Abstract
Background
Egypt is the nation most affected by hepatitis C virus (HCV) infection, following an epidemic of historic proportions. We aimed to characterize the epidemic’s historical evolution and to delineate the role of parenteral antischistosomal therapy (PAT) campaigns in transmission.
Methods
A mathematical model was constructed and analysed in order to understand HCV-transmission dynamics. The model was fitted to Egypt’s Demographic and Health Survey data and to a systematic database of HCV-prevalence data.
Results
The incidence rate peaked in 1966 at 15.7 infections per 1000 person-years—a period of time that coincides with the PAT campaigns—and rapidly declined thereafter, beginning the mid-1990s. The annual number of new infections peaked in 1993 at 581 200 (with rapid demographic growth), leading to a high-incidence-cohort effect, and declined to 67 800 by 2018. The number of individuals ever infected (1950–2018) was 16.4 million, with HCV prevalence peaking in 1979. The number of individuals ever exposed to PAT was 8.3 million; however, of these individuals, 7.3 million were alive in 1980 and only 3.5 million alive in 2018. The number of individuals ever infected due to PAT exposure was 963 900, with 850 200 individuals alive in 1980 and only 389 800 alive in 2018. The proportion of PAT-attributed prevalent infections peaked at 19.9% in 1972, declining to 5.5% by 2018.
Conclusions
PAT campaigns played an important role in HCV transmission, yet explain only 6% of infections—they appear to be a manifestation, rather than a cause, of the epidemic. A possible driver of the epidemic could be the mass expansion of inadequate-quality healthcare during PAT campaigns and subsequent decades. Despite a historic toll, the epidemic has been rapidly diminishing since the mid-1990s.
Keywords: Egypt, Middle East and North Africa, hepatitis C virus, parenteral antischistosomal therapy, mathematical model, prevalence, incidence
Key Messages
Egypt is the nation most affected by hepatitis C virus (HCV) infection. Key drivers of this epidemic, particularly the historical role of parenteral antischistosomal therapy (PAT) campaigns, remain poorly understood.
Using an analytical mathematical modelling approach, we provided a systematic characterization of the level and trend of the epidemic’s historical evolution and a detailed quantitative assessment of the historical role of PAT exposure in HCV transmission.
The results affirm that Egypt has endured an HCV epidemic of historic proportions, although PAT campaigns explain only 6% of the cumulative number of infections that have occurred in Egypt from 1950 until the present.
These findings have implications towards understanding the determinants of generalized HCV epidemics, several of which are observed globally, but which remain poorly understood and are subject to much research interest.
Introduction
Viral hepatitis is the seventh leading cause of mortality, exceeding that of HIV, tuberculosis and malaria.1 Half of all viral hepatitis-related deaths are caused by hepatitis C virus (HCV) infection,1 whose sequelae include hepatitis, fibrosis, cirrhosis and liver cancer.1–3 HCV antibody (Ab) prevalence is estimated to be ∼1% for the global population,4 in stark contrast to Egypt, which is estimated to have a prevalence of 10%5–8—possibly the highest prevalence worldwide.8–10
The era of mass campaigns of parenteral antischistosomal therapy (PAT) is believed to be the main driver of this epidemic.5,11 Millions of people received intravenous injections of tartar emetic as treatment for schistosomiasis between the 1950s and early 1980s, particularly in rural areas.5,7,8 An oral drug, praziquantel, was subsequently introduced in the 1980s, replacing the standard of care.5 Based on indirect evidence and risk-factor analyses of multiple cross-sectional surveys,5,6,8,11–33 it is believed that reuse of glass syringes and lax infection-control practices during these campaigns created a large reservoir of HCV infection in the population.5,8,10,34 Whereas the current infection levels in Egypt are well characterized with quality data,5–9,35–37 the historical role of PAT in the epidemic remains poorly characterized.6–8
Against this background, we aimed to provide a detailed quantitative assessment of the historical role of PAT exposure in HCV transmission in Egypt. In addition to estimating the proportion of incident and prevalent infections strictly attributed to PAT exposure, we estimated the past levels and trends of HCV Ab prevalence, incidence rate, annual number of new infections, PAT exposure and HCV Ab prevalence among those who were exposed to PAT. Accordingly, we provided a comprehensive characterization of the world’s most distinguished HCV epidemic and delineated the role of PAT campaigns in this epidemic.
A major strength of this study is that it is anchored on nationally representative and population-based HCV and PAT-exposure data, and an exhaustive database of HCV Ab-prevalence data. This study used prevalence measures recently extracted through a systematic review of HCV infection in Egypt.6 The present study also accounted for the intricate and rapidly evolving demography of the Egyptian population over the past few decades.
Methods
Mathematical model
A dynamical mathematical model was constructed by extending and adapting earlier models9,38 to describe HCV transmission in the population of Egypt. The model explicitly incorporated HCV acquisition through PAT exposure. The population that has not been exposed to PAT could acquire HCV infection only through modes of exposure other than PAT exposure. Meanwhile, the population that has been exposed to PAT could acquire HCV infection either through PAT exposure or through other modes of exposure (model equations in the Supplementary data are available at IJE online). A descriptive diagram of the model is shown in Supplementary Figure 1, available as Supplementary data at IJE online.
The model structured the population by age group, status of infection, stage of infection and infection risk of exposure. Infection natural history consisted of three stages including primary acute infection, secondary acute infection and chronic infection.9,38,39 Egypt’s population was disaggregated into 13 age groups based on available HCV Ab-prevalence data: 0–4, 5–9, 10–14, 15–19, 20–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59 and >60 years old.
To represent the variability in the infection risk of exposure, five risk groups (other than PAT exposure) were modelled in the population. The mixing between the various risk groups and age groups was dictated by mixing matrices. The force of infection was determined by the iatrogenic contact rate, infection-transmission probability per contact, risk-group mixing and age-group mixing. HCV treatment scale-up was not incorporated in the model, as the modelling was focused on the historical evolution of the epidemic before treatment onset. The impact of HCV treatment on the epidemic in Egypt has already been assessed in our previous study.9
A detailed description of the model is shown in Supplementary Section 1, available as Supplementary data at IJE online. Further details on this type of modelling approach can be found in earlier publications.9,38
Epidemiologic measures
Different epidemiologic measures were used to describe the epidemic and its trends, with a focus on the role of PAT exposure in the epidemic. These measures are listed in Table 1 along with their definitions.
Table 1.
Epidemiologic measures used to characterize the HCV epidemic and its trends, with a focus on the role of PAT exposure in the HCV epidemic of Egypt
| Measure | Definition |
|---|---|
| HCV antibody (Ab) prevalence | Proportion of a given population who are positive for HCV Ab, i.e. proportion of the population who have ever been infected with HCV |
| Annual number of new HCV infections (also known as HCV incidence) | Number of new HCV infections in a given population over the duration of a year |
| HCV-incidence rate (also known as force of infection or hazard rate of infection) | Number of new HCV infections per person-time of the at-risk population, i.e. HCV incidence divided by the population size of the at-risk susceptible population |
| Population proportion of PAT exposure | Proportion of the population who have ever been exposed to PAT |
| Proportion of prevalent infections strictly attributed to PAT | Number of living individuals who have ever been infected by HCV due to PAT exposure divided by the total number of living individuals who have ever been infected by HCV regardless of the mode of transmission |
| Proportion of new HCV infections strictly attributed to PAT | HCV incidence due to PAT exposure divided by the total HCV incidence regardless of the mode of transmission |
HCV, hepatitis C virus; PAT, parenteral antischistosomal therapy; Ab, antibody.
Data sources and model fitting
Current data for HCV transmission and natural history were used to parameterize the model (Supplementary Table 1, available as Supplementary data at IJE online). Historical demographics (1950–2018) were extracted from the United Nations Population Division database.40 HCV and PAT-exposure input epidemiological data were estimated from the 2008 and 2015 Egypt Demographic and Health Surveys (EDHS).10,34 These data include the age-specific HCV Ab prevalence, population proportion of PAT exposure, HCV Ab prevalence among those exposed to PAT and the proportion of individuals exposed to PAT among those who were HCV Ab-positive.
The time series of HCV Ab prevalence was generated using 259 systematically extracted Ab-prevalence data points obtained through a systematic review of HCV infection in Egypt.6 These data were measured on different populations over 1990–2016, with the populations categorized as general populations, populations at intermediate risk, high-risk clinical populations, special clinical populations and populations with liver-related conditions.6
The prevalence measures (in those other than the general population) may not be representative of the general population and thus were used to determine only the HCV Ab-prevalence trend (not prevalence level). Specifically, these measures were converted into a corresponding prevalence trend by multiplying each measure in each specific population category by a factor (labelled as the ‘anchoring factor’). The temporal variation of HCV Ab prevalence in the population was determined by fitting the anchored prevalence datapoints over 1990–2016, thereby determining the anchoring factors, as well as fitting the 2008 and 2015 EDHS data10,34 (Supplementary Figure 5, available as Supplementary data at IJE online). The fitting of the EDHS data was set at 100% relative weight, since these are the best-quality nationally representative probability-based data in Egypt, whereas the fitting to the anchored prevalence datapoints was set at 10% relative weight.
The model was fitted to data by utilizing a generalized Lognormal-Gaussian function for the effective rate of infectious contacts (Supplementary Figure 2, available as Supplementary data at IJE online) and using a nonlinear least-square fitting method implemented in MATLAB®41 using the Nelder–Mead simplex algorithm.42 The formula for the rate of exposure to PAT can be found in Supplementary Section 1, available as Supplementary data at IJE online.
Further details on data sources and model fitting are shown in Supplementary Section 2, available as Supplementary data at IJE online.
Uncertainty analysis and sensitivity analyses
A multivariable uncertainty analysis was implemented to derive the uncertainty ranges of the predicted outcomes. A total of 1000 runs of the model were conducted utilizing Latin Hypercube sampling from a multidimensional distribution of the model’s structural parameters, with 25% uncertainty around each parameter point estimate. The means and associated 95% uncertainty intervals (UIs) were reported for the key indicators that characterize the role of PAT exposure in the HCV epidemic. Further details on the uncertainty analysis are shown in Supplementary Section 2, available as Supplementary data at IJE online.
We conducted a sensitivity analysis to assess the impact of under-reporting or over-reporting of PAT exposure by adjusting the self-reported PAT-exposure data by ±50%. We also conducted a sensitivity analysis to assess the impact of HCV disease-related mortality on the model predictions. This was done by incorporating the relative risk of HCV disease-related mortality stratified by age in the model based on the mortality data of a prospective study from Egypt.43
Results
The model generated robust fits to the various data sources. Supplementary Figure 3, available as Supplementary data at IJE online, shows the fit to Egypt’s demographics. Supplementary Figure 4, available as Supplementary data at IJE online, shows the fit to the different HCV and PAT-exposure indicators as extracted from the 2008 and 2015 EDHS data.10,34Supplementary Figure 5, available as Supplementary data at IJE online, shows the anchored fit to the HCV Ab-prevalence data of the systematic review.6
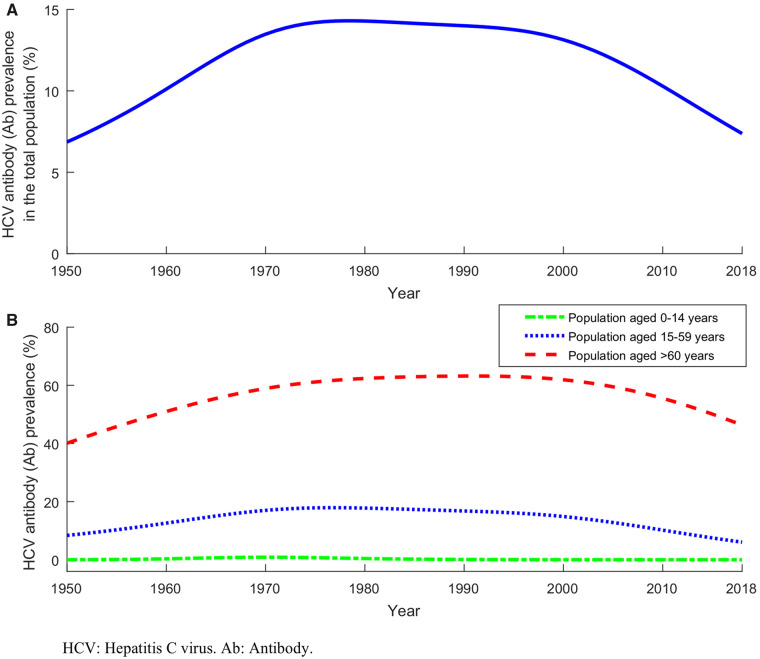
Figure 1 reports model predictions for the trend in HCV Ab prevalence in the total population of Egypt including all age groups (Figure 1A) and trends in prevalence in children (0–14 years), adults (15–59 years) and older adults (>60 years) (Figure 1B). HCV prevalence was rising for several decades and reached its peak in 1979, but was projected to decline slowly thereafter. HCV prevalence by age group was similar to that in the total population, but the peak shifted to later years for the older age groups. The earliest peak was in 1970 for children and the latest in 1991 for older adults.
Figure 1.
Historical trend of HCV antibody (Ab) prevalence in Egypt. (A) Estimated HCV Ab prevalence in the total population between 1950 and 2018. (B) Estimated HCV Ab prevalence in children (0–14 years), adults (15–59 years) and older adults (>60 years) between 1950 and 2018.
The HCV-incidence rate in the total population and the annual number of new HCV infections (HCV incidence) can be seen in Figure 2A and B, respectively. The incidence rate peaked in 1966 at 15.7 infections per 1000 person-years and rapidly declined thereafter, starting in mid-1990. The incidence was estimated at 8.0 per 1000 person-years in 2000, but at only 0.7 per 1000 person-years in 2018. Meanwhile, with the rapid demographic growth in Egypt (Supplementary Figure 3, available as Supplementary data at IJE online), the annual number of new infections peaked much later in 1993 at 581 200 and declined thereafter down to 491 200 in 2000 and 67 800 in 2018. The cumulative number of individuals who have ever been infected by HCV since 1950 was 16.4 million by 2018, but the number of these individuals alive in 1980 was 4.7 million, 8.6 million in 2000 and 7.1 million in 2018 (Table 2).
Figure 2.
Historical trend of HCV incidence in Egypt. (A) Estimated HCV-incidence rate in the total population of Egypt between 1950 and 2018. (B) Estimated annual number of new HCV infections in the total population of Egypt between 1950 and 2018.
Table 2.
Key predicted indicators relating to PAT exposure and HCV infection in Egypt
| Indicator | 1950 (95% UI) | 1960 (95% UI) | 1970 (95% UI) | 1980 (95% UI) | 1990 (95% UI) | 2000 (95% UI) | 2010 (95% UI) | 2018 (95% UI) |
|---|---|---|---|---|---|---|---|---|
| Number of individuals who have ever been exposed to PAT (millions) | 0.043 (0.042–0.045) | 1.39 (1.36–1.43) | 6.04 (5.91–6.17) | 8.18 (8.01–8.36) | 8.32 (8.15–8.50) | 8.32 (8.15–8.50) | 8.32 (8.15–8.50) | 8.32 (8.15–8.50) |
| Number of individuals who have ever been exposed to PAT and are currently alive (millions) | 0.038 (0.037–0.039) | 1.29 (1.26–1.32) | 5.65 (5.53–5.78) | 7.29 (7.14–7.45) | 6.61 (6.47–6.75) | 5.61 (5.49–5.73) | 4.48 (4.38–4.58) | 3.54(3.47–3.62) |
| Number of individuals who have ever been infected by HCV since 1950 (millions) | – | 1.34 (0.68–2.64) | 3.77 (2.38–5.96) | 6.34 (4.36–9.22) | 9.61 (7.55–12.22) | 13.54 (11.94–15.35) | 15.82 (14.30–17.52) | 16.38 (14.83–18.09) |
| Number of individuals who have ever been infected by HCV since 1950 and are currently alive (millions) | – | 1.09 (0.54–2.22) | 3.01 (1.88–4.83) | 4.73 (3.23–6.92) | 6.62 (5.31–8.25) | 8.62 (7.88–9.44) | 8.55 (7.83–9.32) | 7.08 (6.52–7.68) |
| Number of individuals who have ever been infected by HCV strictly due to PAT exposure (thousands) | 4.6 (4.0–5.4) | 150.1 (129.1–176.4) | 687.4 (586.8–805.2) | 947.9 (808.9–1110.6) | 963.8 (822.5–1129.3) | 963.9 (822.6–1129.4) | 963.9 (822.6–1129.4) | 963.9 (822.6–1129.4) |
| Number of individuals who have ever been infected by HCV strictly due to PAT exposure and are currently alive (thousands) | 4.1 (3.6–4.8) | 140.9 (120.6–164.6) | 646.4 (553.2–755.2) | 850.2 (729.5–990.8) | 767.9 (660.8–892.5) | 642.0 (553.6–744.6) | 499.8 (431.3–579.2) | 389.8 (336.6–451.5) |
| Number of individuals who have ever been infected by HCV, and also have ever been exposed to PAT, and are currently alive (millions) | 0.006 (0.005–0.007) | 0.23 (0.17–0.31) | 1.22 (0.93–1.59) | 1.88 (1.47–2.40) | 2.16 (1.84–2.52) | 2.27 (2.15–2.39) | 1.91 (1.83–2.00) | 1.48 (1.41–1.53) |
PAT, parenteral antischistosomal therapy; HCV, hepatitis C virus; UI, uncertainty interval.
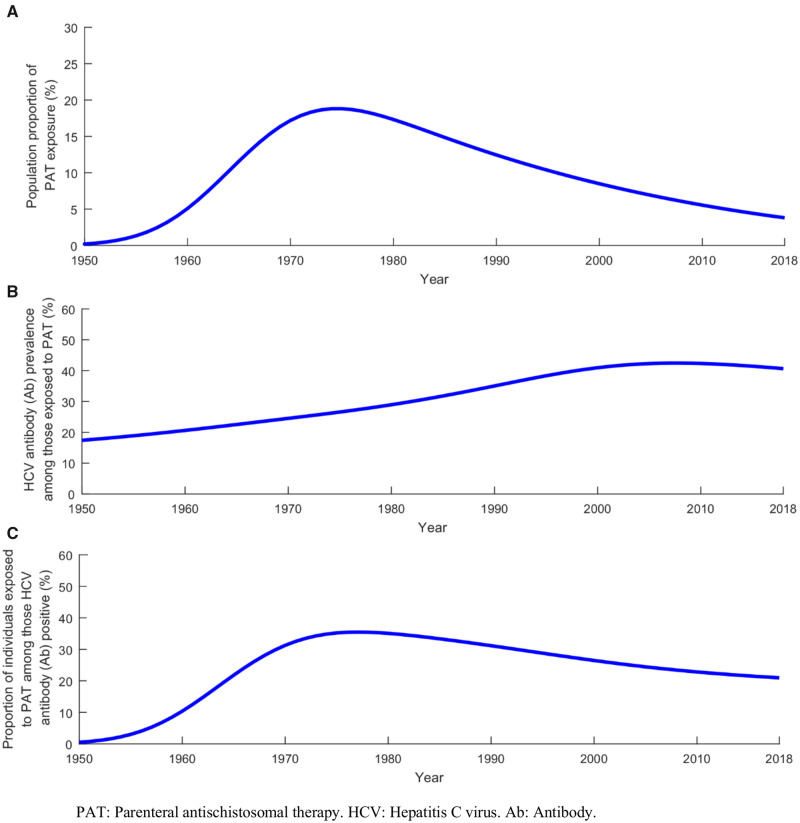
Table 2 and Figures 3 and 4 report key indicators that characterize the historical role of PAT exposure in HCV transmission. The population proportion of PAT exposure was increasing with the mass campaigns and estimated at 0.2% in 1950, 5.1% in 1960 and 17.2% in 1970 (Figure 3A). It reached a peak of 18.8% in 1974 and declined thereafter to 8.5% by 2000 and 3.8% by 2018 (Figure 3A). The cumulative number of individuals who have ever been exposed to PAT was 8.3 million by 2018, but the number of them who were alive was 7.3 million in 1980, 5.6 million in 2000 and only 3.5 million in 2018 (Table 2).
Figure 3.
Temporal evolution of PAT exposure and the HCV epidemic in Egypt. (A) Projected population proportion of PAT exposure in the total population between 1950 and 2018. (B) Projected HCV antibody (Ab) prevalence in those exposed to PAT between 1950 and 2018. (C) Projected proportion of individuals exposed to PAT among those who were HCV Ab-positive between 1950 and 2018.
Figure 4.
Role of PAT exposure in the Egypt HCV epidemic. (A) Estimated proportion of the prevalent HCV infections that were strictly attributed to PAT exposure between 1950 and 2018. (B) Estimated proportion of the new (incident) HCV infections that were strictly attributed to PAT exposure between 1950 and 2018. (C) Estimated age distribution of the number of living individuals who had acquired HCV strictly through PAT exposure by 2018.
HCV Ab prevalence in those exposed to PAT peaked in 2008 at 42.4% and declined slowly thereafter to 40.6% in 2018 (Figure 3B). The number of HCV-prevalent infections (regardless of whether attributed to PAT or not) among those who have ever been exposed to PAT and alive was estimated at 1.9 million in 1980, 2.3 million in 2000 and 1.5 million in 2018 (Table 2). The proportion of individuals exposed to PAT among the total HCV Ab-positive population was growing and reached a peak of 35.5% in 1977, but declined thereafter to 26.5% by 2000 and 21.0% by 2018 (Figure 3C).
The proportion of prevalent infections strictly attributed to PAT exposure was increasing and reached a peak of 19.9% in 1972, but declined thereafter to 7.4% by 2000 and 5.5% by 2018 (Figure 4A). The cumulative number of individuals who have ever been infected strictly due to PAT exposure was 963 900 by 2018, but the number of them who were alive was 850 200 in 1980, 642 000 in 2000 and only 389 800 in 2018 (Table 2).
The proportion of new (incident) HCV infections strictly attributed to PAT exposure peaked in 1965 at 21.4%, declined thereafter and eventually vanished with the end of PAT campaigns in the early 1980s (Figure 4B). In 2018, all HCV infections attributed to PAT exposure were among those >35 years of age (Figure 4C). Notably, the majority of them, totalling 200 280, were among those >60 years of age—highlighting the advanced ageing of the cohort that acquired HCV during PAT campaigns.
The multivariable uncertainty analysis supported the validity of our results and findings for the role of PAT campaigns in the HCV epidemic (Table 2, Figure 4 and Supplementary Figure 6, available as Supplementary data at IJE online). Even with large variations in input parameters, the role of PAT exposure remained qualitatively invariable.
Supplementary Figures 7 and 8, available as Supplementary data at IJE online, show the results of the sensitivity analyses for the role of PAT campaigns in the HCV epidemic. The analyses affirmed our conclusion that PAT exposure explains only a minority of HCV infections in Egypt.
Discussion
Egypt has endured an HCV epidemic of historic proportions,5–8 possibly representing the world's largest iatrogenic transmission of blood-borne pathogens known to date.5 Using an analytical approach anchored on extensive quality data for HCV and PAT exposure, we aimed to characterize the historical evolution of this epidemic and to delineate the role of PAT campaigns. We found that PAT exposure played an important role in transmission at the time of the campaigns, between the 1950s and early 1980s. However, cumulatively up to the present, PAT exposure has explained only 5.9% of the cumulative number of 16.4 million infections that have occurred in Egypt since 1950 (Table 2). Notably, among those who are HCV Ab-positive today, only 5.5% were infected through PAT exposure, the majority of whom are already >60 years of age. These findings demonstrate that, despite the role of PAT, the vast majority of infections have been acquired through other modes of transmission, explaining the observed weak spatial association between HCV prevalence and PAT exposure seen in the spatial scan statistical analyses.7
Strikingly, even during the peak of the PAT campaigns, <25% of the incidence could be explained by PAT exposure (Figure 4B). Even for those exposed to PAT in earlier times but alive after the year 2000, most infections were acquired through other modes of transmission, sometimes during the several decades of their lifetime. These findings, following multiple studies implicating healthcare exposures (other than PAT) in HCV acquisition,6,8 demonstrate that PAT campaigns were not strictly the cause of the epidemic, as much as a ‘symptom’ of the less-than-optimal quality of healthcare and limited awareness of blood-borne infection control, at the time of the campaigns and in subsequent decades. The reuse of syringes and lax sterilization practices may have been common well beyond PAT campaigns and up to recent times.6,8,44
With all the evidence on HCV epidemiology6–8 and infection control44 being Egyptian in context, it seems most consistent and logical to hypothesize that the cause of the epidemic is the mass expansion of healthcare services and infrastructure in Egypt, which accelerated during the PAT campaigns and subsequent decades, but whose implementation did not adequately adhere to the best practices of infection control. Much of the transmission may have occurred through formal and informal healthcare procedures,6,8 resulting in a series of clustered micro-epidemics. These micro-epidemics represent large and small outbreaks of different intensities, as opposed to one large epidemic.7
Demography is perhaps the most underappreciated factor in understanding the epidemic. Egypt has been witnessing a rapid growth in population size over the decades, with the population increasing 3-fold since 1965,40 coinciding with the height of the PAT campaigns and associated HCV incidence. The mortality rate was also high at this time, but has declined steadily in recent decades, leading to major increases in life expectancy.40 Accordingly, most of those who acquired HCV through PAT exposure are no longer alive today (Table 2). Even though the HCV-incidence rate declined substantially after the peak of the PAT era (Figure 2A), it remained considerable at a time when the population was increasing rapidly (along with its life expectancy), leading to a large number of incident infections and subsequently prevalent infections (Figure 2B). Most infected individuals alive today acquired their infection after the PAT campaigns were phased out in the early 1980s. This further demonstrates that PAT exposure is just one driver of incidence, but most incident infections appear to be related to broader healthcare practices.
The modelled epidemic history highlights several notable findings. Whereas the HCV-incidence rate (i.e. the likelihood of infection for a given person) was highest during the peak of the PAT era in the 1960s (Figure 2A), the incidence (annual number of new infections) was the highest more than two decades later in around 1990 (Figure 2B)—the decline in the incidence rate following the PAT era was more than compensated for by the rapid population growth from the 1970s to the 1990s. Since 1993, both the incidence rate and the incidence have been declining very rapidly (Figure 2), likely due to blood screening, improved injection safety and infection control following the identification of the virus in 198945,46 and discovery of the epidemic in Egypt in the early 1990s.47,48
Another notable finding is that there are two ‘cohort effects’ in the Egypt epidemic, best seen in the two incidence peaks in Figure 2B. The first cohort is the well-known and characterized ‘PAT-era cohort’,5 whereas the second is that of the rapid population growth and high-incidence era between the 1970s and the 1990s. This latter cohort, labelled here as the ‘high-incidence cohort’, constitutes the most infected people alive today in Egypt.
There are limitations in this study. A sophisticated dynamical modelling approach was used to capture transmission dynamics that was refined and perfected over a series of applications,9,38,49 although model projections are contingent on the quality and representativeness of the input data. PAT-exposure data were extracted from the EDHS databases.10,34 Whereas these surveys are of high quality and are nationally representative, PAT exposure was measured through self-reporting and thus could be prone to reporting or recall bias. Nevertheless, the conducted sensitivity analysis affirmed our findings even in the presence of a large reporting bias in the PAT-exposure data (Supplementary Figure 7, available as Supplementary data at IJE online).
Whereas a large database of systematically extracted HCV Ab-prevalence data was used to fit the model (practically every available measure in the literature6), fitting the model to the era before the discovery of the epidemic hinged on the availability of age-specific prevalence data to capture historical exposure over time. Therefore, the uncertainty in the estimates increases as the projections go further back in time, with less age-cohort data becoming available. Despite this wide uncertainty in the earlier years, the uncertainty analysis supported our predictions that PAT exposure contributed to only a minority of infections (Supplementary Figure 6, available as Supplementary data at IJE online).
Disease-related mortality was not incorporated in the model, but the relative risk of mortality with infection is relatively too small to appreciably affect the projections.9,43,50 Even if it did, it would have further lowered the estimates for the role of PAT exposure in the epidemic. Indeed, this was the case, as demonstrated by the conducted sensitivity analysis incorporating HCV-disease-related mortality (Supplementary Figure 8, available as Supplementary data at IJE online).
Despite these limitations, a major strength of this study is that it provided a comprehensive characterization of the epidemic’s historical evolution that is anchored on an extensive and quality foundation of epidemiologic evidence and incorporated the intricate demography of the Egyptian population. Even by factoring in the uncertainty in the modelled projections (Figure 4), the conclusions on the role of PAT exposure were invariable.
To sum up, even though PAT campaigns played an important role in HCV transmission in Egypt, they explain only 6% of the infections that occurred since 1950. Most infections have been acquired through other modes of transmission, sometime over the past few decades. PAT campaigns were not the main cause of the epidemic—the ultimate driver of the epidemic is presumably the mass expansion of healthcare services and infrastructure that accelerated during the PAT campaigns and subsequent decades, but whose implementation did not adequately adhere to the best practices of infection control. Whereas broad healthcare practices may have driven the epidemic, demographic trends amplified it, leading to an immense expansion and a high-incidence-cohort effect for those who acquired the infection between the 1970s and the 1990s. Despite this historical adversity, the epidemic has been rapidly diminishing since the mid-1990s, with curtailed incidence at present.
Supplementary Data
Supplementary data are available at IJE online.
Author Contributions
H.A. designed the mathematical model, conducted the analyses and wrote the first draft of the paper. H.C. and S.P.K. contributed through statistical analysis, literature searches and model parameterization. L.J.A. conceived and led the design of the study and model, analyses and drafting of the article. All authors have read and approved the final manuscript.
Funding
This publication was made possible by NPRP grant numbers 9-040-3-008 and 12S-0216-190094 from the Qatar National Research Fund (a member of Qatar Foundation). The findings achieved herein are solely the responsibility of the authors. The authors are also grateful for infrastructure support provided by the Biostatistics, Epidemiology, and Biomathematics Research Core at Weill Cornell Medicine-Qatar.
Conflict of interest
None declared.
Supplementary Material
References
- 1. Stanaway JD, Flaxman AD, Naghavi M. et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet 2016;388:1081–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maheshwari A, Ray S, Thuluvath PJ.. Acute hepatitis C. Lancet 2008;372:321–32. [DOI] [PubMed] [Google Scholar]
- 3. Shepard CW, Finelli L, Alter MJ.. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis 2005;5:558–67. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. The Global Viral Hepatitis Report. Geneva: World Health Organization, 2017.
- 5. Frank C, Mohamed MK, Strickland GT. et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet 2000;355:887–91. [DOI] [PubMed] [Google Scholar]
- 6. Kouyoumjian SP, Chemaitelly H, Abu-Raddad LJ.. Characterizing hepatitis C virus epidemiology in Egypt: systematic reviews, meta-analyses, and meta-regressions. Sci Rep 2018;8:1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cuadros DF, Branscum AJ, Miller FD, Abu-Raddad LJ.. Abu-Raddad LJ. Spatial epidemiology of hepatitis C virus infection in Egypt: analyses and implications. Hepatology 2014;60:1150–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mohamoud YA, Mumtaz GR, Riome S, Miller D, Abu-Raddad LJ.. The epidemiology of hepatitis C virus in Egypt: a systematic review and data synthesis. BMC Infect Dis 2013;13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ayoub HH, Abu-Raddad LJ.. Impact of treatment on hepatitis C virus transmission and incidence in Egypt: a case for treatment as prevention. J Viral Hepat 2017;24:486–95. [DOI] [PubMed] [Google Scholar]
- 10.Ministry of Health and Population [Egypt], El-Zanaty and Associates [Egypt], ICF International. Egypt Health Issues Survey 2015 Cairo: Ministry of Health and Population, 2015.
- 11. Strickland GT. Liver disease in Egypt: hepatitis C superseded schistosomiasis as a result of iatrogenic and biological factors. Hepatology 2006;43:915–22. [DOI] [PubMed] [Google Scholar]
- 12. Nafeh MA, Medhat A, Shehata M. et al. Hepatitis C in a community in Upper Egypt: I. Cross-sectional survey. Am J Trop Med Hyg 2000;63:236–41. [PubMed] [Google Scholar]
- 13. Habib M, Mohamed MK, Abdel AF. et al. Hepatitis C virus infection in a community in the Nile Delta: risk factors for seropositivity. Hepatology 2001;33:248–53. [DOI] [PubMed] [Google Scholar]
- 14. Tanaka Y, Agha S, Saudy N. et al. Exponential spread of hepatitis C virus genotype 4a in Egypt. J Mol Evol 2004;58:191–95. [DOI] [PubMed] [Google Scholar]
- 15. Pybus OG, Drummond AJ, Nakano T, Robertson BH, Rambaut A.. The epidemiology and iatrogenic transmission of hepatitis C virus in Egypt: a Bayesian coalescent approach. Mol Biol Evol 2003;20:381–87. [DOI] [PubMed] [Google Scholar]
- 16. Okasha O, Munier A, Delarocque-Astagneau E. et al. Hepatitis C virus infection and risk factors in health-care workers at Ain Shams University Hospitals, Cairo, Egypt. East Mediterr Health J 2015;21:199–212. [DOI] [PubMed] [Google Scholar]
- 17. Derbala M, Chandra P, Amer A, et al. Epidemiology of HCV in Egypt according to age: parenteral antischistosomal therapy: the accused innocent. 23rd Conference of the Asian Pacific Association for the Study of the Liver, APASL Springer, New York, 2014.
- 18. Edris A, Nour MO, Zedan OO, Mansour AE, Ghandour AA, Omran T.. Seroprevalence and risk factors for hepatitis B and C virus infection in Damietta Governorate, Egypt. East Mediterr Health J 2014;20:605–13. [PubMed] [Google Scholar]
- 19. Zuure FR, Bouman J, Martens M. et al. Screening for hepatitis B and C in first-generation Egyptian migrants living in the Netherlands. Liver Int 2013;33:727–38. [DOI] [PubMed] [Google Scholar]
- 20. Mohamed MK, Bakr I, El-Hoseiny M. et al. HCV-related morbidity in a rural community of Egypt. J Med Virol 2006;78:1185–89. [DOI] [PubMed] [Google Scholar]
- 21. Stoszek SK, Abdel-Hamid M, Narooz S. et al. Prevalence of and risk factors for hepatitis C in rural pregnant Egyptian women. Trans R Soc Trop Med Hyg 2006;100:102–07. [DOI] [PubMed] [Google Scholar]
- 22. Arafa N, El Hoseiny M, Rekacewicz C. et al. Changing pattern of hepatitis C virus spread in rural areas of Egypt. J Hepatol 2005;43:418–24. [DOI] [PubMed] [Google Scholar]
- 23. el-Sadawy M, Ragab H, el-Toukhy H. et al. Hepatitis C virus infection at Sharkia Governorate, Egypt: seroprevalence and associated risk factors. J Egypt Soc Parasitol 2004;34:367–84. [PubMed] [Google Scholar]
- 24. Strickland GT, Elhefni H, Salman T. et al. Role of hepatitis C infection in chronic liver disease in Egypt. Am J Trop Med Hyg 2002;67:436–42. [DOI] [PubMed] [Google Scholar]
- 25. Abdel-Aziz F, Habib M, Mohamed MK. et al. Hepatitis C virus (HCV) infection in a community in the Nile Delta: population description and HCV prevalence. Hepatology 2000;32:111–15. [DOI] [PubMed] [Google Scholar]
- 26. Angelico M, Renganathan E, Gandin C. et al. Chronic liver disease in the Alexandria governorate, Egypt: contribution of schistosomiasis and hepatitis virus infections. J Hepatol 1997;26:236–43. [DOI] [PubMed] [Google Scholar]
- 27. El-Sayed HF, Abaza SM, Mehanna S, Winch PJ.. The prevalence of hepatitis B and C infections among immigrants to a newly reclaimed area endemic for Schistosoma mansoni in Sinai, Egypt. Acta Trop 1997;68:229–37. [DOI] [PubMed] [Google Scholar]
- 28. El-Zayadi A, Khalifa AA, El-Misiery A, Naser AM, Dabbous H, Aboul-Ezz AA.. Evaluation of risk factors for intrafamilial transmission of HCV infection in Egypt. J Egypt Public Health Assoc 1997;72:33–51. [PubMed] [Google Scholar]
- 29. Mohamed MK, Hussein MH, Massoud AA. et al. Study of the risk factors for viral hepatitis C infection among Egyptians applying for work abroad. J Egypt Public Health Assoc 1996;71:113–47. [PubMed] [Google Scholar]
- 30. Quinti I, Renganathan E, El Ghazzawi E. et al. Seroprevalence of HIV and HCV infections in Alexandria, Egypt. Zentralbl Fur Bakteriol 1995;283:239–44. [DOI] [PubMed] [Google Scholar]
- 31. Bassily S. International Conference on Schistosomiasis. The Srp 1993-Le Caire, 14-18 Fevrier 1993. Hepatite B, Hepatite C Et Bilharziose. Med Chir Dig 1993;22:441–42. [Google Scholar]
- 32. Zakaria S, Esmat G, Al Boraey Y. et al. A community-based study of viral hepatitis infection in Giza Governorate, Egypt: seroprevalence, risk factors and associated morbidity. Med J Cairo Univ 2005;73:899. [Google Scholar]
- 33. Mahmud S, Chemaitelly HS, Kouyoumjian SP, Al Kanaani Z, Abu‐Raddad LJ.. Key associations for hepatitis C virus genotypes in the Middle East and North Africa. J Med Virol 2019;92:386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. El-Zanaty F, Way A.. Egypt Demographic and Health Survey 2008 Cairo: Ministry of Health, El-Zanaty and Associates, and Macro International, 2009.
- 35. Benova L, Awad SF, Miller FD, Abu-Raddad LJ.. Estimation of hepatitis C virus infections resulting from vertical transmission in Egypt. Hepatology 2015;61:834–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miller FD, Abu-Raddad LJ.. Evidence of intense ongoing endemic transmission of hepatitis C virus in Egypt. Proc Natl Acad Sci U S A 2010;107:14757–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miller FD, Abu-Raddad LJ.. Quantifying current hepatitis C virus incidence in Egypt. J Viral Hepat 2013;20:666–67. [DOI] [PubMed] [Google Scholar]
- 38. Ayoub HH, Al Kanaani Z, Abu-Raddad LJ.. Characterizing the temporal evolution of the hepatitis C virus epidemic in Pakistan. J Viral Hepat 2018;25:670–79. [DOI] [PubMed] [Google Scholar]
- 39. Hajarizadeh B, Grebely J, Dore GJ.. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol 2013;10:553–62. [DOI] [PubMed] [Google Scholar]
- 40.United Nations Department of Economic and Social Affairs. World Population Prospects, the 2015 Revision New York: United Nations Department of Economic and Social Affairs, 2015.
- 41.MATLAB® The Language of Technical Computing. Natick: The MathWorks, Inc, 2015. [Google Scholar]
- 42. Lagarias JC, Reeds JA, Wright MH, Wright PE.. Convergence properties of the Nelder--Mead simplex method in low dimensions. SIAM J Optim 1998;9:112–47. [Google Scholar]
- 43. Mostafa A, Shimakawa Y, Medhat A. et al. Excess mortality rate associated with hepatitis C virus infection: a community-based cohort study in rural Egypt. J Hepatol 2016;64:1240–46. [DOI] [PubMed] [Google Scholar]
- 44. Talaat M, Kandeel A, Rasslan O. et al. Evolution of infection control in Egypt: achievements and challenges. Am J Infect Control 2006;34:193–200. [DOI] [PubMed] [Google Scholar]
- 45. Choo Q-L, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M.. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 1989;244:359–62. [DOI] [PubMed] [Google Scholar]
- 46. Kuo G, Choo Q, Alter H. et al. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science 1989;244:362–64. [DOI] [PubMed] [Google Scholar]
- 47. Saeed AA, Al-Admawi AM, Al-Rasheed A. et al. Hepatitis C virus infection in Egyptian volunteer blood donors in Riyadh. Lancet 1991;338:459–60. [DOI] [PubMed] [Google Scholar]
- 48. Kamel MA, Ghaffar YA, Wasef MA, Wright M, Clark LC, Miller FD.. High HCV prevalence in Egyptian blood donors. Lancet 1992;340:427. [DOI] [PubMed] [Google Scholar]
- 49. Ayoub HH, Chemaitelly H, Omori R, Abu-Raddad LJ.. Hepatitis C virus infection spontaneous clearance: has it been underestimated? Int J Infect Dis 2018;75:60–66. [DOI] [PubMed] [Google Scholar]
- 50. Wise M, Bialek S, Finelli L, Bell BP, Sorvillo F.. Changing trends in hepatitis C-related mortality in the United States, 1995–2004. Hepatology 2007;47:1128–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.