Highlights
-
•
Posttraumatic stress disorder (PTSD) shows altered effective connectivity dynamics.
-
•
Modeling between the periaqueductal gray (PAG) and the default mode network (DMN).
-
•
In PTSD, stronger excitatory effective connectivity from the PAG towards the DMN.
-
•
Trauma-related/neutral stimulus modulations to effective connectivity are compared.
-
•
In PTSD, trauma-related stimulus modulations differ significantly to the controls.
Keywords: Posttraumatic stress disorder, Dynamic causal modeling, Periaqueductal gray, Default mode network, Trauma
Abstract
Self-related processes define assorted self-relevant or social-cognitive functions that allow us to gather insight and to draw inferences related to our own mental conditions. Self-related processes are mediated by the default mode network (DMN), which, critically, shows altered functionality in individuals with posttraumatic stress disorder (PTSD). In PTSD, the midbrain periaqueductal gray (PAG) demonstrates stronger functional connectivity with the DMN [i.e., precuneus (PCN), medial prefrontal cortex (mPFC)] as compared to healthy individuals during subliminal, trauma-related stimulus processing. Here, we analyzed the directed functional connectivity between the PAG and the PCN, as well as between the PAG and the mPFC to more explicitly characterize the functional connectivity we have observed previously on the corresponding sample and paradigm. We evaluated three models varying with regard to context-dependent modulatory directions (i.e., bi-directional, bottom-up, top-down) among individuals with PTSD (n = 26) and healthy participants (n = 20), where Bayesian model selection was used to identify the most optimal model for each group. We then compared the effective connectivity strength for each parameter across the models and between our groups using Bayesian model averaging. Bi-directional models were found to be favoured across both groups. In PTSD, we revealed the PAG to show stronger excitatory effective connectivity to the PCN, as well as to the mPFC as compared to controls. In PTSD, we further demonstrated that PAG-mediated effective connectivity to the PCN, as well as to the mPFC were modulated more strongly during subliminal, trauma-related stimulus conditions as compared to controls. Clinical disturbances towards self-related processes are reported widely by participants with PTSD during trauma-related stimulus processing, where altered functional connectivity directed by the PAG to the DMN may elucidate experiential links between self- and trauma-related processing in traumatized individuals.
1. Introduction
Traumatic experiences can have a severe affect on the sense of self, where traumatized individuals remain often tortured by thoughts that echo strongly negative core beliefs about themselves, or can experience somatically-based alterations in self-identity. Clinical disturbances towards self-related processes are evidenced by statements, like, “I do not know myself anymore,” “I will never be able to feel normal emotions again,” or, “I feel as though my body does not belong to me” (Foa et al., 1999, Bernstein and Putnam, 1986, Dell, 2006). Statements such as these are recited often by individuals with posttraumatic stress disorder (PTSD), which underscores the vulnerability the sense of self has in regard to trauma (for a review, see Frewen et al., 2020). In PTSD, individuals who report the greatest symptom severity are more likely to reveal an apparent link between self- and trauma-related processing (Berntsen and Rubin, 2007). Related, when participants with PTSD are asked to characterize a self-defining memory, they state more often a trauma-related memory as compared to trauma-exposed healthy individuals (Sutherland and Bryant, 2005). Clinical disturbances in self-related processes have been described robustly in participants with PTSD (for a review, see Frewen and Lanius, 2006, Lanius et al., 2011), where these disturbances are thought to arise from intrinsic brain networks (Conway and Pleydell-Pearce, 2000, Qin and Northoff, 2011).
Self-related processes are mediated predominantly by the default mode network (DMN), which refers to a large-scale, intrinsic brain network distributed across the cortical mid-line and comprised primarily by the posterior cingulate cortex, the precuneus (PCN), as well as the medial prefrontal cortex (mPFC) (Greicius et al., 2003, Spreng et al., 2009, Qin and Northoff, 2011; for a review, see Raichle, 2015). The DMN is recruited during rest principally, but also demonstrates activity during internally-guided cognition, which includes autobiographical memory and self-referential processes (Greicius and Menon, 2004, Fransson, 2005). Self-referential processes describe various self-relevant or social-cognitive functions that allow us to gather insight and to draw inferences about the mental and the physical states of ourselves and others (Greicius et al., 2003). In PTSD as compared to healthy individuals, both the PCN and the mPFC demonstrate disrupted functional connectivity with the DMN during rest (Bluhm et al., 2009, DiGangi et al., 2016, Reuveni et al., 2016, Wu et al., 2011, Qin et al., 2012; Akiki et al., 2018; for a review, see Wang et al., 2016, Koch et al., 2016, Barredo et al., 2018; Akiki et al., 2017). Furthermore, reductions in DMN functional connectivity are found to be related to greater symptom severity in participants with PTSD (Bluhm et al., 2009, Sripada et al., 2012, Qin et al., 2012, Shang et al., 2014; for a review, see Kennis et al., 2016, Wang et al., 2016, Barredo et al., 2018). Traumatized individuals who display the strongest symptom severity are more likely to show the clinical disturbances in self-related processes that were mentioned prior (Cloitre et al., 1997, Frewen et al., 2017, Qin et al., 2012, Nicholson et al., 2020; for a review, see van der Kolk et al., 2005, Frewen et al., 2008). In PTSD, clinical disturbances towards self-related processes are observed both at rest, as well as during trauma-related stimulus conditions (Lanius et al., 2011, Sutherland and Bryant, 2005), where the latter may be mediated by the DMN as well.
In PTSD, traumatized individuals describe clinically a link between self- and trauma-related processing (Berntsen and Rubin, 2007), where these links may be mediated by aberrant functional connectivity across distributed neural systems. Here, Terpou et al. (2019a) have described an interaction between the midbrain periaqueductal gray (PAG) and the DMN in participants with PTSD during subliminal, trauma-related stimulus conditions. The PAG refers to the gray matter located around the cerebral aqueduct of the midbrain, which, when activated, can elicit evolutionarily conserved defense responses that function to quell or to escape an impending threat (e.g., fight, flight, faint; De Oca et al., 1998, Brandão et al., 2008, Fenster et al., 2018; for a review, see Keay and Bandler, 2014). Interestingly, the PAG reveals stronger activity in participants with PTSD as compared to controls during subliminal, trauma-related stimulus conditions (Terpou et al., 2019b, Rabellino et al., 2016, Felmingham et al., 2008), where the PAG is thought to mediate, in part, threat-evoked physiological changes (for a review, see Kozlowska et al., 2015, Terpou et al., 2019c). In PTSD, Terpou et al. (2019a) revealed increased functional connectivity between the PAG and the PCN, as well as between the PAG and the mPFC during subliminal, trauma-related stimulus conditions as compared to controls. Trauma-related stimulus conditions were compared to neutral stimulus conditions, where both conditions were presented subliminally in order to prevent participants from exercising avoidance techniques. Avoidance can reduce neurophysiological responses, but only during stimulus conditions where participants are consciously aware of the content presented. Stronger functional connectivity between the PAG and the DMN wastaken as evidence linking both self- and trauma-related processing under subliminal conditions in participants with PTSD, where the proposed study now aims to characterize the directed functional connectivity observed by Terpou et al. (2019a) across the same participant sample and paradigm we analyzed previously.
These findings by Terpou et al. (2019a) are intriguing both in regard to the unanticipated functional connectivity revealed between the PAG and the DMN, as well as the context by which the findings were generated, namely – subliminal, trauma-related stimulus conditions. However, we have yet to study the effective connectivity dynamics between the PAG and the DMN across the corresponding sample, where effective connectivity has the advantage to measure the directed functional connectivity between two regions. Hence, we implemented dynamic causal modeling (DCM) to estimate the directionality across network interactions between the PAG and the PCN, as well as between the PAG and the mPFC in participants with PTSD and healthy controls during subliminal, neutral and subliminal, trauma-related stimulus conditions. Specifically, we sought to determine whether these stimulus conditions modulate functional connectivity between the PAG and the DMN predominantly via bi-directional, bottom-up, or top-down effective connectivity. Nicholson et al. (2017) have documented previously greater bottom-up, PAG-mediated effective connectivity to the mPFC in participants with PTSD who displayed a more traditionalsymptom pattern as compared to participants with PTSD who displayed a dissociative symptom pattern principally. Findings by Nicholson et al. (2017) were observed during rest and generated on a subject sample that did not overlap with the participant sample (or paradigm) characterized in the present study. Accordingly, we hypothesized that the participants with PTSD in the current sample would show stronger condition-dependent modulations in effective connectivity in the bottom-up direction, a pattern that would suggest the PAG is driving the aberrant functional connectivity observed with the DMN. By contrast, we hypothesized that the healthy participants would demonstrate greater condition-dependent modulations in effective connectivity bi-directionally; however, we caution that healthy individuals did not display strong functional connectivity between the PAG and the DMN in Terpou et al. (2019a), and thus were not the primary focus to characterize in the present study. Additionally, we sought to determine the group-specific strengths in effective connectivity between the PAG and the PCN, as well as between the PAG and the mPFC. Identification of the effective connectivity strengths, as well as the excitatory and the inhibitory characteristics of the network interactions, would indeed afford a stronger understanding of the functional dynamics that may mediate the intrinsic links between self- and trauma-related processing in individuals with PTSD.
2. Methods
2.1. Participants
Our study was reviewed by the Health Sciences Research Ethics Board of Western University and adhered to the standards set out by Canada’s Tri-Council Policy in accordance with the Code of Ethics of the World Medical Association (i.e., Declaration of Helsinki). Our sample included 46 participants recruited by the London Health Services Centre via referrals from family physicians, community clinics, mental health professionals, and local advertisements. Twenty-six participants met criteria for a primary PTSD diagnosis and the remaining twenty participants were included as healthy control subjects. Written and informed consent was received by all participants. Analyses discussed in the present paper are novel; however, data generated on the present sample have been analyzed in previous publications (Rabellino et al., 2015, Rabellino et al., 2016, Rabellino et al., 2017, Terpou et al., 2019a, Terpou et al., 2019b). Scanning began on March 29, 2011 and concluded on November 12, 2013.
Exclusion criteria included incompatibilities with scanning conditions, previous neurologic and development illness, comorbid schizophrenia or bipolar disorder, alcohol or substance abuse, a history of head trauma, or pregnancy during scan. Diagnoses were determined using a Clinician Administered PTSD Scale (CAPS-IV (cut-off score > 50 for PTSD diagnosis); Blake et al., 1995) as well as the Structured Clinical Interview for DSM-IV Axis-I disorders (SCID-I; First, 2015). Healthy controls were permitted if they did not meet any current or lifetime criteria for an Axis-I psychiatric disorder. Participants with PTSD were medication free for at least six weeks prior to scanning. The Childhood Trauma Questionnaire (CTQ; Bernstein et al., 2003) and the Multiscale Dissociation Inventory (MDI; Briere et al., 2005) were administered as well to characterize our clinical sample further. The State-Trait Anxiety Inventory (STAI; Spielberger, 2010), the Responses to Script Driven Imagery Questionnaire (RSDI; Hopper et al., 2007), and the Clinician Administered Dissociative States Scale (CADSS; Bremner et al., 1998) were administered after each scanning session to provide information on subject symptom states for individuals with PTSD and healthy individuals.
2.2. Experimental paradigm
Paradigm and stimulus presentation durations were based on other previously published methods (Felmingham et al., 2008, Rabellino et al., 2016, Williams et al., 2006). Stimuli had a subliminal and a supraliminal display over two consecutive sessions that were separated by a two-minute rest period and were counterbalanced across subjects. Whereas subliminal stimuli were presented for 16 ms and followed by a backward mask, supraliminal stimuli were presented for 500 ms. Stimuli consisted of both threat (i.e., fearful faces and trauma-related words) and neutral (i.e., neutral faces and neutral words) material, presented in a pseudo-randomized block design. Word stimuli were subject-specific, with trauma-related words generated in reference to a trauma memory, or, in the case of controls, an aversive memory. Neutral words were selected on the basis that they had not elicited a strong positive or a strong negative reaction during a pre-scan exposure to the words. Trauma-related and neutral words were matched for syllable and for letter length. For a detailed description of the subliminal-supraliminal threat paradigm, please see Supplemental Information.
2.3. Data acquisition
Functional magnetic resonance imaging (fMRI) was conducted using a 3.0 T whole-body MRI scanner (Siemens Biograph mMR, Siemens Medical Solutions, Erlangen, Germany) with a 32-channel phased-array head coil. T1-weighted anatomical images were collected with 1 mm isotropic resolution (MP-RAGE, TR/TE/TI = 2300 ms/2.98 ms/900 ms, FA 9°, FOV = 256 mm × 240 mm × 192 mm, acceleration factor = 4, total acquisition time = 192 s). For blood-oxygen-level dependent fMRI, transverse imaging slices covering the whole-brain were prescribed parallel to the anterior commissure-posterior commissure (AC-PC) line. Data were acquired using a gradient echo planar imaging (EPI) sequence (single-shot, blipped) with an interleaved slice acquisition order and tridimensional prospective acquisition correction (3D PACE) and an isotropic resolution of 2 mm [(FOV = 192 mm × 192 mm × 128 mm (94 × 94 matrix, 64 slices), TR/TE = 3000 ms/20 ms, FA = 90° (FOV = Field of View, TR = Repetition Time, TE = Echo Time, FA = Flip Angle)].
Preprocessing and statistical analyses were conducted on Statistical Parametric Mapping (SPM12, Wellcome Trust Centre for Neuroimaging, London, UK: http://www.fil.ion.ucl.ac.uk/sp) within MATLAB 9.2 software (R2017a, Mathworks Inc., MA). Preprocessing protocols for both whole-brain as well as the partial-brain space as provided by the spatially-unbiased infratentorial template toolbox (SUIT; Diedrichsen, 2006) are detailed further in the Supplemental Information.
2.4. Dynamic causal modeling
DCM estimates the directionality of the functional dependencies that exist across an underlying dynamical system (for a review, see Friston et al., 2003). DCM allows for inferences to be made about the architecture of distributed brain networks in terms of the effective connectivity shown by the network as well as their condition-dependent modulations (Kiebel et al., 2007). DCM is a model-driven Bayesian approach, where network architectures of plausible models are specified a priori and are then evaluated on their ability to explain observed neural responses with Bayesian model selection (BMS) (Stephan et al., 2009). DCM also offers an ability to compare the strength of model connectivity parameters using Bayesian model averaging (BMA) (Friston et al., 2007, Stephan et al., 2010). Although group comparisons can be conducted within either conventional statistical or Bayesian frameworks, we implemented conventional statistics in the present study.
2.4.1. First-level: Time series extraction
Coordinate locations of three brain regions that we have revealed formerly to demonstrate group differences in functional connectivity between participants with PTSD and healthy individuals during subliminal, trauma-related (as compared to neutral) stimulus conditions were selected (Terpou et al., 2019a): the PAG, the PCN, and the mPFC. Notably, there are structural connections to facilitate, in part, inter-nodal network dynamics between the PAG, the PCN, and the mPFC (Linnman et al., 2012, Menant et al., 2016, Ezra et al., 2015). Whereas time series of the PAG were extracted from subject-specific general linear models (GLMs) computed in SUIT-space, time series of the PCN and the mPFC were extracted from subject-specific GLMs created in whole-brain space. Here, SUIT-space refers to the partial-brain space implemented in the SUIT toolbox, which, through improved normalization procedures, afforded a greater signal resolution and hence signal extraction of the PAG across the included participants (Diedrichsen et al., 2009).
First-level GLMs modeled the stimulus condition onsets and included an artifact detection regressor and realignment parameters as multiple regressors. Time series were extracted from a contrast that modeled both subliminal, neutral and subliminal, trauma-related stimulus conditions. Coordinates and sizes of the spheres for the time series were as follows: PAG ([x: 0; y: −32; z: −11 (mm)]; sphere size: 6 mm), PCN ([x: 6; y: −52; z: 30 (mm)]; sphere size: 8 mm), and mPFC ([x: 0; y: 60; z: −2 (mm)]; sphere size: 8 mm). Extracted time series were permitted to vary slightly from these coordinates and were inspected individually to assure that the relocated volumes-of-interest remained in the proper anatomical location. Whereas a ±2 mm variation in each coordinate plane was allowed for the PAG, a ±3 mm variation in each coordinate plane was allowed for the PCN and the mPFC. Smaller sphere sizes and variations were used for the PAG to account for the size of the structure and to limit the potential signal interference of neighbouring midbrain structures. Each eigenvariate extracted from the PAG were examined to confirm that the signal variation was explained mainly by the set volume-of-interest (>75% explained; see Supplemental Information).
2.4.2. First-level: Neural model specification
2.4.2.1. Modeling specifications
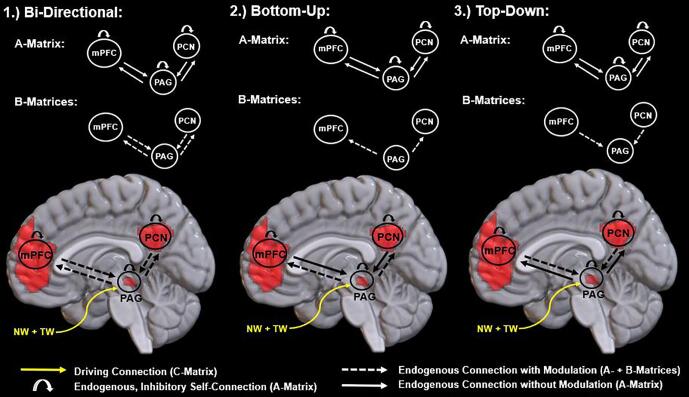
Subliminal, neutral and subliminal, trauma-related stimulus onsets were imported from the GLMs into the DCM framework. In line with relevant scanning parameters, slice timing was set to 2 s. Bilinear, one-state model terms were selected, where the models did not include stochastic effects or mean centre-input. Each DCM modeled fMRI in the time domain, where we assessed condition-specific, time-varying fluctuations in effective connectivity due to our experimental context. We specified three models for every participant (Fig. 1). Each model had a different modulatory direction, which were used to parameterize changes in effective connectivity that were generated by the stimulus conditions. Models included a bi-directional, a bottom-up, and a top-down model.
Fig. 1.
The above illustration details the three specified models. On the top, we illustrate the endogenous and the modulatory model connectivity parameters as specified in the A-matrix and the B-matrix, respectively. On the bottom, we superimpose these model connectivity parameters (as well as the C-matrix) onto template masks that give a relative indication of the coordinate locations that correspond to the various network nodes. Note that neither template masks nor circles represent actual coordinate locations or sizes of the spheres used for signal extraction and are intended for graphic illustration only. On the very bottom, we provide a legend for these model connectivity parameters.
Abbreviations: PAG: periaqueductal gray; PCN: precuneus; mPFC: medial prefrontal cortex; NW: neutral condition; TW: trauma-related condition.
2.4.2.2. Direct connections (C-matrix)
C-matrices specify the direct connections and were held constant across our models, where each stimulus condition had a direct input into the network at the PAG. The selection was based on the relationship between the PAG and the superior colliculus, where the superior colliculus receives visual information from the retina (Liddell et al., 2005, Tamietto and de Gelder, 2010), discriminates between threat and non-threat stimuli (Liddell et al., 2005), and innervates the PAG subsequently (Grofová et al., 1978, Keay and Bandler, 2014). Taken together, stimulus conditions are likely to have onset into the network at the PAG and not at the level of the PCN or the mPFC.
2.4.2.3. Endogenous connections (A-matrix)
Endogenous connections were specified in the A-matrix and did not differ across the models or across the stimulus conditions. Whereas endogenous connections between the PAG and the PCN, as well as between the PAG and the mPFC were modeled, endogenous connections between the PCN and the mPFC were not modeled. We restricted the model space to address specifically the key research question, which was to characterize the directional connectivity between the PAG and the DMN (i.e., PCN, mPFC) as a direct follow-up to the findings reported by Terpou et al. (2019a). The PCN and the mPFC were included in the present study by virtue of the functional connectivity each node shared with the PAG. Hence, we did not model the endogenous connection between the PCN and the mPFC to provide the most parsimonious model space. Moreover, Rabellino et al. (2015) have revealed that the PCN displays reduced functional connectivity with the DMN during subliminal, threat-related stimulus processing in the same participant sample and paradigm analyzed here. In turn, we omitted the endogenous connection between the PCN and the mPFC to focus on the network interactions yet to be characterized. Additionally, endogenous, inhibitory self-connections were modeled for the PAG, the PCN, and the mPFC across the models. Each model then had the same seven endogenous connections, which included three inhibitory self-connections, and the two bi-directional connections between the PAG and the PCN, as well as between the PAG and the mPFC.
2.4.2.4. Modulatory connections (B-matrix)
Modulatory connections were specified in the B-matrix and were the only model parameters that were varied across the three models (Fig. 1). Modulatory connections exert influence over the endogenous connections, where different B-matrices can be specified for each stimulus condition across a given model. Each model included endogenous connections that were modulated to have the same condition-dependent modulations across the stimulus conditions. Bi-directional models had both neutral and trauma-related stimulus conditions modulating the endogenous connections between the PAG and the PCN, as well as between the PAG and the mPFC in both directions. Bottom-up models had the stimulus conditions modulating the endogenous connections from the PAG to the PCN, as well as from the PAG to the mPFC. Conversely, top-down models had the stimulus conditions modulating the endogenous connections from the PCN to the PAG, as well as from the mPFC to the PAG.
2.4.3. Second-level: Group comparisons
2.4.3.1. Bayesian model selection
Following the specification and the estimation of the three models across participants, a random-effects BMS was conducted in SPM12. BMS evaluates the evidence for each model and identifies the model that best accounts for the data, where winning models are identified when they exhibit relatively high exceedance probabilities. Exceedance probability is a measure of how likely it is that a given model is more frequent than the other models at explaining the data in the comparison test (Stephan et al., 2009). Exceedance probabilities quantify the properties of a good model to allow for comparisons between competing models (Stephen et al., 2010). In BMS, each model is evaluated with respect to its accuracy (i.e., how well the model parameters predicted the observed data) and its complexity (i.e., how much divergence the model parameters exerted from the model priors) (Friston et al., 2007, Stephan et al., 2009). Highly accurate and minimally complex models have strong model evidence and are hence more likely to be generalizable (Stephan et al., 2010).
2.4.3.2. Bayesian model averaging
BMA was also conducted across the models within each group, where BMA reflects the weighted average of each model parameter across subjects and models, weighted by the models’ posterior probabilities. Means and standard deviations of BMA parameter estimates were recorded for every parameter, where these values can be interpreted as the evidence for the connection strength of a parameter. Means and standard deviations were used to conduct independent t-tests in which we Bonferroni-corrected for multiple comparisons (p = 0.05/17).
3. Results
3.1. Demographics and clinical measures
Independent t-tests conducted across the demographic measures did not reveal significant group differences. As expected for clinical measures, participants with PTSD scored significantly higher on total scores for the CAPS, MDI, CTQ, and RSDI as compared to controls (see Table 1).
Table 1.
Clinical and Demographic Information.
| Measure | PTSD (n = 26) M ± SD |
Healthy Controls (n = 20) M ± SD |
χ2 p |
t-Test p |
|---|---|---|---|---|
| Years of Age | 38.8 ± 12.2 | 32.5 ± 11.6 | – | 0.088 |
| Sex (n) | Male = 11, Female = 15 | Male = 10, Female = 10 | 0.604 | – |
| Employment Status (n) | Employed = 18, Unemployed = 7 | Employed = 17, Unemployed = 3 | 0.297 | – |
| CAPS Total | 70.6 ± 11.9 | 0.94 ± 2.9 | – | <0.001 |
| CTQ – Emotional Abuse | 14.5 ± 6.1 | 6.8 ± 3.1 | – | <0.001 |
| CTQ – Physical Abuse | 10.1 ± 6.4 | 5.7 ± 1.6 | – | 0.004 |
| CTQ – Sexual Abuse | 13.4 ± 7.8 | 5.3 ± 1.1 | – | <0.001 |
| CTQ – Emotional Neglect | 13.5 ± 5.9 | 8.8 ± 4.2 | – | 0.004 |
| CTQ – Physical Neglect | 10.2 ± 4.7 | 6.8 ± 2.7 | – | 0.006 |
| MDI Total | 58.8 ± 21.6 | 33.7 ± 3.8 | – | <0.001 |
| MDI – Depersonalization | 7.8 ± 4.1 | – | – | – |
| MDI – Derealization | 9.5 ± 4.5 | – | – | – |
| MDI – Dep./Der. | 8.7 ± 4.1 | – | – | – |
| BDI | 24.0 ± 6.7 | – | – | – |
| CADSS Total | 4.3 ± 2.6 | – | – | – |
| STAI Total | 6.2 ± 2.5 | – | – | – |
| RSDI Total | 4.1 ± 1.8 | – | – | – |
| RSDI – Distress | 2.2 ± 0.9 | 1.0 ± 0.0 | – | <0.001 |
| RSDI – Reliving | 2.0 ± 1.0 | 1.0 ± 0.0 | – | 0.001 |
| RSDI – Avoidance Thoughts | 1.9 ± 0.8 | 1.1 ± 0.3 | – | 0.001 |
| Axis-I Comorbidities (current [past]) frequency | Major Depressive Disorder (8[9]) | |||
| Dysthymic Disorder (0[3]) | ||||
| Agoraphobia w/o PD (3) | ||||
| Social Phobia (4) | ||||
| Specific Phobia (2) | ||||
| OCD (1[1]) | ||||
| Eating Disorders (1[1]) | ||||
| Somatoform Disorder (6) | ||||
| Lifetime Alcohol Abuse or Dependence [16] |
Table 1: Age, sex, trait scores (CAPS Total, CTQ, MDI (Total, Dep, Der, Dep/Der), BDI, CADSS, STAI, RSDI (Total, Distress, Reliving, Avoidance Thoughts), as well as the comorbidities for participants with PTSD and healthy individuals as mean values plus/minus standard deviations.
Abbreviations: CAPS: Clinician Administered PTSD Scale; CTQ: Childhood Trauma Questionnaire; MDI: Multiscale Dissociation Inventory [Dep: Depersonalization Subscale; Der: Derealization Subscale; Dep/Der: Depersonalization and Derealization Subscales Averaged]; BDI: Beck’s Depression Inventory; CADSS: Clinician Administered Dissociative States Scale; STAI: State-Trait Anxiety Inventory; RSDI: Responses to Script Driven Imagery; PD: Panic Disorder; OCD: Obsessive-Compulsive Disorder.
3.2. Bayesian model selection
The BMS analysis favoured bi-directional models for both groups. Exceedance probabilities for the bi-directional models of the control and the PTSD group were 0.935 and 0.969, respectively. These are above common thresholds to report model superiority (i.e., > 0.85–0.90; Stephan et al., 2009) and suggest bi-directional modulations are favoured across the current experimental conditions.
3.3. Bayesian model averaging
3.3.1. Direct connections (C-matrix)
Subliminal, neutral and subliminal, trauma-related stimulus conditions revealed greater parameter estimates for driving inputs directed to the PAG in participants with PTSD as compared to controls (see Table 2).
Table 2.
Mean/Standard Deviation of BMA Parameter Estimates
| Matrices (Condition) | Model Parameters | Mean (in HZ) |
Standard Deviation |
Effect Size |
t-Tests (df = 44) |
|||
|---|---|---|---|---|---|---|---|---|
| Controls | PTSD | Controls | PTSD | Cohen’s d | t-statistic | p-value | ||
| C(NW) | PAG | −0.1371 | −0.2863 | 0.0469 | 0.0472 | 3.1732 | 113.612 | < 0.001 |
| C(TW) | PAG | −0.2433 | −0.3223 | 0.0490 | 0.0493 | 1.6134 | 30.729 | < 0.001 |
| A | PAG → PAG | −0.0362 | −0.0377 | 0.0275 | 0.0240 | 0.0582 | 0.0389 | 0.844 |
| PCN → PCN | −0.0155 | −0.0119 | 0.0281 | 0.0242 | −0.1376 | 0.2175 | 0.643 | |
| mPFC → mPFC | −0.0223 | −0.0104 | 0.0278 | 0.0248 | −0.4524 | 2.3432 | 0.133 | |
| PAG → PCN | 0.0016 | 0.0362 | 0.0222 | 0.0211 | −1.5981 | 29.055 | < 0.001 | |
| PAG → mPFC | 0.0116 | 0.0352 | 0.0251 | 0.0225 | −0.9916 | 44.237 | < 0.001 | |
| PCN → PAG | 0.0261 | 0.0422 | 0.0261 | 0.0228 | −0.6585 | 5.0324 | 0.031 | |
| mPFC → PAG | −0.0004 | 0.0251 | 0.0256 | 0.0227 | −1.0547 | 13.131 | 0.002 | |
| B(NW) | PAG → PCN | 0.2135 | 0.4256 | 0.1768 | 0.1641 | −1.2447 | 17.670 | < 0.001 |
| PAG → mPFC | 0.1435 | 0.2297 | 0.1944 | 0.1788 | −0.4619 | 2.4358 | 0.126 | |
| PCN → PAG | 0.1204 | 0.1789 | 0.2124 | 0.1863 | −0.2934 | 0.9869 | 0.326 | |
| mPFC → PAG | 0.1021 | 0.1335 | 0.2096 | 0.1831 | −0.1599 | 0.2951 | 0.589 | |
| B(TW) | PAG → PCN | 0.3287 | 0.5069 | 0.1774 | 0.1591 | −1.0591 | 12.833 | <0.001 |
| PAG → mPFC | −0.0236 | 0.1841 | 0.1941 | 0.1756 | −1.1236 | 14.419 | <0.001 | |
| PCN → PAG | −0.0361 | 0.1381 | 0.2101 | 0.1862 | −0.8791 | 8.8502 | 0.005 | |
| mPFC → PAG | −0.0402 | 0.0623 | 0.2112 | 0.1824 | −0.5208 | 3.1119 | 0.085 | |
Table 2: Means and standard deviation values for BMA model parameters are represented for each group. For endogenous connections, whereas positive parameter values indicate that an increase in activity in the one region results in an increase in the rate of change in the activity of the connected region, negative parameter values indicate that an increase in activity of the one region results in a decrease in the rate of change in activity of the connected region. Bold font represents significance at p ≤ 0.0029.
Abbreviations: PTSD: posttraumatic stress disorder; C(NW): neutral stimulus condition direct connections; C(TW): trauma-related stimulus condition direct connections; A: endogenous connections; B(NW): neutral stimulus condition modulatory connections; B(TW): trauma-related stimulus condition modulatory connections; PAG: periaqueductal gray; PCN: precuneus; mPFC: medial prefrontal cortex.
3.3.2. Endogenous connections (A-matrix)
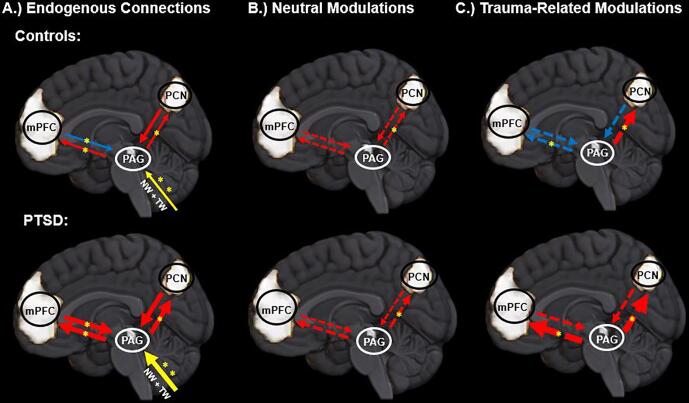
In PTSD, stronger excitatory effective connectivity from the PAG to the PCN, as well as from the PAG to the mPFC were demonstrated as compared to healthy controls (see Table 2). In PTSD, stronger excitatory effective connectivity from the mPFC to the PAG were also revealed as compared to controls, where controls featured a weak inhibitory connection for the parameter. No differences were found across endogenous, inhibitory self-connections for the PAG, the PCN, or the mPFC.
3.3.3. Modulatory connections (B-matrix)
In PTSD as compared to the control group, stronger modulation to the subliminal, neutral stimulus conditions were revealed, where neutral stimulus conditions prompted greater increases in the rate of change in effective connectivity from the PAG to the PCN. In PTSD as compared to controls, stronger modulation to the subliminal, trauma-related stimulus conditions were revealed as well, where trauma-related stimulus conditions led to greater increases in the rate of change in effective connectivity from the PAG to the PCN, and from the PAG to the mPFC (see Table 2). In controls, trauma-related stimulus conditions prompted increases as well as decreases in the rate of change in effective connectivity from the PAG to the PCN, and from the PAG to the mPFC, respectively.
4. Discussion
4.1. Overview
We sought to characterize the effective connectivity dynamics between the PAG and the PCN, as well as between the PAG and the mPFC during both subliminal, neutral and subliminal, trauma-related stimulus conditions in participants with PTSD as compared to healthy individuals. Critically, we found stronger excitatory effective connectivity from the PAG to the PCN, as well as between the PAG and the mPFC bi-directionally in individuals with PTSD as compared to healthy controls (Fig. 2A). Additionally, we revealed that the effective connectivity from the PAG to the PCN, as well as from the PAG to the mPFC were modulated more strongly in participants with PTSD as compared to healthy controls during subliminal, trauma-related stimulus conditions (Fig. 2C). Accordingly, bottom-up, or PAG-mediated functional connectivity to the DMN contributed more to our group differences, where subliminal, trauma-related stimulus conditions were revealed to lead to stronger increases in the rate of change in the effective connectivity. These findings may assist to explain linkages between self- and trauma-related processing in individuals with PTSD.
Fig. 2.
Top and bottom images illustrate the group-specific effective connectivity dynamics for the control and the PTSD group, respectively. Asterisks denote the particular parameter surpassed significance in group comparisons. Network nodes are included in the circles and the lines represent the connections between the nodes. Solid and dashed lines indicate an endogenous and a modulatory connection, respectively. Yellow, blue, and red lines indicate a direct, an inhibitory (or decrease), or an excitatory (or increase) connection, respectively. The size of the line gives a relative indication of the strength of the underlying model connectivity parameter.
Abbreviations: PAG: periaqueductal gray; PCN: precuneus; mPFC: medial prefrontal cortex; NW: neutral stimulus condition; TW: trauma-related stimulus condition; PTSD: posttraumatic stress disorder.
4.2. Endogenous connections (A-matrix)
Endogenous connectivity from the PAG to the PCN, from the PAG to the mPFC, as well as from the mPFC to the PAG showed greater excitatory effective connectivity in participants with PTSD as compared to controls, which we interpret here as the PAG-mediated recruitment of the DMN.
Nicholson et al. (2018) reported similarly an increase in DMN recruitment in participants with PTSD during trauma-related stimulus conditions across a real-time neurofeedback protocol. In particular, the DMN revealed stronger recruitment while viewing trauma-related stimuli as compared to viewing neutral words in participants with PTSD. Related, Nicholson et al. revealed also the PAG to be incorporated functionally within the salience network during the data-driven identification of the intrinsic connectivity networks. Switching between the intrinsic connectivity networks is mediated by the salience network and thought to be modulated by the anterior insula (Menon and Uddin, 2010, Seeley et al., 2007, Sridharan et al., 2008). In PTSD, Harricharan et al. (2016) have reported greater resting-state functional connectivity between the anterior insula and the PAG as compared to healthy individuals. Furthermore, Daniels et al. (2010) revealed that the intrinsic connectivity networks feature a dysregulated equilibrium in PTSD, where individuals do not inhibit appropriately the DMN during a working memory task. Accordingly, the PAG may be contributing to the aberrant recruitment of the DMN in traumatized individuals. Here, we found that the PAG demonstrates greater bottom-up, excitatory effective connectivity during subliminal, trauma-related stimulus conditions in participants with PTSD as compared to healthy individuals. These findings are in keeping with Nicholson et al. (2017), where stronger bottom-up, excitatory effective connectivity from the PAG to the mPFC were displayed during rest in participants with PTSD who presented with typical symptom patterns as compared to participants with PTSD who presented with more dissociative symptom patterns. These dynamics provide an early signal that PAG-mediated recruitment of the DMN – shown here during subliminal, trauma-related stimulus conditions – may support, in part, the apparent links between self- and trauma-related processing.
4.3. Modulatory connections (B-matrix)
4.3.1. Subliminal, trauma-related stimulus conditions
Subliminal, trauma-related stimulus conditions modulated effective connectivity more strongly in participants with PTSD as compared to healthy controls, where greater increases in effective connectivity from the PAG to the PCN, as well as from the PAG to the mPFC were revealed. Trauma-related stimulus conditions are used often to re-establish certain elements of a trauma memory (Elsesser et al., 2005, Liberzon et al., 1999, Halligan et al., 2006), where the PCN and the mPFC are thought to contribute to self-related (as well as visual imagery) processes and memory-related construction, respectively (for a review, see Cabeza and St Jacques, 2007, Svoboda et al., 2006). In PTSD as compared to controls, the PCN and the mPFC display stronger and lesser activity during trauma-related stimulus conditions, respectively (for a review, see Sartory et al., 2013, Thome et al., 2019). Enhanced activity in the PCN (as well as the posterior parietal cortices more generally) support reliving experiences during trauma-related stimulus processing in participants with PTSD (for a review, see Brewin, 2015). Reliving experiences are thought to re-establish the physiological, or visceral conditions encountered by the traumatized individual during trauma-related encoding (Rubin et al., 2004). Physiological changes are coordinated, in part, by the PAG (Brandão et al., 2008), where these changes may be provoked during trauma-related stimulus conditions in PTSD.
Subliminal stimulus conditions are used principally to evoke responses across subcortical systems, which may help explain why the PAG showed stronger excitatory effective connectivity to the PCN, as well as to the mPFC in participants with PTSD. Moreover, effective connectivity from the PAG to the PCN, as well as from the PAG to the mPFC were modulated more strongly in PTSD as compared to controls during trauma-related stimulus conditions. Subliminal, trauma-related stimulus conditions may then lead to PAG-mediated functional connectivity to the DMN in participants with PTSD. Indeed, Nicholson et al. (2017) have demonstrated similarly stronger bottom-up, or PAG-mediated effective connectivity to the mPFC in participants with PTSD who presented with common symptom patterns as compared to participants with PTSD who presented with more dissociative symptom patterns; however, these results were shown during rest, where individuals with PTSD demonstrate reduced DMN functional connectivity. Here, trauma-related stimulus conditions appear to drive stronger bottom-up, or PAG-mediated effective connectivity, where these patterns may serve to re-establish varying physiological sensations related to the trauma(s). Following the re-established trauma-related sensations, traumatized individuals may bring online reliving sensations, which are mediated largely by the DMN and instantiated by increased visual imagery processes and a putative bias to engage with a trauma-related memory from a self-related perspective in PTSD, in particular, whilst the memory remains unprocessed (van der Kolk, 2015).
4.3.2. Subliminal, neutral stimulus conditions
Subliminal, neutral stimulus conditions also modulated the effective connectivity from the PAG to the PCN more strongly in participants with PTSD as compared to controls. Stronger condition-dependent modulations to the neutral stimulus conditions may support an attention threat bias in participants with PTSD. Traumatized individuals generally exhibit stronger startle responses and emotion dysregulation during trauma-related stimulus conditions (Fani et al., 2012a, Fani et al., 2012b, Naim et al., 2015), but these responses are documented as well under neutral stimulus conditions (Felmingham et al., 2003, Pineles et al., 2009, Litz et al., 2000; for a review, see Weber 2008). Attention threat biases are often indexed indirectly via autonomic responses (e.g., heart rate, blood pressure, skin conductance), which are mediated, in part, by the PAG (for a review, see Terpou et al., 2019c). Moreover, subliminal stimulus conditions are used to elicit activity across evolutionarily conserved, fast-responding midbrain systems (Liddell et al., 2005). In PTSD, neutral stimulus conditions may have then been misidentified to be threatening, which can assist to explain the stronger modulations to neutral stimulus conditions revealed here.
4.4. Limitations and future directions
Our sample size was relatively small, thus precluding the authors to investigate the differences between participants with PTSD who meet or do not meet criteria for the dissociative subtype of the disorder. In PTSD, the dissociative subtype differs from the typical symptom pattern in both its clinical and neural characteristics (Lanius et al., 2010, Steuwe et al., 2012, Wolf et al., 2012), where the mPFC is involved considerably in differentiating between these diagnoses (Nicholson et al., 2019). Secondly, neutral and trauma-related words were not matched for frequency in the English language, which may have introduced novelty effects for the trauma-related words in the event that the words were less common as compared to the neutral words. Thirdly, we did not remove effects related to cardiac or respiratory activity by adjusting data to a contrast during eigenvariate extraction. Hence, DCMs may have been required to explain noise or confounds in the data via task-related processes, which would have reduced the accuracy of the model parameter estimates. Fourthly, subliminal stimulus durations were in keeping with standard procedures (Felmingham et al., 2008, Williams et al., 2006, Rabellino et al., 2016); however, we did not verify whether every individual perceived each stimulus subliminally. Lastly, we remind readers that our findings were generated from the same participant sample and paradigm as analyzed by Terpou et al. (2019a). Consequentially, we urge caution during the generalization of these findings to other samples and paradigms. We encourage future researchers to examine the network interactions across the DMN, where fully-connected models may uncover different effective connectivity dynamics during rest, as well as during similar threat- or trauma-related stimulus conditions in participants with PTSD.
4.5. Conclusion
Here, we explored the effective connectivity dynamics between the PAG and the PCN, as well as between the PAG and the mPFC during subliminal, neutral, as well as subliminal, trauma-related stimulus conditions in participants with PTSD as compared to healthy individuals. In PTSD, we revealed the PAG to display stronger bottom-up, excitatory effective connectivity to the PCN and to the mPFC, where effective connectivity between these model parameters were also modulated more strongly during subliminal, trauma-related stimulus conditions as compared to controls. It remains unclear whether these effective connectivity dynamics occur during other experimental contexts; however, we present evidence to understand further the phenomenological disturbances towards self-related processes that are reported by participants with PTSD during trauma-related processing. Future research evaluating the effective connectivity between the PAG and the DMN during rest are warranted critically. We discuss these findings in regard to the different elements expressed during trauma-related reliving, where the PAG and the DMN are thought to mediate physiological sensations related to trauma and self-related perspectives, respectively. We find evidence that the former drives the latter, which does beg intrigue into whether other network-related alterations in traumatized individuals are driven by subcortical systems that remain poorly described in the PTSD literature.
Funding
Research funding was provided by the Canadian Institutes of Health Research [Grant Numbers: 137150, 97914, 365726], the Canadian Institute for Military and Veterans Health Research [Grant Number: #W7714-145967/001/SV TA-27], IBM and SOSCIP. Authors acknowledge gratefully infrastructure funding from the Canada Foundation for Innovation Grant [Grant Number: 31724] (Jean Théberge) and the Lawson Health Research Institute start-up funding (Jean Théberge). Ruth A. Lanius is supported by the Harris-Woodman Chair in Psyche and Soma; Margaret C. McKinnon is supported by the Homewood Chair in Mental Health and Trauma; Andrew A. Nicholson is supported by the Marie Skłodowska-Curie Individual Fellowship from the European Research Commission [Grant Number: 897709]; and Braeden A. Terpou is funded by the Jonathan and Joshua Memorial Graduate Scholarship, as well as an Ontario Graduate Scholarships (OGS). Funding sources were not involved in the study design; collection, analysis, or interpretation of the data; preparation or writing of the final report; or in the decision to submit the article for publication.
CRediT authorship contribution statement
Braeden A. Terpou: Conceptualization, Methodology, Validation, Formal analysis, Writing - original draft, Writing - review & editing, Funding acquisition, Visualization. Maria Densmore: Methodology, Software, Investigation, Data curation, Resources, Writing - review & editing. Jean Théberge: Methodology, Software, Resources, Funding acquisition. Paul Frewen: Resources, Writing - review & editing. Margaret C. McKinnon: Resources, Writing - review & editing, Funding acquisition. Andrew A. Nicholson: Conceptualization, Methodology, Software, Resources, Writing - review & editing, Supervision. Ruth A. Lanius: Conceptualization, Investigation, Resources, Writing - review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102345.
Contributor Information
Braeden A. Terpou, Email: bterpou@uwo.ca.
Maria Densmore, Email: mdensmor@lawsonimaging.ca.
Jean Théberge, Email: jtheberge@lawsonimaging.ca.
Paul Frewen, Email: pfrewen@uwo.ca.
Margaret C. McKinnon, Email: mckinno@mcmaster.ca.
Andrew A. Nicholson, Email: dr.andrewnicholson@gmail.com.
Ruth A. Lanius, Email: ruth.lanius@lhsc.on.ca.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Akiki Teddy J., Averill Christopher L., Abdallah Chadi G. A Network-Based Neurobiological Model of PTSD: Evidence From Structural and Functional Neuroimaging Studies. Current Psychiatry Reports. 2017;19(11):1–10. doi: 10.1038/srep27131. 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiki Teddy J., Averill Christopher L., Wrocklage Kristen M., Scott Cobb J., Averill Lynnette A., Schweinsburg Brian, Alexander-Bloch Aaron, Martini Brenda, Southwick Steven M., Krystal John H., Abdallah Chadi G. Default mode network abnormalities in posttraumatic stress disorder: A novel network-restricted topology approach. NeuroImage. 2018;176(1):489–498. doi: 10.1016/j.neuroimage.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barredo J., Aiken E., Van ’T Wout-Frank M., Greenberg B.D., Carpenter L.L., Philip N.S. Network functional architecture and aberrant functional connectivity in post-traumatic stress disorder: A clinical application of network convergence. Brain Connect. 2018;8(9):549–557. doi: 10.1089/brain.2018.0634. [DOI] [PubMed] [Google Scholar]
- Bernstein D.P., Stein J.A., Newcomb M.D., Walker E., Pogge D., Ahluvalia T., Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27(2):169–190. doi: 10.1016/S0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Bernstein E.M., Putnam F.W. Development, reliability, and validity of a dissociation scale. J. Nerv. Ment. Dis. 1986;174(12):727–733. doi: 10.1097/00005053-198612000-00004. [DOI] [PubMed] [Google Scholar]
- Berntsen D., Rubin D.C. When a trauma becomes a key to identity: enhanced integration of trauma memories predicts posttraumatic stress disorder symptoms. Appl. Cognitive Psychol. 2007;21(4):417–431. doi: 10.1002/acp.1290. [DOI] [Google Scholar]
- Blake D.D., Weathers F.W., Nagy L.M., Kaloupek D.G., Gusman F.D., Charney D.S., Keane T.M. The development of a Clinician-Administered PTSD Scale. J. Trauma Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bluhm R.L., Williamson P.C., Osuch E.A., Frewen P.A., Stevens T.K., Boksman K., Lanius R.A. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J. Psychiatry Neurosci. 2009;34(3):187–194. doi: 10.5194/bg-13-4673-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandão M.L., Zanoveli J.M., Ruiz-Martinez R.C., Oliveira L.C., Landeira-Fernandez J. Different patterns of freezing behavior organized in the periaqueductal gray of rats: association with different types of anxiety. Behav. Brain Res. 2008;188(1):1–13. doi: 10.1016/j.bbr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Bremner J.D., Krystal J.H., Putnam F.W., Southwick S.M., Marmar C., Charney D.S., Mazure C.M. Measurement of dissociative states with the clinician-administered dissociative states scale (CADSS) J. Trauma. Stress. 1998;11(1):125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- Brewin C.R. Re-experiencing traumatic events in PTSD: New avenues in research on intrusive memories and flashbacks. Eur. J. Psychotraumatol. 2015;6(3):765–783. doi: 10.3402/ejpt.v6.27180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briere J., Weathers F.W., Runtz M. Is dissociation a multidimensional construct? Data from the Multiscale Dissociation Inventory. J. Trauma. Stress. 2005;18(3):221–231. doi: 10.1002/jts.20024. [DOI] [PubMed] [Google Scholar]
- Cabeza R., St Jacques P. Functional neuroimaging of autobiographical memory. Trends Cognitive Sci. 2007;11(5):219–227. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Cloitre M., Scarvalone P., Difede J.A. Posttraumatic stress disorder, self- and interpersonal dysfunction among sexually retraumatized women. J. Trauma Stress. 1997;10(3):437–452. doi: 10.1023/A:1024893305226. [DOI] [PubMed] [Google Scholar]
- Conway M.A., Pleydell-Pearce C.W. The construction of autobiographical memories in the self-memory system. Psychol. Rev. 2000;107(2):261–288. doi: 10.1037/0033-295X.107.2.261. [DOI] [PubMed] [Google Scholar]
- Daniels J.K., Mcfarlane A.C., Bluhm R.L., Moores K.A., Richard Clark C., Shaw M.E., Lanius R.A. Switching between executive and default mode networks in posttraumatic stress disorder: Alterations in functional connectivity. J. Psychiatry Neurosci. 2010;35(4):258–266. doi: 10.1503/jpn.090010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell P.F. The multidimensional inventory of dissociation (MID) a comprehensive measure of pathological dissociation. J. Trauma Dissoc. 2006;7(2):77–106. doi: 10.1300/J229v07n02_06. [DOI] [PubMed] [Google Scholar]
- De Oca B.M., DeCola J.P., Maren S., Fanselow M.S. Distinct regions of the periaqueductal gray are involved in the acquisition and expression of defensive responses. J. Neurosci. 1998;18(9):3426–3442. doi: 10.1523/JNEUROSCI.18-09-03426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. NeuroImage. 2006;33(1):127–138. doi: 10.1016/j.neuroimage.2006.05.056. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J., Balsters J.H., Flavell J., Cussans E., Ramnani N. A probabilistic MR atlas of the human cerebellum. NeuroImage. 2009;46(1):39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- DiGangi J.A., Tadayyon A., Fitzgerald D.A., Rabinak C.A., Kennedy A., Klumpp H., Phan K.L. Reduced default mode network connectivity following combat trauma. Neurosci. Lett. 2016;615:37–43. doi: 10.1016/j.neulet.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsesser K., Sartory G., Tackenberg A. Initial symptoms and reactions to trauma-related stimuli and the development of posttraumatic stress disorder. Depress. Anxiety. 2005;21(2):61–70. doi: 10.1002/da.20047. [DOI] [PubMed] [Google Scholar]
- Ezra M., Faull O.K., Jbabdi S., Pattinson K.T.S. Connectivity-based segmentation of the periaqueductal gray matter in human with brainstem optimized diffusion MRI. Hum. Brain Mapp. 2015;36(9):3459–3471. doi: 10.1002/hbm.22855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N., Jovanovic T., Ely T.D., Bradley B., Gutman D., Tone E.B., Ressler K.J. Neural correlates of attention bias to threat in post-traumatic stress disorder. Biol. Psychol. 2012;90(2):134–142. doi: 10.1016/j.biopsycho.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N., Tone E.B., Phifer J., Norrholm S.D., Bradley B., Ressler K.J., Jovanovic T. Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychol. Med. 2012;42(03):533–543. doi: 10.1017/S0033291711001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham K., Bryant R.A., Gordon E. Processing angry and neutral faces in post-traumatic stress disorder: an event-related potentials study. NeuroReport. 2003;14(5):777–780. doi: 10.1097/00001756-200304150-00024. [DOI] [PubMed] [Google Scholar]
- Felmingham K., Kemp A.H., Williams L., Falconer E., Olivieri G., Peduto A., Bryant R. Dissociative responses to conscious and non-conscious fear impact underlying brain function in post-traumatic stress disorder. Psychol. Med. 2008;38(12):1771–1780. doi: 10.1017/S0033291708002742. [DOI] [PubMed] [Google Scholar]
- Fenster R.J., Lebois L.A.M., Ressler K.J., Suh J. Brain circuit dysfunction in post-traumatic stress disorder: From mouse to man. Nat. Rev. Neurosci. 2018;19(9):535–551. doi: 10.1038/s41583-018-0039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B. The Encyclopedia of Clinical Psychology. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2015. Structured Clinical Interview for the DSM (SCID) pp. 1–6. 10.1002/9781118625392.wbecp351. [Google Scholar]
- Foa E.B., Tolin D.F., Ehlers A., Clark D.M., Orsillo S.M. The posttraumatic cognitions inventory (PTCI): development and validation. Psychol. Assess. 1999;11(3):303–314. doi: 10.1037/1040-3590.11.3.303. [DOI] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: An fMRI investigation of the resting-state default mode of brain function hypothesis. Hum. Brain Mapp. 2005;26(1):15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen P.A., Dozois D.J.A., Neufeld R.W.J., Lanius R.A. Meta-analysis of alexithymia in posttraumatic stress disorder. J. Trauma Stress. 2008;21(2):243–246. doi: 10.1002/jts.20320. [DOI] [PubMed] [Google Scholar]
- Frewen P.A., Lanius R.A. Toward a psychobiology of posttraumatic self-dysregulation: reexperiencing, hyperarousal, dissociation, and emotional numbing. Ann. N. Y. Acad. Sci. 2006;1071:110–124. doi: 10.1196/annals.1364.010. [DOI] [PubMed] [Google Scholar]
- Frewen P.A., Schroeter M.L., Riva G., Cipresso P., Fairfield B., Padulo C., Northoff G. Neuroimaging the consciousness of self: Review, and conceptual-methodological framework. Neurosci. Biobehav. Rev. 2020 doi: 10.1016/j.neubiorev.2020.01.023. [DOI] [PubMed] [Google Scholar]
- Frewen P., Thornley E., Rabellino D., Lanius R. Neuroimaging the traumatized self: FMRI reveals altered response in cortical midline structures and occipital cortex during visual and verbal self- and other-referential processing in women with PTSD. Eur. J. Psychotraumatol. 2017;8(1):1314164. doi: 10.1080/20008198.2017.1314164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Harrison L., Penny W. Dynamic causal modelling. NeuroImage. 2003;19(4):1273–1302. doi: 10.1016/S1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Mattout J., Trujillo-Barreto N., Ashburner J., Penny W. Variational free energy and the Laplace approximation. NeuroImage. 2007;34(1):220–234. doi: 10.1016/j.neuroimage.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U.S.A. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J. Cognit. Neurosci. 2004;16(9):1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Grofová I., Ottersen O.P., Rinvik E. Mesencephalic and diencephalic afferents to the superior colliculus and periaqueductal gray substance demonstrated by retrograde axonal transport of horseradish peroxidase in the cat. Brain Res. 1978;146(2):205–220. doi: 10.1016/0006-8993(78)90969-1. [DOI] [PubMed] [Google Scholar]
- Halligan S.L., Michael T., Wilhelm F.H., Clark D.M., Ehlers A. Reduced heart rate responding to trauma reliving in trauma survivors with PTSD: correlates and consequences. J. Trauma. Stress. 2006;19(5):721–734. doi: 10.1002/jts.20167. [DOI] [PubMed] [Google Scholar]
- Harricharan S., Rabellino D., Frewen P.A., Densmore M., Théberge J., McKinnon M.C., Lanius R.A. fMRI functional connectivity of the periaqueductal gray in PTSD and its dissociative subtype. Brain Behav. 2016;6(12) doi: 10.1002/brb3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper J.W., Frewen P.A., Sack M., Lanius R.A., van der Kolk B.A. The responses to script-driven imagery scale (RSDI): assessment of state posttraumatic symptoms for psychobiological and treatment research. J. Psychopathol. Behav. Assess. 2007;29(4):249–268. doi: 10.1007/s10862-007-9046-0. [DOI] [Google Scholar]
- Keay, K.A., Bandler, R., 2014. Periaqueductal Gray. The Rat Nervous System: Fourth Edition, 207–221. https://doi.org/10.1016/B978-0-12-374245-2.00010-3.
- Kennis M., Van Rooij S.J.H., Van Den Heuvel M.P., Kahn R.S., Geuze E. Functional network topology associated with posttraumatic stress disorder in veterans. NeuroImage: Clinical. 2016;10(2):302–309. doi: 10.1016/j.nicl.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebel S.J., Klöppel S., Weiskopf N., Friston K.J. Dynamic causal modeling: a generative model of slice timing in fMRI. NeuroImage. 2007;34(4):1487–1496. doi: 10.1016/j.neuroimage.2006.10.026. [DOI] [PubMed] [Google Scholar]
- Koch S.B.J., van Zuiden M., Nawijn L., Frijling J.L., Veltman D.J., Olff M. Aberrant resting-state brain activity in posttraumatic stress disorder: a meta-analysis and systematic review. Depress. Anxiety. 2016;33(7):592–605. doi: 10.1002/da.22478. [DOI] [PubMed] [Google Scholar]
- Kozlowska K., Walker P., McLean L., Carrive P. Fear and the defense cascade. Harvard Rev. Psychiatry. 2015;23(4):263–287. doi: 10.1097/HRP.0000000000000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius R.A., Bluhm R.L., Frewen P.A. How understanding the neurobiology of complex post-traumatic stress disorder can inform clinical practice: a social cognitive and affective neuroscience approach. Acta Psychiatr. Scand. 2011;124(5):331–348. doi: 10.1111/j.1600-0447.2011.01755.x. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Vermetten E., Loewenstein R.J., Brand B., Schmahl C., Bremner J.D., Spiegel D. Emotion modulation in PTSD: clinical and neurobiological evidence for a dissociative subtype. Am. J. Psychiatry. 2010;167(6):640–647. doi: 10.1176/appi.ajp.2009.09081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I., Taylor S.F., Amdur R., Jung T.D., Chamberlain K.R., Minoshima S., Fig L.M. Brain activation in PTSD in response to trauma-related stimuli. Biol. Psychiatry. 1999;45(3):817–826. doi: 10.1016/S0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- Liddell B.J., Brown K.J., Kemp A.H., Barton M.J., Das P., Peduto A., Williams L.M. A direct brainstem-amygdala-cortical “alarm” system for subliminal signals of fear. NeuroImage. 2005;24(1):235–243. doi: 10.1016/j.neuroimage.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Linnman C., Moulton E.A., Barmettler G., Becerra L., Borsook D. Neuroimaging of the periaqueductal gray: State of the field. NeuroImage. 2012;60(1):505–522. doi: 10.1016/j.neuroimage.2011.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litz B.T., Orsillo S.M., Kaloupek D., Weathers F. Emotional processing in posttraumatic stress disorder. J. Abnorm. Psychol. 2000;109(1):26–39. doi: 10.1037//0021-843x.109.1.26. [DOI] [PubMed] [Google Scholar]
- Menant O., Andersson F., Zelena D., Chaillou E. The benefits of magnetic resonance imaging methods to extend the knowledge of the anatomical organisation of the periaqueductal gray in mammals. J. Chem. Neuroanat. 2016;77:110–120. doi: 10.1016/j.jchemneu.2016.06.003. [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim R., Abend R., Wald I., Eldar S., Levi O., Fruchter E., Bar-Haim Y. Threat-related attention bias variability and posttraumatic stress. Am. J. Psychiatry. 2015;172(12):1242–1250. doi: 10.1176/appi.ajp.2015.14121579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A.A., Densmore M., McKinnon M.C., Neufeld R.W.J., Frewen P.A., Théberge J., Lanius R.A. Machine learning multivariate pattern analysis predicts classification of posttraumatic stress disorder and its dissociative subtype: a multimodal neuroimaging approach. Psychol. Med. 2019;49(12):2049–2059. doi: 10.1017/S0033291718002866. [DOI] [PubMed] [Google Scholar]
- Nicholson A.A., Friston K.J., Zeidman P., Harricharan S., McKinnon M.C., Densmore M., Lanius R.A. Dynamic causal modeling in PTSD and its dissociative subtype: Bottom-up versus top-down processing within fear and emotion regulation circuitry. Hum. Brain Mapp. 2017;38(11):5551–5561. doi: 10.1002/hbm.23748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A.A., Rabellino D., Densmore M., Frewen P.A., Paret C., Kluetsch R., Lanius R.A. Intrinsic connectivity network dynamics in PTSD during amygdala downregulation using real-time fMRI neurofeedback: a preliminary analysis. Hum. Brain Mapp. 2018;39(11):4258–4275. doi: 10.1002/hbm.24244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A.A., Ros T., Jetly R., Lanius R.A. Regulating posttraumatic stress disorder symptoms with neurofeedback: regaining control of the mind. J. Military, Veteran Family Health. 2020;6(S1):3–15. doi: 10.3138/jmvfh.2019-0032. [DOI] [Google Scholar]
- Pineles S.L., Shipherd J.C., Mostoufi S.M., Abramovitz S.M., Yovel I. Attentional biases in PTSD: more evidence for interference. Behav. Res. Ther. 2009;47(12):1050–1057. doi: 10.1016/j.brat.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Qin P., Liu Y., Shi J., Wang Y., Duncan N., Gong Q., Northoff G. Dissociation between anterior and posterior cortical regions during self-specificity and familiarity: a combined fMRI-meta-analytic study. Hum. Brain Mapp. 2012;33(1):154–164. doi: 10.1002/hbm.21201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P., Northoff G. How is our self related to midline regions and the default-mode network? NeuroImage. 2011;57(3):1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Rabellino D., Densmore M., Frewen P.A., Théberge J., Lanius R.A. The innate alarm circuit in post-traumatic stress disorder: conscious and subconscious processing of fear- and trauma-related cues. Psychiatry Res. – Neuroimaging. 2016;248:142–150. doi: 10.1016/j.pscychresns.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Rabellino D., D’Andrea W., Siegle G., Frewen P.A., Minshew R., Densmore M., Lanius R.A. Neural correlates of heart rate variability in PTSD during sub- and supraliminal processing of trauma-related cues. Hum. Brain Mapp. 2017;38(10):4898–4907. doi: 10.1002/hbm.23702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino D., Tursich M., Frewen P.A., Daniels J.K., Densmore M., Théberge J., Lanius R.A. Intrinsic connectivity networks in post-traumatic stress disorder during sub- and supraliminal processing of threat-related stimuli. Acta Psychiatr. Scand. 2015;132(5):365–378. doi: 10.1111/acps.12418. [DOI] [PubMed] [Google Scholar]
- Raichle M.E. The brain’s default mode network. Annu. Rev. Neurosci. 2015;38(1):433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Reuveni I., Bonne O., Giesser R., Shragai T., Lazarovits G., Isserles M., Levin N. Anatomical and functional connectivity in the default mode network of post-traumatic stress disorder patients after civilian and military-related trauma. Hum. Brain Mapp. 2016;37(2):589–599. doi: 10.1002/hbm.23051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D.C., Feldman M.E., Beckham J.C. Reliving, emotions, and fragmentation in the autobiographical memories of veterans diagnosed with PTSD. Appl. Cognitive Psychol. 2004;18(1):17–35. doi: 10.1002/acp.950. [DOI] [Google Scholar]
- Sartory G., Cwik J., Knuppertz H., Schürholt B., Lebens M., Seitz R.J., Schulze R. In search of the trauma memory: A meta-analysis of functional neuroimaging studies of symptom provocation in posttraumatic stress disorder (PTSD) PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0058150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J., Lui S., Meng Y., Zhu H., Qiu C., Gong Q., Zhang W. Alterations in low-level perceptual networks related to clinical severity in PTSD after an earthquake: a resting-state fMRI study. PLoS ONE. 2014;9(5) doi: 10.1371/journal.pone.0096834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger, C.D., 2010. State-Trait Anxiety Inventory. In: The Corsini Encyclopedia of Psychology. Hoboken, NJ, USA: John Wiley & Sons, Inc. https://doi.org/10.1002/9780470479216.corpsy0943.
- Spreng R.N., Mar R.A., Kim A.S.N. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J. Cognit. Neurosci. 2009;21(3):489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Sridharan D., Levitin D.J., Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. PNAS. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada R.K., King A.P., Welsh R.C., Garfinkel S.N., Wang X., Sripada C.S., Liberzon I. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom. Med. 2012;74(9):904–911. doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan K.E., Penny W.D., Daunizeau J., Moran R.J., Friston K.J. Bayesian model selection for group studies. NeuroImage. 2009;46(4):1004–1017. doi: 10.1016/j.neuroimage.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan K.E., Penny W.D., Moran R.J., den Ouden H.E.M., Daunizeau J., Friston K.J. Ten simple rules for dynamic causal modeling. NeuroImage. 2010;49(4):3099–3109. doi: 10.1016/j.neuroimage.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuwe C., Lanius R.A., Frewen P.A. Evidence for a dissociative subtype of PTSD by latent profile and confirmatory factor analyses in a civilian sample. Depress. Anxiety. 2012;29(8):689–700. doi: 10.1002/da.21944. [DOI] [PubMed] [Google Scholar]
- Sutherland K., Bryant R.A. Self-defining memories in post-traumatic stress disorder. Br. J. Clin. Psychol. 2005;44(4):591–598. doi: 10.1348/014466505X64081. [DOI] [PubMed] [Google Scholar]
- Svoboda E., McKinnon M.C., Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44(12):2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamietto M., de Gelder B. Neural bases of the non-conscious perception of emotional signals. Nat. Rev. Neurosci. 2010;11(10):697–709. doi: 10.1038/nrn2889. [DOI] [PubMed] [Google Scholar]
- Terpou B.A., Densmore M., Théberge J., Thome J., Frewen P., McKinnon M.C., Lanius R.A. The threatful self: midbrain functional connectivity to cortical midline and parietal regions during subliminal trauma-related processing in PTSD. Chronic Stress. 2019;3 doi: 10.1177/2470547019871369. 247054701987136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpou B.A., Densmore M., Thome J., Frewen P., McKinnon M.C., Lanius R.A. The innate alarm system and subliminal threat presentation in posttraumatic stress disorder: neuroimaging of the midbrain and cerebellum. Chronic Stress. 2019;3(1) doi: 10.1177/2470547018821496. 247054701882149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpou B.A., Harricharan S., McKinnon M.C., Frewen P., Jetly R., Lanius R.A. The effects of trauma on brain and body: a unifying role for the midbrain periaqueductal gray. J. Neurosci. Res. 2019;97(9):1110–1140. doi: 10.1002/jnr.24447. [DOI] [PubMed] [Google Scholar]
- Thome J., Terpou B.A., McKinnon M.C., Lanius R.A. The neural correlates of trauma-related autobiographical memory in posttraumatic stress disorder: a meta-analysis. Depress. Anxiety. 2019;37(4):321–345. doi: 10.1002/da.22977. [DOI] [PubMed] [Google Scholar]
- van der Kolk B.A. The body keeps the score: Brain, mind, and body in the healing of trauma. Viking. 2015 [Google Scholar]
- van der Kolk B.A., Roth S., Pelcovitz D., Sunday S., Spinazzola J. Disorders of extreme stress: The empirical foundation of a complex adaptation to trauma. J. Trauma. Stress. 2005;18(5):389–399. doi: 10.1002/jts.20047. [DOI] [PubMed] [Google Scholar]
- Wang T., Liu J., Zhang J., Zhan W., Li L., Wu M., Gong Q. Altered resting-state functional activity in posttraumatic stress disorder: a quantitative meta-analysis. Sci. Rep. 2016;6(1):1–14. doi: 10.1038/srep27131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber D.L. Information processing bias in post-traumatic stress disorder. Open Neuroimag. J. 2008;2:29–51. doi: 10.2174/1874440000802010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L.M., Liddell B.J., Kemp A.H., Bryant R.A., Meares R.A., Peduto A.S., Kemp A.H. Amygdala–prefrontal dissociation of subliminal and supraliminal fear. Hum. Brain Mapp. 2006;27:652–661. doi: 10.1002/hbm.20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf E.J., Miller M.W., Reardon A.F., Ryabchenko K.A., Castillo D., Freund R. A latent class analysis of dissociation and posttraumatic stress disorder: evidence for a dissociative subtype. Arch. Gen. Psychiatry. 2012;69(7):698–705. doi: 10.1001/archgenpsychiatry.2011.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R.Z., Zhang J.R., Qiu C.J., Meng Y.J., Zhu H.R., Gong Q.Y., Zhang W. Study on resting-state default mode network in patients with posttraumatic stress disorder after the earthquake. J. Sichuan Univ. (Medical Science Edition) 2011;42(3):397–400. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.