Synopsis
Ikaros zinc finger 1 (IKZF1 or Ikaros) is a hematopoietic zinc finger DNA-binding transcription factor that acts as a critical regulator of lymphocyte and myeloid differentiation. Somatic mutations in IKAROS are frequent and a sign of poor prognosis in B-cell progenitor acute lymphoblastic leukemia (ALL). More recently, loss-of-function germline heterozygous mutations in IKAROS have been described as causative of two distinct primary immunodeficiency (PID)/inborn error of immunity (IEI) diseases: mutations acting by haploinsufficiency present with a common variable immune deficiency (CVID)-like phenotype mainly characterized by increased susceptibility to infections, while mutations acting in a dominant negative fashion present with a combined immunodeficiency (CID) phenotype with high prevalence of Pneumocystis jirovecii pneumonia. Pathophysiology, clinical and laboratory manifestations of IKAROS-associated diseases in PID patients are reviewed here.
Keywords: IKAROS, immunodeficiency, antibody deficiency, common-variable immunodeficiency, combined immunodeficiency, haploinsufficiency, dominant negative, gene dosage
Introduction
Ikaros zinc finger 1 (IKZF1 or Ikaros) is a hematopoietic zinc finger DNA-binding transcription factor that acts as a critical regulator at multiple stages of lymphocyte differentiation, from early multipotent precursors to mature effector cells1–3. Regulatory functions in myeloid differentiation have also been ascribed for Ikaros4. Knowledge of Ikaros involvement in human disease was initially limited to its tumor suppressive activity, as somatic mutations in IKAROS are frequently observed in B-cell progenitor acute lymphoblastic leukemia (ALL), conferring a poor prognosis5,6. More recently, a large screening of presumed sporadic cases of B-ALL in childhood identified germline heterozygous mutations in IKAROS in 0.9% of patients7.
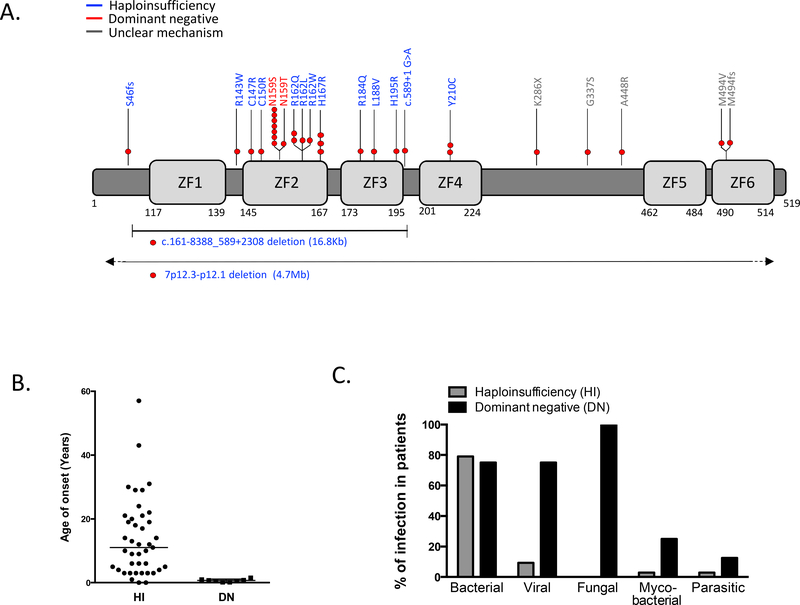
In 2012, Goldman et al8 described a germline heterozygous allelic variant in IKAROS (p.Y210C) leading to immunodeficiency in an infant born with congenital pancytopenia and selective lymphopenia characterized by absence of B and natural killer cells with normal numbers of T cells. In 2016, Kuehn et al9, studying six probands with common variable immunodeficiency (CVID) and decreased numbers of B cells, identified six additional germline heterozygous mutations in IKZF1 in 29 individuals from six families. Several reports followed these two initial descriptions and, to date, 22 germline heterozygous allelic variants in IKAROS have been reported in the setting of primary immunodeficiency (PID)8–19 (Figure 1A).
Figure 1.
IKAROS mutations and clinical features.
A. Schematic of the human IKAROS protein and the location of mutations. The numbers represent the amino acid location based on IKZF1 transcript variant 1 (NM_006060). Different molecular mechanisms of IKAROS mutations are shown in blue (haploinsufficiency, HI) and red (dominant negative, DN) color. Red circles indicate the number of families carrying the same mutation.
B. Age of onset of clinical manifestations in patients with IKAROS mutations. Horizontal bars represent median values.
C. Infectious agents among symptomatic cases of IKAROS-associated primary immunodeficiency by mechanism of action.
Ikaros belongs to the so-called zinc finger (ZF) family of proteins that also include Helios/IKZF2, Aiolos/IKZF3, Eos/IKZF4 and Pegasus/IKZF5. All of them share a similar structure characterized by the presence of two sets of highly conserved C2H2-type ZF motifs. The ZFs located N-terminal to these proteins mediate binding to specific DNA sequences located throughout the genome. The second set of ZFs are C-terminal located and enable the protein-to-protein Ikaros family members interaction as homodimers (e.g., IKZF1/IKZF1) or heterodimers (e.g. IKZF1/IKZF2). Several Ikaros family members undergo alternative splicing, e.g., IKZF1 has at least 17 isoforms, adding to the function complexity of these transcription factors (reviewed in ref4,20 ).
The main isoform of IKAROS (isoform 1) consists of an N-terminal DNA-binding domain (DBD) comprising 4 ZF (ZF1–4) and a C-terminal dimerization domain (DD), where two more zinc fingers are located (ZF5–6). Reported IKZF1 variants associated with immunodeficiency primarily cluster around the DBD, mostly affecting critical to DNA binding ZF2–3 (Figure 1A). Variants located between the DBD and DD (i.e., p.K286X, p.G337S, and p.A448R), or directly affecting the DD (p.M494V and p. M494fsX86) have also been reported in CVID patients17. Among these variants, p.K286X and p.M494fsX86 are highly likely to primarily affect IKAROS dimerization and secondarily impact on DNA binding or pericentromeric targeting. However, because the pathophysiology of the above-mentioned variants has not yet been completely elucidated, in this review we focused our attention on variants with a proven defect on Ikaros-dependent functions (DNA binding and pericentromeric targeting), or directly affecting protein expression.
IKAROS protein expression in lymphocytes was not affected by single amino acid mutants but was reduced to approximately half in patients carrying large deletions or a frameshift mutation leading to an early stop codon (i.e., 4.7 Mb deletion and S46AfsX14)9,12. However, regardless of normal protein expression in patients with missense mutations, in-vitro functional assessment revealed that all mutants behaved in a loss-of-function manner, demonstrated by their inability to bind target DNA sequences and to localize to pericentromeric heterochromatin (PC-HC) 8–13,15–17.
Mutants are categorized in two groups depending on: a) how they impact the DNA binding to PC-HC, and b) their effect over the wild type (WT) allele: heterozygous haploinsufficiency (HI) mutations do not bind target DNA on PC-HC per se, and do not affect the function of the WT allele; on the other hand, heterozygous dominant negative (DN) mutations do not bind target DNA on PC-HC per se either, but they negatively affect the WT allele function. Fifteen unique HI variants have been described8–10,12,14–18. Only two missense variants affecting the same amino acid (N159) have been described to act in a DN manner in humans10,11,13,19. HI and DN variants result in distinct clinical and immunological phenotypes that will be reviewed in this article.
Haploinsufficiency allelic variants in IKZF1
Clinical presentation
Germline heterozygous allelic variants in IKZF1 acting through a DNA binding to PC-HC HI mechanism have been reported in 59 individuals (30 male) from 19 families 8–10,12,14–18 (Figure 1A). Clinical manifestations have been described in 43 patients (22 male) with a median age of onset of 11 years (ranging from less than one to 57 years of age) (Figure 1B). Almost one fourth of IKZF1 HI mutants were identified in asymptomatic carriers (27.1% 16/59) suggesting incomplete penetrance of the disease. However, given the wide range of age of initial presentation observed in symptomatic cases, later onset of symptoms cannot be ruled out.
Among the symptomatic patients, bacterial infections were the most frequent clinical manifestation, reported in 79.1% (34/43) of the cases (Figure 1C). The respiratory tract was the mainly affected system, with both upper and lower respiratory tract involvement. Otitis was reported in 23.3% of cases. Streptococcus pneumoniae was the most commonly identified pathogen. Patients experienced recurrent episodes of sinopulmonary infections with variable severity. Bronchiectasis have not been described, but chest computed tomography data were scarce in the reports8–10,12,14–18.
Sepsis (Streptococcus pneumoniae, Haemophilus influenzae, and Enterococcus gallinarum) was reported in five patients (two of whom following splenectomy). Four patients, including the two splenectomized, had a history of bacterial meningitis due to Streptococcus pneumoniae and Enterococcus gallinarum infections. Less commonly affected sites for bacterial infections were the skin (3 patients) and the hip joint (1 patient). Chronic or recurrent diarrhea have been described in 4 patients (Clostridium difficile and Blastocystis hominis identified in one patient and Giardia lamblia in another). Mycobacterial infection has been reported only in one patient who presented with skin nodules positive for Mycobacterium chelonae after receiving chemotherapy for leukemia treatment. Because of myelosuppression, this patient required a bone marrow transplant (BMT) to control his infection9.
Viral infections were described in four patients (9.3% of symptomatic cases), generally not severe (herpes simplex virus causing recurrent herpes labialis in three, human papillomavirus causing warts in hands/feet in two, and mumps meningitis in one)9,10,18. Fungal infections have not been described in IKZF1 haploinsufficiency (Figure 1C). Autoimmunity/immune dysregulation was the second most common clinical manifestation described, affecting 39.5% (17/43) of the symptomatic cases. Gender bias was not observed among patients presenting with autoimmunity. Autoimmune conditions started predominantly in childhood, with a median age of clinical presentation of 11 years. Idiopathic thrombocytopenic purpura (ITP) was the most frequently reported (6 patients), followed by systemic lupus erythematosus (SLE - 3 patients)9,10,12,15–18. Other autoimmune manifestations reported are described in table 1.
Table 1.
Autoimmune/ immune dysregulation manifestations in IKAROS haploinsufficiency
| Frequency (% of total mutation carriers) | |
|---|---|
| Immune thrombocytopenia | 6 (10) |
| Arthritis | 4 (7) |
| Reactive arthritis (2) | |
| Juvenile idiopathic arthritis (1) | |
| Seronegative arthritis (1) | |
| Systemic lupus erythematosus | 3 (5) |
| Antiphospholipid syndrome | 3 (5) |
| Myasthenia gravis | 1 (2) |
| IgA vasculitis | 1 (2) |
| Urticaria | 1 (2) |
| Hashimoto thyroiditis | 1 (2) |
| Psoriasis-like disease | 1 (2) |
Two patients developed B-cell acute lymphoblastic leukemia (B-ALL) at 3 and 5 years of age, respectively9. A third patient was diagnosed with a solid pseudopapillary pancreas tumor at the age of 2214.
Interestingly, a distinct presentation of premature birth and nonautoimmune congenital pancytopenia has been documented in two unrelated patients sharing the same IKZF1 variant (p.Y210C)8,10. To this date, this variant is the sole reported in ZF4 of IKAROS. The variant was also detected in an asymptomatic brother of one of the probands. This genotype-phenotype correlation suggests unique functions of ZF4 in hematopoiesis, but more cases are needed to further understand the connection.
Laboratory phenotype
Decreased serum IgG levels and reduced B-cell counts in peripheral blood were the predominant findings, present in 87.8% (36/41) and 82.9% (34/41) of the symptomatic cases. Among asymptomatic mutation carriers whose laboratorial information was available, 63.6% (7/11) showed a reduction in serum IgG levels, and 42% (5/12) had low B-cell counts, suggesting that immunological penetrance was observed without clinical manifestation. Longitudinal data from patients who started follow-up at an early age documented a progressive decline in peripheral B-cell counts as well as serum immunoglobulin levels 8–10,12,14–18.
Serum levels of other immunoglobulin isotypes were also reduced (low IgM in 75.7% 28/37, low IgA in 83.3% 30/36). Antibody responses to protein as well as polysaccharide vaccines were reduced or absent in most patients tested8–10,12,14–18.
In contrast to the B-cell compartment, total T cell numbers were normal or high in 55.6% (20/36) and 38.9% (14/36) of the patients, respectively. Interestingly, there was a trend towards increased CD8+ T cells, resulting in an inverted CD4:CD8 ratio in 63.4% (26/41) of the patients. This was statistically more frequent among patients with protein positive missense mutations than among individual with IKZF1 full gene deletion8. Memory T cell differentiation, including Treg and Tfh cells, and proliferation studies, when performed were mostly normal; however, high and low values have also been reported8–10,12,14–18. Natural killer cell numbers were reduced in 30% (6/20) of patients
Bone marrow studies from a subset of IKZF1 HI patients revealed a profound decrease in B-cell lineage precursors or hematogones. Frequencies of hematopoietic stem cells and common lymphoid progenitors were variable. The maturation profile within the B-cell lineage also varied, with some patients exhibiting normal development while others showed a partial-to-almost-complete block at different stages. A very early arrest on B-cell development defined by a marked decrease in pro-B cells (based on the co-expression of CD34 and CD19) and even earlier precursors (pre-pro B cells expressing surface CD34 and cytoplasmic TdT in the absence of CD19), was characteristically seen in some of these patients 9,10,12 Moreover, Kuehn et al found normal numbers of CD138+ plasma cells in the bone marrow aspirates from 2 IKZF1 HI patients. The authors hypothesized that the presence of plasma cells in the context of a B-cell maturation arrest may reflect the progressive nature of the disease and may also explain the remnant production of functional antibodies earlier in life9.
Treatments and outcomes
Most patients were treated with broad spectrum antimicrobial therapy only during acute infectious episodes. Immunoglobulin replacement, either intravenously or subcutaneously, was the main prophylactic intervention, administered to 56% (19/34) of patients with a history of recurrent or severe bacterial infections. Consistent with a predominantly humoral deficiency, most patients experienced a reduction in frequency and severity of infections after initiation of immunoglobulin substitution8–10,12,14–18.
Immune thrombocytopenia was managed with multiple therapeutic approaches including corticosteroids, high dose intravenous immunoglobulin, anti-IgD therapy, and rituximab. One patient required splenectomy. Patients with antiphospholipid syndrome received anticoagulation and steroids. Immunosuppressant therapies for SLE included methylprednisolone pulse, hydroxychloroquine, and mycophenolate mofetil9,10,12,15–18.
Hematopoietic stem cell transplantation (HSCT) was indicated for the two patients with nonautoimmune congenital pancytopenia, but only preformed in one. The transplanted patient passed away on day 40 post-HSCT due to multiorgan failure, whereas the second patient experienced a spontaneous hematologic recovery after one month of birth and did not require HSCT. Despite the overall improvement, his low B cells and hypogammaglobulinemia persisted8,10.
Out of the 59 IKZF1 HI mutation carriers reported, three are deceased: two pediatric and one adult patient8,9. In addition to the above-mentioned infant with pancytopenia who died shortly after HSCT, the second pediatric patient died at 5 years of age because of relapsed acute lymphoblastic leukemia. The third patient died due to pneumonia at the age of 74 years.
Dominant negative allelic variants in IKZF1
Clinical presentation
Germline dominant allelic variants in IKZF1 acting through a DN mechanism have been reported in 8 unrelated patients (1 male). No asymptomatic cases have been described. Initial clinical symptoms presented early in life in all patients, ranging from 2 months to 1.5 years of age10,11,13,19 (Figure 1B).
The clinical phenotype was characterized by recurrent and severe infections, caused by a broad range of pathogens10,11,13,19. All patients presented at least one episode of Pneumocystis jirovecii pneumonia (PJP) within the first two years of life. Two patients experienced multiple episodes of PJP. Recurrent bacterial sinopulmonary infections were reported in 6/8 patients (Figure 1C). One patient had a history of Streptococcus pneumoniae meningitis. Three patients reported recurrent otitis media and one patient skin abscesses. Two cases of mycobacterial infections have been described. One patient had pulmonary Mycobacterium avium complex and a second patient developed Mycobacterium avium intracellulare lymphadenitis after HSCT. Recurrent or severe viral infections were reported in two thirds of patients (6/8), including respiratory syncytial virus (RSV), adenovirus, influenza, HSV, and molluscum contagiosum. Three patients reported chickenpox without complications. In addition to PJP, both superficial (recurrent oral candidiasis) and invasive (pulmonary aspergillosis, Candida parapsillosis fungemia) fungal infections have been described. One patient had Cryptosporidium species cholangitis that led to cirrhosis.
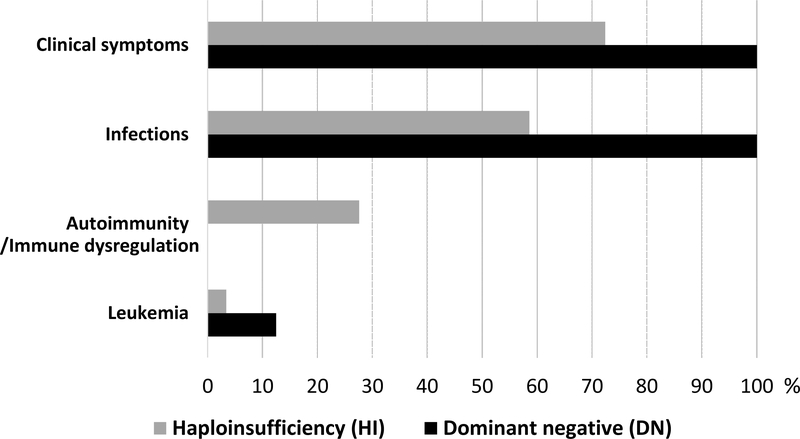
One patient developed T-cell acute lymphoblastic leukemia at the age of 13 years11. Autoimmunity, immune dysregulation or allergic manifestations have not been observed among the reported cases. (Figure 2).
Figure 2.
Clinical presentation of IKAROS-associated primary immunodeficiency by mechanism of action. Percentage indicates penetrance of clinical disease in IKAROS mutation carriers.
Laboratory phenotype
All patients presented with profound hypogammaglobulinemia involving all major isotypes and nearly absence of peripheral blood B cells10,11,13,19. Bone marrow studies performed in two DN patients found, similarly to what was described for the HI patients8, an early B cell developmental arrest with absence of early B-cell precursors. However, in opposition to the HI patients, plasma cells were virtually absent, suggesting an earlier in life and more severe B-cell maturation arrest in patients carrying IKZF1 DN mutations13.
T-cell counts were variable among patients. Phenotypically, a large predominance of naïve T cells presenting features of recent thymic emigrants (i.e., CD31+) and Th0 phenotype (intracellular IL-2 was detected, but IL-4, IFNg, FoxP3 or IL-17, were almost absent), along with a reduction in memory/effector T cells was characteristically observed in all but one patient. Moreover, in vitro, patients’ T cells failed to acquire a memory phenotype despite appropriate stimulation. TCR driven proliferation was normal in response to strong stimuli, but markedly impaired upon low dose stimulation and this defect could not be rescued by addition of IL-2. NK cell counts were normal in most patients (71%)10,11,13,19.
In addition to adaptive immunity defects, disease progression showed to include myeloid abnormalities, such as eosinopenia and neutropenia, that were distinctively detected in most patients. In vitro studies also demonstrated monocyte dysfunction13. Interestingly, the lack of B cells, antibodies, IgE, memory/effector T cells and eosinophils may play a protective role towards developing allergic and autoimmune/immune dysregulated manifestations in these patients.
Treatments and outcomes
Because of the earlier and more severe nature of infections seen in this group of patients, all were treated with aggressive antimicrobials regimens and immunoglobulin replacement since very early in life 10,11,13,19 One patient developed bronchiectasis despite maintenance of appropriate IgG trough levels10. Furthermore, due to the life-threatening characteristic of this allelic variant, several patients underwent HSCT based on their clinical/immunophenotypic presentation, being their genetic diagnosis only retrospectively confirmed.
Kellner et al19 reported the detailed HSCT outcomes in four patients with DN IKZF1 mutations. One of the patients reported in this series had previously rejected an unconditioned haploidentical CD34+ HSCT at 10 months of age recommended due to combined immunodeficiency. She underwent a second transplant after being diagnosed with T-ALL at the age of 13 years. All four patients achieved greater than 99% donor chimerism. Infectious complications after transplant included Epstein-Barr virus (EBV) reactivation in one, cytomegalovirus (CMV) viremia in two, and mycobacterial lymphadenitis in one patient. Grade II, skin stage 3 and gastrointestinal tract stage 1, acute graft-versus-host disease (GVHD) was observed in only one patient who responded well to standard treatment. One patient with prior history of Cryptosporidium cholangitis progressed to liver failure and died approximately one year after HSCT. At most recent follow-up reported (ranging from 1 to 7 years) the three surviving patients had no signs of chronic GVHD, were off immunoglobulin replacement and antimicrobial prophylaxis. Neutropenia was noticed in one patient and mild thrombocytopenia in a second one. Although this is small cohort, HSCT appears as a valid curative option for IKZF1 DN disease, an early onset, severe, progressive immunodeficiency with T, B and myeloid involvement, and increased susceptibility to hematologic malignancies.
Conclusions
As a crucial regulator of hematopoiesis, it is of no surprise that IKAROS-associated diseases go beyond cancer predisposition. At least two main categories of primary immunodeficiencies are caused by autosomal dominant germline mutations in IKZF1. Heterozygous IKAROS variants acting by HI and impacting on DNA binding to PC-HC result in a predominantly antibody deficiency characterized by a CVID-like phenotype of recurrent sinopulmonary infections, hypogammaglobulinemia and defective vaccine responses with low peripheral B-cell counts and increased risk of autoimmunity and cancer. On the other hand, defects dependent on heterozygous DN mutations affecting DNA binding to PC-HC result in an early onset combined immunodeficiency with recurrent and severe bacterial, viral, and fungal infections and a particular susceptibility to pneumocystis pneumonia. In the latter scenario, the findings of profoundly low B-cell count and predominance of naïve T-cells serve as diagnostic cues.
The differences in immunologic involvement and clinical phenotypes between HI and DN allelic variants affecting DNA binding to PC-HC, suggest an IKZF1 gene dosage and type of mutation effect. Patients with HI mutations due to a single gene full deletion seemed to have a less extended immunologic lineage impact than those with single gene missense mutations. While patients with single gene full deletions make ~50% of WT/WT IKZF1 homodimers (all produced by their remaining WT allele) and show an almost exclusive B-cell impact, patients carrying single gene missense mutations form ~25% of WT/WT homodimers (being the rest, ~50% WT/Mutant, and ~25% Mutant/Mutant) and present with a B-cell plus a CD8+ T-cell and dendritic cell involvement9,21. Moreover, patients carrying the more “aggressive” DN mutations, not only display a deleterious effect on B-cell development, but also on T cells (arrested at a naïve/Th0 stage with virtually no Th1/Th2/Th17/Treg commitment, and defective TCR signaling), and myeloid cells (neutropenia, eosinopenia, and monocyte dysfunction)13. In other words, and based on HI and DN patients’ clinical immune phenotypes, B cells appear to be the most and myeloid cells (in general) the least sensitive lineages to IKAROS gene dosage 9,13,21,22.
So far, both somatic as well as germline IKZF1 genetic defects have been described to be associated with different clinical phenotypes impacting on the hematopoietic compartment. A combination of malignant lymphoproliferative diseases (e.g., leukemias), central cytopenias (e.g., B-cell lymphopenia), autoimmunity/immune dysregulation (e.g., ITP and SLE), and, immunodeficiency manifestations characterized by increased infectious disease susceptibility, all with variable degrees of expressivity, have been associated with those mutations. However, while in this review we addressed the pathophysiology, clinical and laboratory manifestations, as well as penetrance and expressivity of genetic variants affecting IKAROS’ N-terminal ZFs directly involved in DNA binding to PC-HC, it is very likely that this description only represents a partial view of the evolving picture of IKAROS-associated diseases. Defects affecting the C-terminal ZFs directly involved in protein dimerization have not been described to date, neither mutations behaving as gain-of-function or displaying neomorphic activities. Although this hypothesis could sound as far-fetching, a scenario depicting multiple allelic variants and immune phenotypes associated with a single gene, has been already described for other transcription factors as in signal transducer and activator of transcription (STAT) 1 and STAT323–25. Unless these yet-to-be described IKZF1 changes are intrinsically incompatible with life (not suggested by the animal models representing some of these defects), it is possible that such “experiments of nature”26 will be diagnosed in the future. In order for that to happen, unbiased genetic diagnostic approaches to evaluate patients with PID/inborn errors of immunity (IEI) must be pursued, in opposition to a clinical manifestations-based candidate gene diagnosis strategy, that already proved us wrong multiple times. Furthermore, testing all symptomatic and asymptomatic relatives to the index cases in a systematic way should be pursued as the proper way of determining the spectrum, penetrance and expressivity of these diseases.
Key Points.
Heterozygous germline mutations in IKAROS cause immunodeficiency through at least two different mechanisms
Haploinsufficiency mutations cause a CVID-like phenotype with a B-cell deficiency, infections and also increased risk of autoimmunity/immune dysregulation and B-ALL; penetrance does not seem to be complete
Dominant negative mutations cause a fully penetrant early-onset CID with T, B and myeloid cells defects, and increased susceptibility to pneumocystis pneumonia and T-ALL
Acknowledgement
This work was supported by the Intramural Research Program, National Institutes of Health Clinical Center and National Institute of Allergy and Infectious Diseases. The content of this article does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Georgopoulos K, Moore DD, Derfler B. Ikaros, an early lymphoid-specific transcription factor and a putative mediator for T cell commitment. Science. 1992;258(5083):808–812. [DOI] [PubMed] [Google Scholar]
- 2.Georgopoulos K, Bigby M, Wang JH, et al. The Ikaros gene is required for the development of all lymphoid lineages. Cell. 1994;79(1):143–156. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida T, Georgopoulos K. Ikaros fingers on lymphocyte differentiation. Int J Hematol. 2014;100(3):220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francis OL, Payne JL, Su RJ, Payne KJ. Regulator of myeloid differentiation and function: The secret life of Ikaros. World J Biol Chem. 2011;2(6):119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kastner P, Dupuis A, Gaub MP, Herbrecht R, Lutz P, Chan S. Function of Ikaros as a tumor suppressor in B cell acute lymphoblastic leukemia. Am J Blood Res. 2013;3(1):1–13. [PMC free article] [PubMed] [Google Scholar]
- 6.Mullighan CG, Su X, Zhang J, et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009;360(5):470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Churchman ML, Qian M, Te Kronnie G, et al. Germline Genetic IKZF1 Variation and Predisposition to Childhood Acute Lymphoblastic Leukemia. Cancer Cell. 2018;33(5):937–948.e938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldman FD, Gurel Z, Al-Zubeidi D, et al. Congenital pancytopenia and absence of B lymphocytes in a neonate with a mutation in the Ikaros gene. Pediatr Blood Cancer. 2012;58(4):591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuehn HS, Boisson B, Cunningham-Rundles C, et al. Loss of B Cells in Patients with Heterozygous Mutations in IKAROS. N Engl J Med. 2016;374(11):1032–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoshino A, Okada S, Yoshida K, et al. Abnormal hematopoiesis and autoimmunity in human subjects with germline IKZF1 mutations. J Allergy Clin Immunol. 2017;140(1):223–231. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida N, Sakaguchi H, Muramatsu H, et al. Germline IKAROS mutation associated with primary immunodeficiency that progressed to T-cell acute lymphoblastic leukemia. Leukemia. 2017;31(5):1221–1223. [DOI] [PubMed] [Google Scholar]
- 12.Bogaert DJ, Kuehn HS, Bonroy C, et al. A novel IKAROS haploinsufficiency kindred with unexpectedly late and variable B-cell maturation defects. J Allergy Clin Immunol. 2018;141(1):432–435.e437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boutboul D, Kuehn HS, Van de Wyngaert Z, et al. Dominant-negative IKZF1 mutations cause a T, B, and myeloid cell combined immunodeficiency. J Clin Invest. 2018;128(7):3071–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen QY, Wang XC, Wang WJ, Zhou QH, Liu DR, Wang Y. B-cell Deficiency: A De Novo IKZF1 Patient and Review of the Literature. J Investig Allergol Clin Immunol. 2018;28(1):53–56. [DOI] [PubMed] [Google Scholar]
- 15.Sriaroon P, Chang Y, Ujhazi B, et al. Familial Immune Thrombocytopenia Associated With a Novel Variant in IKZF1. FrontPediatr. 2019;7:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Nieuwenhove E, Garcia-Perez JE, Helsen C, et al. A kindred with mutant IKAROS and autoimmunity. J Allergy Clin Immunol. 2018;142(2):699–702.e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eskandarian Z, Fliegauf M, Bulashevska A, et al. Assessing the Functional Relevance of Variants in the IKAROS Family Zinc Finger Protein 1 (IKZF1) in a Cohort of Patients with Primary Immunodeficiency. Front Immunol. 2019;10:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dieudonné Y, Guffroy A, Vollmer O, Carapito R, Korganow AS. IKZF1 Loss-of-Function Variant Causes Autoimmunity and Severe Familial Antiphospholipid Syndrome. J Clin Immunol. 2019;39(4):353–357. [DOI] [PubMed] [Google Scholar]
- 19.Kellner ES, Krupski C, Kuehn HS, et al. Allogeneic hematopoietic stem cell transplant outcomes for patients with dominant negative IKZF1/IKAROS mutations. J Allergy Clin Immunol. 2019;144(1):339–342. [DOI] [PubMed] [Google Scholar]
- 20.John LB, Ward AC. The Ikaros gene family: transcriptional regulators of hematopoiesis and immunity. Mol Immunol. 2011;48(9–10):1272–1278. [DOI] [PubMed] [Google Scholar]
- 21.Cytlak U, Resteu A, Bogaert D, et al. Ikaros family zinc finger 1 regulates dendritic cell development and function in humans. Nat Commun. 2018;9(1):1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdulhay N, Fiorini C, Kumanovics A, et al. Normal hematologic parameters and fetal hemoglobin silencing with heterozygous IKZF1 mutations. Blood. 2016;128(16):2100–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olbrich P, Freeman AF. STAT1 and STAT3 mutations: important lessons for clinical immunologists. Expert Rev Clin Immunol. 2018;14(12):1029–1041. [DOI] [PubMed] [Google Scholar]
- 24.Jhamnani RD, Rosenzweig SD. An update on gain-of-function mutations in primary immunodeficiency diseases. Curr Opin Allergy Clin Immunol. 2017;17(6):391–397. [DOI] [PubMed] [Google Scholar]
- 25.Shahmarvand N, Nagy A, Shahryari J, Ohgami RS. Mutations in the signal transducer and activator of transcription family of genes in cancer. CancerSci. 2018;109(4):926–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Good RA, Zak SJ. Disturbances in gamma globulin synthesis as experiments of nature. Pediatrics. 1956;18(1):109–149.. [PubMed] [Google Scholar]