Abstract
Binary or Bin toxin produced by Lysinibacillus sphaericus is composed of BinA (42 kDa) and BinB (51 kDa) subunits. These work together to exert maximal toxicity against mosquito larvae via pore formation and induction of apoptosis. The C-terminal domains in both subunits are homologous to those of aerolysin-type β pore-forming toxins, including parasporin-2 (PS2). The latter is one of the Bacillus thuringiensis toxins that exhibits specific cytotoxicity against human cancer cells. The present study investigates the possible anticancer activity of Bin toxin using PS2 as a control. We demonstrate that treatment with a high concentration of trypsin-activated Bin inhibits cell proliferation in human cancer cells A549, Caco-2, HepG2, HK-1 and KKU-M055. In the most susceptible cells, HK-1, Bin toxin exposure led to morphological alterations, decreased migration, decreased adhesion activity and apoptosis induction. Although these effects necessitated high concentrations, they suggest that Bin toxin may be optimized as a novel potential cancer-therapeutic agent.
Keywords: Lysinibacillus sphaericus, Bin toxin, Parasporin-2, Anticancer effect, Human cancer cells, Apoptosis
Introduction
Binary toxin or Bin toxin is a mosquito larvicidal toxin produced by Lysinibacillus sphaericus (Ls) during sporulation. Bin toxin is composed of BinA (42 kDa) and BinB (51 kDa) subunits. Maximum activity against mosquito larvae is achieved when both subunits are present at equimolar amounts (Baumann et al. 1988; Berry 2012; Berry and Hindley 1987; Hindley and Berry 1987). The specificity of Bin toxin depends on the binding of BinB to the corresponding receptor in epithelial membranes of midgut cells, whereas BinA is proposed to be a toxic component (Charles et al. 1997; Lekakarn et al. 2015). After mosquito larvae are fed with Bin toxin at different doses, the midgut epithelial cells show several morphological characteristics of apoptosis such as mitochondrial swelling, chromatin condensation, cytoplasmic vacuolization, apoptotic cell formation as well as the activation of caspase-3 and caspase-9, suggesting that Bin toxin induces apoptosis via an intrinsic or mitochondrial pathway (Tangsongcharoen et al. 2015, 2017).
The three-dimensional structures of protoxins BinA and BinB and activated BinB have been solved by X-ray crystallography (Colletier 2016; Srisucharitpanit et al. 2014). The N-terminal domain is globular, and based on its structural similarities with sugar-binding proteins or lectins, it is proposed to be responsible for receptor recognition. The C-terminal domains of BinA and BinB show partial homology with those of aerolysin-type β pore-forming toxins including parasporin-2 (PS2) (Colletier 2016; Srisucharitpanit et al. 2014). The latter is produced by Bacillus thuringiensis (Bt) and is toxic to human cancer cells but non-toxic to normal cells. Cytological and biochemical observations suggest that PS2 is a pore-forming toxin (Akiba 2009). Similarly, activated BinA and BinB interact with membranes and form ion channels (Chooduang et al. 2018). These support the classification of both PS2 and Bin toxin as aerolysin-type-β-pore-forming toxins.
Although PS2 primarily changes the concentration of ions across target cell membranes, it also induced cell cycle arrest and caspase-dependent apoptotic cell death in various human cancer cell lines (Brasseur et al. 2015). Due to the structural and functional similarity between Bin proteins and PS2, we hypothesize that Bin toxin may also be toxic to human lung cancer cells. Indeed, anticancer activity was previously reported for Bin proteins extracted from L. sphaericus IAB872 (Luo et al. 2014), but whether the observed cytotoxicity was contributed by the BinA/BinB complex or any of the two subunits, in particular, was not described. Thus, the present study investigates the anticancer activity of Bin toxin, whether as individual subunits or as a mixture, against various human cancer cells. Bin-treated cancer cells showed morphological alterations, decreased cell migration and cell adhesion activity and apoptosis induction. Taken together, the findings in this study pave the way for the potential development of Bin toxin as a future cancer-therapeutic agent.
Materials and methods
Protein preparation
BinA and BinB proteins were produced as His-tagged proteins from E. coli BL21 (DE3) pLysS containing pRSET C-binA and pET28-binB, respectively and purified as described previously (Srisucharitpanit et al. 2012). Purified His-tagged BinA and BinB were activated with trypsin using a trypsin-to-toxin mass ratio of 1:10 and incubated at 37 °C for 2 h. The reactions were stopped by adding phenylmethylsulfonyl fluoride (PMSF) and the trypsin-activated proteins were purified by size-exclusion chromatography (Superdex 200 h 10/30 column, GE healthcare Life Science). The full-length parasporin-2 gene was de novo synthesized (GenScript Company, USA) based on the available protein sequence of parasporin-2 (PS2) (NCBI accession number AB099515.1) and cloned into pET-28b (+) to express as a His-tagged fusion protein using E. coli BL21(DE3) pLysS as a host strain. E. coli cells expressing His-tagged PS2 were grown in LB medium supplemented with 50 μg/mL of kanamycin and 34 μg/mL of chloramphenicol. Expression of His-tagged PS2 was induced by adding 0.2 mM IPTG to an exponential growth culture, and further incubated for 5 h at 18 °C with shaking at 250 rpm. Cells were harvested by centrifugation at 8000g at 4 °C for 10 min and cell pellet containing the protein inclusions was collected. The PS2 inclusions were dissolved with carbonate buffer (56 mM Na2CO3 pH 11.4), followed by centrifugation at 12,000g at 4 °C for 1 h to separate soluble and insoluble fractions. The soluble protein fraction was passed through HisTrapTM FF 5 mL column (GE Healthcare Life science) that had been equilibrated with the carbonate buffer and the unbound proteins were washed with carbonate buffer containing 25 mM imidazole. The bound protein was eluted with carbonate buffer containing 100 mM imidazole. Protein-containing fractions were pooled and concentrated by ultrafiltration at 4 °C using Amicon ultra centrifugal column (10-kDa cutoff). Purified His-tagged PS2 was activated with proteinase K using a proteinase K-to-toxin mass ratio of 1:100 and incubated at 37 °C for 30 min. The reactions were stopped by PMSF addition and the proteinase K-activated PS2 was purified by size-exclusion chromatography (Superdex 200 h 10/30 column, GE healthcare Life Science). Protein quality and quantity were estimated by SDS-PAGE and a Bradford assay, respectively.
Cell cultures
Human cancer cell lines including cholangiocarcinoma (KKU-M055) were purchased from the Japanese Collection of Research Bioresources Cell Bank (JCRB Cell Bank). Hepatocellular carcinoma (HepG2), epithelial lung carcinoma (A549), colon carcinoma (Caco-2), and nasopharyngeal carcinoma cells (HK-1) were kindly provided by Asst. Prof. Supeecha Kumkate, Dr. Patompon Wongtrakoongate, Dr. Amornrat Aroonnual and Prof. Maria Li Lung, respectively. KKU-M055 and A549 cells were cultured in Ham’s F12 medium (Gibco). HepG2 and Caco-2 were cultured in DMEM medium (Gibco). HK-1 cells were cultured in RPMI medium (Gibco). All cultured cells were cultured in the specified media supplemented with 10% fetal bovine serum and 1% penicillin streptomycin and were maintained in a 5% CO2 incubator at 37 °C.
Cell viability assay
Cells were seeded into the 96-well plates at 10,000 cells/well for 48 h before incubation with 500 μg/mL of BinA, BinB, BinA/BinB mixture (250 μg/mL of BinA and BinB), bovine serum albumin (BSA), and PS2 (20 μg/mL). After incubation for 48 h, cell morphologies were observed under an inverted microscope with 10 × objective lens and photographed with a digital camera. Then the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (Invitrogen) was added to the treated cells at a final concentration of 0.05 mg/mL and incubated at 37 °C for 3 h to generate formazan crystals. Finally, 100 μL DMSO (Merck) was added to dissolve the crystals and measured the absorbance at 540 nm using a microplate-spectrophotometer (Beckman Coulter).
Cell migration and adhesion assays
Cell migration was observed with a wound healing assay, based on the ability of cells to move into a scratched area (wound) of monolayer of cells so that they heal the wound. HK-1 cells were seeded at 100,000 cells/well in a 24-well plate and incubated for 48 h. Seeded cells were washed with PBS before being treated with Bin toxin (100 μg/mL), PS2 (2 μg/mL) and PBS (negative control) for 24 h. Treated cells were washed with PBS buffer, then cell monolayers were mechanically wounded with a 200 μL sterile pipette tip followed by adding migrating medium (0.1% fetal bovine serum, 1% penicillin streptomycin in RPMI). Cell migration was observed under a light microscope at the same position for 18 h. The percentage of cell migration was analyzed from different areas at 0 h and 18 h and compared with control treatment. Cell adhesion was detected via cell-Matrigel interaction. HK-1 cells after treated with Bin toxin (200 μg/mL) and PS2 (3 μg/mL) for 24 h were trypsinized and reseeded into Matrigel-coated plate (Corning™) and incubated for 48 h. Then, treated cells were washed with PBS buffer and non-adherent cells were removed by gently washing. Finally, the attached cells were measured using MTT assay. The percentage of cell adhesion was calculated from the absorbance at 540 nm compared with control treatment.
Flow cytometry
Apoptosis of Bin-treated HK-1 cells was evaluated using flow cytometry and Annexin V-FITC apoptosis kit (BD Biosciences). Briefly, HK-1 cells were treated with Bin toxin for 24 h, then trypsinized and harvested from a 6-well plate. The collected cells were washed with cold PBS buffer for 2 times followed by resuspension in Annexin V binding buffer (BD Biosciences). Then, approximately 105 cells were stained with FITC conjugated Annexin V and propidium iodide (PI) for 15 min at room temperature in dark. The stained cells were analysed using a BD Accuri C6 Plus flow cytometer (BD Biosciences). Apoptosis was measured as recommended by the manufacturer of the annexin V-FITC Detection Kit (BD Biosciences). Data were retrieved and analyzed using BD Accuri C6 Plus software (BD Biosciences).
Statistical analysis
All experiments were performed using at least three independent replicates. All data are represented as mean ± standard error of the mean (SEM) and analysed using the SPSS 24 software (SPSS Inc., Chicago, USA). One-way ANOVA test was used for the statistical analysis of multiple groups. Statistical significance was defined as P < 0.05.
Results
Bin toxin-induced morphological changes and inhibited cell proliferation of human cancer cells
To compare the anticancer activity of Bin and PS2 toxins, the full-length recombinant BinA, BinB, and PS2 proteins N-terminally-tagged with a hexa-histidine were expressed in E. coli BL21(DH3) pLysS. Their molecular masses were 42, 51, and 37 kDa, respectively, as expected (Fig. 1). Both BinA and BinB were expressed as soluble proteins using modified conditions described previously (Srisucharitpanit et al. 2012) whereas PS2 was expressed as inclusions, as in the native bacterial strain (Brasseur et al. 2015).
Fig. 1.
SDS-PAGE analysis of recombinant protein expression and activation. The recombinant BinA (a, lane 1), BinB (a, lane 3), and PS2 (b, lane 1) proteins were expressed as protoxin forms with molecular masses of 42, 51, and 37 kDa, respectively. BinA and BinB protoxins were activated by trypsin digestion, whereas PS2 inclusion protein was solubilized and activated by proteinase K digestion, giving the protein fragments of about 40 kDa for BinA (a, lane 2), 45 kDa for BinB (a, lane 4), and 30 kDa for PS2 (b, lane 2)
Since the cytotoxic activities of Bin and PS2 toxins require trypsin and proteinase K activation, respectively (Broadwell et al. 1990; Ito 2004), purified protoxins BinA and BinB were activated with trypsin to generate the expected protein fragments of about 40 and 45 kDa for BinA and BinB, respectively. Similarly, PS2 protein inclusions were solubilized in alkaline buffer followed by purification and activated by proteinase K to generate the 30-kDa active form (Fig. 1).
Either trypsin-activated Bin (500 µg/mL) or proteinase K-activated PS2 (20 µg/mL) was added to various human cancer cells from diverse tissues, e.g., A549, Caco-2, HepG2, HK-1 and KKU-M055, and morphological cell alterations were monitored using inverted microscopy. Cell shrinkage in all cell types was observed after treatment with either BinB or PS2, but it was less pronounced when using BinA and BinA/BinB mixture. However, in Caco-2 and HepG2 cells, these two latter treatments led to cell vacuolization and cell clumping, respectively. None of the above morphological changes were detected in cells treated with PBS or BSA (Fig. 2a) or protoxins of either Bin or PS2 (data not shown).
Fig. 2.
Morphological changes and cytotoxicity of various human cancer cells after exposure to activated Bin and PS2 proteins. A549, Caco-2, HK-1, HepG2, and KKU-M055 cells were treated with Bin toxin (BinA, BinB, BinA/BinB mixture) at 500 µg/mL compared to BSA (500 µg/mL), PBS (negative control), and PS2 (20 µg/mL) and morphological changes were observed under an inverted microscope with 10 × objective lens after 48 h of incubation (a) and cell viability was determined via MTT assay and percentage of cell viability is represented as a bar chart b. Data represent the mean of three independent experiments ± S.E.M. with n = 3. *P < 0.05; **P < 0.01, ***P < 0.001 compared to cells treated with PBS as a negative control
Next, the effect of toxin treatment on cell proliferation was assessed via an MTT assay. Proteinase K activated-PS2 (20 µg/mL) showed strong cytotoxicity against most tested human cancer cells (Fig. 2b). However, inhibition of cell proliferation by Bin toxin required high concentration (500 µg/mL) and the response was cell-dependent. HK-1 cells were the most sensitive to BinB and BinA/BinB treatment, with inhibition rates of 90.3% and 85.4%, respectively (Fig. 2b), therefore, HK-1 cell line was selected for further experiments. Dose–response experiments showed that only BinB protein exhibited a slight cytotoxic effect to HK-1 cells, with an IC50 value of 235 µg/mL, whereas no obvious cytotoxic effect was observed caused by BinA or the BinA/BinB mixture. In contrast, PS2 was highly cytotoxic to HK-1 cells, with IC50 of 0.52 µg/mL (data not shown).
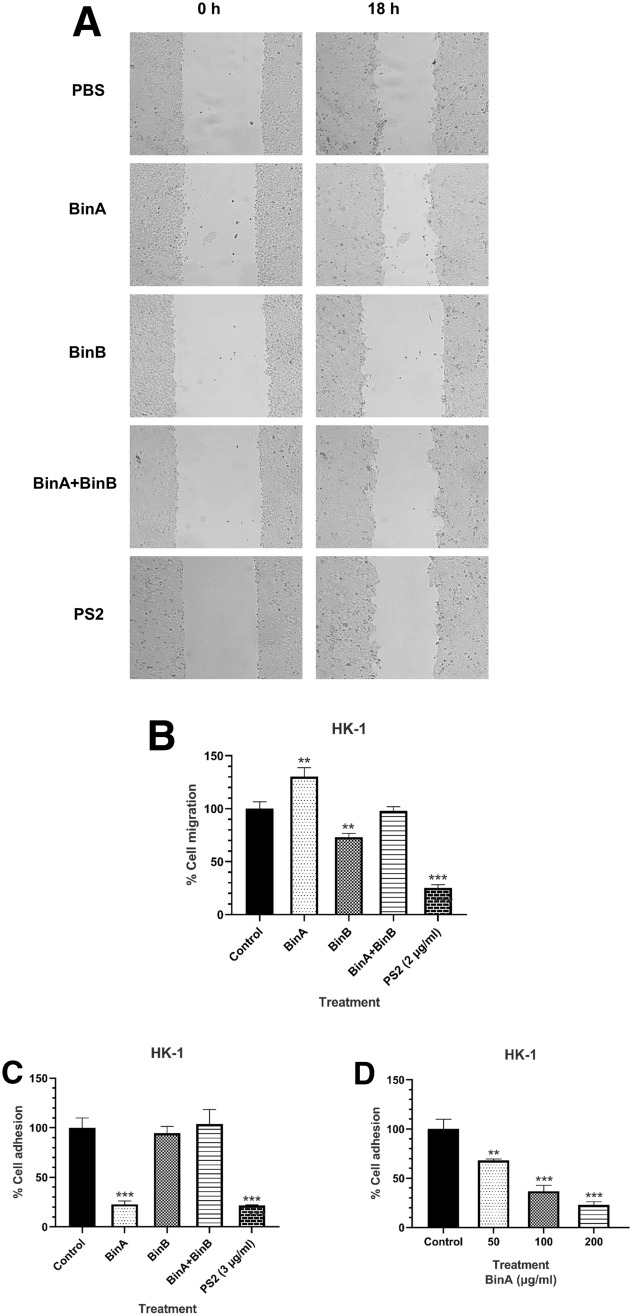
Bin toxin inhibited cancer cell migration and adhesion
HK-1 cells were also tested for migration and adhesion after Bin and PS2 toxin exposure. HK-1 cells were treated with Bin toxin at the sub-IC50 concentrations of 100 and 200 µg/mL for cell migration and cell adhesion, respectively. For cell migration, BinB and BinA proteins exhibited a slightly inhibitory and a stimulating effect, respectively, whereas the mixture BinA/BinB did not produce any effect, similar to PBS treatment. For cell adhesion, BinA produced a small inhibitory effect in a concentration-dependent manner (Fig. 3c, d), but neither BinB nor the mixture of BinA/BinB could elicit any inhibitory effect. In contrast, PS2 induced the strongest inhibitory effects on both cell migration and cell adhesion (Fig. 3b, c). These findings demonstrate that both Bin and PS2 toxins can inhibit HK-1 cells migration and adhesion, albeit at different levels.
Fig. 3.
Effects of Bin toxin on HK-1 cell migration and adhesion. HK-1 cells were treated with Bin toxin at sub-IC50 concentrations and then were used for migration/adhesion assays. Wound space was created by scratching the cell culture after toxin treatment for different time points and the scratched area was then imaged under a phase-contrast microscope (a) and the percentage of cell migration was calculated by comparison of the relative wound area change of initial treatment and 18 h treatment with untreated cells (b). The effect of Bin toxin on cell adhesion was monitored by measuring the cell viability via MTT assay of attached cells on a Matrigel-coated plate after incubated with different protein samples for 48 h (c). d Dose responses on the adhesion of HK-1 cells after BinA treatment. Data represent the mean of three independent experiments ± S.E.M. with n = 3. *P < 0.05; **P < 0.01, ***P < 0.001 compared to cells treated with PBS as a negative control
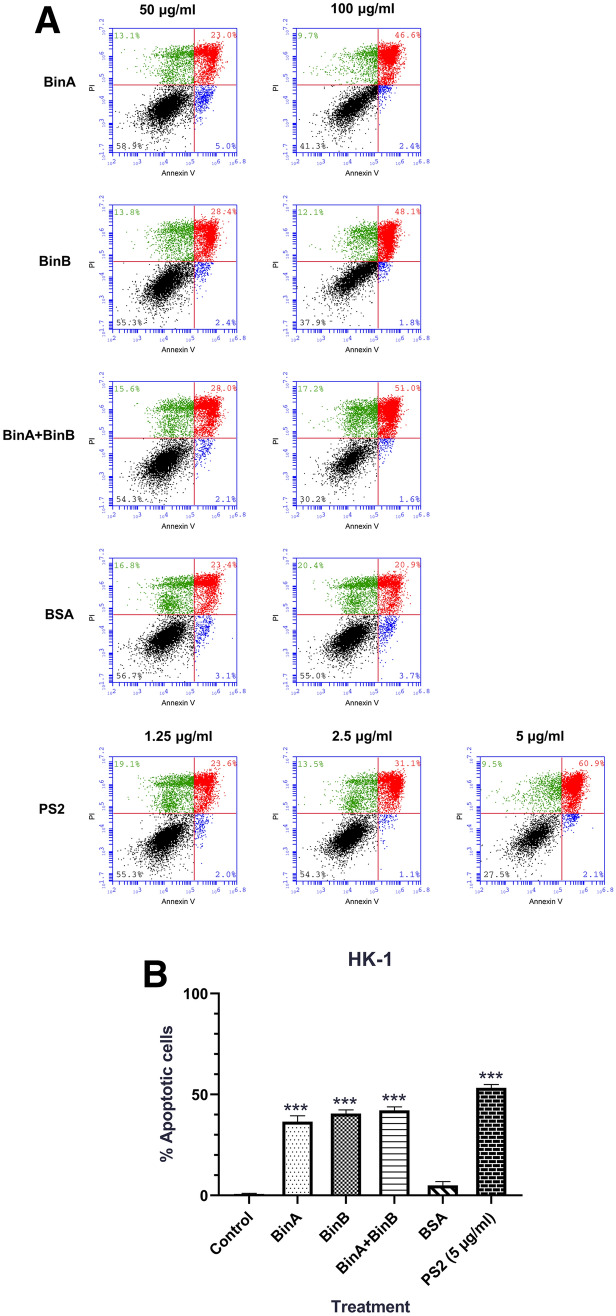
Bin toxin induced cancer cell apoptosis in HK-1 cell line
To investigate further the underlying mechanism of cell cytotoxicity, Bin-treated HK-1 cells were assessed for cell apoptosis by staining with FITC conjugated Annexin V and propidium iodide (PI) followed by flow cytometry. Following treatment with a high concentration (100 µg/mL) of either BinA or BinB protein, both early and late apoptosis was observed in Annexin‐V/PI-stained cells. However, the mixture of BinA/BinB did not induce apoptosis (Fig. 4a, b). PS2 showed strong apoptosis induction at low concentration (5 µg/mL) with 63% apoptotic cells compared to PBS treatment (Fig. 4b).
Fig. 4.
Apoptosis analysis of HK-1 cells after Bin and PS2 exposure using flow cytometry assay. HK-1 cells were treated with BinA, BinB and combination of BinA and BinB (at concentrations of 50 and 100 μg/mL) compared with PS2 (at concentrations of 1.25, 2.5, and 5 μg/mL) and BSA (at concentrations of 50 and 100 μg/mL). After 24 h of incubation, cells were stained with Annexin V-FITC and PI (Annexin V-FITC apoptosis kit: BD Biosciences) followed by flow cytometry analysis. a Dot plots of Annexin-V/PI staining are shown in different sample groups, presenting intact cells at lower-left quadrant, early apoptotic cells at lower-right quadrant, late apoptotic or necrotic cells at upper-right quadrant, and necrotic cells at upper-left quadrant. b Bar chart showing the percentage of total apoptotic cells after treatment with Bin (100 μg/mL) and PS2 (5 μg/mL) using PBS as a baseline control. Data represent the mean of three independent experiments ± S.E.M. with n = 3. *P < 0.05; **P < 0.01, ***P < 0.001 compared to cells treated with PBS as a negative control
Discussion
The structures of BinA and BinB, in either protoxin or activated form, share structural homology with aerolysin-type β pore-forming toxins (Colletier 2016; Srisucharitpanit et al. 2014). Among the members of aerolysin-type β pore-forming toxins, parasporin-2 or PS2 from B. thuringiensis has been suggested as a new therapeutic agent for the treatment of cancer due to the strong and selective cytotoxicity against human cancer cells without affecting normal cells (Brasseur et al. 2015; Kitada 2006). It is thus of interest to explore whether Bin toxin is cytotoxic against human cancer cells. Our results show that Caco-2, HepG2, HK-1 and KKU-M055 are susceptible to Bin toxin. Although the cytotoxicity assays were performed using a high concentration (500 µg/mL), exposure to bovine serum albumin (BSA) at the same concentration did not affect cell viability or morphology. This indicates that the observed cytotoxic effects of Bin toxin are specific to Bin activity, and not merely the result of osmotic imbalance that may interfere with cell homeostasis and proliferation.
Herein we found that BinB alone was toxic against A549, Caco-2, HepG2, HK-1 and KKU-M055 cells, although at a concentration much higher than the one used against mosquito larvae (Baumann et al. 1988; Berry 2012; Berry and Hindley 1987; Hindley and Berry 1987). In A549 cells, the mixture of BinA/BinB was slightly more toxic than BinB alone, but the difference is not significant. In general, we found that the mixture of BinA/BinB produced no effect in cells when compared to BinA or BinB. It has been reported that BinA and BinB, after trypsin activation, form a heterodimeric complex in solution (Surya et al. 2016). This complex may be toxic against insect cells that have the specific Bin receptor, but not toxic against cancer cells. This makes sense if high toxicity requires previous interaction with a receptor. When cancer cells are exposed to the Bin toxin, it is the individual subunits that are more toxic whereas in the mixture, either of the subunits is inactivated by complex formation. We have reported previously that when Culex larval gut cells are exposed to either BinB or BinA alone, BinB is detected both on the cell membrane and inside the cytoplasm, whereas BinA is detected only on the cell membrane. However, when exposed to both subunits, BinA and BinB co-localized both on the cell surface and in the cytoplasm, indicating that BinB plays an important role in the translocation and internalization of BinA into susceptible larval gut cells (Lekakarn et al. 2015). Thus, the stronger cytotoxicity of BinB towards human cancer cells than BinA may be attributed by the higher capability of BinB to translocate and internalize into the target cancer cells, whereas in the formation of a complex BinA/BinB may have the effect of inactivating BinB, in the absence of a specific receptor.
Although the structures of BinA and BinB are highly similar, the different susceptibility of cancer cells when challenged with different Bin components may be attributed by the diverse parts of their structures. One of the major structural differences of BinA and BinB is the presence of a longer insertion peptide and aromatic amino acids in the N-terminal domain of BinB (Colletier 2016). This part of BinB may play an important role in receptor binding possibly via glycoprotein or glycolipid recognition at the plasma membrane of cancer cells. PS2 was much more cytotoxic than Bin toxin against several human cancer cells, including HepG2 and Caco-2, tested in the previous studies (Brasseur et al. 2015; Kitada 2006; Luo et al. 2014) and A549, HK-1, and KKU-M055, tested herein. Since Bin toxin is highly specific to certain mosquito species and this specificity depends on cell membrane receptor recognition, it is likely that the lower toxicity observed in Bin toxin is due to its lower binding affinity to cancer cell receptors. The receptors of Bin toxin have been identified as α-glucosidases and found in three species: Cpm1 for Culex pipiens maltase 1 (Darboux et al. 2001; Silva-Filha et al. 1999), Cqm1 for Cx. quinquefasciatus maltase 1 (Romao et al. 2006) and Agm3 for An. gambiae maltase 3 (Opota et al. 2008). Aedes aegypti larvae also possess an orthologue of the Bin toxin receptor, Aam1 protein (Ae. aegypti maltase 1), but it is not able to bind to Bin toxin (Ferreira et al. 2010). These receptors are membrane proteins bound to the mosquito gut epithelium through a GPI anchor, and differences in these receptors are believed to be the crucial factor in determining specificity. The specific receptors and lipid composition of cancer cells susceptible to PS2 and Bin toxins are unknown. For PS2 toxin, lipids, GPI-anchored proteins or sugar chains might be indispensable for toxin association, oligomerization, translocation and cytocidal activity. The PS2 toxin appears to bind specifically to the plasma membrane of susceptible cells, causing a rapid increase in membrane permeability (Kitada 2006). Previous study reported that GPI anchored-proteins expressed on cancer cell membrane surface serve as a specificity determinant of PS2 (Abe et al. 2017). It is thus likely that different GPI-anchored proteins present on cancer cell membranes also play a crucial role in determining the selective cytotoxicity of Bin toxin against cancer cells. As both PS2 and Bin toxins recognize a limited number of cancer cells, the future determination of the specific receptors on cancer cells will contribute to the fundamental mechanism of cytotoxicity and applications as anticancer therapies.
In addition, Bin toxin could induce morphological changes of treated cells including cell shrinkage, vacuolization and cell clumping probably by the effects of toxin on the osmotic imbalance through pore formation, internalization and cytoskeleton interference (Sekimata et al. 1999). Moreover, after Bin internalization inside the epithelial gut cells of mosquito larvae, some morphological changes characteristics of apoptosis as well as caspase-3/-9 activation were observed, suggesting that Bin can induce apoptosis via intrinsic pathway (Tangsongcharoen et al. 2015). Thus, the internalization of Bin toxin components into the cancer cells as well as intracellular localization need to be elucidated to gain insights into the underlying mechanism of anticancer activity.
In addition, following individual BinA and BinB treatments, cell apoptosis was observed by flow cytometry. However, the BinA/BinB mixture did not induce cell apoptosis. We also found that PS2 induced apoptosis in HK-1 cells which is consistent with a previous report (Brasseur et al. 2015). It is also possible that apoptosis may be caused by the ability of Bin and PS2 to form small channels in the cancer cell membranes as previously reported in aerolysin-induced apoptosis of T lymphomas via small channel formation (Nelson et al. 1999). Moreover, BinB toxin showed antimigration and antiadhesion activities, whereas BinA showed strong antiadhesion activity but promoting cell migration in HK-1 cells. The discrepancy of these activities between BinA and BinB is unclear and remains to be investigated further.
In conclusion, Bin toxin, when used at high concentration, induces cytotoxicity towards various human cancer cells and cell morphology alteration, inhibits cell proliferation, and possibly induces apoptosis in HK-1 cells. Although the toxicity of Bin toxin towards human cancer cells is much lower than that of PS2 toxin, it may be possible to engineer its sequence so that it is capable of binding to specific types of cancer cells.
Acknowledgements
This work was supported by Thailand Research Fund, Mahidol University and Synchrotron Light Research Institute (Public Organization) (BRG5980016) (to PB), Thailand Graduated Institute of Science and Technology (TGIST) (to WC).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abe Y, Inoue H, Ashida H, Maeda Y, Kinoshita T, Kitada S. Glycan region of GPI anchored-protein is required for cytocidal oligomerization of an anticancer parasporin-2, Cry46Aa1 protein, from Bacillus thuringiensis strain A1547. J Invertebr Pathol. 2017;142:71–81. doi: 10.1016/j.jip.2016.11.008. [DOI] [PubMed] [Google Scholar]
- Akiba T, et al. Crystal structure of the parasporin-2 Bacillus thuringiensis toxin that recognizes cancer cells. J Mol Biol. 2009;386:121–133. doi: 10.1016/j.jmb.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Baumann L, Broadwell AH, Baumann P. Sequence analysis of the mosquitocidal toxin genes encoding 51.4- and 41.9-kilodalton proteins from Bacillus sphaericus 2362 and 2297. J Bacteriol. 1988;170:2045–2050. doi: 10.1128/JB.170.5.2045-2050.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C. The bacterium, Lysinibacillus sphaericus, as an insect pathogen. J Invertebr Pathol. 2012;109:1–10. doi: 10.1016/j.jip.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Berry C, Hindley J. Bacillus sphaericus strain 2362: identification and nucleotide sequence of the 41.9 kDa toxin gene. Nucleic Acids Res. 1987;15:5891. doi: 10.1093/nar/15.14.5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasseur K, Auger P, Asselin E, Parent S, Cote JC, Sirois M. Parasporin-2 from a new Bacillus thuringiensis 4r2 strain induces caspases activation and apoptosis in human cancer cells. PLoS ONE. 2015;10:e0135106. doi: 10.1371/journal.pone.0135106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwell AH, Baumann L, Baumann P. The 42- and 51-kilodalton mosquitocidal proteins of Bacillus sphaericus 2362: construction of recombinants with enhanced expression and in vivo studies of processing and toxicity. J Bacteriol. 1990;172:2217–2223. doi: 10.1128/JB.172.5.2217-2223.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles JF, Silva-Filha MH, Nielsen-LeRoux C, Humphreys MJ, Berry C. Binding of the 51- and 42-kDa individual components from the Bacillus sphaericus crystal toxin to mosquito larval midgut membranes from Culex and Anopheles sp. (Diptera: Culicidae) FEMS Microbiol Lett. 1997;156:153–159. doi: 10.1016/S0378-1097(97)00419-9. [DOI] [PubMed] [Google Scholar]
- Chooduang S, Surya W, Torres J, Boonserm P. An aromatic cluster in Lysinibacillus sphaericus BinB involved in toxicity and proper in-membrane folding. Arch Biochem Biophys. 2018;660:29–35. doi: 10.1016/j.abb.2018.10.006. [DOI] [PubMed] [Google Scholar]
- Colletier JP, et al. De novo phasing with X-ray laser reveals mosquito larvicide BinAB structure. Nature. 2016;539:43–47. doi: 10.1038/nature19825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darboux I, Nielsen-LeRoux C, Charles JF, Pauron D. The receptor of Bacillus sphaericus binary toxin in Culex pipiens (Diptera: Culicidae) midgut: molecular cloning and expression. Insect Biochem Mol Biol. 2001;31:981–990. doi: 10.1016/S0965-1748(01)00046-7. [DOI] [PubMed] [Google Scholar]
- Ferreira LM, Romao TP, de-Melo-Neto OP, Silva-Filha MH. The orthologue to the Cpm1/Cqm1 receptor in Aedes aegypti is expressed as a midgut GPI-anchored alpha-glucosidase, which does not bind to the insecticidal binary toxin. Insect Biochem Mol Biol. 2010;40:604–610. doi: 10.1016/j.ibmb.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Hindley J, Berry C. Identification, cloning and sequence analysis of the Bacillus sphaericus 1593 41.9 kD larvicidal toxin gene. Mol Microbiol. 1987;1:187–194. doi: 10.1111/j.1365-2958.1987.tb00511.x. [DOI] [PubMed] [Google Scholar]
- Ito A, et al. A Bacillus thuringiensis crystal protein with selective cytocidal action to human cells. J Biol Chem. 2004;279:21282–21286. doi: 10.1074/jbc.M401881200. [DOI] [PubMed] [Google Scholar]
- Kitada S, et al. Cytocidal actions of parasporin-2, an anti-tumor crystal toxin from Bacillus thuringiensis. J Biol Chem. 2006;281:26350–26360. doi: 10.1074/jbc.M602589200. [DOI] [PubMed] [Google Scholar]
- Lekakarn H, Promdonkoy B, Boonserm P. Interaction of Lysinibacillus sphaericus binary toxin with mosquito larval gut cells: binding and internalization. J Invertebr Pathol. 2015;132:125–131. doi: 10.1016/j.jip.2015.09.010. [DOI] [PubMed] [Google Scholar]
- Luo W, Liu C, Zhang R, He J, Han B. Anticancer activity of binary toxins from Lysinibacillus sphaericus IAB872 against human lung cancer cell line A549. Nat Prod Commun. 2014;9:107–110. [PubMed] [Google Scholar]
- Nelson KL, Brodsky RA, Buckley JT. Channels formed by subnanomolar concentrations of the toxin aerolysin trigger apoptosis of T lymphomas. Cell Microbiol. 1999;1:69–74. doi: 10.1046/j.1462-5822.1999.00009.x. [DOI] [PubMed] [Google Scholar]
- Opota O, Charles JF, Warot S, Pauron D, Darboux I. Identification and characterization of the receptor for the Bacillus sphaericus binary toxin in the malaria vector mosquito, Anopheles gambiae. Comp Biochem Physiol. 2008;149:419–427. doi: 10.1016/j.cbpb.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Romao TP, de Melo Chalegre KD, Key S, Ayres CF, Fontes de Oliveira CM, de-Melo-Neto OP, Silva-Filha MH. A second independent resistance mechanism to Bacillus sphaericus binary toxin targets its alpha-glucosidase receptor in Culex quinquefasciatus. FEBS J. 2006;273:1556–1568. doi: 10.1111/j.1742-4658.2006.05177.x. [DOI] [PubMed] [Google Scholar]
- Sekimata M, Kabuyama Y, Emori Y, Homma Y. Morphological changes and detachment of adherent cells induced by p122, a GTPase-activating protein for Rho. J Biol Chem. 1999;274:17757–17762. doi: 10.1074/jbc.274.25.17757. [DOI] [PubMed] [Google Scholar]
- Silva-Filha MH, Nielsen-LeRoux C, Charles JF. Identification of the receptor for Bacillus sphaericus crystal toxin in the brush border membrane of the mosquito Culex pipiens (Diptera: Culicidae) Insect Biochem Mol Biol. 1999;29:711–721. doi: 10.1016/s0965-1748(99)00047-8. [DOI] [PubMed] [Google Scholar]
- Srisucharitpanit K, Inchana P, Rungrod A, Promdonkoy B, Boonserm P. Expression and purification of the active soluble form of Bacillus sphaericus binary toxin for structural analysis. Protein Expr Purif. 2012;82:368–372. doi: 10.1016/j.pep.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Srisucharitpanit K, Yao M, Promdonkoy B, Chimnaronk S, Tanaka I, Boonserm P. Crystal structure of BinB: a receptor binding component of the binary toxin from Lysinibacillus sphaericus. Proteins. 2014;82:2703–2712. doi: 10.1002/prot.24636. [DOI] [PubMed] [Google Scholar]
- Surya W, Chooduang S, Choong YK, Torres J, Boonserm P. Binary toxin subunits of Lysinibacillus sphaericus are monomeric and form heterodimers after in vitro activation. PLoS ONE. 2016;11:e0158356. doi: 10.1371/journal.pone.0158356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangsongcharoen C, Chomanee N, Promdonkoy B, Boonserm P. Lysinibacillus sphaericus binary toxin induces apoptosis in susceptible Culex quinquefasciatus larvae. J Invertebr Pathol. 2015;128:57–63. doi: 10.1016/j.jip.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Tangsongcharoen C, Jupatanakul N, Promdonkoy B, Dimopoulos G, Boonserm P. Molecular analysis of Culex quinquefasciatus larvae responses to Lysinibacillus sphaericus Bin toxin. PLoS ONE. 2017;12:e0175473. doi: 10.1371/journal.pone.0175473. [DOI] [PMC free article] [PubMed] [Google Scholar]