is noteworthy that dysfunctional mitochondria also showed lower levels of mitochondrial uniporter protein MCU, although other members of uniporter complex such as MICU1 and EMRE are affected in a variable, cell-specific manner. In this regard, cells with mitochondrial defect show impaired uniporter complex and altered Ca2+ clearance as shown by Mootha's group by shRNA-mediated KD of MCU1 uniporter protein (Bick et al., 2012; Kamer and Mootha, 2015). Taken together, the results of this study provide mechanistic details of MtRS with respect to altered Ca2+ release from ER and altered Ca2+ clearance from mitochondria as the basis of Cn activation at the point of initiation of MtRS.
Limitations of the Study
Although we present evidence for the upregulation of MtRS marker genes in both MCU1-depleted and MCU1-deleted mtDNA-depleted cells, a more comprehensive proteomic analysis and gene expression analyses would have been more informative. We plan to carry out these analyses in our future experiments. The FKBP binding to tetrameric RyR channel protein can be affected either by reduced steady-state levels of FKBP proteins or PKA-mediated phosphorylation of RyR channel proteins. Based on lower steady-state levels of FKBP, we suggested the former possibility. However, we cannot exclude the possibility of RyR subunit phosphorylation, which may also be the reason for the reduced affinity for the FKBP12, and this needs to be investigated. The Ca2+ leak or mishandling due to dysregulation of RyR channels has been well documented in cardiac and skeletal myopathies, as discussed earlier in the article. In this respect, it would be interesting to study the possible activation of MtRS and its role in these muscle disease models.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Narayan Avadhani (narayan@vet.upenn.edu).
Materials Availability
All unique/stable reagents generated in this study are available from the Lead Contact with a completed Materials Transfer Agreement.
Date and Code Availability
No custom code, software, or algorithm was used in this research.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank members of the Avadhani and Hajnoczky laboratories for useful discussions and suggestions. This work was supported by National Institutes of Health (NIH), United States grants CA-22762 and GM34883 and an endowment from the Harriet Ellison Woodward trust.
Author Contributions
N.G.A., G.H., and G.C. conceptualized the project and obtained research support; A.R.C., S.S., and G.C. carried out the experiments; A.R.C. and S.S. analyzed the data; N.G.A., S.S., and A.R.C. wrote the paper; N.G.A., A.R.C., G.H., and G.C. edited the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: August 21, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101370.
Supplemental Information
References
- Ahern G.P., Junankar P.R., Dulhunty A.F. Single channel activity of the ryanodine receptor calcium release channel is modulated by FK-506. FEBS Lett. 1994;352:369–374. doi: 10.1016/0014-5793(94)01001-3. [DOI] [PubMed] [Google Scholar]
- Amuthan G., Biswas G., Ananadatheerthavarada H.K., Vijayasarathy C., Shephard H.M., Avadhani N.G. Mitochondrial stress-induced calcium signaling, phenotypic changes and invasive behavior in human lung carcinoma A549 cells. Oncogene. 2002;21:7839–7849. doi: 10.1038/sj.onc.1205983. [DOI] [PubMed] [Google Scholar]
- Amuthan G., Biswas G., Zhang S.Y., Klein-Szanto A., Vijayasarathy C., Avadhani N.G. Mitochondria-to-nucleus stress signaling induces phenotypic changes, tumor progression and cell invasion. EMBO J. 2001;20:1910–1920. doi: 10.1093/emboj/20.8.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnould T., Vankoningsloo S., Renard P., Houbion A., Ninane N., Demazy C., Remacle J., Raes M. CREB activation induced by mitochondrial dysfunction is a new signaling pathway that impairs cell proliferation. EMBO J. 2002;21:53–63. doi: 10.1093/emboj/21.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagur R., Hajnoczky G. Intracellular Ca(2+) sensing: its role in calcium homeostasis and signaling. Mol. Cell. 2017;66:780–788. doi: 10.1016/j.molcel.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman J.M., Perocchi F., Girgis H.S., Plovanich M., Belcher-Timme C.A., Sancak Y., Bao X.R., Strittmatter L., Goldberger O., Bogorad R.L. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger A.M., Mongillo M., Marks A.R. Stressed out: the skeletal muscle ryanodine receptor as a target of stress. J. Clin. Invest. 2008;118:445–453. doi: 10.1172/JCI34006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger A.M., Reiken S., Carlson C., Mongillo M., Liu X., Rothman L., Matecki S., Lacampagne A., Marks A.R. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat. Med. 2009;15:325–330. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick A.G., Calvo S.E., Mootha V.K. Evolutionary diversity of the mitochondrial calcium uniporter. Science. 2012;336:886. doi: 10.1126/science.1214977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas G., Adebanjo O.A., Freedman B.D., Anandatheerthavarada H.K., Vijayasarathy C., Zaidi M., Kotlikoff M., Avadhani N.G. Retrograde Ca2+ signaling in C2C12 skeletal myocytes in response to mitochondrial genetic and metabolic stress: a novel mode of inter-organelle crosstalk. EMBO J. 1999;18:522–533. doi: 10.1093/emboj/18.3.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas G., Anandatheerthavarada H.K., Avadhani N.G. Mechanism of mitochondrial stress-induced resistance to apoptosis in mitochondrial DNA-depleted C2C12 myocytes. Cell Death Differ. 2005;12:266–278. doi: 10.1038/sj.cdd.4401553. [DOI] [PubMed] [Google Scholar]
- Biswas G., Guha M., Avadhani N.G. Mitochondria-to-nucleus stress signaling in mammalian cells: nature of nuclear gene targets, transcription regulation, and induced resistance to apoptosis. Gene. 2005;354:132–139. doi: 10.1016/j.gene.2005.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas G., Srinivasan S., Anandatheerthavarada H.K., Avadhani N.G. Dioxin-mediated tumor progression through activation of mitochondria-to-nucleus stress signaling. Proc. Natl. Acad. Sci. U S A. 2008;105:186–191. doi: 10.1073/pnas.0706183104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas G., Tang W., Sondheimer N., Guha M., Bansal S., Avadhani N.G. A distinctive physiological role for IkappaBbeta in the propagation of mitochondrial respiratory stress signaling. J. Biol. Chem. 2008;283:12586–12594. doi: 10.1074/jbc.M710481200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brillantes A.B., Ondrias K., Scott A., Kobrinsky E., Ondriasova E., Moschella M.C., Jayaraman T., Landers M., Ehrlich B.E., Marks A.R. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell. 1994;77:513–523. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Butow R.A., Avadhani N.G. Mitochondrial signaling: the retrograde response. Mol. Cell. 2004;14:1–15. doi: 10.1016/s1097-2765(04)00179-0. [DOI] [PubMed] [Google Scholar]
- Carden T., Singh B., Mooga V., Bajpai P., Singh K.K. Epigenetic modification of miR-663 controls mitochondria-to-nucleus retrograde signaling and tumor progression. J. Biol. Chem. 2017;292:20694–20706. doi: 10.1074/jbc.M117.797001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas C., Miller R.A., Smith I., Bui T., Molgo J., Muller M., Vais H., Cheung K.H., Yang J., Parker I. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelu M.G., Danila C.I., Gilman C.P., Hamilton S.L. Regulation of ryanodine receptors by FK506 binding proteins. Trends Cardiovasc. Med. 2004;14:227–234. doi: 10.1016/j.tcm.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Chowdhury A.R., Long A., Fuchs S.Y., Rustgi A., Avadhani N.G. Mitochondrial stress-induced p53 attenuates HIF-1alpha activity by physical association and enhanced ubiquitination. Oncogene. 2017;36:397–409. doi: 10.1038/onc.2016.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D.E. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Demaurex N., Nunes P. The role of STIM and ORAI proteins in phagocytic immune cells. Am. J. Physiol. Cell Physiol. 2016;310:C496–C508. doi: 10.1152/ajpcell.00360.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desideri E., Vegliante R., Ciriolo M.R. Mitochondrial dysfunctions in cancer: genetic defects and oncogenic signaling impinging on TCA cycle activity. Cancer Lett. 2015;356:217–223. doi: 10.1016/j.canlet.2014.02.023. [DOI] [PubMed] [Google Scholar]
- Dirksen R.T., Avila G. Altered ryanodine receptor function in central core disease: leaky or uncoupled Ca(2+) release channels? Trends Cardiovasc. Med. 2002;12:189–197. doi: 10.1016/s1050-1738(02)00163-9. [DOI] [PubMed] [Google Scholar]
- Duchen M.R. Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death. J. Physiol. 1999;516(Pt 1):1–17. doi: 10.1111/j.1469-7793.1999.001aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H., Shen L., Chen T., He J., Ding Z., Wei J., Qu J., Chen G., Lu J., Bai Y. Cancer type-specific modulation of mitochondrial haplogroups in breast, colorectal and thyroid cancer. BMC Cancer. 2010;10:421. doi: 10.1186/1471-2407-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C. ER-mitochondria communication. How privileged? Physiol. (Bethesda) 2007;22:261–268. doi: 10.1152/physiol.00017.2007. [DOI] [PubMed] [Google Scholar]
- Friedman J.R., Nunnari J. Mitochondrial form and function. Nature. 2014;505:335–343. doi: 10.1038/nature12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R.M., Glaves J.P., Huan T., Li L., Sykes B.D., Schultz M.C. Rewiring AMPK and mitochondrial retrograde signaling for metabolic control of aging and histone acetylation in respiratory-defective cells. Cell Rep. 2014;7:565–574. doi: 10.1016/j.celrep.2014.03.029. [DOI] [PubMed] [Google Scholar]
- Giorgi C., De Stefani D., Bononi A., Rizzuto R., Pinton P. Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int. J. Biochem. Cell Biol. 2009;41:1817–1827. doi: 10.1016/j.biocel.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffart S., Wiesner R.J. Regulation and co-ordination of nuclear gene expression during mitochondrial biogenesis. Exp. Physiol. 2003;88:33–40. doi: 10.1113/eph8802500. [DOI] [PubMed] [Google Scholar]
- Green D.R., Wang R. Calcium and energy: making the cake and eating it too? Cell. 2010;142:200–202. doi: 10.1016/j.cell.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Guha M., Avadhani N.G. Mitochondrial retrograde signaling at the crossroads of tumor bioenergetics, genetics and epigenetics. Mitochondrion. 2013;13:577–591. doi: 10.1016/j.mito.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha M., Pan H., Fang J.K., Avadhani N.G. Heterogeneous nuclear ribonucleoprotein A2 is a common transcriptional coactivator in the nuclear transcription response to mitochondrial respiratory stress. Mol. Biol. Cell. 2009;20:4107–4119. doi: 10.1091/mbc.E09-04-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha M., Srinivasan S., Biswas G., Avadhani N.G. Activation of a novel calcineurin-mediated insulin-like growth factor-1 receptor pathway, altered metabolism, and tumor cell invasion in cells subjected to mitochondrial respiratory stress. J. Biol. Chem. 2007;282:14536–14546. doi: 10.1074/jbc.M611693200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha M., Srinivasan S., Johnson F.B., Ruthel G., Guja K., Garcia-Diaz M., Kaufman B.A., Glineburg M.R., Fang J., Nakagawa H. hnRNPA2 mediated acetylation reduces telomere length in response to mitochondrial dysfunction. PLoS One. 2018;13:e0206897. doi: 10.1371/journal.pone.0206897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Wu J., Dressman D.C., Iacobuzio-Donahue C., Markowitz S.D., Velculescu V.E., Diaz L.A., Jr., Kinzler K.W., Vogelstein B., Papadopoulos N. Heteroplasmic mitochondrial DNA mutations in normal and tumour cells. Nature. 2010;464:610–614. doi: 10.1038/nature08802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K., Takenaga K., Akimoto M., Koshikawa N., Yamaguchi A., Imanishi H., Nakada K., Honma Y., Hayashi J. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- Ivarsson N., Mattsson C.M., Cheng A.J., Bruton J.D., Ekblom B., Lanner J.T., Westerblad H. SR Ca(2+) leak in skeletal muscle fibers acts as an intracellular signal to increase fatigue resistance. J. Gen. Physiol. 2019;151:567–577. doi: 10.1085/jgp.201812152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamer K.J., Mootha V.K. The molecular era of the mitochondrial calcium uniporter. Nat. Rev. Mol. Cell Biol. 2015;16:545–553. doi: 10.1038/nrm4039. [DOI] [PubMed] [Google Scholar]
- Klee C.B., Crouch T.H., Krinks M.H. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc. Natl. Acad. Sci. U S A. 1979;76:6270–6273. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammucari C., Gherardi G., Rizzuto R. Structure, activity regulation, and role of the mitochondrial calcium uniporter in health and disease. Front. Oncol. 2017;7:139. doi: 10.3389/fonc.2017.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx S.O., Reiken S., Hisamatsu Y., Jayaraman T., Burkhoff D., Rosemblit N., Marks A.R. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- Meissner G. Regulation of ryanodine receptor ion channels through posttranslational modifications. Curr. Top. Membr. 2010;66:91–113. doi: 10.1016/S1063-5823(10)66005-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra J., Jhun B.S., Hurst S., J O.U., Csordas G., Sheu S.S. The mitochondrial Ca(2+) uniporter: structure, function, and pharmacology. Handb Exp. Pharmacol. 2017;240:129–156. doi: 10.1007/164_2017_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah I.N., Stambuk R.A., Casida J.E. Ca2+-activated ryanodine binding: mechanisms of sensitivity and intensity modulation by Mg2+, caffeine, and adenine nucleotides. Mol. Pharmacol. 1987;31:232–238. [PubMed] [Google Scholar]
- Rizzuto R., De Stefani D., Raffaello A., Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- Srinivasan S., Avadhani N.G. Hypoxia-mediated mitochondrial stress in RAW264.7 cells induces osteoclast-like TRAP-positive cells. Ann. N. Y Acad. Sci. 2007;1117:51–61. doi: 10.1196/annals.1402.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S., Guha M., Dong D.W., Whelan K.A., Ruthel G., Uchikado Y., Natsugoe S., Nakagawa H., Avadhani N.G. Disruption of cytochrome c oxidase function induces the Warburg effect and metabolic reprogramming. Oncogene. 2016;35:1585–1595. doi: 10.1038/onc.2015.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait S.W., Green D.R. Mitochondria and cell signalling. J. Cell Sci. 2012;125:807–815. doi: 10.1242/jcs.099234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C.W., Tovey S.C. IP(3) receptors: toward understanding their activation. Cold Spring Harb Perspect. Biol. 2010;2:a004010. doi: 10.1101/cshperspect.a004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visch H.J., Koopman W.J., Zeegers D., van Emst-de Vries S.E., van Kuppeveld F.J., van den Heuvel L.W., Smeitink J.A., Willems P.H. Ca2+-mobilizing agonists increase mitochondrial ATP production to accelerate cytosolic Ca2+ removal: aberrations in human complex I deficiency. Am. J. Physiol. Cell Physiol. 2006;291:C308–C316. doi: 10.1152/ajpcell.00561.2005. [DOI] [PubMed] [Google Scholar]
- Yang D., Kim J. Mitochondrial retrograde signalling and metabolic alterations in the tumour microenvironment. Cells. 2019;8:275. doi: 10.3390/cells8030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong C.Q.Y., Tang B.L. A mitochondrial encoded messenger at the nucleus. Cells. 2018;7:105. doi: 10.3390/cells7080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.